Investigation of the Interaction Between Angiotensin-Converting Enzyme (ACE) and ACE-Inhibitory Tripeptide from Casein
Abstract
1. Introduction
2. Results
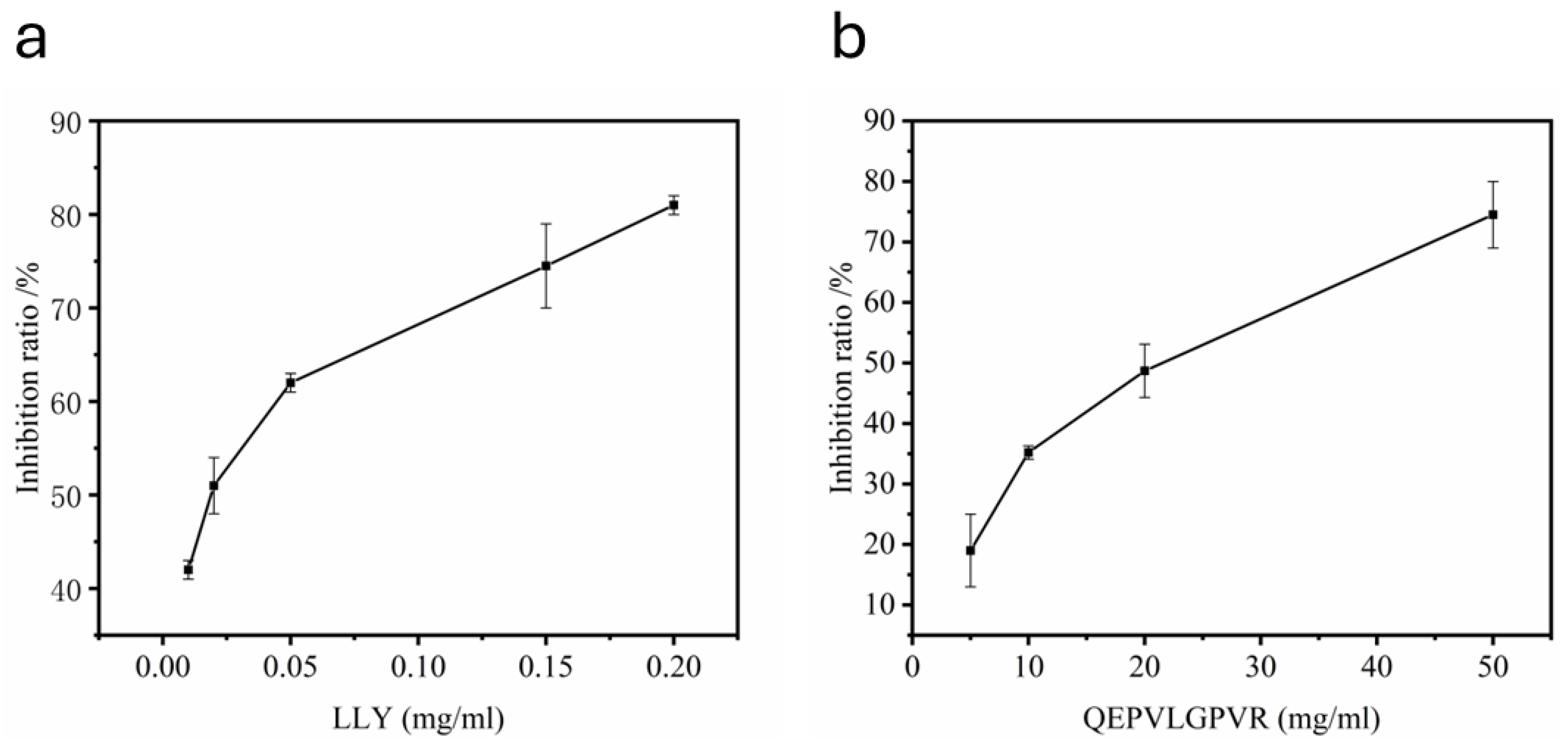
2.1. Evaluation of Half Maximal Concentration—IC50
2.2. Stability Analysis
2.3. Inhibitory Pattern of LLY on ACE
2.4. Molecular Docking Analysis
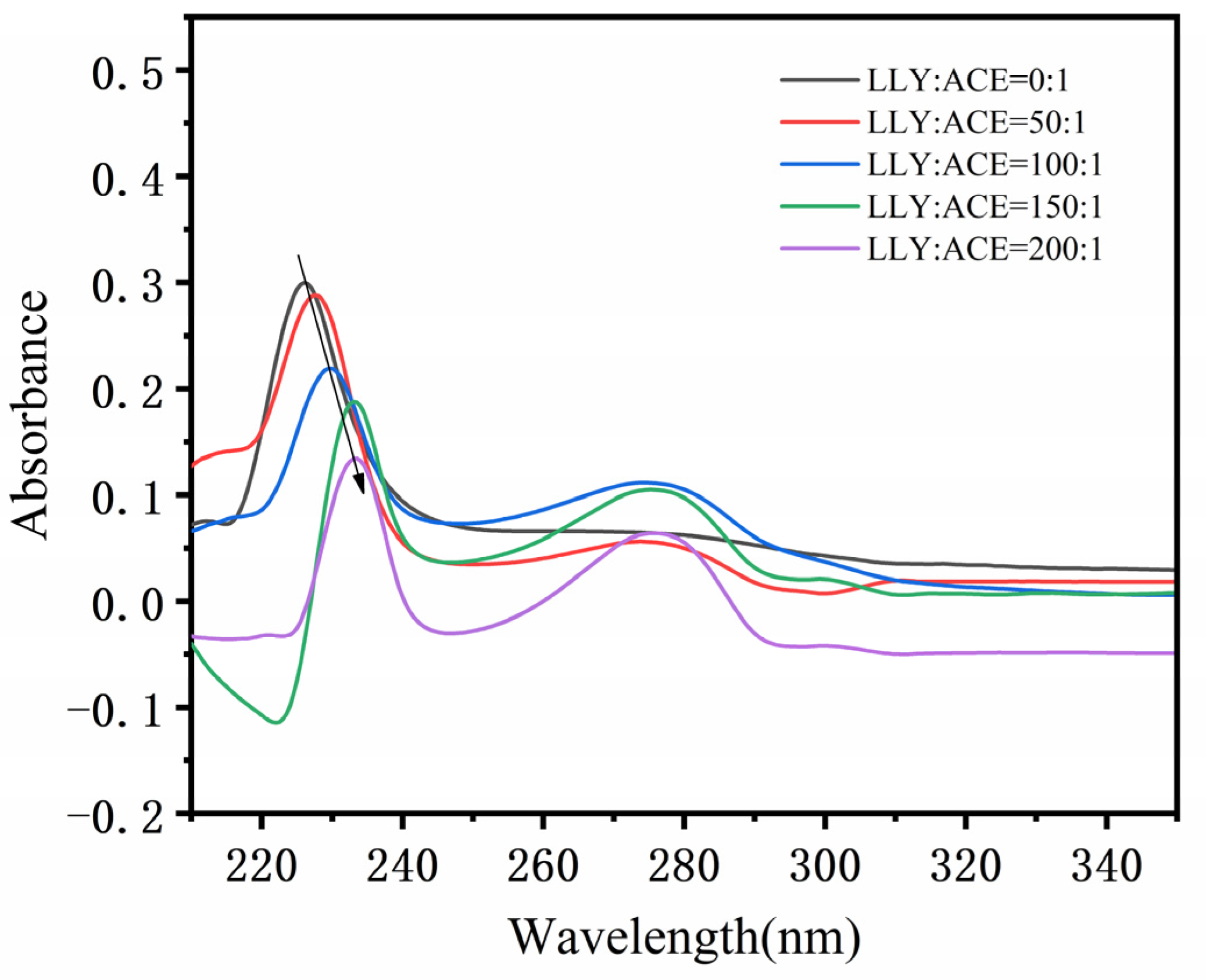
2.5. UV Spectral Analysis
2.6. Fluorescence Spectral Analysis
2.6.1. Fluorescence Spectrum
2.6.2. The Three-Dimensional Fluorescence Spectra
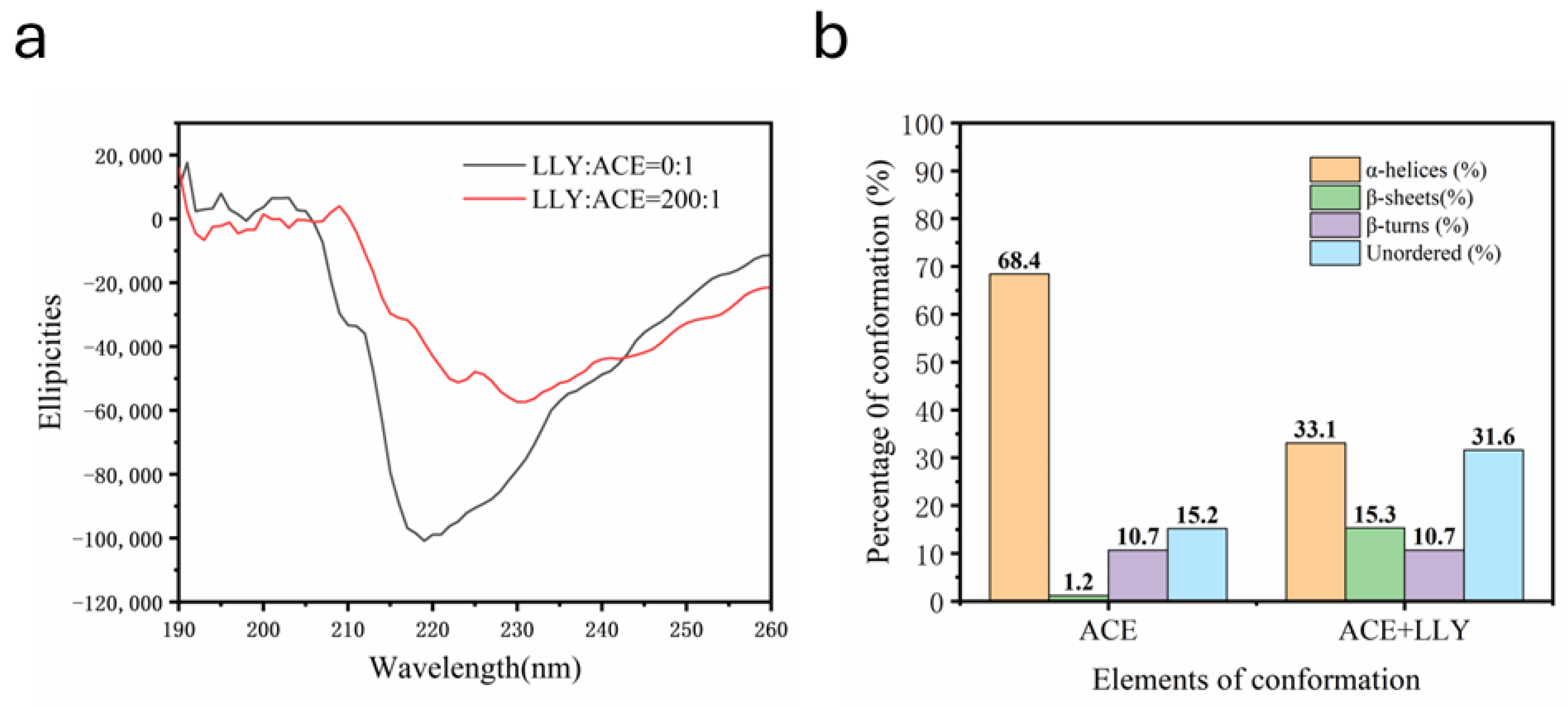
2.7. CD Chromatographic Analysis
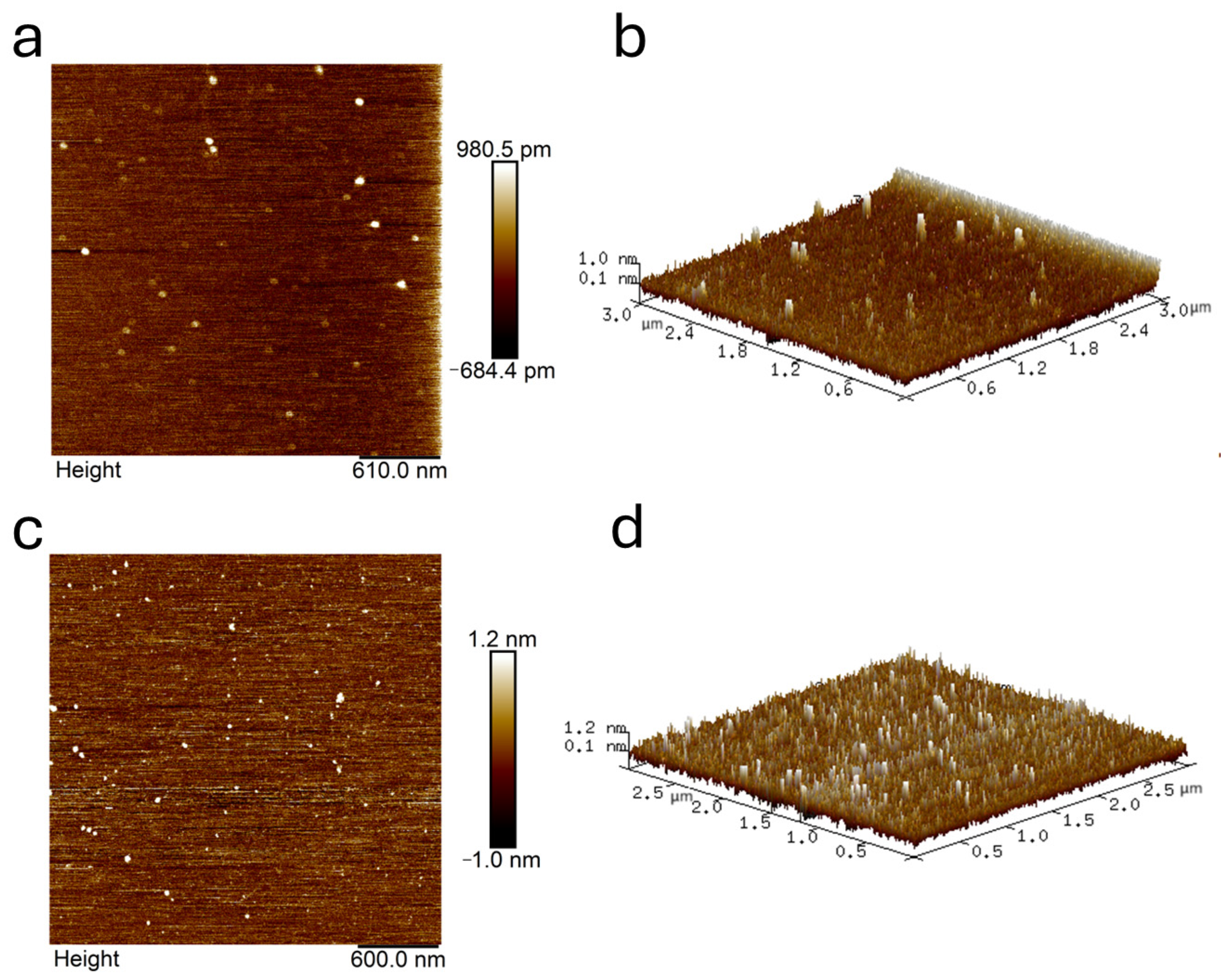
2.8. AFM Analysis
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Determination of Half-Maximal Concentration IC50
4.3. Stability Evaluation of ACE-Inhibitory Peptide LLY
4.4. ACE Inhibition Kinetics
4.5. Molecular Docking
4.6. Spectral Measurement
4.6.1. UV Spectral Measurement
4.6.2. Fluorescence Spectrum Measurements
4.6.3. CD Spectroscopic Measurements
4.7. AFM Measurement
4.8. Statistical Analysis
5. Conclusions
6. Future and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Report on Hypertension: The Race Against a Silent Killer; World Health Organization: Geneva, Switzerland, 2023; pp. 1–276. [Google Scholar]
- Drummond, G.R.; Vinh, A.; Guzik, T.J.; Sobey, C.G. Immune mechanisms of hypertension. Nat. Rev. Immunol. 2019, 19, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Barzideh, Z.; Latiff, A.A.; Gan, C.Y.; Abedin, M.Z.; Alias, A.K. ACE Inhibitory and Antioxidant Activities of Collagen Hydrolysates from the Ribbon Jellyfish (Chrysaora sp.). Food Technol. Biotechnol. 2014, 52, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, R.; Ghasemi, A.-s.; Dehghanbanadaki, N.; Mehralitabar, H. Computational exploration of naturally derived peptides inhibitory mechanisms against ACE enzyme, from interactions to structural-dynamics. Biochem. Biophys. Res. Commun. 2024, 735, 150812. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kang, S.-G.; Jaiswal, L.; Li, J.; Choi, J.-H.; Moon, S.-M.; Cho, J.-Y.; Ham, K.-S. Identification of four new angiotensin I-converting enzyme inhibitory peptides from fermented anchovy sauce. Appl. Biol. Chem. 2016, 59, 25–31. [Google Scholar] [CrossRef]
- Ahmad, H.; Khan, H.; Haque, S.; Ahmad, S.; Srivastava, N.; Khan, A. Angiotensin-Converting Enzyme and Hypertension: A Systemic Analysis of Various ACE Inhibitors, Their Side Effects, and Bioactive Peptides as a Putative Therapy for Hypertension. J. Renin-Angiotensin-Aldosterone Syst. 2023, 2023, 7890188. [Google Scholar] [CrossRef]
- Wang, C.; Tu, M.; Wu, D.; Chen, H.; Chen, C.; Wang, Z.; Jiang, L. Identification of an ACE-Inhibitory Peptide from Walnut Protein and Its Evaluation of the Inhibitory Mechanism. Int. J. Mol. Sci. 2018, 19, 1156. [Google Scholar] [CrossRef]
- Danilov, S.M.; Tovsky, S.I.; Schwartz, D.E.; Dull, R.O. ACE Phenotyping as a Guide Toward Personalized Therapy With ACE Inhibitors. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 374–386. [Google Scholar] [CrossRef]
- Hanif, K.; Bid, H.K.; Konwar, R. Reinventing the ACE inhibitors: Some old and new implications of ACE inhibition. Hypertens. Res. 2010, 33, 11–21. [Google Scholar] [CrossRef]
- Priyanto, A.D.; Doerksen, R.J.; Chang, C.-I.; Sung, W.-C.; Widjanarko, S.B.; Kusnadi, J.; Lin, Y.-C.; Wang, T.-C.; Hsu, J.-L. Screening, discovery, and characterization of angiotensin-I converting enzyme inhibitory peptides derived from proteolytic hydrolysate of bitter melon seed proteins. J. Proteom. 2015, 128, 424–435. [Google Scholar] [CrossRef]
- He, R.; Malomo, S.A.; Girgih, A.T.; Ju, X.; Aluko, R.E. Glycinyl-histidinyl-serine (GHS), a novel rapeseed protein-derived peptide has blood pressure-lowering effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2013, 61, 8396–8402. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, C.; Sun-Waterhouse, D.; Zhao, T.; Waterhouse, G.I.N.; Zhao, M.; Su, G. Identification of post-digestion angiotensin-I converting enzyme (ACE) inhibitory peptides from soybean protein Isolate: Their production conditions and in silico molecular docking with ACE. Food Chem. 2021, 345, 128855. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Qian, Z.-J.; Kim, S.-K. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2010, 118, 96–102. [Google Scholar] [CrossRef]
- Liu, M.; Du, M.; Zhang, Y.; Xu, W.; Wang, C.; Wang, K.; Zhang, L. Purification and identification of an ACE inhibitory peptide from walnut protein. J. Agric. Food Chem. 2013, 61, 4097–4100. [Google Scholar] [CrossRef] [PubMed]
- Memarpoor-Yazdi, M.; Zare-Zardini, H.; Mogharrab, N.; Navapour, L. Purification, Characterization and Mechanistic Evaluation of Angiotensin Converting Enzyme Inhibitory Peptides Derived from Zizyphus Jujuba Fruit. Sci. Rep. 2020, 10, 3976. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, H.; Zhang, M. Isolation, identification, and structure-activity relationship of novel ACE inhibitory peptides from earthworm protein in vitro gastrointestinal digestion product. Food Biosci. 2023, 55, 103010. [Google Scholar] [CrossRef]
- Miguel, M.; Contreras, M.M.; Recio, I.; Aleixandre, A. ACE-inhibitory and antihypertensive properties of a bovine casein hydrolysate. Food Chem. 2009, 112, 211–214. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Tian, B.; Brodkorb, A.; Huo, G.C. Production, analysis and in vivo evaluation of novel angiotensin-I-converting enzyme inhibitory peptides from bovine casein. Food Chem. 2010, 123, 779–786. [Google Scholar] [CrossRef]
- Cadée, J.A.; Chang, C.Y.; Chen, C.W.; Huang, C.N.; Chen, S.L.; Wang, C.K. Bovine casein hydrolysate (C12 Peptide) reduces blood pressure in prehypertensive subjects. Am. J. Hypertens. 2007, 20, 1–5. [Google Scholar] [CrossRef]
- Tu, M.L.; Liu, H.X.; Zhang, R.; Chen, H.; Mao, F.J.; Cheng, S.Z.; Lu, W.H.; Du, M. Analysis and Evaluation of the Inhibitory Mechanism of a Novel Angiotensin-I-Converting Enzyme Inhibitory Peptide Derived from Casein Hydrolysate. J. Agric. Food Chem. 2018, 66, 4139–4144. [Google Scholar] [CrossRef]
- Liu, P.; Lan, X.; Yaseen, M.; Chai, K.; Zhou, L.; Sun, J.; Lan, P.; Tong, Z.; Liao, D.; Sun, L. Immobilized metal affinity chromatography matrix modified by poly (ethylene glycol) methyl ether for purification of angiotensin I-converting enzyme inhibitory peptide from casein hydrolysate. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2020, 1143, 122042. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of Angiotensin I-converting enzyme inhibitory peptides: Quantitative structure-activity relationship study of di- and tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Terashima, M.; Oe, M.; Ogura, K.; Matsumura, S. Inhibition strength of short peptides derived from an ACE inhibitory peptide. J. Agric. Food Chem. 2011, 59, 11234–11237. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.H.; Ma, H.L.; Luo, L.; Yin, X.L. Angiotensin I-converting enzyme (ACE) inhibitory peptide derived from Tenebrio molitor (L.) larva protein hydrolysate. Eur. Food Res. Technol. 2013, 236, 681–689. [Google Scholar] [CrossRef]
- Friedman, D.I.; Amidon, G.L. Characterization of the intestinal transport parameters for small peptide drugs. J. Control. Release 1990, 13, 141–146. [Google Scholar] [CrossRef]
- Fitzgerald, R.J.; Murray, B.A. Bioactive peptides and lactic fermentations. Int. J. Dairy Technol. 2006, 59, 118–125. [Google Scholar] [CrossRef]
- Rubak, Y.T.; Nuraida, L.; Iswantini, D.; Prangdimurti, E. Angiotensin-I-converting enzyme inhibitory peptides in milk fermented by indigenous lactic acid bacteria. Vet. World 2020, 13, 345–353. [Google Scholar] [CrossRef]
- Zhu, Q.; Xue, J.; Wang, P.; Wang, X.; Zhang, J.; Fang, X.; He, Z.; Wu, F. Identification of a Novel ACE Inhibitory Hexapeptide from Camellia Seed Cake and Evaluation of Its Stability. Foods 2023, 12, 501. [Google Scholar] [CrossRef]
- Hu, Y.B.; Peng, J.B.; Gu, S.; Pei, J.F.; Zou, Z.M. Molecular Docking in Xin-Ke-Shu Preparation’s Multi-Target Effect on Coronary Heart Disease. Acta Phys. Chim. Sin. 2012, 28, 1257–1264. [Google Scholar] [CrossRef]
- Feng, X.; Liao, D.; Sun, L.; Feng, S.; Wu, S.; Lan, P.; Wang, Z.; Lan, X. Exploration of interaction between angiotensin I-converting enzyme (ACE) and the inhibitory peptide from Wakame (Undaria pinnatifida). Int. J. Biol. Macromol. 2022, 204, 193–203. [Google Scholar] [CrossRef]
- Lan, X.; Sun, L.; Muhammad, Y.; Wang, Z.; Liu, H.; Sun, J.; Zhou, L.; Feng, X.; Liao, D.; Wang, S. Studies on the Interaction between Angiotensin-Converting Enzyme (ACE) and ACE Inhibitory Peptide from Saurida elongata. J. Agric. Food Chem. 2018, 66, 13414–13422. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Huang, H.-Y.; Zhou, L.; Yang, C.; Zhou, J.-H.; Liu, Z.-M. Study on the conformation changes of Lysozyme induced by Hypocrellin A: The mechanism investigation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Shuib, A.S.; Abdullah, N. Structural characteristics and antihypertensive effects of angiotensin-I-converting enzyme inhibitory peptides in the renin-angiotensin and kallikrein kinin systems. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chen, Q.; Zhang, T.; Liu, M.; Duan, S.; Sun, X. The Antihypertensive Effects and Potential Molecular Mechanism of Microalgal Angiotensin I-Converting Enzyme Inhibitor-Like Peptides: A Mini Review. Int. J. Mol. Sci. 2021, 22, 4068. [Google Scholar] [CrossRef]
- Kheeree, N.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. ACE inhibitory peptides derived from de-fatted lemon basil seeds: Optimization, purification, identification, structure–activity relationship and molecular docking analysis. Food Funct. 2020, 11, 8161–8178. [Google Scholar] [CrossRef]
- Yin, Z.; Yan, R.; Jiang, Y.; Feng, S.; Sun, H.; Sun, J.; Zhao, D.; Li, H.; Wang, B.; Zhang, N. Identification of peptides in Qingke baijiu and evaluation of its angiotensin converting enzyme (ACE) inhibitory activity and stability. Food Chem. 2022, 395, 133551. [Google Scholar] [CrossRef]
- Wang, K.; Luo, Q.; Hong, H.; Liu, H.; Luo, Y. Novel antioxidant and ACE inhibitory peptide identified from Arthrospira platensis protein and stability against thermal/pH treatments and simulated gastrointestinal digestion. Food Res. Int. 2021, 139, 109908. [Google Scholar] [CrossRef]
- Zheng, Y.; Shi, P.; Li, Y.; Zhuang, Y.; You, L.; Liu, L.; Wang, W. A novel ACE-inhibitory hexapeptide from camellia glutelin-2 hydrolysates: Identification, characterization and stability profiles under different food processing conditions. Food Sci. Technol. 2021, 147, 111682. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, S.; Wang, L.; Huang, D.; Chen, S. Identification and characterization of an angiotensin-I converting enzyme inhibitory peptide from enzymatic hydrolysate of rape (Brassica napus L.) bee pollen. Food Sci. Technol. 2021, 147, 111502. [Google Scholar] [CrossRef]
- Li, J.; Hu, H.; Chen, X.; Zhu, H.; Zhang, W.; Tai, Z.; Yu, X.; He, Q. A novel ACE inhibitory peptide from Douchi hydrolysate: Stability, inhibition mechanism, and antihypertensive potential in spontaneously hypertensive rats. Food Chem. 2024, 460, 140734. [Google Scholar] [CrossRef]
- Urbizo-Reyes, U.; Liceaga, A.M.; Reddivari, L.; Kim, K.-H.; Anderson, J.M. Enzyme kinetics, molecular docking, and in silico characterization of canary seed (Phalaris canariensis L.) peptides with ACE and pancreatic lipase inhibitory activity. J. Funct. Foods 2022, 88, 104892. [Google Scholar] [CrossRef]
- Lau, C.C.; Abdullah, N.; Shuib, A.S.; Aminudin, N. Novel angiotensin I-converting enzyme inhibitory peptides derived from edible mushroom Agaricus bisporus (JE Lange) Imbach identified by LC-MS/MS. Food Chem. 2014, 148, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Hu, J.E.; Cui, J.Z.; Bai, X.F.; Du, Y.G.; Miyaguchi, Y.; Lin, B.C. Purification and identification of a ACE inhibitory peptide from oyster proteins hydrolysate and the anti hypertensive effect of hydrolysate in spontaneously hypertensive rats. Food Chem. 2008, 111, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. Food Sci. Technol. 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Wu, Q.; Du, J.; Jia, J.; Kuang, C. Production of ACE inhibitory peptides from sweet sorghum grain protein using alcalase: Hydrolysis kinetic, purification and molecular docking study. Food Chem. 2016, 199, 140–149. [Google Scholar] [CrossRef]
- Fu, Y.; Alashi, A.M.; Young, J.F.; Therkildsen, M.; Aluko, R.E. Enzyme inhibition kinetics and molecular interactions of patatin peptides with angiotensin I-converting enzyme and renin. Int. J. Biol. Macromol. 2017, 101, 207–213. [Google Scholar] [CrossRef]
- Zaharuddin, N.D.; Barkia, I.; Wan Ibadullah, W.Z.; Zarei, M.; Saari, N. Identification, molecular docking, and kinetic studies of six novel angiotensin-I-converting enzyme (ACE) inhibitory peptides derived from Kenaf (Hibiscus cannabinus L.) seed. Int. J. Biol. Macromol. 2022, 220, 1512–1522. [Google Scholar] [CrossRef]
- Chamata, Y.; Watson, K.A.; Jauregi, P. Whey-Derived Peptides Interactions with ACE by Molecular Docking as a Potential Predictive Tool of atural ACE Inhibitors. Int. J. Mol. Sci. 2020, 21, 864. [Google Scholar] [CrossRef]
- Ling, Y.; Sun, L.; Zhuang, L. Preparation and identification of novel inhibitory angiotensin-I-converting enzyme peptides from tilapia skin gelatin hydrolysates: Inhibition kinetics and molecular docking. Food Funct. 2018, 9, 5251–5259. [Google Scholar] [CrossRef]
- Guo, B.; Hu, X.; Wu, J.; Chen, R.; Dai, T.; Liu, Y.; Luo, S.; Liu, C. Soluble starch/whey protein isolate complex-stabilized high internal phase emulsion: Interaction and stability. Food Hydrocoll. 2021, 111, 106377. [Google Scholar] [CrossRef]
- Sengupta, B.; Chakraborty, S.; Crawford, M.; Taylor, J.M.; Blackmon, L.E.; Biswas, P.K.; Kramer, W.H. Characterization of diadzein–hemoglobin binding using optical spectroscopy and molecular dynamics simulations. Int. J. Biol. Macromol. 2012, 51, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Dufrêne, Y.F.; Ando, T.; Garcia, R.; Alsteens, D.; Martinez-Martin, D.; Engel, A.; Gerber, C.; Müller, D.J. Imaging modes of atomic force microscopy for application in molecular and cell biology. Nat. Nanotechnol. 2017, 12, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Heble, A.Y.; Zhang, S. Multiparametric AFM insights into electron transport mechanisms in biomemristors. Mater. Today Phys. 2024, 44, 101429. [Google Scholar] [CrossRef]
- Zeng, X.-H.; Du, H.; Zhao, H.-M.; Xiang, L.; Feng, N.-X.; Li, H.; Li, Y.-W.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H.; et al. Insights into the binding interaction of substrate with catechol 2,3-dioxygenase from biophysics point of view. J. Hazard. Mater. 2020, 391, 122211. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, X.; Huang, W.; Wang, C.; He, Q. A novel angiotensin-converting enzyme inhibitory peptide from rabbit meat protein hydrolysate: Identification, molecular mechanism, and antihypertensive effect in vivo. Food Funct. 2021, 12, 12077–12086. [Google Scholar] [CrossRef]
- Lan, X.; Liao, D.; Wu, S.; Wang, F.; Sun, J.; Tong, Z. Rapid purification and characterization of angiotensin converting enzyme inhibitory peptides from lizard fish protein hydrolysates with magnetic affinity separation. Food Chem. 2015, 182, 136–142. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, W.; Zhang, Y.-Q.; Dai, Z.-Y. A novel angiotensin-converting enzyme (ACE) inhibitory peptide from tilapia skin: Preparation, identification and its potential antihypertensive mechanism. Food Chem. 2024, 430, 137074. [Google Scholar] [CrossRef]
- Xie, J.; Chen, X.; Wu, J.; Zhang, Y.; Zhou, Y.; Zhang, L.; Tang, Y.-J.; Wei, D. Antihypertensive Effects, Molecular Docking Study, and Isothermal Titration Calorimetry Assay of Angiotensin I-Converting Enzyme Inhibitory Peptides from Chlorella vulgaris. J. Agric. Food Chem. 2018, 66, 1359–1368. [Google Scholar] [CrossRef]
- Feng, X.; Liao, D.; Sun, L.; Wu, S.; Lan, P.; Wang, Z.; Li, C.; Zhou, Q.; Lu, Y.; Lan, X. Affinity Purification of Angiotensin Converting Enzyme Inhibitory Peptides from Wakame (Undaria Pinnatifida) Using Immobilized ACE on Magnetic Metal Organic Frameworks. Mar. Drugs 2021, 19, 177. [Google Scholar] [CrossRef]
- Wang, R.; Lu, Y.; Wang, S. Comparative Evaluation of 11 Scoring Functions for Molecular Docking. J. Med. Chem. 2003, 46, 2287–2303. [Google Scholar] [CrossRef]




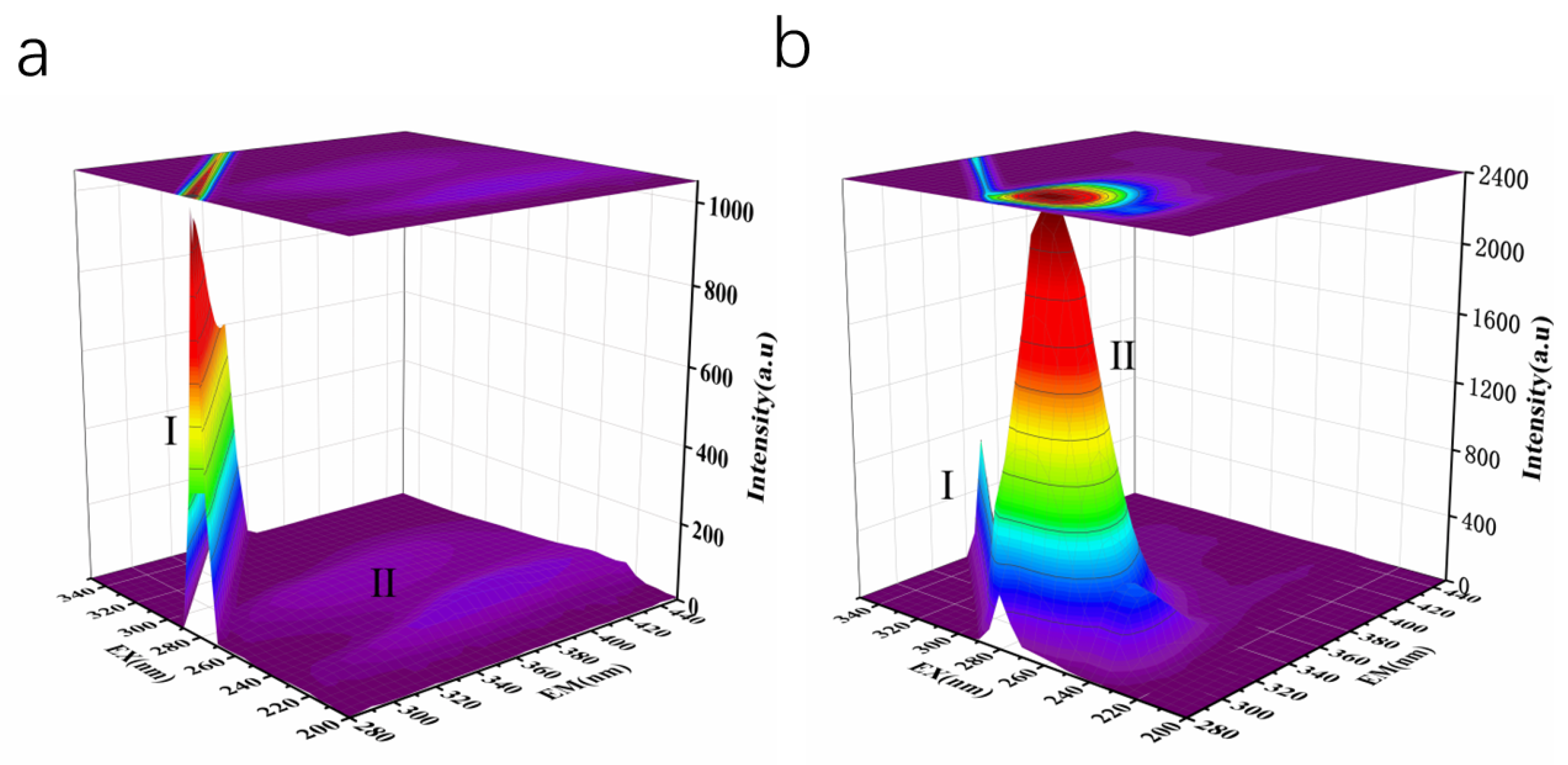
| System | Peak No | Peak Position [λex/λem(nm/nm)] | Intensity |
|---|---|---|---|
| ACE | I | 280/280 | 1029 |
| II | 275/305 | 43.49 | |
| ACE + LLY | I | 290/290 | 734.5 |
| II | 275/305 | 2368 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Xie, T.; Cai, M.; Xu, X.; Li, M.; Liu, P.; Lan, X. Investigation of the Interaction Between Angiotensin-Converting Enzyme (ACE) and ACE-Inhibitory Tripeptide from Casein. Int. J. Mol. Sci. 2024, 25, 13021. https://doi.org/10.3390/ijms252313021
Yang C, Xie T, Cai M, Xu X, Li M, Liu P, Lan X. Investigation of the Interaction Between Angiotensin-Converting Enzyme (ACE) and ACE-Inhibitory Tripeptide from Casein. International Journal of Molecular Sciences. 2024; 25(23):13021. https://doi.org/10.3390/ijms252313021
Chicago/Turabian StyleYang, Cuicui, Tianzhao Xie, Mengmeng Cai, Xiaoting Xu, Muzijun Li, Pengru Liu, and Xiongdiao Lan. 2024. "Investigation of the Interaction Between Angiotensin-Converting Enzyme (ACE) and ACE-Inhibitory Tripeptide from Casein" International Journal of Molecular Sciences 25, no. 23: 13021. https://doi.org/10.3390/ijms252313021
APA StyleYang, C., Xie, T., Cai, M., Xu, X., Li, M., Liu, P., & Lan, X. (2024). Investigation of the Interaction Between Angiotensin-Converting Enzyme (ACE) and ACE-Inhibitory Tripeptide from Casein. International Journal of Molecular Sciences, 25(23), 13021. https://doi.org/10.3390/ijms252313021






