Diacylglycerol Kinases and Its Role in Lipid Metabolism and Related Diseases
Abstract
1. Introduction
2. Types of Diacylglycerol Kinase and Their Structural Features
3. Expression and Subcellular Localization of DGKs
4. DGKs in Lipid Metabolism and Signal Transduction
5. Physiological Functions of DGKs
6. Inhibitors of DGKs
7. Future Prospectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Yang, Y.; Lee, M.; Fairn, G.D. Phospholipid subcellular localization and dynamics. J. Biol. Chem. 2018, 293, 6230–6240. [Google Scholar] [CrossRef]
- Wang, H.; Airola, M.V.; Reue, K. How lipid droplets “TAG” along: Glycerolipid synthetic enzymes and lipid storage. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1131–1145. [Google Scholar] [CrossRef]
- Sahu, U.; Villa, E.; Reczek, C.R.; Zhao, Z.B.; O’Hara, B.P.; Torno, M.D.; Mishra, R.; Shannon, W.D.; Asara, J.M.; Gao, P.; et al. Pyrimidines maintain mitochondrial pyruvate oxidation to support de novo lipogenesis. Science 2024, 383, 1484–1492. [Google Scholar] [CrossRef]
- Possik, E.; Klein, L.L.; Sanjab, P.; Zhu, R.; Cote, L.; Bai, Y.; Zhang, D.; Sun, H.; Al-Mass, A.; Oppong, A.; et al. Glycerol 3-phosphate phosphatase/PGPH-2 counters metabolic stress and promotes healthy aging via a glycogen sensing-AMPK-HLH-30-autophagy axis in C. elegans. Nat. Commun. 2023, 14, 5214. [Google Scholar] [CrossRef]
- Yu, J.; Loh, K.; Song, Z.Y.; Yang, H.Q.; Zhang, Y.; Lin, S. Update on glycerol-3-phosphate acyltransferases: The roles in the development of insulin resistance. Nutr. Diabetes 2018, 8, 34. [Google Scholar] [CrossRef]
- Garg, A.; Agarwal, A.K. Lipodystrophies: Disorders of adipose tissue biology. Biochim. Biophys. Acta 2009, 1791, 507–513. [Google Scholar] [CrossRef]
- Mardian, E.B.; Bradley, R.M.; Aristizabal Henao, J.J.; Marvyn, P.M.; Moes, K.A.; Bombardier, E.; Tupling, A.R.; Stark, K.D.; Duncan, R.E. Agpat4/Lpaatdelta deficiency highlights the molecular heterogeneity of epididymal and perirenal white adipose depots. J. Lipid Res. 2017, 58, 2037–2050. [Google Scholar] [CrossRef]
- Tanaka, Y.; Shimanaka, Y.; Caddeo, A.; Kubo, T.; Mao, Y.; Kubota, T.; Kubota, N.; Yamauchi, T.; Mancina, R.M.; Baselli, G.; et al. LPIAT1/MBOAT7 depletion increases triglyceride synthesis fueled by high phosphatidylinositol turnover. Gut 2021, 70, 180–193. [Google Scholar] [CrossRef]
- Carman, G.M.; Han, G.S. Regulation of phospholipid synthesis in yeast. J. Lipid Res. 2009, 50, S69–S73. [Google Scholar] [CrossRef]
- Valentine, W.J.; Yanagida, K.; Kawana, H.; Kono, N.; Noda, N.N.; Aoki, J.; Shindou, H. Update and nomenclature proposal for mammalian lysophospholipid acyltransferases, which create membrane phospholipid diversity. J. Biol. Chem. 2022, 298, 101470. [Google Scholar] [CrossRef]
- Lim, S.A.; Su, W.; Chapman, N.M.; Chi, H. Lipid metabolism in T cell signaling and function. Nat. Chem. Biol. 2022, 18, 470–481. [Google Scholar] [CrossRef]
- Mutlu, A.S.; Duffy, J.; Wang, M.C. Lipid metabolism and lipid signals in aging and longevity. Dev. Cell 2021, 56, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, K.; Nzirorera, C.; Kienesberger, P.C. Lipid metabolism and signaling in cardiac lipotoxicity. Biochim. Biophys. Acta 2016, 1861, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Bustos, V.; Partridge, L. Good Ol’ Fat: Links between Lipid Signaling and Longevity. Trends Biochem. Sci. 2017, 42, 812–823. [Google Scholar] [CrossRef]
- DeBose-Boyd, R.A.; Ye, J. SREBPs in Lipid Metabolism, Insulin Signaling, and Beyond. Trends Biochem. Sci. 2018, 43, 358–368. [Google Scholar] [CrossRef]
- Balla, T. Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiol. Rev. 2013, 93, 1019–1137. [Google Scholar] [CrossRef] [PubMed]
- Sakane, F.; Mizuno, S.; Takahashi, D.; Sakai, H. Where do substrates of diacylglycerol kinases come from? Diacylglycerol kinases utilize diacylglycerol species supplied from phosphatidylinositol turnover-independent pathways. Adv. Biol. Regul. 2018, 67, 101–108. [Google Scholar] [CrossRef]
- Jenkins, G.M.; Frohman, M.A. Phospholipase D: A lipid centric review. Cell. Mol. Life Sci. 2005, 62, 2305–2316. [Google Scholar] [CrossRef]
- Zhong, X.P.; Guo, R.; Zhou, H.; Liu, C.; Wan, C.K. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol. Rev. 2008, 224, 249–264. [Google Scholar] [CrossRef]
- Topham, M.K. Signaling roles of diacylglycerol kinases. J. Cell. Biochem. 2006, 97, 474–484. [Google Scholar] [CrossRef]
- Baldanzi, G.; Ragnoli, B.; Malerba, M. Potential role of diacylglycerol kinases in immune-mediated diseases. Clin. Sci. 2020, 134, 1637–1658. [Google Scholar] [CrossRef]
- Sim, J.A.; Kim, J.; Yang, D. Beyond Lipid Signaling: Pleiotropic Effects of Diacylglycerol Kinases in Cellular Signaling. Int. J. Mol. Sci. 2020, 21, 6861. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Prescott, S.M.; Topham, M.K. Association of diacylglycerol kinase zeta with protein kinase C alpha: Spatial regulation of diacylglycerol signaling. J. Cell Biol. 2003, 160, 929–937. [Google Scholar] [CrossRef]
- Regier, D.S.; Higbee, J.; Lund, K.M.; Sakane, F.; Prescott, S.M.; Topham, M.K. Diacylglycerol kinase iota regulates Ras guanyl-releasing protein 3 and inhibits Rap1 signaling. Proc. Natl. Acad. Sci. USA 2005, 102, 7595–7600. [Google Scholar] [CrossRef] [PubMed]
- Avila-Flores, A.; Santos, T.; Rincon, E.; Merida, I. Modulation of the mammalian target of rapamycin pathway by diacylglycerol kinase-produced phosphatidic acid. J. Biol. Chem. 2005, 280, 10091–10099. [Google Scholar] [CrossRef] [PubMed]
- Wichroski, M.; Benci, J.; Liu, S.Q.; Chupak, L.; Fang, J.; Cao, C.; Wang, C.; Onorato, J.; Qiu, H.; Shan, Y.; et al. DGKalpha/zeta inhibitors combine with PD-1 checkpoint therapy to promote T cell-mediated antitumor immunity. Sci. Transl. Med. 2023, 15, eadh1892. [Google Scholar] [CrossRef] [PubMed]
- Zadoorian, A.; Du, X.; Yang, H. Lipid droplet biogenesis and functions in health and disease. Nat. Rev. Endocrinol. 2023, 19, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Mathiowetz, A.J.; Olzmann, J.A. Lipid droplets and cellular lipid flux. Nat. Cell Biol. 2024, 26, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T. Biosynthesis and biology of mammalian GPI-anchored proteins. Open Biol. 2020, 10, 190290. [Google Scholar] [CrossRef]
- Turban, S.; Hajduch, E. Protein kinase C isoforms: Mediators of reactive lipid metabolites in the development of insulin resistance. FEBS Lett. 2011, 585, 269–274. [Google Scholar] [CrossRef]
- Hernandez-Lara, M.A.; Yadav, S.K.; Conaway, S., Jr.; Shah, S.D.; Penn, R.B.; Deshpande, D.A. Crosstalk between diacylglycerol kinase and protein kinase A in the regulation of airway smooth muscle cell proliferation. Respir. Res. 2023, 24, 155. [Google Scholar] [CrossRef]
- Griner, E.M.; Kazanietz, M.G. Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer 2007, 7, 281–294. [Google Scholar] [CrossRef]
- Aulakh, S.S.; Bozelli, J.C., Jr.; Epand, R.M. Exploring the AlphaFold Predicted Conformational Properties of Human Diacylglycerol Kinases. J. Phys. Chem. B 2022, 126, 7172–7183. [Google Scholar] [CrossRef]
- Topham, M.K.; Prescott, S.M. Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. J. Biol. Chem. 1999, 274, 11447–11450. [Google Scholar] [CrossRef]
- Gupta, R.S.; Epand, R.M. Phylogenetic analysis of the diacylglycerol kinase family of proteins and identification of multiple highly-specific conserved inserts and deletions within the catalytic domain that are distinctive characteristics of different classes of DGK homologs. PLoS ONE 2017, 12, e0182758. [Google Scholar] [CrossRef]
- Zambo, B.; Gogl, G.; Morlet, B.; Eberling, P.; Negroni, L.; Moine, H.; Trave, G. Comparative analysis of PDZ-binding motifs in the diacylglycerol kinase family. FEBS J. 2024, 291, 690–704. [Google Scholar] [CrossRef]
- Krishna, S.; Zhong, X.P. Regulation of Lipid Signaling by Diacylglycerol Kinases during T Cell Development and Function. Front. Immunol. 2013, 4, 178. [Google Scholar] [CrossRef]
- Sakane, F.; Mizuno, S.; Komenoi, S. Diacylglycerol Kinases as Emerging Potential Drug Targets for a Variety of Diseases: An Update. Front. Cell Dev. Biol. 2016, 4, 82. [Google Scholar] [CrossRef]
- Sanjuan, M.A.; Jones, D.R.; Izquierdo, M.; Merida, I. Role of diacylglycerol kinase alpha in the attenuation of receptor signaling. J. Cell Biol. 2001, 153, 207–220. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, W.; Hawes, J.W.; Walsh, J.P. A domain with homology to neuronal calcium sensors is required for calcium-dependent activation of diacylglycerol kinase alpha. J. Biol. Chem. 2000, 275, 34092–34099. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamamoto, T.; Sakai, H.; Sakane, F. Calcium negatively regulates an intramolecular interaction between the N-terminal recoverin homology and EF-hand motif domains and the C-terminal C1 and catalytic domains of diacylglycerol kinase alpha. Biochem. Biophys. Res. Commun. 2012, 423, 571–576. [Google Scholar] [CrossRef]
- Merino, E.; Sanjuan, M.A.; Moraga, I.; Cipres, A.; Merida, I. Role of the diacylglycerol kinase alpha-conserved domains in membrane targeting in intact T cells. J. Biol. Chem. 2007, 282, 35396–35404. [Google Scholar] [CrossRef]
- Yamada, K.; Sakane, F.; Matsushima, N.; Kanoh, H. EF-hand motifs of alpha, beta and gamma isoforms of diacylglycerol kinase bind calcium with different affinities and conformational changes. Biochem. J. 1997, 321 Pt 1, 59–64. [Google Scholar] [CrossRef]
- Tanino, F.; Maeda, Y.; Sakai, H.; Sakane, F. Induction of filopodia-like protrusions in N1E-115 neuroblastoma cells by diacylglycerol kinase gamma independent of its enzymatic activity: Potential novel function of the C-terminal region containing the catalytic domain of diacylglycerol kinase gamma. Mol. Cell. Biochem. 2013, 373, 85–93. [Google Scholar] [CrossRef]
- Shulga, Y.V.; Topham, M.K.; Epand, R.M. Regulation and functions of diacylglycerol kinases. Chem. Rev. 2011, 111, 6186–61208. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Imai, S.; Sakane, F.; Kanoh, H. Phorbol ester-regulated oligomerization of diacylglycerol kinase delta linked to its phosphorylation and translocation. J. Biol. Chem. 2002, 277, 35323–35332. [Google Scholar] [CrossRef]
- Knight, M.J.; Joubert, M.K.; Plotkowski, M.L.; Kropat, J.; Gingery, M.; Sakane, F.; Merchant, S.S.; Bowie, J.U. Zinc binding drives sheet formation by the SAM domain of diacylglycerol kinase delta. Biochemistry 2010, 49, 9667–9676. [Google Scholar] [CrossRef]
- Imai, S.; Kai, M.; Yasuda, S.; Kanoh, H.; Sakane, F. Identification and characterization of a novel human type II diacylglycerol kinase, DGK kappa. J. Biol. Chem. 2005, 280, 39870–39881. [Google Scholar] [CrossRef]
- Tang, W.; Bunting, M.; Zimmerman, G.A.; McIntyre, T.M.; Prescott, S.M. Molecular cloning of a novel human diacylglycerol kinase highly selective for arachidonate-containing substrates. FASEB J. 1996, 10, L49. [Google Scholar] [CrossRef]
- Tanimura, A.; Yamazaki, M.; Hashimotodani, Y.; Uchigashima, M.; Kawata, S.; Abe, M.; Kita, Y.; Hashimoto, K.; Shimizu, T.; Watanabe, M.; et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 2010, 65, 320–327. [Google Scholar] [CrossRef]
- Traynor-Kaplan, A.; Kruse, M.; Dickson, E.J.; Dai, G.; Vivas, O.; Yu, H.; Whittington, D.; Hille, B. Fatty-acyl chain profiles of cellular phosphoinositides. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 513–522. [Google Scholar] [CrossRef]
- Bozelli, J.C., Jr.; Yune, J.; Aulakh, S.S.; Cao, Z.; Fernandes, A.; Seitova, A.; Tong, Y.; Schreier, S.; Epand, R.M. Human Diacylglycerol Kinase epsilon N-Terminal Segment Regulates the Phosphatidylinositol Cycle, Controlling the Rate but Not the Acyl Chain Composition of Its Lipid Intermediates. ACS Chem. Biol. 2022, 17, 2495–2506. [Google Scholar] [CrossRef]
- Rodriguez de Turco, E.B.; Tang, W.; Topham, M.K.; Sakane, F.; Marcheselli, V.L.; Chen, C.; Taketomi, A.; Prescott, S.M.; Bazan, N.G. Diacylglycerol kinase epsilon regulates seizure susceptibility and long-term potentiation through arachidonoyl-inositol lipid signaling. Proc. Natl. Acad. Sci. USA 2001, 98, 4740–4745. [Google Scholar] [CrossRef]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.J.; et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Traczyk, G.; Hromada-Judycka, A.; Swiatkowska, A.; Wisniewska, J.; Ciesielska, A.; Kwiatkowska, K. Diacylglycerol kinase-epsilon is S-palmitoylated on cysteine in the cytoplasmic end of its N-terminal transmembrane fragment. J. Lipid Res. 2024, 65, 100480. [Google Scholar] [CrossRef]
- Topham, M.K.; Bunting, M.; Zimmerman, G.A.; McIntyre, T.M.; Blackshear, P.J.; Prescott, S.M. Protein kinase C regulates the nuclear localization of diacylglycerol kinase-zeta. Nature 1998, 394, 697–700. [Google Scholar] [CrossRef]
- Santos, T.; Carrasco, S.; Jones, D.R.; Merida, I.; Eguinoa, A. Dynamics of diacylglycerol kinase zeta translocation in living T-cells. Study of the structural domain requirements for translocation and activity. J. Biol. Chem. 2002, 277, 30300–30309. [Google Scholar] [CrossRef]
- Rincón, E.; Santos, T.; Avila-Flores, A.; Albar, J.P.; Lalioti, V.; Lei, C.; Hong, W.; Mérida, I. Proteomics identification of sorting nexin 27 as a diacylglycerol kinase ζ-associated protein: New diacylglycerol kinase roles in endocytic recycling. Mol. Cell. Proteom. 2007, 6, 1073–1087. [Google Scholar] [CrossRef]
- Hogan, A.; Shepherd, L.; Chabot, J.; Quenneville, S.; Prescott, S.M.; Topham, M.K.; Gee, S.H. Interaction of γ1-syntrophin with diacylglycerol kinase-ζ: Regulation of nuclear localization by PDZ interactions. J. Biol. Chem. 2001, 276, 26526–26533. [Google Scholar] [CrossRef] [PubMed]
- Abramovici, H.; Hogan, A.B.; Obagi, C.; Topham, M.K.; Gee, S.H. Diacylglycerol kinase-ζ localization in skeletal muscle is regulated by phosphorylation and interaction with syntrophins. Mol. Biol. Cell 2003, 14, 4499–4511. [Google Scholar] [CrossRef]
- Abramovici, H.; Gee, S.H. Morphological changes and spatial regulation of diacylglycerol kinase-ζ, syntrophins, and Rac1 during myoblast fusion. Cell Motil. Cytoskel 2007, 64, 549–567. [Google Scholar] [CrossRef]
- Liu, Z.T.; Chang, G.Q.; Leibowitz, S.F. Diacylglycerol kinase zeta in hypothalamus interacts with long form leptin receptor: Relation to dietary fat and body weight regulation. Obes. Res. 2001, 9, 111s. [Google Scholar]
- Kim, K.; Yang, J.H.; Zhong, X.P.; Kim, M.H.; Kim, Y.S.; Lee, H.W.; Han, S.; Choi, J.; Han, K.; Seo, J.; et al. Synaptic removal of diacylglycerol by DGKζ and PSD-95 regulates dendritic spine maintenance. EMBO J. 2009, 28, 1170–1179. [Google Scholar] [CrossRef]
- Los, A.P.; van Baal, J.; de Widt, J.; Divecha, N.; van Blitterswijk, W.J. Structure-activity relationship of diacylglycerol kinase θ. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2004, 1636, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Barbernitz, M.X.; Devine, L.R.; Cole, R.N.; Raben, D.M. The role of N-terminal phosphorylation of DGK-theta. J. Lipid Res. 2024, 65, 100506. [Google Scholar] [CrossRef]
- Zheng, Z.G.; Xu, Y.Y.; Liu, W.P.; Zhang, Y.; Zhang, C.; Liu, H.L.; Zhang, X.Y.; Liu, R.Z.; Zhang, Y.P.; Shi, M.Y.; et al. Discovery of a potent allosteric activator of DGKQ that ameliorates obesity-induced insulin resistance via the sn-1,2-DAG-PKCepsilon signaling axis. Cell Metab. 2023, 35, 101–117 e111. [Google Scholar] [CrossRef]
- van Blitterswijk, W.J.; Houssa, B. Properties and functions of diacylglycerol kinases. Cell. Signal. 2000, 12, 595–605. [Google Scholar] [CrossRef]
- Centonze, S.; Baldanzi, G. Diacylglycerol Kinases in Signal Transduction. Int. J. Mol. Sci. 2022, 23, 8423. [Google Scholar] [CrossRef]
- Merida, I.; Andrada, E.; Gharbi, S.I.; Avila-Flores, A. Redundant and specialized roles for diacylglycerol kinases alpha and zeta in the control of T cell functions. Sci. Signal. 2015, 8, re6. [Google Scholar] [CrossRef] [PubMed]
- Sakane, F.; Imai, S.; Kai, M.; Yasuda, S.; Kanoh, H. Diacylglycerol kinases as emerging potential drug targets for a variety of diseases. Curr. Drug Targets 2008, 9, 626–640. [Google Scholar] [CrossRef]
- Merida, I.; Arranz-Nicolas, J.; Torres-Ayuso, P.; Avila-Flores, A. Diacylglycerol Kinase Malfunction in Human Disease and the Search for Specific Inhibitors. Handb. Exp. Pharmacol. 2020, 259, 133–162. [Google Scholar]
- Kang, H.; Lee, H.; Kim, K.; Shin, E.; Kim, B.; Kang, J.; Kim, B.; Lee, J.S.; Lee, J.M.; Youn, H.; et al. DGKB mediates radioresistance by regulating DGAT1-dependent lipotoxicity in glioblastoma. Cell Rep. Med. 2023, 4, 100880. [Google Scholar] [CrossRef] [PubMed]
- Ishisaka, M.; Hara, H. The roles of diacylglycerol kinases in the central nervous system: Review of genetic studies in mice. J. Pharmacol. Sci. 2014, 124, 336–343. [Google Scholar] [CrossRef]
- Krishna, S.; Zhong, X. Role of diacylglycerol kinases in T cell development and function. Crit. Rev. Immunol. 2013, 33, 97–118. [Google Scholar] [CrossRef]
- Chen, S.S.; Hu, Z.; Zhong, X.P. Diacylglycerol Kinases in T Cell Tolerance and Effector Function. Front. Cell Dev. Biol. 2016, 4, 130. [Google Scholar] [CrossRef]
- Xie, D.; Zhang, S.; Chen, P.; Deng, W.; Pan, Y.; Xie, J.; Wang, J.; Liao, B.; Sleasman, J.W.; Zhong, X.P. Negative control of diacylglycerol kinase zeta-mediated inhibition of T cell receptor signaling by nuclear sequestration in mice. Eur. J. Immunol. 2020, 50, 1729–1745. [Google Scholar] [CrossRef]
- Yamamoto, M.; Tanaka, T.; Hozumi, Y.; Saino-Saito, S.; Nakano, T.; Tajima, K.; Kato, T.; Goto, K. Expression of mRNAs for the diacylglycerol kinase family in immune cells during an inflammatory reaction. Biomed. Res. 2014, 35, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wattenberg, B.W.; Pitson, S.M.; Raben, D.M. The sphingosine and diacylglycerol kinase superfamily of signaling kinases: Localization as a key to signaling function. J. Lipid Res. 2006, 47, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Naslavsky, N.; Caplan, S. Diacylglycerol kinases in membrane trafficking. Cell. Logist. 2015, 5, e1078431. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Lam, S.M.; Chen, L.; Gao, Y.; Wang, W.; Xu, Y.; Tan, T.; Yu, H.; Zhang, M.; et al. Low-input lipidomics reveals lipid metabolism remodelling during early mammalian embryo development. Nat. Cell Biol. 2024, 26, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, M.J.; Llorente, A.; Petan, T.; Khnykin, D.; Popa, I.; Nikolac Perkovic, M.; Konjevod, M.; Jaganjac, M. The expanding organelle lipidomes: Current knowledge and challenges. Cell. Mol. Life Sci. 2023, 80, 237. [Google Scholar] [CrossRef]
- Jackson, C.L.; Walch, L.; Verbavatz, J.M. Lipids and Their Trafficking: An Integral Part of Cellular Organization. Dev. Cell 2016, 39, 139–153. [Google Scholar] [CrossRef]
- Eichmann, T.O.; Lass, A. DAG tales: The multiple faces of diacylglycerol--stereochemistry, metabolism, and signaling. Cell. Mol. Life Sci. 2015, 72, 3931–3952. [Google Scholar] [CrossRef]
- Muro, E.; Atilla-Gokcumen, G.E.; Eggert, U.S. Lipids in cell biology: How can we understand them better? Mol. Biol. Cell 2014, 25, 1819–1823. [Google Scholar] [CrossRef]
- Ganesan, S.; Shabits, B.N.; Zaremberg, V. Tracking Diacylglycerol and Phosphatidic Acid Pools in Budding Yeast. Lipid Insights 2015, 8, 75–85. [Google Scholar] [CrossRef]
- Brindley, D.N.; Pilquil, C.; Sariahmetoglu, M.; Reue, K. Phosphatidate degradation: Phosphatidate phosphatases (lipins) and lipid phosphate phosphatases. Biochim. Biophys. Acta 2009, 1791, 956–961. [Google Scholar] [CrossRef]
- Zhang, P.; Csaki, L.S.; Ronquillo, E.; Baufeld, L.J.; Lin, J.Y.; Gutierrez, A.; Dwyer, J.R.; Brindley, D.N.; Fong, L.G.; Tontonoz, P.; et al. Lipin 2/3 phosphatidic acid phosphatases maintain phospholipid homeostasis to regulate chylomicron synthesis. J. Clin. Investig. 2019, 129, 281–295. [Google Scholar] [CrossRef]
- Nakano, T.; Goto, K. Diacylglycerol Kinase epsilon in Adipose Tissues: A Crosstalk Between Signal Transduction and Energy Metabolism. Front. Physiol. 2022, 13, 815085. [Google Scholar] [CrossRef]
- Shulga, Y.V.; Topham, M.K.; Epand, R.M. Study of arachidonoyl specificity in two enzymes of the PI cycle. J. Mol. Biol. 2011, 409, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Fujita, M. Biosynthesis of GPI-anchored proteins: Special emphasis on GPI lipid remodeling. J. Lipid Res. 2016, 57, 6–24. [Google Scholar] [CrossRef]
- Blunsom, N.J.; Cockcroft, S. Phosphatidylinositol synthesis at the endoplasmic reticulum. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158471. [Google Scholar] [CrossRef]
- Zechner, R.; Madeo, F.; Kratky, D. Cytosolic lipolysis and lipophagy: Two sides of the same coin. Nat. Rev. Mol. Cell Biol. 2017, 18, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Posor, Y.; Jang, W.; Haucke, V. Phosphoinositides as membrane organizers. Nat. Rev. Mol. Cell Biol. 2022, 23, 797–816. [Google Scholar] [CrossRef]
- Chen, G.; Harwood, J.L.; Lemieux, M.J.; Stone, S.J.; Weselake, R.J. Acyl-CoA:diacylglycerol acyltransferase: Properties, physiological roles, metabolic engineering and intentional control. Prog. Lipid Res. 2022, 88, 101181. [Google Scholar]
- Mueller, D.L. Linking diacylglycerol kinase to T cell anergy. Nat. Immunol. 2006, 7, 1132–1134. [Google Scholar] [CrossRef]
- Olenchock, B.A.; Guo, R.; Carpenter, J.H.; Jordan, M.; Topham, M.K.; Koretzky, G.A.; Zhong, X.P. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat. Immunol. 2006, 7, 1174–1181. [Google Scholar] [CrossRef]
- Foster, D.A. Phosphatidic acid signaling to mTOR: Signals for the survival of human cancer cells. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2009, 1791, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.Y.; Kim, Y.Y.; Yu, H.S.; Lee, M.; Kim, S.; Lee, J. CRISPR/Cas9-Mediated Knockout of DGK Improves Antitumor Activities of Human T Cells. Cancer Res. 2018, 78, 4692–4703. [Google Scholar] [CrossRef]
- Arranz-Nicolas, J.; Martin-Salgado, M.; Adan-Barrientos, I.; Liebana, R.; Del Carmen Moreno-Ortiz, M.; Leitner, J.; Steinberger, P.; Avila-Flores, A.; Merida, I. Diacylglycerol kinase alpha inhibition cooperates with PD-1-targeted therapies to restore the T cell activation program. Cancer Immunol. Immunother. 2021, 70, 3277–3289. [Google Scholar] [CrossRef]
- Arranz-Nicolas, J.; Ogando, J.; Soutar, D.; Arcos-Perez, R.; Meraviglia-Crivelli, D.; Manes, S.; Merida, I.; Avila-Flores, A. Diacylglycerol kinase alpha inactivation is an integral component of the costimulatory pathway that amplifies TCR signals. Cancer Immunol. Immunother. 2018, 67, 965–980. [Google Scholar] [CrossRef]
- Zhong, X.P.; Hainey, E.A.; Olenchock, B.A.; Zhao, H.; Topham, M.K.; Koretzky, G.A. Regulation of T cell receptor-induced activation of the Ras-ERK pathway by diacylglycerol kinase zeta. J. Biol. Chem. 2002, 277, 31089–31098. [Google Scholar] [CrossRef]
- Manigat, L.C.; Granade, M.E.; Taori, S.; Miller, C.A.; Vass, L.R.; Zhong, X.P.; Harris, T.E.; Purow, B.W. Loss of Diacylglycerol Kinase alpha Enhances Macrophage Responsiveness. Front. Immunol. 2021, 12, 722469. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, M.A.; Pradet-Balade, B.; Jones, D.R.; Martinez, A.C.; Stone, J.C.; Garcia-Sanz, J.A.; Merida, I. T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: A novel mechanism for Ras attenuation. J. Immunol. 2003, 170, 2877–2883. [Google Scholar] [CrossRef]
- Valdor, R.; Macian, F. Induction and stability of the anergic phenotype in T cells. Semin. Immunol. 2013, 25, 313–320. [Google Scholar] [CrossRef]
- Chauveau, A.; Le Floc’h, A.; Bantilan, N.S.; Koretzky, G.A.; Huse, M. Diacylglycerol kinase alpha establishes T cell polarity by shaping diacylglycerol accumulation at the immunological synapse. Sci. Signal. 2014, 7, ra82. [Google Scholar] [CrossRef]
- Hansell, N.K.; Halford, G.S.; Andrews, G.; Shum, D.H.; Harris, S.E.; Davies, G.; Franic, S.; Christoforou, A.; Zietsch, B.; Painter, J.; et al. Genetic basis of a cognitive complexity metric. PLoS ONE 2015, 10, e0123886. [Google Scholar] [CrossRef]
- Kai, M.; Yamamoto, E.; Sato, A.; Yamano, H.O.; Niinuma, T.; Kitajima, H.; Harada, T.; Aoki, H.; Maruyama, R.; Toyota, M.; et al. Epigenetic silencing of diacylglycerol kinase gamma in colorectal cancer. Mol. Carcinog. 2017, 56, 1743–1752. [Google Scholar] [CrossRef]
- Leach, N.T.; Sun, Y.; Michaud, S.; Zheng, Y.; Ligon, K.L.; Ligon, A.H.; Sander, T.; Korf, B.R.; Lu, W.; Harris, D.J.; et al. Disruption of diacylglycerol kinase delta (DGKD) associated with seizures in humans and mice. Am. J. Hum. Genet. 2007, 80, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Baum, A.E.; Akula, N.; Cabanero, M.; Cardona, I.; Corona, W.; Klemens, B.; Schulze, T.G.; Cichon, S.; Rietschel, M.; Nothen, M.M.; et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol. Psychiatry 2008, 13, 197–207. [Google Scholar] [CrossRef] [PubMed]
- van der Zanden, L.F.; van Rooij, I.A.; Feitz, W.F.; Knight, J.; Donders, A.R.; Renkema, K.Y.; Bongers, E.M.; Vermeulen, S.H.; Kiemeney, L.A.; Veltman, J.A.; et al. Common variants in DGKK are strongly associated with risk of hypospadias. Nat. Genet. 2011, 43, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Tabet, R.; Moutin, E.; Becker, J.A.; Heintz, D.; Fouillen, L.; Flatter, E.; Krezel, W.; Alunni, V.; Koebel, P.; Dembele, D.; et al. Fragile X Mental Retardation Protein (FMRP) controls diacylglycerol kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2016, 113, E3619–E3628. [Google Scholar] [CrossRef]
- Lemaire, M.; Fremeaux-Bacchi, V.; Schaefer, F.; Choi, M.; Tang, W.H.; Le Quintrec, M.; Fakhouri, F.; Taque, S.; Nobili, F.; Martinez, F.; et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat. Genet. 2013, 45, 531–536. [Google Scholar] [CrossRef]
- Ozaltin, F.; Li, B.; Rauhauser, A.; An, S.W.; Soylemezoglu, O.; Gonul, I.I.; Taskiran, E.Z.; Ibsirlioglu, T.; Korkmaz, E.; Bilginer, Y.; et al. DGKE variants cause a glomerular microangiopathy that mimics membranoproliferative GN. J. Am. Soc. Nephrol. 2013, 24, 377–384. [Google Scholar] [CrossRef]
- Quaggin, S.E. DGKE and atypical HUS. Nat. Genet. 2013, 45, 475–476. [Google Scholar] [CrossRef]
- Westland, R.; Bodria, M.; Carrea, A.; Lata, S.; Scolari, F.; Fremeaux-Bacchi, V.; D’Agati, V.D.; Lifton, R.P.; Gharavi, A.G.; Ghiggeri, G.M.; et al. Phenotypic expansion of DGKE-associated diseases. J. Am. Soc. Nephrol. 2014, 25, 1408–1414. [Google Scholar] [CrossRef]
- Palma, L.M.P.; Vaisbich-Guimaraes, M.H.; Sridharan, M.; Tran, C.L.; Sethi, S. Thrombotic microangiopathy in children. Pediatr. Nephrol. 2022, 37, 1967–1980. [Google Scholar] [CrossRef]
- Dai, X.; Ma, Y.; Lin, Q.; Tang, H.; Chen, R.; Zhu, Y.; Shen, Y.; Cui, N.; Hong, Z.; Li, Y.; et al. Clinical features and management of atypical hemolytic uremic syndrome patient with DGKE gene variants: A case report. Front. Pediatr. 2023, 11, 1162974. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kato, H.; Ikeda, Y.; Nangaku, M. Pathogenesis of Atypical Hemolytic Uremic Syndrome. J. Atheroscler. Thromb. 2019, 26, 99–110. [Google Scholar] [CrossRef]
- Zhu, J.; Chaki, M.; Lu, D.; Ren, C.; Wang, S.S.; Rauhauser, A.; Li, B.; Zimmerman, S.; Jun, B.; Du, Y.; et al. Loss of diacylglycerol kinase epsilon in mice causes endothelial distress and impairs glomerular Cox-2 and PGE2 production. Am. J. Physiol.-Ren. Physiol. 2016, 310, F895–F908. [Google Scholar] [CrossRef]
- Bruneau, S.; Neel, M.; Roumenina, L.T.; Frimat, M.; Laurent, L.; Fremeaux-Bacchi, V.; Fakhouri, F. Loss of DGKepsilon induces endothelial cell activation and death independently of complement activation. Blood 2015, 125, 1038–1046. [Google Scholar] [CrossRef]
- Bezdicka, M.; Pavlicek, P.; Blahova, K.; Hacek, J.; Zieg, J. Various phenotypes of disease associated with mutated DGKE gene. Eur. J. Med. Genet. 2020, 63, 103953. [Google Scholar] [CrossRef]
- Basak, R.; Wang, X.; Keane, C.; Woroniecki, R. Atypical presentation of atypical haemolytic uraemic syndrome. BMJ Case Rep. 2018, 2018, bcr-2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ding, Q.; Dai, D.F.; Padhy, B.; Nayak, M.K.; Li, C.; Purvis, M.; Jin, H.; Shu, C.; Chauhan, A.K.; et al. Loss of diacylglycerol kinase epsilon causes thrombotic microangiopathy by impairing endothelial VEGFA signaling. JCI Insight 2021, 6, e146959. [Google Scholar] [CrossRef]
- Torres-Ayuso, P.; Tello-Lafoz, M.; Merida, I.; Avila-Flores, A. Diacylglycerol kinase-zeta regulates mTORC1 and lipogenic metabolism in cancer cells through SREBP-1. Oncogenesis 2015, 4, e164. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Wu, C.; Zhang, J.; Liu, J.; Zhang, X.; Hao, P.; Zhao, S.; Zhang, Z. Loss of Diacylglycerol Kinase-Zeta Inhibits Cell Proliferation and Survival in Human Gliomas. Mol. Neurobiol. 2016, 53, 5425–5435. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Mulatz, K.; Ard, R.; Nguyen, T.; Gee, S.H. Increased diacylglycerol kinase zeta expression in human metastatic colon cancer cells augments Rho GTPase activity and contributes to enhanced invasion. BMC Cancer 2014, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Kondo, H. Molecular-Cloning and Expression of a 90-Kda Diacylglycerol Kinase That Predominantly Localizes in Neurons. Proc. Natl. Acad. Sci. USA 1993, 90, 7598–7602. [Google Scholar] [CrossRef]
- Adachi, N.; Oyasu, M.; Taniguchi, T.; Yamaguchi, Y.; Takenaka, R.; Shirai, Y.; Saito, N. Immunocytochemical localization of a neuron-specific diacylglycerol kinase β and γ in the developing rat brain. Mol. Brain Res. 2005, 139, 288–299. [Google Scholar] [CrossRef]
- Hozumi, Y.; Fukaya, M.; Adachi, N.; Saito, N.; Otani, K.; Kondo, H.; Watanabe, M.; Goto, K. Diacylglycerol kinase accumulates on the perisynaptic site of medium spiny neurons in the striatum. Eur. J. Neurosci. 2008, 28, 2409–2422. [Google Scholar] [CrossRef]
- Kakefuda, K.; Oyagi, A.; Ishisaka, M.; Tsuruma, K.; Shimazawa, M.; Yokota, K.; Shirai, Y.; Horie, K.; Saito, N.; Takeda, J.; et al. Diacylglycerol kinase beta knockout mice exhibit lithium-sensitive behavioral abnormalities. PLoS ONE 2010, 5, e13447. [Google Scholar] [CrossRef]
- Shirai, Y.; Kouzuki, T.; Kakefuda, K.; Moriguchi, S.; Oyagi, A.; Horie, K.; Morita, S.Y.; Shimazawa, M.; Fukunaga, K.; Takeda, J.; et al. Essential role of neuron-enriched diacylglycerol kinase (DGK), DGKbeta in neurite spine formation, contributing to cognitive function. PLoS ONE 2010, 5, e11602. [Google Scholar] [CrossRef]
- Maeda, Y.; Shibata, K.; Akiyama, R.; Murakami, Y.; Takao, S.; Murakami, C.; Takahashi, D.; Sakai, H.; Sakane, F. Diacylglycerol kinase beta induces filopodium formation via its C1, catalytic and carboxy-terminal domains and interacts with the Rac1-GTPase-activating protein, beta2-chimaerin. Biochem. Biophys. Res. Commun. 2018, 504, 54–60. [Google Scholar] [CrossRef]
- Nakai, H.; Tsumagari, R.; Maruo, K.; Nakashima, A.; Kikkawa, U.; Ueda, S.; Yamanoue, M.; Saito, N.; Takei, N.; Shirai, Y. mTORC1 is involved in DGKbeta-induced neurite outgrowth and spinogenesis. Neurochem. Int. 2020, 134, 104645. [Google Scholar] [CrossRef]
- Goto, K.; Funayama, M.; Kondo, H. Cloning and expression of a cytoskeleton-associated diacylglycerol kinase that is dominantly expressed in cerebellum. Proc. Natl. Acad. Sci. USA 1994, 91, 13042–13046. [Google Scholar] [CrossRef]
- Hozumi, Y.; Nakano, T.; Goto, K. Cellular expression and subcellular localization of diacylglycerol kinase gamma in rat brain. Biomed. Res. 2021, 42, 33–42. [Google Scholar] [CrossRef]
- Tsumagari, R.; Kakizawa, S.; Kikunaga, S.; Fujihara, Y.; Ueda, S.; Yamanoue, M.; Saito, N.; Ikawa, M.; Shirai, Y. DGKgamma Knock-Out Mice Show Impairments in Cerebellar Motor Coordination, LTD, and the Dendritic Development of Purkinje Cells through the Activation of PKCgamma. eNeuro 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, J.; Zhou, S.; Yao, F.; Zhang, R.; You, W.; Dai, J.; Yu, K.; Zhang, Y.; Baheti, T.; et al. Endothelial DGKG promotes tumor angiogenesis and immune evasion in hepatocellular carcinoma. J. Hepatol. 2024, 80, 82–98. [Google Scholar] [CrossRef]
- Manneras-Holm, L.; Kirchner, H.; Bjornholm, M.; Chibalin, A.V.; Zierath, J.R. mRNA expression of diacylglycerol kinase isoforms in insulin-sensitive tissues: Effects of obesity and insulin resistance. Physiol. Rep. 2015, 3, e12372. [Google Scholar] [CrossRef]
- Chibalin, A.V.; Leng, Y.; Vieira, E.; Krook, A.; Bjornholm, M.; Long, Y.C.; Kotova, O.; Zhong, Z.; Sakane, F.; Steiler, T.; et al. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell 2008, 132, 375–386. [Google Scholar] [CrossRef]
- Wada, Y.; Sakiyama, S.; Sakai, H.; Sakane, F. Myristic Acid Enhances Diacylglycerol Kinase delta-Dependent Glucose Uptake in Myotubes. Lipids 2016, 51, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Murakami, C.; Hoshino, F.; Murakami, Y.; Sakane, F. Diacylglycerol kinase delta destabilizes serotonin transporter protein through the ubiquitin-proteasome system. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158608. [Google Scholar]
- Weber, H.; Kittel-Schneider, S.; Gessner, A.; Domschke, K.; Neuner, M.; Jacob, C.P.; Buttenschon, H.N.; Boreatti-Hummer, A.; Volkert, J.; Herterich, S.; et al. Cross-disorder analysis of bipolar risk genes: Further evidence of DGKH as a risk gene for bipolar disorder, but also unipolar depression and adult ADHD. Neuropsychopharmacology 2011, 36, 2076–2085. [Google Scholar] [CrossRef]
- Badner, J.A.; Gershon, E.S. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol. Psychiatry 2002, 7, 405–411. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, T.; Li, T.; Li, Y.; Chen, P.; Zhao, Q.; Liu, J.; Li, J.; Feng, G.; He, L.; et al. Common SNPs and haplotypes in DGKH are associated with bipolar disorder and schizophrenia in the Chinese Han population. Mol. Psychiatry 2011, 16, 473–475. [Google Scholar] [CrossRef]
- Isozaki, T.; Komenoi, S.; Lu, Q.; Usuki, T.; Tomokata, S.; Matsutomo, D.; Sakai, H.; Bando, K.; Kiyonari, H.; Sakane, F. Deficiency of diacylglycerol kinase eta induces lithium-sensitive mania-like behavior. J. Neurochem. 2016, 138, 448–456. [Google Scholar] [CrossRef]
- Komenoi, S.; Suzuki, Y.; Asami, M.; Murakami, C.; Hoshino, F.; Chiba, S.; Takahashi, D.; Kado, S.; Sakane, F. Microarray analysis of gene expression in the diacylglycerol kinase eta knockout mouse brain. Biochem. Biophys. Rep. 2019, 19, 100660. [Google Scholar]
- Yasuda, S.; Kai, M.; Imai, S.; Takeishi, K.; Taketomi, A.; Toyota, M.; Kanoh, H.; Sakane, F. Diacylglycerol kinase eta augments C-Raf activity and B-Raf/C-Raf heterodimerization. J. Biol. Chem. 2009, 284, 29559–29570. [Google Scholar] [CrossRef]
- Suzuki, Y.; Asami, M.; Takahashi, D.; Sakane, F. Diacylglycerol kinase eta colocalizes and interacts with apoptosis signal-regulating kinase 3 in response to osmotic shock. Biochem. Biophys. Rep. 2021, 26, 101006. [Google Scholar]
- Asami, M.; Suzuki, Y.; Sakane, F. Dopamine and the phosphorylated dopamine transporter are increased in the diacylglycerol kinase eta-knockout mouse brain. FEBS Lett. 2021, 595, 1313–1321. [Google Scholar] [CrossRef]
- Sakai, H.; Murakami, C.; Usuki, T.; Lu, Q.; Matsumoto, K.I.; Urano, T.; Sakane, F. Diacylglycerol kinase eta regulates C2C12 myoblast proliferation through the mTOR signaling pathway. Biochimie 2020, 177, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Habbas, K.; Cakil, O.; Zambo, B.; Tabet, R.; Riet, F.; Dembele, D.; Mandel, J.L.; Hocquemiller, M.; Laufer, R.; Piguet, F.; et al. AAV-delivered diacylglycerol kinase DGKk achieves long-term rescue of fragile X syndrome mouse model. EMBO Mol. Med. 2022, 14, e14649. [Google Scholar] [CrossRef] [PubMed]
- Hozyasz, K.K.; Mostowska, A.; Kowal, A.; Mydlak, D.; Tsibulski, A.; Jagodzinski, P.P. Further Evidence of the Association of the Diacylglycerol Kinase Kappa (DGKK) Gene With Hypospadias. Urol. J. 2018, 15, 272–276. [Google Scholar]
- Tu, G.W.; Zhang, Y.; Ma, J.F.; Hou, J.Y.; Hao, G.W.; Su, Y.; Luo, J.C.; Sheng, L.; Luo, Z. Extracellular vesicles derived from CD4+ T cells carry DGKK to promote sepsis-induced lung injury by regulating oxidative stress and inflammation. Cell. Mol. Biol. Lett. 2023, 28, 24. [Google Scholar] [CrossRef]
- Lung, M.; Shulga, Y.V.; Ivanova, P.T.; Myers, D.S.; Milne, S.B.; Brown, H.A.; Topham, M.K.; Epand, R.M. Diacylglycerol kinase epsilon is selective for both acyl chains of phosphatidic acid or diacylglycerol. J. Biol. Chem. 2009, 284, 31062–31073. [Google Scholar] [CrossRef]
- Zhang, N.; Li, B.; Al-Ramahi, I.; Cong, X.; Held, J.M.; Kim, E.; Botas, J.; Gibson, B.W.; Ellerby, L.M. Inhibition of lipid signaling enzyme diacylglycerol kinase epsilon attenuates mutant huntingtin toxicity. J. Biol. Chem. 2012, 287, 21204–21213. [Google Scholar] [CrossRef]
- Nakano, T.; Seino, K.; Wakabayashi, I.; Stafforini, D.M.; Topham, M.K.; Goto, K. Deletion of diacylglycerol kinase epsilon confers susceptibility to obesity via reduced lipolytic activity in murine adipocytes. FASEB J. 2018, 32, 4121–4131. [Google Scholar] [CrossRef] [PubMed]
- Alabdulqader, M.; Alfakeeh, K. A patient with a homozygous diacylglycerol kinase epsilon (DGKE) gene mutation with atypical haemolytic uraemic syndrome and low C3 responded well to eculizumab: A case report. BMC Nephrol. 2021, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, Z.; Zhang, Y.; Zuo, F.; Du, J.; Wang, M.; Hu, M.; Sun, Y.; Wang, X.; Liu, M.; et al. Diacylglycerol kinase epsilon protects against renal ischemia/reperfusion injury in mice through Kruppel-like factor 15/klotho pathway. Ren. Fail. 2022, 44, 902–913. [Google Scholar] [CrossRef]
- Singh, B.K.; Lu, W.; Paustian, A.M.S.; Ge, M.Q.; Koziol-White, C.J.; Flayer, C.H.; Killingbeck, S.S.; Wang, N.D.; Dong, X.Z.; Riese, M.J.; et al. Diacylglycerol kinase ζ promotes allergic airway inflammation and airway hyperresponsiveness through distinct mechanisms. Sci. Signal. 2019, 12, eaax3332. [Google Scholar] [CrossRef]
- Singh, B.K.; Yokoyama, Y.; Tanaka, Y.; Laczko, D.; Deshpande, D.A.; Kambayashi, T. Diacylglycerol kinase zeta deficiency attenuates papain-induced type 2 airway inflammation. Cell. Immunol. 2023, 393, 104780. [Google Scholar] [CrossRef]
- Arranz-Nicolas, J.; Martin-Salgado, M.; Rodriguez-Rodriguez, C.; Liebana, R.; Moreno-Ortiz, M.C.; Leitner, J.; Steinberger, P.; Avila-Flores, A.; Merida, I. Diacylglycerol kinase zeta limits IL-2-dependent control of PD-1 expression in tumor-infiltrating T lymphocytes. J. Immunother. Cancer 2020, 8, e001521. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, H.; Li, X.; Liu, Q.; Liu, Y.; Hou, Y.; Jin, W. DGKZ promotes TGFbeta signaling pathway and metastasis in triple-negative breast cancer by suppressing lipid raft-dependent endocytosis of TGFbetaR2. Cell Death Dis. 2022, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhao, J.; Luo, C.; Zhu, Z. Overexpression of DGKI in Gastric Cancer Predicts Poor Prognosis. Front. Med. 2020, 7, 320. [Google Scholar] [CrossRef]
- Bartsch, V.B.; Niehaus, J.K.; Taylor-Blake, B.; Zylka, M.J. Enhanced histamine-induced itch in diacylglycerol kinase iota knockout mice. PLoS ONE 2019, 14, e0217819. [Google Scholar] [CrossRef]
- Bartsch, V.B.; Lord, J.S.; Diering, G.H.; Zylka, M.J. Mania- and anxiety-like behavior and impaired maternal care in female diacylglycerol kinase eta and iota double knockout mice. Genes. Brain Behav. 2020, 19, e12570. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, H.L.; Tu-Sekine, B.; Volk, L.; Anggono, V.; Huganir, R.L.; Raben, D.M. DGKθ Catalytic Activity Is Required for Efficient Recycling of Presynaptic Vesicles at Excitatory Synapses. Cell Rep. 2016, 14, 200–207. [Google Scholar] [CrossRef]
- Tu-Sekine, B.; Ostroski, M.; Raben, D.M. Modulation of diacylglycerol kinase θ activity by α-thrombin and phospholipids. Biochemistry 2007, 46, 924–932. [Google Scholar] [CrossRef]
- Tu-Sekine, B.; Raben, D.M. Characterization of cellular DGK-theta. Adv. Enzym. Regul. 2010, 50, 81–94. [Google Scholar] [CrossRef][Green Version]
- Barber, C.N.; Goldschmidt, H.L.; Ma, Q.Q.; Devine, L.R.; Cole, R.N.; Huganir, R.L.; Raben, D.M. Identification of Synaptic DGKθ Interactors That Stimulate DGKθ Activity. Front. Synaptic Neurosci. 2022, 14, 855673. [Google Scholar] [CrossRef]
- Lim, J.L.; Ng, E.Y.; Lim, S.Y.; Tan, A.H.; Abdul-Aziz, Z.; Ibrahim, K.A.; Gopalai, A.A.; Tay, Y.W.; Vijayanathan, Y.; Toh, T.S.; et al. Association study of MCCC1/LAMP3 and DGKQ variants with Parkinson’s disease in patients of Malay ancestry. Neurol. Sci. 2021, 42, 4203–4207. [Google Scholar] [CrossRef]
- Boroda, S.; Niccum, M.; Raje, V.; Purow, B.W.; Harris, T.E. Dual activities of ritanserin and R59022 as DGKalpha inhibitors and serotonin receptor antagonists. Biochem. Pharmacol. 2017, 123, 29–39. [Google Scholar] [CrossRef]
- Sato, M.; Liu, K.; Sasaki, S.; Kunii, N.; Sakai, H.; Mizuno, H.; Saga, H.; Sakane, F. Evaluations of the selectivities of the diacylglycerol kinase inhibitors R59022 and R59949 among diacylglycerol kinase isozymes using a new non-radioactive assay method. Pharmacology 2013, 92, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhong, M.; Hu, Y.; Pan, G.; Yao, J.; Tang, Y.; Duan, H.; Jiang, Y.; Shan, W.; Lin, J.; et al. Ritanserin suppresses acute myeloid leukemia by inhibiting DGKalpha to downregulate phospholipase D and the Jak-Stat/MAPK pathway. Discov. Oncol. 2023, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Velnati, S.; Ruffo, E.; Massarotti, A.; Talmon, M.; Varma, K.S.S.; Gesu, A.; Fresu, L.G.; Snow, A.L.; Bertoni, A.; Capello, D.; et al. Identification of a novel DGKalpha inhibitor for XLP-1 therapy by virtual screening. Eur. J. Med. Chem. 2019, 164, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Velnati, S.; Massarotti, A.; Antona, A.; Talmon, M.; Fresu, L.G.; Galetto, A.S.; Capello, D.; Bertoni, A.; Mercalli, V.; Graziani, A.; et al. Structure activity relationship studies on Amb639752: Toward the identification of a common pharmacophoric structure for DGKalpha inhibitors. J. Enzym. Inhib. Med. Chem. 2020, 35, 96–108. [Google Scholar] [CrossRef]
- Abdel-Magid, A.F. Cancer Immunotherapy through the Inhibition of Diacylglycerol Kinases Alpha and Zeta. ACS Med. Chem. Lett. 2020, 11, 1083–1085. [Google Scholar] [CrossRef]
- Liu, K.; Kunii, N.; Sakuma, M.; Yamaki, A.; Mizuno, S.; Sato, M.; Sakai, H.; Kado, S.; Kumagai, K.; Kojima, H.; et al. A novel diacylglycerol kinase alpha-selective inhibitor, CU-3, induces cancer cell apoptosis and enhances immune response. J. Lipid Res. 2016, 57, 368–379. [Google Scholar] [CrossRef]
- Murakami, Y.; Murakami, C.; Hoshino, F.; Lu, Q.; Akiyama, R.; Yamaki, A.; Takahashi, D.; Sakane, F. Palmitic acid- and/or palmitoleic acid-containing phosphatidic acids are generated by diacylglycerol kinase alpha in starved Jurkat T cells. Biochem. Biophys. Res. Commun. 2020, 525, 1054–1060. [Google Scholar] [CrossRef]
- Yamaki, A.; Akiyama, R.; Murakami, C.; Takao, S.; Murakami, Y.; Mizuno, S.; Takahashi, D.; Kado, S.; Taketomi, A.; Shirai, Y.; et al. Diacylglycerol kinase alpha-selective inhibitors induce apoptosis and reduce viability of melanoma and several other cancer cell lines. J. Cell. Biochem. 2019, 120, 10043–10056. [Google Scholar] [CrossRef] [PubMed]
- Okumura, M.; Yokoyama, Y.; Yoshida, T.; Okada, Y.; Takizawa, M.; Ikeda, O.; Kambayashi, T. The diacylglycerol kinase zeta inhibitor ASP1570 augments natural killer cell function. Int. Immunopharmacol. 2023, 125, 111145. [Google Scholar] [CrossRef]
- Noessner, E. DGK-alpha: A Checkpoint in Cancer-Mediated Immuno-Inhibition and Target for Immunotherapy. Front. Cell Dev. Biol. 2017, 5, 16. [Google Scholar] [CrossRef]
- Singaram, I.; Sharma, A.; Pant, S.; Lihan, M.; Park, M.J.; Pergande, M.; Buwaneka, P.; Hu, Y.; Mahmud, N.; Kim, Y.M.; et al. Targeting lipid-protein interaction to treat Syk-mediated acute myeloid leukemia. Nat. Chem. Biol. 2023, 19, 239–250. [Google Scholar] [CrossRef]
- Nakano, T.; Matsui, H.; Tanaka, T.; Hozumi, Y.; Iseki, K.; Kawamae, K.; Goto, K. Arachidonoyl-Specific Diacylglycerol Kinase epsilon and the Endoplasmic Reticulum. Front. Cell Dev. Biol. 2016, 4, 132. [Google Scholar] [CrossRef]

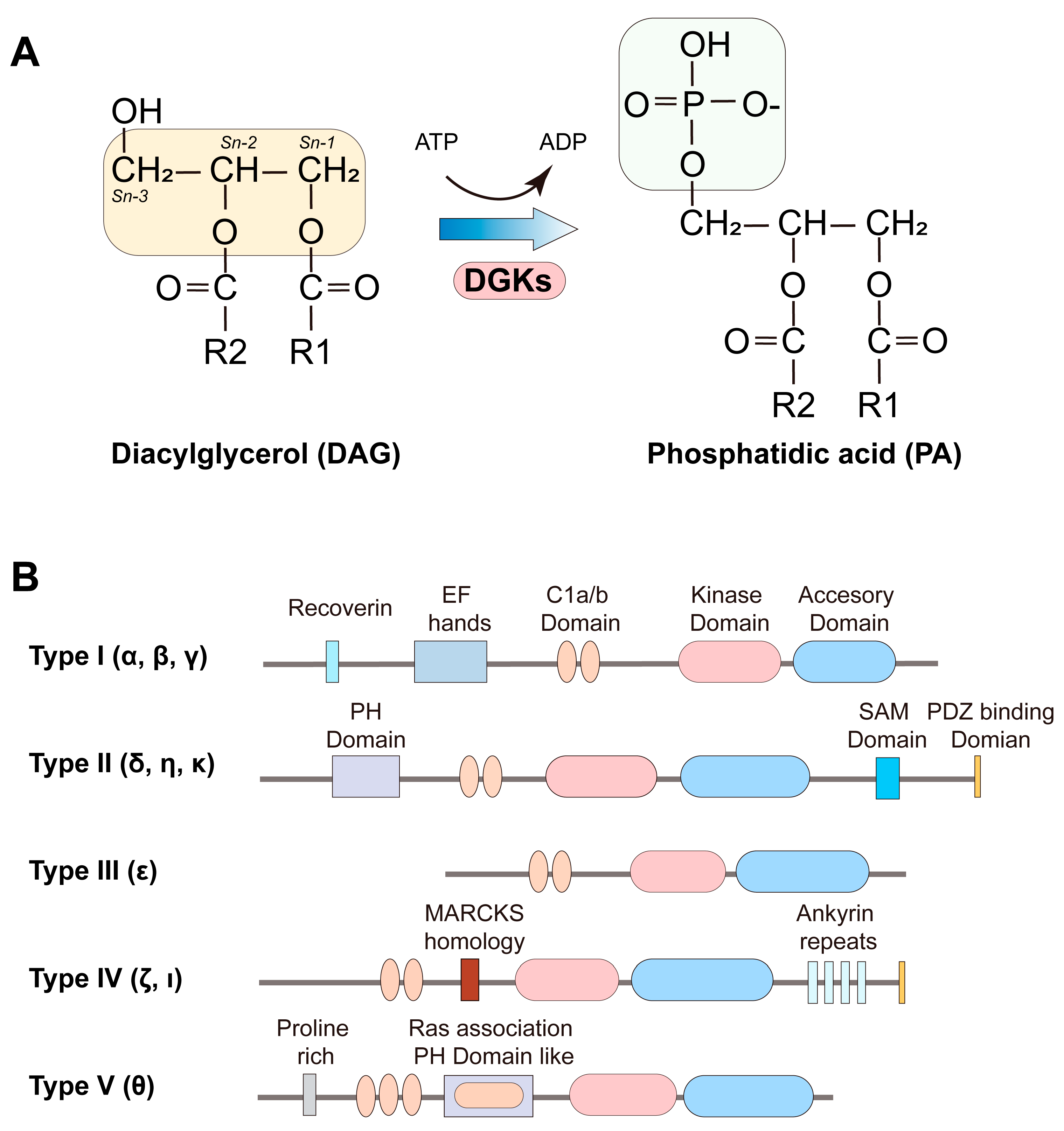

| Gene | Substrate Specificity | Main Subcellular Localization | |
|---|---|---|---|
| DGKα | DGKA | Non-specific | Cytoplasmic |
| DGKβ | DGKB | Non-specific | Postsynaptic membrane, Cytoplasmic |
| DGKγ | DGKG | Non-specific | Golgi apparatus, Cytoplasm |
| DGKδ | DGKD | Non-specific | Plasma membrane |
| DGKη | DGKH | Non-specific | Plasma membrane |
| DGKκ | DGKK | Non-specific | Plasma membrane |
| DGKε | DGKE | sn-2-arachidonoyl (20:4)-DG | Endoplasmic reticulum |
| DGKζ | DGKZ | Non-specific | Nucleus and Plasma membrane |
| DGKι | DGKI | Non-specific | Cytoplasm and nucleus |
| DGKθ | DGKQ | Non-specific | Plasma membrane and nucleus |
| Related Disease | Symptoms | Related References | |
|---|---|---|---|
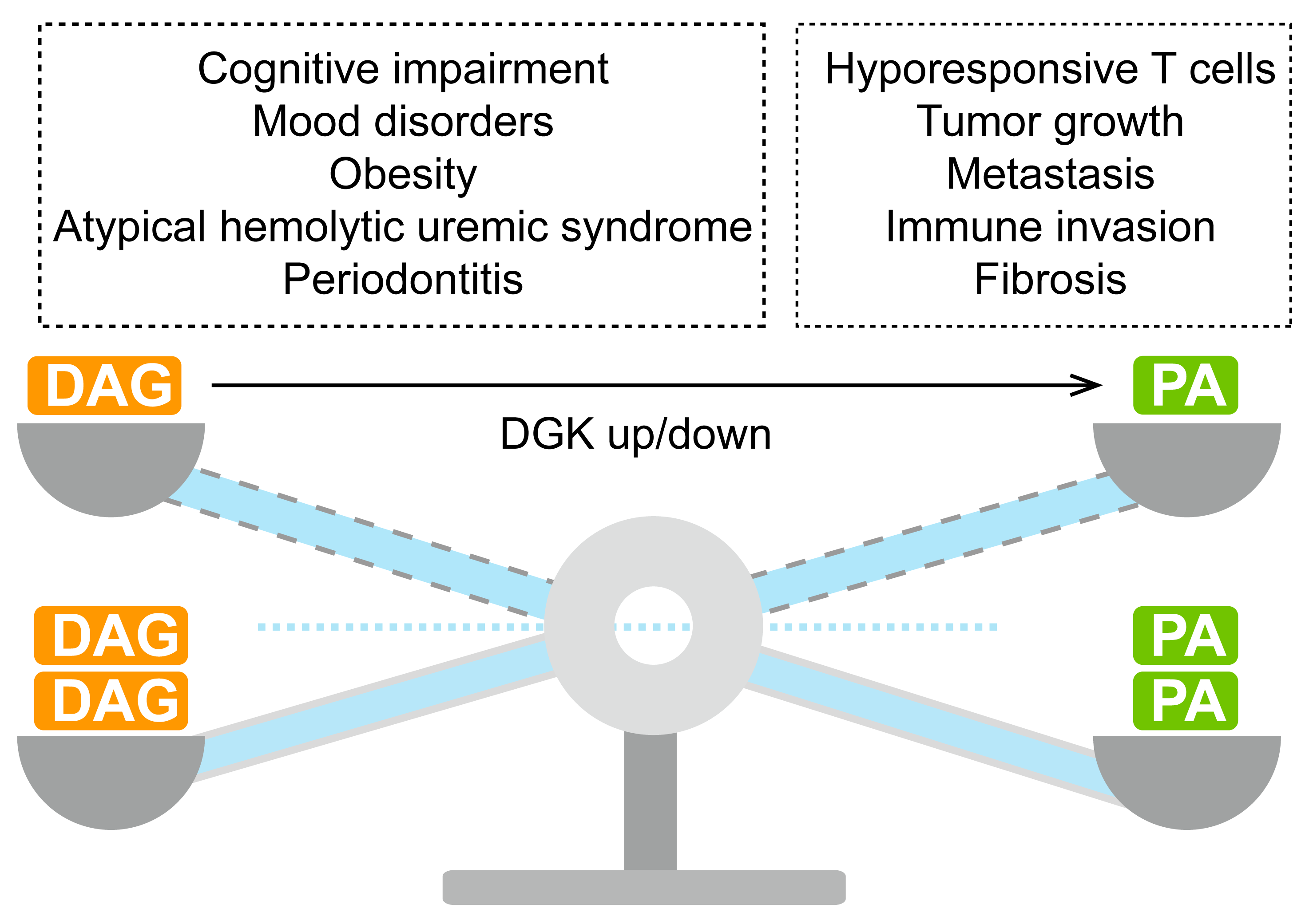
| DGKα | T cell dysfunction; cancer. | T cell hypofunctionality;Tumor growth, invasion and drug resistance; Fibrosis. | [100,104,105,106] |
| DGKβ | Mood disorder. | Cognitive impairment; mania-like behavior. | [107] |
| DGKγ | Colon cancer. | Migration and invasion. | [108] |
| DGKδ | Diabetes; obesity. | Seizures, capillary abnormality, developmental delay, infantile hypotonia and obesity. | [109] |
| DGKη | Bipolar disorder. | Bipolar disorder. | [110] |
| DGKκ | Hypospadias. | Intellectual disability; autism. | [111,112] |
| DGKε | aHUS. | Thrombotic microangiopathy, hemoglobin, microangiopathy. | [113,114,115,116,117,118,119,120,121,122,123,124] |
| DGKζ | Colon cancer and glioma. | Tumor growth and invasion. | [125,126,127] |
| DGKι | |||
| DGKθ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yang, Z.; Zhou, X.; Li, Z.; Hideki, N. Diacylglycerol Kinases and Its Role in Lipid Metabolism and Related Diseases. Int. J. Mol. Sci. 2024, 25, 13207. https://doi.org/10.3390/ijms252313207
Liu Y, Yang Z, Zhou X, Li Z, Hideki N. Diacylglycerol Kinases and Its Role in Lipid Metabolism and Related Diseases. International Journal of Molecular Sciences. 2024; 25(23):13207. https://doi.org/10.3390/ijms252313207
Chicago/Turabian StyleLiu, Yishi, Zehui Yang, Xiaoman Zhou, Zijie Li, and Nakanishi Hideki. 2024. "Diacylglycerol Kinases and Its Role in Lipid Metabolism and Related Diseases" International Journal of Molecular Sciences 25, no. 23: 13207. https://doi.org/10.3390/ijms252313207
APA StyleLiu, Y., Yang, Z., Zhou, X., Li, Z., & Hideki, N. (2024). Diacylglycerol Kinases and Its Role in Lipid Metabolism and Related Diseases. International Journal of Molecular Sciences, 25(23), 13207. https://doi.org/10.3390/ijms252313207








