Anemia and Mineral Bone Disorder in Kidney Disease Patients: The Role of FGF-23 and Other Related Factors
Abstract
1. Introduction
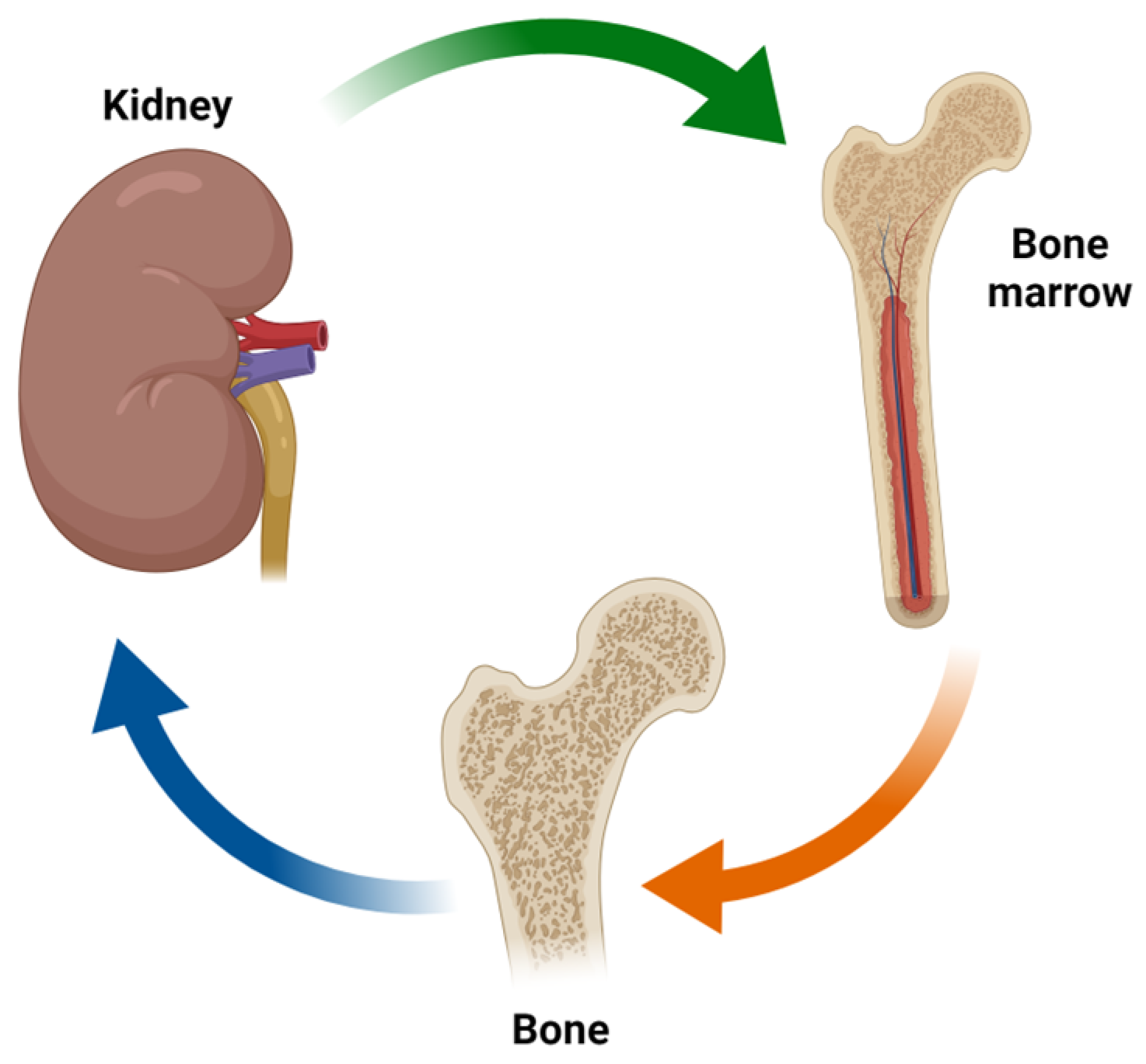
2. Factors Involved in the Kidney/Bone Marrow/Bone Axis
2.1. EPO: Synthesis, Regulation and Pathways
2.2. CKD-MBD and Anemia: Different Patterns of Presentation
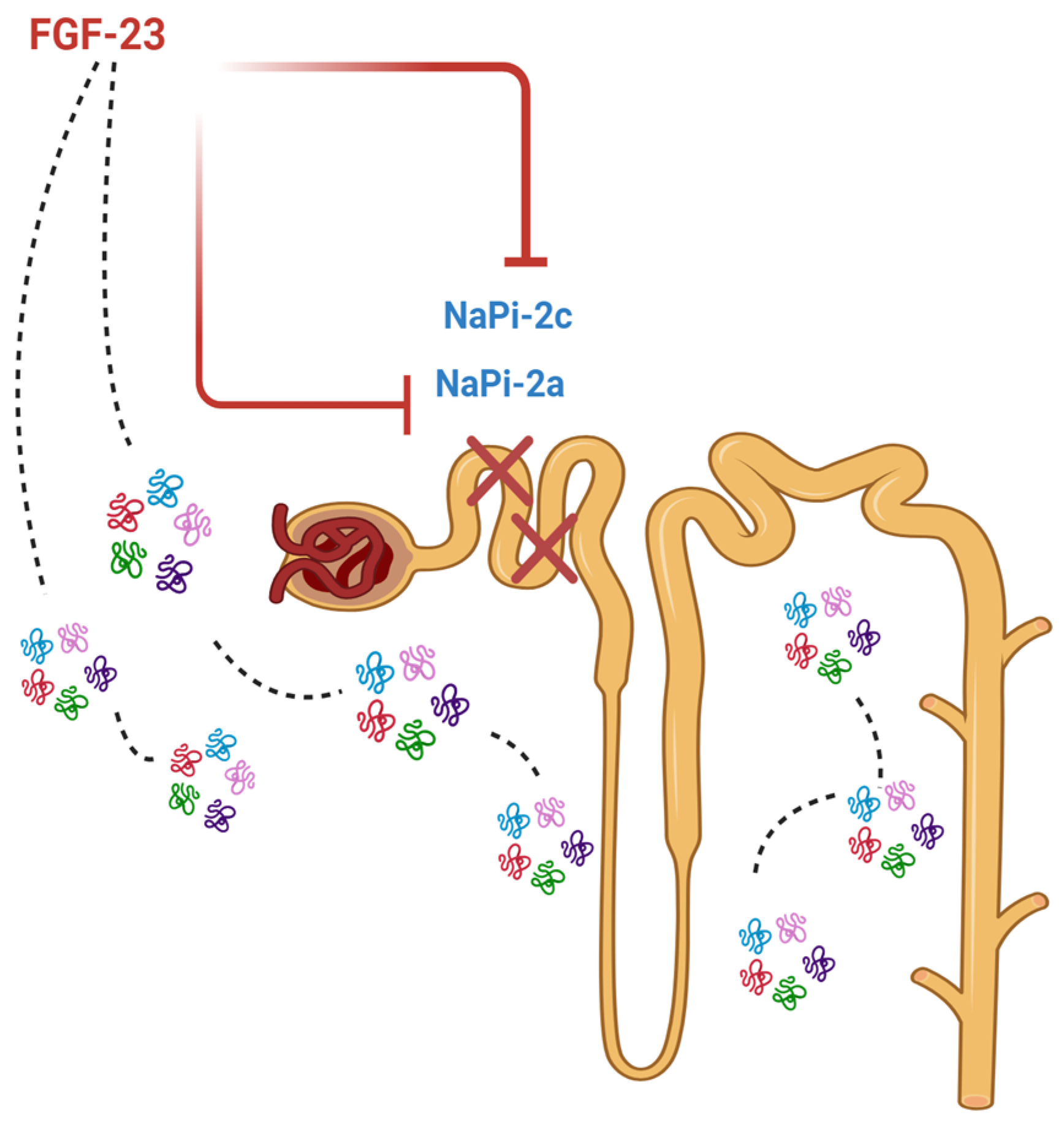
2.3. FGF-23: Production and Main Actions
2.4. Vitamin D and Anemia
2.5. FGF-23 and Iron Deficiency
2.6. FGF-23 and Epo
2.7. FGF-23 and ESA Hyporesponsiveness
2.8. FGF-23 and HIFs
2.9. Klotho and Anemia
3. New Biomarkers of Mineral Bone Disease
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Jelkmann, W. Physiology and pharmacology of erythropoietin. Transfus. Med. Hemother. 2013, 40, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Cernaro, V.; Coppolino, G.; Visconti, L.; Rivoli, L.; Lacquaniti, A.; Santoro, D.; Buemi, A.; Loddo, S.; Buemi, M. Erythropoiesis and chronic kidney disease-related anemia: From physiology to new therapeutic advancements. Med. Res. Rev. 2019, 39, 427–460. [Google Scholar] [CrossRef] [PubMed]
- Fandrey, J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Physiology 2004, 286, R977–R988. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Wang, G.L.; Semenza, G.L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 1995, 270, 1230–1237. [Google Scholar] [CrossRef]
- Kewley, R.J.; Whitelaw, M.L.; Chapman-Smith, A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int. J. Biochem. Cell Biol. 2004, 36, 189–204. [Google Scholar] [CrossRef]
- Semenza, G.L. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 1999, 15, 551–578. [Google Scholar] [CrossRef]
- Wenger, R.H.; Stiehl, D.P.; Camenisch, G. Integration of oxygen signaling at the consensus HRE. Sci. STKE 2005, 2005, re12. [Google Scholar] [CrossRef]
- Crugliano, G.; Serra, R.; Ielapi, N.; Battaglia, Y.; Coppolino, G.; Bolignano, D.; Bracale, U.M.; Pisani, A.; Faga, T.; Michael, A.; et al. Hypoxia-Inducible Factor Stabilizers in End Stage Kidney Disease: “Can the Promise Be Kept”? Int. J. Mol. Sci. 2021, 22, 12590. [Google Scholar] [CrossRef]
- Haase, V.H. Hypoxic regulation of erythropoiesis and iron metabolism. Am. J. Physiol. Ren. Physiol. 2010, 299, F1–F13. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014, 9, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Schofield, C.J.; Ratcliffe, P.J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 2004, 5, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood 2009, 114, 2015–2019. [Google Scholar] [CrossRef]
- Barbour, H.; Nkwe, N.S.; Estavoyer, B.; Messmer, C.; Gushul-Leclaire, M.; Villot, R.; Uriarte, M.; Boulay, K.; Hlayhel, S.; Farhat, B.; et al. An inventory of crosstalk between ubiquitination and other post-translational modifications in orchestrating cellular processes. iScience 2023, 26, 106276. [Google Scholar] [CrossRef]
- Peyssonnaux, C.; Nizet, V.; Johnson, R.S. Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle 2008, 7, 28–32. [Google Scholar] [CrossRef]
- Sinclair, A.M.; Coxon, A.; McCaffery, I.; Kaufman, S.; Paweletz, K.; Liu, L.; Busse, L.; Swift, S.; Elliott, S.; Begley, C.G. Functional erythropoietin receptor is undetectable in endothelial, cardiac, neuronal, and renal cells. Blood 2010, 115, 4264–4272. [Google Scholar] [CrossRef]
- Elliott, S.; Busse, L.; Swift, S.; McCaffery, I.; Rossi, J.; Kassner, P.; Begley, C.G. Lack of expression and function of erythropoietin receptors in the kidney. Nephrol. Dial. Transplant. 2011, 27, 2733–2745. [Google Scholar] [CrossRef][Green Version]
- Elliott, S.; Sinclair, A.M. The effect of erythropoietin on normal and neoplastic cells. Biologics 2012, 6, 163–189. [Google Scholar] [CrossRef]
- Frank, S.J. Minireview: Receptor Dimerization in GH and Erythropoietin Action—It Takes Two to Tango, but How? Endocrinology 2002, 143, 2–10. [Google Scholar] [CrossRef]
- Andreucci, M.; Provenzano, M.; Faga, T.; Gagliardi, I.; Pisani, A.; Perticone, M.; Coppolino, G.; De Sarro, G.; Serra, R.; Michael, A. Darbepoetin alfa reduces cell death due to radiocontrast media in human renal proximal tubular cells. Toxicol. Rep. 2021, 8, 816–821. [Google Scholar] [CrossRef] [PubMed]
- El-Maadawy, W.H.; Hassan, M.; Hafiz, E.; Badawy, M.H.; Eldahshan, S.; AbuSeada, A.; El-Shazly, M.A.M.; Ghareeb, M.A. Co-treatment with Esculin and erythropoietin protects against renal ischemia-reperfusion injury via P2X7 receptor inhibition and PI3K/Akt activation. Sci. Rep. 2022, 12, 6239. [Google Scholar] [CrossRef] [PubMed]
- Asker, M.E.; Ali, S.I.; Mohamed, S.H.; Abdelaleem, R.M.A.; Younis, N.N. The efficacy of bone marrow-derived mesenchymal stem cells and/or erythropoietin in ameliorating kidney damage in gamma irradiated rats: Role of non-hematopoietic erythropoietin anti-apoptotic signaling. Life Sci. 2021, 275, 119388. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Nishino, T.; Obata, Y.; Nakazawa, M.; Furusu, A.; Abe, K.; Miyazaki, M.; Koji, T.; Kohno, S. Recombinant human erythropoietin attenuates renal tubulointerstitial injury in murine adriamycin-induced nephropathy. J. Nephrol. 2013, 26, 527–533. [Google Scholar] [CrossRef]
- Cassis, P.; Azzollini, N.; Solini, S.; Mister, M.; Aiello, S.; Cugini, D.; Scudeletti, P.; Gagliardini, E.; Abbate, M.; Gallon, L.; et al. Both darbepoetin alfa and carbamylated erythropoietin prevent kidney graft dysfunction due to ischemia/reperfusion in rats. Transplantation 2011, 92, 271–279. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, D.; Na, N.; Li, H.; Miao, B.; Hong, L.; Huang, Z. Renoprotective effect of erythropoietin via modulation of the STAT6/MAPK/NF-κB pathway in ischemia/reperfusion injury after renal transplantation. Int. J. Mol. Med. 2018, 41, 25–32. [Google Scholar] [CrossRef]
- Conrad, K.P.; Benyo, D.F.; Westerhausen-Larsen, A.; Miles, T.M. Expression of erythropoietin by the human placenta. FASEB J. 1996, 10, 760–768. [Google Scholar] [CrossRef]
- Kobayashi, T.; Yanase, H.; Iwanaga, T.; Sasaki, R.; Nagao, M. Epididymis is a novel site of erythropoietin production in mouse reproductive organs. Biochem. Biophys. Res. Commun. 2002, 296, 145–151. [Google Scholar] [CrossRef]
- Buemi, M.; Senatore, M.; Gallo, G.C.; Crascì, E.; Campo, S.; Sturiale, A.; Coppolino, G.; Bolignano, D.; Frisina, N. Pulmonary hypertension and erythropoietin. Kidney Blood Press. Res. 2007, 30, 248–252. [Google Scholar] [CrossRef]
- Dame, C.; Bartmann, P.; Wolber, E.-M.; Fahnenstich, H.; Hofmann, D.; Fandrey, J. Erythropoietin gene expression in different areas of the developing human central nervous system. Dev. Brain Res. 2000, 125, 69–74. [Google Scholar] [CrossRef]
- Yu, X.; Shacka, J.J.; Eells, J.B.; Suarez-Quian, C.; Przygodzki, R.M.; Beleslin-Cokic, B.; Lin, C.S.; Nikodem, V.M.; Hempstead, B.; Flanders, K.C.; et al. Erythropoietin receptor signalling is required for normal brain development. Development 2002, 129, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Bernaudin, M.; Marti, H.H.; Roussel, S.; Divoux, D.; Nouvelot, A.; MacKenzie, E.T.; Petit, E. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 1999, 19, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, M.; Wen, T.C.; Matsuda, S.; Masuda, S.; Morishita, E.; Nagao, M.; Sasaki, R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc. Natl. Acad. Sci. USA 1998, 95, 4635–4640. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Nagao, M.; Takahata, K.; Konishi, Y.; Gallyas, F., Jr.; Tabira, T.; Sasaki, R. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J. Biol. Chem. 1993, 268, 11208–11216. [Google Scholar] [CrossRef]
- Sirén, A.L.; Fratelli, M.; Brines, M.; Goemans, C.; Casagrande, S.; Lewczuk, P.; Keenan, S.; Gleiter, C.; Pasquali, C.; Capobianco, A.; et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc. Natl. Acad. Sci. USA 2001, 98, 4044–4049. [Google Scholar] [CrossRef]
- Noguchi, C.T.; Asavaritikrai, P.; Teng, R.; Jia, Y. Role of erythropoietin in the brain. Crit. Rev. Oncol. Hematol. 2007, 64, 159–171. [Google Scholar] [CrossRef]
- Tan, C.C.; Eckardt, K.U.; Firth, J.D.; Ratcliffe, P.J. Feedback modulation of renal and hepatic erythropoietin mRNA in response to graded anemia and hypoxia. Am. J. Physiol.-Ren. Physiol. 1992, 263, F474–F481. [Google Scholar] [CrossRef]
- Tan, C.C.; Eckardt, K.U.; Firth, J.D.; Ratcliffe, P.J. Erythropoietin expression in primary rat Sertoli and peritubular myoid cells. Blood 2001, 98, 2872–2874. [Google Scholar]
- Yasuda, Y.; Masuda, S.; Chikuma, M.; Inoue, K.; Nagao, M.; Sasaki, R. Estrogen-dependent production of erythropoietin in uterus and its implication in uterine angiogenesis. J. Biol. Chem. 1998, 273, 25381–25387. [Google Scholar] [CrossRef]
- Masuda, S.; Kobayashi, T.; Chikuma, M.; Nagao, M.; Sasaki, R.; Haase, V.H.; Rabie, T.; Marti, H.H.; Fandrey, J.; Mukundan, H.; et al. The oviduct produces erythropoietin in an estrogen- and oxygen-dependent manner. Am. J. Physiol. Metab. 2000, 278, E1038–E1044. [Google Scholar] [CrossRef]
- Yu, A.Y.; Shimoda, L.A.; Iyer, N.V.; Huso, D.L.; Sun, X.; McWilliams, R.; Beaty, T.; Sham, J.S.; Wiener, C.M.; Sylvester, J.T.; et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J. Clin. Investig. 1999, 103, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Westgren, M.; Ek, S.; Remberger, M.; Ringden, O.; Stangenberg, M. Cytokines in fetal blood and amniotic fluid in Rh-immunized pregnancies. Obstet. Gynecol. 1995, 86, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Koury, M.J.; Bondurant, M.C.; Graber, S.E.; Sawyer, S.T. Erythropoietin messenger RNA levels in developing mice and transfer of 125I-erythropoietin by the placenta. J. Clin. Investig. 1988, 82, 154–159. [Google Scholar] [CrossRef] [PubMed]
- E Juul, S.; Ledbetter, D.J.; Joyce, A.E.; Dame, C.; Christensen, R.D.; Zhao, Y.; DeMarco, V. Erythropoietin acts as a trophic factor in neonatal rat intestine. Gut 2001, 49, 182–189. [Google Scholar] [CrossRef]
- Fandrey, J.; Bunn, H.F. In vivo and in vitro regulation of erythropoietin mRNA: Measurement by competitive polymerase chain reaction. Blood 1993, 81, 617–623. [Google Scholar] [CrossRef]
- Wu, H.; Lee, S.H.; Gao, J.; Liu, X.; Iruela-Arispe, M.L. Inactivation of erythropoietin leads to defects in cardiac morphogenesis. Development 1999, 126, 3597–3605. [Google Scholar] [CrossRef]
- Moe, S.; Drüeke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006, 69, 1945–1953. [Google Scholar] [CrossRef]
- Russo, D.; Battaglia, Y. Clinical Significance of FGF-23 in Patients with CKD. Int. J. Nephrol. 2011, 2011, 364890. [Google Scholar] [CrossRef]
- Chiu, Y.-W.; Mehrotra, R. Phosphorus and coronarycalcification in predialysis patients. Kidney Int. 2010, 78, 818. [Google Scholar] [CrossRef][Green Version]
- Fang, Y.; Ginsberg, C.; Sugatani, T.; Monier-Faugere, M.-C.; Malluche, H.; Hruska, K.A. Early chronic kidney disease-mineral bone disorder stimulates vascular calcification. Kidney Int. 2014, 85, 142–150. [Google Scholar] [CrossRef]
- Fang, Y.; Ginsberg, C.; Sugatani, T.; Monier-Faugere, M.-C.; Malluche, H.; Hruska, K.A. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone 2009, 45, 1161–1168. [Google Scholar]
- Sabbagh, Y.; Graciolli, F.G.; O’Brien, S.; Tang, W.; dos Reis, L.M.; Ryan, S.; Phillips, L.; Boulanger, J.; Song, W.; Bracken, C.; et al. Repression of osteocyte Wnt/β-catenin signaling is an early event in the progression of renal osteodystrophy. J. Bone Miner. Res. 2012, 27, 1757–1772. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.B.; Cancela, A.L.; Graciolli, F.G.; Dos Reis, L.M.; Draibe, S.A.; Cuppari, L.; Carvalho, A.B.; Jorgetti, V.; Canziani, M.E.; Moysés, R.M. Early control of PTH and FGF23 in normophosphatemic CKD patients: A new target in CKD-MBD therapy? Clin. J. Am. Soc. Nephrol. 2010, 5, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Wahl, P.; Vargas, G.S.; Gutiérrez, O.M.; Scialla, J.; Xie, H.; Appleby, D.; Nessel, L.; Bellovich, K.; Chen, J.; et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011, 79, 1370–1378. [Google Scholar] [CrossRef]
- Isakova, T.; Cai, X.; Lee, J.; Mehta, R.; Zhang, X.; Yang, W.; Nessel, L.; Anderson, A.H.; Lo, J.; Porter, A.; et al. Longitudinal Evolution of Markers of Mineral Metabolism in Patients with CKD: The Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2020, 75, 235–244. [Google Scholar] [CrossRef]
- Spasovski, G.B.; Bervoets, A.R.J.; Behets, G.; Ivanovski, N.; Sikole, A.; Dams, G.; Couttenye, M.; De Broe, M.E.; D’Haese, P.C. Spectrum of renal bone disease in end-stage renal failure patients not yet on dialysis. Nephrol. Dial. Transpl. 2003, 18, 1159–1166. [Google Scholar] [CrossRef]
- Hernandez, D.; Concepcion, M.T.; Lorenzo, V.; Martinez, M.E.; Rodriguez, A.; De Bonis, E.; Gonzalez-Posada, J.M.; Felsenfeld, A.J.; Rodriguez, M.; Torres, A. Adynamic bone disease with negative aluminium staining in predialysis patients: Prevalence and evolution after maintenance dialysis. Nephrol. Dial. Transpl. 1994, 9, 517–523. [Google Scholar] [CrossRef]
- Shin, S.K.; Kim, D.H.; Kim, H.S.; Shin, K.T.; Ma, K.A.; Kim, S.J.; Kwak, Y.S.; Ha, S.K.; Sherrard, D.J. Renal osteodystrophy in pre-dialysis patients: Ethnic difference? Perit. Dial. Int. 1999, 19 (Suppl. S2), S402–S407. [Google Scholar] [CrossRef]
- Inaba, M.; Okuno, S.; Nagasue, K.; Otoshi, T.; Kurioka, Y.; Maekawa, K.; Kumeda, Y.; Imanishi, Y.; Ishimura, E.; Ohta, T.; et al. Impaired secretion of parathyroid hormone is coherent to diabetic hemodialyzed patients. Am. J. Kidney Dis. 2001, 38 (Suppl. S1), S139–S142. [Google Scholar] [CrossRef]
- Vincenti, F.; Arnaud, S.B.; Recker, R.; Genant, H.; Amend, W.J.; Feduska, N.J.; Salvatierra, O. Parathyroid and bone response of the diabetic patient to uremia. Kidney Int. 1984, 25, 677–682. [Google Scholar] [CrossRef]
- Inaba, M.; Okuno, S.; Kumeda, Y.; Yamakawa, T.; Ishimura, E.; Nishizawa, Y. Increased incidence of vertebral fracture in older female hemodialyzed patients with type 2 diabetes mellitus. Calcif. Tissue Int. 2005, 76, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Lukowsky, L.R.; Molnar, M.Z.; Zaritsky, J.J.; Sim, J.J.; Mucsi, I.; Kovesdy, C.P.; Kalantar-Zadeh, K. Mineral and bone disorders and survival in hemodialysis patients with and without polycystic kidney disease. Nephrol. Dial. Transplant. 2012, 27, 2899–2907. [Google Scholar] [CrossRef] [PubMed]
- Gitomer, B.; Pereira, R.; Salusky, I.B.; Stoneback, J.W.; Isakova, T.; Cai, X.; Dalrymple, L.S.; Ofsthun, N.; You, Z.; Malluche, H.H.; et al. Mineral bone disease in autosomal dominant polycystic kidney disease. Kidney Int. 2021, 99, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.K.; Dash, S.C.; Tiwari, S.C.; Agarwal, S.K.; Saxena, S.; Fishbane, S. Bone histology in patients with nephrotic syndrome and normal renal function. Kidney Int. 1999, 55, 1912–1919. [Google Scholar] [CrossRef][Green Version]
- Abbott, K.C.; Agodoa, L.Y. Polycystic kidney disease in patients on the renal transplant waiting list: Trends in hematocrit and survival. BMC Nephrol. 2002, 3, 7. [Google Scholar] [CrossRef][Green Version]
- Chandra, M.; Miller, M.E.; Garcia, J.F.; Mossey, R.T.; McVicar, M. Serum immunoreactive erythropoietin levels in patients with polycystic kidney disease as compared with other hemodialysis patients. Nephron 1985, 39, 26–29. [Google Scholar] [CrossRef]
- Verdalles, U.; Abad, S.; Vega, A.; Caro, C.R.; Ampuero, J.; Jofre, R.; Lopez-Gomez, J.M. Factors related to the absence of anemia in hemodialysis patients. Blood Purif. 2011, 32, 69–74. [Google Scholar] [CrossRef]
- de Almeida, E.A.F.; Alho, I.; Marques, F.; Thiran, C.; Bicho, M.P.; Prata, M. Haemoglobin and erythropoietin levels in polycystic kidney disease. Nephrol. Dial. Transplant. 2007, 23, 412–413. [Google Scholar] [CrossRef][Green Version]
- Eckardt, K.U.; Möllmann, M.; Neumann, R.; Brunkhorst, R.; Burger, H.U.; Lonnemann, G.; Scholz, H.; Keusch, G.; Buchholz, B.; Frei, U. Erythropoietin in polycystic kidneys. J. Clin. Investig. 1989, 84, 1160–1166. [Google Scholar] [CrossRef]
- Bernhardt, W.M.; Wiesener, M.S.; Weidemann, A.; Schmitt, R.; Weichert, W.; Lechler, P.; Campean, V.; Ong, A.C.M.; Willam, C.; Gretz, N.; et al. Involvement of hypoxia-inducible transcription factors in polycystic kidney disease. Am. J. Pathol. 2007, 170, 830–842. [Google Scholar] [CrossRef]
- Liu, S.; Quarles, L.D. How fibroblast growth factor 23 works. J. Am. Soc. Nephrol. 2007, 18, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Lavi-Moshayoff, V.; Wasserman, G.; Meir, T.; Silver, J.; Naveh-Many, T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am. J. Physiol.-Ren. Physiol. 2010, 299, F882–F889. [Google Scholar] [CrossRef] [PubMed]
- López, I.; Rodríguez-Ortiz, M.E.; Almadén, Y.; Guerrero, F.; de Oca, A.M.; Pineda, C.; Shalhoub, V.; Rodríguez, M.; Aguilera-Tejero, E. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 2011, 80, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Quinn, S.J.; Thomsen, A.R.B.; Pang, J.L.; Kantham, L.; Bräuner-Osborne, H.; Pollak, M.; Goltzman, D.; Brown, E.M. Interactions between calcium and phosphorus in the regulation of the production of fibroblast growth factor 23 in vivo. Am. J. Physiol.-Endocrinol. Metab. 2013, 304, E310–E320. [Google Scholar] [CrossRef]
- Imanishi, Y.; Inaba, M.; Nakatsuka, K.; Nagasue, K.; Okuno, S.; Yoshihara, A.; Miura, M.; Miyauchi, A.; Kobayashi, K.; Miki, T.; et al. FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int. 2004, 65, 1943–1946. [Google Scholar] [CrossRef]
- Larsson, T.; Nisbeth, U.; Ljunggren, Ö.; Jüppner, H.; Jonsson, K.B. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003, 64, 2272–2279. [Google Scholar] [CrossRef]
- Kakitani, M.; Yamazaki, Y.; Hasegawa, H.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Tomizuka, K.; Yamashita, T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Investig. 2004, 113, 561–568. [Google Scholar]
- Krajisnik, T.; Björklund, P.; Marsell, R.; Ljunggren, O.; Åkerström, G.; Jonsson, K.B.; Westin, G.; Larsson, T.E. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J. Endocrinol. 2007, 195, 125–131. [Google Scholar] [CrossRef]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.-C.; Sloan, A.; Isakova, T.; Gutiérrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef]
- Murali, S.K.; Roschger, P.; Zeitz, U.; Klaushofer, K.; Andrukhova, O.; Erben, R.G. FGF23 Regulates Bone Mineralization in a 1,25(OH)2 D3 and Klotho-Independent Manner. J. Bone Miner. Res. 2016, 31, 129–142. [Google Scholar] [CrossRef]
- Château, M.-T.; Araiz, C.; Descamps, S.; Galas, S. Klotho interferes with a novel FGF-signalling pathway and insulin/Igf-like signalling to improve longevity and stress resistance in Caenorhabditis elegans. Aging 2010, 2, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, O.; Imura, A.; Iwano, A.; Freund, J.N.; Henrissat, B.; Fujimori, T.; Nabeshima, Y. Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J. Biol. Chem. 2004, 279, 9777–9784. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, I.; Yamazaki, Y.; Shimada, T.; Iijima, K.; Hasegawa, H.; Okawa, K.; Fujita, T.; Fukumoto, S.; Yamashita, T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006, 444, 770–774. [Google Scholar] [CrossRef]
- Grabner, A.; Amaral, A.P.; Schramm, K.; Singh, S.; Sloan, A.; Yanucil, C.; Li, J.; Shehadeh, L.A.; Hare, J.M.; David, V.; et al. Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell Metab. 2015, 22, 1020–1032. [Google Scholar] [CrossRef]
- Martin, A.; David, V.; Quarles, L.D. Regulation and function of the FGF23/klotho endocrine pathways. Physiol. Rev. 2012, 92, 131–155. [Google Scholar] [CrossRef]
- Ho, B.B.; Bergwitz, C. FGF23 signalling and physiology. J. Mol. Endocrinol. 2021, 66, R23–R32. [Google Scholar] [CrossRef]
- Miyamoto, K.-I.; Ito, M.; Tatsumi, S.; Kuwahata, M.; Segawa, H. New Aspect of Renal Phosphate Reabsorption: The Type IIc Sodium-Dependent Phosphate Transporter. Am. J. Nephrol. 2007, 27, 503–515. [Google Scholar] [CrossRef]
- Saito, H.; Kusano, K.; Kinosaki, M.; Ito, H.; Hirata, M.; Segawa, H.; Miyamoto, K.; Fukushima, N. Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J. Biol. Chem. 2003, 278, 2206–2211. [Google Scholar] [CrossRef]
- Bai, X.-Y.; Miao, D.; Goltzman, D.; Karaplis, A.C. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J. Biol. Chem. 2003, 278, 9843–9849. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 Is a Potent Regulator of Vitamin D Metabolism and Phosphate Homeostasis*. J. Bone Miner. Res. 2009, 19, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, O.; Isakova, T.; Rhee, E.; Shah, A.; Holmes, J.; Collerone, G.; Juppner, H.; Wolf, M. Fibroblast Growth Factor-23 Mitigates Hyperphosphatemia but Accentuates Calcitriol Deficiency in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2005, 16, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Portale, A.A.; Wolf, M.; Jüppner, H.; Messinger, S.; Kumar, J.; Wesseling-Perry, K.; Schwartz, G.J.; Furth, S.L.; Warady, B.A.; Salusky, I.B. Disordered FGF23 and Mineral Metabolism in Children with CKD. Bone Res. 2014, 9, 344–353. [Google Scholar] [CrossRef]
- Courbon, G.; Francis, C.; Gerber, C.; Neuburg, S.; Wang, X.; Lynch, E.; Isakova, T.; Babitt, J.L.; Wolf, M.; Martin, A.; et al. Lipocalin 2 stimulates bone fibroblast growth factor 23 production in chronic kidney disease. Bone Res. 2021, 9, 35. [Google Scholar] [CrossRef]
- Agoro, R.; White, K.E. Anemia and fibroblast growth factor 23 elevation in chronic kidney disease: Homeostatic interactions and emerging therapeutics. Curr. Opin. Nephrol. Hypertens. 2022, 31, 320–325. [Google Scholar] [CrossRef]
- Souma, N.; Isakova, T.; Lipiszko, D.; Sacco, R.L.; Elkind, M.S.V.; DeRosa, J.T.; Silverberg, S.J.; Mendez, A.J.; Dong, C.; Wright, C.B.; et al. Fibroblast Growth Factor 23 and Cause-Specific Mortality in the General Population: The Northern Manhattan Study. J. Clin. Endocrinol. Metab. 2016, 101, 3779–3786. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Mannstadt, M.; Isakova, T.; Rauh-Hain, J.A.; Tamez, H.; Shah, A.; Smith, K.; Lee, H.; Thadhani, R.; Jüppner, H.; et al. Fibroblast Growth Factor 23 and Mortality among Patients Undergoing Hemodialysis. N. Engl. J. Med. 2008, 359, 584–592. [Google Scholar] [CrossRef]
- Baia, L.C.; Humalda, J.K.; Vervloet, M.G.; Navis, G.; Bakker, S.J.; de Borst, M.H. Fibroblast Growth Factor 23 and Cardiovascular Mortality after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2013, 8, 1968–1978. [Google Scholar] [CrossRef]
- Isakova, T.; Xie, H.; Yang, W.; Xie, D.; Anderson, A.H.; Scialla, J.; Wahl, P.; Gutiérrez, O.M.; Steigerwalt, S.; He, J.; et al. Fibroblast Growth Factor 23 and Risks of Mortality and End-Stage Renal Disease in Patients with Chronic Kidney Disease. JAMA 2011, 305, 2432–2439. [Google Scholar] [CrossRef]
- Edmonston, D.; Fuchs, M.A.; Burke, E.J.; Isakova, T.; Wolf, M.; Appel, L.J.; Chen, J.; Cohen, D.L.; Feldman, H.I.; Go, A.S.; et al. Klotho and Clinical Outcomes in CKD: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2024, 84, 349–360.e1. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, Y.; Bellasi, A.; Esposito, P.; Bortoluzzi, A.; Rotondi, S.; Andreucci, M.; Fiorini, F.; Russo, D.; Storari, A. The Impact of Cholecaciferol Supplementation on Bone Mineral Density in Long-Term Kidney Transplant Recipients. Biomolecules 2023, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, Y.; Bellasi, A.; Bortoluzzi, A.; Tondolo, F.; Esposito, P.; Provenzano, M.; Russo, D.; Andreucci, M.; Cianciolo, G.; Storari, A. Bone Mineral Density Changes in Long-Term Kidney Transplant Recipients: A Real-Life Cohort Study of Native Vitamin D Supplementation. Nutrients 2022, 14, 323. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, Y.; Cojocaru, E.; Fiorini, F.; Granata, A.; Esposito, P.; Russo, L.; Bortoluzzi, A.; Storari, A.; Russo, D. Vitamin D in kidney transplant recipients. Clin. Nephrol. 2020, 93, 57–64. [Google Scholar] [CrossRef]
- Kiss, Z.; Ambrus, C.; Almasi, C.; Berta, K.; Deak, G.; Horonyi, P.; Kiss, I.; Lakatos, P.; Marton, A.; Molnar, M.Z.; et al. Serum 25(OH)-Cholecalciferol Concentration Is Associated with Hemoglobin Level and Erythropoietin Resistance in Patients on Maintenance Hemodialysis. Nephron Clin. Pract. 2010, 117, 373–378. [Google Scholar] [CrossRef]
- Patel, N.M.; Gutiérrez, O.M.; Andress, D.L.; Coyne, D.W.; Levin, A.; Wolf, M. Vitamin D deficiency and anemia in early chronic kidney disease. Kidney Int. 2010, 77, 715–720. [Google Scholar] [CrossRef][Green Version]
- Bolignano, D.; D’arrigo, G.; Pisano, A.; Coppolino, G. Pentoxifylline for Anemia in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0134104. [Google Scholar] [CrossRef]
- Mihaila, S. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Maedica 2010, 5, 228. [Google Scholar]
- Wolf, M.; Shah, A.; Gutierrez, O.; Ankers, E.; Monroy, M.; Tamez, H.; Steele, D.; Chang, Y.; Camargo, C.; Tonelli, M.; et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007, 72, 1004–1013. [Google Scholar] [CrossRef]
- Icardi, A.; Paoletti, E.; De Nicola, L.; Mazzaferro, S.; Russo, R.; Cozzolino, M. Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency: The potential role of inflammation. Nephrol. Dial. Transplant. 2013, 28, 1672–1679. [Google Scholar] [CrossRef]
- Matias, P.J.; Laranjinha, I.; Ávila, G.; Azevedo, A.; Jorge, C.; Ferreira, C.; Aires, I.; Amaral, T.; Gil, C.; Ferreira, A. Cholecalciferol Supplementation in Hemodialysis Patients: Effects on Mineral Metabolism, Inflammation, and Cardiac Dimension Parameters. Semin. Dial 2010, 5, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Zaritsky, J.J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Zavala, K.; Nayak, A.; Wesseling-Perry, K.; Westerman, M.; Hollis, B.W.; et al. Suppression of Iron-Regulatory Hepcidin by Vitamin D. J. Am. Soc. Nephrol. 2014, 25, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Zaritsky, J. Hepcidin for Clinicians. Clin. J. Am. Soc. Nephrol. 2009, 4, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Barminko, J.; Reinholt, B.M.; Emmanuelli, A.; Lejeune, A.N.; Baron, M.H. Activation of the vitamin D receptor transcription factor stimulates the growth of definitive erythroid progenitors. Blood Adv. 2018, 2, 1207–1219. [Google Scholar] [CrossRef]
- Obi, Y.; Yamaguchi, S.; Hamano, T.; Sakaguchi, Y.; Shimomura, A.; Namba-Hamano, T.; Mikami, S.; Nishi, O.; Tanaka, M.; Kamoto, A.; et al. Effect of cholecalciferol on serum hepcidin and parameters of anaemia and CKD-MBD among haemodialysis patients: A randomized clinical trial. Sci. Rep. 2020, 10, 15500. [Google Scholar] [CrossRef]
- Farrow, E.G.; Yu, X.; Summers, L.J.; Davis, S.I.; Fleet, J.C.; Allen, M.R.; Robling, A.G.; Stayrook, K.R.; Jideonwo, V.; Magers, M.J.; et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc. Natl. Acad. Sci. USA 2011, 108, E1146–E1155. [Google Scholar] [CrossRef]
- Lewerin, C.; Ljunggren, Ö.; Nilsson-Ehle, H.; Karlsson, M.K.; Herlitz, H.; Lorentzon, M.; Ohlsson, C.; Mellström, D. Low serum iron is associated with high serum intact FGF23 in elderly men: The Swedish MrOS study. Bone 2017, 98, 1–8. [Google Scholar] [CrossRef]
- Mehta, R.; Cai, X.; Hodakowski, A.; Lee, J.; Leonard, M.; Ricardo, A.; Chen, J.; Hamm, L.; Sondheimer, J.; Dobre, M.; et al. Fibroblast Growth Factor 23 and Anemia in the Chronic Renal Insufficiency Cohort Study. Clin. J. Am. Soc. Nephrol. 2017, 12, 1795–1803. [Google Scholar] [CrossRef]
- Eisenga, M.F.; van Londen, M.; Leaf, D.E.; Nolte, I.M.; Navis, G.; Bakker, S.J.; de Borst, M.H.; Gaillard, C.A. C-Terminal Fibroblast Growth Factor 23, Iron Deficiency, and Mortality in Renal Transplant Recipients. J. Am. Soc. Nephrol. 2017, 28, 3639–3646. [Google Scholar] [CrossRef]
- Wolf, M.; White, K.E. Coupling fibroblast growth factor 23 production and cleavage: Iron deficiency, rickets, and kidney disease. Curr. Opin. Nephrol. Hypertens. 2014, 23, 411–419. [Google Scholar] [CrossRef]
- McMahon, S.; Otsuki, T.; Yoshida, Y.; Nagura, C.; Kitai, M.; Shibahara, N.; Tomita, H.; Maruyama, T.; Abe, M. Hypoxia-enhanced Expression of the Proprotein Convertase Furin Is Mediated by Hypoxia-inducible Factor-1: Impact on the bioactivation of proproteins*. J. Biol. Chem. 2005, 280, 6561–6569. [Google Scholar] [CrossRef] [PubMed]
- David, V.; Francis, C.; Babitt, J.L. Ironing out the cross talk between FGF23 and inflammation. Am. J. Physiol.-Ren. Physiol. 2017, 312, F1–F8. [Google Scholar] [CrossRef] [PubMed]
- Eisenga, M.F.; Emans, M.E.; van der Putten, K.; Cramer, M.J.; Diepenbroek, A.; Velthuis, B.K.; Doevendans, P.A.; Verhaar, M.C.; Joles, J.A.; Bakker, S.J.L.; et al. Epoetin Beta and C-Terminal Fibroblast Growth Factor 23 in Patients with Chronic Heart Failure and Chronic Kidney Disease. J. Am. Hear. Assoc. 2019, 8, e011130. [Google Scholar] [CrossRef] [PubMed]
- Fukao, W.; Hasuike, Y.; Yamakawa, T.; Toyoda, K.; Aichi, M.; Masachika, S.; Kantou, M.; Takahishi, S.; Iwasaki, T.; Yahiro, M.; et al. Oral Versus Intravenous Iron Supplementation for the Treatment of Iron Deficiency Anemia in Patients on Maintenance Hemodialysis—Effect on Fibroblast Growth Factor-23 Metabolism. J. Ren. Nutr. 2018, 28, 270–277. [Google Scholar]
- Shima, H.; Miya, K.; Okada, K.; Minakuchi, J.; Kawashima, S. Sucroferric oxyhydroxide decreases serum phosphorus level and fibroblast growth factor 23 and improves renal anemia in hemodialysis patients. BMC Res. Notes 2018, 11, 363. [Google Scholar] [CrossRef]
- Maruyama, N.; Otsuki, T.; Yoshida, Y.; Nagura, C.; Kitai, M.; Shibahara, N.; Tomita, H.; Maruyama, T.; Abe, M. Ferric Citrate Decreases Fibroblast Growth Factor 23 and Improves Erythropoietin Responsiveness in Hemodialysis Patients. Am. J. Nephrol. 2018, 47, 406–414. [Google Scholar] [CrossRef]
- Imel, E.A.; Biggin, A.; Schindeler, A.; Munns, C.F. FGF23, Hypophosphatemia, and Emerging Treatments. JBMR Plus 2019, 3, e10190. [Google Scholar] [CrossRef]
- Wolf, M.; Koch, T.A.; Bregman, D.B. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J. Bone Miner. Res. 2013, 28, 1793–1803. [Google Scholar] [CrossRef]
- Noonan, M.L.; Clinkenbeard, E.L.; Ni, P.; Swallow, E.A.; Tippen, S.P.; Agoro, R.; Allen, M.R.; White, K.E. Erythropoietin and a hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHDi) lowers FGF23 in a model of chronic kidney disease (CKD). Physiol. Rep. 2020, 8, e14434. [Google Scholar] [CrossRef]
- Babitt, J.L.; Lin, H.Y. Mechanisms of Anemia in CKD. J. Am. Soc. Nephrol. 2012, 23, 1631–1634. [Google Scholar] [CrossRef]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Sofue, T.; Nakagawa, N.; Kanda, E.; Nagasu, H.; Matsushita, K.; Nangaku, M.; Maruyama, S.; Wada, T.; Terada, Y.; Yamagata, K.; et al. Prevalence of anemia in patients with chronic kidney disease in Japan: A nationwide, cross-sectional cohort study using data from the Japan Chronic Kidney Disease Database (J-CKD-DB). PLoS ONE 2020, 15, e0236132. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.M.; Streja, E.; Kalantar-Zadeh, K. Burden of Anemia in Chronic Kidney Disease: Beyond Erythropoietin. Adv. Ther. 2021, 38, 52–75. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, F.O.; Story, K.; Firanek, C.; Mendelssohn, D.; Barre, P.; Takano, T.; Soroka, S.; Mujais, S. Health-Related Quality of Life and Hemoglobin Levels in Chronic Kidney Disease Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 33–38. [Google Scholar] [CrossRef]
- Lamerato, L.; James, G.; van Haalen, H.; Hedman, K.; Sloand, J.A.; Tang, A.; Wittbrodt, E.T.; Yee, J. Epidemiology and outcomes in patients with anemia of CKD not on dialysis from a large US healthcare system database: A retrospective observational study. BMC Nephrol. 2022, 23, 166. [Google Scholar] [CrossRef]
- Eschbach, J.W.; Abdulhadi, M.H.; Browne, J.K.; Delano, B.G.; Downing, M.R.; Egrie, J.C.; Evans, R.W.; Friedman, E.A.; Graber, S.E.; Haley, N.R.; et al. Recombinant Human Erythropoietin in Anemic Patients with End-Stage Renal Disease. Ann. Intern. Med. 1989, 111, 992–1000. [Google Scholar] [CrossRef]
- Rabadi, S.; Udo, I.; Leaf, D.E.; Waikar, S.S.; Christov, M. Acute blood loss stimulates fibroblast growth factor 23 production. Am. J. Physiol. Ren. Physiol. 2018, 314, F132–F139. [Google Scholar] [CrossRef]
- Daryadel, A.; Bettoni, C.; Haider, T.; Imenez Silva, P.H.; Schnitzbauer, U.; Pastor-Arroyo, E.M.; Wenger, R.H.; Gassmann, M.; Wagner, C.A. Erythropoietin stimulates fibroblast growth factor 23 (FGF23) in mice and men. Pflüg. Arch. 2018, 470, 1569–1582. [Google Scholar] [CrossRef]
- Coe, L.M.; Madathil, S.V.; Casu, C.; Lanske, B.; Rivella, S.; Sitara, D. FGF-23 Is a Negative Regulator of Prenatal and Postnatal Erythropoiesis*. J. Biol. Chem. 2014, 289, 9795–9810. [Google Scholar] [CrossRef]
- Agoro, R.; Montagna, A.; Goetz, R.; Aligbe, O.; Singh, G.; Coe, L.M.; Mohammadi, M.; Rivella, S.; Sitara, D. Inhibition of fibroblast growth factor 23 (FGF23) signaling rescues renal anemia. FASEB J. 2018, 32, 3752–3764. [Google Scholar] [CrossRef]
- Hanudel, M.; Eisenga, M.; Rappaport, M.; Chua, K.J.; Qiao, B.; Jung, C.-L.; Gabayan, V.; Gales, B.; Ramos, G.; Jong, M.; et al. Effects of erythropoietin on fibroblast growth factor 23 in mice and humans. Nephrol. Dial. Transplant. 2018, 34, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, K.; Braam, B.; Jie, K.E.; Gaillard, C.A. Mechanisms of Disease: Erythropoietin resistance in patients with both heart and kidney failure. Nat. Clin. Pract. Nephrol. 2008, 4, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Clinkenbeard, E.L.; Hanudel, M.R.; Stayrook, K.R.; Appaiah, H.N.; Farrow, E.G.; Cass, T.A.; Summers, L.J.; Ip, C.S.; Hum, J.M.; Thomas, J.C.; et al. Erythropoietin stimulates murine and human fibroblast growth factor-23, revealing novel roles for bone and bone marrow. Haematologica 2017, 102, e427–e430. [Google Scholar] [CrossRef] [PubMed]
- Flamme, I.; Ellinghaus, P.; Urrego, D.; Krüger, T. FGF23 expression in rodents is directly induced via erythropoietin after inhibition of hypoxia inducible factor proline hydroxylase. PLoS ONE 2017, 12, e0186979. [Google Scholar] [CrossRef]
- Toro, L.; Barrientos, V.; León, P.; Rojas, M.; Gonzalez, M.; González-Ibáñez, A.; Illanes, S.; Sugikawa, K.; Abarzúa, N.; Bascuñán, C.; et al. Erythropoietin induces bone marrow and plasma fibroblast growth factor 23 during acute kidney injury. Kidney Int. 2018, 93, 1131–1141. [Google Scholar] [CrossRef]
- Tagliabracci, V.S.; Engel, J.L.; Wiley, S.E.; Xiao, J.; Gonzalez, D.J.; Appaiah, H.N.; Koller, A.; Nizet, V.; White, K.E.; Dixon, J.E. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc. Natl. Acad. Sci. USA 2014, 111, 5520–5525. [Google Scholar] [CrossRef]
- Yamamoto, H.; Ramos-Molina, B.; Lick, A.N.; Prideaux, M.; Albornoz, V.; Bonewald, L.; Lindberg, I. Posttranslational processing of FGF23 in osteocytes during the osteoblast to osteocyte transition. Bone 2016, 84, 120–130. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.V.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Mendoza, J.M.; Isakova, T.; Ricardo, A.C.; Xie, H.; Navaneethan, S.D.; Anderson, A.H.; Bazzano, L.A.; Xie, D.; Kretzler, M.; Nessel, L.; et al. Fibroblast Growth Factor 23 and Inflammation in CKD. Clin. J. Am. Soc. Nephrol. 2012, 7, 1155–1162. [Google Scholar] [CrossRef]
- Hanks, L.J.; Casazza, K.; Judd, S.E.; Jenny, N.S.; Gutiérrez, O.M. Associations of Fibroblast Growth Factor-23 with Markers of Inflammation, Insulin Resistance and Obesity in Adults. PLoS ONE 2015, 10, e0122885. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Wijenayaka, A.R.; Prideaux, M.; Kogawa, M.; Ormsby, R.T.; Evdokiou, A.; Bonewald, L.F.; Findlay, D.M.; Atkins, G.J. Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol. Cell. Endocrinol. 2015, 399, 208–218. [Google Scholar] [CrossRef] [PubMed]
- David, V.; Martin, A.; Isakova, T.; Spaulding, C.; Qi, L.; Ramirez, V.; Zumbrennen-Bullough, K.B.; Sun, C.C.; Lin, H.Y.; Babitt, J.L.; et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016, 89, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Zhao, J.; Fuller, D.S.; Hanafusa, N.; Hasegawa, T.; Fujino, H.; Nomura, T.; Zee, J.; Young, E.; Robinson, B.M.; et al. Association of erythropoietin resistance and fibroblast growth factor 23 in dialysis patients: Results from the Japanese Dialysis Outcomes and Practice Patterns Study. Nephrology 2021, 26, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Kim, H.; An, S.Y.; Lee, M.; Cha, M.-U.; Park, J.T.; Yoo, T.-H.; Lee, K.-B.; Kim, Y.-H.; Sung, S.-A.; et al. Circulating Fibroblast Growth Factor-23 Levels are Associated with an Increased Risk of Anemia Development in Patients with Nondialysis Chronic Kidney Disease. Sci. Rep. 2018, 8, 7294. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; McAllister, C.J.; Lehn, R.S.; Lee, G.H.; Nissenson, A.R.; Kopple, J.D. Effect of malnutrition-inflammation complex syndrome on EPO hyporesponsiveness in maintenance hemodialysis patients. Am. J. Kidney Dis. 2003, 42, 761–773. [Google Scholar] [CrossRef]
- Drüeke, T.B.; Eckardt, K.U. Role of secondary hyperparathyroidism in erythropoietin resistance of chronic renal failure patients. Nephrol. Dial. Transpl. 2002, 17 (Suppl. S5), 28–31. [Google Scholar] [CrossRef]
- Mpio, I.; Boumendjel, N.; Karaaslan, H.; Arkouche, W.; Lenz, A.; Cardozo, C.; Cardozo, J.; Pastural-Thaunat, M.; Fouque, D.; Silou, J.; et al. Secondary hyperparathyroidism and anemia. Effects of a calcimimetic on the control of anemia in chronic hemodialysed patients. Pilot Study. Néphrologie Thérapeutique 2011, 7, 229–236. [Google Scholar] [CrossRef]
- Fusaro, M.; D’angelo, A.; Naso, A.; Frigo, A.C.; Miozzo, D.; Gallieni, M.; Calò, L.A. Treatment with calcimimetic (cinacalcet) alters epoetin dosage requirements in dialysis patients: Preliminary report. Ren. Fail. 2011, 33, 732–735. [Google Scholar] [CrossRef]
- Torun, D.; Yildiz, I.; Micozkadioglu, H.; Nursal, G.N.; Yigit, F.; Ozelsancak, R. The effects of cinacalcet treatment on bone mineral metabolism, anemia parameters, left ventricular mass index and parathyroid gland volume in hemodialysis patients with severe secondary hyperparathyroidism. Saudi J. Kidney Dis. Transplant. 2016, 27, 15–22. [Google Scholar] [CrossRef]
- Battistella, M.; Richardson, R.M.; Bargman, J.M.; Chan, C.T. Improved parathyroid hormone control by cinacalcet is associated with reduction in darbepoetin requirement in patients with end-stage renal disease. Clin. Nephrol. 2011, 76, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Yoshida, K.; Fukuma, S.; Ito, K.; Matsushita, K.; Fukagawa, M.; Fukuhara, S.; Akizawa, T. Effects of Secondary Hyperparathyroidism Treatment on Improvement in Anemia: Results from the MBD-5D Study. PLoS ONE 2016, 11, e0164865. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Komaba, H.; Nakanishi, S.; Fujimori, A.; Fukagawa, M. Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol. Dial. Transplant. 2011, 27, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Miikkulainen, P.; Högel, H.; Rantanen, K.; Suomi, T.; Kouvonen, P.; Elo, L.L.; Jaakkola, P.M. HIF prolyl hydroxylase PHD3 regulates translational machinery and glucose metabolism in clear cell renal cell carcinoma. Cancer Metab. 2017, 5, 5. [Google Scholar] [CrossRef]
- Sanghani, N.S.; Haase, V.H. Hypoxia-Inducible Factor Activators in Renal Anemia: Current Clinical Experience. Adv. Chronic Kidney Dis. 2019, 26, 253–266. [Google Scholar] [CrossRef]
- Provenzano, R.; Besarab, A.; Wright, S.; Dua, S.; Zeig, S.; Nguyen, P.; Poole, L.; Saikali, K.G.; Saha, G.; Hemmerich, S.; et al. Roxadustat (FG-4592) Versus Epoetin Alfa for Anemia in Patients Receiving Maintenance Hemodialysis: A Phase 2, Randomized, 6- to 19-Week, Open-Label, Active-Comparator, Dose-Ranging, Safety and Exploratory Efficacy Study. Am. J. Kidney Dis. 2016, 67, 912–924. [Google Scholar] [CrossRef]
- Holdstock, L.; Meadowcroft, A.M.; Maier, R.; Johnson, B.M.; Jones, D.; Rastogi, A.; Zeig, S.; Lepore, J.J.; Cobitz, A.R. Four-Week Studies of Oral Hypoxia-Inducible Factor-Prolyl Hydroxylase Inhibitor GSK1278863 for Treatment of Anemia. Am. Soc. Nephrol. 2016, 27, 1234–1244. [Google Scholar] [CrossRef]
- Zhang, Q.; Doucet, M.; Tomlinson, R.E.; Han, X.; Quarles, L.D.; Collins, M.T.; Clemens, T.L. The hypoxia-inducible factor-1α activates ectopic production of fibroblast growth factor 23 in tumor-induced osteomalacia. Bone Res. 2016, 4, 16011. [Google Scholar] [CrossRef]
- Landau, D.; London, L.; Bandach, I.; Segev, Y. The hypoxia inducible factor/erythropoietin (EPO)/EPO receptor pathway is disturbed in a rat model of chronic kidney disease related anemia. PLoS ONE 2018, 13, e0196684. [Google Scholar] [CrossRef]
- Yoshida, S.; Saito, T.; Shibagaki, K.; Hirao, K.; Yuza, T.; Tomosugi, N.; Honda, H. Changes of biomarkers for erythropoiesis, iron metabolism, and FGF23 by supplementation with roxadustat in patients on hemodialysis. Sci. Rep. 2023, 13, 3181. [Google Scholar] [CrossRef]
- Sugiura, H.; Yoshida, T.; Mitobe, M.; Shiohira, S.; Nitta, K.; Tsuchiya, K. Recombinant human erythropoietin mitigates reductions in renal klotho expression. Am. J. Nephrol. 2010, 32, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Leone, F.; Lofaro, D.; Gigliotti, P.; Perri, A.; Vizza, D.; Toteda, G.; Lupinacci, S.; Armentano, F.; Papalia, T.; Bonofiglio, R. Soluble Klotho levels in adult renal transplant recipients are modulated by recombinant human erythropoietin. J. Nephrol. 2014, 27, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Sari, F.; Inci, A.; Dolu, S.; Ellidag, H.Y.; Cetinkaya, R.; Ersoy, F.F. High serum soluble α-Klotho levels in patients with autosomal dominant polycystic kidney disease. J. Investig. Med. 2017, 65, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Civitelli, R.; Armamento-Villareal, R.; Napoli, N. Bone turnover markers: Understanding their value in clinical trials and clinical practice. Osteoporos. Int. 2009, 20, 843–851. [Google Scholar] [CrossRef]
- Nickolas, T.L.; Cremers, S.; Zhang, A.; Thomas, V.; Stein, E.; Cohen, A.; Chauncey, R.; Nikkel, L.; Yin, M.T.; Liu, X.S.; et al. Discriminants of prevalent fractures in chronic kidney disease. J. Am. Soc. Nephrol. 2011, 22, 1560–1572. [Google Scholar] [CrossRef]
- Bauer, D.; Krege, J.; Lane, N.; Leary, E.; Libanati, C.; Miller, P.; Myers, G.; Silverman, S.; Vesper, H.W.; Lee, D.; et al. National Bone Health Alliance Bone Turnover Marker Project: Current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporos. Int. 2012, 23, 2425–2433. [Google Scholar] [CrossRef]
- Ferreira, M.A. Diagnosis of renal osteodystrophy: When and how to use biochemical markers and non-invasive methods; when bone biopsy is needed. Nephrol. Dial. Transpl. 2000, 15 (Suppl. S5), 8–14. [Google Scholar] [CrossRef]
- Leeming, D.J.; Alexandersen, P.; Karsdal, M.A.; Qvist, P.; Schaller, S.; Tankó, L.B. An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur. J. Clin. Pharmacol. 2006, 62, 781–792. [Google Scholar] [CrossRef]
- Behets, G.J.; Viaene, L.; Meijers, B.; Blocki, F.; Brandenburg, V.M.; Verhulst, A.; D’haese, P.C.; Evenepoel, P. Circulating levels of sclerostin but not DKK1 associate with laboratory parameters of CKD-MBD. PLoS ONE 2017, 12, e0176411. [Google Scholar] [CrossRef]
- Baron, R.; Rawadi, G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 2007, 148, 2635–2643. [Google Scholar] [CrossRef]
- Winkler, D.G.; Sutherland, M.K.; Geoghegan, J.C.; Yu, C.; Hayes, T.; Skonier, J.E.; Shpektor, D.; Jonas, M.; Kovacevich, B.R.; Staehling-Hampton, K.; et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003, 22, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Kusu, N.; Laurikkala, J.; Imanishi, M.; Usui, H.; Konishi, M.; Miyake, A.; Thesleff, I.; Itoh, N. Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J. Biol. Chem. 2003, 278, 24113–24117. [Google Scholar] [CrossRef] [PubMed]
- Pinzone, J.J.; Hall, B.M.; Thudi, N.K.; Vonau, M.; Qiang, Y.-W.; Rosol, T.J.; Shaughnessy, J.D., Jr. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 2009, 113, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, V.M.; Kramann, R.; Koos, R.; Krüger, T.; Schurgers, L.; Mühlenbruch, G.; Hübner, S.; Gladziwa, U.; Drechsler, C.; Ketteler, M. Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: A cross-sectional study. BMC Nephrol. 2013, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, V.M.; D’haese, P.; Deck, A.; Mekahli, D.; Meijers, B.; Neven, E.; Evenepoel, P. From skeletal to cardiovascular disease in 12 steps-the evolution of sclerostin as a major player in CKD-MBD. Pediatr. Nephrol. 2016, 31, 195–206. [Google Scholar] [CrossRef]
- Cejka, D.; Herberth, J.; Branscum, A.J.; Fardo, D.W.; Monier-Faugere, M.-C.; Diarra, D.; Haas, M.; Malluche, H.H. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin. J. Am. Soc. Nephrol. 2011, 6, 877–882. [Google Scholar] [CrossRef]
- Delanaye, P.; Krzesinski, J.-M.; Warling, X.; Moonen, M.; Smelten, N.; Médart, L.; Bruyere, O.; Reginster, J.-Y.; Pottel, H.; Cavalier, E. Clinical and biological determinants of sclerostin plasma concentration in hemodialysis patients. Nephron Clin. Pract. 2014, 128, 127–134. [Google Scholar] [CrossRef]
- Viaene, L.; Behets, G.J.; Claes, K.; Meijers, B.; Blocki, F.; Brandenburg, V.; Evenepoel, P.; D’Haese, P.C. Sclerostin: Another bone-related protein related to all-cause mortality in haemodialysis? Nephrol. Dial. Transpl. 2013, 28, 3024–3030. [Google Scholar] [CrossRef]
- Kanbay, M.; Siriopol, D.; Saglam, M.; Kurt, Y.G.; Gok, M.; Cetinkaya, H.; Karaman, M.; Unal, H.U.; Oguz, Y.; Sari, S.; et al. Serum sclerostin and adverse outcomes in nondialyzed chronic kidney disease patients. J. Clin. Endocrinol. Metab. 2014, 99, E1854–E1861. [Google Scholar] [CrossRef]
- Gonçalves, F.L.C.; Elias, R.M.; dos Reis, L.M.; Graciolli, F.G.; Zampieri, F.G.; Oliveira, R.B.; Jorgetti, V.; Moysés, R.M. Serum sclerostin is an independent predictor of mortality in hemodialysis patients. BMC Nephrol. 2014, 15, 190. [Google Scholar] [CrossRef]
- Drechsler, C.; Evenepoel, P.; Vervloet, M.G.; Wanner, C.; Ketteler, M.; Marx, N.; Floege, J.; Dekker, F.W.; Brandenburg, V.M. High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: Results from the NECOSAD study. Nephrol. Dial. Transpl. 2015, 30, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-Q.; Lin, S.; Xu, P.-C.; Zheng, Z.-F.; Jia, J.-Y. Serum osteoprotegerin measurement for early diagnosis of chronic kidney disease-mineral and bone disorder. Nephrology 2011, 16, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Gršković, A.; Ćelić, T.; Španjol, J.; Markić, D.; Devčić, B.; Bobinac, D.; Rački, S. Osteoprotegerin as an Early Sign of Chronic Kidney Disease-Mineral and Bone Disorder. Acta Clin. Croat. 2023, 62 (Suppl. S2), 46–52. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, J.; Stopiński, M.; Mucha, K.; Pac, M.; Gołębiowski, M.; A Niewczas, M.; Pączek, L.; Foroncewicz, B. Circulating Osteoprotegerin in Chronic Kidney Disease and All-Cause Mortality. Int. J. Gen. Med. 2021, 14, 2413–2420. [Google Scholar] [CrossRef]
- Jirak, P.; Stechemesser, L.; Moré, E.; Franzen, M.; Topf, A.; Mirna, M.; Paar, V.; Pistulli, R.; Kretzschmar, D.; Wernly, B.; et al. Chapter Three—Clinical Implications of Fetuin-A. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 79–130. [Google Scholar]
- Hamano, T.; Matsui, I.; Mikami, S.; Tomida, K.; Fujii, N.; Imai, E.; Rakugi, H.; Isaka, Y. Fetuin-mineral complex reflects extraosseous calcification stress in CKD. J. Am. Soc. Nephrol. 2010, 21, 1998–2007. [Google Scholar] [CrossRef]
- Koos, R.; Brandenburg, V.; Mahnken, A.H.; Mühlenbruch, G.; Stanzel, S.; Günther, R.W.; Floege, J.; Jahnen-Dechent, W.; Kelm, M.; Kühl, H.P. Association of fetuin-A levels with the progression of aortic valve calcification in non-dialyzed patients. Eur. Hear. J. 2009, 30, 2054–2061. [Google Scholar] [CrossRef]
- Sevinc, C.; Yilmaz, G.; Ustundag, S. The relationship between calcification inhibitor levels in chronic kidney disease and the development of atherosclerosis. Ren. Fail. 2021, 43, 1349–1358. [Google Scholar] [CrossRef]
- Cottone, S.; Lorito, M.C.; Riccobene, R.; Nardi, E.; Mule’, G.; Buscemi, S.; Geraci, C.; Guarneri, M.; Arsena, R.; Cerasola, G. Oxidative stress, inflammation and cardiovascular disease in chronic renal failure. J. Nephrol. 2008, 21, 175–179. [Google Scholar]
- Cottone, S.; Nardi, E.; Mulè, G.; Vadalà, A.; Lorito, M.; Riccobene, R.; Palermo, A.; Arsena, R.; Guarneri, M.; Cerasola, G. Association between biomarkers of inflammation and left ventricular hypertrophy in moderate chronic kidney disease. Clin. Nephrol. 2007, 67, 209–216. [Google Scholar] [CrossRef]
- Ketteler, M.; Bongartz, P.; Westenfeld, R.; Wildberger, J.E.; Mahnken, A.H.; Böhm, R.; Metzger, T.; Wanner, C.; Jahnen-Dechent, W.; Floege, J. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: A cross-sectional study. Lancet 2003, 361, 827–833. [Google Scholar] [CrossRef]
- Zhou, Z.; Ji, Y.; Ju, H.; Chen, H.; Sun, M. Circulating Fetuin-A and Risk of All-Cause Mortality in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Front. Physiol. 2019, 10, 966. [Google Scholar] [CrossRef]
- Nitta, K.; Hanafusa, N.; Akiyama, K.; Kawaguchi, Y.; Tsuchiya, K. Chronic Kidney Disease—Mineral and Bone Disorder (CKD-MBD), from Bench to Bedside. Kidney Dial. 2023, 3, 46–55. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carullo, N.; Sorbo, D.; Faga, T.; Pugliese, S.; Zicarelli, M.T.; Costa, D.; Ielapi, N.; Battaglia, Y.; Pisani, A.; Coppolino, G.; et al. Anemia and Mineral Bone Disorder in Kidney Disease Patients: The Role of FGF-23 and Other Related Factors. Int. J. Mol. Sci. 2024, 25, 12838. https://doi.org/10.3390/ijms252312838
Carullo N, Sorbo D, Faga T, Pugliese S, Zicarelli MT, Costa D, Ielapi N, Battaglia Y, Pisani A, Coppolino G, et al. Anemia and Mineral Bone Disorder in Kidney Disease Patients: The Role of FGF-23 and Other Related Factors. International Journal of Molecular Sciences. 2024; 25(23):12838. https://doi.org/10.3390/ijms252312838
Chicago/Turabian StyleCarullo, Nazareno, David Sorbo, Teresa Faga, Sara Pugliese, Maria Teresa Zicarelli, Davide Costa, Nicola Ielapi, Yuri Battaglia, Antonio Pisani, Giuseppe Coppolino, and et al. 2024. "Anemia and Mineral Bone Disorder in Kidney Disease Patients: The Role of FGF-23 and Other Related Factors" International Journal of Molecular Sciences 25, no. 23: 12838. https://doi.org/10.3390/ijms252312838
APA StyleCarullo, N., Sorbo, D., Faga, T., Pugliese, S., Zicarelli, M. T., Costa, D., Ielapi, N., Battaglia, Y., Pisani, A., Coppolino, G., Bolignano, D., Michael, A., Serra, R., & Andreucci, M. (2024). Anemia and Mineral Bone Disorder in Kidney Disease Patients: The Role of FGF-23 and Other Related Factors. International Journal of Molecular Sciences, 25(23), 12838. https://doi.org/10.3390/ijms252312838










