Inhibitory Effects of Gliadin Hydrolysates on BACE1 Expression and APP Processing to Prevent Aβ Aggregation
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of Proteases on the Hydrolysis of Protein Substrates
2.2. Inhibitory Activity of Protein Hydrolysates on BACE1
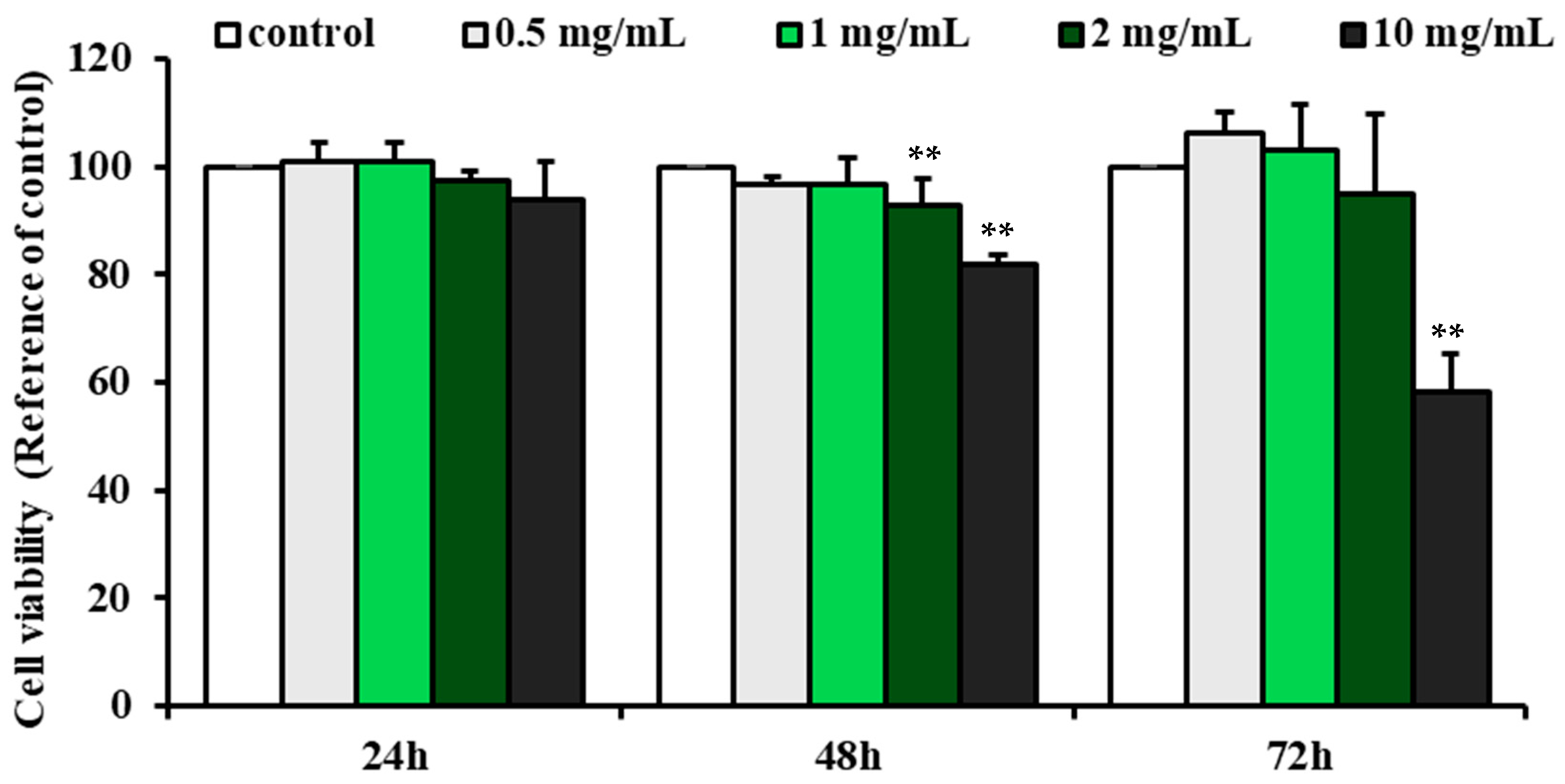
2.3. Effect of G-Bro on the Viability of N2a/PS/APP Cells
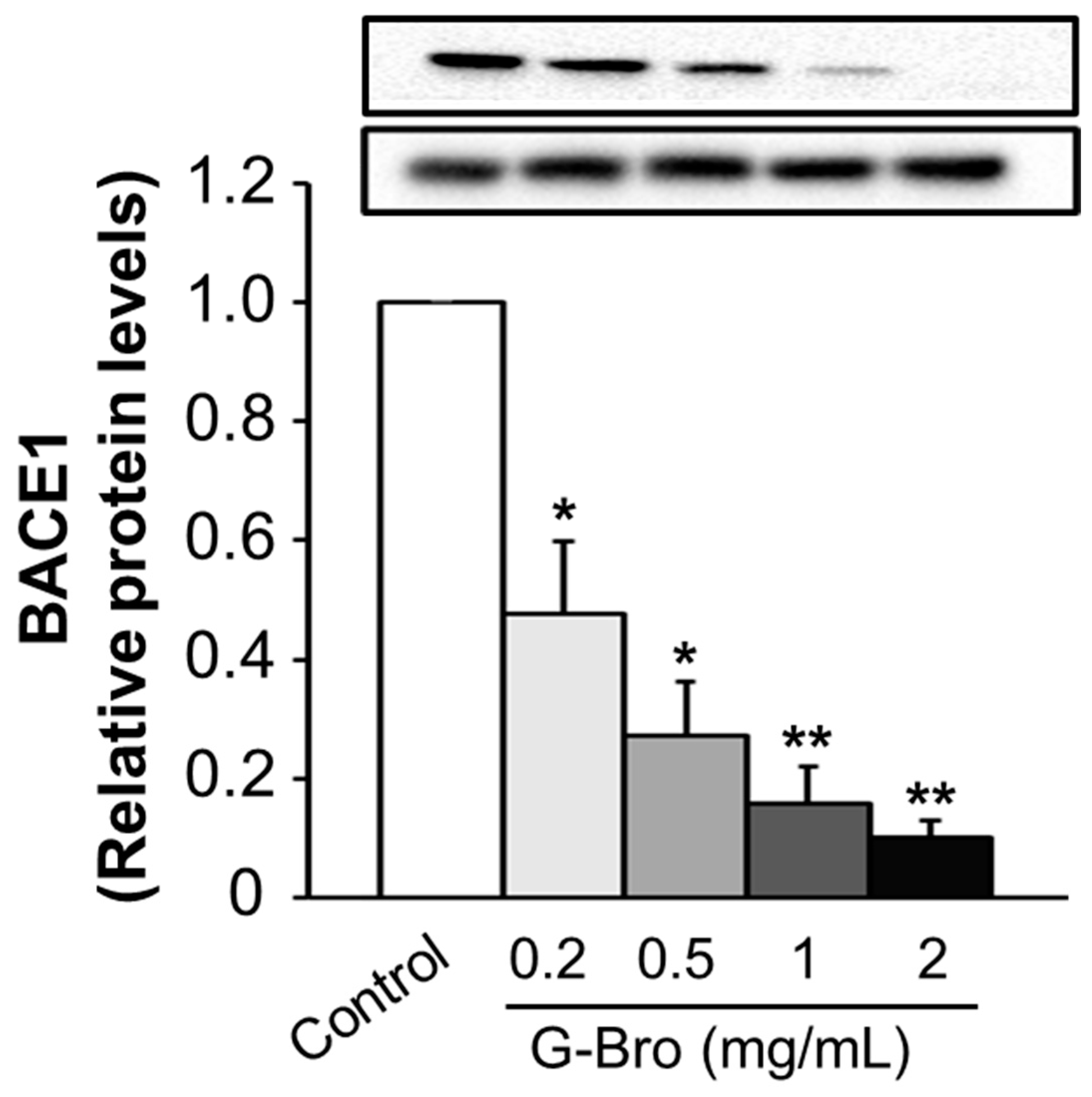
2.4. Inhibitory Effect of G-Bro on BACE1 Protein Expression
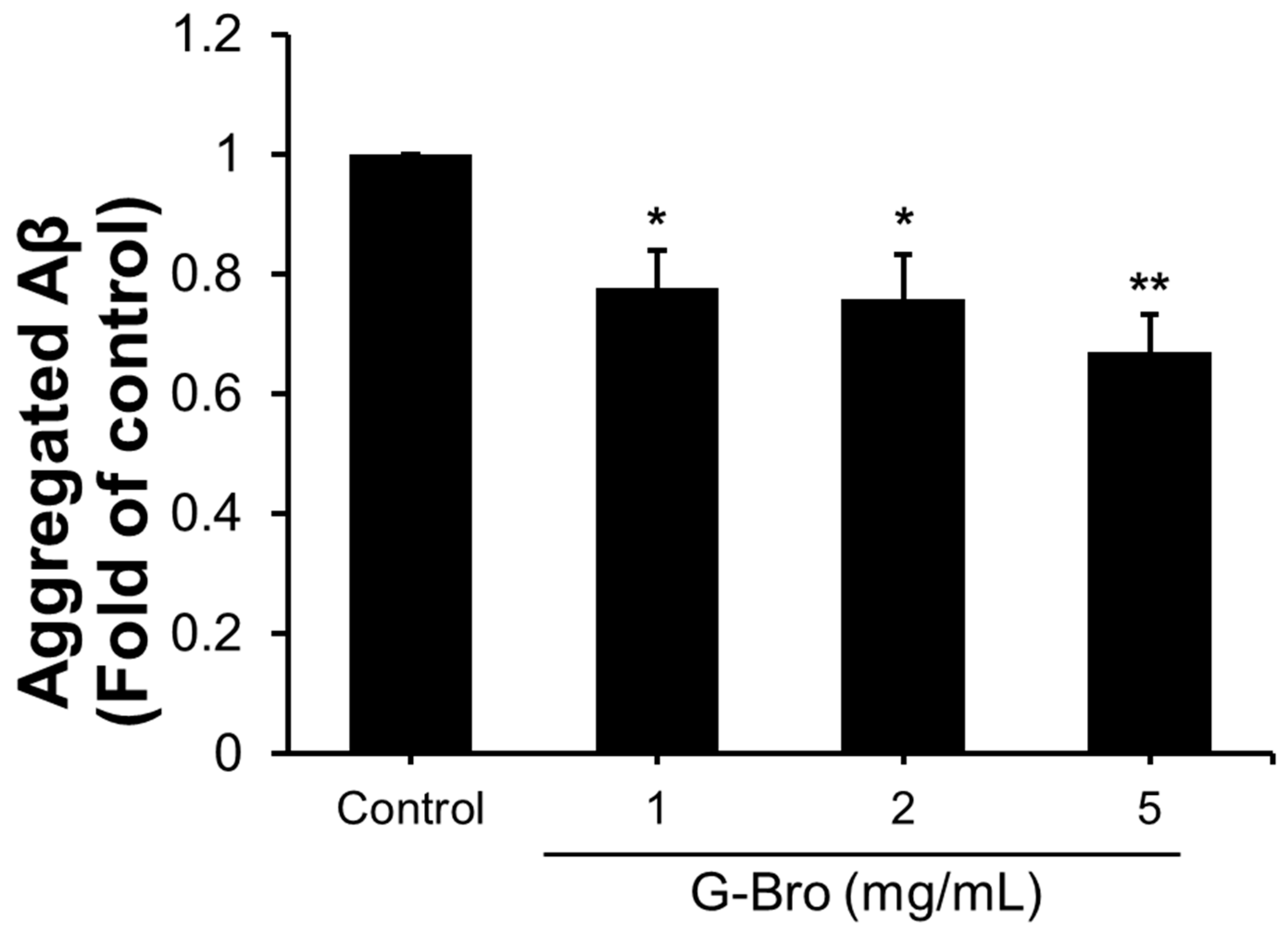
2.5. Effects of G-Bro on sAPP Production and Aβ Aggregation
2.6. Identification of G-Bro Peptides by nanoUHPLC-ESI-Q TOF Mass Spectrometry
3. Materials and Methods
3.1. Study Design
3.2. Materials
3.3. Preparation of Protein Hydrolysates
3.4. BACE1 Activity Assay
3.5. Cell Viability
3.6. β-Secretase (BACE1) Protein Expression
3.7. The Levels of sAPP and Aβ
3.8. Peptide Identification of Protein Hydrolysates
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adem, M.A.; Decourt, B.; Sabbagh, M.N. Pharmacological Approaches Using Diabetic Drugs Repurposed for Alzheimer’s Disease. Biomedicines 2024, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Pal, P.; Gupta, S.K. Advances in Alzheimer’s disease: A multifaceted review of potential therapies and diagnostic techniques for early detection. Neurochem. Int. 2024, 177, 105761. [Google Scholar] [CrossRef] [PubMed]
- Raymond, R.D.D. Biomedicines in Longevity and Aging The Quest to Resist Biological Decline. BioMedicine 2024, 14, 2. [Google Scholar] [CrossRef]
- Seo, D.-O.; Holtzman, D.M. Current understanding of the Alzheimer’s disease-associated microbiome and therapeutic strategies. Exp. Mol. Med. 2024, 56, 86–94. [Google Scholar] [CrossRef]
- Sharma, M.; Tanwar, A.K.; Purohit, P.K.; Pal, P.; Kumar, D.; Vaidya, S.; Prajapati, S.K.; Kumar, A.; Dhama, N.; Kumar, S. Regulatory roles of microRNAs in modulating mitochondrial dynamics, amyloid beta fibrillation, microglial activation, and cholinergic signaling: Implications for Alzheimer’s disease pathogenesis. Neurosci. Biobehav. Rev. 2024, 161, 105685. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Haziyihan, A.; Zhao, W.; Chen, Y.; Chao, H. Trajectory of brain-derived amyloid beta in Alzheimer’s disease: Where is it coming from and where is it going? Transl. Neurodegener. 2024, 13, 42. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Perche, F.; Ikegami, M.; Uchida, S.; Kataoka, K.; Itaka, K. Messenger RNA-based therapeutics for brain diseases: An animal study for augmenting clearance of beta-amyloid by intracerebral administration of neprilysin mRNA loaded in polyplex nanomicelles. J. Control. Release 2016, 235, 268–275. [Google Scholar] [CrossRef]
- Arya, R.; Jain, S.; Paliwal, S.; Madan, K.; Sharma, S.; Mishra, A.; Tiwari, P.; Kadiri, S.K. BACE1 inhibitors: A promising therapeutic approach for the management of Alzheimer’s disease. Asian Pac. J. Trop. Biomed. 2024, 14, 369–381. [Google Scholar] [CrossRef]
- Coimbra, J.R.; Resende, R.; Custódio, J.; Salvador, J.A.; Santos, A.E. BACE1 Inhibitors for Alzheimer’s Disease: Current Challenges and Future Perspectives. J. Alzheimer’s Dis. 2024, 101, 1–26. [Google Scholar] [CrossRef]
- Carmona-Iragui, M.; O’Connor, A.; Llibre-Guerra, J.; Lao, P.; Ashton, N.J.; Fortea, J.; Sánchez-Valle, R. Clinical and research application of fluid biomarkers in autosomal dominant Alzheimer’s disease and Down syndrome. EBioMedicine 2024, 108, 105327. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef] [PubMed]
- Prado, P.C.; Lima, J.A.; Hamerski, L.; Rennó, M.N. Natural Products with BACE1 and GSK3β Inhibitory Activity. Mini-Rev. Med. Chem. 2023, 23, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Alrouji, M.; Alhumaydhi, F.A.; Al Abdulmonem, W.; Sharaf, S.E.; Shahwan, M.; Majarisi, T.; Atiya, A.; Shamsi, A. Identifying β-secretase 1 (BACE1) inhibitors from plant-based compounds: An approach targeting Alzheimer’s therapeutics employing molecular docking and dynamics simulation. Mol. Divers. 2023, 28, 2967–2980. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.B.; Jung, E.-J.; Jo, K.; Suh, H.J.; Choi, H.-S. Neuroprotective effect of whey protein hydrolysate containing leucine-aspartate-isoleucine-glutamine-lysine on HT22 cells in hydrogen peroxide–induced oxidative stress. J. Dairy Sci. 2024, 107, 2620–2632. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, A.; Zhu, L.; Zhuang, C.; Zhao, Q.; Zou, Y. Peptide inhibitors targeting Ras and Ras-associated protein–protein interactions. Eur. J. Med. Chem. 2024, 116878. [Google Scholar] [CrossRef]
- Nemati, M.; Shahosseini, S.R.; Ariaii, P. Review of fish protein hydrolysates: Production methods, antioxidant and antimicrobial activity and nanoencapsulation. Food Sci. Biotechnol. 2024, 33, 1789–1803. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Kuang, Y.; Wang, N. Amino acids biostimulants and protein hydrolysates in agricultural sciences. Plants 2024, 13, 210. [Google Scholar] [CrossRef]
- Dou, P.; Li, X.; Zou, X.; Wang, K.; Yao, L.; Sun, Z.; Hong, H.; Luo, Y.; Tan, Y. Antihypertensive effects of whey protein hydrolysate involve reshaping the gut microbiome in spontaneously hypertension rats. Food Sci. Hum. Wellness 2024, 13, 1974–1986. [Google Scholar] [CrossRef]
- Ding, N.; Meng, H.; Wu, C.; Hong, H.; Luo, Y.; Tan, Y. Targeting brain health: Whey protein hydrolysate intervention enhances cognitive function in middle-aged mice. Food Biosci. 2024, 57, 103460. [Google Scholar] [CrossRef]
- Thomas, C.; Kingshott, R.N.; Allott, K.M.; Tang, J.C.; Dunn, R.; Fraser, W.D.; Thorley, J.; Virgilio, N.; Prawitt, J.; Hogervorst, E. Collagen peptide supplementation before bedtime reduces sleep fragmentation and improves cognitive function in physically active males with sleep complaints. Eur. J. Nutr. 2024, 63, 323–335. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, P.; Zhang, Y.; Wang, X.; Yue, C.; Bai, C.; Wu, W.; Zhang, Y.; Zhao, Z. Measurement of degree of hydrolysis and molecular weight distribution of protein hydrolysates by liquid chromatography-mass spectrometry. Talanta 2024, 268, 125347. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Zhang, J.; Chen, Q.; Xie, Y.; Zhang, M.; Qu, J.; Tan, Y.; Diao, Y.; Wang, Y.; Zhang, Y. Biodegradation of poly (ethylene terephthalate) through PETase surface-display: From function to structure. J. Hazard. Mater. 2024, 461, 132632. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Sarabandi, K.; Peighambardoust, S.H.; Sarabandi, R.; Kafil, H.S.; Hesarinejad, M.A. Enzymatic modification of cold pressed coconut meal protein: Nutritional, functional and biological properties. Sustain. Food Technol. 2024, 2, 1545–1557. [Google Scholar] [CrossRef]
- Yeerong, K.; Chantawannakul, P.; Anuchapreeda, S.; Wangtueai, S.; Chaiyana, W. Optimization of Hydrolysis Conditions, Isolation, and Identification of Biologically Active Peptides Derived from Acheta domesticus for Antioxidant and Collagenase Inhibition. Antioxidants 2024, 13, 367. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, L.; Udenigwe, C.C.; Lin, L.; Zhao, M. Enzymatic characterization and synergistic effects of protease combinations on DPP-IV inhibitory peptide release from bovine casein. Food Biosci. 2024, 60, 104276. [Google Scholar] [CrossRef]
- Nuñez, S.M.; Valencia, P.; Solís, T.; Valdivia, S.; Cárdenas, C.; Guzman, F.; Pinto, M.; Almonacid, S. Enzymatic Hydrolysis of Salmon Frame Proteins Using a Sequential Batch Operational Strategy: An Improvement in Water-Holding Capacity. Foods 2024, 13, 1378. [Google Scholar] [CrossRef] [PubMed]
- Abdelhedi, O.; Mora, L.; Jridi, M.; Toldrá, F.; Nasri, M. Proteolysis coupled with membrane separation for the isolation of bioactive peptides from defatted smooth hound byproduct proteins. Waste Biomass Valorizat. 2024, 15, 1959–1974. [Google Scholar] [CrossRef]
- Dent, T.; Campanella, O.; Maleky, F. Enzymatic hydrolysis of soy and chickpea protein with Alcalase and Flavourzyme and formation of hydrogen bond mediated insoluble aggregates. Curr. Res. Food Sci. 2023, 6, 100487. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, R.; Xue, J.; Yang, X.; Lin, D. Characterization of bacterial cellulose nanofibers/soy protein isolate complex particles for Pickering emulsion gels: The effect of protein structure changes induced by pH. Int. J. Biol. Macromol. 2023, 226, 254–266. [Google Scholar] [CrossRef]
- Cochereau, R.; Voisin, H.; Solé-Jamault, V.; Novales, B.; Davy, J.; Jamme, F.; Renard, D.; Boire, A. Influence of pH and lipid membrane on the liquid–liquid phase separation of wheat γ-gliadin in aqueous conditions. J. Colloid Interface Sci. 2024, 668, 252–263. [Google Scholar] [CrossRef]
- Rani, M.; Siddiqi, R.A.; Sharma, R.; Gill, B.S.; Sogi, D.S. Functional and structural properties of gliadin as influenced by pH, extraction protocols, and wheat cultivars. Int. J. Biol. Macromol. 2023, 234, 123484. [Google Scholar] [CrossRef] [PubMed]
- Iram, F.; Shahid, M.; Ansari, J.; Ashraf, G.M.; Hassan, M.I.; Islam, A. Navigating the Maze of Alzheimer’s Disease by Exploring BACE1: Discovery, Current Scenario, and Future Prospects. Ageing Res. Rev. 2024, 98, 102342. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; McClements, D.J. Bioactive functional ingredients from aquatic origin: A review of recent progress in marine-derived nutraceuticals. Crit. Rev. Food Sci. Nutr. 2022, 62, 1242–1269. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, A.U.; Abuine, R.; Palanisamy, S.; Lee, J.K.; Byun, H.-G. Characterization and purification of β− secretase inhibitory peptides fraction from sea cucumber (Holothuria spinifera) enzymatic hydrolysates. Process Biochem. 2021, 111, 86–96. [Google Scholar] [CrossRef]
- Zhong, H.; Jin, Y.; Hussain, M.; Liu, X.; Feng, F.; Guan, R. Recent advances of hepatoprotective peptides: Production, structure, mechanisms, and interactions with intestinal microbiota. Food Biosci. 2024, 58, 103744. [Google Scholar] [CrossRef]
- Martínez, A. Synthesis, in vitro activity, and molecular docking of caffeic acid and resveratrol derivatives against Alzheimer’s disease-related enzymes. Med. Chem. Res. 2024, 33, 1681–1697. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Yu, Y.; Sun, P.; Fan, Z.; Zhang, W.S.; Feng, C.Q. Royal jelly peptides: Potential inhibitors of β-secretase in N2a/APP695swe cells. Sci. Rep. 2019, 9, 168. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Z.; Peng, Y.; Xu, B. Insights into prevention mechanisms of bioactive components from healthy diets against Alzheimer’s disease. J. Nutr. Biochem. 2023, 119, 109397. [Google Scholar] [CrossRef]
- He, Z.; Li, X.; Wang, Z.; Cao, Y.; Han, S.; Li, N.; Cai, J.; Cheng, S.; Liu, Q. Protective effects of luteolin against amyloid beta-induced oxidative stress and mitochondrial impairments through peroxisome proliferator-activated receptor γ-dependent mechanism in Alzheimer’s disease. Redox Biol. 2023, 66, 102848. [Google Scholar] [CrossRef]
- Jayawickreme, D.K.; Ekwosi, C.; Anand, A.; Andres-Mach, M.; Wlaź, P.; Socała, K. Luteolin for neurodegenerative diseases: A review. Pharmacol. Rep. 2024, 76, 644–664. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Y. The role of natural flavonoids on neuroinflammation as a therapeutic target for Alzheimer’s disease: A narrative review. Neural Regen. Res. 2023, 18, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gao, W.; Pan, D.; Ma, Y.; Zhang, R.; Cao, Y. Puerarin modulation of CENPA affects downstream PLK1 and CCNB1 expression to inhibit bladder cancer cell proliferation. Tradit. Med. Res. 2024, 9, 52. [Google Scholar] [CrossRef]
- Chen, M.; Wu, B.; Huang, Y.; Wang, W.; Zheng, Y.; Shabbir, S.; Liu, P.; Dai, Y.; Xia, M.; Hu, G. Transcription factor shapes chromosomal conformation and regulates gene expression in bacterial adaptation. Nucleic Acids Res. 2024, 52, 5643–5657. [Google Scholar] [CrossRef]
- Roy, R.; Paul, S. Illustrating the effect of small molecules derived from natural resources on amyloid peptides. J. Phys. Chem. B 2023, 127, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Lux, J.; Azarkh, M.; Fitzner, L.; Keppler, J.K.; Schwarz, K.; Drescher, M.; Steffen-Heins, A. Amyloid aggregation of spin-labeled β-lactoglobulin. Part II: Identification of spin-labeled protein and peptide sequences after amyloid aggregation. Food Hydrocoll. 2020, 112, 106174. [Google Scholar] [CrossRef]
- Zabłocka, A.; Sokołowska, A.; Macała, J.; Bartoszewska, M.; Mitkiewicz, M.; Janusz, M.; Wilusz, T.; Polanowski, A. Colostral proline-rich polypeptide complexes. Comparative study of the antioxidant properties, cytokine-inducing activity, and nitric oxide release of preparations produced by a laboratory and a large-scale method. Int. J. Pept. Res. Ther. 2020, 26, 685–694. [Google Scholar] [CrossRef]
- Kanchi, P.K.; Dasmahapatra, A.K. Destabilization of the Alzheimer’s amyloid-β peptide by a proline-rich β-sheet breaker peptide: A molecular dynamics simulation study. J. Mol. Model. 2021, 27, 356. [Google Scholar] [CrossRef]
- Daniecki, N.J.; Bhatt, M.R.; Yap, G.P.; Zondlo, N.J. Proline C− H Bonds as Loci for Proline Assembly via C− H/O Interactions. ChemBioChem 2022, 23, e202200409. [Google Scholar] [CrossRef]
- Milardi, D.; Gazit, E.; Radford, S.E.; Xu, Y.; Gallardo, R.U.; Caflisch, A.; Westermark, G.T.; Westermark, P.; Rosa, C.L.; Ramamoorthy, A. Proteostasis of islet amyloid polypeptide: A molecular perspective of risk factors and protective strategies for type II diabetes. Chem. Rev. 2021, 121, 1845–1893. [Google Scholar] [CrossRef]
- Nguyen, K.V.; Gendrault, J.-L.; Wolff, C.-M. Poly-l-lysine dissolves fibrillar aggregation of the Alzheimer β-amyloid peptide in vitro. Biochem. Biophys. Res. Commun. 2002, 291, 764–768. [Google Scholar] [CrossRef]
- Zhu, H.; Nytka, M.; Vu, T.N.K.; Lemr, K.; Tureček, F. Photochemical and Collision-Induced Cross-Linking in Stereochemically Distinct Scaffolds of Peptides and Nitrile Imines in Gas-Phase Ions. J. Am. Soc. Mass Spectrom. 2024, 35, 3070–3088. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, A.C.; Das, B.K.; Saveri, P.; Mani, E.; Deshpande, A.P.; Shanmugam, G. Efficiency Enhancement in Peptide Hydrogelators: The Crucial Role of Side Chain Hydrogen Bonding Over Aromatic π–π Interactions. Langmuir 2024, 40, 24405–24418. [Google Scholar] [CrossRef] [PubMed]
- Polańska, O.; Szulc, N.; Stottko, R.; Olek, M.; Nadwodna, J.; Gąsior-Głogowska, M.; Szefczyk, M. Challenges in Peptide Solubilization–Amyloids Case Study. Chem. Rec. 2024, 24, e202400053. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Tabassum, S.; Yano, S.; Abdullah, F.B.; Wang, R.; Ikeue, T.; Nagai, A. A Cationic Zn-Phthalocyanine Turns Alzheimer’s Amyloid β Aggregates into Non-Toxic Oligomers and Inhibits Neurotoxicity in Culture. Int. J. Mol. Sci. 2024, 25, 8931. [Google Scholar] [CrossRef] [PubMed]
- Pariary, R.; Shome, G.; Kalita, S.; Kalita, S.; Roy, A.; Harikishore, A.; Jana, K.; Senapati, D.; Mandal, B.; Mandal, A.K. Peptide-Based Strategies: Combating Alzheimer’s Amyloid β Aggregation through Ergonomic Design and Fibril Disruption. Biochemistry 2024, 63, 2397–2413. [Google Scholar] [CrossRef]
- Li, W.B.; Xu, L.L.; Wang, S.L.; Wang, Y.Y.; Pan, Y.C.; Shi, L.Q.; Guo, D.S. Co-Assembled Nanoparticles toward Multi-Target Combinational Therapy of Alzheimer’s Disease by Making Full Use of Molecular Recognition and Self-Assembly. Adv. Mater. 2024, 36, 2401918. [Google Scholar] [CrossRef]
- Juković, M.; Ratkaj, I.; Kalafatovic, D.; Bradshaw, N.J. Amyloids, amorphous aggregates and assemblies of peptides—Assessing aggregation. Biophys. Chem. 2024, 308, 107202. [Google Scholar] [CrossRef]
- Zhao, D.; Zhou, Y.; Xing, F.; Wang, H.; Zhou, J. Porous organic cage as an inhibitor of Aβ42 peptide: A simulation study. Phys. Chem. Chem. Phys. 2024. [Google Scholar] [CrossRef]
- Rosales Hernández, M.C.; Olvera-Valdez, M.; Velazquez Toledano, J.; Mendieta Wejebe, J.E.; Fragoso Morales, L.G.; Cruz, A. Exploring the Benzazoles Derivatives as Pharmacophores for AChE, BACE1, and as Anti-Aβ Aggregation to Find Multitarget Compounds against Alzheimer’s Disease. Molecules 2024, 29, 4780. [Google Scholar] [CrossRef]
- Liu, H.; Cui, Y.; Zhao, X.; Wei, L.; Wang, X.; Shen, N.; Odom, T.; Li, X.; Lawless, W.; Karunarathne, K. Helical sulfonyl-γ-AApeptides modulating Aβ oligomerization and cytotoxicity by recognizing Aβ helix. Proc. Natl. Acad. Sci. USA 2024, 121, e2311733121. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Wang, X.; Feng, Y.; Jiang, J.; Bi, J. Innovative insights into the enzymatic hydrolysis of salmon milt: Structural and functional analysis influenced by protease type and enzymolysis time. Food Chem. 2025, 463, 141154. [Google Scholar] [CrossRef] [PubMed]
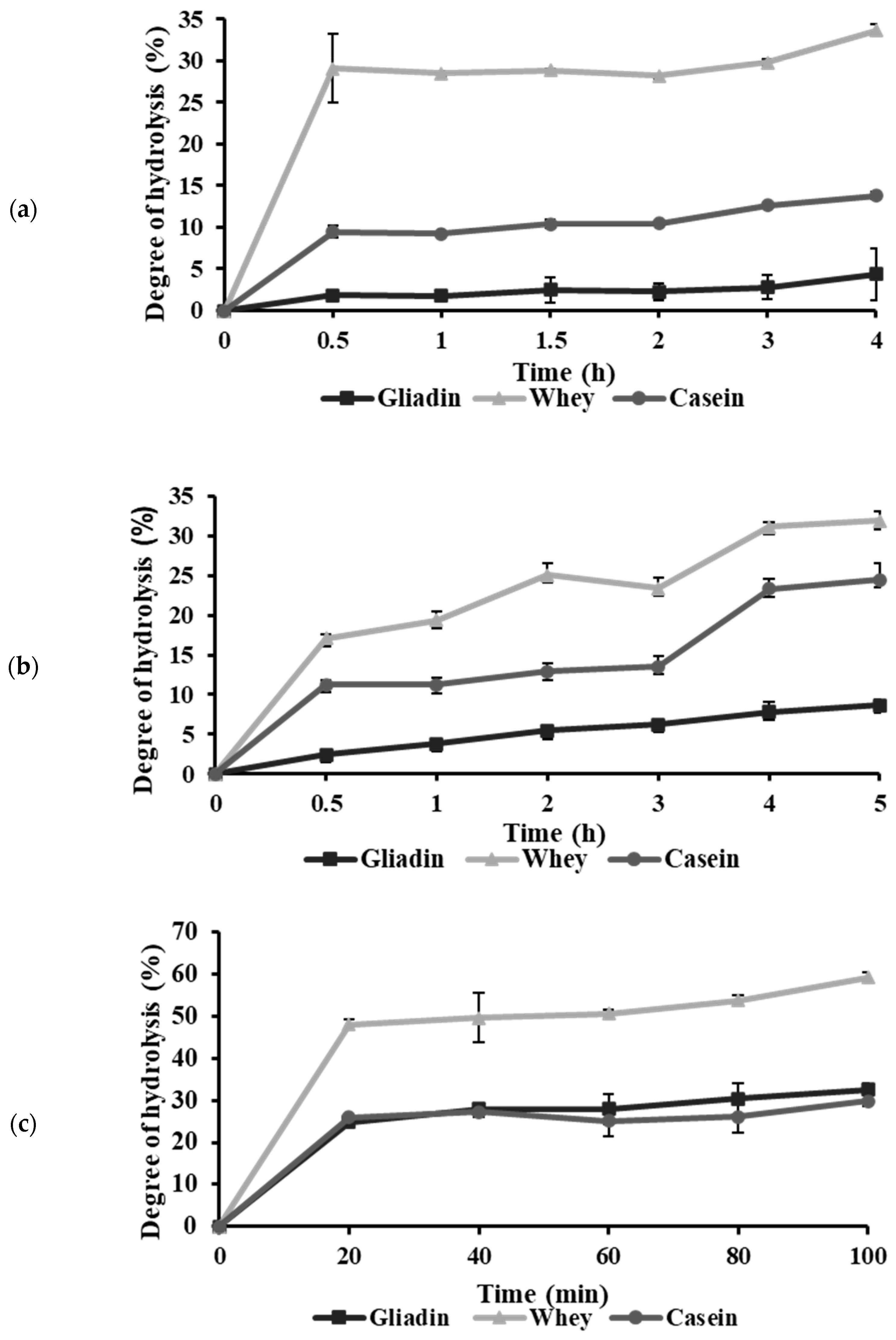


| Abbreviation | Protein | Enzyme | Optimal Hydrolysis Duration | DH (%) |
|---|---|---|---|---|
| G-Pap | Gliadin | Papain | 2 h | 1.56 |
| W-Pap | Whey | Papain | 2 h | 28.19 |
| C-Pap | Casein | Papain | 2 h | 10.41 |
| G-Bro | Gliadin | Bromelain | 3 h | 4.58 |
| W-Bro | Whey | Bromelain | 3 h | 25.09 |
| C-Bro | Casein | Bromelain | 3 h | 13.69 |
| G-The | Gliadin | Thermolysin | 1 h | 28.27 |
| W-The | Whey | Thermolysin | 1 h | 51.27 |
| C-The | Casein | Thermolysin | 1 h | 26.41 |
| Protein Hydrolysates | Equation | R2 | IC50 (mg/mL) |
|---|---|---|---|
| G-Pap | y = 46.3x + 18.9 | 0.974 | 0.672 |
| W-Pap | y = 51.5x + 14.3 | 0.996 | 0.693 |
| C-Pap | y = 17.9In(x) + 42.3 | 0.929 | NA |
| G-Bro | y = 54.6x + 27.7 | 0.931 | 0.408 |
| W-Bro | y = 49.5x + 23.7 | 0.950 | 0.533 |
| C-Bro | y = 37.9x + 15 | 0.971 | 0.923 |
| G-The | y = −107x + 7.86 | 0.983 | NA |
| W-The | y = −187x + 6.76 | 0.906 | NA |
| C-The | y = −531x + 89.14 | 0.977 | NA |
| Peptide No | m/z Meas. | Rt [min] | Score | Range | Sequence |
|---|---|---|---|---|---|
| 1 | 1095.5222 | 19.45 | 81.25 | 130–168 | SPQRPGQGQQPGQGQQGYYPTSPQQPGQWQQPEQGQPRY |
| 2 | 686.3484 | 15.36 | 27.47 | 426–433 | GAAGEPGK |
| 3 | 766.4068 | 21.22 | 59.75 | 21–40 | VRVPVPQLQPQNPSQQQPQK |
| 4 | 857.4509 | 43.51 | 21.15 | 534–540 | ENQILLK |
| 5 | 1063.6136 | 39.33 | 20.97 | 1615–1622 | SRRYLLKK |
| 6 | 879.4480 | 36.82 | 35.77 | 248–255 | DIMGVSNK |
| 7 | 996.6132 | 30.76 | 40.96 | 16–25 | LVGALVLPSK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Hsieh, C.-H.; Lai, P.-Y.; Huang, C.-W.; Chung, Y.-H.; Huang, S.-M.; Hsu, K.-C. Inhibitory Effects of Gliadin Hydrolysates on BACE1 Expression and APP Processing to Prevent Aβ Aggregation. Int. J. Mol. Sci. 2024, 25, 13212. https://doi.org/10.3390/ijms252313212
Lin C-Y, Hsieh C-H, Lai P-Y, Huang C-W, Chung Y-H, Huang S-M, Hsu K-C. Inhibitory Effects of Gliadin Hydrolysates on BACE1 Expression and APP Processing to Prevent Aβ Aggregation. International Journal of Molecular Sciences. 2024; 25(23):13212. https://doi.org/10.3390/ijms252313212
Chicago/Turabian StyleLin, Chin-Yu, Cheng-Hong Hsieh, Pei-Yu Lai, Ching-Wei Huang, Yung-Hui Chung, Shang-Ming Huang, and Kuo-Chiang Hsu. 2024. "Inhibitory Effects of Gliadin Hydrolysates on BACE1 Expression and APP Processing to Prevent Aβ Aggregation" International Journal of Molecular Sciences 25, no. 23: 13212. https://doi.org/10.3390/ijms252313212
APA StyleLin, C.-Y., Hsieh, C.-H., Lai, P.-Y., Huang, C.-W., Chung, Y.-H., Huang, S.-M., & Hsu, K.-C. (2024). Inhibitory Effects of Gliadin Hydrolysates on BACE1 Expression and APP Processing to Prevent Aβ Aggregation. International Journal of Molecular Sciences, 25(23), 13212. https://doi.org/10.3390/ijms252313212








