Unlocking the Potential of Pyrrole: Recent Advances in New Pyrrole-Containing Compounds with Antibacterial Potential
Abstract
1. Introduction
1.1. The Role of Nitrogen Heterocycles in the Discovery of New Antibacterials
1.2. Aim of This Work
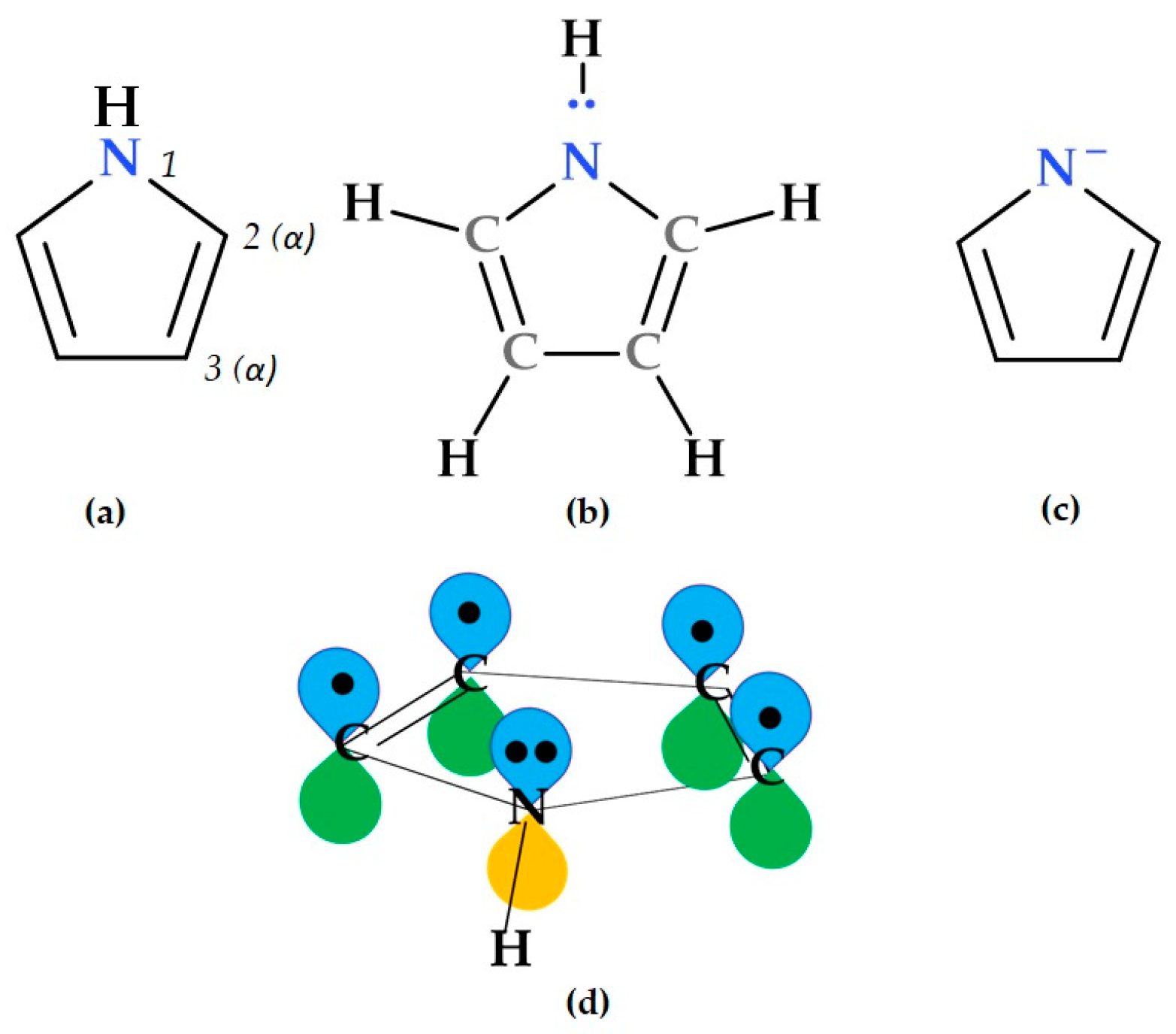
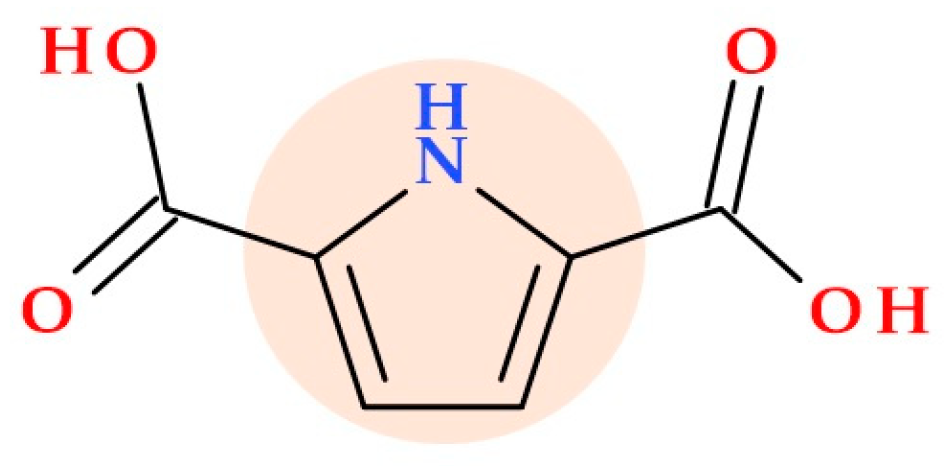
2. Short Description of the Pyrrole Heterocycle
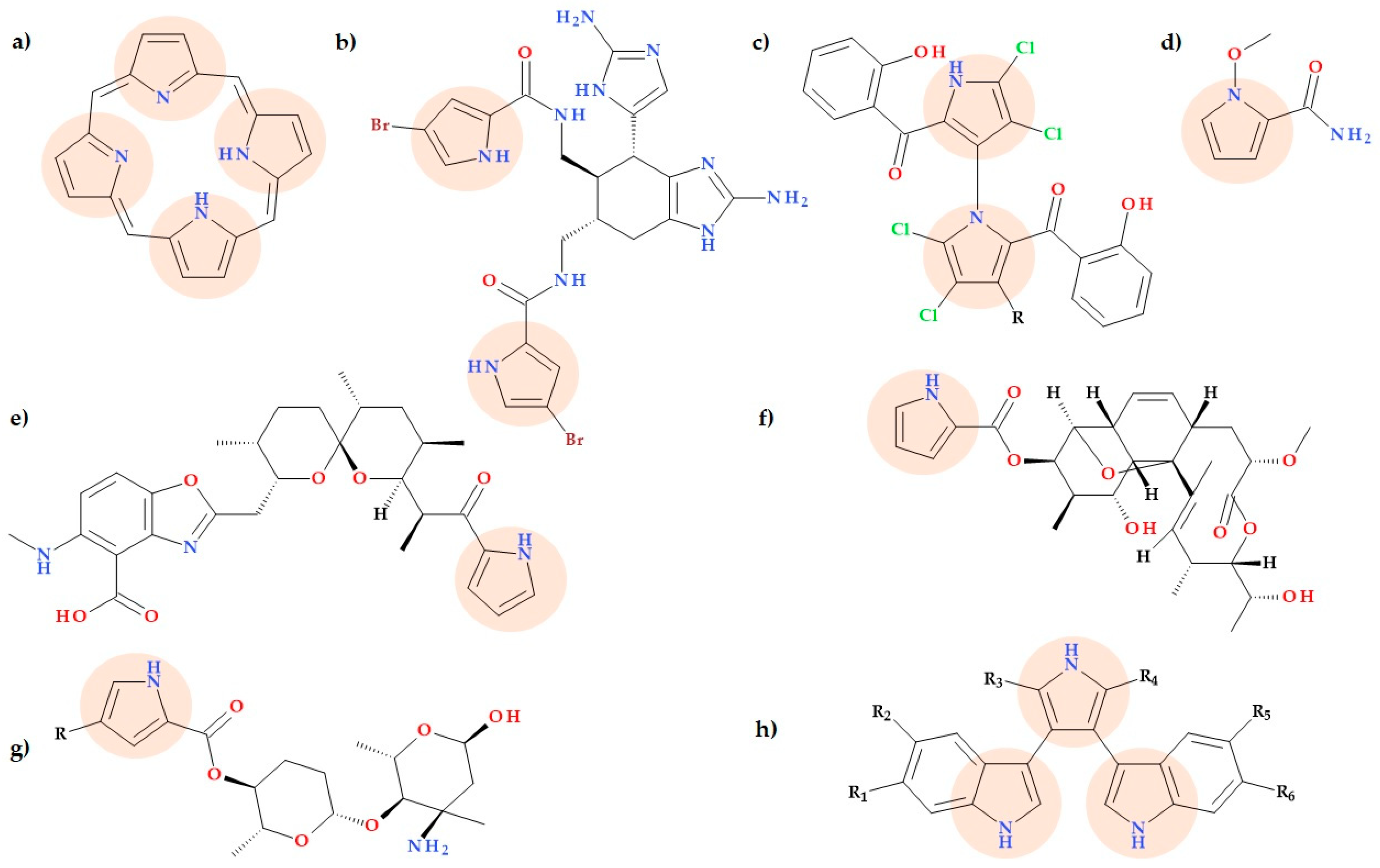
3. Natural Compounds Containing Pyrrole Heterocycle
3.1. Calcimycin
3.2. Lynamycins
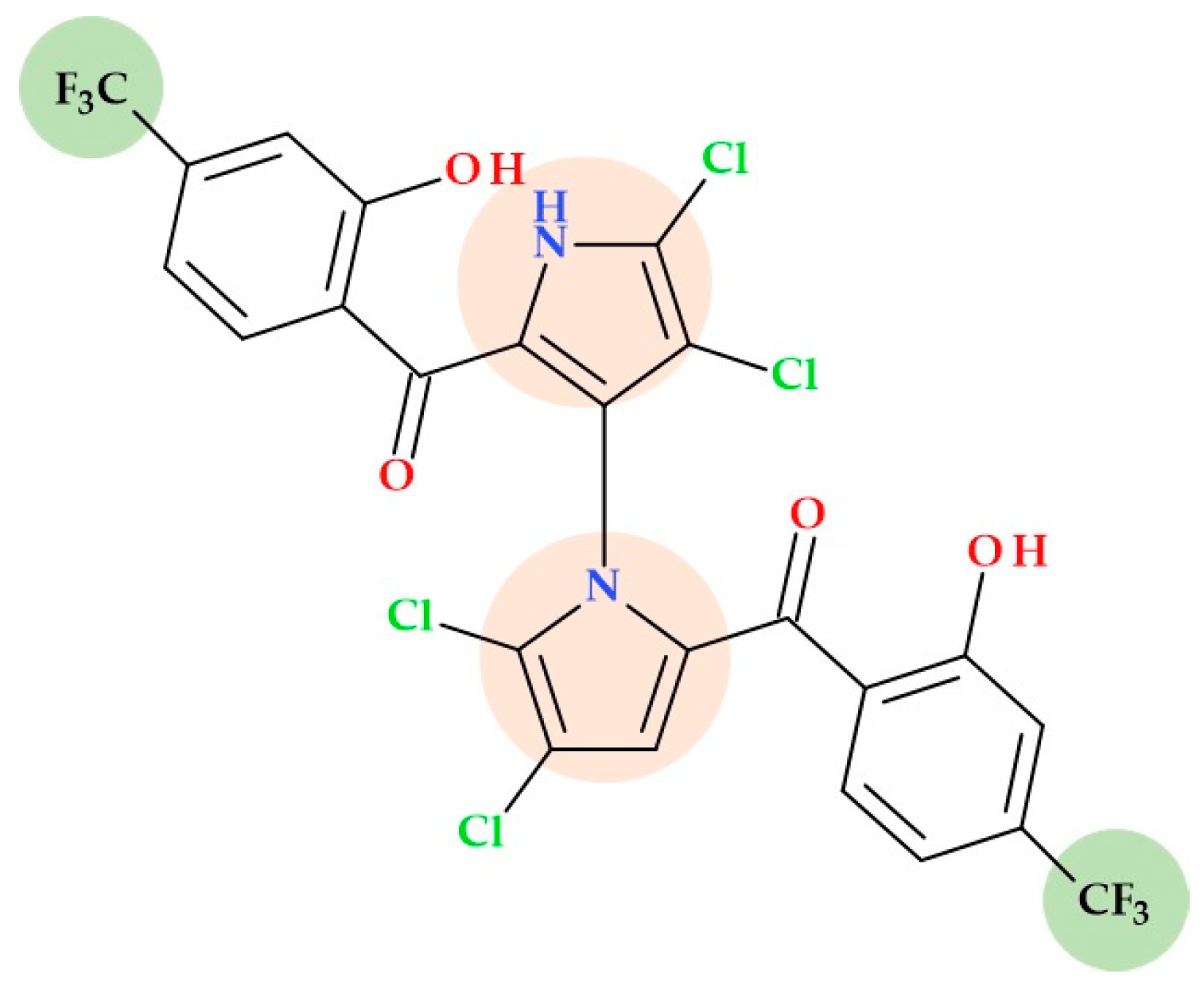
3.3. Marinopyrroles
3.4. 1-Methoxypyrrole-2-carboxamide
3.5. Nargenicin
3.6. Phallusialides A-E
3.7. Phenethylamine Alkaloids
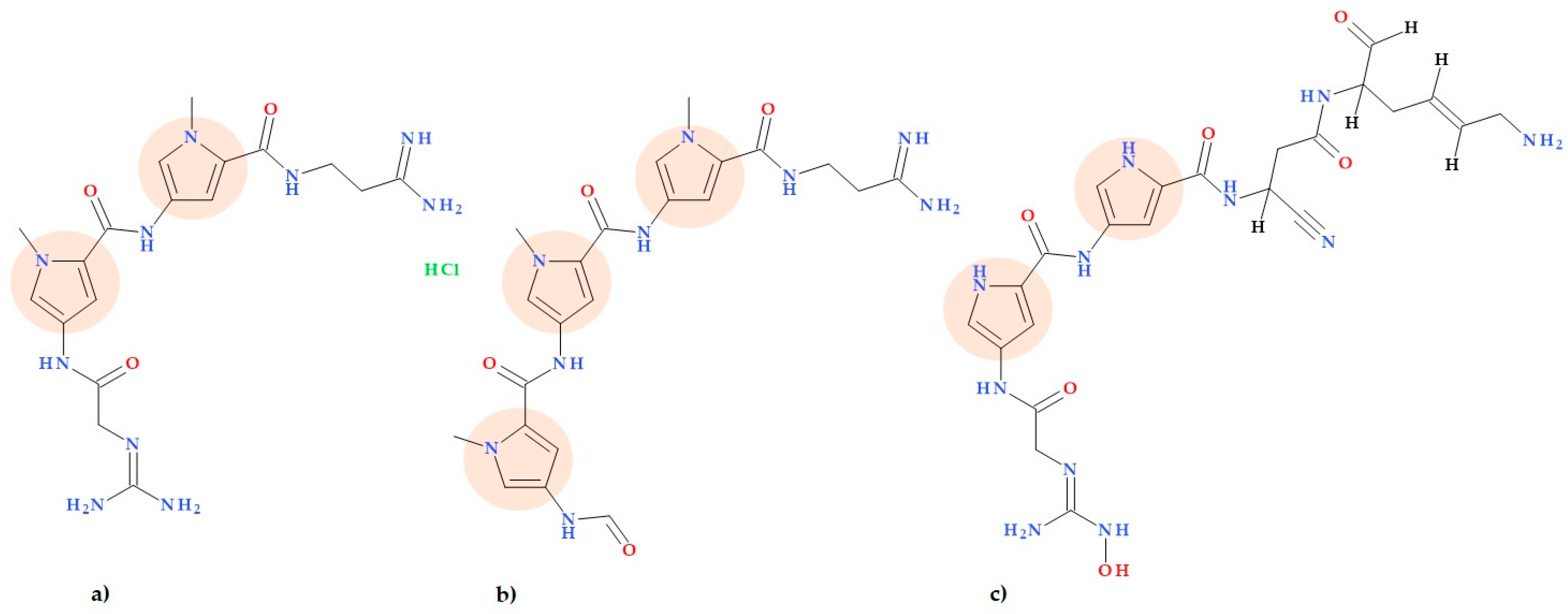
3.8. Pyrrolamides
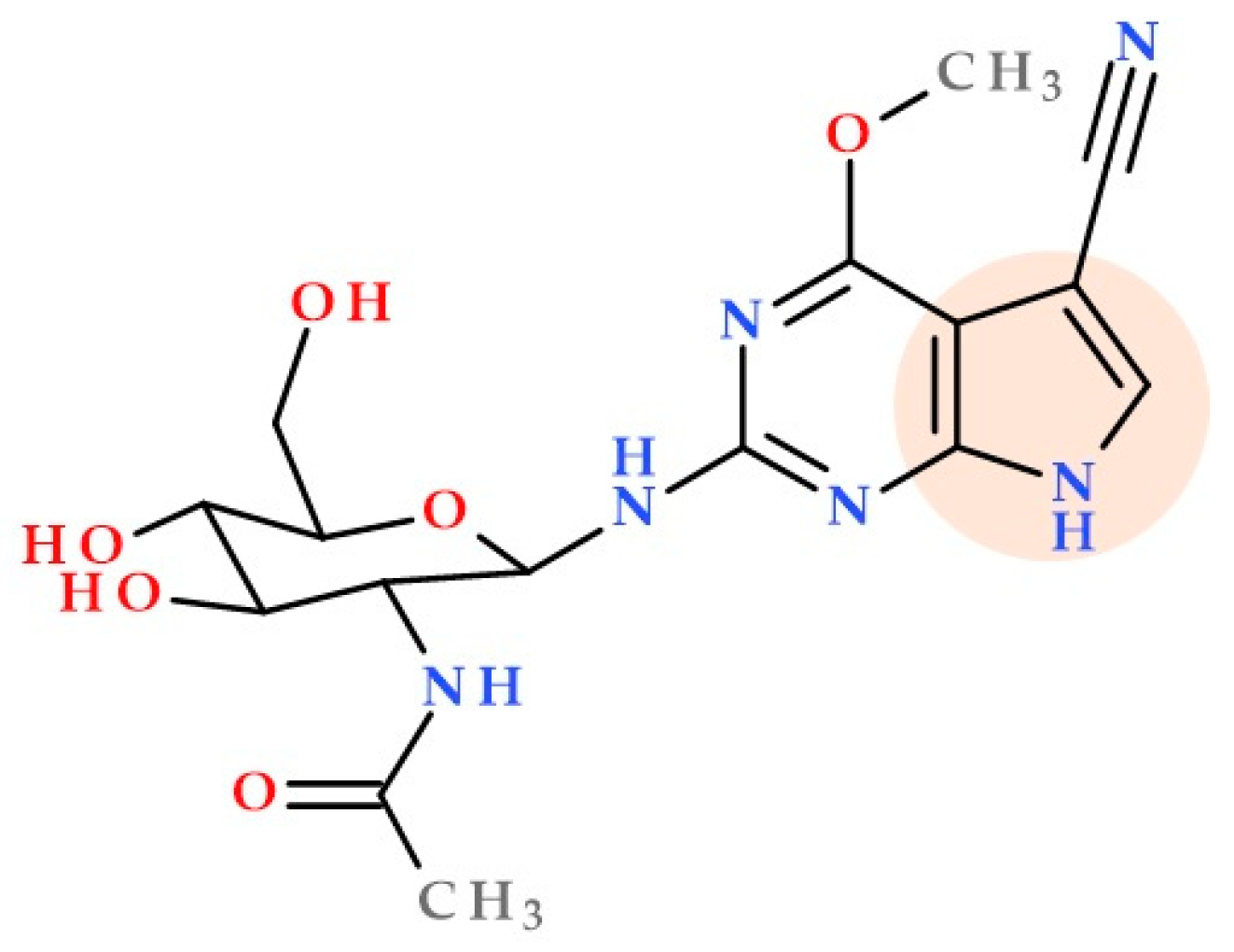
3.9. Pyrrolo-Pyrimidine Derivatives
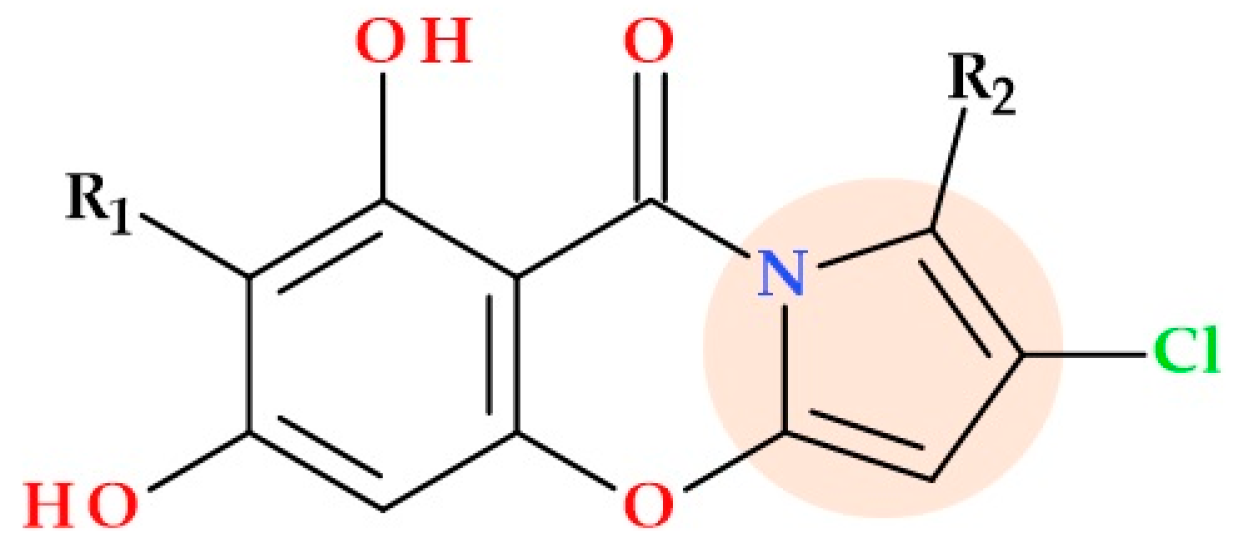
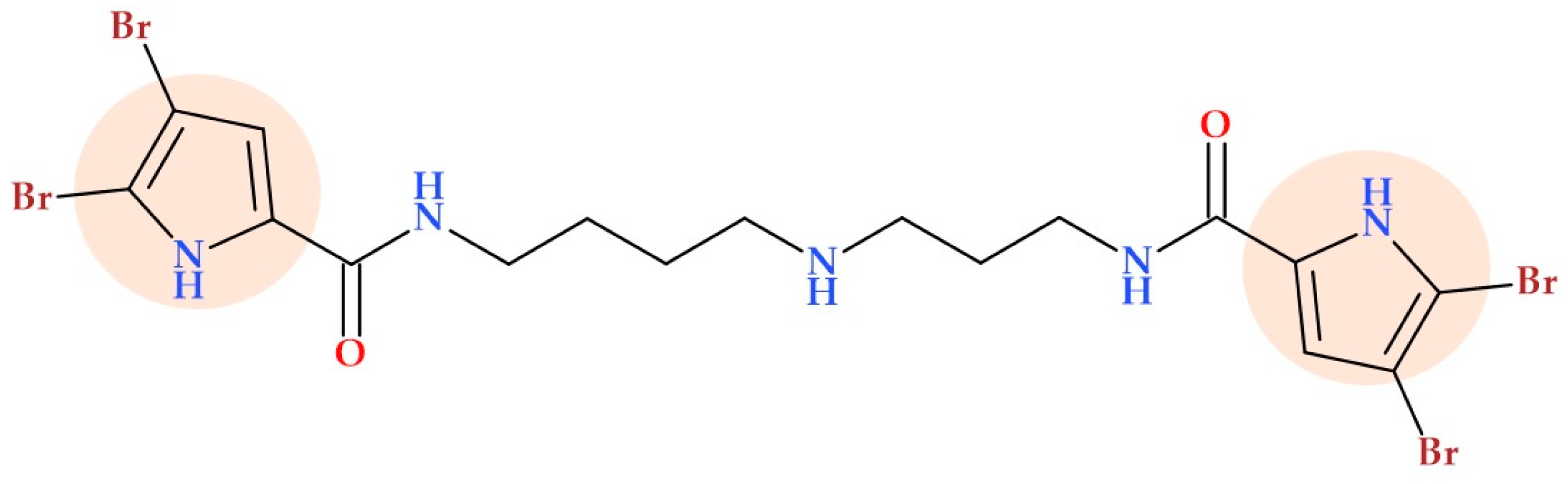
3.10. Pyrrollomycines
3.11. PT22
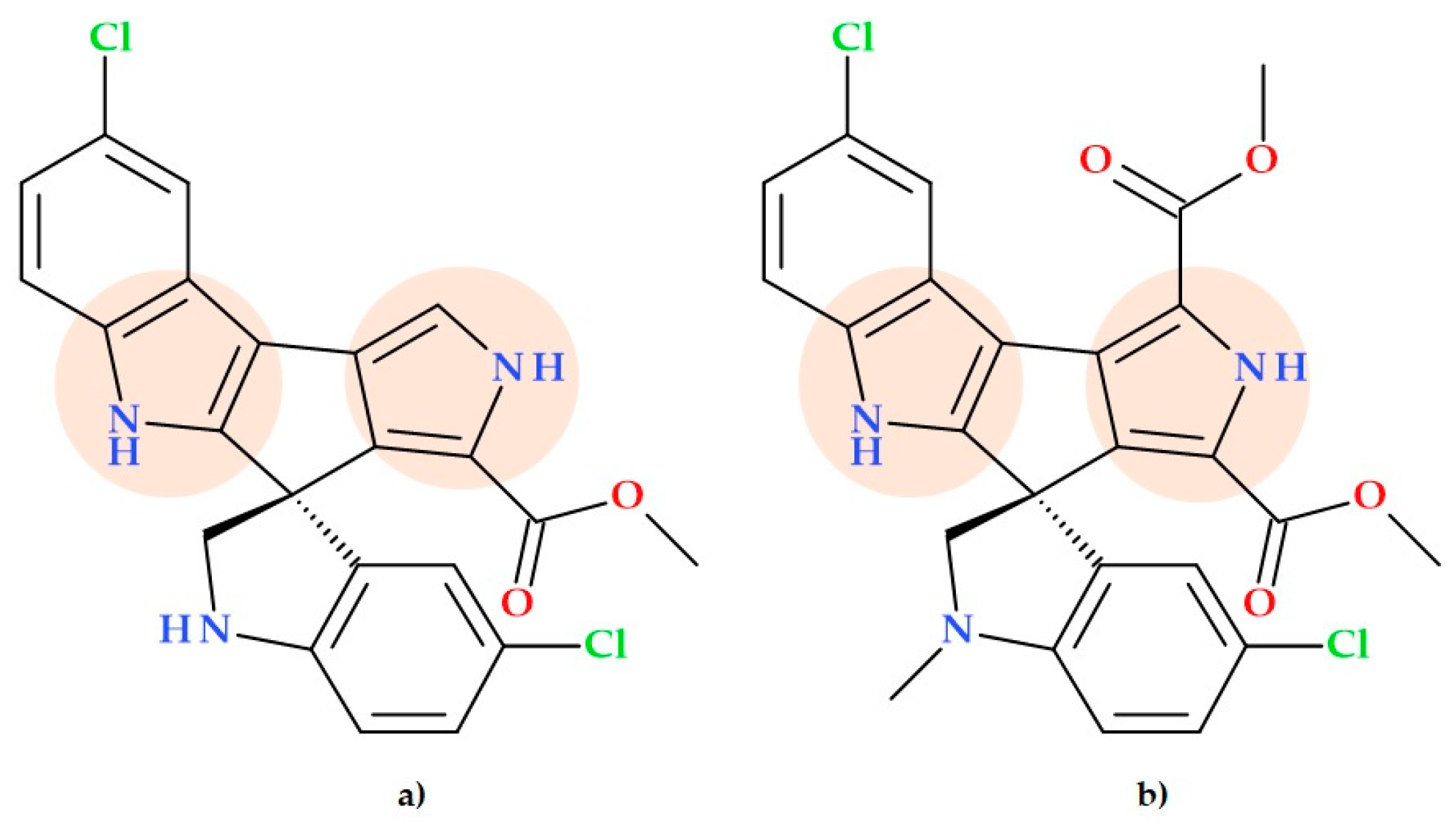
3.12. Spiroindimycins
3.13. Spirotetronate Polyketides
3.14. Streptopyrroles
4. Drugs Containing Pyrrole Heterocycle
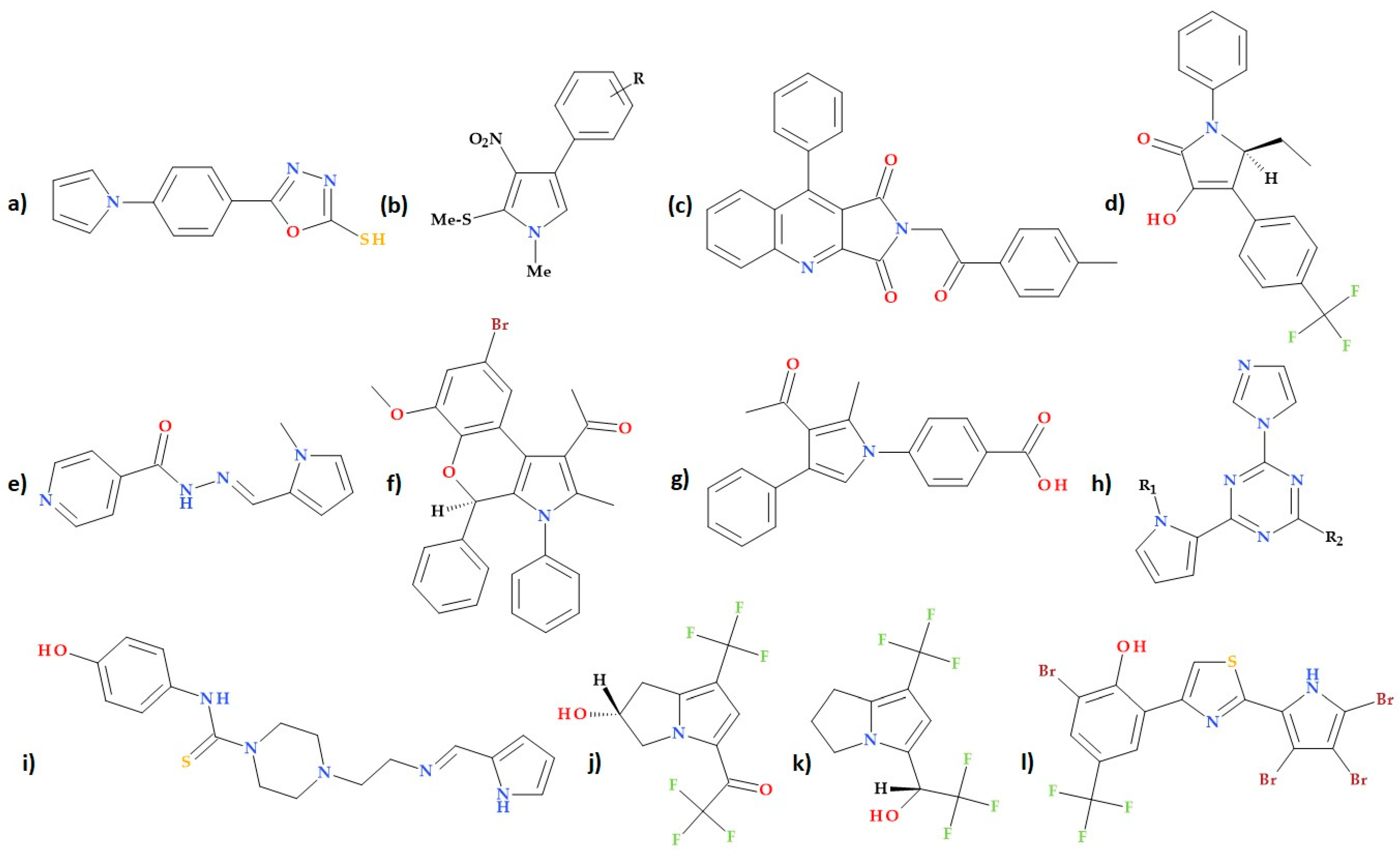
5. New Antibacterial Agents Containing Pyrrole in Their Molecular Structure
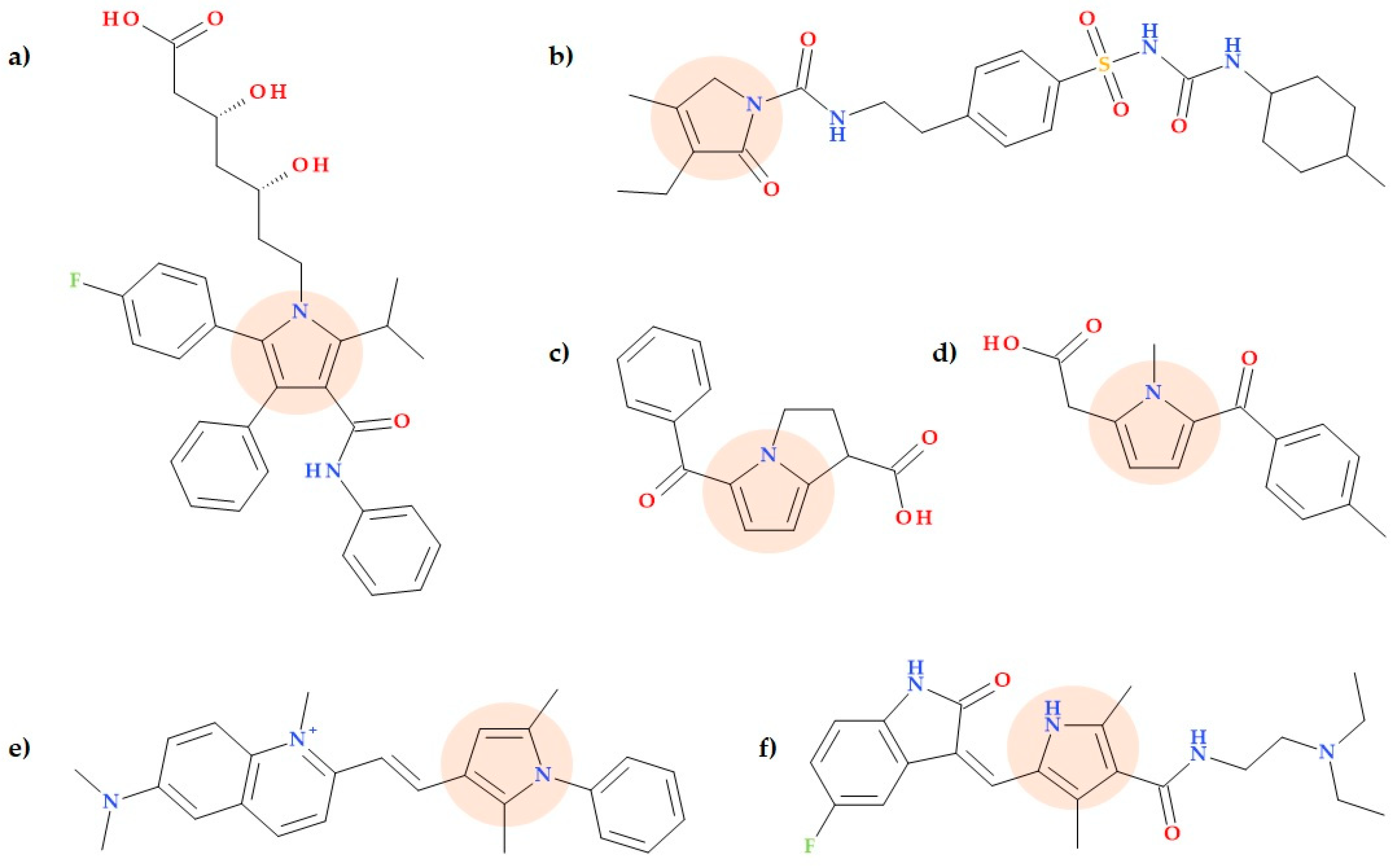
5.1. Alkaloids
5.2. Armeniaspirols and Derivatives
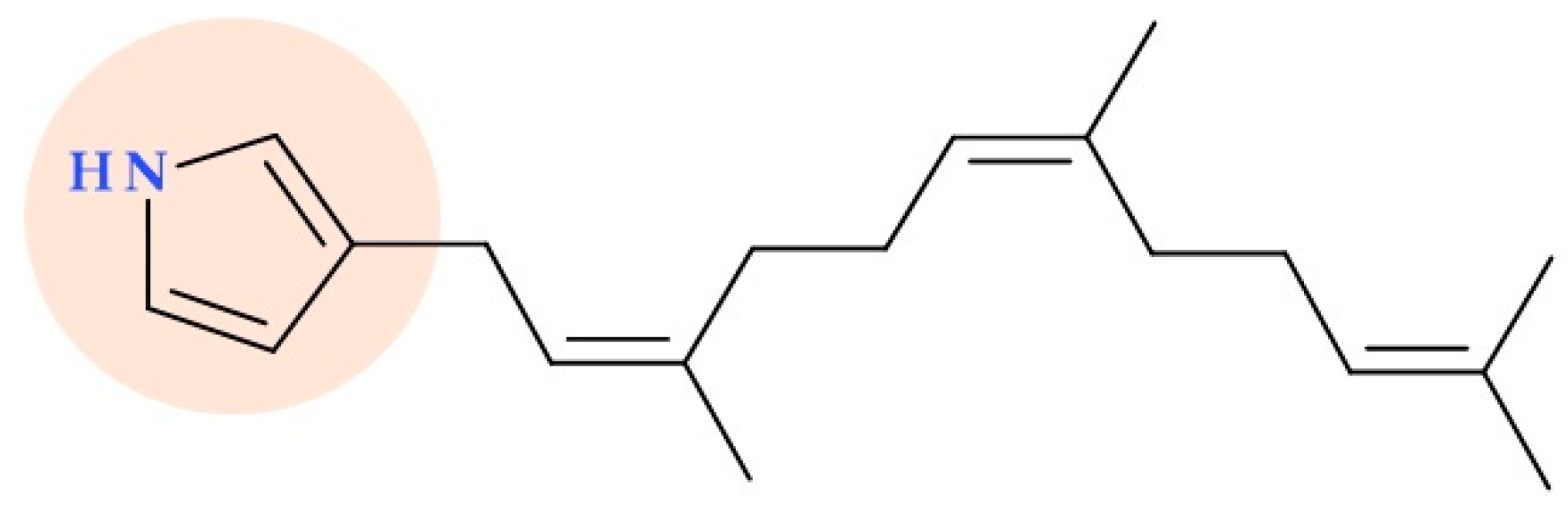
5.3. 3-Farnesylpyrrole
5.4. Marinopyrrole Derivatives
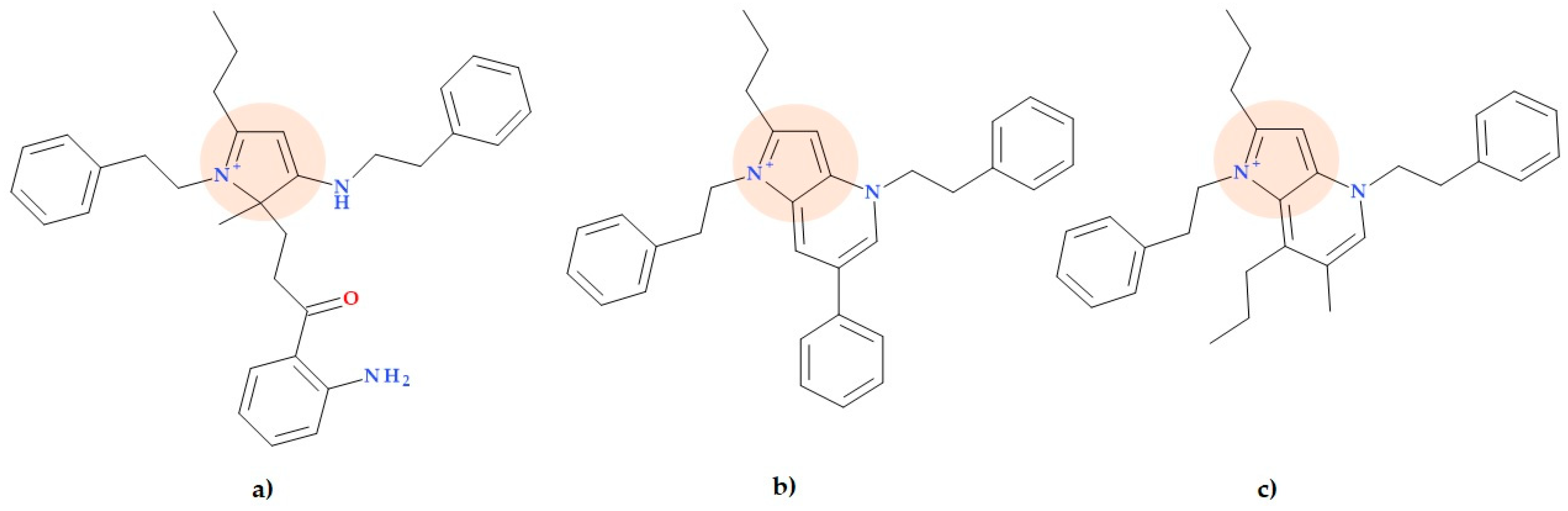
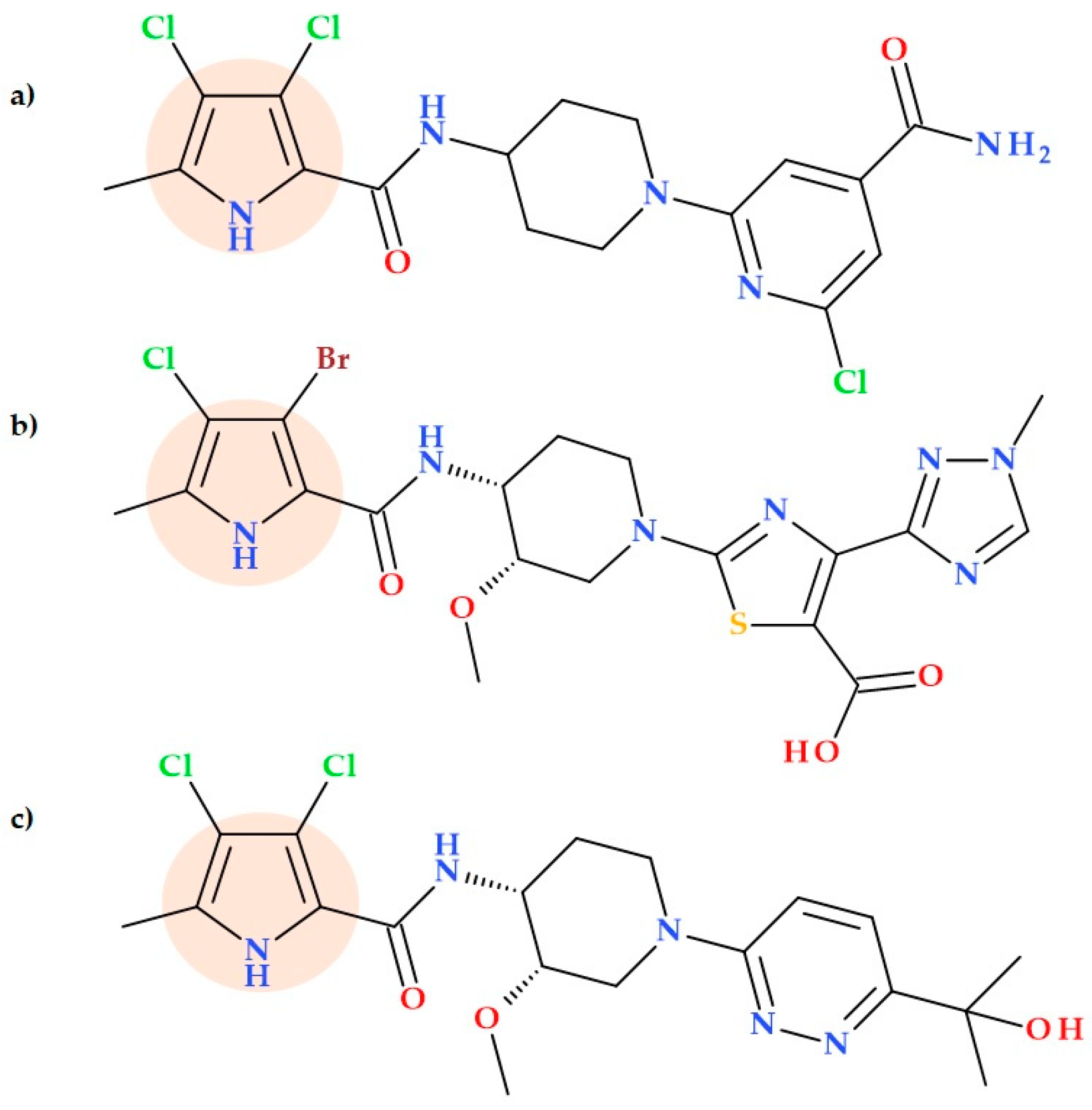
5.5. Pyrrolamides
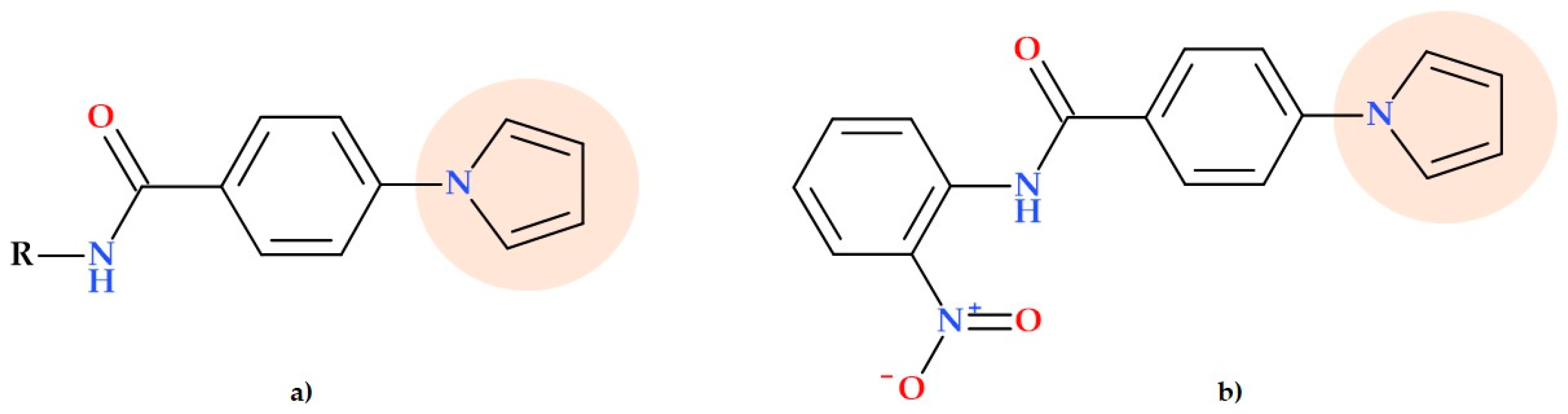
5.6. Pyrrolyl Benzamide Derivatives
5.7. Pyrrole-2-carboxylate and Pyrrole-2-carboxamide Derivatives
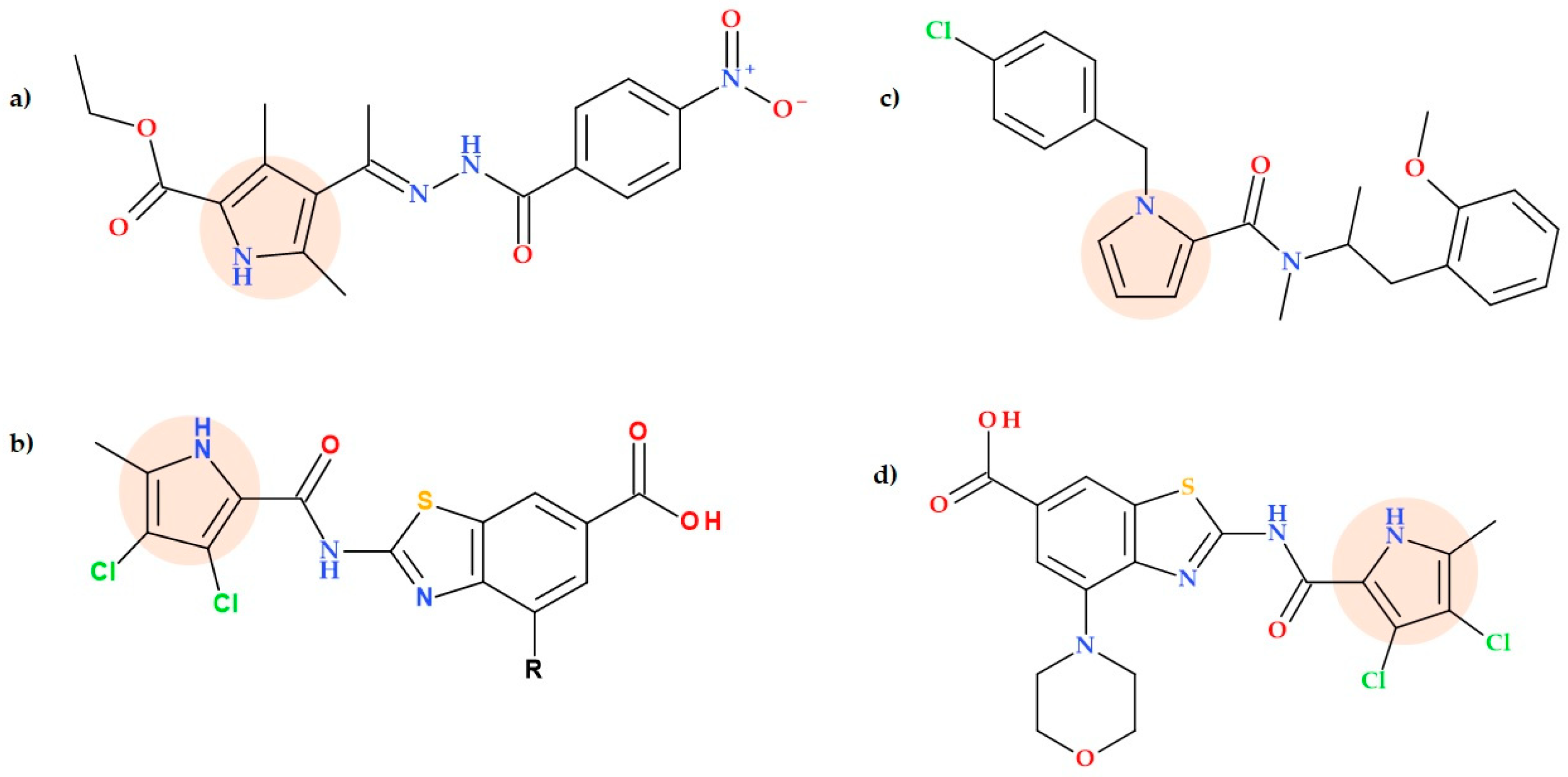
- -
- 1-(4-Chlorobenzyl)-N-(4-methoxybenzyl)-1H-pyrrole-2-carboxamide;
- -
- 1-(4-Chlorobenzyl)-N-(4-iodobenzyl)-1H-pyrrole-2-carboxamide;
- -
- 1-(4-Chlorobenzyl)-N-(1-(2-methoxyphenyl)propan-2-yl)-N-methyl-1H-pyrrole-2-carboxamide;
- -
- 1’-(1-(4-Chlorobenzyl)-1H-pyrrole-2-carbonyl)spiro[chroman2,4’-piperidin]-4-one;
- -
- 7-Bromo-1’-(1-(4-chlorobenzyl)-1H-pyrrole-2-carbonyl)spiro[chroman-2,4’-piperidin]-4-one;
- -
- Tert-butyl 4-(1-(4-chlorobenzyl)-1H-pyrrole-2-carboxamido)piperidine-1-carboxylate;
- -
- 1-(4-Chlorobenzyl)-N-cyclohexyl-1H-pyrrole-2-carboxamide.
5.8. Pyrrole-3-carboxaldehyde Derivatives
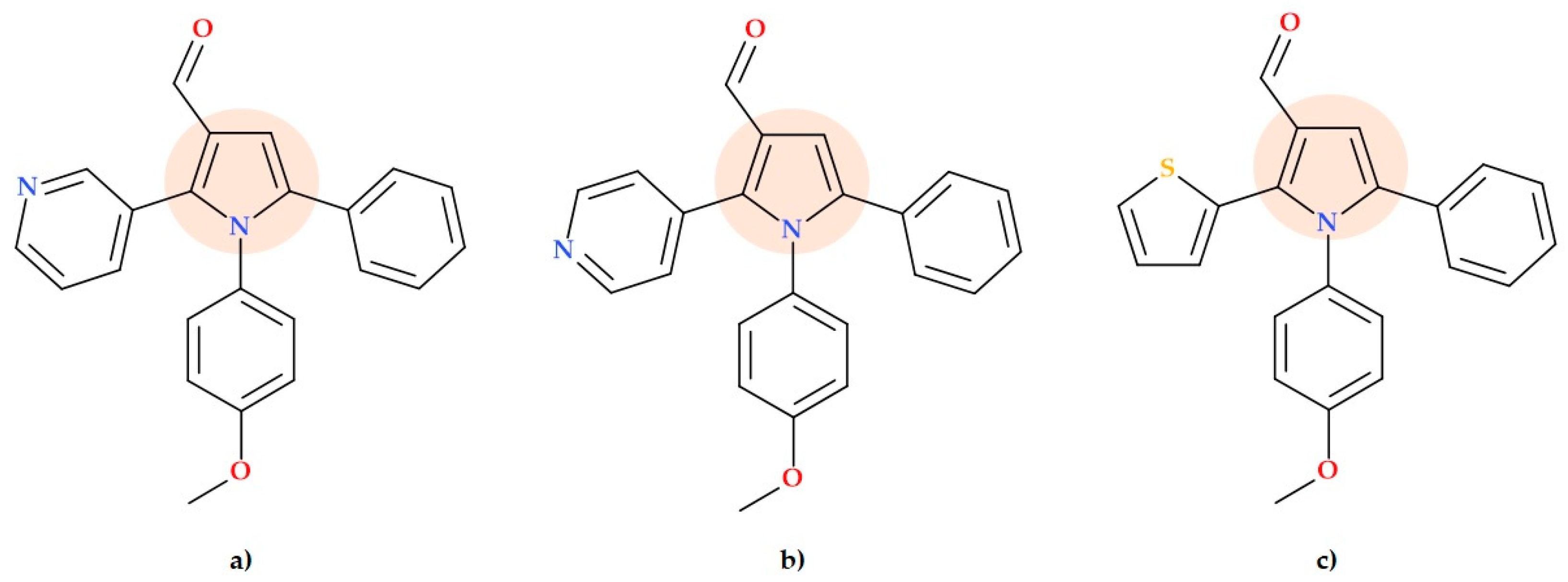
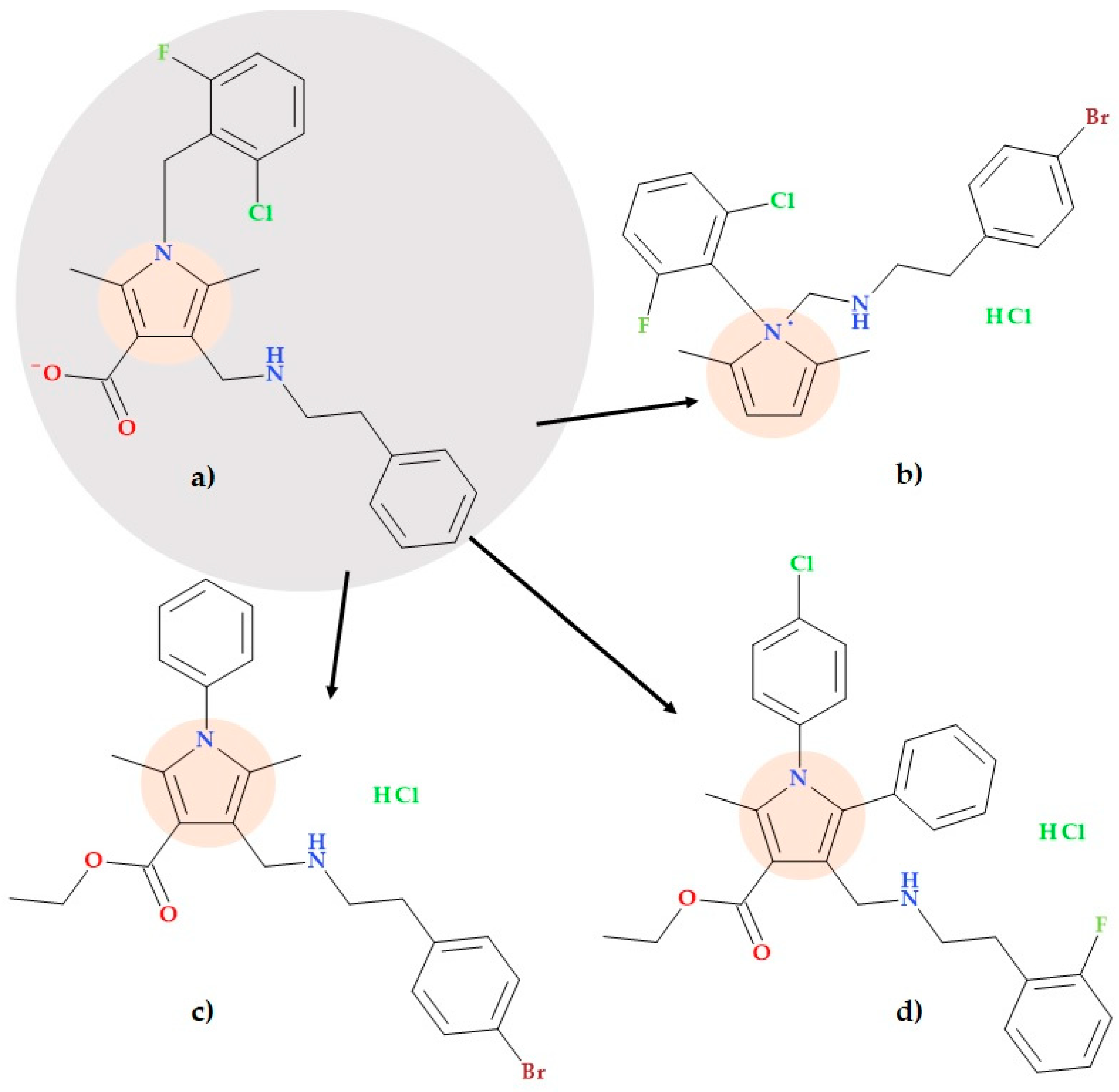
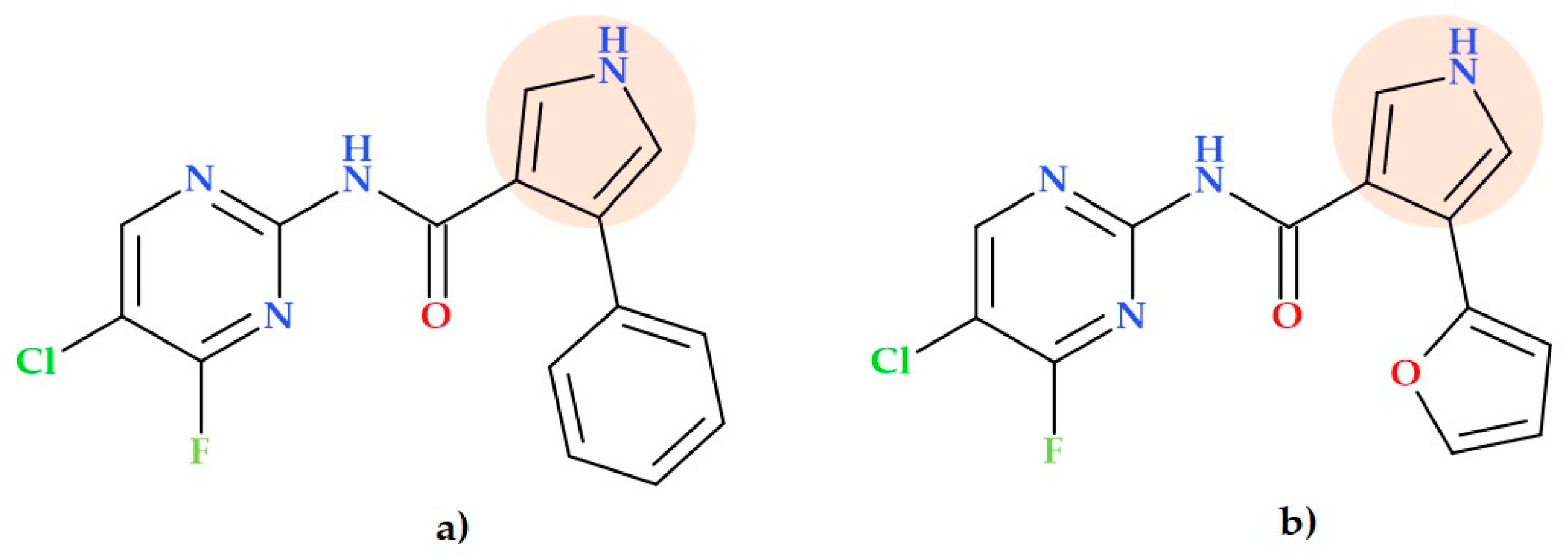
5.9. Pyrrole-3-carboxylates
- -
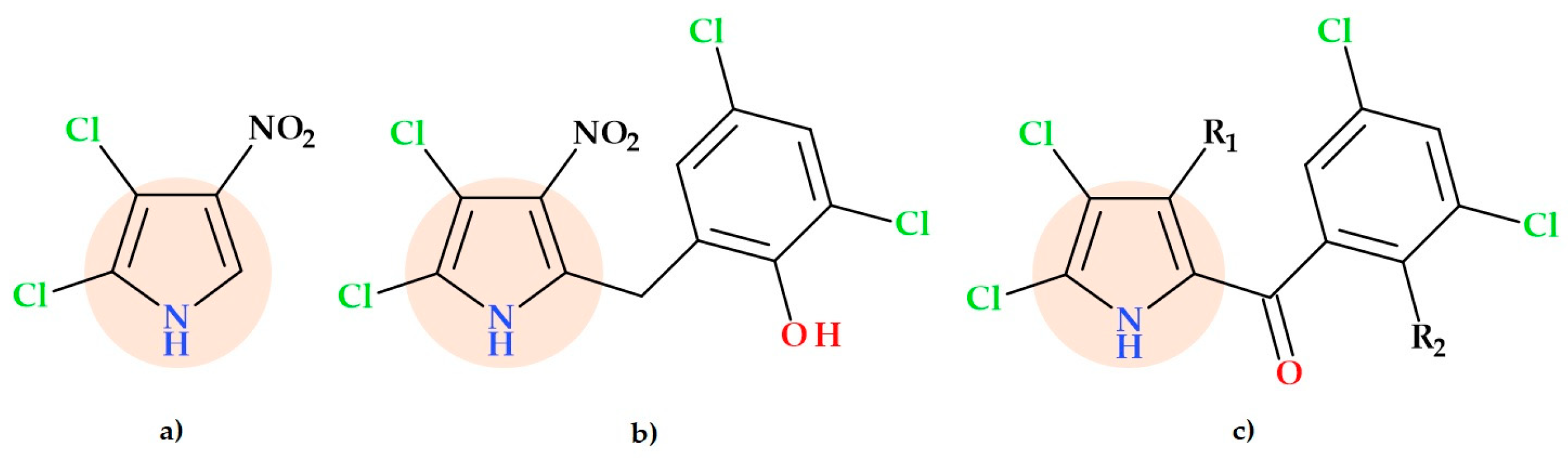
- 2-(4-Bromophenyl)-N-((1-(2-chloro-6-fluorophenyl)-2,5-dimethyl-1H-pyrrolyl)methyl)ethan-1-amine hydrochloride (Figure 19b);
- -
- Ethyl 4-(((4-bromophenethyl)amino)methyl)-2,5-dimethyl-1-phenyl-1H-pyrrole-3-carboxylate hydrochloride (Figure 19c);
- -
- Ethyl 1-(4-chlorophenyl)-4-(((2-fluorophenethyl)amino)methyl)-2-methyl-5-phenyl-1H-pyrrole-3-carboxylate hydrochloride (Figure 19d).
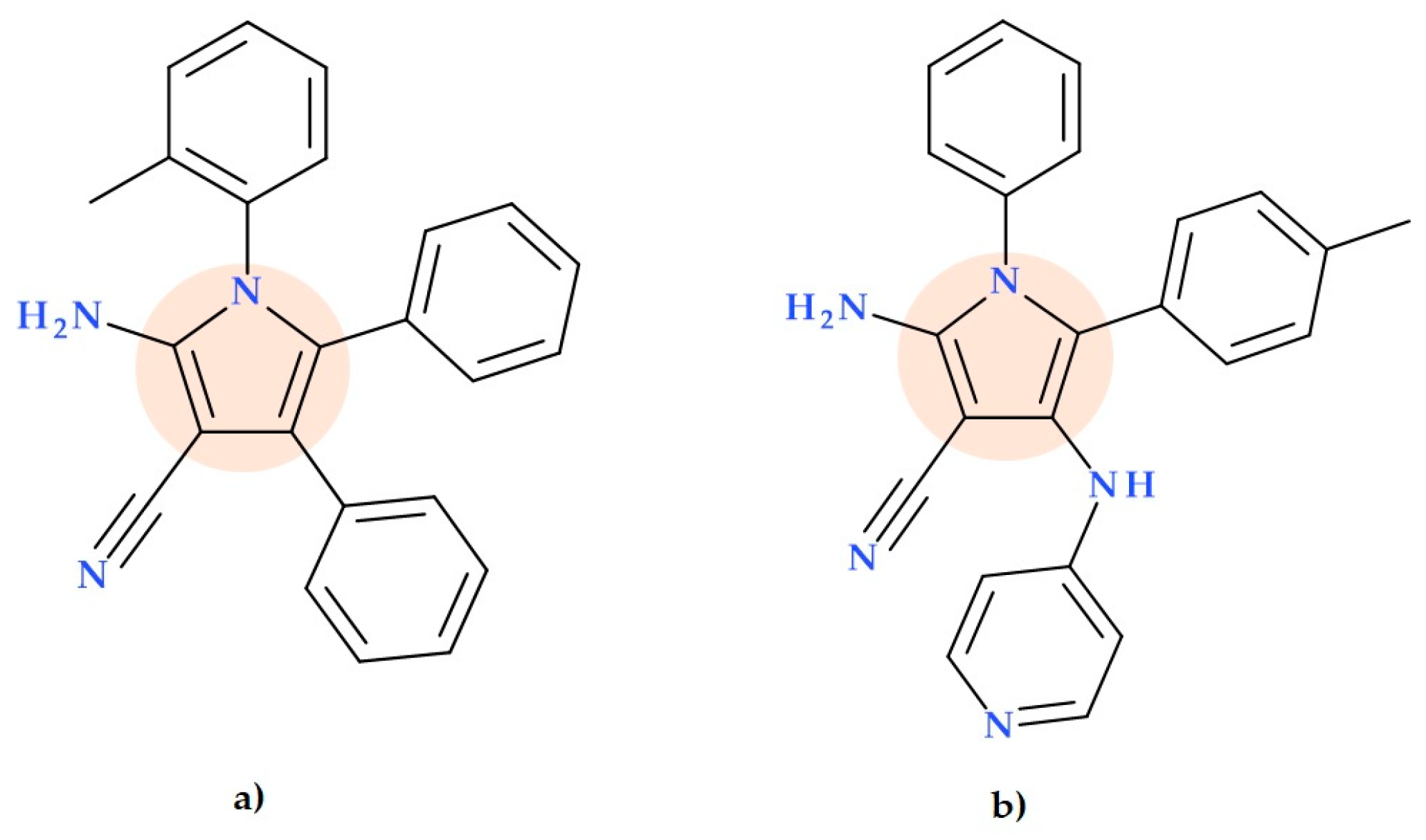
5.10. Pyrrole-3-carbonitriles
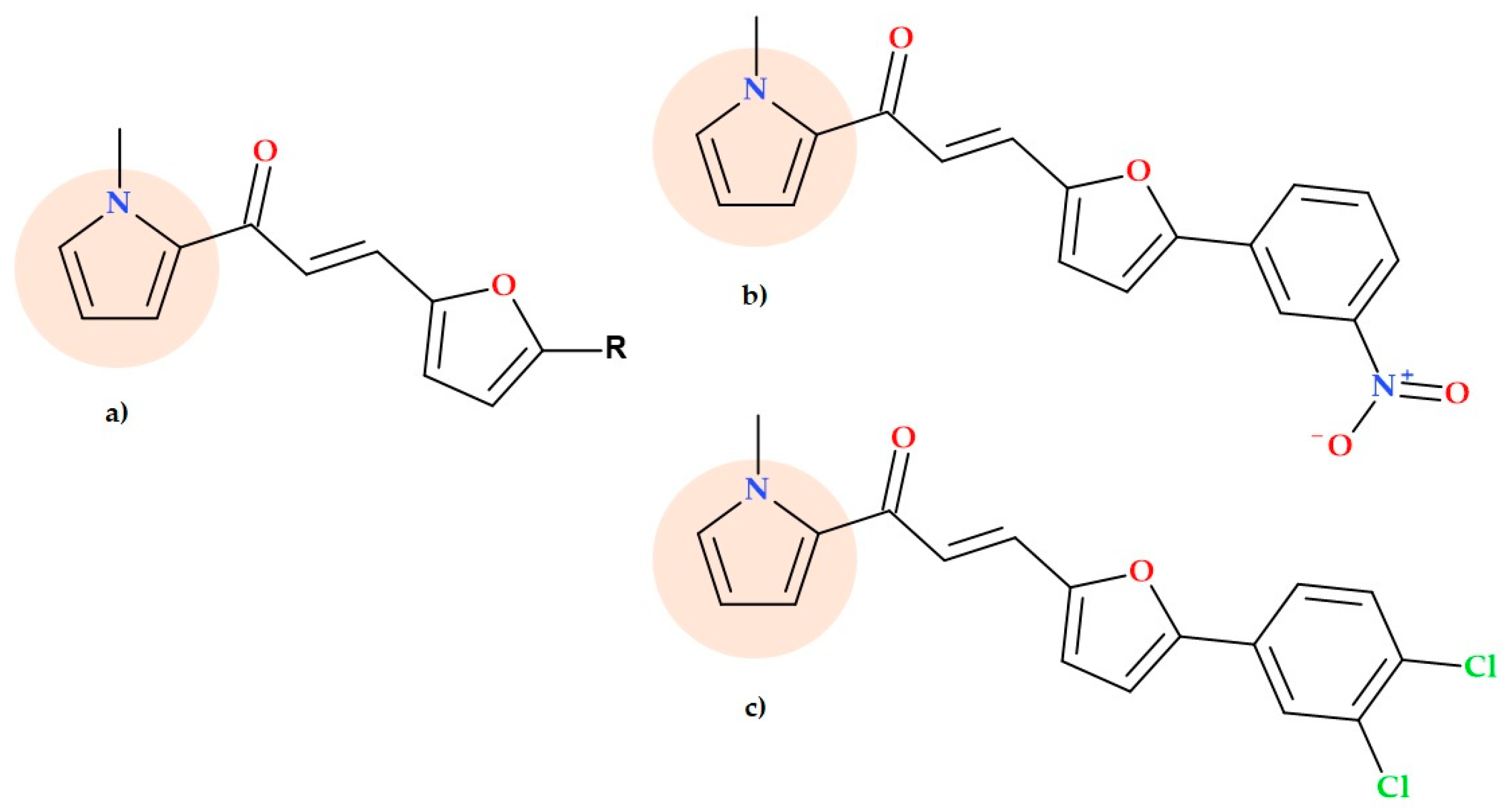
5.11. Pyrrole-Based Chalcone Derivatives
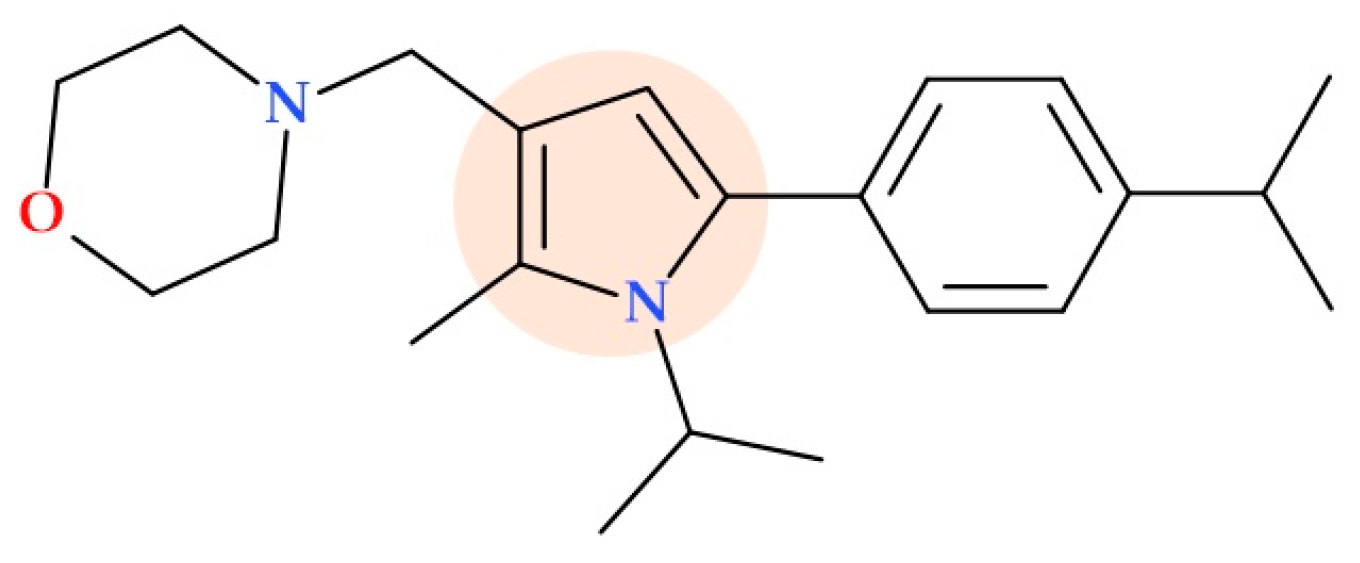
5.12. Pyrrole Morpholine Derivatives
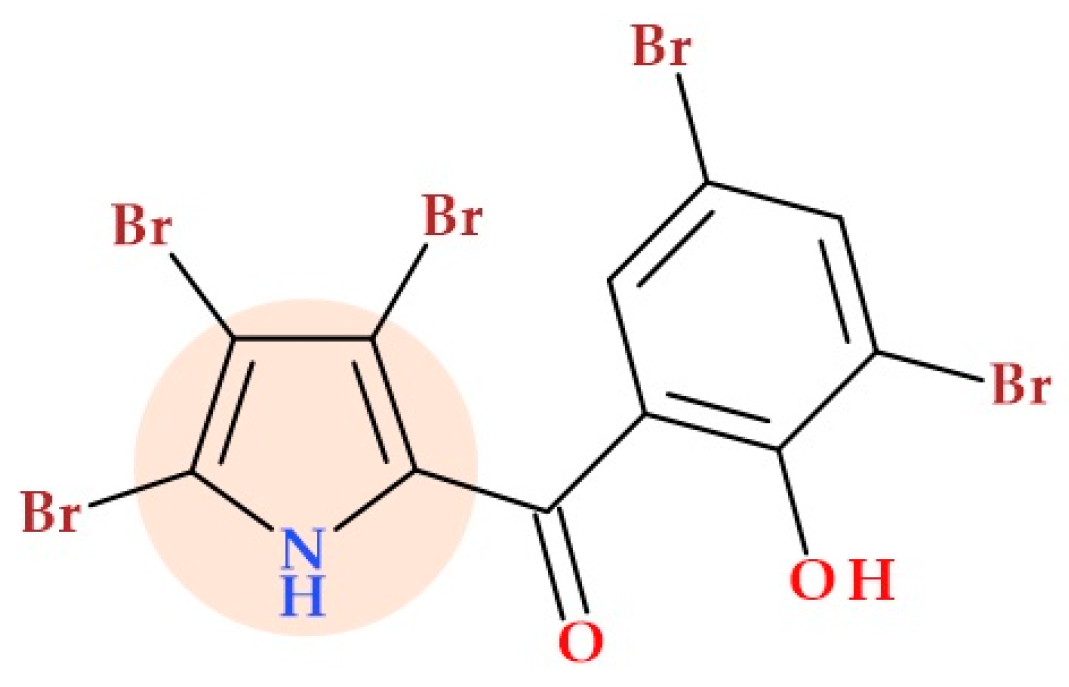
5.13. Pyrrolomycin Derivatives
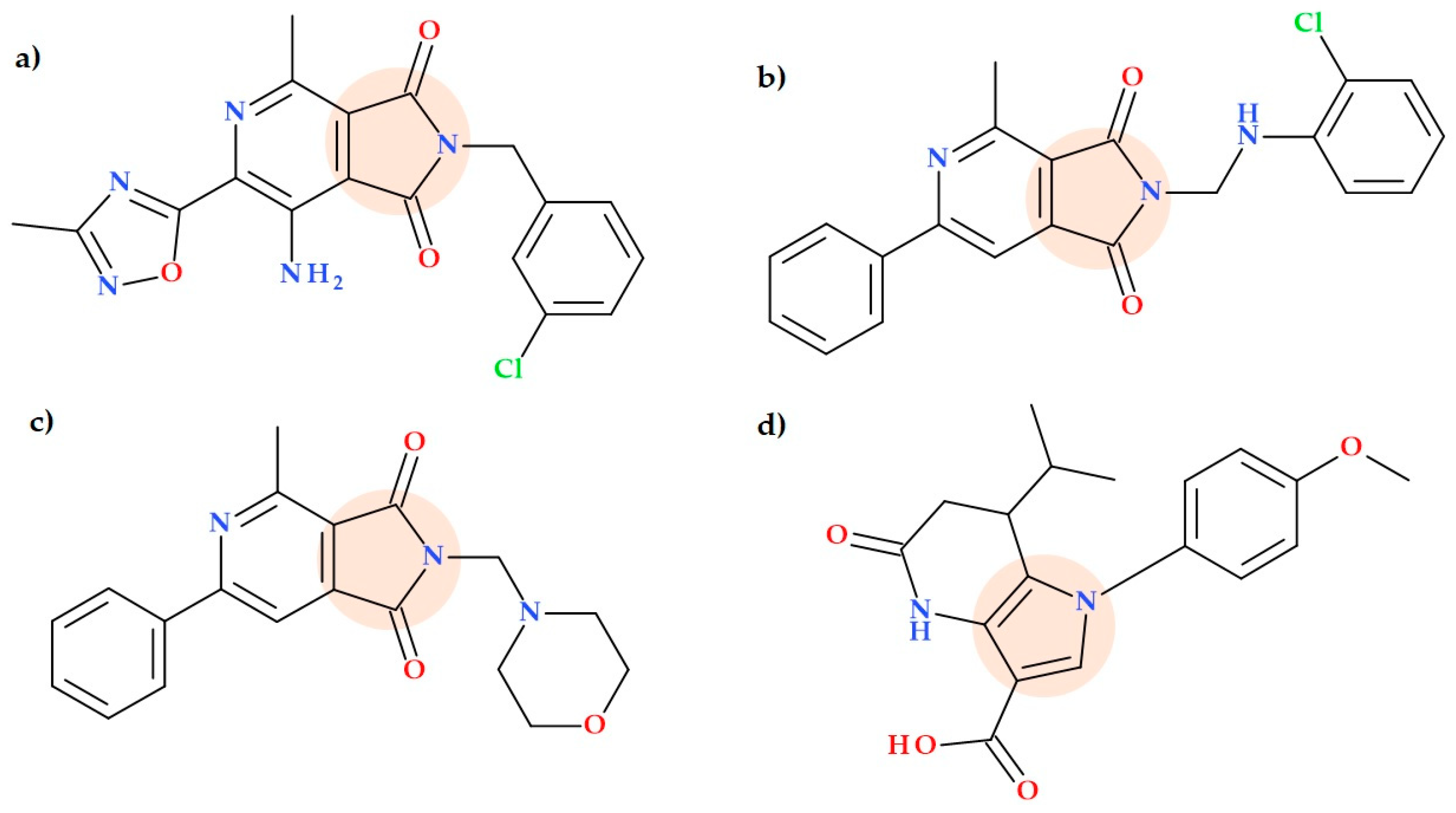
5.14. Pyrrolo-Pyridine Derivatives
5.15. Pyrrolyl Pyrimidine Derivatives
5.16. Pyrrolo-Pyrimidine Derivatives
5.17. Pyrrole Polymers
5.18. Other Pyrrole-Containing Compounds
5.19. Hybrids and Conjugates
5.19.1. Curcumin-Based Pyrrole Conjugates
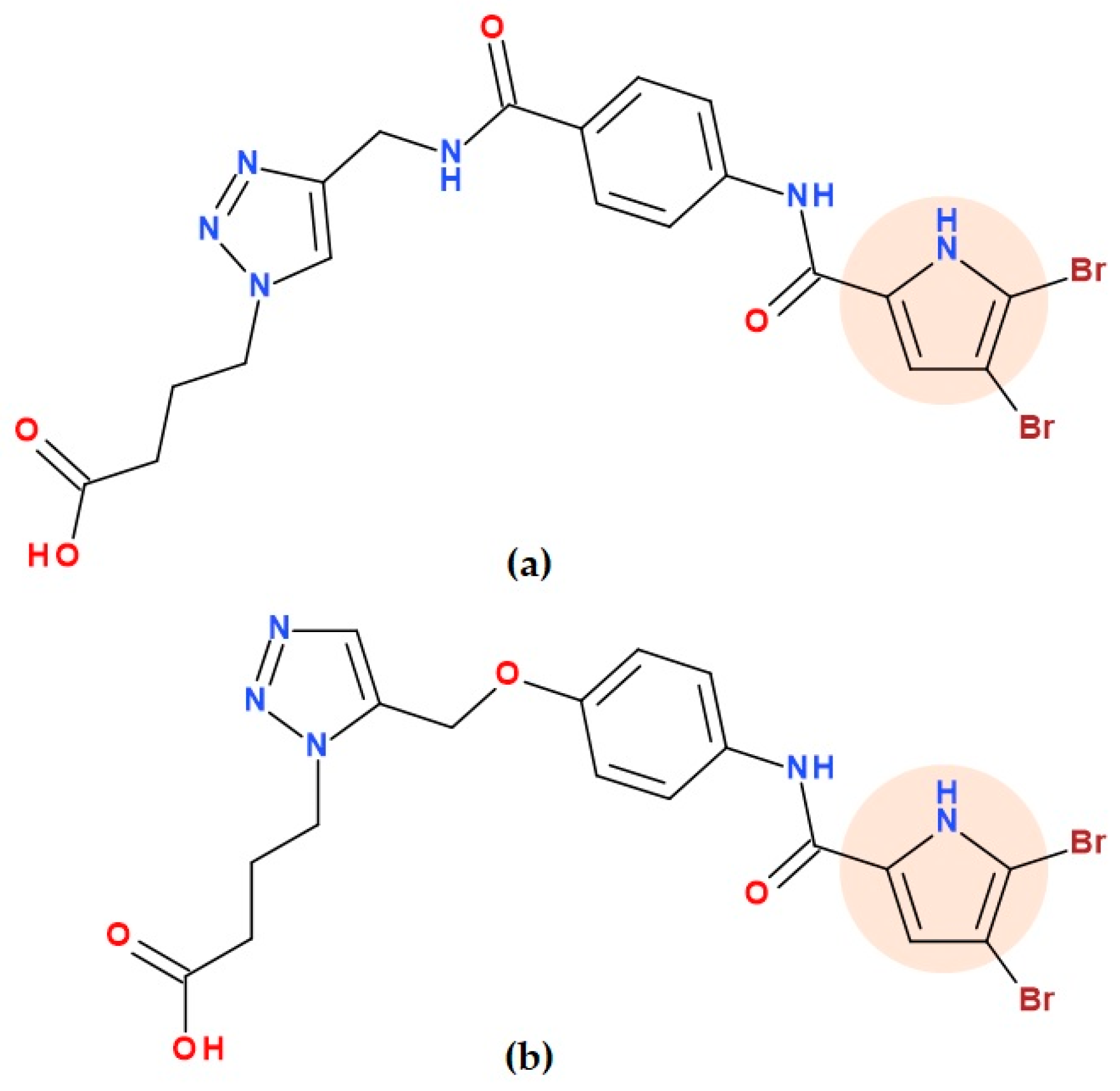
5.19.2. 4,5-Dibromo-N-phenyl-1H-pyrrole-2-carboxamide Hybrids
5.19.3. Hybrids of Sulfonamides Containing the Pyrrole Heterocycle
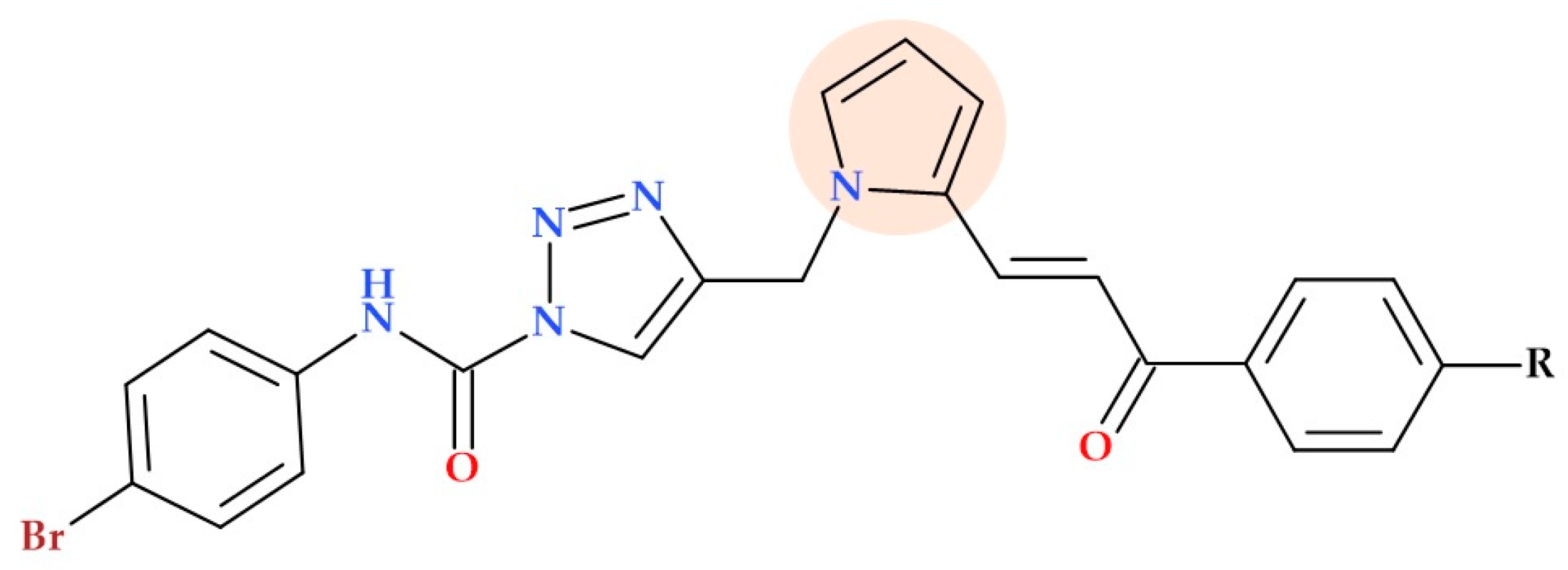
5.19.4. Pyrrole-1,2,3-triazole Hybrids
5.19.5. Silatran Pyrrole-2-carboxamide Hybrids
5.20. Metal Complexes
Copper Complexes with Pyrrole Hydrazone Derivatives
6. Considerations Regarding the Development of New Pyrrole-Based Antibacterials
6.1. Optimizing the Molecular Structure to Increase the Antibacterial Activity
6.1.1. Activity Against Gram-Positive Pathogens
6.1.2. Activity Against Mycobacterium tuberculosis
6.1.3. Activity Against Gram-Negative Pathogens
6.1.4. Activity Against Gram-Positive and Gram-Negative Pathogens
6.2. Possible Mechanisms of Action and Target Enzymes
7. Materials and Methods
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gomtsyan, A. Heterocycles in Drugs and Drug Discovery. Chem. Heterocycl. Compd. 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Asif, M.; Alghamdi, S. An Overview on Biological Importance of Pyrrolone and Pyrrolidinone Derivatives as Promising Scaffolds. Russ. J. Org. Chem. 2021, 57, 1700–1718. [Google Scholar] [CrossRef]
- FDA Novel Drug Approvals at FDA. Available online: https://www.fda.gov/drugs/development-approval-process-drugs/novel-drug-approvals-fda (accessed on 9 October 2024).
- Yao, J.; Zou, P.; Cui, Y.; Quan, L.; Gao, C.; Li, Z.; Gong, W.; Yang, M. Recent Advances in Strategies to Combat Bacterial Drug Resistance: Antimicrobial Materials and Drug Delivery Systems. Pharmaceutics 2023, 15, 1188. [Google Scholar] [CrossRef] [PubMed]
- Habboush, Y.; Guzman, N. Antibiotic Resistance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Rusu, A.; Moga, I.-M.; Uncu, L.; Hancu, G. The Role of Five-Membered Heterocycles in the Molecular Structure of Antibacterial Drugs Used in Therapy. Pharmaceutics 2023, 15, 2554. [Google Scholar] [CrossRef]
- Gupta, R.R.; Kumar, M.; Gupta, V. Heterocyclic Chemistry: Volume II: Five-Membered Heterocycles; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-662-07757-3. [Google Scholar]
- Eicher, T.; Hauptmann, S. The Chemistry of Heterocycles: Structure, Reactions, Syntheses and Applications; Wiley: Hoboken, NJ, USA, 2002; ISBN 978-3-527-30887-3. [Google Scholar]
- Chemaxon Marvin. Available online: https://chemaxon.com/marvin (accessed on 22 July 2024).
- Katritzky, A.R.; Ramsden, C.A.; Joule, J.A.; Zhdankin, V.V. Handbook of Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 978-0-08-095844-6. [Google Scholar]
- Ji Ram, V.; Sethi, A.; Nath, M.; Pratap, R. Chapter 5—Five-Membered Heterocycles. In The Chemistry of Heterocycles; Ji Ram, V., Sethi, A., Nath, M., Pratap, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–478. ISBN 978-0-08-101033-4. [Google Scholar]
- Watson, D. Pharmaceutical Chemistry, 1st ed.; Churchill Livingstone: London, UK, 2011; ISBN 978-0-443-07232-1. [Google Scholar]
- NIH PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 12 July 2023).
- Bhardwaj, V.; Gumber, D.; Abbot, V.; Dhiman, S.; Sharma, P. Pyrrole: A Resourceful Small Molecule in Key Medicinal Hetero-Aromatics. RSC Adv. 2015, 5, 15233–15266. [Google Scholar] [CrossRef]
- Jeelan Basha, N.; Basavarajaiah, S.M.; Shyamsunder, K. Therapeutic Potential of Pyrrole and Pyrrolidine Analogs: An Update. Mol. Divers. 2022, 26, 2915–2937. [Google Scholar] [CrossRef]
- Pech-Puch, D.; Pérez-Povedano, M.; Martinez-Guitian, M.; Lasarte-Monterrubio, C.; Vázquez-Ucha, J.C.; Bou, G.; Rodríguez, J.; Beceiro, A.; Jimenez, C. In Vitro and In Vivo Assessment of the Efficacy of Bromoageliferin, an Alkaloid Isolated from the Sponge Agelas Dilatata, against Pseudomonas aeruginosa. Mar. Drugs 2020, 18, 326. [Google Scholar] [CrossRef]
- Al-Mourabit, A.; Zancanella, M.A.; Tilvi, S.; Romo, D. Biosynthesis, Asymmetric Synthesis, and Pharmacology, Including Cellular Targets, of the Pyrrole-2-Aminoimidazole Marine Alkaloids. Nat. Prod. Rep. 2011, 28, 1229–1260. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Yan, J.; Jia, J.; Xue, J.; Qu, X.; Hu, Y.; Deng, Z.; Bi, H.; Zhu, D. Characterization of the Biosynthetic Gene Cluster for the Antibiotic Armeniaspirols in Streptomyces armeniacus. J. Nat. Prod. 2019, 82, 318–323. [Google Scholar] [CrossRef]
- Pal, S.; Manjunath, B.; Ghorai, S.; Sasmal, S. Chapter Two—Benzoxazole Alkaloids: Occurrence, Chemistry, and Biology. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 79, pp. 71–137. [Google Scholar]
- Liu, C.M.; Hermann, T.E.; Liu, M.; Bull, D.N.; Palleroni, N.J.; Prosser, B.L.; Westley, J.W.; Miller, P.A. X-14547A, a New Ionophorous Antibiotic Produced by Streptomyces Antibioticus NRRL 8167. Discovery, Fermentation, Biological Properties and Taxonomy of the Producing Culture. J. Antibiot. 1979, 32, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Neha; Ranjan, P.; Das, P. Calcimycin Mediates Apoptosis in Breast and Cervical Cancer Cell Lines by Inducing Intracellular Calcium Levels in a P2RX4-Dependent Manner. Biochim. Biophys. Acta Gen. Subj. 2024, 1868, 130535. [Google Scholar] [CrossRef] [PubMed]
- McArthur, K.A.; Mitchell, S.S.; Tsueng, G.; Rheingold, A.; White, D.J.; Grodberg, J.; Lam, K.S.; Potts, B.C.M. Lynamicins A−E, Chlorinated Bisindole Pyrrole Antibiotics from a Novel Marine Actinomycete. J. Nat. Prod. 2008, 71, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Sigala, I.; Ganidis, G.; Thysiadis, S.; Zografos, A.L.; Giannakouros, T.; Sarli, V.; Nikolakaki, E. Lynamicin D an Antimicrobial Natural Product Affects Splicing by Inducing the Expression of SR Protein Kinase 1. Bioorg. Med. Chem. 2017, 25, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Seipp, K.; Geske, L.; Opatz, T. Marine Pyrrole Alkaloids. Mar. Drugs 2021, 19, 514. [Google Scholar] [CrossRef]
- Li, R. Marinopyrroles: Unique Drug Discoveries Based on Marine Natural Products. Med. Res. Rev. 2016, 36, 169–189. [Google Scholar] [CrossRef]
- Lindel, T. Chapter Three—Chemistry and Biology of the Pyrrole–Imidazole Alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 77, pp. 117–219. [Google Scholar]
- Singh, K.S.; Majik, M.S. Chapter 10—Pyrrole-Derived Alkaloids of Marine Sponges and Their Biological Properties. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 62, pp. 377–409. [Google Scholar]
- Lu, W.-Y.; Li, H.-J.; Li, Q.-Y.; Wu, Y.-C. Application of Marine Natural Products in Drug Research. Bioorg. Med. Chem. 2021, 35, 116058. [Google Scholar] [CrossRef]
- Singh, N.; Singh, S.; Kohli, S.; Singh, A.; Asiki, H.; Rathee, G.; Chandra, R.; Anderson, E.A. Recent Progress in the Total Synthesis of Pyrrole-Containing Natural Products (2011–2020). Org. Chem. Front. 2021, 8, 5550–5573. [Google Scholar] [CrossRef]
- Li, C.-S.; Liu, L.-T.; Yang, L.; Li, J.; Dong, X. Chemistry and Bioactivity of Marine-Derived Bisabolane Sesquiterpenoids: A Review. Front. Chem. 2022, 10, 881767. [Google Scholar] [CrossRef]
- Hughes, C.C.; Prieto-Davo, A.; Jensen, P.R.; Fenical, W. The Marinopyrroles, Antibiotics of an Unprecedented Structure Class from a Marine Streptomyces sp. Org. Lett. 2008, 10, 629. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, Y.; Song, H.; Pan, L.; Li, J.; Qin, Y.; Li, R. Marinopyrrole Derivatives as Potential Antibiotic Agents against Methicillin-Resistant Staphylococcus aureus (II). Mar. Drugs 2013, 11, 2927–2948. [Google Scholar] [CrossRef] [PubMed]
- Castro-Falcon, G.; Straetener, J.; Bornikoel, J.; Reimer, D.; Purdy, T.; Schempp, F.; Liu, D.; Linington, R.; Brötz-Oesterhelt, H.; Hughes, C. Antibacterial Marinopyrroles and Pseudilins Act as Protonophores. ACS Chem. Biol. 2023, 19, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Wattanasuepsin, W.; Intra, B.; Euanorasetr, J.; Watanabe, Y.; Mingma, R.; Fukasawa, W.; Mori, M.; Matsumoto, A.; Shiomi, K.; Panbangred, W. 1-Methoxypyrrole-2-Carboxamide—A New Pyrrole Compound Isolated from Streptomyces griseocarneus SWW368. J. Gen. Appl. Microbiol. 2017, 63, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Pidot, S.J.; Herisse, M.; Sharkey, L.; Atkin, L.; Porter, J.L.; Seemann, T.; Howden, B.P.; Rizzacasa, M.A.; Stinear, T.P. Biosynthesis and Ether-Bridge Formation in Nargenicin Macrolides. Angew. Chem. 2019, 131, 4036–4041. [Google Scholar] [CrossRef]
- Pidot, S.J.; Rizzacasa, M.A. The Nargenicin Family of Oxa-Bridged Macrolide Antibiotics. Chem.-A Eur. J. 2020, 26, 2780–2792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Braun, D.R.; Chanana, S.; Rajski, S.R.; Bugni, T.S. Phallusialides A–E, Pyrrole-Derived Alkaloids Discovered from a Marine-Derived Micromonospora sp. Bacterium Using MS-Based Metabolomics Approaches. J. Nat. Prod. 2019, 82, 3432–3439. [Google Scholar] [CrossRef]
- Wang, L.; Linares-Otoya, V.; Liu, Y.; Mettal, U.; Marner, M.; Armas-Mantilla, L.; Willbold, S.; Kurtán, T.; Linares-Otoya, L.; Schäberle, T.F. Discovery and Biosynthesis of Antimicrobial Phenethylamine Alkaloids from the Marine Flavobacterium Tenacibaculum discolor Sv11. J. Nat. Prod. 2022, 85, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Huang, S.; Deng, Z.; Zhao, C.; Yu, Y. Mining of the Pyrrolamide Antibiotics Analogs in Streptomyces netropsis Reveals the Amidohydrolase-Dependent “Iterative Strategy” Underlying the Pyrrole Polymerization. PLoS ONE 2014, 9, e99077. [Google Scholar] [CrossRef]
- Eakin, A.E.; Green, O.; Hales, N.; Walkup, G.K.; Bist, S.; Singh, A.; Mullen, G.; Bryant, J.; Embrey, K.; Gao, N.; et al. Pyrrolamide DNA Gyrase Inhibitors: Fragment-Based Nuclear Magnetic Resonance Screening To Identify Antibacterial Agents. Antimicrob. Agents Chemother. 2012, 56, 1240–1246. [Google Scholar] [CrossRef]
- Shuai, H.; Myronovskyi, M.; Nadmid, S.; Luzhetskyy, A. Identification of a Biosynthetic Gene Cluster Responsible for the Production of a New Pyrrolopyrimidine Natural Product—Huimycin. Biomolecules 2020, 10, 1074. [Google Scholar] [CrossRef]
- Cascioferro, S.; Raimondi, M.V.; Cusimano, M.G.; Raffa, D.; Maggio, B.; Daidone, G.; Schillaci, D. Pharmaceutical Potential of Synthetic and Natural Pyrrolomycins. Molecules 2015, 20, 21658–21671. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.V.; Presentato, A.; Li Petri, G.; Buttacavoli, M.; Ribaudo, A.; De Caro, V.; Alduina, R.; Cancemi, P. New Synthetic Nitro-Pyrrolomycins as Promising Antibacterial and Anticancer Agents. Antibiotics 2020, 9, 292. [Google Scholar] [CrossRef]
- Valderrama, K.; Pradel, E.; Firsov, A.M.; Drobecq, H.; Bauderlique-le Roy, H.; Villemagne, B.; Antonenko, Y.N.; Hartkoorn, R.C. Pyrrolomycins Are Potent Natural Protonophores. Antimicrob. Agents Chemother. 2019, 63, e01450-19. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Zeng, Y.; Wang, W.; Tang, S.; Jia, A. 1H-Pyrrole-2,5-Dicarboxylic Acid, a Quorum Sensing Inhibitor from One Endophytic Fungus in Areca catechu L., Acts as Antibiotic Accelerant against Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2024, 14, 1413728. [Google Scholar] [CrossRef]
- Saurav, K.; Zhang, W.; Saha, S.; Zhang, H.; Li, S.; Zhang, Q.; Wu, Z.; Zhang, G.; Zhu, Y.; Verma, G. In Silico Molecular Docking, Preclinical Evaluation of Spiroindimicins A-D, Lynamicin A and D Isolated from Deep Marine Sea Derived Streptomyces sp. SCSIO 03032. Interdiscip. Sci. Comput. Life Sci. 2014, 6, 187–196. [Google Scholar] [CrossRef]
- Ching, K.-C.; Chin, E.J.; Wibowo, M.; Tan, Z.Y.; Yang, L.-K.; Seow, D.C.; Leong, C.-Y.; Ng, V.W.; Ng, S.-B.; Kanagasundaram, Y. Antibacterial Spirotetronate Polyketides from an Actinomadura sp. Strain A30804. Molecules 2022, 27, 8196. [Google Scholar] [CrossRef]
- Heo, C.-S.; Kang, J.S.; Kwon, J.-H.; Anh, C.V.; Shin, H.J. Pyrrole-Containing Alkaloids from a Marine-Derived Actinobacterium Streptomyces zhaozhouensis and Their Antimicrobial and Cytotoxic Activities. Mar. Drugs 2023, 21, 167. [Google Scholar] [CrossRef]
- Ganesh, B.H.; Raj, A.G.; Aruchamy, B.; Nanjan, P.; Drago, C.; Ramani, P. Pyrrole: A Decisive Scaffold for the Development of Therapeutic Agents and Structure-Activity Relationship. ChemMedChem 2024, 19, e202300447. [Google Scholar] [CrossRef] [PubMed]
- Beale, J.M., Jr.; Block, J.H. (Eds.) Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th ed.; Wolters Kluwer Health: Baltimore, MD, USA, 2010; ISBN 978-0-7817-7929-6. [Google Scholar]
- DrugBank DrugBank|Powering Health Insights with Structured Drug Data. Available online: https://www.drugbank.com/ (accessed on 11 July 2023).
- Gunderson, E.L.; Bryant, C.; Bulman, C.A.; Fischer, C.; Luo, M.; Vogel, I.; Lim, K.-C.; Jawahar, S.; Tricoche, N.; Voronin, D.; et al. Pyrvinium Pamoate and Structural Analogs Are Early Macrofilaricide Leads. Pharmaceuticals 2022, 15, 189. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Sadek, I. Sunitinib: The Antiangiogenic Effects and Beyond. OncoTargets Ther. 2016, 9, 5495–5505. [Google Scholar] [CrossRef] [PubMed]
- ChEMBL Database. Available online: https://www.ebi.ac.uk/chembl/ (accessed on 2 April 2024).
- Wójcicka, A.; Redzicka, A. An Overview of the Biological Activity of Pyrrolo [3,4-c]Pyridine Derivatives. Pharmaceuticals 2021, 14, 354. [Google Scholar] [CrossRef]
- Shi, T.; Yin, G.; Wang, X.; Xiong, Y.; Peng, Y.; Li, S.; Zeng, Y.; Wang, Z. Recent Advances in the Syntheses of Pyrroles. Green Synth. Catal. 2023, 4, 20–34. [Google Scholar] [CrossRef]
- Aatif, M.; Raza, M.A.; Javed, K.; Nashre-ul-Islam, S.M.; Farhan, M.; Alam, M.W. Potential Nitrogen-Based Heterocyclic Compounds for Treating Infectious Diseases: A Literature Review. Antibiotics 2022, 11, 1750. [Google Scholar] [CrossRef] [PubMed]
- Li Petri, G.; Spanò, V.; Spatola, R.; Holl, R.; Raimondi, M.V.; Barraja, P.; Montalbano, A. Bioactive Pyrrole-Based Compounds with Target Selectivity. Eur. J. Med. Chem. 2020, 208, 112783. [Google Scholar] [CrossRef]
- Barker, D.; Lee, S.; Varnava, K.G.; Sparrow, K.; van Rensburg, M.; Deed, R.C.; Cadelis, M.M.; Li, S.A.; Copp, B.R.; Sarojini, V.; et al. Synthesis and Antibacterial Analysis of Analogues of the Marine Alkaloid Pseudoceratidine. Molecules 2020, 25, 2713. [Google Scholar] [CrossRef]
- Couturier, C.; Bauer, A.; Rey, A.; Schroif-Dufour, C.; Broenstrup, M. Armeniaspiroles, a New Class of Antibacterials: Antibacterial Activities and Total Synthesis of 5-Chloro-Armeniaspirole A. Bioorganic Med. Chem. Lett. 2012, 22, 6292–6296. [Google Scholar] [CrossRef]
- Kwon, H.C.; Espindola, A.P.D.M.; Park, J.-S.; Prieto-Davó, A.; Rose, M.; Jensen, P.R.; Fenical, W. Nitropyrrolins A−E, Cytotoxic Farnesyl-α-Nitropyrroles from a Marine-Derived Bacterium within the Actinomycete Family Streptomycetaceae. J. Nat. Prod. 2010, 73, 2047–2052. [Google Scholar] [CrossRef]
- Patel, A.; Shah, H.; Shah, U.; Bambharoliya, T.; Patel, M.; Panchal, I.; Parikh, V.; Nagani, A.; Patel, H.; Vaghasiya, J.; et al. A Review on the Synthetic Approach of Marinopyrroles: A Natural Antitumor Agent from the Ocean. Lett. Org. Chem. 2021, 18, 251–264. [Google Scholar] [CrossRef]
- Sherer, B.A.; Hull, K.; Green, O.; Basarab, G.; Hauck, S.; Hill, P.; Loch, J.T.; Mullen, G.; Bist, S.; Bryant, J.; et al. Pyrrolamide DNA Gyrase Inhibitors: Optimization of Antibacterial Activity and Efficacy. Bioorg. Med. Chem. Lett. 2011, 21, 7416–7420. [Google Scholar] [CrossRef] [PubMed]
- Hameed, P.S.; Solapure, S.; Mukherjee, K.; Nandi, V.; Waterson, D.; Shandil, R.; Balganesh, M.; Sambandamurthy, V.K.; Raichurkar, A.K.; Deshpande, A.; et al. Optimization of Pyrrolamides as Mycobacterial GyrB ATPase Inhibitors: Structure-Activity Relationship and In Vivo Efficacy in a Mouse Model of Tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 61–70. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, J.; Zhang, J.; Han, Z.; Hu, Y.; Shao, H.-H.; Li, T.; Xia, J.; Lei, K.; Wang, W.; et al. Discovery and Druggability Evaluation of Pyrrolamide-Type GyrB/ParE Inhibitor against Drug-Resistant Bacterial Infection. Acta Pharm. Sin. B 2023, 13, 4945–4962. [Google Scholar] [CrossRef] [PubMed]
- Basarab, G.S.; Hill, P.J.; Garner, C.E.; Hull, K.; Green, O.; Sherer, B.A.; Dangel, P.B.; Manchester, J.I.; Bist, S.; Hauck, S.; et al. Optimization of Pyrrolamide Topoisomerase II Inhibitors Toward Identification of an Antibacterial Clinical Candidate (AZD5099). J. Med. Chem. 2014, 57, 6060–6082. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.D.; Dixit, S.R.; Basha, J.; Kulkarni, V.H.; Aminabhavi, T.M.; Nadagouda, M.N.; Lherbet, C. Pharmacophore Mapping, Molecular Docking, Chemical Synthesis of Some Novel Pyrrolyl Benzamide Derivatives and Evaluation of Their Inhibitory Activity against Enoyl-ACP Reductase (InhA) and Mycobacterium Tuberculosis. Bioorg. Chem. 2018, 81, 440–453. [Google Scholar] [CrossRef]
- Rawat, P.; Gautam, A.; Singh, R.N. Synthesis, Spectral, Structural and Antimicrobial Activities of Ethyl-4-{[-(1-(2-(4-Nitrobenzoyl)Hydrazono)Ethyl]}-3,5-Dimethyl-1H-Pyrrole-2-Carboxylate. J. Mol. Struct. 2022, 1255, 132405. [Google Scholar] [CrossRef]
- Mane, Y.D.; Surwase, S.M.; Biradar, D.O.; Sarnikar, Y.P.; Jawle, B.H.; Shinde, V.S.; Khade, B.C. Design and Synthesis of Diverse Pyrrole-2-Carboxamide Derivatives as a Potent Antibacterial Agents. J. Heterocycl. Chem. 2017, 54, 2627–2634. [Google Scholar] [CrossRef]
- Nyerges, A.; Tomašič, T.; Durcik, M.; Revesz, T.; Szili, P.; Draskovits, G.; Bogar, F.; Skok, Ž.; Zidar, N.; Ilaš, J.; et al. Rational Design of Balanced Dual-Targeting Antibiotics with Limited Resistance. PLoS Biol. 2020, 18, e3000819. [Google Scholar] [CrossRef]
- Durcik, M.; Cotman, A.E.; Toplak, Ž.; Možina, Š.; Skok, Ž.; Szili, P.E.; Czikkely, M.; Maharramov, E.; Vu, T.H.; Piras, M.V.; et al. New Dual Inhibitors of Bacterial Topoisomerases with Broad-Spectrum Antibacterial Activity and In Vivo Efficacy against Vancomycin-Intermediate Staphylococcus aureus. J. Med. Chem. 2023, 66, 3968–3994. [Google Scholar] [CrossRef]
- Mir, N.A.; Ramaraju, P.; Vanaparthi, S.; Choudhary, S.; Singh, R.P.; Sharma, P.; Kant, R.; Singh, R.; Sankaranarayanan, M.; Kumar, I. Sequential Multicomponent Catalytic Synthesis of Pyrrole-3-Carboxaldehydes: Evaluation of Antibacterial and Antifungal Activities along with Docking Studies. New J. Chem. 2020, 44, 16329–16339. [Google Scholar] [CrossRef]
- Massa, S.; Di Santo, R.; Mai, A.; Botta, M.; Artico, M.; Panico, S.; Simonetti, G. Research on Antibacterial and Antifungal Agents. XIII. Synthesis and Antimicrobial Activity of 1-Arylmethyl-4-Aryl-1H-Pyrrole-3-Carboxylic Acids. Farmaco 1990, 45, 833–846. [Google Scholar]
- Liu, J.; Ren, Z.; Fan, L.; Wei, J.; Tang, X.; Xu, X.; Yang, D. Design, Synthesis, Biological Evaluation, Structure-Activity Relationship, and Toxicity of Clinafloxacin-Azole Conjugates as Novel Antitubercular Agents. Bioorg. Med. Chem. 2019, 27, 175–187. [Google Scholar] [CrossRef]
- Grozav, A.M.; Chornous, V.O.; Diichuk, I.V.; Kemskyi, S.V.; Yakovychuk, N.D.; Fedoriv, M.Z.; Vovk, M.V. Synthesis and Biological Evaluation of O-Acyloximes of 5-Chloro-4-Formyl-1H-Pyrrol-3-Carboxylates as Antimicrobial Agents. Biopolym. Cell 2022, 38, 48–57. [Google Scholar] [CrossRef]
- Mohamed, M.S.; El-Domany, R.A.; Abd El-Hameed, R.H. Synthesis of Certain Pyrrole Derivatives as Antimicrobial Agents. Acta Pharm. 2009, 59, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Hilmy, K.M.H.; Kishk, F.N.M.; Shahen, E.B.A.; Sobh, E.A.; Hawata, M.A. New Pyrrole Derivatives as DNA Gyrase and 14α-Demethylase Inhibitors: Design, Synthesis, Antimicrobial Evaluation, and Molecular Docking. Drug Dev. Res. 2023, 84, 1204–1230. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, A.; Altıntop, M.D.; Sever, B.; Gençer, H.K.; Kapkaç, H.A.; Atlı, Ö.; Baysal, M. A New Series of Pyrrole-Based Chalcones: Synthesis and Evaluation of Antimicrobial Activity, Cytotoxicity, and Genotoxicity. Molecules 2017, 22, 2112. [Google Scholar] [CrossRef] [PubMed]
- Mezgebe, K.; Melaku, Y.; Mulugeta, E. Synthesis and Pharmacological Activities of Chalcone and Its Derivatives Bearing N-Heterocyclic Scaffolds: A Review. ACS Omega 2023, 8, 19194–19211. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R.K. Morpholine as Ubiquitous Pharmacophore in Medicinal Chemistry: Deep Insight into the Structure-Activity Relationship (SAR). Bioorg. Chem. 2020, 96, 103578. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, S.M.R.; Farhadi, T.; Ganjparvar, M. Linezolid: A Review of Its Properties, Function, and Use in Critical Care. Drug Des. Dev. Ther. 2018, 12, 1759–1767. [Google Scholar] [CrossRef]
- Poce, G.; Cocozza, M.; Alfonso, S.; Consalvi, S.; Venditti, G.; Fernandez-Menendez, R.; Bates, R.H.; Barros Aguirre, D.; Ballell, L.; De Logu, A.; et al. In Vivo Potent BM635 Analogue with Improved Drug-like Properties. Eur. J. Med. Chem. 2018, 145, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.V.; Listro, R.; Cusimano, M.G.; La Franca, M.; Faddetta, T.; Gallo, G.; Schillaci, D.; Collina, S.; Leonchiks, A.; Barone, G. Pyrrolomycins as Antimicrobial Agents. Microwave-Assisted Organic Synthesis and Insights into Their Antimicrobial Mechanism of Action. Bioorg. Med. Chem. 2019, 27, 721–728. [Google Scholar] [CrossRef]
- Petruso, S.; Bonanno, S.; Caronna, S.; Ciofalo, M.; Maggio, B.; Schillaci, D. Oxidative Halogenation of Substituted Pyrroles with Cu(II). Part IV. Bromination of 2-(2′-Hydroxybenzoyl)Pyrrole. A New Synthesis of Bioactive Analogs of Monodeoxypyoluteorin. J. Heterocycl. Chem. 1994, 31, 941–945. [Google Scholar] [CrossRef]
- van der Westhuyzen, R.; Winks, S.; Wilson, C.R.; Boyle, G.A.; Gessner, R.K.; Soares de Melo, C.; Taylor, D.; de Kock, C.; Njoroge, M.; Brunschwig, C.; et al. Pyrrolo [3,4-c]Pyridine-1,3(2H)-Diones: A Novel Antimycobacterial Class Targeting Mycobacterial Respiration. J. Med. Chem. 2015, 58, 9371–9381. [Google Scholar] [CrossRef]
- Wojcicka, A.; Becan, L.; Junka, A.; Brtoszewicz, M.; Secewicz, A.; Trynda, J.; Wietrzyk, J. Synthesis and Biological Activity of Novel 6-Phenyl-1H-Pyrrolo [3,4-c]Pyridine-1,3-Dione Derivatives. Acta Pol. Pharm. 2017, 74, 435–443. [Google Scholar] [PubMed]
- Veselov, M.S.; Ivanenkov, Y.A.; Yamidanov, R.S.; Osterman, I.A.; Sergiev, P.V.; Aladinskiy, V.A.; Aladinskaya, A.V.; Terentiev, V.A.; Ayginin, A.A.; Skvortsov, D.A.; et al. Identification of Pyrrolo-Pyridine Derivatives as Novel Class of Antibacterials. Mol. Divers. 2020, 24, 233–239. [Google Scholar] [CrossRef]
- Syamaiah, K.; Mallikarjuna Reddy, G.; Padmavathi, V.; Padmaja, A. Synthesis and Antimicrobial Activity of Some New Amido/Sulfonamido-Linked 3,4-Disubstituted Pyrroles. Med. Chem. Res. 2014, 23, 3287–3297. [Google Scholar] [CrossRef]
- Pathania, S.; Rawal, R.K. Pyrrolopyrimidines: An Update on Recent Advancements in Their Medicinal Attributes. Eur. J. Med. Chem. 2018, 157, 503–526. [Google Scholar] [CrossRef] [PubMed]
- Fatahala, S.S.; Mohamed, M.S.; Sabry, J.Y.; Mansour, Y.E.E.-D. Synthesis Strategies and Medicinal Value of Pyrrole and Its Fused Heterocyclic Compounds. Med. Chem. 2022, 18, 1013–1043. [Google Scholar] [CrossRef] [PubMed]
- Shiva Raju, K.; AnkiReddy, S.; Sabitha, G.; Siva Krishna, V.; Sriram, D.; Bharathi Reddy, K.; Rao Sagurthi, S. Synthesis and Biological Evaluation of 1H-Pyrrolo [2,3-d]Pyrimidine-1,2,3-Triazole Derivatives as Novel Anti-Tubercular Agents. Bioorg. Med. Chem. Lett. 2019, 29, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Hilmy, K.; Tag, M.; Aish, E.; Elsafty, M.; Attia, H. Synthesis and Biological Evaluation of Pyrrolo [2,3-d]Pyrimidine Derivatives as a Novel Class of Antimicrobial and Antiviral Agents. Russ. J. Org. Chem. 2021, 57, 430–439. [Google Scholar] [CrossRef]
- Jesumoroti, O.J.; Beteck, R.M.; Jordaan, A.; Warner, D.F.; Legoabe, L.J. Exploration of 4-Aminopyrrolo [2,3-d]Pyrimidine as Antitubercular Agents. Mol. Divers. 2023, 27, 753–765. [Google Scholar] [CrossRef]
- Abd El-Hameed, R.H.; Sayed, A.I.; Mahmoud Ali, S.; Mosa, M.A.; Khoder, Z.M.; Fatahala, S.S. Synthesis of Novel Pyrroles and Fused Pyrroles as Antifungal and Antibacterial Agents. J. Enzym. Inhib. Med. Chem. 2021, 36, 2183–2198. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Sharma, K.; Luong, J.H.T.; Gedanken, A. Antibacterial Activities of Microwave-Assisted Synthesized Polypyrrole/Chitosan and Poly (Pyrrole-N-(1-Naphthyl) Ethylenediamine) Stimulated by C-Dots. Carbohydr. Polym. 2020, 243, 116474. [Google Scholar] [CrossRef] [PubMed]
- Elibal, F.; Gumustekin, S.; Ozkazanc, H.; Ozkazanc, E. Poly(N-Methylpyrrole) with High Antibacterial Activity Synthesized via Interfacial Polymerization Method. J. Mol. Struct. 2021, 1242, 130712. [Google Scholar] [CrossRef]
- Joshi, S.D.; Vagdevi, H.M.; Vaidya, V.P.; Gadaginamath, G.S. Synthesis of New 4-Pyrrol-1-Yl Benzoic Acid Hydrazide Analogs and Some Derived Oxadiazole, Triazole and Pyrrole Ring Systems: A Novel Class of Potential Antibacterial and Antitubercular Agents. Eur. J. Med. Chem. 2008, 43, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Balachandra, B.; Shanmugam, S.; Muneeswaran, T.; Ramakritinan, M. Iodine Catalyzed One-Pot Synthesis of Highly Substituted N-Methyl Pyrroles via [3 + 2] Annulation and Their in Vitro Evaluation as Antibacterial Agents. RSC Adv. 2015, 5, 64781–64789. [Google Scholar] [CrossRef]
- Hosseyni Largani, T.; Imanzadeh, G.; Zahri, S.; Noroozi Pesyan, N.; Şahin, E. A Facile Synthesis and Antibacterial Activity of Novel Pyrrolo [3,4-b]Quinolin-2(3H)-Yl)Benzamides. Green Chem. Lett. Rev. 2017, 10, 387–392. [Google Scholar] [CrossRef]
- Cusumano, A.Q.; Pierce, J.G. 3-Hydroxy-1,5-Dihydro-2H-Pyrrol-2-Ones as Novel Antibacterial Scaffolds against Methicillin-Resistant Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2018, 28, 2732–2735. [Google Scholar] [CrossRef]
- Volynets, G.P.; Tukalo, M.A.; Bdzhola, V.G.; Derkach, N.M.; Gumeniuk, M.I.; Tarnavskiy, S.S.; Yarmoluk, S.M. Novel Isoniazid Derivative as Promising Antituberculosis Agent. Future Microbiol. 2020, 15, 869–879. [Google Scholar] [CrossRef]
- Baral, N.; Mishra, D.R.; Mishra, N.P.; Mohapatra, S.; Raiguru, B.P.; Panda, P.; Nayak, S.; Nayak, M.; Kumar, P.S. Microwave-Assisted Rapid and Efficient Synthesis of Chromene-Fused Pyrrole Derivatives through Multicomponent Reaction and Evaluation of Antibacterial Activity with Molecular Docking Investigation. J. Heterocycl. Chem. 2020, 57, 575–589. [Google Scholar] [CrossRef]
- Akbaslar, D.; Giray, E.S.; Algul, O. Revisit to the Synthesis of 1,2,3,4-Tetrasubstituted Pyrrole Derivatives in Lactic Acid Media as a Green Solvent and Catalyst. Mol. Divers. 2021, 25, 2321–2338. [Google Scholar] [CrossRef] [PubMed]
- Green, K.D.; Pang, A.H.; Thamban Chandrika, N.; Garzan, A.; Baughn, A.D.; Tsodikov, O.V.; Garneau-Tsodikova, S. Discovery and Optimization of 6-(1-Substituted Pyrrole-2-Yl)-s-Triazine Containing Compounds as Antibacterial Agents. ACS Infect. Dis. 2022, 8, 757–767. [Google Scholar] [CrossRef]
- Velu, B.; Thirunarayanan, G.; Kulanthaivel, S. Design and Synthesis of Potential Pyrrole Coupled Carbothioamide Derivatives and Its Antibacterial Studies against Superbug MRSA. Biointerface Res. Appl. Chem. 2023, 13, 1–39. [Google Scholar]
- Hodyna, D.; Klipkov, A.; Kachaeva, M.; Shulha, Y.; Gerus, I.; Metelytsia, L.; Kovalishyn, V. In Silico Design and In Vitro Assessment of Bicyclic Trifluoromethylated Pyrroles as New Antibacterial and Antifungal Agents. Chem. Biodivers. 2024, 21, e202400638. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, H.; Chen, F.; He, Q.; Zhang, X.; Lan, L.; Yang, C. Design, Synthesis and Biological Evaluation of Thiazolyl-Halogenated Pyrroles or Pyrazoles as Novel Antibacterial and Antibiofilm Agents. Eur. J. Med. Chem. 2024, 268, 116221. [Google Scholar] [CrossRef]
- Bremner, J.B.; Ambrus, J.I.; Samosorn, S. Dual Action-Based Approaches to Antibacterial Agents. Curr. Med. Chem. 2007, 14, 1459–1477. [Google Scholar] [CrossRef]
- Lungu, I.-A.; Moldovan, O.-L.; Biriș, V.; Rusu, A. Fluoroquinolones Hybrid Molecules as Promising Antibacterial Agents in the Fight against Antibacterial Resistance. Pharmaceutics 2022, 14, 1749. [Google Scholar] [CrossRef]
- Gupta, V.; Datta, P. Next-Generation Strategy for Treating Drug Resistant Bacteria: Antibiotic Hybrids. Indian J. Med. Res. 2019, 149, 97–106. [Google Scholar] [CrossRef]
- Gogoi, N.G.; Rahman, A.; Dutta, P.; Saikia, J.; Baruah, A.; Handique, J.G. Design, Synthesis, Biological Evaluation and in Silico Studies of Curcumin Pyrrole Conjugates. Chem. Biodivers. 2024, 21, e202301605. [Google Scholar] [CrossRef]
- Merugu, S.R.; Mokoena, S.; Obakachi, V.A.; Shaik, B.B.; Kushawaha, B.; Kushwaha, N.D.; Ike, B.W.; Palkar, M.B.; Bonde, C.G.; Ganai, A.M.; et al. Novel 4,5-Dibromo-N-Phenyl-1H-Pyrrole-2-Carboxamide Hybrids as Promising DNA Gyrase Inhibitors: Design, Synthesis and Antimicrobial Evaluation. J. Mol. Struct. 2024, 1302, 137359. [Google Scholar] [CrossRef]
- Gaffer, H.E.; Mahmoud, S.A.; El-Sedik, M.S.; Aysha, T.; Abdel-Rhman, M.H.; Abdel-latif, E. Synthesis, Molecular Modelling, and Antibacterial Evaluation of New Sulfonamide-Dyes Based Pyrrole Compounds. Sci. Rep. 2024, 14, 10973. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, A.; Lal, K.; Singh, M.B.; Kumari, K. Facile Synthesis, Antimicrobial Screening and Docking Studies of Pyrrole-Triazole Hybrids as Potential Antimicrobial Agents. Res. Chem. Intermed. 2023, 49, 1311–1326. [Google Scholar] [CrossRef]
- Adamovich, S.N.; Sadykov, E.K.; Ushakov, I.A.; Oborina, E.N.; Belovezhets, L.A. Antibacterial Activity of New Silatrane Pyrrole-2-Carboxamide Hybrids. Mendeleev Commun. 2021, 31, 204–206. [Google Scholar] [CrossRef]
- Joshi, S.D.; Kumar, D.; Dixit, S.R.; Tigadi, N.; More, U.A.; Lherbet, C.; Aminabhavi, T.M.; Yang, K.S. Synthesis, Characterization and Antitubercular Activities of Novel Pyrrolyl Hydrazones and Their Cu-Complexes. Eur. J. Med. Chem. 2016, 121, 21–39. [Google Scholar] [CrossRef]
- Champoux, J.J. DNA Topoisomerases: Structure, Function, and Mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef]
- Hamid, H. InhA Inhibitors as Potential Antitubercular Agents. Orient. J. Chem. 2016, 32, 59–75. [Google Scholar] [CrossRef][Green Version]
- Zrelovs, N.; Kurbatska, V.; Rudevica, Z.; Leonchiks, A.; Fridmanis, D. Sorting out the Superbugs: Potential of Sortase A Inhibitors among Other Antimicrobial Strategies to Tackle the Problem of Antibiotic Resistance. Antibiotics 2021, 10, 164. [Google Scholar] [CrossRef]
- Su, C.-C.; Klenotic, P.A.; Bolla, J.R.; Purdy, G.E.; Robinson, C.V.; Yu, E.W. MmpL3 Is a Lipid Transporter That Binds Trehalose Monomycolate and Phosphatidylethanolamine. Proc. Natl. Acad. Sci. USA 2019, 116, 11241–11246. [Google Scholar] [CrossRef]
- Systèmes, D. BIOVIA Draw for Academics Thank You. Available online: https://discover.3ds.com/biovia-draw-academic-thank-you (accessed on 26 July 2024).






| Nitrogen Five-Member Heterocycle | Antibacterial Compound | FDA Approval Year | Antibacterial Class (Generation) |
|---|---|---|---|
| Pyrrolidine (in a bicycle) | Finafloxacin | 2014 | Fluoroquinolones |
| Pyrrolidine | Cefepime | 1996 | Cephalosporins (4th generation) |
| Meropenem | 1996 | Carbapenems | |
| Ertapenem | 2001 | Carbapenems | |
| Gemifloxacin | 2003 | Fluoroquinolones | |
| Ceftobiprole | 2009 | Cephalosporins (5th generation) | |
| Doripenem | 2014 | Carbapenems | |
| Eravacycline | 2018 | Tetracyclines | |
| Gemifloxacin | 2018 | Fluoroquinolones | |
| Cefidorocol | 2019 | Cephalosporins (5th generation) | |
| Pyrrolidine and 1,2,3-Triazole | Cefepime and Enmetazobactam | 2024 | Cephalosporins (4th generation) and beta-lactamase inhibitor |
| 1,2,3-Triazole | Tazobactam | 1992 | Beta-lactamase inhibitor |
| Tetrazole | Cefoperazone | 1981 | Cephalosporins (3rd generation) |
| Cefotiam | 1981 | Cephalosporins (2nd generation) | |
| Latamoxef/Moxalactam | 1982 | Oxacephem cephalosporins (1st generation) | |
| Cefonicid | 1983 | Cephalosporins (2nd generation) | |
| Cefotetan | 1987 | Cephalosporins (3rd generation) | |
| Tedizolid | 2014 | Oxazolidinones |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, A.; Oancea, O.-L.; Tanase, C.; Uncu, L. Unlocking the Potential of Pyrrole: Recent Advances in New Pyrrole-Containing Compounds with Antibacterial Potential. Int. J. Mol. Sci. 2024, 25, 12873. https://doi.org/10.3390/ijms252312873
Rusu A, Oancea O-L, Tanase C, Uncu L. Unlocking the Potential of Pyrrole: Recent Advances in New Pyrrole-Containing Compounds with Antibacterial Potential. International Journal of Molecular Sciences. 2024; 25(23):12873. https://doi.org/10.3390/ijms252312873
Chicago/Turabian StyleRusu, Aura, Octavia-Laura Oancea, Corneliu Tanase, and Livia Uncu. 2024. "Unlocking the Potential of Pyrrole: Recent Advances in New Pyrrole-Containing Compounds with Antibacterial Potential" International Journal of Molecular Sciences 25, no. 23: 12873. https://doi.org/10.3390/ijms252312873
APA StyleRusu, A., Oancea, O.-L., Tanase, C., & Uncu, L. (2024). Unlocking the Potential of Pyrrole: Recent Advances in New Pyrrole-Containing Compounds with Antibacterial Potential. International Journal of Molecular Sciences, 25(23), 12873. https://doi.org/10.3390/ijms252312873









