Chemical Characterization and Assessment of the Neuroprotective Potential of Euphrasia officinalis
Abstract
1. Introduction
2. Results
2.1. EOEA and EOB Are Rich in Phenolics and Flavonoids
2.2. EOEA and EOB Are Rich in the Identified Polyphenols
2.3. EOEA and EOB Show Remarkable Antioxidant Potential
2.4. EO Extracts Demonstrate Anticholinesterase Activity
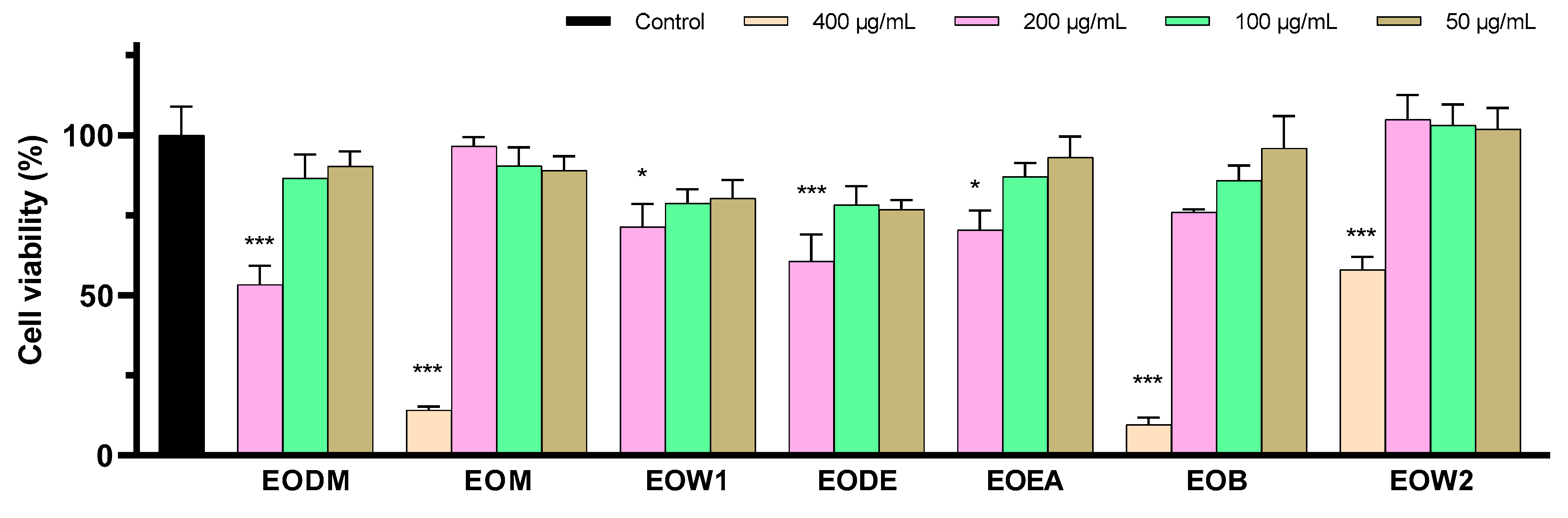
2.5. Five EO Extracts Exhibit Important Anti-Neurotoxic Potential
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
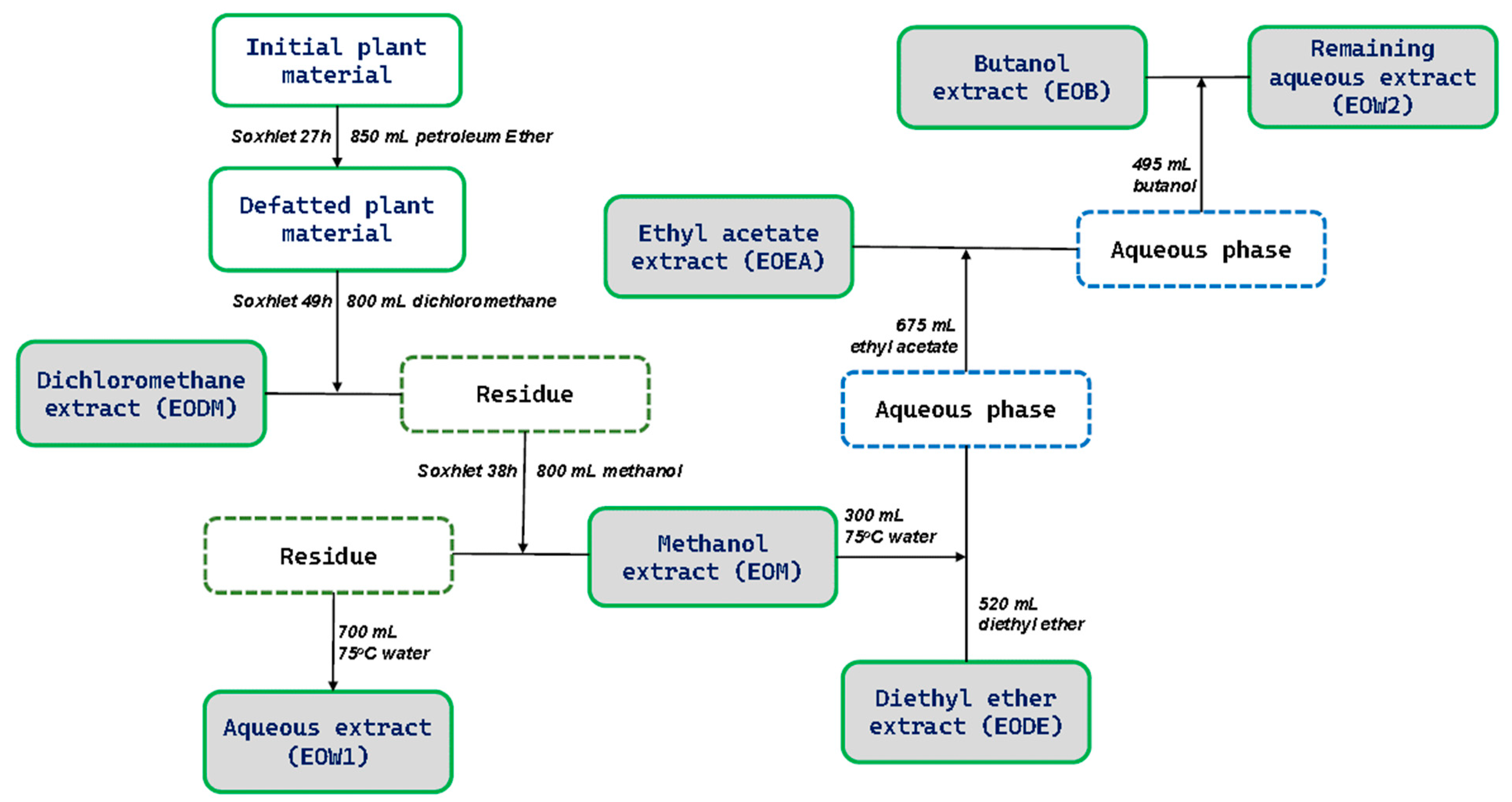
4.3. Preparation of EO Extracts
4.4. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) Determination
4.5. Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS)
4.6. DPPH Radical Scavenging Activity Assay
4.7. Ferric Reducing Antioxidant Power (FRAP) Assay
4.8. Cell Culture
4.9. Intracellular Oxidative Stress Evaluation
4.10. Acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE) Activity Inhibitory Assay
- ∆A = absorbance of a sample or a solvent control well at t = 10 min minus absorbance at t = 0.
4.11. Peptides Preparation
4.12. MTT Cell Viability Assay
4.13. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Möller, H.J.; Graeber, M.B. The case described by Alois Alzheimer in 1911. Historical and conceptual perspectives based on the clinical record and neurohistological sections. Eur. Arch. Psychiatry Clin. Neurosci. 1998, 248, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Rosa-Neto, P.; Morais, J.A.; Webster, C. World Alzheimer Report 2021: Journey Through the Diagnosis of Dementia; Alzheimer’s Disease International: London, UK, 2021. [Google Scholar]
- Giannouli, V.; Tsolaki, M. Financial Incapacity of Patients with Mild Alzheimer’s Disease: What Neurologists Need to Know about Where the Impairment Lies. Neurol. Int. 2022, 14, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.M.; Dillon, C.; MacHnicki, G.; Allegri, R.F. The economic cost of Alzheimer’s disease: Family or public health burden? Dement. Neuropsychol. 2010, 4, 262. [Google Scholar] [CrossRef]
- Mileski, M.; Lee, K.; Bourquard, C.; Cavazos, B.; Dusek, K.; Kimbrough, K.; Sweeney, L.; McClay, R. Preventing the Abuse of Residents with Dementia or Alzheimer’s Disease in The Long-Term Care Setting: A Systematic Review. Clin. Interv. Aging 2019, 14, 1797–1815. [Google Scholar] [CrossRef]
- Tuzimski, T.; Petruczynik, A. Determination of Anti-Alzheimer’s Disease Activity of Selected Plant Ingredients. Molecules 2022, 27, 3222. [Google Scholar] [CrossRef]
- Olloquequi, J.; Ettcheto, M.; Cano, A.; Sanchez-López, E.; Carrasco, M.; Espinosa, T.; Beas-Zarate, C.; Gudiño-Cabrera, G.; Ureña-Guerrero, M.E.; Verdaguer, E.; et al. Impact of New Drugs for Therapeutic Intervention in Alzheimer’s Disease. Front. Biosci. 2022, 27, 146. [Google Scholar] [CrossRef]
- Heidebrink, J.L.; Paulson, H.L. Lessons Learned from Approval of Aducanumab for Alzheimer’s Disease. Annu. Rev. Med. 2024, 75, 99–111. [Google Scholar] [CrossRef]
- Dyer, O. Aduhelm: Biogen abandons Alzheimer’s drug after controversial approval left it unfunded by Medicare. BMJ 2024, 384, q281. [Google Scholar] [CrossRef] [PubMed]
- Vejandla, B.; Savani, S.; Appalaneni, R.; Veeravalli, R.S.; Gude, S.S. Alzheimer’s Disease: The Past, Present, and Future of a Globally Progressive Disease. Cureus 2024, 16, e51705. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease (review). Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, M.; Di Marino, S.; Florenzano, F.; Ciotta, M.T.; Nori, S.L.; Rodriquez, M.; Sorrentino, G.; D’Ursi, A.M.; Scrima, M. β-Amyloid-acetylcholine molecular interaction: New role of cholinergic mediators in anti-Alzheimer therapy? Future Med. Chem. 2016, 8, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zang, W.J.; Wang, H.; Zhao, M.; Yu, X.J.; He, X.; Miao, Y.; Zhou, J. Acetylcholine promotes ROS detoxification against hypoxia/reoxygenation-induced oxidative stress through FoxO3a/PGC-1α dependent superoxide dismutase. Cell. Physiol. Biochem. 2014, 34, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Sagal, J.P.; Colombres, M. Acetylcholinesterase Interaction with Alzheimer Amyloid β. In Alzheimer’s Disease. Subcellular Biochemistry; Harris, J.R., Fahrenholz, F., Eds.; Springer: Boston, MA, USA, 2005; pp. 299–317. [Google Scholar] [CrossRef]
- Bhat, B.A.; Almilaibary, A.; Mir, R.A.; Aljarallah, B.M.; Mir, W.R.; Ahmad, F.; Mir, M.A. Natural Therapeutics in Aid of Treating Alzheimer’s Disease: A Green Gateway Toward Ending Quest for Treating Neurological Disorders. Front. Neurosci. 2022, 16, 884345. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, B.; Zhao, J.; Wang, C.; Wang, Z.; Liu, A.; Du, G. Medicine-Food Herbs against Alzheimer’s Disease: A Review of Their Traditional Functional Features, Substance Basis, Clinical Practices and Mechanisms of Action. Molecules 2022, 27, 901. [Google Scholar] [CrossRef]
- Paduch, R.; Woźniak, A.; Niedziela, P.; Rejdak, R. Assessment of Eyebright (Euphrasia officinalis L.) Extract Activity in Relation to Human Corneal Cells Using In Vitro Tests. Balkan Med. J. 2014, 31, 29. [Google Scholar] [CrossRef]
- Liu, Y.; Hwang, E.; Ngo, H.T.T.; Perumalsamy, H.; Kim, Y.J.; Li, L.; Yi, T.H. Protective Effects of Euphrasia officinalis Extract against Ultraviolet B-Induced Photoaging in Normal Human Dermal Fibroblasts. Int. J. Mol. Sci. 2018, 19, 3327. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; D’Ambrosio, M.; Luceri, C. Pharmacological activities of an eye drop containing Matricaria chamomilla and Euphrasia officinalis extracts in UVB-induced oxidative stress and inflammation of human corneal cells. J. Photochem. Photobiol. B 2017, 173, 618–625. [Google Scholar] [CrossRef]
- Porchezhian, E.; Ansari, S.H.; Shreedharan, N.K.K. Antihyperglycemic activity of Euphrasia officinale leaves. Fitoterapia 2000, 71, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Benedec, D.; Oniga, I.; Hanganu, D.; Vlase, A.M.; Ielciu, I.; Crișan, G.; Fiţ, N.; Niculae, M.; Bab, T.; Pall, E.; et al. Revealing the Phenolic Composition and the Antioxidant, Antimicrobial and Antiproliferative Activities of Two Euphrasia sp. Extracts. Plants 2024, 13, 1790. [Google Scholar] [CrossRef] [PubMed]
- Howard, M. Traditional Folk Remedies: A Comprehensive Herbal; Century: New York, NY, USA, 1987; p. 229. [Google Scholar]
- Mediaeval Ophthalmic Treatment in use to day. Br. J. Ophthalmol. 1930, 14, 413. [CrossRef] [PubMed][Green Version]
- Jahn, H. Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci. 2013, 15, 445. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Anand, U.; Nandy, S.; Oleksak, P.; Qusti, S.; Alshammari, E.M.; El-Saber Batiha, G.; Koshy, E.P.; Dey, A. Herbal drugs and natural bioactive products as potential therapeutics: A review on pro-cognitives and brain boosters perspectives. Saudi Pharm. J. SPJ 2021, 29, 879. [Google Scholar] [CrossRef]
- Sinyor, B.; Mineo, J.; Ochner, C. Alzheimer’s Disease, Inflammation, and the Role of Antioxidants. J. Alzheimer’s Dis. Rep. 2020, 4, 175. [Google Scholar] [CrossRef]
- Teixeira, R.; Silva, L.R. Bioactive compounds and in vitro biological activity of Euphrasia rostkoviana Hayne extracts. Ind. Crops Prod. 2013, 50, 680–689. [Google Scholar] [CrossRef]
- Jafri, S.A.A.; Khalid, Z.M.; Khan, M.Z.; Jogezai, N.U. Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan. Open Chem. 2022, 20, 1337–1356. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753. [Google Scholar] [CrossRef]
- Sahab Uddin, M.; Tanvir Kabir, M.; Niaz, K.; Jeandet, P.; Clément, C.; Mathew, B.; Rauf, A.; Rengasamy, K.R.R.; Sobarzo-Sánchez, E.; Ashraf, G.M.; et al. Molecular Insight into the Therapeutic Promise of Flavonoids against Alzheimer’s Disease. Molecules 2020, 25, 1267. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, D.S.P.; Anna, L.; Albert, K.I. A study on the antioxidant and antimicrobial activities of pressurized-liquid extracts of Clinopodium vulgare and Sideritis scardica. Agro Food Ind. Hi-Tech 2014, 25, 55–58. [Google Scholar]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gai, Z.; Gui, T.; Chen, J.; Chen, Q.; Li, Y. Antioxidant Effects of Protocatechuic Acid and Protocatechuic Aldehyde: Old Wine in a New Bottle. Evid. Based Complement. Alternat. Med. 2021, 2021, 6139308. [Google Scholar] [CrossRef]
- Velika, B.; Kron, I. Antioxidant properties of benzoic acid derivatives against Superoxide radical. Free. Radic. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Pavlíková, N. Caffeic Acid and Diseases—Mechanisms of Action. Int. J. Mol. Sci. 2023, 24, 588. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Xing, R.; Qi, F.; Dong, J.; Li, D.; Tian, X.; Yu, B.; Huang, M.; Zhang, L.; et al. Syringic acid demonstrates promising protective effect against tau fibrillization and cytotoxicity through regulation of endoplasmic reticulum stress-mediated pathway as a prelude to Alzheimer’s disease. Int. J. Biol. Macromol. 2021, 192, 491–497. [Google Scholar] [CrossRef]
- Parthiban, A.; Sachithanandam, V.; Lalitha, P.; Elumalai, D.; Asha, R.N.; Jeyakumar, T.C.; Muthukumaran, J.; Jain, M.; Jayabal, K.; Mageswaran, T.; et al. Isolation and biological evaluation 7-hydroxy flavone from Avicennia officinalis L: Insights from extensive in vitro, DFT, molecular docking and molecular dynamics simulation studies. J. Biomol. Struct. Dyn. 2023, 41, 2848–2860. [Google Scholar] [CrossRef]
- Lim, H.S.; Kim, O.S.; Kim, B.Y.; Jeong, S.J. Apigetrin from Scutellaria baicalensis Georgi Inhibits Neuroinflammation in BV-2 Microglia and Exerts Neuroprotective Effect in HT22 Hippocampal Cells. J. Med. Food 2016, 19, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Ververis, A.; Ioannou, K.; Kyriakou, S.; Violaki, N.; Panayiotidis, M.I.; Plioukas, M.; Christodoulou, K. Sideritis scardica Extracts Demonstrate Neuroprotective Activity against Aβ25–35 Toxicity. Plants 2023, 12, 1716. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Berbel, I.; Espargaró, A.; Viayna, A.; Caballero, A.B.; Busquets, M.A.; Gámez, P.; Luque, F.J.; Sabaté, R. Three to Tango: Inhibitory Effect of Quercetin and Apigenin on Acetylcholinesterase, Amyloid-β Aggregation and Acetylcholinesterase-Amyloid Interaction. Pharmaceutics 2022, 14, 2342. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, Y.M.; Park, H.J.; Sohn, H.S.; Jung, H.A. The effects of C-glycosylation of luteolin on its antioxidant, anti-Alzheimer’s disease, anti-diabetic, and anti-inflammatory activities. Arch. Pharm. Res. 2014, 37, 1354–1363. [Google Scholar] [CrossRef]
- Szwajgier, D. Anticholinesterase activity of selected phenolic acids and flavonoids—Interaction testing in model solutions. Ann. Agric. Environ. Med. 2015, 22, 690–694. [Google Scholar] [CrossRef]
- Ververis, A.; Savvidou, G.; Ioannou, K.; Nicolaou, P.; Christodoulou, K.; Plioukas, M. Greek Sage Exhibits Neuroprotective Activity against Amyloid Beta-Induced Toxicity. Evid. Based Complement. Altern. Med. 2020, 2020, 2975284. [Google Scholar] [CrossRef]
- Ben Mansour, R.; Ksouri, W.M.; Cluzet, S.; Krisa, S.; Richard, T.; Ksouri, R. Assessment of Antioxidant Activity and Neuroprotective Capacity on PC12 Cell Line of Frankenia thymifolia and Related Phenolic LC-MS/MS Identification. Evid. Based Complement. Alternat. Med. 2016, 2016, 2843463. [Google Scholar] [CrossRef]
- de Souza, S.B.; Ferreira, R.S.; dos Santos, C.C.; Castro e Silva, J.H.; Pereira, E.P.; de Almeida, M.M.A.; do Nascimento, R.P.; de Sampaio Schitine, C.; de Oliveira, J.V.R.; dos Santos, B.L.; et al. Neuroprotection induced by coumarins in central nervous system disease models. In Natural Molecules in Neuroprotection and Neurotoxicity; De Oliveira, M.R., Ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 1411–1440. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Meng, S.; Ma, T.; Wan, L.; Xu, S. Chlorogenic Acid Alleviates Aβ25-35-Induced Autophagy and Cognitive Impairment via the mTOR/TFEB Signaling Pathway. Drug Des. Devel. Ther. 2020, 14, 1705–1716. [Google Scholar] [CrossRef]
- Xu, B.; Mo, X.; Chen, J.; Yu, H.; Liu, Y. Myricetin Inhibits α-Synuclein Amyloid Aggregation by Delaying the Liquid-to-Solid Phase Transition. Chembiochem 2022, 23, e202200216. [Google Scholar] [CrossRef]
- Chiu, Y.-J.; Teng, Y.-S.; Chen, C.-M.; Sun, Y.-C.; Hsieh-Li, H.M.; Chang, K.-H.; Lee-Chen, G.-J. A Neuroprotective Action of Quercetin and Apigenin through Inhibiting Aggregation of Aβ and Activation of TRKB Signaling in a Cellular Experiment. Biomol. Ther. 2023, 31, 285. [Google Scholar] [CrossRef]
- Wang, S.W.; Wang, Y.J.; Su, Y.J.; Zhou, W.W.; Yang, S.G.; Zhang, R.; Zhao, M.; Li, Y.N.; Zhang, Z.P.; Zhan, D.W.; et al. Rutin inhibits β-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proinflammatory cytokines. Neurotoxicology 2012, 33, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Alsadat, A.M.; Nikbakht, F.; Hossein Nia, H.; Golab, F.; Khadem, Y.; Barati, M.; Vazifekhah, S. GSK-3β as a target for apigenin-induced neuroprotection against Aβ 25-35 in a rat model of Alzheimer’s disease. Neuropeptides 2021, 90, 102200. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gao, M.; Qiang, G.F.; Zhang, T.T.; Lan, X.; Ying, J.; Du, G.H. The anti-amnesic effects of luteolin against amyloid β25-35 peptide-induced toxicity in mice involve the protection of neurovascular unit. Neuroscience 2009, 162, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Park, J.S.; Kang, M.H.; Lee, H.J.; Ali, J.; Tahir, M.; Choe, K.; Kim, M.O. Caffeic Acid, a Polyphenolic Micronutrient Rescues Mice Brains against Aβ-Induced Neurodegeneration and Memory Impairment. Antioxidants 2023, 12, 1284. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, T.C.; Winter, A.N.; Punessen, N.C.; Linseman, D.A. Procyanidin B2 Protects Neurons from Oxidative, Nitrosative, and Excitotoxic Stress. Antioxidants 2017, 6, 77. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Álvarez-Fernández, M.A.; Cerezo, A.B.; Richard, T.; Troncoso, A.M.; Garcia-Parrilla, M.C. Protocatechuic Acid: Inhibition of Fibril Formation, Destabilization of Preformed Fibrils of Amyloid-β and α-Synuclein, and Neuroprotection. J. Agric. Food Chem. 2016, 64, 7722–7732. [Google Scholar] [CrossRef]
- Yu, M.; Chen, X.; Liu, J.; Ma, Q.; Zhuo, Z.; Chen, H.; Zhou, L.; Yang, S.; Zheng, L.; Ning, C.; et al. Gallic acid disruption of Aβ1-42 aggregation rescues cognitive decline of APP/PS1 double transgenic mouse. Neurobiol. Dis. 2019, 124, 67–80. [Google Scholar] [CrossRef]
- Nagori, K.; Pradhan, M.; Nakhate, K.T. Ethyl gallate ameliorates diabetes-induced Alzheimer’s disease-like phenotype in rats via activation of α7 nicotinic receptors and mitigation of oxidative stress. Biochem. Biophys. Res. Commun. 2024, 737, 150925. [Google Scholar] [CrossRef]
- Salamanova, E.; Atanasova, M.; Dimitrov, I.; Doytchinova, I. Effects of Curcumin and Ferulic Acid on the Folding of Amyloid-β Peptide. Molecules 2021, 26, 2815. [Google Scholar] [CrossRef] [PubMed]
- Mugundhan, V.; Arthanari, A.; Parthasarathy, P.R. Protective Effect of Ferulic Acid on Acetylcholinesterase and Amyloid Beta Peptide Plaque Formation in Alzheimer’s Disease: An In Vitro Study. Cureus 2024, 16, e54103. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Percaccio, E.; Gullì, M.; Romano, A.; Vitalone, A.; Mazzanti, G.; Gaetani, S.; Di Sotto, A. Recent Advances in the Neuroprotective Properties of Ferulic Acid in Alzheimer’s Disease: A Narrative Review. Nutrients 2022, 14, 3709. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Shikha, D.; Thakur, M.; Aneja, A. Functionality of apigenin as a potent antioxidant with emphasis on bioavailability, metabolism, action mechanism and in vitro and in vivo studies: A review. J. Food Biochem. 2022, 46, e13950. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, Z.; Sadeer, N.B.; Hussain, M.; Mahwish; Alsagaby, S.A.; Imran, M.; Mumtaz, T.; Umar, M.; Tauseef, A.; Al Abdulmonem, W.; et al. Therapeutical properties of apigenin: A review on the experimental evidence and basic mechanisms. Int. J. Food Prop. 2023, 26, 1914–1939. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.L.; Liu, R.; Li, X.X.; Li, J.F.; Zhang, L. Neuroprotective, Anti-Amyloidogenic and Neurotrophic Effects of Apigenin in an Alzheimer’s Disease Mouse Model. Molecules 2013, 18, 9949. [Google Scholar] [CrossRef]
- Pravin, B.; Nanaware, V.; Ashwini, B.; Wondmie, G.F.; Jardan, Y.A.B.; Bourhia, M. Assessing the antioxidant properties of Naringin and Rutin and investigating their oxidative DNA damage effects in breast cancer. Sci. Rep. 2024, 14, 15314. [Google Scholar] [CrossRef] [PubMed]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative Properties and Effect of Quercetin and Its Glycosylated Form (Rutin) on Acetylcholinesterase and Butyrylcholinesterase Activities. J. Evid. Based Complement. Altern. Med. 2016, 21, NP11–NP17. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Li, L.J.; Dong, Q.X.; Zhu, J.; Huang, Y.R.; Hou, S.J.; Yu, X.L.; Liu, R.T. Rutin prevents tau pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J. Neuroinflammation 2021, 18, 131. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869. [Google Scholar] [CrossRef]
- Nasb, M.; Tao, W.; Chen, N. Alzheimer’s Disease Puzzle: Delving into Pathogenesis Hypotheses. Aging Dis. 2024, 15, 43–73. [Google Scholar] [CrossRef]
- WFO (2024): World Flora Online. Published on the Internet. Available online: http://www.worldfloraonline.org (accessed on 16 October 2024).
- Scalbert, A.; Monties, B.; Janin, G. Tannins in Wood: Comparison of Different Estimation Methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Ververis, A.; Kyriakou, S.; Ioannou, K.; Chatzopoulou, P.S.; Panayiotidis, M.I.; Plioukas, M.; Christodoulou, K. Chemical Profiling and Antioxidant and Anti-Amyloid Capacities of Salvia fruticosa Extracts from Greece. Plants 2023, 12, 3191. [Google Scholar] [CrossRef] [PubMed]
- Parejo, I.; Codina, C.; Petrakis, C.; Kefalas, P. Evaluation of scavenging activity assessed by Co(II)/EDTA-induced luminol chemiluminescence and DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical assay. J. Pharmacol. Toxicol. Methods 2000, 44, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef]



| EXTRACT | EODM | EOM | EOW1 | EODE | EOEA | EOB | EOW2 |
|---|---|---|---|---|---|---|---|
| Benzoic acid derivatives (μg/g of dry extract) | |||||||
| m-hydroxy benzoic acid | n.d. | 1.25 ± 0.10 a | 1.34 ± 0.02 a | 6.12 ± 0.54 b | 45.55 ± 2.32 d | 24.32 ± 0.99 c | n.d. |
| p-hydroxy benzoic acid | 1.32 ± 0.01 a | 6.16 ± 0.23 c | n.d. | n.d. | 64.54 ± 3.14 e | 40.43 ± 2.21 d | 3.45 ± 0.15 b |
| Protocatechuic acid | n.d. | 4.44 ± 1.23 a | 4.21 ± 0.29 a | 3.33 ± 0.12 a | 112.36 ± 9.87 c | 55.48 ± 2.45 b | n.d. |
| Vanillin | n.d. | 0.60 ± 0.01 b | 0.26 ± 0.01 a | 0.21 ± 0.01 a | 11.65 ± 0.22 c | 12.32 ± 1.03 c | n.d. |
| Gentisic acid | n.d. | 1.84 ± 0.04 b | 4.43 ± 0.22 d | 3.41 ± 0.22 c | 8.45 ± 0.46 e | n.d. | 0.55 ± 0.03 a |
| Gallic acid derivatives (μg/g of dry extract) | |||||||
| Gallic acid | 0.95 ± 0.56 a | 86.36 ± 2.15 f | 6.45 ± 0.03 c | 3.21 ± 0.01 b | 245.65 ± 2.14 g | 64.14 ± 1.21 e | 11.48 ± 0.89 d |
| Ethyl gallate | n.d. | 24.13 ± 1.03 d | 12.12 ± 0.10 c | 6.08 ± 0.24 b | 149.56 ± 4.13 f | 60.15 ± 1.98 e | 0.96 ± 0.01 a |
| Syringic acid | n.d. | 3.94 ± 0.13 a | n.d. | n.d. | 47.31 ± 2.19 c | 9.98 ± 2.14 b | 4.96 ± 0.36 a |
| Cinnamic acid derivatives (μg/g of dry extract) | |||||||
| Ferulic acid | 4.89 ± 0.17 b | 35.65 ± 2.36 d | 3.60 ± 0.13 a | 20.21 ± 1.74 c | 978.98 ± 12.65 f | 645.55 ± 11.11 e | n.d. |
| Ferulic acid ethyl ester | 0.84 ± 0.01 a | 3.65 ± 0.14 c | n.d. | 2.68 ± 0.02 b | 64.18 ± 2.94 e | 25.12 ± 1.48 d | n.d. |
| Caffeic acid | 5.49 ± 0.23 b | 13.62 ± 0.97 d | 14.18 ± 0.06 d | 10.98 ± 0.02 c | 179.92 ± 11.11 f | 100.98 ± 2.32 e | 1.14 ± 0.01 a |
| Dihydrocaffeic acid | n.d. | 41.10 ± 2.61 c | n.d. | 26.87 ± 1.12 b | 348.98 ± 20.07 e | 73.18 ± 6.01 d | 2.14 ± 0.01 a |
| Trans-cinnamaldehyde | n.d. | 4.87 ± 0.12 a | n.d. | 5.67 ± 0.48 a | 21.55 ± 1.78 c | 10.21 ± 0.10 b | n.d. |
| Trans-cinnamyl alcohol | 0.21 ± 0.01 a | n.d. | 0.48 ± 0.01 b | 0.22 ± 0.01 a | n.d. | 6.54 ± 0.01 c | n.d. |
| m-coumaric acid | n.d. | 8.90 ± 0.32 b | n.d. | 5.51 ± 0.26 a | 34.98 ± 1.40 d | 12.38 ± 1.01 c | n.d. |
| p-coumaric acid | n.d. | 7.78 ± 0.28 b | n.d. | 6.18 ± 0.36 a | n.d. | 5.24 ± 0.24 a | n.d. |
| Chlorogenic acid | 4.11 ± 0.23 b | 41.21 ± 0.94 f | 7.18 ± 0.36 c | 21.53 ± 1.47 e | 148.98 ± 10.41 g | 14.46 ± 0.11 d | 2.14 ± 0.02 a |
| Coumarin derivatives (μg/g of dry extract) | |||||||
| Coumarin | 2.12 ± 0.01 b | 6.48 ± 0.51 c | 19.26 ± 1.29 d | 5.13 ± 0.27 c | 22.13 ± 0.61 d | n.d. | 1.11 ± 0.01 a |
| m-hydroxycoumarin | n.d. | n.d. | 10.95 ± 0.31 b | n.d. | 12.48 ± 0.58 c | 9.00 ± 0.04 b | 2.14 ± 0.01 a |
| p-hydroxycoumarin | 9.19 ± 0.67 b | n.d. | n.d. | n.d. | n.d. | 5.11 ± 0.02 a | n.d. |
| 7- hydroxycoumarin | 1.86 ± 0.07 a | 14.04 ± 1.02 d | 12.01 ± 0.13 d | 8.42 ± 0.26 b | 13.32 ± 1.04 d | 10.48 ± 1.00 c | n.d. |
| Osthol | 6.12 ± 0.13 d | n.d. | n.d. | 2.21 ± 0.01 c | n.d. | 1.11 ± 0.01 b | 0.69 ± 0.01 a |
| Phenolic derivatives (μg/g of dry extract) | |||||||
| Eugenol | n.d. | 1.40 ± 0.01 b | n.d. | 0.48 ± 0.01 a | 5.14 ± 0.14 c | 1.21 ± 0.10 b | n.d. |
| Total Phenolic Content (TPC) (mg of gallic acid eq/g of extract) | |||||||
| 22.51 ± 1.01 a | 86.56 ± 3.54 c | 91.64 ± 3.78 c | 98.10 ± 2.01 d | 405.98 ± 16.54 f | 236.89 ± 12.68 e | 47.13 ± 1.54 b | |
| Furanocoumarin derivatives (μg/g of dry extract) | |||||||
| Isopimpinellin | n.d. | 0.58 ± 0.02 a | n.d. | n.d. | 4.12 ± 0.32 b | n.d. | n.d. |
| Xanthotoxin | 0.21 ± 0.01 a | 0.92 ± 0.03 b | n.d. | n.d. | 2.98 ± 0.24 d | 1.48 ± 0.08 c | n.d. |
| Xanthotoxol | 0.11 ± 0.01 a | n.d. | n.d. | n.d. | 12.98 ± 1.03 c | 5.33 ± 0.17 b | n.d. |
| Flavanone derivatives (μg/g of dry extract) | |||||||
| 2′-hydroxyflavanone | n.d. | n.d. | n.d. | 6.45 ± 0.45 a | 45.68 ± 3.05 c | 24.12 ± 1.58 b | 5.38 ± 0.13 a |
| 7-hydroxyflavanone | 1.44 ± 0.01 a | 3.00 ± 0.14 b | n.d. | 14.48 ± 1.21 d | 678.18 ± 26.18 f | 357.98 ± 15.93 e | 10.42 ± 0.70 c |
| 4′-methoxyflavanone | n.d. | n.d. | n.d. | 26.26 ± 2.02 a | 345.16 ± 19.45 c | 197.64 ± 11.99 b | n.d. |
| Naringin | n.d. | 2.96 ± 0.11 b | 1.89 ± 0.03 a | 3.24 ± 0.11 b | 8.35 ± 0.61 d | 4.21 ± 0.36 b, c | n.d. |
| Flavone derivatives (μg/g of dry extract) | |||||||
| Apigenin | 4.62 ± 0.26 b | 12.28 ± 0.13 c | n.d. | 348.15 ± 15.48 d | 897.48 ± 36.24 f | 657.98 ± 27.13 e | 3.21 ± 0.24 a |
| Apigenin-7-O-glucoside | n.d. | n.d. | n.d. | 210.69 ± 15.97 a | 743.14 ± 17.48 c | 555.47 ± 15.87 b | n.d. |
| Luteolin | n.d. | n.d. | 0.22 ± 0.01 a | 73.85 ± 6.12 b | 169.18 ± 12.12 d | 97.15 ± 3.37 c | n.d. |
| Luteolin-7-O-glucoside | 1.82 ± 0.11 a | 8.53 ± 0.69 c | 3.77 ± 0.13 b | 16.98 ± 1.21 d | 49.98 ± 3.15 e | 14.69 ± 1.21 d | n.d. |
| Flavonol derivatives (μg/g of dry extract) | |||||||
| Isorhamnetin | 2.64 ± 0.02 a | 2.65 ± 0.10 a | n.d. | 2.54 ± 0.11 a | 67.98 ± 5.50 c | 41.24 ± 2.40 b | n.d. |
| Quercetin-3-O-rhamnoside | n.d. | 0.64 ± 0.02 a | n.d. | n.d. | 456.13 ± 13.44 c | 98.45 ± 6.47 b | n.d. |
| Quercetin-3-O-rutinoside | n.d. | n.d. | 0.68 ± 0.03 a | n.d. | 947.98 ± 46.98 c | 236.79 ± 14.44 b | n.d. |
| Quercetin-3-O-galactoside | n.d. | n.d. | 0.020 ± 0.001 a | n.d. | 6.54 ± 0.21 c | 0.98 ± 0.01 b | n.d. |
| Myricetin-3-O-galactoside | 1.22 ± 0.01 a | 2.13 ± 0.01 b | n.d. | 2.01 ± 0.10 b | 183.31 ± 10.18 e | 54.48 ± 2.34 d | 3.21 ± 0.24 c |
| Myricetin-3-O-rhamnoside | 0.16 ± 0.01 a | 0.20 ± 0.01 a | 1.47 ± 0.01 b | n.d. | 16.65 ± 0.24 d | 10.98 ± 0.10 c | 1.00 ± 0.001 b |
| Kaempferol | n.d. | 1.29 ± 0.03 a | n.d. | n.d. | 54.98 ± 3.69 c | 21.21 ± 1.53 b | n.d. |
| Kaempferol-3-O-rutinoside | n.d. | 11.48 ± 0.41 c | n.d. | 9.87 ± 0.48 b | 96.12 ± 4.56 d | n.d. | 4.98 ± 0.08 a |
| Procyanidins (μg/g of dry extract) | |||||||
| Procyanidin-B2 | 0.21 ± 0.01 a | n.d. | 44.65 ± 2.31 d | n.d. | 5.65 ± 0.12 b | n.d. | 10.48 ± 1.06 c |
| Total Flavonoid Content (TFC) (μmol catechin eq/g of extract) | |||||||
| 24.65 ± 1.23 a | 548.26 ± 2.45 e | 136.66 ± 6.15 c | 230.56 ± 7.98 d | 766.98 ± 10.13 g | 651.33 ± 12.21 f | 82.11 ± 2.17 b | |
| Total Flavonoid Content (TFC) (μmol rutin eq/g of extract) | |||||||
| 20.15 ± 1.11 a | 845.37 ± 9.87 e | 187.98 ± 8.88 c | 285.47 ± 16.69 d | 988.12 ± 13.32 f | 979.98 ± 14.54 f | 91.52 ± 6.14 b | |
| EODM | EOM | EOW1 | EODE | EOEA | EOB | EOW2 | ||
|---|---|---|---|---|---|---|---|---|
| DPPH• | EC50 (mg extract/mg DPPH•) | 14.18 ± 1.91 b | 0.42 ± 0.01 a | 0.39 ± 0.02 a | 0.50 ± 0.02 a | 0.23 ± 0.01 a | 0.73 ± 0.04 a | 0.66 ± 0.03 a |
| AE | 0.07 | 2.36 | 2.59 | 2.00 | 4.43 | 1.38 | 1.51 | |
| FRAP | μmol AAE/g | 57.18 ± 3.67 a | 724.44 ± 18.76 d | 364.29 ± 3.38 bc | 439.82 ± 9.14 c | 2992.48 ± 256.74 f | 1192.21 ± 41.66 e | 106.44 ± 17.84 ab |
| μmol TEAC/g | 73.72 ± 6.37 a | 793.92 ± 25.68 c | 528.34 ± 42.99 abc | 604.38 ± 52.65 bc | 3658.90 ± 430.45 e | 1523.40 ± 125.42 d | 171.80 ± 7.04 ab | |
| DCFDA | IC50 (μg/mL) | 16.05 ± 3.61 b | 21.00 ± 7.89 bc | 33.34 ± 5.18 c | 27.37 ± 1.83 bc | 2.80 ± 2.28 a | 3.38 ± 2.26 a | 31.28 ± 5.16 c |
| EODM | EOM | EOW1 | EODE | EOEA | EOB | EOW2 | Donepezil | ||
|---|---|---|---|---|---|---|---|---|---|
| AChE | IC25 (μg/mL) | 760.70 ± 39.02 bc | 848.19 ± 69.21 c | >1000 | >1000 | 542.42 ± 71.41 b | 274.29 ± 14.56 a | >1000 | 4.17 ± 0.16 ng/mL |
| IC50 (μg/mL) | >1000 | >1000 | >1000 | >1000 | >1000 | 646.93 ± 33.03 | >1000 | 10.16 ± 0.16 ng/mL | |
| Inhibition % | 33.27 ± 5.83 bc | 34.15 ± 4.34 bc | 11.07 ± 3.73 a | 23.27 ± 3.50 ab | 49.70 ± 2.46 c | 66.93 ± 1.42 d | n.d. | 76.16 ± 0.51 | |
| BChE | IC50 (μg/mL) | 267.82 ± 45.70 a | 615.95 ± 101.86 b | >1000 | 315.75 ± 62.21 a | 276.79 ± 41.74 a | 288.02 ± 33.19 a | >1000 | 1.26 ± 0.03 |
| Inhibition % | 72.48 ± 0.84 cd | 63.81 ± 3.92 c | 4.28 ± 1.12 a | 34.96 ± 8.81 b | 87.13 ± 6.46 de | 97.58 ± 2.42 e | 7.56 ± 2.43 a | 84.09 ± 1.55 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ververis, A.; Kyriakou, S.; Paraskeva, H.; Panayiotidis, M.I.; Plioukas, M.; Christodoulou, K. Chemical Characterization and Assessment of the Neuroprotective Potential of Euphrasia officinalis. Int. J. Mol. Sci. 2024, 25, 12902. https://doi.org/10.3390/ijms252312902
Ververis A, Kyriakou S, Paraskeva H, Panayiotidis MI, Plioukas M, Christodoulou K. Chemical Characterization and Assessment of the Neuroprotective Potential of Euphrasia officinalis. International Journal of Molecular Sciences. 2024; 25(23):12902. https://doi.org/10.3390/ijms252312902
Chicago/Turabian StyleVerveris, Antonis, Sotiris Kyriakou, Hariklia Paraskeva, Mihalis I. Panayiotidis, Michael Plioukas, and Kyproula Christodoulou. 2024. "Chemical Characterization and Assessment of the Neuroprotective Potential of Euphrasia officinalis" International Journal of Molecular Sciences 25, no. 23: 12902. https://doi.org/10.3390/ijms252312902
APA StyleVerveris, A., Kyriakou, S., Paraskeva, H., Panayiotidis, M. I., Plioukas, M., & Christodoulou, K. (2024). Chemical Characterization and Assessment of the Neuroprotective Potential of Euphrasia officinalis. International Journal of Molecular Sciences, 25(23), 12902. https://doi.org/10.3390/ijms252312902







