Calcium Ions in the Physiology and Pathology of the Central Nervous System
Abstract
1. Introduction
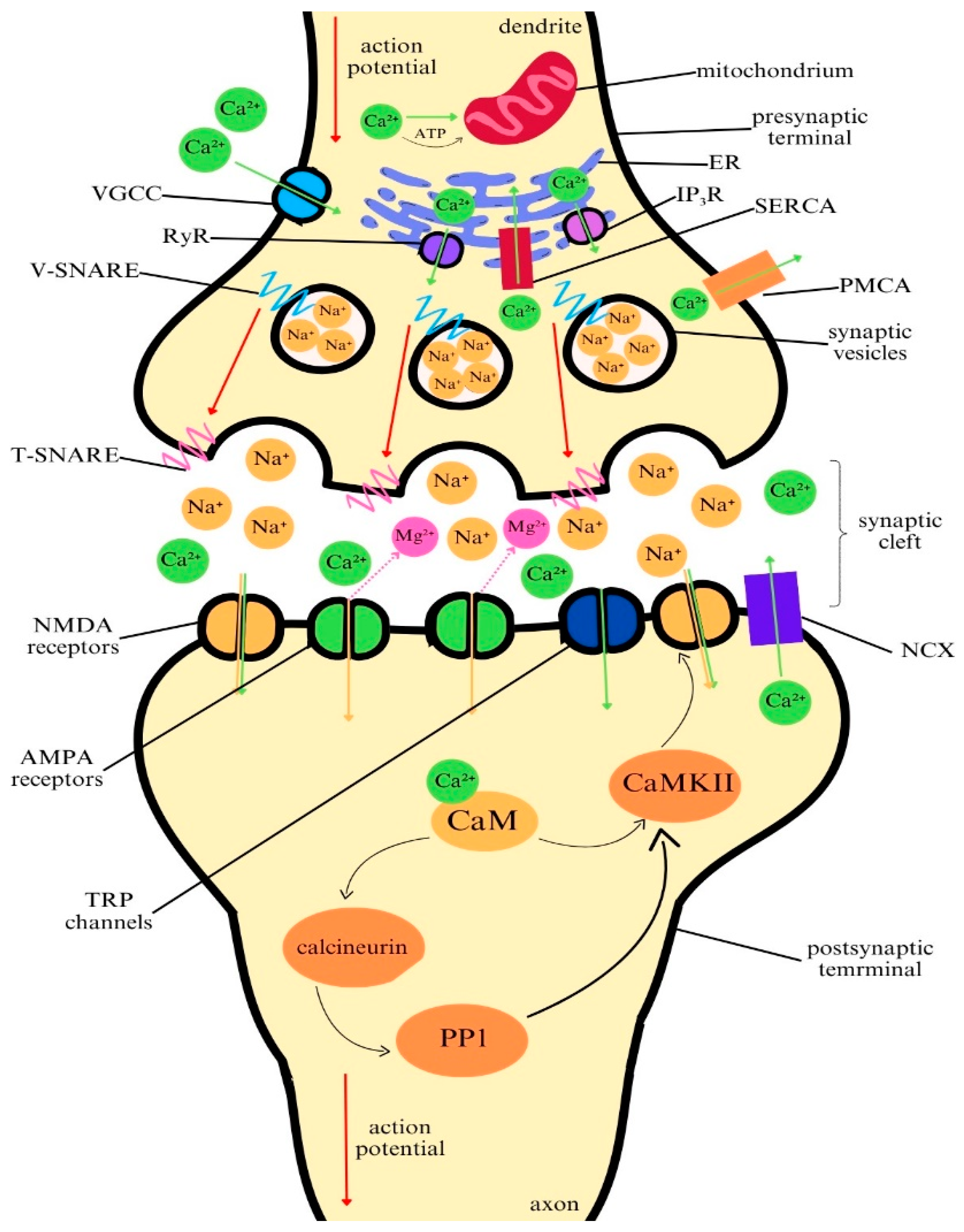
2. The Role of Calcium Ions in the Functioning of the Central Nervous System
2.1. The Role of Calcium in Neuronal Communication
2.2. Calcium as a Second Messenger in Signal Transduction
2.3. Calcium Homeostasis in Neurons and Astrocytes
3. Channelopathies Related to Impaired Transport of Calcium Ions in Neurology
3.1. CACNA Gene Family-Related Channelopathies
3.1.1. CACNA1A Gene
3.1.2. CACNA1B Gene
3.1.3. Other CACNA Family Genes
3.2. TRP Gene Family-Related Channelopathies
4. The Role of Calcium Ions in Pathology Central Nervous System
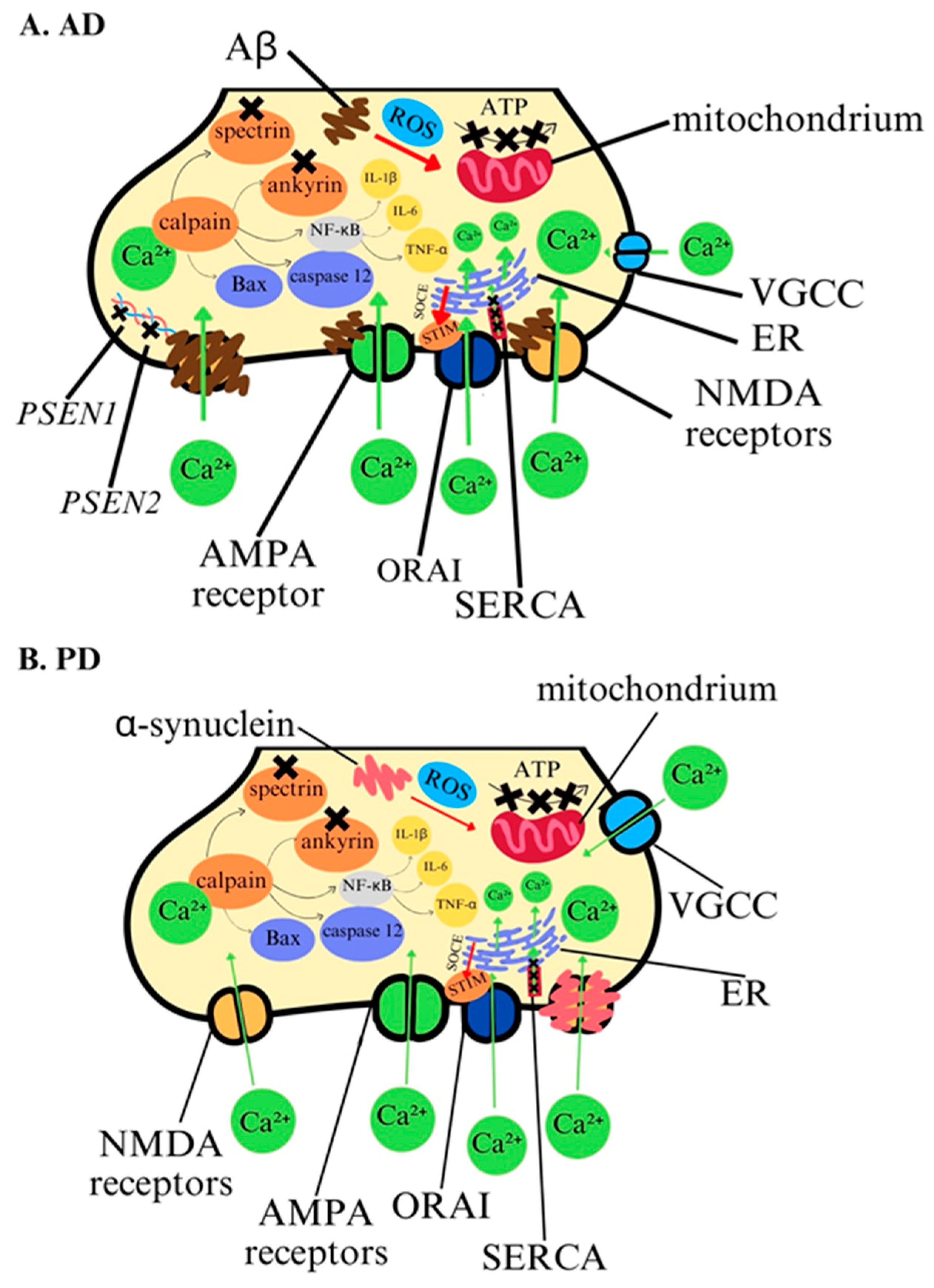
4.1. Calcium Ions in Pathology of Alzheimer’s Disease
4.2. Calcium Ions in Pathology of Parkinson’s Disease
4.3. Calcium Ions in Pathology of Huntington’s Disease
4.4. Calcium Ions in Epilepsy
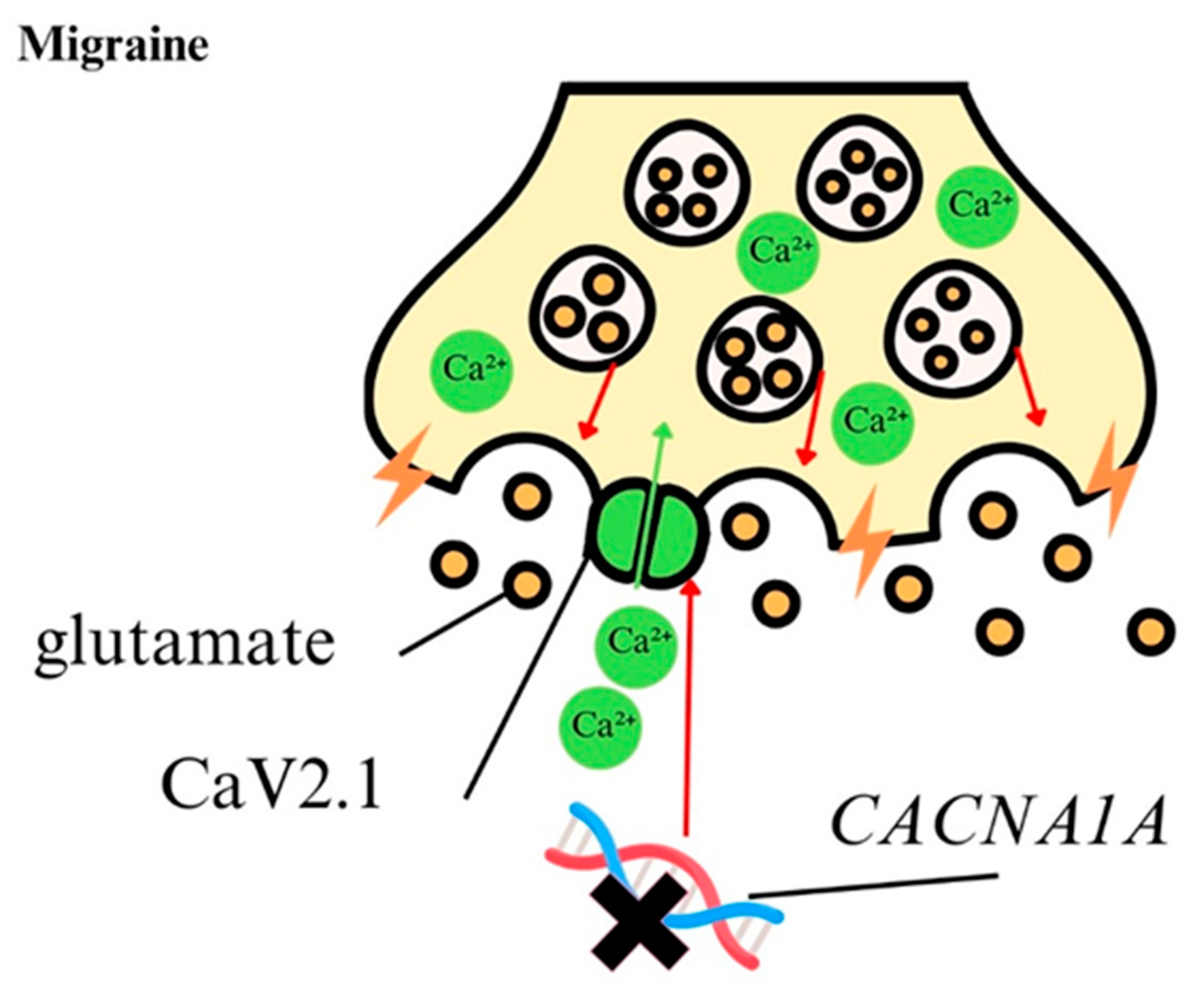
4.5. Calcium Ions in Migraine
| Neurological Disease | Biochemical Changes | Cellular Changes | Genetic Factors | Clinical Symptoms | Diagnostic/ Therapeutic Factor | References |
|---|---|---|---|---|---|---|
| Alzheimer’s disease (AD) | Calpain activation, Calcium-permeable receptors; AMPA NMDA VGCC, apoptosis induction. | Mitochondrial and endoplasmic reticulum dysfunction, oxidative stress generation, neuronal degeneration, beta-amyloid aggregation, excitotoxicity. | Mutation in AD-causal genes; PSEN1 PSEN2 leading to amyloid cascade. | Cognitive decline. | Calcium ion antagonists-dihydropyridine to inhibit calcium overload, calcium ion inhibitor-curcumin, restoring mitochondrial calcium homeostasis-aduhelm? | [68,69] [72,73,74,75,76,77,78,79] [148,149,150] |
| Parkinson’s disease (PD) | Calcium-permeable receptors; AMPA NMDA VGCC, calpain activation, apoptosis induction. | Alpha-synuclein aggregation, Mitochondrial and endoplasmic reticulum dysfunction, oxidative stress generation, dopaminergic neurons degeneration in the substantia nigra. excitotoxicity. | Mutation in PARK genes leading to Lewy bodies aggregation. | Motor dysfunction. | Calcium channel blockers- Isradipine. | [80,81,82,83] [151,152] [153,154,155,156] |
| Huntington’s disease (HD) | Calcium-permeable receptors; AMPA NMDA VGCC, calpain activation, apoptosis induction, store-operated calcium entry (SOCE) mechanism activation. | Mutant huntingtin (mHtt) interacts directly with calcium-binding proteins calbindin and parvalbumin, Mitochondrial and endoplasmic reticulum dysfunction, oxidative stress generation. | Mutation in HTT gene, expanded CAG repeat. | Motor dysfunction, cognitive decline. | Compound stabilizing SOCE-tetrahydro-carbazoles as candidate for targeted HD therapy, potential targets for maintaining calcium homeostasis- RyR, IP3R. | [92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108] [157,158,159,160] |
| Epilepsy | Store-operated calcium entry (SOCE) mechanism activation, excessive glutamate release, excitotoxicity | Endoplasmic reticulum dysfunction, promotes synchronized neuronal firing. | Channelopathies of CaV subunits. | Seizure susceptibility. | Potential targets for preventing epileptic seizures-CaBPs like calmodulin, parvalbumin, calretinin, calbindin. | [113,114,115,116,117,118,119,120,121] [161,162,163,164,165,166,167,168,169] |
| Migraine | Excessive glutamate release, excitotoxicity. | Cortical spreading depression (CSD) genering. | Channelopathies of CaV subunits. | Migraine attack. | Calcium channels blockers- flunarizine, verapamil, inhibitor of ASIC1a- amiloride, tarantula toxin. | [8,133,134,135,136] [144,145,146,147] [170,171,172,173,174] |
5. Potential Role of Calcium Ions in Diagnostics and Therapy of Neurological Diseases
5.1. Calcium Ions as Diagnostic and Therapeutic Target in Alzheimer’s Disease
5.2. Calcium Ions as Diagnostic and Therapeutic Target in Parkinson’s Disease
5.3. Calcium Ions as Diagnostic and Therapeutic Target in Huntington’s Disease
5.4. Calcium Ions as Diagnostic and Therapeutic Target in Epilepsy
5.5. Calcium Ions as Diagnostic and Therapeutic Target in Migraine
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seibert, M.J.; Evans, C.S.; Stanley, K.S.; Wu, Z.; Chapman, E.R. Synaptotagmin 9 modulates spontaneous neurotransmitter release in striatal neurons by regulating substance P secretion. J. Neurosci. 2023, 43, 1475–1491. [Google Scholar] [CrossRef]
- Guan, P.P.; Cao, L.L.; Wang, P. Elevating the levels of calcium ions exacerbate Alzheimer’s disease via inducing the production and aggregation of β-amyloid protein and phosphorylated tau. Int. J. Mol. Sci. 2021, 22, 5900. [Google Scholar] [CrossRef] [PubMed]
- Baracaldo-Santamaría, D.; Avendaño-Lopez, S.S.; Ariza-Salamanca, D.F.; Rodriguez-Giraldo, M.; Calderon-Ospina, C.A.; González-Reyes, R.E.; Nava-Mesa, M.O. Role of calcium modulation in the pathophysiology and treatment of Alzheimer’s disease. Int. J. Mol. Sci. 2023, 24, 9067. [Google Scholar] [CrossRef] [PubMed]
- Matuz-Mares, D.; González-Andrade, M.; Araiza-Villanueva, M.G.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Mitochondrial calcium: Effects of its imbalance in disease. Antioxidants 2022, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Leandrou, E.; Emmanouilidou, E.; Vekrellis, K. Voltage-gated calcium channels and α-synuclein: Implications in Parkinson’s disease. Front. Mol. Neurosci. 2019, 12, 237. [Google Scholar] [CrossRef] [PubMed]
- Intihar, T.A.; Martinez, E.A.; Gomez-Pastor, R. Mitochondrial dysfunction in Huntington’s disease; Interplay between HSF1, p53 and PGC-1α transcription factors. Front. Cell. Neurosci. 2019, 13, 103. [Google Scholar] [CrossRef]
- Kapadia, K.; Trojniak, A.E.; Guzmán Rodríguez, K.B.; Klus, N.J.; Huntley, C.; McDonald, P.; Roy, A.; Frankowski, K.J.; Aubé, J.; Muma, N.A. Small-molecule disruptors of mutant huntingtin-calmodulin protein-protein interaction attenuate deleterious effects of mutant huntingtin. ACS Chem. Neurosci. 2022, 13, 2315–2337. [Google Scholar] [CrossRef]
- Kowalska, M.; Prendecki, M.; Piekut, T.; Kozubski, W.; Dorszewska, J. Migraine: Calcium channels and glia. Int. J. Mol. Sci. 2021, 22, 2688. [Google Scholar] [CrossRef]
- Wattiez, A.S.; Sowers, L.P.; Russo, A.F. Calcitonin gene-related peptide (CGRP): Role in migraine pathophysiology and therapeutic targeting. Expert Opin. Ther. Targets 2020, 24, 91–100. [Google Scholar] [CrossRef]
- Proft, J.; Rzhepetskyy, Y.; Lazniewska, J.; Zhang, F.X.; Cain, S.M.; Snutch, T.P.; Zamponi, G.W.; Weiss, N. The Cacna1h mutation in the GAERS model of absence epilepsy enhances T-type Ca2+ currents by altering calnexin-dependent trafficking of Cav3.2 channels. Sci. Rep. 2017, 7, 11513. [Google Scholar] [CrossRef]
- Chen, T.S.; Huang, T.H.; Lai, M.C.; Huang, C.W. The role of glutamate receptors in epilepsy. Biomedicines 2023, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wei, Z.H.; Liu, C.; Li, G.Y.; Qiao, X.Z.; Gan, Y.J.; Zhang, C.C.; Deng, Y.C. Genetic variations in GABA metabolism and epilepsy. Seizure 2022, 101, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Chiozzi, P.; Sarti, A.C.; Sanz, J.M.; Giuliani, A.L.; Adinolfi, E.; Vultaggio-Poma, V.; Falzoni, S.; Di Virgilio, F. Amyloid β-dependent mitochondrial toxicity in mouse microglia requires P2X7 receptor expression and is prevented by nimodipine. Sci. Rep. 2019, 9, 6475. [Google Scholar] [CrossRef]
- Singh, A.; Verma, P.; Raju, A.; Mohanakumar, K.P. Nimodipine attenuates the parkinsonian neurotoxin, MPTP-induced changes in the calcium binding proteins, calpain and calbindin. J. Chem. Neuroanat. 2019, 95, 89–94. [Google Scholar] [CrossRef]
- Khalil, A.; Saleh, S.; Nassar, N. Nimodipine: A novel protective calcium channel blocker against 3-nitropropionic acid induced Huntington’s disease. FASEB J. 2015, 29, 771–774. [Google Scholar] [CrossRef]
- Carson, L.; Kui, C.; Smith, G.; Dixit, A.K. The Effect of the 2019 novel coronavirus pandemic on stroke and tia patient admissions: Perspectives and risk factors. J. Clin. Med. 2021, 10, 1357. [Google Scholar] [CrossRef]
- Cutrer, F.M.; Moyer, A.M.; Atkinson, E.J.; Wang, L.; Tian, S.; Wu, Y.; Garza, I.; Robertson, C.E.; Huebert, C.A.; Moore, B.E.; et al. Genetic variants related to successful migraine prophylaxis with verapamil. Mol. Genet. Genom. Med. 2021, 9, e1680. [Google Scholar] [CrossRef]
- Godoy, L.D.; Prizon, T.; Rossignoli, M.T.; Leite, J.P.; Liberato, J.L. Parvalbumin role in epilepsy and psychiatric comorbidities: From mechanism to intervention. Front. Integr. Neurosci. 2022, 16, 765324. [Google Scholar] [CrossRef]
- Mark, M.D.; Schwitalla, J.C.; Groemmke, M.; Herlitze, S. Keeping our calcium in balance to maintain our balance. Biochem. Biophys. Res. Commun. 2017, 483, 1040–1050. [Google Scholar] [CrossRef]
- Dolphin, A.C.; Lee, A. Presynaptic calcium channels: Specialized control of synaptic neurotransmitter release. Nat. Rev. Neurosci. 2021, 21, 213. [Google Scholar] [CrossRef]
- Heck, J.; Palmeira Do Amaral, A.C.; Weißbach, S.; El Khallouqi, A.; Bikbaev, A.; Heine, M. More than a pore: How voltage-gated calcium channels act on different levels of neuronal communication regulation. Channels 2021, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Malsam, J.; Söllner, T.H. Organization of SNAREs within the golgi stack. Cold Spring Harb. Perspect. Biol. 2021, 3, a005249. [Google Scholar] [CrossRef] [PubMed]
- Neher, E.; Sakaba, T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 2008, 59, 861–872. [Google Scholar] [CrossRef]
- Carafoli, E.; Crompton, M. The regulation of intracellular calcium. Curr. Top. Membr. Transp. 1978, 10, 151–216. [Google Scholar]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Stevens, F.C. Calmodulin: An introduction. Can. J. Biochem. Cell Biol. 1983, 61, 906–910. [Google Scholar] [CrossRef]
- Wayman, G.A.; Lee, Y.S.; Tokumitsu, H.; Silva, A.; Soderling, T.R. Calmodulin-kinases: Modulators of neuronal development and plasticity. Neuron 2008, 59, 914. [Google Scholar] [CrossRef] [PubMed]
- Nagase, T.; Ito, K.I.; Kato, K.; Kaneko, K.; Kohda, K.; Matsumoto, M.; Hoshino, A.; Inoue, T.; Fujii, S.; Kato, H.; et al. Long-term potentiation and long-term depression in hippocampal CA1 neurons of mice lacking the IP3 type 1 receptor. Neuroscience 2003, 117, 821–830. [Google Scholar] [CrossRef]
- Connor, J.A.; Petrozzino, J.; Pozzo-Miller, L.D.; Otani, S. Calcium signals in long-term potentiation and long-term depression. Can. J. Physiol. Pharmacol. 1999, 77, 722–734. [Google Scholar] [CrossRef]
- Lüscher, C.; Malenka, R.C. NMDA receptor-dependent long-term potentiation and Long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4, a005710. [Google Scholar] [CrossRef]
- Cooke, S.F.; Bliss, T.V.P. Plasticity in the human central nervous system. Brain 2006, 129, 1659–1673. [Google Scholar] [CrossRef] [PubMed]
- Creamer, T.P. Calcineurin. Cell Commun. Signal. 2020, 18, 137. [Google Scholar] [CrossRef] [PubMed]
- Massey, P.V.; Bashir, Z.I. Long-term depression: Multiple forms and implications for brain function. Trends Neurosci. 2007, 30, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201309. [Google Scholar] [CrossRef]
- Gleichmann, M.; Mattson, M.P. Neuronal calcium homeostasis and dysregulation. Antioxid. Redox Signal. 2011, 14, 1261. [Google Scholar] [CrossRef]
- Bollimuntha, S.; Pani, B.; Singh, B.B. Neuronal store-operated Ca2+ signaling: An overview and its function. Adv. Exp. Med. Biol. 2017, 993, 535. [Google Scholar]
- Duchen, M.R. Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000, 529, 57–68. [Google Scholar] [CrossRef]
- Britzolaki, A.; Saurine, J.; Flaherty, E.; Thelen, C.; Pitychoutis, P.M. The SERCA2: A gatekeeper of neuronal calcium homeostasis in the brain. Cell. Mol. Neurobiol. 2018, 38, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Britzolaki, A.; Saurine, J.; Klocke, B.; Pitychoutis, P.M. A Role for SERCA pumps in the neurobiology of neuropsychiatric and neurodegenerative disorders. Adv. Exp. Med. Biol. 2020, 1131, 131–161. [Google Scholar]
- Khakh, B.S.; McCarthy, K.D. Astrocyte calcium signaling: From observations to functions and the challenges therein. Cold Spring Harb. Perspect. Biol. 2015, 7, a020404. [Google Scholar] [CrossRef]
- Shah, V.N.; Chagot, B.; Chazin, W.J. Calcium-dependent regulation of ion channels. Calcium Bind. Proteins 2006, 1, 203–212. [Google Scholar]
- Hidalgo, P.; Gonzalez-Gutierrez, G.; Garcia-Olivares, J.; Neely, A. The alpha1-beta-subunit interaction that modulates calcium channel activity is reversible and requires a competent alpha-interaction domain. J. Biol. Chem. 2006, 281, 24104–24110. [Google Scholar] [CrossRef]
- Kim, J.B. Channelopathies. Korean J. Pediatr. 2014, 57, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Indelicato, E.; Boesch, S. CACNA1A-Related Channelopathies: Clinical manifestations and treatment options. Handb. Exp. Pharmacol. 2023, 279, 227–248. [Google Scholar] [PubMed]
- Spekker, E.; Nagy-Grócz, G.; Vécsei, L. Ion channel disturbances in migraine headache: Exploring the potential role of the kynurenine system in the context of the trigeminovascular system. Int. J. Mol. Sci. 2023, 24, 16574. [Google Scholar] [CrossRef] [PubMed]
- Koch, N.A.; Sonnenberg, L.; Hedrich, U.B.S.; Lauxmann, S.; Benda, J. Loss or gain of function? Effects of ion channel mutations on neuronal firing depend on the neuron type. Front. Neurol. 2023, 14, 1194811. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, 003947. [Google Scholar] [CrossRef] [PubMed]
- Correa, B.H.M.; Moreira, C.R.; Hildebrand, M.E.; Vieira, L.B. The Role of voltage-gated calcium channels in basal ganglia neurodegenerative disorders. Curr. Neuropharmacol. 2023, 21, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Szymanowicz, O.; Drużdż, A.; Słowikowski, B.; Pawlak, S.; Potocka, E.; Goutor, U.; Konieczny, M.; Ciastoń, M.; Lewandowska, A.; Jagodziński, P.P.; et al. A Review of the CACNA gene family: Its role in neurological disorders. Diseases 2024, 12, 90. [Google Scholar] [CrossRef]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: Multiple processes, complex pathophysiology. J. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef]
- Pietrobon, D. CaV2.1 channelopathies. Pflügers Archiv. Eur. J. Physiol. 2010, 460, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Terpolilli, N.A.; Dolp, R.; Waehner, K.; Schwarzmaier, S.M.; Rumbler, E.; Todorov, B.; Ferrari, M.D.; van den Maagdenberg, A.M.J.M.; Plesnila, N. CaV2.1 channel mutations causing familial hemiplegic migraine type 1 increase the susceptibility for cortical spreading depolarizations and seizures and worsen outcome after experimental traumatic brain injury. Elife 2022, 11, 74923. [Google Scholar] [CrossRef] [PubMed]
- Tottene, A.; Fellin, T.; Pagnutti, S.; Luvisetto, S.; Striessnig, J.; Fletcher, C.; Pietrobon, D. Familial hemiplegic migraine mutations increase Ca(2+) influx through single human CaV2.1 channels and decrease maximal CaV2.1 current density in neurons. Proc. Natl. Acad. Sci. USA 2002, 99, 13284–13289. [Google Scholar] [CrossRef]
- Melliti, K.; Grabner, M.; Seabrook, G.R. The familial hemiplegic migraine mutation R192Q reduces G-protein-mediated inhibition of P/Q-type (Ca(V)2.1) calcium channels expressed in human embryonic kidney cells. J. Physiol. 2003, 546, 337–347. [Google Scholar] [CrossRef]
- Kors, E.E.; Terwindt, G.M.; Vermeulen, F.L.; Fitzsimons, R.B.; Jardine, P.E.; Heywood, P.; Love, S.; van den Maagdenberg, A.M.; Haan, J.; Frants, R.R.; et al. Delayed cerebral edema and fatal coma after minor head trauma: Role of the CACNA1A calcium channel subunit gene and relationship with familial hemiplegic migraine. Ann. Neurol. 2001, 49, 753–760. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, W.; Zhang, S.; Wang, X.; Tang, Z.; Gu, J.; Li, J.; Huang, J. CACNA1B (Cav2.2) Overexpression and its association with clinicopathologic characteristics and unfavorable prognosis in non-small cell lung cancer. Dis. Markers 2017, 2017, 6136401. [Google Scholar] [CrossRef]
- Andrade, A.; Brennecke, A.; Mallat, S.; Brown, J.; Gomez-Rivadeneira, J.; Czepiel, N.; Londrigan, L. Genetic Associations between Voltage-Gated Calcium Channels and Psychiatric Disorders. Int. J. Mol. Sci. 2019, 20, 3537. [Google Scholar] [CrossRef]
- Groen, J.L.; Andrade, A.; Ritz, K.; Jalalzadeh, H.; Haagmans, M.; Bradley, T.E.; Jongejan, A.; Verbeek, D.S.; Nürnberg, P.; Denome, S.; et al. CACNA1B mutation is linked to unique myoclonus-dystonia syndrome. Hum. Mol. Genet. 2015, 24, 987–993. [Google Scholar] [CrossRef]
- Herold, K.G.; Hussey, J.W.; Dick, I.E. CACNA1C-related channelopathies. Handb. Exp. Pharmacol. 2023, 279, 159–181. [Google Scholar]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef]
- Zhu, D.; Yin, J.; Liang, C.; Luo, X.; Lv, D.; Dai, Z.; Xiong, S.; Fu, J.; Li, Y.; Lin, J.; et al. CACNA1C (rs1006737) may be a susceptibility gene for schizophrenia: An updated meta-analysis. Brain Behav. 2019, 9, 01292. [Google Scholar] [CrossRef] [PubMed]
- Helbig, K.; Lauerer-Braun, R.; Bahr, J.; Souza, I.; Myers, C.; Uysal, B.; Schwarz, N.; Gandini, M.; Huang, S.; Keren, B.; et al. De Novo pathogenic variants in CACNA1E cause developmental and epileptic encephalopathy with contractures, macrocephaly, and dyskinesias. Am. J. Hum. Genet. 2018, 103, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Montell, C.; Rubin, G.M. Molecular characterization of the Drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Yang, D. TRPA1-Related Diseases and Applications of Nanotherapy. Int. J. Mol. Sci. 2024, 25, 9234. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Prendecki, M.; Kapelusiak-Pielok, M.; Grzelak, T.; Łagan-Jędrzejczyk, U.; Wiszniewska, M.; Kozubski, W.; Dorszewska, J. Analysis of genetic variants in SCN1A, SCN2A, KCNK18, TRPA1 and STX1A as a possible marker of migraine. Curr. Genomics 2020, 21, 224–236. [Google Scholar] [CrossRef]
- Masood, T.; Lakatos, S.; Rosta, J. Modification of the TRP Channel TRPA1 as a Relevant Factor in Migraine-Related Intracranial Hypersensitivity. Int. J. Mol. Sci. 2023, 24, 5375. [Google Scholar] [CrossRef]
- Shea, T.B.; Ekinci, F.J. Biphasic effect of calcium influx on tau phosphorylation: Involvement of calcium-dependent phosphatase and kinase activities. J. Alzheimers Dis. 1999, 1, 353–360. [Google Scholar] [CrossRef]
- Britti, E.; Ros, J.; Esteras, N.; Abramov, A.Y. Tau inhibits mitochondrial calcium efflux and makes neurons vulnerable to calcium-induced cell death. Cell Calcium 2020, 86, 102150. [Google Scholar] [CrossRef]
- Lerdkrai, C.; Garaschuk, O. Role of presynaptic calcium stores for neural network dysfunction in Alzheimer’s disease. Neural Regen. Res. 2018, 13, 977–978. [Google Scholar]
- Lerdkrai, C.; Asavapanumas, N.; Brawek, B.; Kovalchuk, Y.; Mojtahedi, N.; Del Moral, M.O.; Garaschuk, O. Intracellular Ca2+ stores control in vivo neuronal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, E1279–E1288. [Google Scholar] [CrossRef] [PubMed]
- Taoufik, E.; Kouroupi, G.; Zygogianni, O.; Matsas, R. Synaptic dysfunction in neurodegenerative and neurodevelopmental diseases: An overview of induced pluripotent stem-cell-based disease models. Open Biol. 2018, 8, 180138. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The Amyloid hypothesis of Alzheimer’s disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Kim, S.; Rhim, H. Effects of amyloid-β peptides on voltage-gated L-type Ca(V)1.2 and Ca(V)1.3 Ca(2+) channels. Mol. Cells 2011, 32, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Goussakov, I.; Miller, M.B.; Stutzmann, G.E. NMDA-Mediated Ca(2+) Influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. J. Neurosci. 2010, 30, 12128–12137. [Google Scholar] [CrossRef]
- Guo, C.; Ma, Y.Y. Calcium Permeable-AMPA receptors and excitotoxicity in neurological disorders. Front. Neural Circuits 2021, 15, 711564. [Google Scholar] [CrossRef]
- Anekonda, T.S.; Quinn, J.F.; Harris, C.; Frahler, K.; Wadsworth, T.L.; Woltjer, R.L. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer’s disease. Neurobiol. Dis. 2011, 41, 62–70. [Google Scholar] [CrossRef]
- Shen, H.; Pan, J.; Pan, L.; Zhang, N. TRPC6 Inhibited NMDA current in cultured hippocampal neurons. Neuromol. Med. 2013, 15, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Prikhodko, V.; Chernyuk, D.; Sysoev, Y.; Zernov, N.; Okovityi, S.; Popugaeva, E. Potential drug candidates to treat TRPC6 channel deficiencies in the pathophysiology of Alzheimer’s disease and brain ischemia. Cells 2020, 9, 2351. [Google Scholar] [CrossRef]
- Raffaello, A.; Mammucari, C.; Gherardi, G.; Rizzuto, R. Calcium at the center of cell signaling: Interplay between endoplasmic reticulum, mitochondria, and lysosomes. Trends Biochem. Sci. 2016, 41, 1035–1049. [Google Scholar] [CrossRef]
- Thiel, G.; Schmidt, T.; Rossler, O.G. Ca2+ Microdomains, calcineurin and the regulation of gene transcription. Cells 2021, 10, 875. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; di Porzio, U.; Viggiano, D.; de Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. [Google Scholar] [CrossRef]
- Bastioli, G.; Piccirillo, S.; Graciotti, L.; Carone, M.; Sprega, G.; Taoussi, O.; Preziuso, A.; Castaldo, P. Calcium deregulation in neurodegeneration and neuroinflammation in Parkinson’s Disease: Role of calcium-storing organelles and sodium–calcium exchanger. Cells 2024, 13, 1301. [Google Scholar] [CrossRef] [PubMed]
- Samavarchi Tehrani, S.; Sarfi, M.; Yousefi, T.; Ahangar, A.A.; Gholinia, H.; Ahangar, R.M.; Maniati, M.; Saadat, P. Comparison of the calcium-related factors in Parkinson’s disease patients with healthy individuals. Caspian J. Intern. Med. 2020, 11, 28–33. [Google Scholar] [PubMed]
- Mira, R.G.; Cerpa, W. Building a bridge between NMDAR-mediated excitotoxicity and mitochondrial dysfunction in chronic and acute diseases. Cell. Mol. Neurobiol. 2021, 41, 1413–1430. [Google Scholar] [CrossRef]
- Hasreiter, J.; Goldnagl, L.; Bohm, S.; Kubista, H. Cav1.2 and Cav1.3 L-type calcium channels operate in a similar voltage range but show different coupling to Ca(2+)-dependent conductances in hippocampal neurons. Am. J. Physiol. Cell Physiol. 2014, 306, C1200–C1213. [Google Scholar] [CrossRef][Green Version]
- Jung, J.H.; Na, H.K.; Jeong, S.H.; Chung, S.J.; Yoo, H.S.; Lee, Y.H.; Baik, K.; Kim, S.J.; Sohn, Y.H.; Lee, P.H. Effects of dihydropyridines on the motor and cognitive outcomes of patients with Parkinson’s disease. Mov. Disord. 2023, 38, 843–853. [Google Scholar] [CrossRef]
- Guzman, J.N.; Ilijic, E.; Yang, B.; Sanchez-Padilla, J.; Wokosin, D.; Galtieri, D.; Kondapalli, J.; Schumacker, P.T.; Surmeier, D.J. Systemic isradipine treatment diminishes calcium-dependent mitochondrial oxidant stress. J. Clin. Investig. 2018, 128, 2266–2280. [Google Scholar] [CrossRef] [PubMed]
- Ilijic, E.; Guzman, J.N.; Surmeier, D.J. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol. Dis. 2011, 43, 364–371. [Google Scholar] [CrossRef]
- Lee, H.J.; Jung, Y.H.; Choi, G.E.; Kim, J.S.; Chae, C.W.; Lim, J.R.; Kim, S.Y.; Yoon, J.H.; Cho, J.H.; Lee, S.J.; et al. Urolithin A suppresses high glucose-induced neuronal amyloidogenesis by modulating TGM2-dependent ER-mitochondria contacts and calcium homeostasis. Cell Death Differ. 2021, 28, 184–202. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Fujioka, H.; Liu, J.; Chen, S.; Zhu, X. DJ-1 regulates the integrity and function of ER-mitochondria association through interaction with IP3R3-Grp75-VDAC. Proc. Natl. Acad. Sci. USA 2019, 116, 25322–25328. [Google Scholar] [CrossRef]
- Saris, N.E.; Carafoli, E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry 2005, 70, 187–194. [Google Scholar] [CrossRef]
- Naranjo, J.R.; Mellström, B. Ca2+-dependent transcriptional control of Ca2+ homeostasis. J. Biol. Chem. 2012, 287, 31674–31680. [Google Scholar] [CrossRef]
- Bao, J.; Sharp, A.H.; Wagster, M.V.; Becher, M.; Schilling, G.; Ross, C.A.; Dawson, V.L.; Dawson, T.M. Expansion of polyglutamine repeat in huntingtin leads to abnormal protein interactions involving calmodulin. Proc. Natl. Acad. Sci. USA 1996, 93, 5037–5042. [Google Scholar] [CrossRef]
- Kreutz, M.R.; Naranjo, J.R.; Koch, K.W.; Schwaller, B. The neuronal functions of EF-Hand Ca2+-binding proteins. Front. Mol. Neurosci. 2012, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Dudek, N.L.; Dai, Y.; Muma, N.A. Neuroprotective effects of calmodulin peptide 76-121aa: Disruption of calmodulin binding to mutant huntingtin. Brain Pathol. 2010, 20, 176–189. [Google Scholar] [CrossRef]
- Menzies, F.M.; Garcia-Arencibia, M.; Imarisio, S.; O’Sullivan, N.C.; Ricketts, T.; Kent, B.A.; Rao, M.V.; Lam, W.; Green-Thompson, Z.W.; Nixon, R.A.; et al. Calpain inhibition mediates autophagy-dependent protection against polyglutamine toxicity. Cell Death Differ. 2015, 22, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Valenza, M.; Cattaneo, E. Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol. Rev. 2010, 90, 905–981. [Google Scholar] [CrossRef] [PubMed]
- Luthi-Carter, R.; Hanson, S.A.; Strand, A.D.; Bergstrom, D.A.; Chun, W.; Peters, N.L.; Woods, A.M.; Chan, E.Y.; Kooperberg, C.; Krainc, D.; et al. Dysregulation of gene expression in the R6/2 model of polyglutamine disease: Parallel changes in muscle and brain. Hum. Mol. Genet. 2002, 11, 1911–1926. [Google Scholar] [CrossRef]
- Hodges, A.; Strand, A.D.; Aragaki, A.K.; Kuhn, A.; Sengstag, T.; Hughes, G.; Elliston, L.A.; Hartog, C.; Goldstein, D.R.; Thu, D.; et al. Regional and cellular gene expression changes in human Huntington’s disease brain. Hum. Mol. Genet. 2006, 15, 965–977. [Google Scholar] [CrossRef]
- Kuhn, A.; Goldstein, D.R.; Hodges, A.; Strand, A.D.; Sengstag, T.; Kooperberg, C.; Becanovic, K.; Pouladi, M.A.; Sathasivam, K.; Cha, J.H.; et al. Mutant huntingtin’s effects on striatal gene expression in mice recapitulate changes observed in human Huntington’s disease brain and do not differ with mutant huntingtin length or wild-type huntingtin dosage. Hum. Mol. Genet. 2007, 16, 1845–1861. [Google Scholar] [CrossRef] [PubMed]
- Czeredys, M.; Gruszczynska-Biegala, J.; Schacht, T.; Methner, A.; Kuznicki, J. Expression of genes encoding the calcium signalosome in cellular and transgenic models of Huntington’s disease. Front. Mol. Neurosci. 2013, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Cesca, F.; Bregant, E.; Peterlin, B.; Zadel, M.; Dubsky de Wittenau, G.; Siciliano, G.; Ceravolo, R.; Petrozzi, L.; Pauletto, G.; Verriello, L.; et al. Evaluating the SERCA2 and VEGF mRNAs as potential molecular biomarkers of the onset and progression in Huntington’s disease. PLoS ONE 2015, 10, e0125259. [Google Scholar] [CrossRef]
- Wu, J.; Shih, H.; Vigont, V.; Hrdlicka, L.; Diggins, L.; Singh, C.; Mahoney, M.; Chesworth, R.; Shapiro, G.; Ahlijanian, M.; et al. Neuronal store-operated calcium entry pathway as a novel therapeutic target for Huntington’s disease treatment. Chem. Biol. 2011, 18, 777–793. [Google Scholar] [CrossRef]
- Egorova, P.; Popugaeva, E.; Bezprozvanny, I. Disturbed calcium signaling in spinocerebellar ataxias and Alzheimer’s disease. Semin. Cell Dev. Biol. 2015, 40, 127–133. [Google Scholar] [CrossRef]
- Glushankova, L.N.; Zimina, O.A.; Vigont, V.A.; Mozhaeva, G.N.; Bezprozvanny, I.B.; Kaznacheeva, E.V. Changes in the store-dependent calcium influx in a cellular model of Huntington’s disease. Dokl. Biol. Sci. 2010, 433, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Vigont, V.; Kolobkova, Y.; Skopin, A.; Zimina, O.; Zenin, V.; Glushankova, L.; Kaznacheyeva, E. Both Orai1 and TRPC1 are Involved in excessive store-operated calcium entry in striatal neurons expressing mutant Huntingtin exon. Front. Physiol. 2015, 6, 337. [Google Scholar] [CrossRef]
- Nekrasov, E.D.; Vigont, V.A.; Klyushnikov, S.A.; Lebedeva, O.S.; Vassina, E.M.; Bogomazova, A.N.; Chestkov, I.V.; Semashko, T.A.; Kiseleva, E.; Suldina, L.A.; et al. Manifestation of Huntington’s disease pathology in human induced pluripotent stem cell-derived neurons. Mol. Neurodegener. 2016, 11, 27. [Google Scholar] [CrossRef]
- Tang, T.S.; Slow, E.; Lupu, V.; Stavrovskaya, I.G.; Sugimori, M.; Llinás, R.; Kristal, B.S.; Hayden, M.R.; Bezprozvanny, I. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 2602–2607. [Google Scholar] [CrossRef]
- Costa, V.; Giacomello, M.; Hudec, R.; Lopreiato, R.; Ermak, G.; Lim, D.; Malorni, W.; Davies, K.J.; Carafoli, E.; Scorrano, L. Mitochondrial fission and cristae disruption increase the response of cell models of Huntington’s disease to apoptotic stimuli. EMBO Mol. Med. 2010, 2, 490–503. [Google Scholar] [CrossRef]
- Wang, H.; Lim, P.J.; Karbowski, M.; Monteiro, M.J. Effects of overexpression of huntingtin proteins on mitochondrial integrity. Hum. Mol. Genet. 2009, 18, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Luciani, D.; Gwiazda, K.; Yang, T.; Kalynyak, T.; Bychkivska, Y.; Frey, M.; Jeffrey, K.; Sampaio, A.; Underhill, T.; Johnson, J. Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes 2009, 58, 422–432. [Google Scholar] [CrossRef]
- Berridge, M.; Lipp, P.; Bootman, M. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Simms, B.; Zamponi, G. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Neuron 2014, 82, 24–45. [Google Scholar] [CrossRef]
- Magloczky, Z.; Halasz, P.; Vajda, J.; Czirjak, S.; Freund, T. Loss of Calbindin-D28K immunoreactivity from dentate granule cells in human temporal lobe epilepsy. Neuroscience 1997, 76, 377–385. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, L.; Konopka, H.; Solís, P.; Scevola, L.; Lima, M.; Nunez, C.; Seoane, E.; Oddo, S.; Kochen, S. Depression and temporal lobe epilepsy: Expression pattern of calbindin immunoreactivity in hippocampal dentate gyrus of patients who underwent epilepsy surgery with and without comorbid depression. Behav. Neurol. 2019, 2019, 7396793. [Google Scholar] [CrossRef]
- Sammels, E.; Parys, J.; Missiaen, L.; De Smedt, H.; Bultynck, G. Intracellular Ca2+ storage in health and disease: A dynamic equilibrium. Cell Calcium 2010, 47, 297–314. [Google Scholar] [CrossRef]
- Rajakulendran, S.; Hanna, M. The role of calcium channels in epilepsy. Cold Spring Harb. Perspect. Med. 2016, 6, a022723. [Google Scholar] [CrossRef]
- Tsien, R.; Lipscombe, D.; Madison, D.; Bley, K.; Fox, A. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988, 11, 431–438. [Google Scholar] [CrossRef]
- Wu, Y.; Whiteus, C.; Xu, C.; Hayworth, K.; Weinberg, R.; Hess, H.; De Camilli, P. Contacts between the endoplasmic reticulum and other membranes in neurons. Proc. Natl. Acad. Sci. USA 2017, 114, E4859–E4867. [Google Scholar] [CrossRef]
- Stathopulos, P.; Li, G.; Plevin, M.; Ames, J.; Ikura, M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J. Biol. Chem. 2006, 281, 35855–35862. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zheng, Q.; Tan, H.; Zhang, B.; Li, X.; Yang, Y.; Yu, J.; Liu, Y.; Chai, H.; Wang, X.; et al. TMCO1 is an ER Ca(2+) load-activated Ca(2+) channel. Cell 2016, 165, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Khananshvili, D. The SLC8 gene family of sodium-calcium exchangers (NCX)—Structure, function, and regulation in health and disease. Mol. Aspects Med. 2013, 34, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Lytton, J. Na+/Ca2+ exchangers: Three mammalian gene families control Ca2+ transport. Biochem. J. 2007, 406, 365–382. [Google Scholar] [CrossRef]
- Bozarth, X.; Dines, J.; Cong, Q.; Mirzaa, G.; Foss, K.; Lawrence Merritt, J.; Thies, J.; Mefford, H.; Novotny, E. Expanding clinical phenotype in CACNA1C related disorders: From neonatal onset severe epileptic encephalopathy to late-onset epilepsy. Am. J. Med. Genet. A 2018, 176, 2733–2739. [Google Scholar] [CrossRef]
- Bomben, V.; Aiba, I.; Qian, J.; Mark, M.; Herlitze, S.; Noebels, J. Isolated P/Q calcium channel deletion in layer VI corticothalamic neurons generates absence epilepsy. J. Neurosci. 2016, 36, 405–418. [Google Scholar] [CrossRef]
- Stendel, C.; D’Adamo, M.; Wiessner, M.; Dusl, M.; Cenciarini, M.; Belia, S.; Nematian-Ardestani, E.; Bauer, P.; Senderek, J.; Klopstock, T.; et al. Association of a novel splice site mutation in P/Q-type calcium channels with childhood epilepsy and late-onset slowly progressive non-episodic cerebellar ataxia. Int. J. Mol. Sci. 2020, 21, 3810. [Google Scholar] [CrossRef] [PubMed]
- Imbrici, P.; Jaffe, S.; Eunson, L.; Davies, N.; Herd, C.; Robertson, R.; Kullmann, D.; Hanna, M. Dysfunction of the brain calcium channel CaV2.1 in absence epilepsy and episodic ataxia. Brain 2004, 127, 2682–2692. [Google Scholar] [CrossRef] [PubMed]
- Jouvenceau, A.; Eunson, L.; Spauschus, A.; Ramesh, V.; Zuberi, S.; Kullmann, D.; Hanna, M. Human epilepsy associated with dysfunction of the brain P/Q-type calcium channel. Lancet 2001, 358, 801–807. [Google Scholar] [CrossRef]
- Gorman, K.; Meyer, E.; Grozeva, D.; Spinelli, E.; McTague, A.; Sanchis-Juan, A.; Carss, K.; Bryant, E.; Reich, A.; Schneider, A.; et al. Bi-allelic loss-of-function CACNA1B mutations in progressive epilepsy-dyskinesia. Am. J. Hum. Genet. 2019, 104, 948–956. [Google Scholar] [CrossRef]
- Bidaud, I.; Lory, P. Hallmarks of the channelopathies associated with L-type calcium channels: A focus on the Timothy mutations in Ca(v)1.2 channels. Biochimie 2011, 93, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, H.; Shirah, B.; Samman, A.; Alhazmi, A. CACNA1H epilepsy and hearing loss in a patient with a rare heterozygous variant in the gene. J. Epilepsy Res. 2022, 12, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Tfelt-Hansen, P.C. History of migraine with aura and cortical spreading depression from 1941 and onwards. Cephalalgia 2010, 30, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.; Brennan, K. Cortical spreading depression-new insights and persistent questions. Cephalalgia 2009, 29, 1115–1124. [Google Scholar] [CrossRef]
- Vitale, M.; Tottene, A.; Zarin Zadeh, M.; Brennan, K.C.; Pietrobon, D. Mechanisms of initiation of cortical spreading depression. J. Headache Pain 2023, 24, 105. [Google Scholar] [CrossRef]
- Tottene, A.; Urbani, A.; Pietrobon, D. Role of different voltage-gated Ca2+ channels in cortical spreading depression: Specific requirement of P/Q-type Ca2+ channels. Channels 2011, 14, 110–114. [Google Scholar] [CrossRef]
- Tottene, A.; Conti, R.; Fabbro, A.; Vecchia, D.; Shapovalova, M.; Santello, M.; van den Maagdenberg, A.M.; Ferrari, M.D.; Pietrobon, D. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron 2009, 61, 762–773. [Google Scholar] [CrossRef]
- Grangeon, L.; Lange, K.S.; Waliszewska-Prosół, M.; Onan, D.; Marschollek, K.; Wiels, W.; Mikulenka, P.; Farham, F.; Gollion, C.; Ducros, A.; et al. Genetics of migraine: Where are we now? J. Headache Pain 2023, 24, 12. [Google Scholar] [CrossRef]
- Pietrobon, D. Familial hemiplegic migraine. Neurotherapeutics 2007, 4, 274–284. [Google Scholar] [CrossRef]
- Weyrer, C.; Turecek, J.; Niday, Z.; Liu, P.W.; Nanou, E.; Catterall, W.A.; Bean, B.P.; Regehr, W.G. The Role of CaV2.1 Channel facilitation in synaptic facilitation. Cell Rep. 2019, 26, 2289–2297. [Google Scholar] [CrossRef]
- van den Maagdenberg, A.M.; Pizzorusso, T.; Kaja, S.; Terpolilli, N.; Shapovalova, M.; Hoebeek, F.E.; Barrett, C.F.; Gherardini, L.; van de Ven, R.C.; Todorov, B.; et al. High cortical spreading depression susceptibility and migraine-associated symptoms in Ca(v)2.1 S218L mice. Ann. Neurol. 2010, 67, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Ducros, A.; Denier, C.; Joutel, A.; Vahedi, K.; Michel, A.; Darcel, F.; Madigand, M.; Guerouaou, D.; Tison, F.; Julien, J.; et al. Recurrence of the T666M calcium channel CACNA1A gene mutation in familial hemiplegic migraine with progressive cerebellar ataxia. Am. J. Hum. Genet. 1999, 64, 89–98. [Google Scholar] [CrossRef]
- Maksemous, N.; Harder, A.V.E.; Ibrahim, O.; Vijfhuizen, L.S.; Sutherland, H.; Pelzer, N.; de Boer, I.; Terwindt, G.M.; Lea, R.A.; van den Maagdenberg, A.M.J.M.; et al. Whole exome sequencing of hemiplegic migraine patients shows an increased burden of missense variants in CACNA1H and CACNA1I genes. Mol. Neurobiol. 2023, 60, 3034–3043. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.H.; Kogelman, L.J.A.; Kristensen, D.M.; Chalmer, M.A.; Olesen, J.; Hansen, T.F. Functional gene networks reveal distinct mechanisms segregating in migraine families. Brain J. Neurol. 2020, 143, 2945–2956. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, A.; D’Onofrio, M.; Buzzi, M.G.; Arisi, I.; Grieco, G.S.; Pierelli, F.; Santorelli, F.M.; Schoenen, J. Possible involvement of the CACNA1E gene in migraine: A search for single nucleotide polymorphism in different clinical phenotypes. Headache 2017, 57, 1136–1144. [Google Scholar] [CrossRef]
- Kürtüncü, M.; Kaya, D.; Zuliani, L.; Erdağ, E.; İçöz, S.; Uğurel, E.; Çavuş, F.; Ayşit, N.; Birişik, Ö.; Vincent, A.; et al. CACNA1H antibodies associated with headache with neurological deficits and cerebrospinal fluid lymphocytosis (HaNDL). Cephalalgia 2013, 33, 123–129. [Google Scholar] [CrossRef]
- Chichorro, J.G.; Gambeta, E.; Baggio, D.F.; Zamponi, G.W. Voltage-gated calcium channels as potential therapeutic targets in migraine. J. Pain 2024, 25, 104514. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Chen, Z.T.; Liao, T.Y.; Lin, C.; Shen, H.C.; Wang, Y.H.; Chang, C.W.; Liu, R.S.; Chen, R.P.; Tu, P.H. An intranasally delivered peptide drug ameliorates cognitive decline in Alzheimer transgenic mice. EMBO Mol. Med. 2017, 9, 703–715. [Google Scholar] [CrossRef]
- Daverey, A.; Agrawal, S.K. Neuroprotective effects of Riluzole and Curcumin in human astrocytes and spinal cord white matter hypoxia. Neurosci. Lett. 2020, 738, 135351. [Google Scholar] [CrossRef]
- Kastanenka, K.V.; Bussiere, T.; Shakerdge, N.; Qian, F.; Weinreb, P.H.; Rhodes, K.; Bacskai, B.J. Immunotherapy with aducanumab restores calcium homeostasis in Tg2576 mice. J. Neurosci. 2016, 36, 12549–12558. [Google Scholar] [CrossRef]
- Paul, S.; Mahanta, S. Association of heat-shock proteins in various neurodegenerative disorders: Is it a master key to open the therapeutic door? Mol. Cell. Biochem. 2014, 386, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. The relevance of metals in the pathophysiology of neurodegeneration, pathological considerations. In International Review of Neurobiology; Academic Press: Cambridge, MA, USA, 2013; Volume 110, pp. 1–47. [Google Scholar]
- Kuhnert, N.; Karaköse, H.; Jaiswal, R. Analysis of chlorogenic acids and other hydroxycinnamates in food, plants and pharmacokinetic studies. In Handbook of Analysis of Active Compounds in Functional Foods; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–52. [Google Scholar]
- Küng, C.F.; Lüscher, T.F. Different mechanisms of endothelial dysfunction with aging and hypertension in rat aorta. Hypertension 1995, 25, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Ritz, B.; Rhodes, S.L.; Qian, L.; Schernhammer, E.; Olsen, J.H.; Friis, S. L-type calcium channel blockers and Parkinson disease in Denmark. Ann. Neurol. 2010, 67, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef]
- Wu, J.; Ryskamp, D.A.; Liang, X.; Egorova, P.; Zakharova, O.; Hung, G.; Bezprozvanny, I. Enhanced store-operated calcium entry leads to striatal synaptic loss in a Huntington’s disease mouse model. J. Neurosci. 2016, 36, 125–141. [Google Scholar] [CrossRef]
- Sun, Y.M.; Zhang, Y.B.; Wu, Z.Y. Huntington’s disease: Relationship between phenotype and genotype. Mol. Neurobiol. 2017, 54, 342–348. [Google Scholar] [CrossRef]
- Czeredys, M.; Maciag, F.; Methner, A.; Kuznicki, J. Tetrahydrocarbazoles decrease elevated SOCE in medium spiny neurons from transgenic YAC128 mice, a model of Huntington’s disease. Biochem. Biophys. Res. Commun. 2017, 483, 1194–1205. [Google Scholar] [CrossRef]
- Tang, T.S.; Guo, C.; Wang, H.; Chen, X.; Bezprozvanny, I. Neuroprotective effects of inositol 1,4,5-trisphosphate receptor C-terminal fragment in a Huntington’s disease mouse model. J. Neurosci. 2009, 29, 1257–1266. [Google Scholar] [CrossRef]
- Nejatbakhsh, N.; Feng, Z. Calcium binding protein-mediated regulation of voltagegated calcium channels linked to human diseases. Acta Pharmacol. Sin. 2011, 32, 741–748. [Google Scholar] [CrossRef]
- Ambrosino, P.; Alaimo, A.; Bartollino, S.; Manocchio, L.; De Maria, M.; Mosca, I.; GomisPerez, C.; Alberdi, A.; Scambia, G.; Lesca, G.; et al. Epilepsy-causing mutations in Kv7.2 C-terminus affect binding and functional modulation by calmodulin. Biochim. Biophys. Acta 2015, 1852, 1856–1866. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, J.; Chen, L.; Shen, Y.; Zhao, J.; Xu, C.; Wu, X.; Cheng, H.; Ying, X.; Guo, Y.; et al. Pharmaco-genetic therapeutics targeting parvalbumin neurons attenuate temporal lobe epilepsy. Neurobiol. Dis. 2018, 117, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Toth, K.; Magloczky, Z. The vulnerability of calretinin-containing hippocampal interneurons to temporal lobe epilepsy. Front. Neuroanat. 2014, 8, 100. [Google Scholar]
- Berridge, M.; Bootman, M.; Roderick, H. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Nagerl, U.; Mody, I.; Jeub, M.; Lie, A.; Elger, C.; Beck, H. Surviving granule cells of the sclerotic human hippocampus have reduced Ca(2+) influx because of a loss of calbindin-D(28k) in temporal lobe epilepsy. J. Neurosci. 2000, 20, 1831–1836. [Google Scholar] [CrossRef]
- Yamamoto, A.; Murphy, N.; Schindler, C.; So, N.; Stohr, S.; Taki, W.; Prehn, J.; Henshall, D. Endoplasmic reticulum stress and apoptosis signaling in human temporal lobe epilepsy. J. Neuropathol. Exp. Neurol. 2006, 65, 217–225. [Google Scholar] [CrossRef]
- Shetty, A.; Hattiangady, B. Restoration of calbindin after fetal hippocampal CA3 cell grafting into the injured hippocampus in a rat model of temporal lobe epilepsy. Hippocampus 2007, 17, 943–956. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y. Gene therapy in epilepsy. Biomed. Pharmacother. 2021, 143, 112075. [Google Scholar] [CrossRef] [PubMed]
- Dussor, G. ASICs as therapeutic targets for migraine. Neuropharmacology 2015, 94, 64–71. [Google Scholar] [CrossRef]
- Mazzuca, M.; Heurteaux, C.; Alloui, A.; Diochot, S.; Baron, A.; Voilley, N.; Blondeau, N.; Escoubas, P.; Gélot, A.; Cupo, A.; et al. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat. Neurosci. 2007, 10, 943–945. [Google Scholar] [CrossRef]
- Holland, P.R.; Akerman, S.; Andreou, A.P.; Karsan, N.; Wemmie, J.A.; Goadsby, P.J. Acid-sensing ion channel 1: A novel therapeutic target for migraine with aura. Ann. Neurol. 2012, 72, 559–563. [Google Scholar] [CrossRef]
- Pietrobon, D. Insights into migraine mechanisms and CaV2.1 calcium channel function from mouse models of familial hemiplegic migraine. J. Physiol. 2010, 588, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- LeRoy, A.; Jacova, C.; Young, C. Neuropsychological performance patterns of adult ADHD subtypes. J. Atten. Disord. 2019, 23, 1136–1147. [Google Scholar] [CrossRef]
- Green, K.N.; Smith, I.F.; Laferla, F.M. Role of calcium in the pathogenesis of Alzheimer’s disease and transgenic models. Subcell Biochem. 2007, 45, 507–521. [Google Scholar]
- Calvo-Rodriguez, M.; Hou, S.S.; Snyder, A.C.; Kharitonova, E.K.; Russ, A.N.; Das, S.; Fan, Z.; Muzikansky, A.; Garcia-Alloza, M.; Serrano-Pozo, A.; et al. Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nat. Commun. 2020, 11, 2146. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Rodriguez, M.; Bacskai, B.J. Mitochondria and calcium in Alzheimer’s disease: From cell signaling to neuronal cell death. Trends Neurosci. 2021, 44, 136–151. [Google Scholar] [CrossRef]
- Esteras, N.; Abramov, A.Y. Mitochondrial calcium deregulation in the mechanism of beta-amyloid and tau pathology. Cells 2020, 9, 2135. [Google Scholar] [CrossRef]
- Starkov, A.A.; Beal, F.M. Portal to Alzheimer’s disease. Nat. Med. 2008, 14, 1020–1021. [Google Scholar] [CrossRef]
- Lacampagne, A.; Liu, X.; Reiken, S.; Bussiere, R.; Meli, A.C.; Lauritzen, I.; Teich, A.F.; Zalk, R.; Saint, N.; Arancio, O.; et al. Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer’s disease-like pathologies and cognitive deficits. Acta Neuropathol. 2017, 134, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Leslie, S.N.; Wang, M.; Morozov, Y.M.; Yang, S.; Mentone, S.; Zeiss, C.; Duque, A.; Rakic, P.; Horvath, T.L.; et al. Age-related calcium dysregulation linked with tau pathology and impaired cognition in non-human primates. Alzheimers Dement. 2021, 17, 920–932. [Google Scholar] [CrossRef]
- Cortes, L.; Malva, J.; Rego, A.C.; Pereira, C.F. Calcium signaling in aging and neurodegenerative diseases. Int. J. Mol. Sci 2020, 21, 1125. [Google Scholar] [CrossRef]
- Bezprozvanny, I.; Mattson, M.P. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008, 31, 454–463. [Google Scholar] [CrossRef]
- Liao, W.; Wei, J.; Liu, C.; Luo, H.; Ruan, Y.; Mai, Y.; Yu, Q.; Cao, Z.; Xu, J.; Zheng, D.; et al. Magnesium-L-threonate treats Alzheimer’s disease by modulating the microbiota-gut-brain axis. Neural Regen. Res. 2024, 19, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Rao, A. Store-operated calcium entry: Mechanisms and modulation. Biochem. Biophys. Res. Commun. 2015, 460, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Secondo, A.; Bagetta, G.; Amantea, D. On the role of store-operated calcium entry in acute and chronic neurodegenerative diseases. Front. Mol. Neurosci. 2018, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Zaichick, S.V.; McGrath, K.M.; Caraveo, G. The role of Ca2+ signaling in Parkinson’s disease. Dis. Model. Mech. 2017, 10, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Guzman, J.N.; Sanchez, J.; Schumacker, P.T. Physiological phenotype and vulnerability in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009290. [Google Scholar] [CrossRef]
- Cabral-Costa, J.V.; Vicente-Gutiérrez, C.; Agulla, J.; Lapresa, R.; Elrod, J.W.; Almeida, A.; Bolaños, J.P.; Kowaltowski, A.J. Mitochondrial sodium/calcium exchanger NCLX regulates glycolysis in astrocytes, impacting on cognitive performance. J. Neurochem. 2023, 165, 521–535. [Google Scholar] [CrossRef]
- Bezprozvanny, I.; Hiesinger, P.R. The synaptic maintenance problem: Membrane recycling, Ca2+ homeostasis and late onset degeneration. Mol. Neurodegener. 2013, 8, 23. [Google Scholar] [CrossRef]
- Milnerwood, A.J.; Raymond, L.A. Corticostriatal synaptic function in mouse models of Huntington’s disease: Early effects of huntingtin repeat length and protein load. J. Physiol. 2007, 585, 817–831. [Google Scholar] [CrossRef]
- Milnerwood, A.J.; Raymond, L.A. Early synaptic pathophysiology in neurodegeneration: Insights from Huntington’s disease. Trends Neurosci. 2010, 33, 513–523. [Google Scholar] [CrossRef]
- Suzuki, M.; Nagai, Y.; Wada, K.; Koike, T. Calcium leak through ryanodine receptor is involved in neuronal death induced by mutant huntingtin. Biochem. Biophys. Res. Commun. 2012, 429, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, J.; Lvovskaya, S.; Herndon, E.; Supnet, C.; Bezprozvanny, I. Dantrolene is neuroprotective in Huntington’s disease transgenic mouse model. Mol. Neurodegener. 2011, 6, 81. [Google Scholar] [CrossRef]
- Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 2018, 70, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Vigont, V.A.; Zimina, O.A.; Glushankova, L.N.; Volodina, A.M.; Vasileva, D.Y.; Zimnikova, E.A.; Timaeva, A.A.; Kazantsev, A.G.; Skopin, A.Y.; Kaznacheyeva, E.V. STIM2 mediates excessive store-operated calcium entry in patient-specific ipsc-derived neurons modeling a juvenile form of Huntington’s disease. Front. Cell Dev. Biol. 2021, 9, 625231. [Google Scholar] [CrossRef]
- Egunlusi, A.O.; Malan, S.F.; Palchykov, V.A.; Joubert, J. Calcium modulating effect of polycyclic cages: A suitable therapeutic approach against excitotoxic-induced neurodegeneration. Mini-Rev. Med. Chem. 2024, 24, 1277–1292. [Google Scholar] [CrossRef]
- Wu, X.; Hong, L. Calmodulin interactions with voltage-gated sodium channels. Int. J. Mol. Sci. 2021, 22, 9798. [Google Scholar] [CrossRef] [PubMed]
- Smitherman, T.A.; Burch, R.; Sheikh, H.; Loder, E. The prevalence, impact, and treatment of migraine and SE-vere headaches in the United States: A review of statistics from national surveillance studies. Headache 2013, 53, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Antonaci, F.; Ghiotto, N.; Wu, S.; Pucci, E.; Costa, A. Recent advances in migraine therapy. Springerplus 2016, 5, 637. [Google Scholar] [CrossRef]
- Diener, H.C.; Dodick, D.W.; Goadsby, P.J.; Lipton, R.B.; Olesen, J.; Silberstein, S.D. Chronic migraine classification, characteristics and treatment. Nat. Rev. Neurol. 2012, 8, 162–171. [Google Scholar] [CrossRef]
- Beswick, P. Comprehensive Medicinal Chemistry III; Progress in the discovery of Ca channel blockers for the treatment of pain; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 65–130. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pikor, D.; Hurła, M.; Słowikowski, B.; Szymanowicz, O.; Poszwa, J.; Banaszek, N.; Drelichowska, A.; Jagodziński, P.P.; Kozubski, W.; Dorszewska, J. Calcium Ions in the Physiology and Pathology of the Central Nervous System. Int. J. Mol. Sci. 2024, 25, 13133. https://doi.org/10.3390/ijms252313133
Pikor D, Hurła M, Słowikowski B, Szymanowicz O, Poszwa J, Banaszek N, Drelichowska A, Jagodziński PP, Kozubski W, Dorszewska J. Calcium Ions in the Physiology and Pathology of the Central Nervous System. International Journal of Molecular Sciences. 2024; 25(23):13133. https://doi.org/10.3390/ijms252313133
Chicago/Turabian StylePikor, Damian, Mikołaj Hurła, Bartosz Słowikowski, Oliwia Szymanowicz, Joanna Poszwa, Natalia Banaszek, Alicja Drelichowska, Paweł P. Jagodziński, Wojciech Kozubski, and Jolanta Dorszewska. 2024. "Calcium Ions in the Physiology and Pathology of the Central Nervous System" International Journal of Molecular Sciences 25, no. 23: 13133. https://doi.org/10.3390/ijms252313133
APA StylePikor, D., Hurła, M., Słowikowski, B., Szymanowicz, O., Poszwa, J., Banaszek, N., Drelichowska, A., Jagodziński, P. P., Kozubski, W., & Dorszewska, J. (2024). Calcium Ions in the Physiology and Pathology of the Central Nervous System. International Journal of Molecular Sciences, 25(23), 13133. https://doi.org/10.3390/ijms252313133






