Dictamnus dasycarpus Turcz. Root Bark Improves Skin Barrier Function and Symptoms of Atopic Dermatitis in Mice
Abstract
:1. Introduction
2. Results
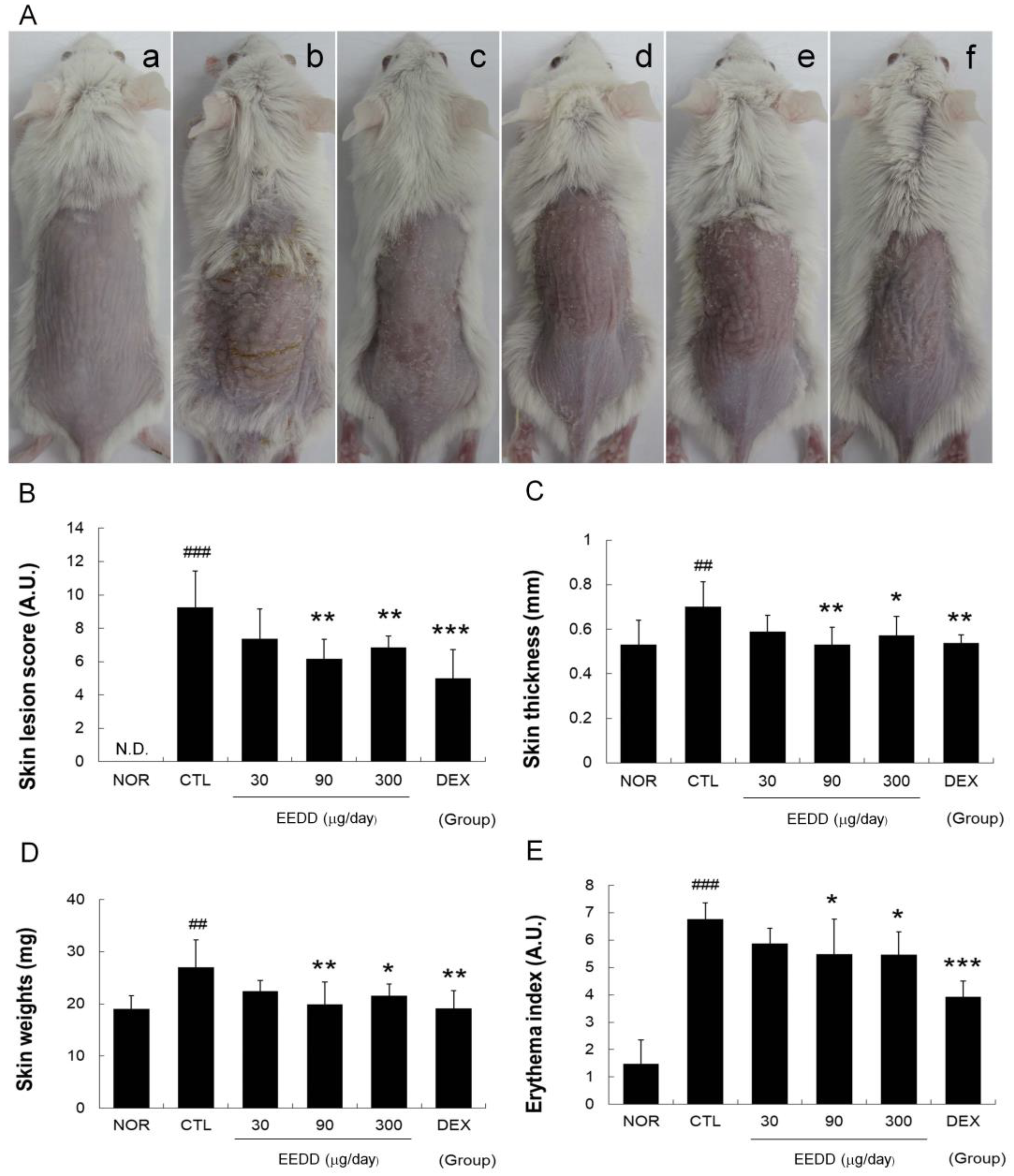
2.1. EEDD Alleviated Skin Lesions and Prevented Increases in Erythema Index, Skin Thickness, and Weight
2.2. EEDD Enhanced Skin Water Content and Water-Holding Capacity
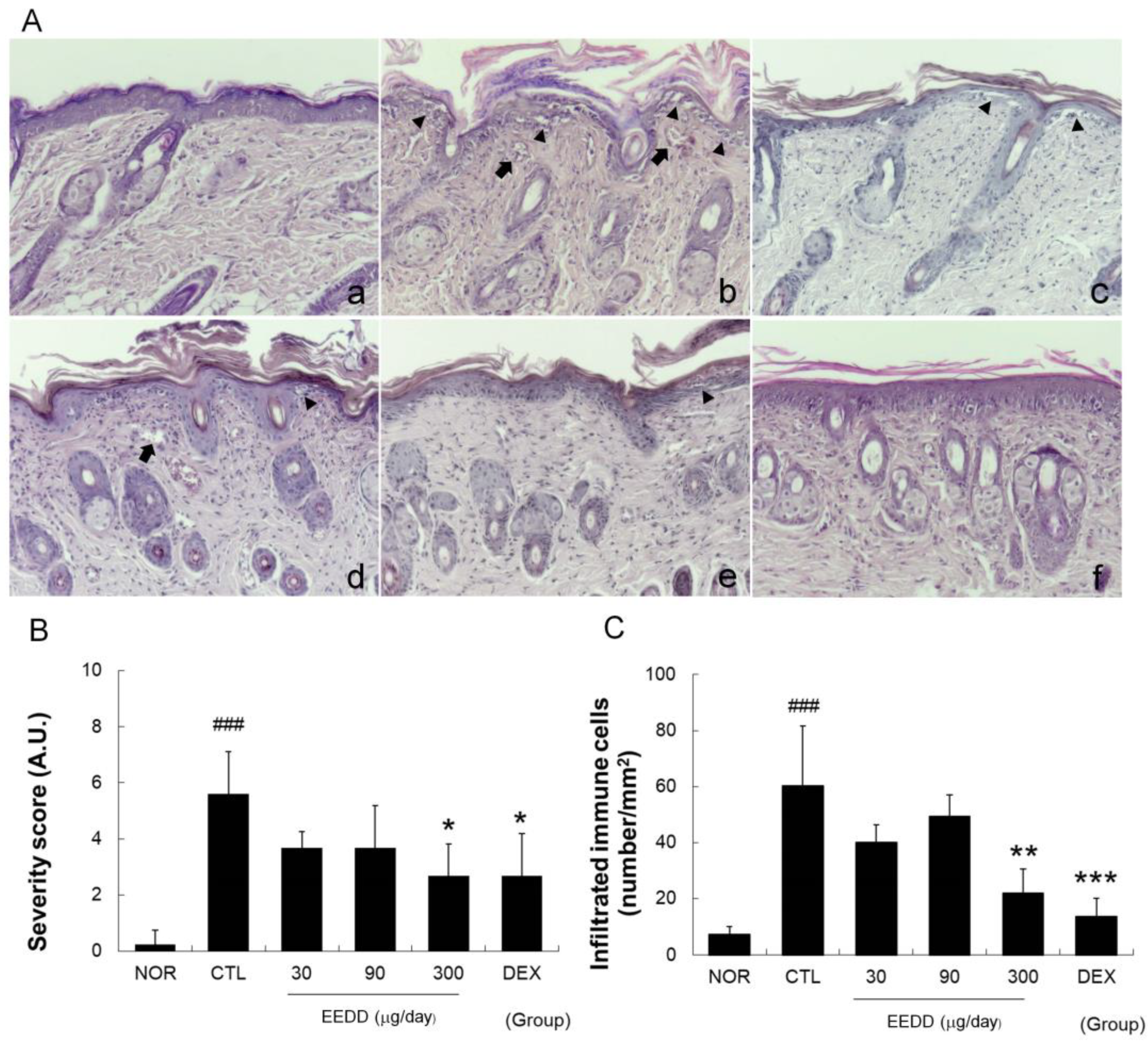
2.3. EEDD Inhibited Histopathological Defects in Inflamed Tissues
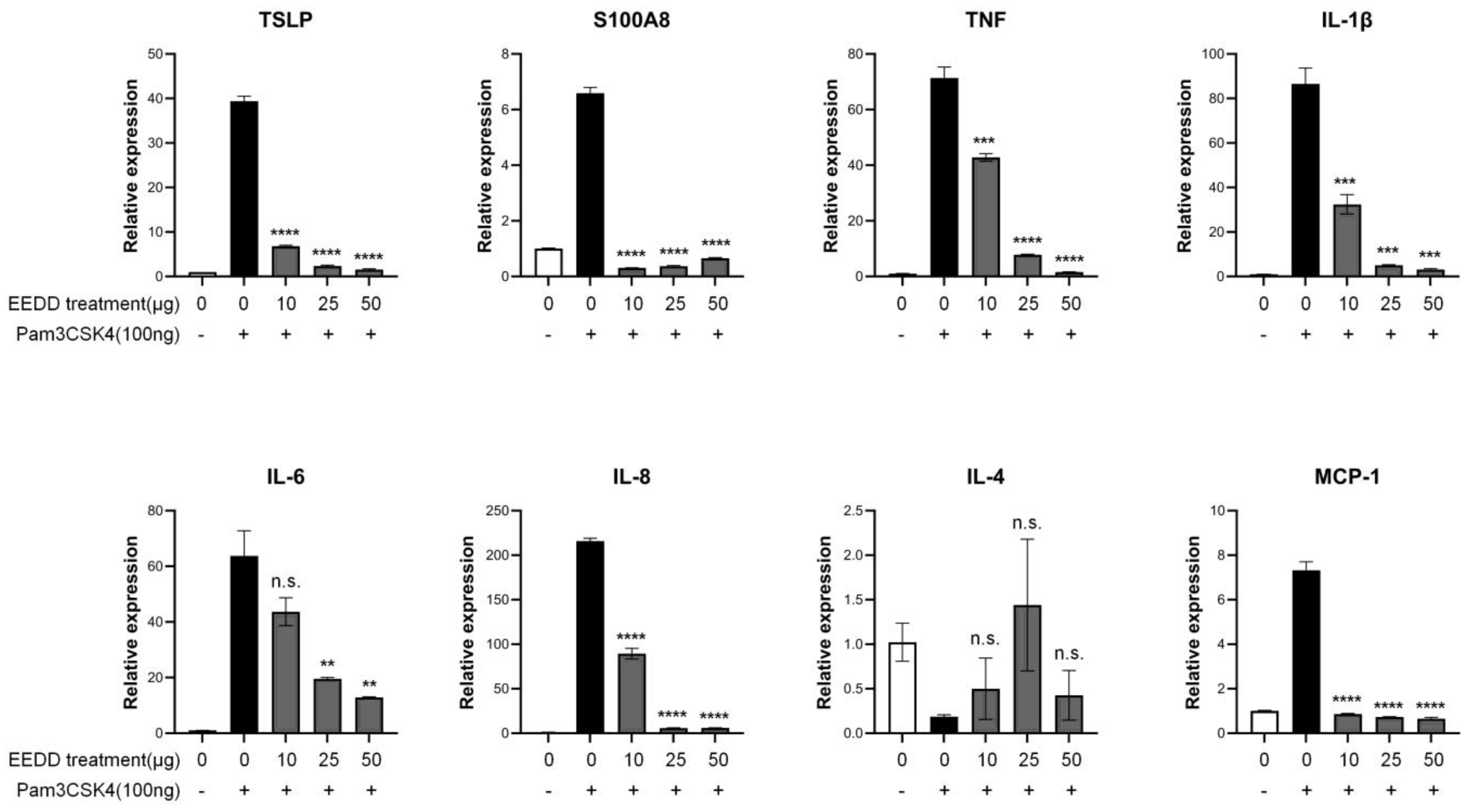
2.4. EEDD Prevented MC903-Induced Increases in TNF-α, IL-2, IL-4, and IL-6 in Inflamed Tissues
2.5. EEDD Suppressed the Expressions of TSLP and Inflammatory Factors
2.6. EEDD Regulated NF-κB (p65) Translocation and the MAPK Pathway
3. Discussion
4. Materials and Methods
4.1. Preparation of the Dictamnus Dasycarpus Root Bark Extract
4.2. Animals
4.3. Induction of AD and Experimental Design
4.4. Assessment of Skin Lesion Severities
4.5. Measurement of Skin Thicknesses, Weight, and Color
4.6. Skin Water Content and Water-Holding Capacity
4.7. Histopathological Examination
4.8. Cytokine Levels
4.9. Cell Culture
4.10. Isolation of Total RNA and RT-qPCR
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novak, N.; Leung, D.Y. Advances in atopic dermatitis. Curr. Opin. Immunol. 2011, 23, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Addor, F.A.; Takaoka, R.; Rivitti, E.A.; Aoki, V. Atopic dermatitis: Correlation between non-damaged skin barrier function and disease activity. Int. J. Dermatol. 2012, 51, 672–676. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, G.M.; Irvine, A.D. The role of filaggrin in the atopic diathesis. Clin. Exp. Allergy 2010, 40, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Skin barrier abnormalities and immune dysfunction in atopic dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Masuda, K.; Tamagawa-Mineoka, R.; Matsunaka, H.; Murakami, Y.; Yamashita, R.; Morita, E.; Katoh, N. Thymic stromal lymphopoietin expression is increased in the horny layer of patients with atopic dermatitis. Clin. Exp. Immunol. 2013, 171, 330–337. [Google Scholar] [CrossRef]
- Kim, J.H.; Bae, H.C.; Ko, N.Y.; Lee, S.H.; Jeong, S.H.; Lee, H.; Ryu, W.I.; Kye, Y.C.; Son, S.W. Thymic stromal lymphopoietin downregulates filaggrin expression by signal transducer and activator of transcription 3 (STAT3) and extracellular signal-regulated kinase (ERK) phosphorylation in keratinocytes. J. Allergy Clin. Immunol. 2015, 136, 205–208.e9. [Google Scholar] [CrossRef]
- Wallmeyer, L.; Dietert, K.; Sochorová, M.; Gruber, A.D.; Kleuser, B.; Vávrová, K.; Hedtrich, S. TSLP is a direct trigger for T cell migration in filaggrin-deficient skin equivalents. Sci. Rep. 2017, 7, 774. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, Z.; Zhai, Y.; Zeng, J.; Li, L.; Wang, D.; Deng, F.; Chang, B.; Zhou, J.; Sun, L. The Role of TSLP in Atopic Dermatitis: From Pathogenetic Molecule to Therapeutical Target. Mediat. Inflamm. 2023, 2023, 7697699. [Google Scholar] [CrossRef]
- Charman, C.R.; Morris, A.D.; Williams, H.C. Topical corticosteroid phobia in patients with atopic eczema. Br. J. Dermatol. 2000, 142, 931–936. [Google Scholar] [CrossRef]
- Kim, C.M.; Shin, M.G.; An, D.G.; Lee, K.S. The Encyclopedia of Oriental Herbal Medicine. Seoul Jeongdam 1997, 1, 1648–1651. [Google Scholar]
- Qin, Y.; Quan, H.-F.; Zhou, X.-R.; Chen, S.-J.; Xia, W.-X.; Li, H.; Huang, H.-L.; Fu, X.-Y.; Dong, L. The traditional uses, phytochemistry, pharmacology and toxicology of Dictamnus dasycarpus: A review. J. Pharm. Pharmacol. 2021, 73, 1571–1591. [Google Scholar] [CrossRef] [PubMed]
- Han, H.Y.; Ryu, M.H.; Lee, G.; Cheon, W.J.; Lee, C.; An, W.G.; Kim, H.; Cho, S.I. Effects of Dictamnus dasycarpus Turcz., root bark on ICAM-1 expression and chemokine productions in vivo and vitro study. J. Ethnopharmacol. 2015, 159, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, M.; Kim, H.; Lee, G.S.; An, W.G.; Cho, S.I. Anti-inflammatory activities of Dictamnus dasycarpus Turcz., root bark on allergic contact dermatitis induced by dinitrofluorobenzene in mice. J. Ethnopharmacol. 2013, 149, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kwon, S.H.; Huh, C.H.; Park, K.C.; Youn, S.W. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: A comprehensive and objective approach. Ski. Res. Technol. 2013, 19, e349–e355. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Amagai, M. Erratum: Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280, Erratum in Int. Immunol. 2017, 29, 243–244. [Google Scholar] [CrossRef]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef]
- Tsakok, T.; Woolf, R.; Smith, C.; Weidinger, S.; Flohr, C. Atopic dermatitis: The skin barrier and beyond. Br. J. Dermatol. 2019, 180, 464–474. [Google Scholar] [CrossRef]
- Rerknimitr, P.; Otsuka, A.; Nakashima, C.; Kabashima, K. The etiopathogenesis of atopic dermatitis: Barrier disruption, immunological derangement, and pruritus. Inflamm. Regen. 2017, 37, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demehri, S.; Morimoto, M.; Holtzman, M.J.; Kopan, R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009, 7, e1000067. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Ojha, U.; Lee, Y.M. Pathological angiogenesis and inflammation in tissues. Arch. Pharmacal Res. 2021, 44, 1–15. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, V.Y.; Chan, L.S. Potential role of angiogenesis and lymphangiogenesis in atopic dermatitis: Evidence from human studies and lessons from an animal model of human disease. In Angiogenesis-Based Dermatology; Springer: London, UK, 2017; pp. 95–122. [Google Scholar]
- Jackson, S.M.; Williams, M.L.; Feingold, K.R.; Elias, P.M. Pathobiology of the stratum corneum. West. J. Med. 1993, 158, 279. [Google Scholar] [PubMed]
- Barker, J.N.; Griffiths, C.E.; Nickoloff, B.J.; Mitra, R.; Dixit, V.M. Keratinocytes as initiators of inflammation. Lancet 1991, 337, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Mestrallet, G.; Rouas-Freiss, N.; LeMaoult, J.; Fortunel, N.O.; Martin, M.T. Skin Immunity and Tolerance: Focus on Epidermal Keratinocytes Expressing HLA-G. Front. Immunol. 2021, 12, 772516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Gu, W.; Sun, B. TH1/TH2 cell differentiation and molecular signals. In T Helper Cell Differentiation and Their Function; Springer: Dordrecht, The Netherlands, 2014; pp. 15–44. [Google Scholar]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef]
- Clahsen, T.; Schaper, F. Interleukin-6 acts in the fashion of a classical chemokine on monocytic cells by inducing integrin activation, cell adhesion, actin polymerization, chemotaxis, and transmigration. J. Leukoc. Biol. 2008, 84, 1521–1529. [Google Scholar] [CrossRef]
- Nedoszytko, B.; Sokołowska-Wojdyło, M.; Ruckemann-Dziurdzińska, K.; Roszkiewicz, J.; Nowicki, R. Review paper Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: Atopic dermatitis, psoriasis and skin mastocytosis. Adv. Dermatol. Allergol./Postępy Dermatol. I Alergol. 2014, 31, 84–91. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Gandhi, D.; Bhandari, S.; Maity, S.; Mahapatra, S.K.; Rajasekaran, S. Activation of ERK/NF-kB Pathways Contributes to the Inflammatory Response in Epithelial Cells and Macrophages Following Manganese Exposure. Biol. Trace Elem. Res. 2024, 1–12. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Agner, T. Basal transepidermal water loss, skin thickness, skin blood flow and skin colour in relation to sodium-lauryl-sulphate-induced irritation in normal skin. Contact Dermat. 1991, 25, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Offner, H.; Subramanian, S.; Parker, S.M.; Wang, C.; Afentoulis, M.E.; Lewis, A.; Vandenbark, A.A.; Hurn, P.D. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J. Immunol. 2006, 176, 6523–6531. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Yang, W.; Park, S.; Yang, J.; Kim, S.; Lyu, J.-H.; Kim, H. The anti-inflammatory and skin barrier function recovery effects of Schisandra chinensis in mice with atopic dermatitis. Medicina 2023, 59, 1353. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.H.; Lee, G.S.; Kim, K.H.; Kim, H.W.; Cho, S.I.; Jeong, S.I.; Kim, H.J.; Ju, Y.S.; Kim, H.K.; Sadikot, R.T.; et al. ent-kaur-16-en-19-oic Acid, isolated from the roots of Aralia continentalis, induces activation of Nrf2. J. Ethnopharmacol. 2011, 137, 1442–1449. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Yang, J.; Sun, K.; Park, S.; Lee, J.; Kim, S.; Lyu, J.H.; Kim, H. Dictamnus dasycarpus Turcz. Root Bark Improves Skin Barrier Function and Symptoms of Atopic Dermatitis in Mice. Int. J. Mol. Sci. 2024, 25, 13178. https://doi.org/10.3390/ijms252313178
Park S, Yang J, Sun K, Park S, Lee J, Kim S, Lyu JH, Kim H. Dictamnus dasycarpus Turcz. Root Bark Improves Skin Barrier Function and Symptoms of Atopic Dermatitis in Mice. International Journal of Molecular Sciences. 2024; 25(23):13178. https://doi.org/10.3390/ijms252313178
Chicago/Turabian StylePark, Sangjun, Jinkyu Yang, Kyoungmin Sun, Seonah Park, Jimi Lee, Soyeon Kim, Ji Hyo Lyu, and Hyungwoo Kim. 2024. "Dictamnus dasycarpus Turcz. Root Bark Improves Skin Barrier Function and Symptoms of Atopic Dermatitis in Mice" International Journal of Molecular Sciences 25, no. 23: 13178. https://doi.org/10.3390/ijms252313178
APA StylePark, S., Yang, J., Sun, K., Park, S., Lee, J., Kim, S., Lyu, J. H., & Kim, H. (2024). Dictamnus dasycarpus Turcz. Root Bark Improves Skin Barrier Function and Symptoms of Atopic Dermatitis in Mice. International Journal of Molecular Sciences, 25(23), 13178. https://doi.org/10.3390/ijms252313178





