Intranasal Immunization with Nasal Immuno-Inducible Sequence-Fused Antigens Elicits Antigen-Specific Antibody Production
Abstract
1. Introduction
2. Results
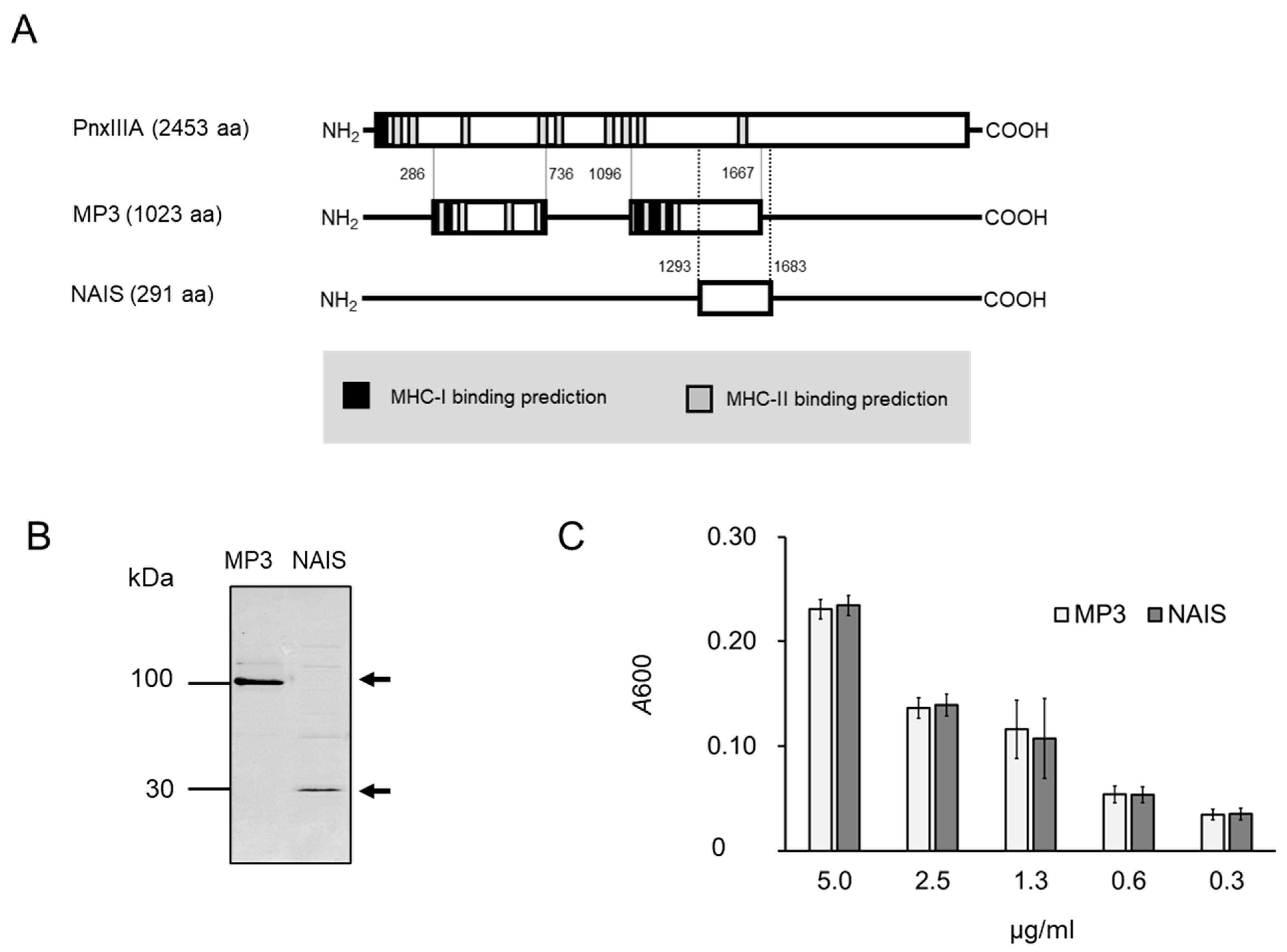
2.1. Vaccine Design and Cytotoxicity
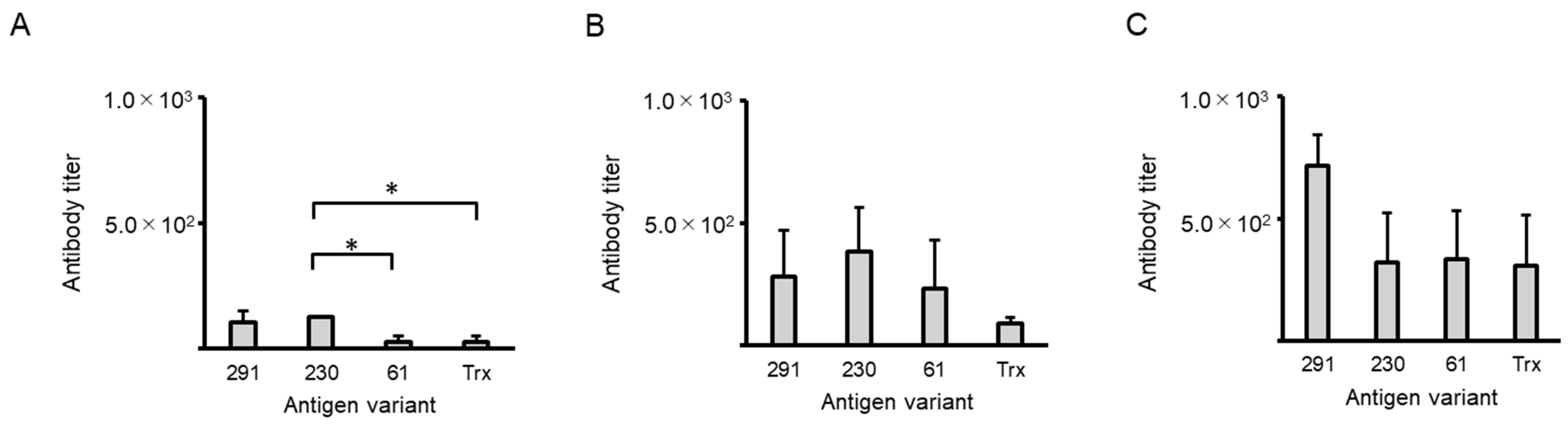
2.2. Antibody Titers Against Trx
2.3. Antibody Titers Against NAIS
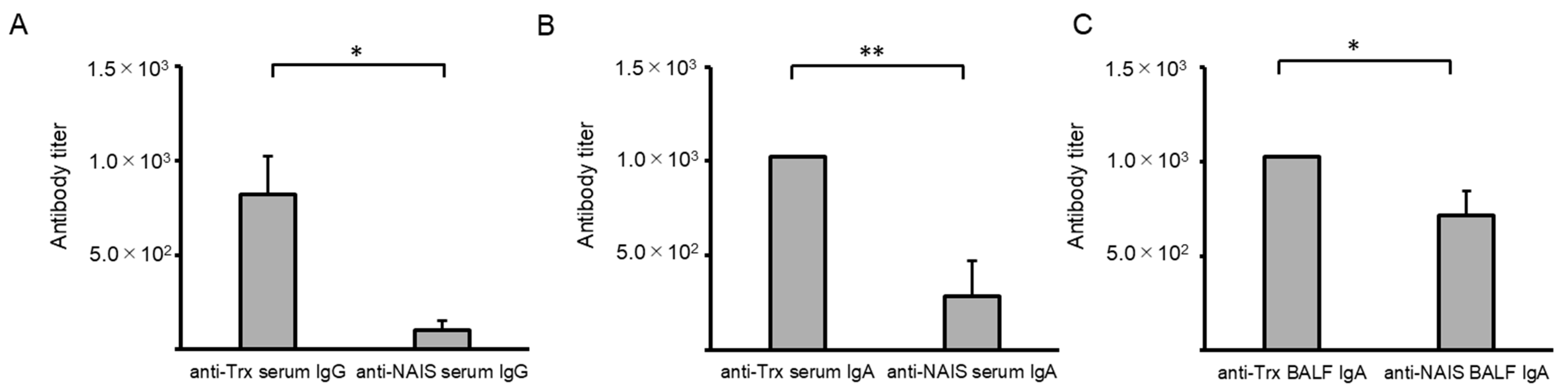
2.4. Comparison of Anti-Trx and Anti-NAIS Antibody Titers
3. Discussion
4. Materials and Methods
4.1. Intranasal Immunization Materials
4.2. Cytotoxicity Assay of NAIS Toward L929 Cells
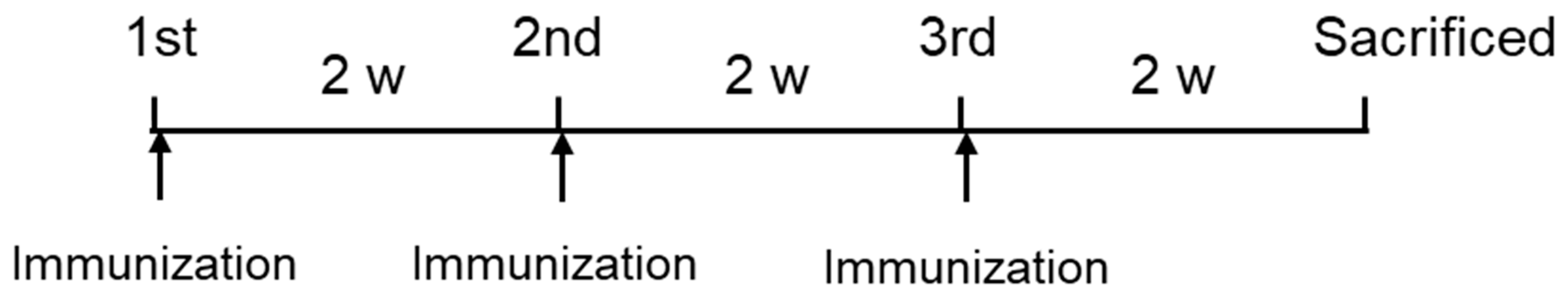
4.3. Intranasal Immunization of NAIS Fused Protein in Mice
4.4. ELISA
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakahashi-Ouchida, R.; Fujihashi, K.; Kurashima, Y.; Yuki, Y.; Kiyono, H. Nasal vaccines: Solutions for respiratory infectious diseases. Trends Mol. Med. 2023, 29, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Kehagia, E.; Papakyriakopoulou, P.; Valsami, G. Advances in intranasal vaccine delivery: A promising non-invasive route of immunization. Vaccine 2023, 41, 3589–3603. [Google Scholar] [CrossRef]
- Carter, N.J.; Curran, M.P. Live attenuated influenza vaccine (FluMist®; Fluenz™): A review of its use in the prevention of seasonal influenza in children and adults. Drugs 2011, 71, 1591–1622. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Ye, J.; Perez, D.R.; Metzger, D.W. Seasonal FluMist Vaccin Induces Cross-React T Cell Immun H1N1 2009 influenza and secondary bacterial infections. J. Immunol. 2011, 186, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Ainai, A.; Suzuki, T.; Hasegawa, H. Intranasal inactivated influenza vaccines for the prevention of seasonal influenza epidemics. Expert. Rev. Vaccines 2018, 17, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.L.; Staats, H.F. Cytokines: The future of intranasal vaccine adjuvants. Clin. Dev. Immunol. 2011, 289597. [Google Scholar] [CrossRef] [PubMed]
- Odelram, H.; Granström, M.; Hedenskog, S.; Duchén, K.; Björkstén, B. Immunoglobulin E and G responses to pertussis toxin after booster immunization in relation to atopy, local reactions and aluminium content of the vaccines. Pediatr. Allergy Immunol. 1994, 5, 118–123. [Google Scholar] [CrossRef]
- Fernández-Tejada, A.; Chea, E.K.; George, C.; Pillarsetty, N.; Gardner, J.R.; Livingston, P.O.; Ragupathi, G.; Lewis, J.S.; Tan, D.S.; Gin, D.Y. Development of a minimal saponin vaccine adjuvant based on QS-21. Nat. Chem. 2014, 6, 635–643. [Google Scholar] [CrossRef]
- Coughlan, L.; Kremer, E.J.; Shayakhmetov, D.M. Adenovirus-based vaccines-a platform for pandemic preparedness against emerging viral pathogens. Mol. Ther. 2022, 30, 1822–1849. [Google Scholar] [CrossRef]
- Filipić, B.; Pantelić, I.; Nikolić, I.; Majhen, D.; Stojić-Vukanić, Z.; Savić, S.; Krajišnik, D. Nanoparticle-Based Adjuvants and Delivery Systems for Modern Vaccines. Vaccines 2023, 11, 1172. [Google Scholar] [CrossRef]
- Sasaki, H.; Ishikawa, H.; Kojima, K.; Itoh, M.; Matsumoto, T.; Itoh, T.; Hosomi, O.; Kawamoto, E. Intranasal immunization with a non-adjuvanted adhesive protein descended from Pasteurella pneumotropica and its preventive efficacy against opportunistic infection in mice. Vaccine 2013, 31, 5729–5735. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Kawamoto, E.; Tanaka, Y.; Sawada, T.; Kunita, S.; Yagami, K. Identification and characterization of hemolysin-like proteins similar to RTX toxin in Pasteurella pneumotropica. J. Bacteriol. 2009, 191, 3698–3705. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Ishikawa, H.; Sato, T.; Sekiguchi, S.; Amao, H.; Kawamoto, E.; Matsumoto, T.; Shirama, K. Molecular and virulence characteristics of an outer membrane-associated RTX exoprotein in Pasteurella pneumotropica. BMC Microbiol. 2011, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Kennedy, S.C.; Lindestam Arlehamn, C.S.; Goldberg, M.F.; Saini, N.K.; Xu, J.; Paul, S.; Hegde, S.S.; Blanchard, J.S.; Chan, J.; et al. Identification of Mycobacterial RplJ/L10 and RpsA/S1 Proteins as Novel Targets for CD4+ T Cells. Infect. Immun. 2017, 85, e01023-16. [Google Scholar] [CrossRef] [PubMed]
- McSorley, S.J.; Ehst, B.D.; Yu, Y.; Gewirtz, A.T. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J. Immunol. 2002 169, 3914–3919. [CrossRef]
- Ishii, K.J.; Uematsu, S.; Akira, S. ‘Toll’ gates for future immunotherapy. Curr. Pharm. Des. 2006, 12, 4135–4142. [Google Scholar] [CrossRef]
- Aguilar, J.C.; Rodríguez, E.G. Vaccine adjuvants revisited. Vaccine 2007, 25, 3752–3762. [Google Scholar] [CrossRef]
- Pasquale, A.D.; Preiss, S.; Silva, F.T.D.; Garçon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Bernal, A.M.; Sosa, F.N.; Todero, M.F.; Montagna, D.R.; Vermeulen, M.E.; Fernández-Brando, R.J.; Ramos, M.V.; Errea, A.J.; Rumbo, M.; Palermo, M.S. Nasal immunization with H7 flagellin protects mice against hemolytic uremic syndrome secondary to Escherichia coli O157:H7 gastrointestinal infection. Front. Cell Infect. Microbiol. 2023, 13, 1143918. [Google Scholar] [CrossRef]
- Detienne, S.; Welsby, I.; Collignon, C.; Wouters, S.; Coccia, M.; Delhaye, S.; Van Maele, L.; Thomas, S.; Swertvaegher, M.; Detavernier, A.; et al. Central role of CD169+ lymph node resident macrophages in the adjuvanticity of the QS-21 component of AS01. Sci. Rep. 2016, 6, 39475. [Google Scholar] [CrossRef] [PubMed]
- Coulter, A.; Harris, R.; Davis, R.; Drane, D.; Cox, J.; Ryan, D.; Sutton, P.; Rockman, S.; Pearse, M. Intranasal vaccination with iscomatrix adjuvanted influenza vaccine. Vaccine 2003, 21, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Pearse, M.J.; Drane, D. ISCOMATRIX adjuvant for antigen delivery. Adv. Drug Deliv. Rev. 2005, 57, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Hegde, V.L.; Yanamandra, A.V.; O’Hara, M.P.; Keegan, B.; Jones, K.M.; Strych, U.; Bottazzi, M.E.; Zhan, B.; Sastry, K.J.; et al. Mucosal vaccination with recombinant Tm-WAP49 protein induces protective humoral and cellular immunity against experimental trichuriasis in AKR mice. Front. Immunol. 2022, 13, 800295. [Google Scholar] [CrossRef] [PubMed]
- Lycke, N.; Lebrero-Fernández, C. ADP-ribosylating enterotoxins as vaccine adjuvants. Curr. Opin. Pharmacol. 2018, 41, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Crothers, J.W.; Norton, E.B. Recent advances in enterotoxin vaccine adjuvants. Curr. Opin. Immunol. 2023, 85, 102398. [Google Scholar] [CrossRef]
- Patel, R.B.; Ye, M.; Carlson, P.M.; Jaquish, A.; Zangl, L.; Ma, B.; Wang, Y.; Arthur, I.; Xie, R.; Brown, R.J.; et al. Development of an in situ cancer vaccine via combinational radiation and bacterial-membrane-coated nanoparticles. Adv. Mater. 2019, 31, e1902626. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, R.; Nie, G. Nanocarriers based on bacterial membrane materials for cancer vaccine delivery. Nat. Protoc. 2022, 17, 2240–2274. [Google Scholar] [CrossRef]
- Gao, X.; Feng, Q.; Wang, J.; Zhao, X. Bacterial outer membrane vesicle-based cancer nanovaccines. Cancer Biol. Med. 2022, 19, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.; Sette, A. Generating quantitative models describing the sequence specificity of biological processes with the stabilized matrix method. BMC Bioinform. 2005, 6, 132. [Google Scholar] [CrossRef]
- Nielsen, M.; Lundegaard, C.; Lund, O. Prediction of MHC class II binding affinity using SMM-align, a novel stabilization matrix alignment method. BMC Bioinform. 2007, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Kunimine, S.; Takai, T.; Kamijo, S.; Maruyama, N.; Kimitsu, T.; Masutani, Y.; Yoshimura, T.; Suchiva, P.; Shimizu, S.; Ogawa, H.; et al. Epicutaneous vaccination with protease inhibitor-treated papain prevents papain-induced Th2-mediated airway inflammation without inducing Th17 in mice. Biochem. Biophys. Res. Commun. 2021, 546, 192–199. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, H.; Suzuki, Y.; Morimoto, K.; Takeda, K.; Uchida, K.; Iyoda, M.; Ishikawa, H. Intranasal Immunization with Nasal Immuno-Inducible Sequence-Fused Antigens Elicits Antigen-Specific Antibody Production. Int. J. Mol. Sci. 2024, 25, 12828. https://doi.org/10.3390/ijms252312828
Sasaki H, Suzuki Y, Morimoto K, Takeda K, Uchida K, Iyoda M, Ishikawa H. Intranasal Immunization with Nasal Immuno-Inducible Sequence-Fused Antigens Elicits Antigen-Specific Antibody Production. International Journal of Molecular Sciences. 2024; 25(23):12828. https://doi.org/10.3390/ijms252312828
Chicago/Turabian StyleSasaki, Hiraku, Yoshio Suzuki, Kodai Morimoto, Kazuyoshi Takeda, Koichiro Uchida, Masayuki Iyoda, and Hiroki Ishikawa. 2024. "Intranasal Immunization with Nasal Immuno-Inducible Sequence-Fused Antigens Elicits Antigen-Specific Antibody Production" International Journal of Molecular Sciences 25, no. 23: 12828. https://doi.org/10.3390/ijms252312828
APA StyleSasaki, H., Suzuki, Y., Morimoto, K., Takeda, K., Uchida, K., Iyoda, M., & Ishikawa, H. (2024). Intranasal Immunization with Nasal Immuno-Inducible Sequence-Fused Antigens Elicits Antigen-Specific Antibody Production. International Journal of Molecular Sciences, 25(23), 12828. https://doi.org/10.3390/ijms252312828







