Application of a Screen-Printed Ion-Selective Electrode Based on Hydrophobic Ti3C2/AuNPs for K+ Determination Across Variable Temperatures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Electrode Preparation Process
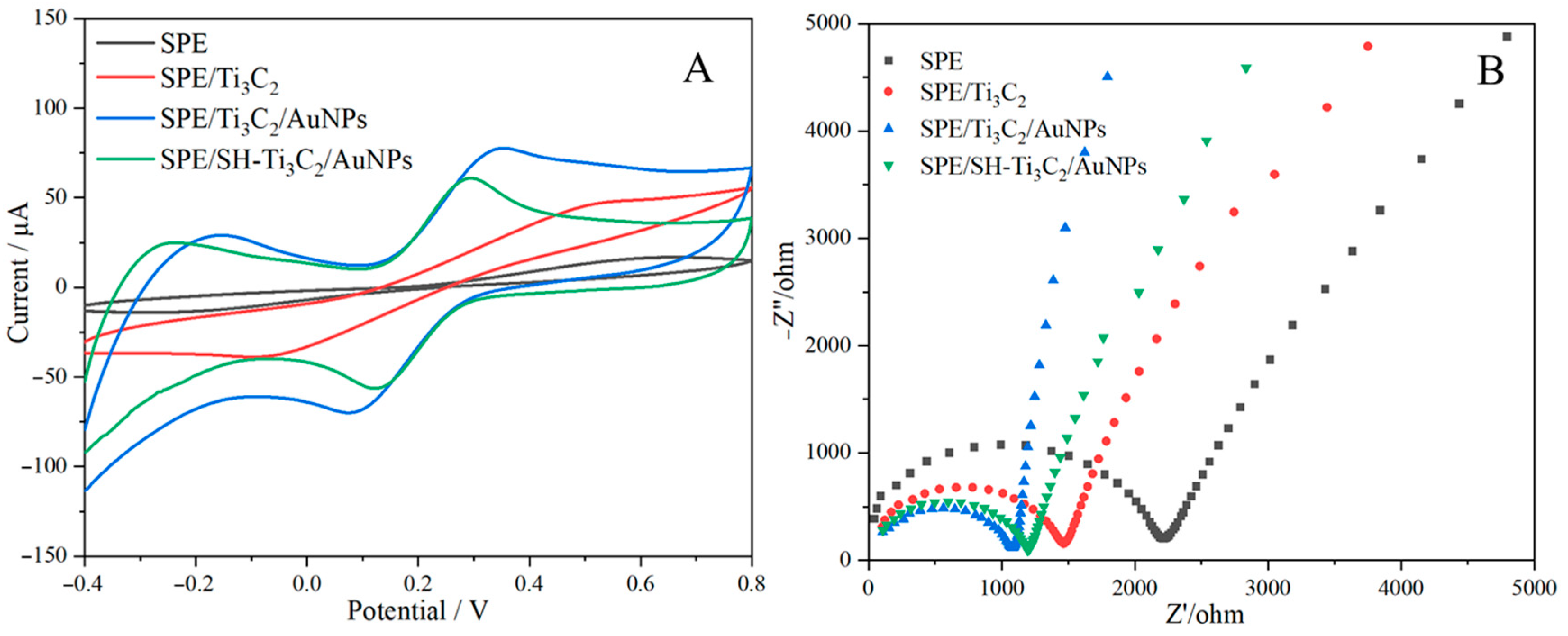
2.2. Electrochemical Characterization of the Fabricated Sensor
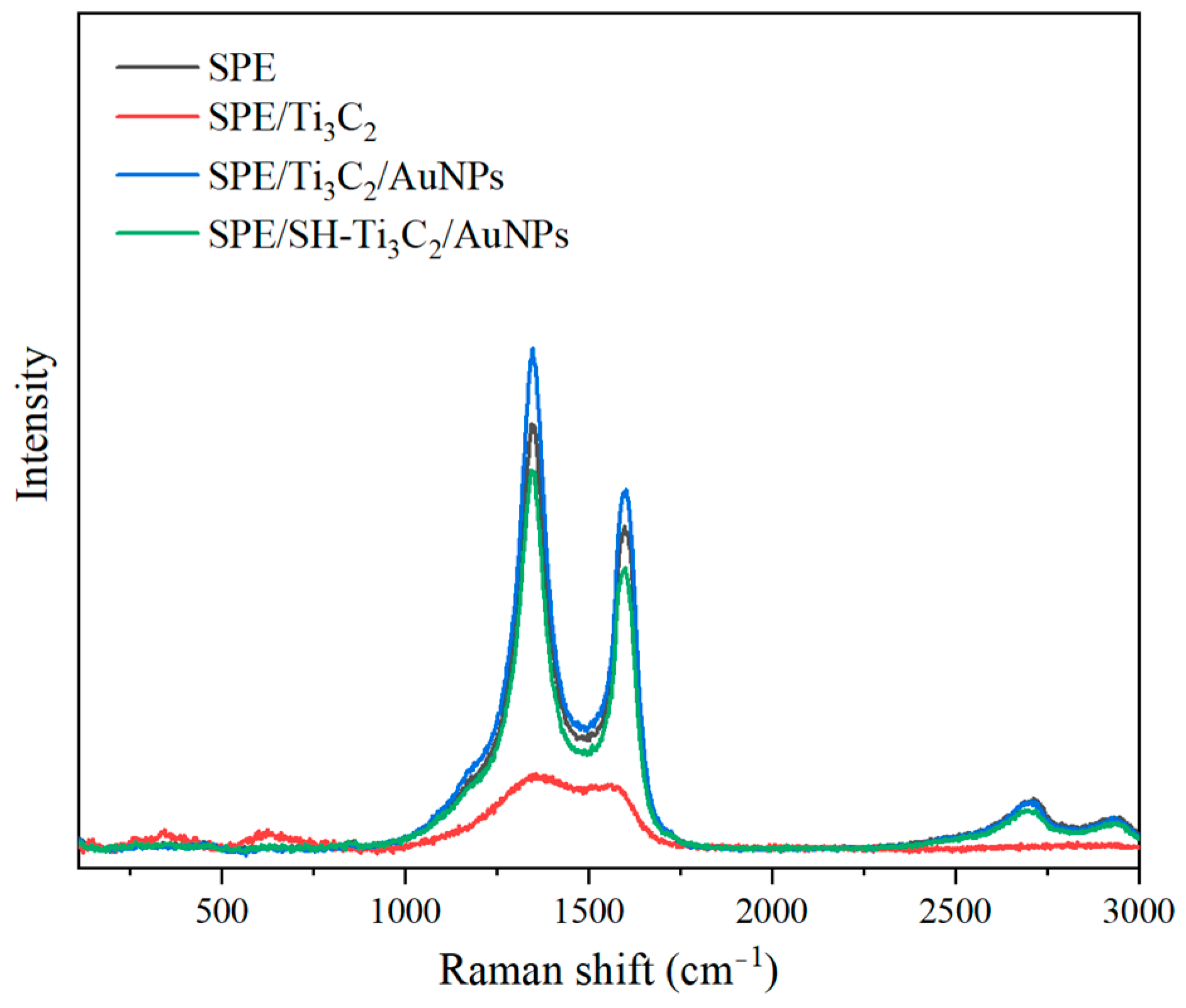
2.3. Raman Spectroscopy
2.4. Test of Contact Angle
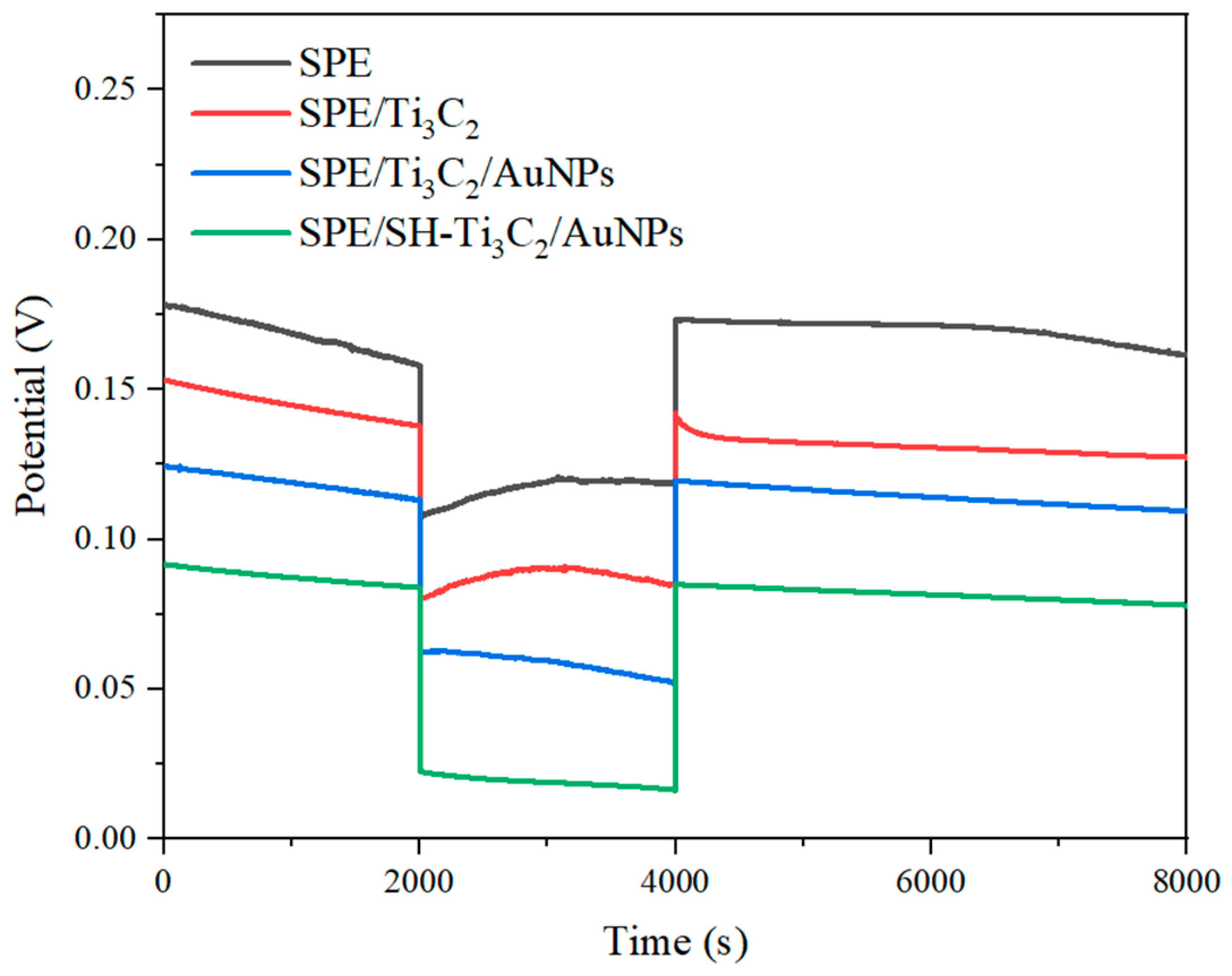
2.5. Water Layer Test
2.6. Chronopotentiometry
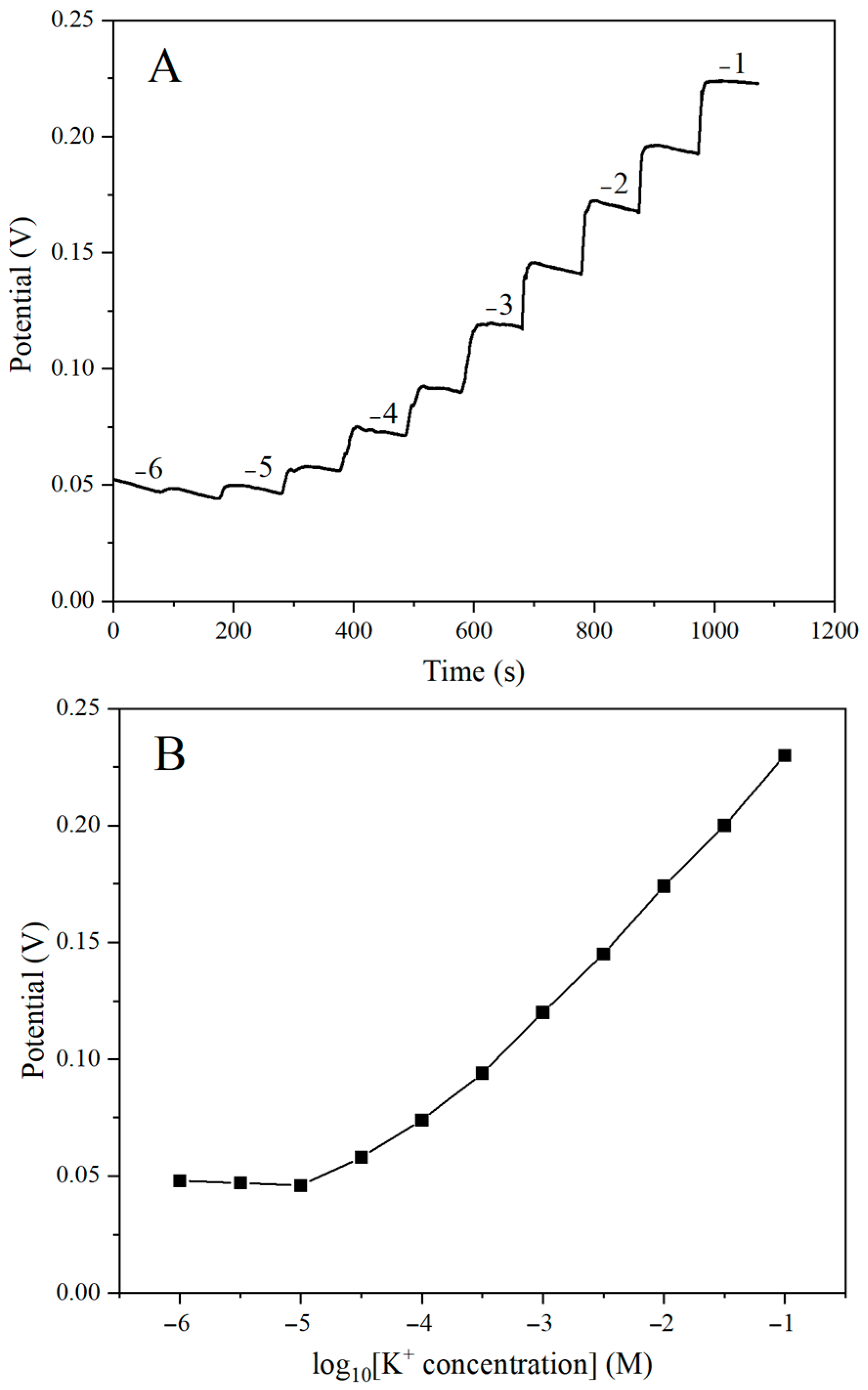
2.7. Potential Characteristic
2.8. Detection Performance of Temperature Sensors
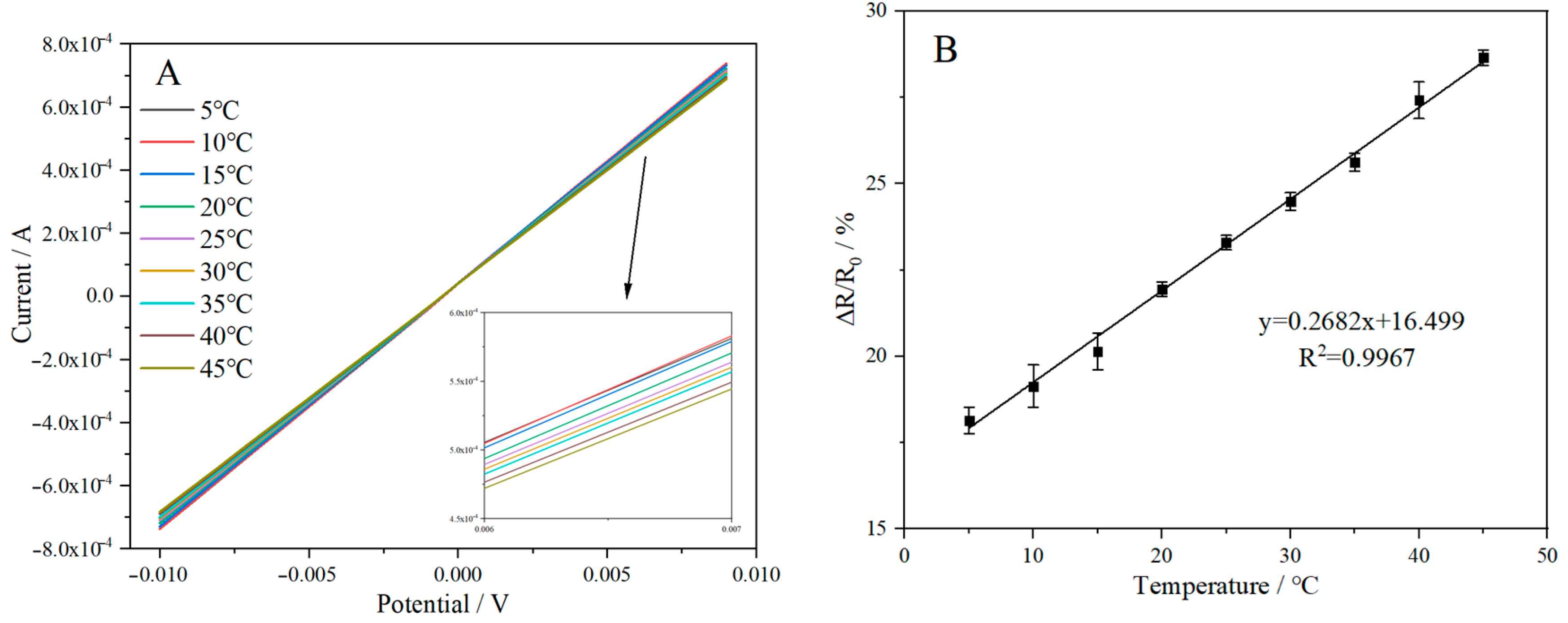
2.8.1. Resistance Rate Response
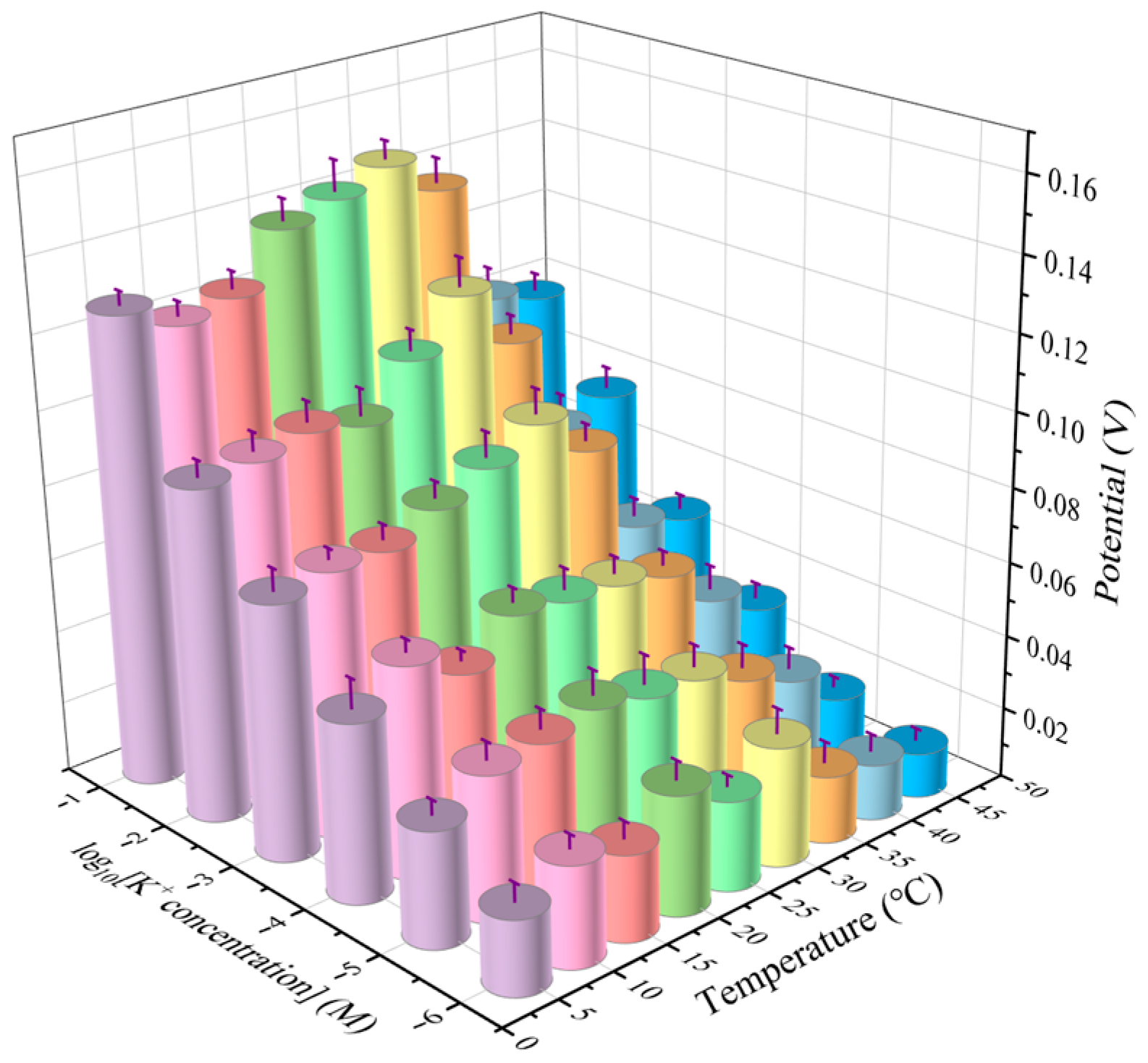
2.8.2. Effect of Temperature and K+ Concentration on Electrical Signal
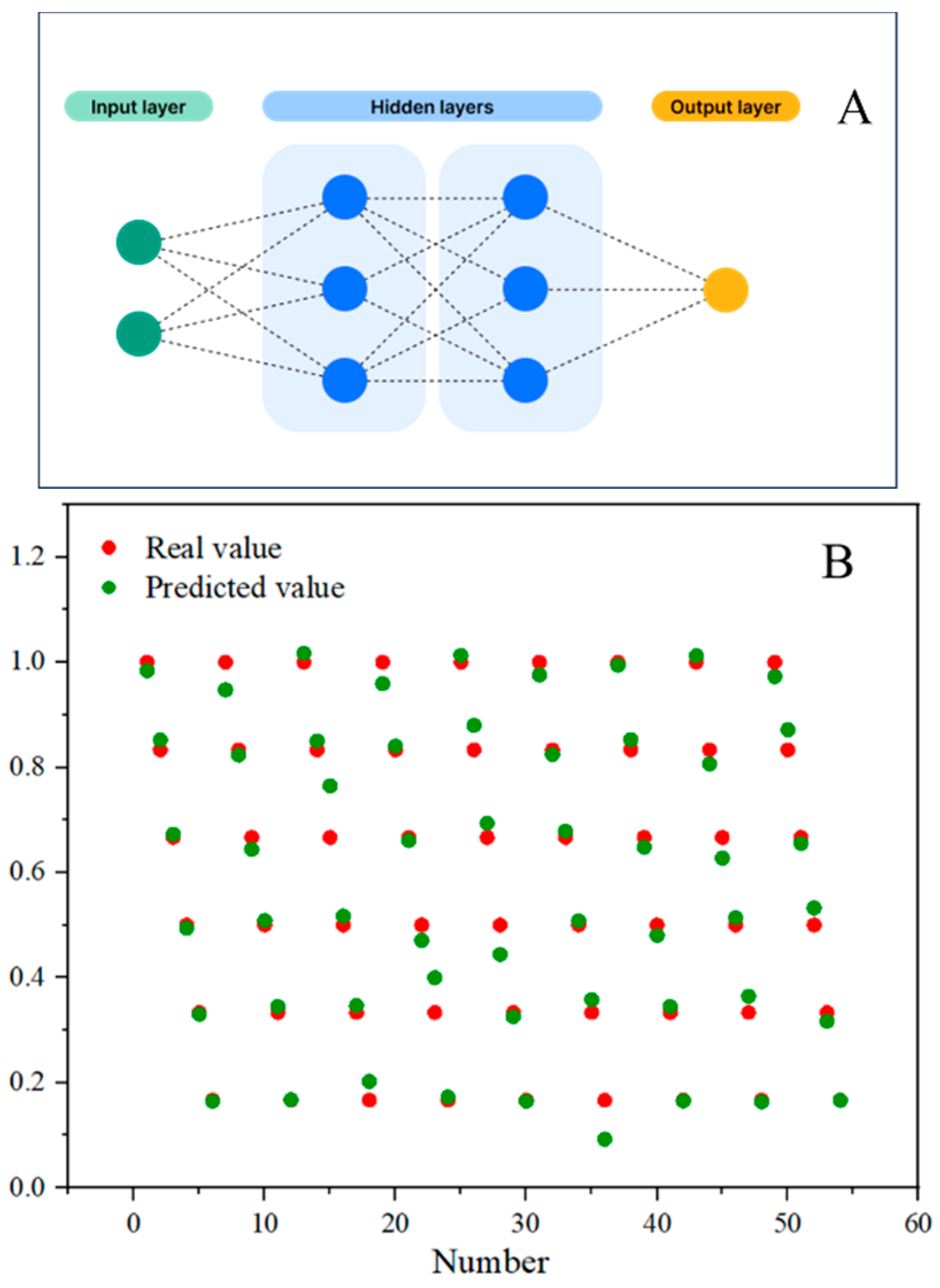
2.8.3. Data Preprocessing
2.8.4. Artificial Neural Network Model
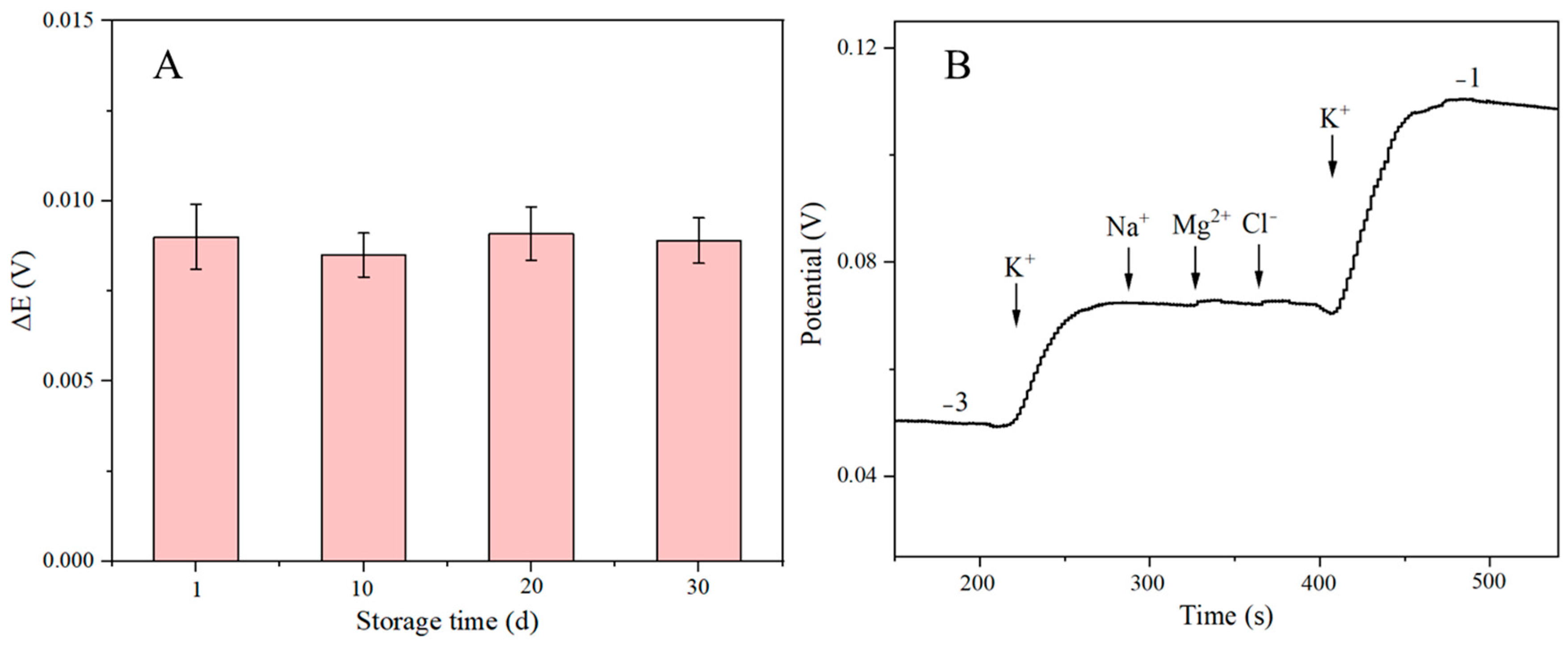
2.9. Stability and Selectivity
2.10. Comparison of Different K-ISEs
2.11. Determination of K+ in Samples
3. Experimental
3.1. Reagents and Materials
3.2. Instruments and Measurements
3.3. Preparation of Electrode
3.4. Preparation of Temperature Sensor
3.5. Sample Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velladurai, C.; Selvaraju, M.; Napolean, R.E. Effects of macro and micro minerals on reproduction in dairy cattle a review. Int. J. Sci. Res. Sci. Technol. 2016, 1, 68–74. [Google Scholar]
- Berg, M.; Plöntzke, J.; Leonhard-Marek, S.; Müller, K.-E.; Röblitz, S. A dynamic model to simulate potassium balance in dairy cows. J. Dairy Sci. 2017, 100, 9799–9814. [Google Scholar] [CrossRef] [PubMed]
- Sejersted, O.M.; Sjøgaard, G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol. Rev. 2000, 80, 1411–1481. [Google Scholar] [CrossRef]
- Numnuam, A.; Chumbimuni-Torres, K.Y.; Xiang, Y.; Bash, R.; Thavarungkul, P.; Kanatharana, P.; Pretsch, E.; Wang, J.; Bakker, E. Aptamer-based potentiometric measurements of proteins using ion-selective microelectrodes. Anal. Chem. 2008, 80, 707–712. [Google Scholar] [CrossRef]
- Michalska, A.J.; Appaih-Kusi, C.; Heng, L.Y.; Walkiewicz, S.; Hall, E.A. An experimental study of membrane materials and inner contacting layers for ion-selective K+ electrodes with a stable response and good dynamic range. Anal. Chem. 2004, 76, 2031–2039. [Google Scholar] [CrossRef]
- Mensah, S.T.; Gonzalez, Y.; Calvo-Marzal, P.; Chumbimuni-Torres, K.Y. Nanomolar detection limits of Cd2+, Ag+, and K+ using paper-strip ion-selective electrodes. Anal. Chem. 2014, 86, 7269–7273. [Google Scholar] [CrossRef]
- Veder, J.-P.; De Marco, R.; Clarke, G.; Chester, R.; Nelson, A.; Prince, K.; Pretsch, E.; Bakker, E. Elimination of undesirable water layers in solid-contact polymeric ion-selective electrodes. Anal. Chem. 2008, 80, 6731–6740. [Google Scholar] [CrossRef]
- Cheng, L.; Li, X.; Zhang, H.; Xiang, Q. Two-dimensional transition metal MXene-based photocatalysts for solar fuel generation. J. Phys. Chem. Lett. 2019, 10, 3488–3494. [Google Scholar] [CrossRef]
- Wu, L.X.; Lu, X.B.; Dhanjai; Wu, Z.S.; Dong, Y.F.; Wang, X.H.; Zheng, S.H.; Chen, J.P. 2D transition metal carbide MXene as a robust biosensing platform for enzyme immobilization and ultrasensitive detection of phenol. Biosens. Bioelectron. 2018, 107, 69–75. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef]
- Zhao, S.L.; Wang, Y.; Dong, J.C.; He, C.T.; Yin, H.J.; An, P.F.; Zhao, K.; Zhang, X.F.; Gao, C.; Zhang, L.J.; et al. Ultrathin metal-organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Zhang, H.X.; Wang, Z.H.; Wang, F.; Zhang, Y.M.; Wang, H.Y.; Liu, Y. In Situ Formation of Gold Nanoparticles Decorated Ti3C2 MXenes Nanoprobe for Highly Sensitive Electrogenerated Chemiluminescence Detection of Exosomes and Their Surface Proteins. Anal. Chem. 2020, 92, 5546–5553. [Google Scholar] [CrossRef]
- Yang, X.; Feng, M.H.; Xia, J.F.; Zhang, F.F.; Wang, Z.H. An electrochemical biosensor based on AuNPs/Ti3C2 MXene three-dimensional nanocomposite for microRNA-155 detection by exonuclease III-aided cascade target recycling. J. Electroanal. Chem. 2020, 878, 114669. [Google Scholar] [CrossRef]
- Jing, X.Y.; Cao, X.Q.; Wang, L.; Lan, T.; Li, Y.Y.; Xie, G.M. DNA-AuNPs based signal amplification for highly sensitive detection of DNA methylation, methyltransferase activity and inhibitor screening. Biosens. Bioelectron. 2014, 58, 40–47. [Google Scholar] [CrossRef]
- Chang, X.; Wu, Q.; Wu, Y.Y.; Xi, X.; Cao, J.R.; Chu, H.Y.; Liu, Q.H.; Li, Y.Y.; Wu, W.; Fang, X.D.; et al. Multifunctional Au Modified Ti3C2-MXene for Photothermal/Enzyme Dynamic/Immune Synergistic Therapy. Nano Lett. 2022, 22, 8321–8330. [Google Scholar] [CrossRef]
- Song, D.D.; Jiang, X.Y.; Li, Y.S.; Lu, X.; Luan, S.R.; Wang, Y.Z.; Li, Y.; Gao, F.M. Metal-organic frameworks-derived MnO/MnO microcuboids with hierarchically ordered nanosheets and Ti3C2 MXene/AuNPs composites for electrochemical pesticide detection. J. Hazard Mater. 2019, 373, 367–376. [Google Scholar] [CrossRef]
- Schreiber, F. Structure and growth of self-assembling monolayers. Prog. Surf. Sci. 2000, 65, 151–256. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Van Geel, P.J. Potential concerns related to using octadecyltrichlorosilane (OTS) in rendering soils and porous ceramics hydrophobic. J. Contam. Hydrol. 2009, 110, 22–33. [Google Scholar] [CrossRef] [PubMed]
- del Torno-de Román, L.; Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Gluconic acid determination in wine by electrochemical biosensing. Sens. Actuators B Chem. 2013, 176, 858–862. [Google Scholar] [CrossRef]
- Metters, J.P.; Tan, F.; Banks, C.E. Screen-printed palladium electroanalytical sensors. J. Solid State Electr. 2013, 17, 1553–1562. [Google Scholar] [CrossRef]
- Vaughan-Jones, R.D.; Kaila, K. The sensitivity of liquid sensor, ion-selective microelectrodes to changes in temperature and solution level. Pflugers Arch. 1986, 406, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Jebakumari, K.A.E.; Murugasenapathi, N.K.; Palanisamy, T. Engineered Two-Dimensional Nanostructures as SERS Substrates for Biomolecule Sensing: A Review. Biosensors 2023, 13, 102. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zeng, Z.X.; Qiao, J.Y.; Dong, S.Q.; Liang, Q.; Shao, S.J. Ultrasensitive determination of nitrite based on electrochemical platform of AuNPs deposited on PDDA-modified MXene nanosheets. Talanta 2021, 221, 121605. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, Y.; Ma, T. Electrostatic self-assembly of 2D delaminated MXene (Ti3C2) onto Ni foam with superior electrochemical performance for supercapacitor. Electrochim. Acta 2019, 305, 164–174. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Kakade, B.A.; Mulla, I.S.; Pillai, V.K. Suppression of electron-transfer characteristics of ferrocene by OTS monolayer on a silicon/electrolyte interface. J. Colloid Interface Sci. 2006, 299, 777–784. [Google Scholar] [CrossRef]
- Kannan, P.; Subramanian, P.; Maiyalagan, T.; Jiang, Z. Cobalt Oxide Porous Nanocubes-Based Electrochemical Immunobiosensing of Hepatitis B Virus DNA in Blood Serum and Urine Samples. Anal. Chem. 2019, 91, 5824–5833. [Google Scholar] [CrossRef]
- Zhao, J.; He, C.; Wu, W.; Yang, H.; Dong, J.; Wen, L.; Hu, Z.; Yang, M.; Hou, C.; Huo, D. MXene-MoS2 heterostructure collaborated with catalyzed hairpin assembly for label-free electrochemical detection of microRNA-21. Talanta 2022, 237, 122927. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.; Metters, J.P.; Kadara, R.O.; Banks, C.E. The fabrication, characterisation and electrochemical investigation of screen-printed graphene electrodes. Phys. Chem. Chem. Phys. 2014, 16, 4598–4611. [Google Scholar] [CrossRef]
- Castaño-Guerrero, Y.; Romaguera-Barcelay, Y.; Moreira, F.T.C.; Brito, W.R.; Fortunato, E.; Sales, M.G.F. Poly (thionine)-modified screen-printed electrodes for CA 19-9 detection and its properties in Raman spectroscopy. Chemosensors 2022, 10, 92. [Google Scholar] [CrossRef]
- Lian, W.; Mai, Y.; Liu, C.; Zhang, L.; Li, S.; Jie, X. Two-dimensional Ti3C2 coating as an emerging protective solid-lubricant for tribology. Ceram. Int. 2018, 44, 20154–20162. [Google Scholar] [CrossRef]
- Luo, Z.Q.; Yu, T.; Kim, K.J.; Ni, Z.H.; You, Y.M.; Lim, S.; Shen, Z.X.; Wang, S.Z.; Lin, J.Y. Thickness-Dependent Reversible Hydrogenation of Graphene Layers. ACS Nano 2009, 3, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, J.E.; Ho, M.; Orendorff, C.J.; Ducey, M.W. Raman spectroscopy of octadecylsilane stationary phase conformational order: Effect of solvent. J. Chromatogr. A 2001, 913, 243–252. [Google Scholar] [CrossRef]
- Zhu, L.; Lv, X.; Li, Z.; Shi, H.; Zhang, Y.; Zhang, L.; Yu, J. All-sealed paper-based electrochemiluminescence platform for on-site determination of lead ions. Biosens. Bioelectron. 2021, 192, 113524. [Google Scholar] [CrossRef]
- Hassan, S.S.; Kamel, A.H.; Fathy, M.A. A novel screen-printed potentiometric electrode with carbon nanotubes/polyaniline transducer and molecularly imprinted polymer for the determination of nalbuphine in pharmaceuticals and biological fluids. Anal. Chim. Acta 2022, 1227, 340239. [Google Scholar] [CrossRef]
- Thuy, N.T.D.; Wang, X.; Zhao, G.; Liang, T.; Zou, Z. A Co3O4 Nanoparticle-Modified Screen-Printed Electrode Sensor for the Detection of Nitrate Ions in Aquaponic Systems. Sensors 2022, 22, 9730. [Google Scholar] [CrossRef]
- Mousavi, Z.; Bobacka, J.; Lewenstam, A.; Ivaska, A. Poly (3,4-ethylenedioxythiophene)(PEDOT) doped with carbon nanotubes as ion-to-electron transducer in polymer membrane-based potassium ion-selective electrodes. J. Electroanal. Chem. 2009, 633, 246–252. [Google Scholar] [CrossRef]
- Shao, Y.Z.; Ying, Y.B.; Ping, J.F. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef]
- Miller, P.R.; Xiao, X.; Brener, I.; Burckel, D.B.; Narayan, R.; Polsky, R. Microneedle-based transdermal sensor for on-chip potentiometric determination of K+. Adv. Healthc. Mater. 2014, 3, 876–881. [Google Scholar] [CrossRef]
- Zahran, E.M.; Gavalas, V.; Valiente, M.; Bachas, L.G. Can Temperature Be Used To Tune the Selectivity of Membrane Ion-Selective Electrodes? Anal. Chem. 2010, 82, 3622–3628. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. A disposable planar paper-based potentiometric ion-sensing platform. Angew. Chem. 2016, 128, 7670–7673. [Google Scholar] [CrossRef]
- Zeng, X.; Yu, S.; Yuan, Q.; Qin, W. Solid-contact K+-selective electrode based on three-dimensional molybdenum sulfide nanoflowers as ion-to-electron transducer. Sens. Actuators B Chem. 2016, 234, 80–83. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Zhou, M.; Gan, S.; Zhang, Q.; Han, D.; Niu, L. All-solid-state potassium-selective electrode using graphene as the solid contact. Analyst 2012, 137, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.T.; Qiu, S.; Chung, H.-J. Potassium ion selective electrode using polyaniline and matrix-supported ion-selective PVC membrane. IEEE Sens. J. 2018, 18, 9081–9087. [Google Scholar] [CrossRef]
- Phoonsawat, K.; Agir, I.; Dungchai, W.; Ozer, T.; Henry, C.S. A smartphone-assisted hybrid sensor for simultaneous potentiometric and distance-based detection of electrolytes. Anal. Chim. Acta 2022, 1226, 340245. [Google Scholar] [CrossRef]
- Hu, J.; Zou, X.U.; Stein, A.; Bühlmann, P. Ion-selective electrodes with colloid-imprinted mesoporous carbon as solid contact. Anal. Chem. 2014, 86, 7111–7118. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Li, P.; Zhang, W.; Lian, K.; Hu, J.; Chen, Y. Preparation and characterization of AuNPs/CNTs-ERGO electrochemical sensors for highly sensitive detection of hydrazine. Talanta 2016, 158, 283–291. [Google Scholar] [CrossRef]





| Sensor | Linear Range (M) | Response Time (s) | Detection Limit (M) | Ref. |
|---|---|---|---|---|
| PG K/ISE | 10−5~10−2 | 20 | 10−5.65 | [38] |
| Valinomycin-doped K-ISE | 10−3.1~10−1 | - | 10−3.1 | [40] |
| MoS2-based K-SC-ISE | 10−5~10−2 | - | 10−5.5 | [41] |
| K-SC-ISE | 10−4.5~10−1 | 10 | 10−4.5 | [42] |
| SPE/PANI/V | 10−5~1 | - | 10−5.8 | [43] |
| K-ISE-dPAD | 10−4~10−1 | - | 10−5 | [44] |
| SPE/OTS-Ti3C2/AuNPs/K-ISM | 10−5~10−1 | 15 | 10−5.2 | This work |
| No. | Added (mM) | Measured (mM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | - | 4.8 ± 0.3 | - | 2.9 |
| 2 | 5 | 9.6 ± 0.4 | 98.0 | 5.8 |
| 3 | 10 | 14.4 ± 0.6 | 97.3 | 6.5 |
| 4 | 15 | 19.1 ± 0.3 | 96.5 | 3.3 |
| 5 | 20 | 24.6 ± 0.6 | 99.2 | 6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Wang, H.; He, Y.; Chen, D.; Chen, R.; Tang, X.; Zhou, M.; Yao, J.; Xiong, B. Application of a Screen-Printed Ion-Selective Electrode Based on Hydrophobic Ti3C2/AuNPs for K+ Determination Across Variable Temperatures. Int. J. Mol. Sci. 2024, 25, 13204. https://doi.org/10.3390/ijms252313204
Yu Z, Wang H, He Y, Chen D, Chen R, Tang X, Zhou M, Yao J, Xiong B. Application of a Screen-Printed Ion-Selective Electrode Based on Hydrophobic Ti3C2/AuNPs for K+ Determination Across Variable Temperatures. International Journal of Molecular Sciences. 2024; 25(23):13204. https://doi.org/10.3390/ijms252313204
Chicago/Turabian StyleYu, Zhixue, Hui Wang, Yue He, Dongfei Chen, Ruipeng Chen, Xiangfang Tang, Mengting Zhou, Junhu Yao, and Benhai Xiong. 2024. "Application of a Screen-Printed Ion-Selective Electrode Based on Hydrophobic Ti3C2/AuNPs for K+ Determination Across Variable Temperatures" International Journal of Molecular Sciences 25, no. 23: 13204. https://doi.org/10.3390/ijms252313204
APA StyleYu, Z., Wang, H., He, Y., Chen, D., Chen, R., Tang, X., Zhou, M., Yao, J., & Xiong, B. (2024). Application of a Screen-Printed Ion-Selective Electrode Based on Hydrophobic Ti3C2/AuNPs for K+ Determination Across Variable Temperatures. International Journal of Molecular Sciences, 25(23), 13204. https://doi.org/10.3390/ijms252313204







