Metabolomics Combined with Transcriptomics Analysis Reveals the Regulation of Flavonoids in the Leaf Color Change of Acer truncatum Bunge
Abstract
:1. Introduction
2. Results
2.1. Variations in Phenotypes and Analysis of Colour Parameters
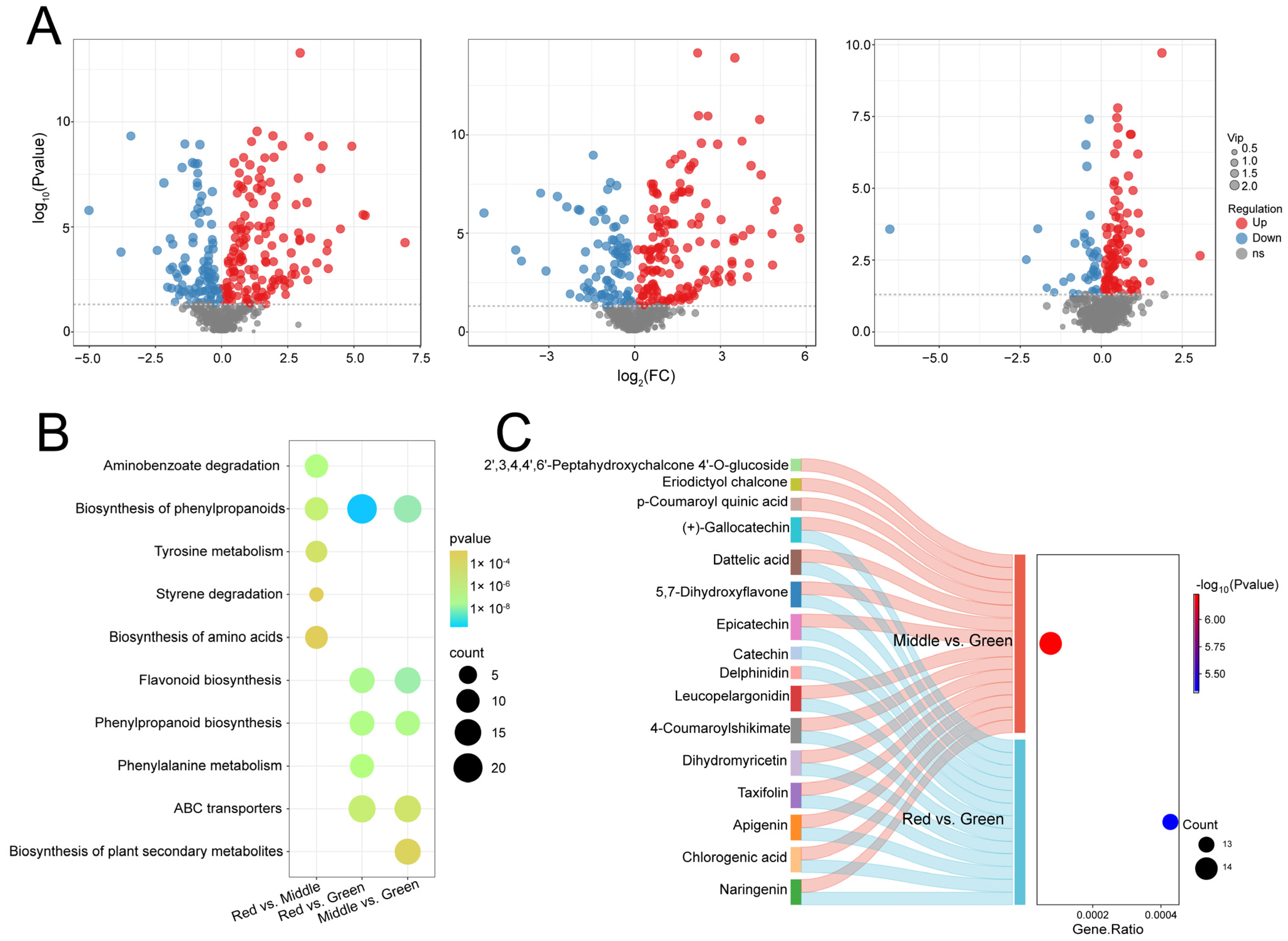
2.2. Analysis of Bioactive Flavonoids
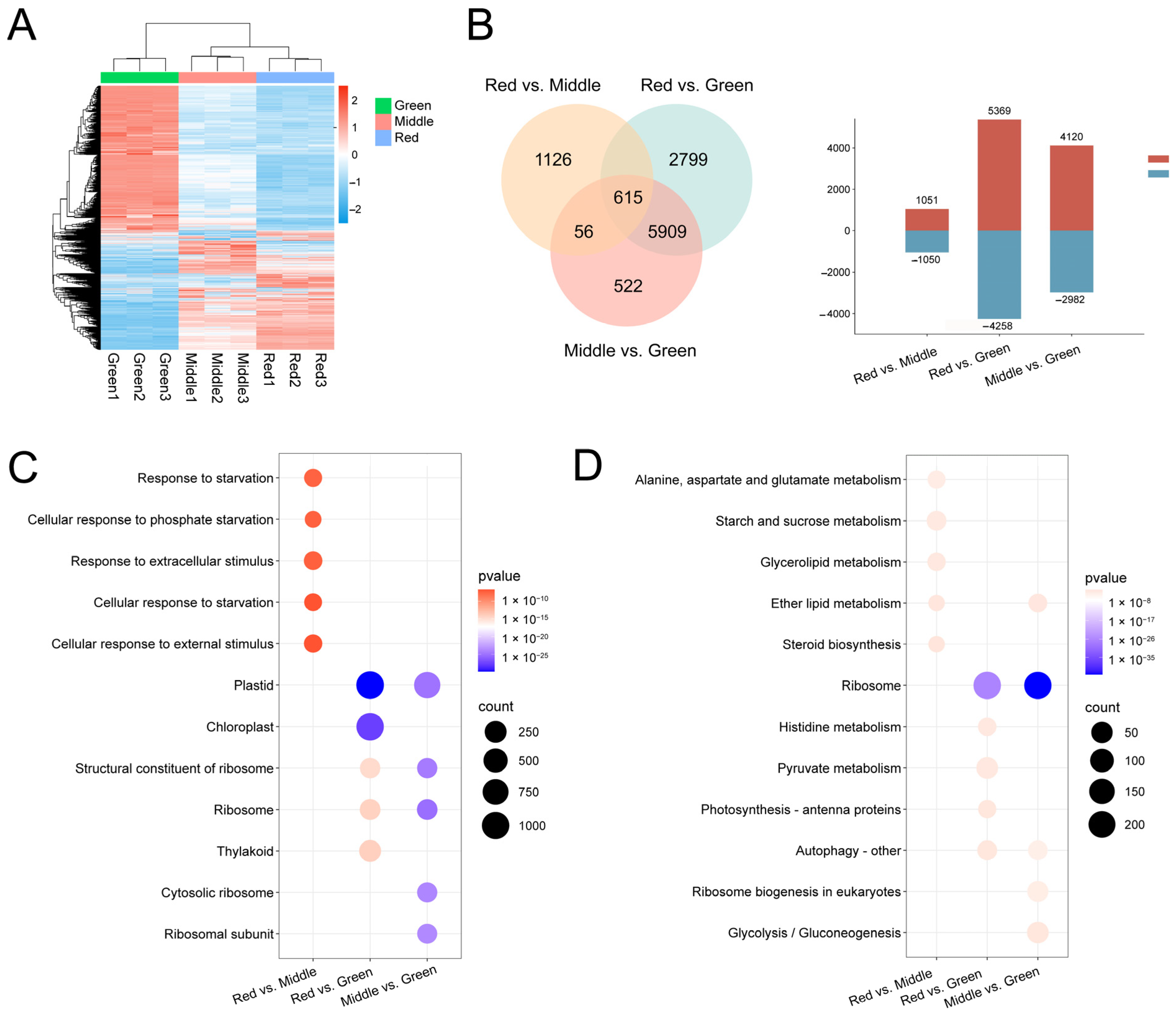
2.3. Analysis of Differentially Expressed Flavonoid Metabolites and KEGG Classification
2.4. Analysis of Transcriptome Results and Functional Annotation
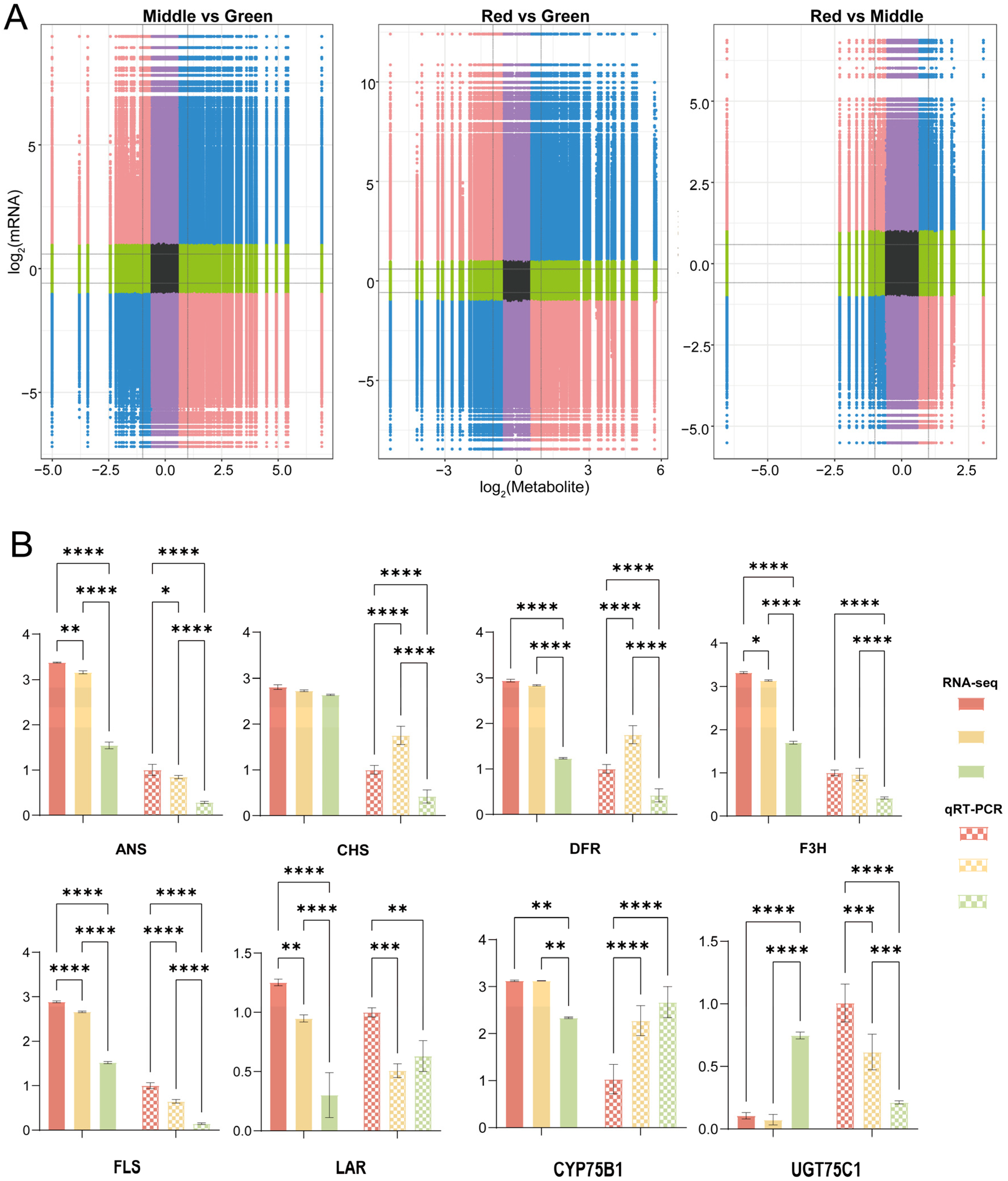
2.5. Flavonoid Biosynthesis in Relation to Genes and Metabolites
2.6. Analysis of Flavonoid and Anthocyanin Biosynthesis
2.7. Analysis of Proteins Encoding Genes for Flavonoids and Anthocyanin Metabolites
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sampling
4.2. Determination of Leaf Colour Parameters
4.3. Extraction of Total Metabolites
4.4. Metabolomics Analysis
4.5. Total RNA Extraction and Transcriptome Sequencing
4.6. Identification and Analysis of DEGs
4.7. qRT-PCR Analysis
4.8. Protein Biology Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhuang, W.; Wang, H.; Liu, T.; Wang, T.; Zhang, F.; Shu, X.; Zhai, H.; Wang, Z. Integrated physiological and genomic analysis reveals structural variations and expression patterns of candidate genes for colored-and green-leaf poplar. Sci. Rep. 2019, 9, 11150. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, X.-R.; Zhao, Y. Leaf color attributes of urban colored-leaf plants. Open Geosci. 2022, 14, 1591–1605. [Google Scholar] [CrossRef]
- Yan, L.; Fang, H.; Liang, Y.; Wang, Y.; Ren, F.; Xie, X.; Wu, D. Transcriptome analyses of Acer Truncatum Bunge seeds to delineate the genes involved in fatty acid metabolism. BMC Genom. 2024, 25, 605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shen, J.; Wu, Y.; Zhang, X.; Zhao, Z.; Wang, J.; Cheng, T.; Zhang, Q.; Pan, H. Comparative transcriptome analysis identified ChlH and POLGAMMA2 in regulating yellow-leaf coloration in Forsythia. Front. Plant Sci. 2022, 13, 1009575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhen, J.; Che, X.; Zhang, K.; Zhang, G.; Yang, H.; Wen, J.; Wang, J.; Wang, J.; He, B.; et al. Transcriptomic and metabolomic analysis of autumn leaf color change in Fraxinus angustifolia. PeerJ 2023, 11, e15319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhan, W.; Sun, A.; Xie, Y.; Han, Z.; Qu, X.; Wang, J.; Zhang, L.; Tian, M.; Pang, X.; et al. Combined transcriptome and metabolome integrated analysis of Acer mandshuricum to reveal candidate genes involved in anthocyanin accumulation. Sci. Rep. 2021, 11, 23148. [Google Scholar] [CrossRef]
- Gao, J.; Ren, R.; Wei, Y.; Jin, J.; Ahmad, S.; Lu, C.; Wu, J.; Zheng, C.; Yang, F.; Zhu, G. Comparative Metabolomic Analysis Reveals Distinct Flavonoid Biosynthesis Regulation for Leaf Color Development of Cymbidium sinense ‘Red Sun’. Int. J. Mol. Sci. 2020, 21, 1869. [Google Scholar] [CrossRef]
- Tan, C.; Yang, J.; Xue, X.; Wei, J.; Li, H.; Li, Z.; Duan, Y. MsMYB62-like as a negative regulator of anthocyanin biosynthesis in Malus spectabilis. Plant Signal. Behav. 2024, 19, 2318509. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, L.; Shang, H.; Chen, Q.; Lu, M.; Mu, D.; Li, X.; Meng, X.; Wu, Y.; Han, X.; et al. Research advances of coloring mechanism regulated by MicroRNAs in plants. Plant Physiol. Biochem. 2024, 215, 109036. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Mao, Y.; Yin, Y.; Shen, X. Physiological analysis and transcriptome sequencing of a delayed-green leaf mutant ‘Duojiao’ of ornamental crabapple (Malus sp.). Physiol. Mol. Biol. Plants 2022, 28, 1833–1848. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Wu, Y.-J.; Sheue, C.-R.; Liao, P.-C.; Chen, Y.-H.; Li, S.-J.; Liu, J.-W.; Chang, H.-T.; Liu, W.-L.; Ko, Y.-Z.; et al. Molecular Basis Underlying Leaf Variegation of a Moth Orchid Mutant (Phalaenopsis aphrodite subsp. formosana). Front. Plant Sci. 2017, 8, 1333. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, C.; Gu, Y.; Cheng, R.; Huang, D.; Liu, X.; Sun, Y. Physiological and transcriptomic analysis of a yellow leaf mutant in watermelon. Sci. Rep. 2023, 13, 9647. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, Y.; Yu, J.; Zhou, H.; Hou, Z.; Wang, X. Molecular and metabolic insights into purplish leaf coloration through the investigation of two mulberry (Morus alba) genotypes. BMC Plant Biol. 2024, 24, 61. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Chen, P.; Chen, J.; Chen, K.; Wang, X.; Abubakar, A.S.; Liu, N.; Yu, C.; Zhu, A. Genomic Survey, Transcriptome, and Metabolome Analysis of Apocynum venetum and Apocynum hendersonii to Reveal Major Flavonoid Biosynthesis Pathways. Metabolites 2019, 9, 296. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, H.; Zhang, Y.; Feng, X.; Dai, Y.; Zhu, P. A novel three-layer module BoMYB1R1-BoMYB4b/BoMIEL1-BoDFR1 regulates anthocyanin accumulation in kale. Plant J. 2024, 119, 1737–1750. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Song, B.; Stöcklin, J.; Armbruster, W.S.; Gao, Y.; Peng, D.; Sun, H. Reversible colour change in leaves enhances pollinator attraction and reproductive success in Saururus chinensis (Saururaceae). Ann. Bot. 2018, 121, 641–650. [Google Scholar] [CrossRef]
- Mais, E.; Alolga, R.N.; Wang, S.-L.; Linus, L.O.; Yin, X.; Qi, L.-W. A comparative UPLC-Q/TOF-MS-based metabolomics approach for distinguishing Zingiber officinale Roscoe of two geographical origins. Food Chem. 2018, 240, 239–244. [Google Scholar] [CrossRef]
- Hein, J.A.; Sherrard, M.E.; Manfredi, K.P.; Abebe, T. The fifth leaf and spike organs of barley (Hordeum vulgare L.) display different physiological and metabolic responses to drought stress. BMC Plant Biol. 2016, 16, 248. [Google Scholar] [CrossRef] [PubMed]
- Rajaprakasam, S.; Shanmugavel, P.; Chockalingam, V.; Jegadeesan, S.; Latha, T.K.S.; Ananthan, S.N.; Muthurajan, R.; Kanagarajan, S. Comparative Metabolomic Profiling of Horse Gram (Macrotyloma uniflorum (Lam.) Verdc.) Genotypes for Horse Gram Yellow Mosaic Virus Resistance. Metabolites 2023, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Carroll, A.J.; Estavillo, G.M.; Rebetzke, G.J.; Pogson, B.J. Wheat drought tolerance in the field is predicted by amino acid responses to glasshouse-imposed drought. J. Exp. Bot. 2019, 70, 4931–4948. [Google Scholar] [CrossRef] [PubMed]
- Manickam, S.; Rajagopalan, V.R.; Kambale, R.; Rajasekaran, R.; Kanagarajan, S.; Muthurajan, R. Plant Metabolomics: Current Initiatives and Future Prospects. Curr. Issues Mol. Biol. 2023, 45, 8894–8906. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Tang, C.; Uyanga, V.A.; Xu, L.; Zhang, F.; Sun, J.; Chen, Y. Flavonoids-targeted metabolomic analysis following rice yellowing. Food Chem. 2024, 430, 136984. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhao, M.; Hu, Y.; Meng, F.; Song, X.; Tigabu, M.; Chiang, V.L.; Sederoff, R.; Ma, W.; et al. Molecular and Metabolic Insights into Anthocyanin Biosynthesis for Leaf Color Change in Chokecherry (Padus virginiana). Int. J. Mol. Sci. 2021, 22, 10697. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Shi, L.; Shen, P.; Li, Y. Leaf color formation mechanisms in Alternanthera bettzickiana elucidated by metabolite and transcriptome analyses. Planta 2022, 255, 59. [Google Scholar] [CrossRef]
- Voss, D.M.; Tang, F.; Riedl, K.M.; Miyagusuku-Cruzado, G.; Yao, S.; Rodriguez-Saona, L.; Hatzakis, E.; Giusti, M.M. Structure elucidation of 4-carboxy-3-deoxyanthocyanidins formed from thermal degradation of hydroxyphenyl-pyranoanthocyanins. New J. Chem. 2023, 47, 20048–20060. [Google Scholar] [CrossRef]
- Sytar, O.; Zivcak, M.; Bruckova, K.; Brestic, M.; Hemmerich, I.; Rauh, C.; Simko, I. Shift in accumulation of flavonoids and phenolic acids in lettuce attributable to changes in ultraviolet radiation and temperature. Sci. Hortic. 2018, 239, 193–204. [Google Scholar] [CrossRef]
- Qian, Q.; Zhang, H.; Zhang, P.; Lan, Z.; Wang, H.; Hou, D. Integrated transcriptomics and metabolomics provide new insights into the leaf coloration of a bamboo variant Phyllostachys violascens var. Flavistriatus. Sci. Hortic. 2024, 334, 113330. [Google Scholar] [CrossRef]
- Gu, C.; Xu, H.; Yuan, Q.; Huang, J.; Yuan, K.; Zhao, Y.; Liu, G.; Zhang, Q.; Jiang, J. Epigenetic Regulation of Anthocyanin Biosynthesis in Betula pendula ‘Purple Rain’. Int. J. Mol. Sci. 2024, 25, 12030. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, R.; Han, H.; Zhang, L.; Wang, F.; Zhang, N.; Dong, R. Transcriptome and Metabolome Combine to Analyze the Mechanism of Leaf Coloration Formation in Aeonium arboreum ‘Pink Sybil’. Plant Mol. Biol. Report. 2024. [Google Scholar] [CrossRef]
- Wang, L.; Qu, L.; Li, H.; Wang, T.; Hu, X.; Yuan, X.; Guo, X. The Effects of Dual Ozone and Drought Stresses on the Photosynthetic Properties of Acer rubrum and A. pictum. Forests 2023, 14, 998. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, B.; Dai, G.; He, X.; Zhou, X.; Huang, T.; Liang, X.; Zhao, J.; Qin, K. Integrated Analysis of Transcriptome and Metabolome Reveals New Insights into the Molecular Mechanism Underlying the Color Differences in Wolfberry (Lycium barbarum). Agronomy 2023, 13, 1926. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.; Han, H.; Ma, Y.; Xie, X.; Feng, Y.; Zhao, Q.; Dong, Y.; Zhao, X.; Gu, H.; et al. Transcriptome and metabolite profiling analyses reveal the molecular mechanism underlying the characteristic accumulation of anthocyanins and flavonols in Fritillaria unibracteata and F. delavayi. Ind. Crops Prod. 2022, 186, 115183. [Google Scholar] [CrossRef]
- Kundan, M.; Gani, U.; Fayaz, M.; Angmo, T.; Kesari, R.; Rahul, V.P.; Gairola, S.; Misra, P. Two R2R3-MYB transcription factors, CsMYB33 and CsMYB78 are involved in the regulation of anthocyanin biosynthesis in Cannabis sativa L. Ind. Crops Prod. 2022, 188, 115546. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, Y.; Vainstein, A.; Chen, S.; Ma, H. Regulation of Fig (Ficus carica L.) Fruit Color: Metabolomic and Transcriptomic Analyses of the Flavonoid Biosynthetic Pathway. Front. Plant Sci. 2017, 8, 1990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zheng, J.; Chen, X.; Shi, X.; Wang, H.; Fu, Q. Comprehensive Analysis of Transcriptome and Metabolome Reveals the Flavonoid Metabolic Pathway Is Associated with Fruit Peel Coloration of Melon. Molecules 2021, 26, 2830. [Google Scholar] [CrossRef]
- Yu, M.; Man, Y.; Lei, R.; Lu, X.; Wang, Y. Metabolomics Study of Flavonoids and Anthocyanin-Related Gene Analysis in Kiwifruit (Actinidia chinensis) and Kiwiberry (Actinidia arguta). Plant Mol. Biol. Report. 2020, 38, 353–369. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Lam, P.Y.; Wang, L.; Lui, A.C.W.; Liu, H.; Takeda-Kimura, Y.; Chen, M.-X.; Zhu, F.-Y.; Zhang, J.; Umezawa, T.; Tobimatsu, Y.; et al. Deficiency in flavonoid biosynthesis genesCHS, CHI, andCHILalters rice flavonoid and lignin profiles. Plant Physiol. 2022, 188, 1993–2011. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, S.B.; Littleton, E.S.; Clark, L.C.; Puller, G.C.; Kojima, S.; Winkel, B.S.J. Mutations that alter Arabidopsis flavonoid metabolism affect the circadian clock. Plant J. 2022, 110, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Yuan, Q.; Xiong, C.; Xu, H.; Hu, B.; Suo, H.; Yang, S.; Hou, X.; Yuan, F.; et al. The bHLH1-DTX35/DFR module regulates pollen fertility by promoting flavonoid biosynthesis in Capsicum annuum L. Hortic. Res. 2022, 9, uhac172. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.S.; Choudhary, N.; Pucker, B. Conserved amino acid residues and gene expression patterns associated with the substrate preferences of the competing enzymes FLS and DFR. PLoS ONE 2024, 19, e0305837. [Google Scholar]
- Gao, H.; Zhang, Y.; Duan, Q.; Ren, Q.; Deng, L.; Huo, Y.; Zhang, B.; Zhen, X. Widely Targeted Metabolomics Analyses Clarify the Biosynthetic Pathways Responsible for Flavonoids in Sweet Potato (Ipomoea batatas (L.) Lam.) Storage Roots. Agriculture 2023, 13, 1955. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, W.; Fu, R.; Zhang, Y. Genome-wide characterization of 2-oxoglutarate and Fe(II)-dependent dioxygenase family genes in tomato during growth cycle and their roles in metabolism. BMC Genom. 2021, 22, 126. [Google Scholar] [CrossRef]
- Farrow, S.C.; Facchini, P.J. Papaverine 7-O-demethylase, a novel 2-oxoglutarate/Fe2+-dependent dioxygenase from opium poppy. FEBS Lett. 2015, 589, 2701–2706. [Google Scholar] [CrossRef]
- Jiang, T.; Cui, A.; Cui, Y.; Cui, R.; Han, M.; Zhang, Y.; Fan, Y.; Huang, H.; Feng, X.; Lei, Y.; et al. Systematic analysis and expression of Gossypium 2ODD superfamily highlight the roles of GhLDOXs responding to alkali and other abiotic stress in cotton. BMC Plant Biol. 2023, 23, 124. [Google Scholar] [CrossRef]
- Wang, H.; Takano, T.; Liu, S. Screening and Evaluation of Saline–Alkaline Tolerant Germplasm of Rice (Oryza sativa L.) in Soda Saline–Alkali Soil. Agronomy 2018, 8, 205. [Google Scholar] [CrossRef]
- Zhu, S.; Cui, M.; Zhao, Q. Characterization of the 2ODD genes of DOXC subfamily and its members involved in flavonoids biosynthesis in Scutellaria baicalensis. BMC Plant Biol. 2024, 24, 804. [Google Scholar] [CrossRef]
- Pei, T.; Zhu, S.; Liao, W.; Fang, Y.; Liu, J.; Kong, Y.; Yan, M.; Cui, M.; Zhao, Q. Gap-free genome assembly and CYP450 gene family analysis reveal the biosynthesis of anthocyanins in Scutellaria baicalensis. Hortic. Res. 2023, 10, uhad235. [Google Scholar] [CrossRef] [PubMed]
- Coupel-Ledru, A.; Westgeest, A.J.; Albasha, R.; Millan, M.; Pallas, B.; Doligez, A.; Flutre, T.; Segura, V.; This, P.; Torregrosa, L.; et al. Clusters of grapevine genes for a burning world. New Phytol. 2024, 242, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, H.; Zhang, W.; Huang, L.; Zhu, J. Transcriptomic Analysis of the Differences in Leaf Color Formation during Stage Transitions in Populus × euramericana ‘Zhonghuahongye’. Agronomy 2022, 12, 2396. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, M.; Liu, Y.; Ding, H.; Du, H.; Sun, Z.; Zhu, J. Metabolome and Transcriptome Analyses Reveal Different Flavonoid Biosynthesis and Chlorophyll Metabolism Profiles between Red Leaf and Green Leaf of Eucommia ulmoides. Forests 2021, 12, 1260. [Google Scholar] [CrossRef]
- Vasilev, N.; Boccard, J.; Lang, G.; Grömping, U.; Fischer, R.; Goepfert, S.; Rudaz, S.; Schillberg, S. Structured plant metabolomics for the simultaneous exploration of multiple factors. Sci. Rep. 2016, 6, 37390. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Yu, R.; Liu, Y.; Liu, J.; Zhang, X.; Gong, Z.; Qu, T. Metabolomics Combined with Transcriptomics Analysis Reveals the Regulation of Flavonoids in the Leaf Color Change of Acer truncatum Bunge. Int. J. Mol. Sci. 2024, 25, 13325. https://doi.org/10.3390/ijms252413325
Sun Y, Yu R, Liu Y, Liu J, Zhang X, Gong Z, Qu T. Metabolomics Combined with Transcriptomics Analysis Reveals the Regulation of Flavonoids in the Leaf Color Change of Acer truncatum Bunge. International Journal of Molecular Sciences. 2024; 25(24):13325. https://doi.org/10.3390/ijms252413325
Chicago/Turabian StyleSun, Yinglun, Ran Yu, Yushan Liu, Jian Liu, Xinyu Zhang, Zaixin Gong, and Tongbao Qu. 2024. "Metabolomics Combined with Transcriptomics Analysis Reveals the Regulation of Flavonoids in the Leaf Color Change of Acer truncatum Bunge" International Journal of Molecular Sciences 25, no. 24: 13325. https://doi.org/10.3390/ijms252413325
APA StyleSun, Y., Yu, R., Liu, Y., Liu, J., Zhang, X., Gong, Z., & Qu, T. (2024). Metabolomics Combined with Transcriptomics Analysis Reveals the Regulation of Flavonoids in the Leaf Color Change of Acer truncatum Bunge. International Journal of Molecular Sciences, 25(24), 13325. https://doi.org/10.3390/ijms252413325





