Interaction of HERVs with PAMPs in Dysregulation of Immune Response Cascade Upon SARS-CoV-2 Infections
Abstract
:1. Introduction
2. The Role of HERVs in Disease
3. Lung Cancer and HERVs: lncRNA Role in Disease Pathogenesis
4. Role of HERVs in Natural Immunity Response
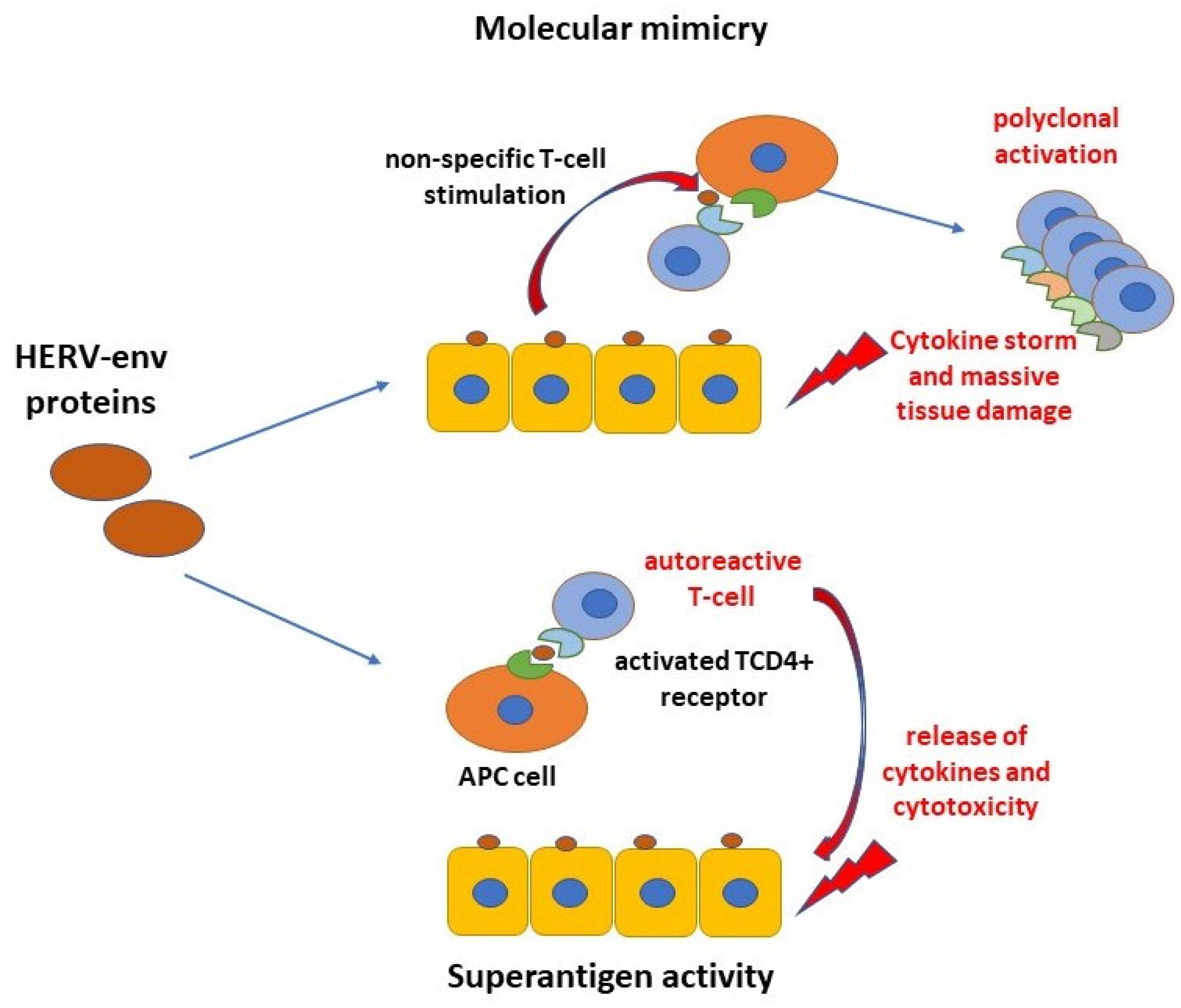
5. Activation of HERV in COVID-19 Patients
6. Implications for HERV Elements Activation Upon COVID-19 Vaccination
7. Conclusive Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Barbulescu, M.; Turner, G.; Seaman, M.I.; Deinard, A.S.; Kidd, K.K.; Lenz, J. Many human endogenous retrovirus K (HERV-K) proviruses are unique to humans. Curr. Biol. 1999, 9, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Vargiu, L.; Rodriguez-Tomé, P.; Sperber, G.O.; Cadeddu, M.; Grandi, N.; Blikstad, V.; Tramontano, E.; Blomberg, J. Classification and characterization of human endogenous retroviruses; mosaic forms are common. Retrovirology 2016, 13, 7. [Google Scholar] [CrossRef]
- Contreras-Galindo, R.; Kaplan, M.H.; Dube, D.; Gonzalez-Hernandez, M.J.; Chan, S.; Meng, F.; Dai, M.; Omenn, G.S.; Gitlin, S.D.; Markovitz, D.M. Human Endogenous Retrovirus Type K (HERV-K) Particles Package and Transmit HERV-K Related Sequences. J. Virol. 2015, 89, 7187–7201. [Google Scholar] [CrossRef]
- Marchi, E.; Kanapin, A.; Magiorkinis, G.; Belshaw, R. Unfixed endogenous retroviral insertions in the human population. J. Virol. 2014, 88, 9529–9537. [Google Scholar] [CrossRef]
- Petrone, V.; Fanelli, M.; Giudice, M.; Toschi, N.; Conti, A.; Maracchioni, C.; Iannetta, M.; Resta, C.; Cipriani, C.; Miele, M.T.; et al. Expression profile of HERVs and inflammatory mediators detected in nasal mucosa as a predictive biomarker of COVID-19 severity. Front. Microbiol. 2023, 14, 1155624. [Google Scholar] [CrossRef]
- Nelson, P.N.; Carnegie, P.R.; Martin, J.; Davari Ejtehadi, H.; Hooley, P.; Roden, D.; Rowland-Jones, S.; Warren, P.; Astley, J.; Murray, P.G. Demystified... Human endogenous retroviruses. Mol. Pathol. 2003, 56, 11–18. [Google Scholar] [CrossRef]
- Durnaoglu, S.; Lee, S.K.; Ahnn, J. Syncytin, envelope protein of human endogenous retrovirus (HERV): No longer ‘fossil’ in human genome. Anim. Cells Syst. 2022, 25, 358–368. [Google Scholar] [CrossRef]
- Rowe, H.M.; Trono, D. Dynamic control of endogenous retroviruses during development. Virology 2011, 411, 273–287. [Google Scholar] [CrossRef]
- Buzdin, A.A.; Prassolov, V.; Garazha, A.V. Friends-Enemies: Endogenous Retroviruses Are Major Transcriptional Regulators of Human DNA. Front. Chem. 2017, 5, 35. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, X.-F.; Chen, T. Human endogenous retroviruses in cancer: Expression, regulation and function. Oncol. Lett. 2021, 21, 121. [Google Scholar] [CrossRef]
- Wildschutte, J.H.; Williams, Z.H.; Montesion, M.; Subramanian, R.P.; Kidd, J.M.; Coffin, J.M. Discovery of unfixed endogenous retrovirus insertions in diverse human populations. Proc. Natl. Acad. Sci. USA 2016, 113, E2326–E2334. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Kusama, K.; Kaneko-Ishino, T.; Nakagawa, S.; Kitao, K.; Miyazawa, T.; Ishino, F. Endogenous Retroviruses and Placental Evolution, Development, and Diversity. Cells 2022, 11, 2458. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Grandi, N.; Tramontano, E.; Dieci, G. Retrotransposons as drivers of mammalian brain evolution. Life 2021, 11, 376. [Google Scholar] [CrossRef]
- Russ, E.; Iordanskiy, S. Endogenous Retroviruses as Modulators of Innate Immunity. Pathogens 2023, 12, 162. [Google Scholar] [CrossRef]
- Balestrieri, E.; Minutolo, A.; Petrone, V.; Fanelli, M.; Iannetta, M.; Malagnino, V.; Zordan, M.; Vitale, P.; Charvet, B.; Horvat, B.; et al. Evidence of the pathogenic HERV-W envelope expression in T lymphocytes in association with the respiratory outcome of COVID-19 patients. EBioMedicine 2021, 66, 103341. [Google Scholar] [CrossRef]
- Li, Y.; Fan, T.; Cui, J. Human endogenous retroviruses in viral disease and therapy. Clin. Transl. Discov. 2022, 2, e38. [Google Scholar] [CrossRef]
- Karimi, A.; Ghadiri-Moghaddam, F.; Valipour, M.; Yahyavi, Y. Effects of prolonged exposure to ELF-EMF on HERVs expression in human melanoma cells. Mol. Biol. Res. Commun. 2022, 11, 67–71. [Google Scholar] [CrossRef]
- Marston, J.L.; Greenig, M.; Singh, M.; Bendall, M.L.; Duarte, R.R.R.; Feschotte, C.; Iñiguez, L.P.; Nixon, D.F. SARS-CoV-2 infection mediates differential expression of human endogenous retroviruses and long interspersed nuclear elements. JCI Insight 2021, 6, e147170. [Google Scholar] [CrossRef]
- Grandi, N.; Tramontano, E. Human endogenous retroviruses are ancient acquired elements still shaping innate immune responses. Front. Immunol. 2018, 9, 2039. [Google Scholar] [CrossRef]
- Durnaoglu, S.; Lee, S.K.; Ahnn, J. Human Endogenous Retroviruses as Gene Expression Regulators: Insights from Animal Models into Human Diseases. Mol. Cells 2021, 44, 861–878. [Google Scholar] [CrossRef]
- Wieland, L.; Schwarz, T.; Engel, K.; Volkmer, I.; Krüger, A.; Tarabuko, A.; Junghans, J.; Kornhuber, M.E.; Hoffmann, F.; Staege, M.S.; et al. Epstein-Barr Virus-Induced Genes and Endogenous Retroviruses in Immortalized B Cells from Patients with Multiple Sclerosis. Cells 2022, 11, 3619. [Google Scholar] [CrossRef]
- Kremer, D.; Gruchot, J.; Weyers, V.; Oldemeier, L.; Göttle, P.; Healy, L.; Ho Jang, J.; Kang TXu, Y.; Volsko, C.; Dutta, R.; et al. pHERV-W envelope protein fuels microglial cell-dependent damage of myelinated axons in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2019, 116, 15216–15225. [Google Scholar] [CrossRef] [PubMed]
- Charvet, B.; Pierquin, J.; Brunel, J.; Gorter, R.; Quétard, C.; Horvat, B.; Amor, S.; Portoukalian, J.; Perron, H. Human endogenous retrovirus type W envelope from multiple sclerosis demyelinating lesions shows unique solubility and antigenic characteristics. Virol. Sin. 2021, 36, 1006–1026. [Google Scholar] [CrossRef] [PubMed]
- Curtin, F.; Perron, H.; Kromminga, A.; Porchet, H.; Lang, A.B. Preclinical and early clinical development of GNbAC1, a humanized IgG4 monoclonal antibody targeting endogenous retroviral MSRV-Env protein. MAbs 2015, 7, 265–275. [Google Scholar] [CrossRef]
- Dai, L.; Del Valle, L.; Miley, W.; Whitby, D.; Ochoa, A.C.; Flemington, E.K.; Qin, Z. Transactivation of human endogenous retrovirus K (HERV-K) by KSHV promotes Kaposi’s Sarcoma development. Oncogene 2018, 37, 4534–4545. [Google Scholar] [CrossRef] [PubMed]
- Mangeney, M.; Renard, M.; Schlecht-Louf, G.; Bouallaga, I.; Heidmann, O.; Letzelter, C.; Richaud, A.; Ducos, B.; Heidmann, T. Placental syncytins: Genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc. Natl. Acad. Sci. USA 2007, 104, 20534–20539. [Google Scholar] [CrossRef]
- Takahashi, A.; Day, N.K.; Luangwedchakarn, V.; Good, R.A.; Haraguchi, S. A retroviral-derived immunosuppressive peptide activates mitogen-activated protein kinases. J. Immunol. 2001, 166, 6771–6775. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Thommen, D.S. Tertiary lymphoid structures in cancer. Science 2022, 375, eabf9419. [Google Scholar] [CrossRef]
- Laumont, C.M.; Banville, A.C.; Gilardi, M.; Hollern, D.P.; Nelson, B.H. Tumour-infiltrating B cells: Immunological mechanisms, clinical impact and therapeutic opportunities. Nat. Rev. Cancer 2022, 22, 414–430. [Google Scholar] [CrossRef]
- Preuss, K.D.; Zwick, C.; Bormann, C.; Neumann, F.; Pfreundschuh, M. Analysis of the B-cell repertoire against antigens expressed by human neoplasms. Immunol. Rev. 2002, 188, 43–50. [Google Scholar] [CrossRef]
- Kassiotis, G.; Stoye, J.P. Immune responses to endogenous retroelements: Taking the bad with the good. Nat. Rev. Immunol. 2016, 16, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, X.; Zhu, Z.; Gorelik, E. Sequence and insertion sites of murine melanoma-associated retrovirus. J. Virol. 1999, 73, 9178–9186. [Google Scholar] [CrossRef] [PubMed]
- Pothlichet, J.; Mangeney, M.; Heidmann, T. Mobility and integration sites of a murine C57BL/6 melanoma endogenous retrovirus involved in tumor progression in vivo. Int. J. Cancer 2006, 119, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Ottina, E.; Levy, P.; Eksmond, U.; Merkenschlager, J.; Young, G.R.; Roels, J.; Stoye, J.P.; Tüting, T.; Calado, D.P.; Kassiotis, G. Restoration of endogenous retrovirus infectivity impacts mouse cancer models. Cancer Immunol. Res. 2018, 6, 1292–1300. [Google Scholar] [CrossRef]
- Global Cancer Facts & Figures 2021 American Cancer Society. 2021. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf (accessed on 15 July 2021).
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Gabriel, U.; Steidler, A.; Trojan, L.; Michel, M.S.; Seifarth, W.; Fabarius, A. Smoking increases transcription of human endogenous retroviruses in a newly established in vitro cell model and in normal urothelium. AIDS Res. Hum. Retroviruses 2010, 26, 883–888. [Google Scholar] [CrossRef]
- Kahyo, T.; Tao, H.; Shinmura, K.; Yamada, H.; Mori, H.; Funai, K.; Kurabe, N.; Suzuki, M.; Tanahashi, M.; Niwa, H.; et al. Identification and association study with lung cancer for novel insertion polymorphisms of human endogenous retrovirus. Carcinogenesis 2013, 34, 2531–2538. [Google Scholar] [CrossRef]
- Deakin, C.T.; Cornish, G.H.; Ng, K.W.; Faulkner, N.; Bolland, W.; Hope, J.; Rosa, A.; Harvey, R.; Hussain, S.; Earl, C.; et al. Favorable antibody responses to human coronaviruses in children and adolescents with autoimmune rheumatic diseases. Med 2021, 2, 1093–1109.e6. [Google Scholar] [CrossRef]
- Tokuyama, M.; Gunn, B.M.; Venkataraman, A.; Kong, Y.; Kang, I.; Rakib, T.; Townsend, M.J.; Costenbader, K.H.; Alter, G.; Iwasaki, A. Antibodies against human endogenous retrovirus K102 envelope activate neutrophils in systemic lupus erythematosus. J. Exp. Med. 2021, 218, e20191766. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, B.; Sun, L.; Yan, X.; Xu, J. Identification of candidate genes or microRNAs associated with the lymph node metastasis of SCLC. Cancer Cell Int. 2018, 18, 161. [Google Scholar] [CrossRef]
- Wei, Y.; Yan, Z.; Wu, C.; Zhang, Q.; Zhu, Y.; Li, K.; Xu, Y. Integrated analysis of dosage effect lncRNAs in lung adenocarcinoma based on comprehensive network. Oncotarget 2017, 8, 71430–71446. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Boumelha, J.; Enfield, K.S.S.; Almagro, J.; Cha, H.; Pich, O.; Karasaki, T.; Moore, D.A.; Salgado, R.; Sivakumar, M.; et al. Antibodies against endogenous retroviruses promote lung cancer immunotherapy. Nature 2023, 616, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, P.; Mulholland, K.A.; Hu, H.; Park, J.; Sheng, X.; Abedini, A.; Liu, H.; Vassalotti, A.; Wu, J.; Susztak, K. Increased levels of endogenous retroviruses trigger fibroinflammation and play a role in kidney disease development. Nat Commun 2023, 14, 559. [Google Scholar] [CrossRef] [PubMed]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science 2016, 351, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Sugimoto, R.; Nakaoka, H.; Yamada, S.; Kimura, T.; Hayano, T.; Inoue, I. Systematic identification and characterization of regulatory elements derived from human endogenous retroviruses. PLoS Genet. 2017, 13, e1006883. [Google Scholar] [CrossRef]
- Rolland, A.; Jouvin-Marche, E.; Viret, C.; Faure, M.; Perron, H.; Marche, P.N. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J. Immunol. 2006, 176, 7636–7644. [Google Scholar] [CrossRef]
- Serra, C.; Mameli, G.; Arru, G.; Sotgiu, S.; Rosati, G.; Dolei, A. In vitro modulation of the multiple sclerosis (MS)-associated retrovirus by cytokines: Implications for MS pathogenesis. J. Neurovirol. 2003, 9, 637–643. [Google Scholar] [CrossRef]
- Ng, K.W.; Attig, J.; Bolland, W.; Young, G.R.; Major, J.; Wrobel, A.G.; Gamblin, S.; Wack, A.; Kassiotis, G. Tissue-specific and interferon-inducible expression of nonfunctional ACE2 through endogenous retroelement co-option. Nat. Genet. 2020, 52, 1294–1302. [Google Scholar] [CrossRef]
- Grandi, N.; Tramontano, E. HERV Envelope Proteins: Physiological Role and Pathogenic Potential in Cancer and Autoimmunity. Front. Microbiol. 2018, 9, 462. [Google Scholar] [CrossRef]
- Mikhalkevich, N.; O’Carroll, I.P.; Tkavc, R.; Lund, K.; Sukumar, G.; Dalgard, C.L.; Johnson, K.R.; Li, W.; Wang, T.; Nath, A.; et al. Response of human macrophages to gamma radiation is mediated via expression of endogenous retroviruses. PLOS Pathog. 2021, 17, e1009305. [Google Scholar] [CrossRef]
- Domingo, J.L. An updated review of the scientific literature on the origin of SARS-CoV-2. Environ. Res. 2022, 215 Pt 1, 114131. [Google Scholar] [CrossRef]
- Bruttel, V.; Washburne, A.; VanDongen, A. Endonuclease fingerprint indicates a synthetic origin of SARS-CoV-2. BioRxiv 2023. [Google Scholar] [CrossRef]
- V‘kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Mohamadian, M.; Chiti, H.; Shoghli, A.; Biglari, S.; Parsamanesh, N.; Esmaeilzadeh, A. COVID-19: Virology, biology and novel laboratory diagnosis. J. Gene Med. 2021, 23, e3303. [Google Scholar] [CrossRef]
- Que, Y.; Hu, C.; Wan, K.; Hu, P.; Wang, R.; Luo, J.; Li, T.; Ping, R.; Hu, Q.; Sun, Y.; et al. Cytokine release syndrome in COVID-19: A major mechanism of morbidity and mortality. Int. Rev. Immunol. 2022, 41, 217–230. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Jaleel, A. Post-Acute Sequelae of COVID-19: What to do Next? Pak. J. Med. Dent. 2023, 12, 3–4. [Google Scholar] [CrossRef]
- Paladino, L.; Vitale, A.M.; Caruso Bavisotto, C.; Conway de Macario, E.; Cappello, F.; Macario, A.J.L.; Gammazza, A.M. The Role of Molecular Chaperones in Virus Infection and Implications for Understanding and Treating COVID-19. J. Clin. Med. 2020, 9, 3518. [Google Scholar] [CrossRef]
- Galeotti, C.; Bayry, J. Autoimmune and inflammatory diseases following COVID-19. Nat. Rev. Rheumatol. 2020, 16, 413–414. [Google Scholar] [CrossRef]
- Liu, Y.; Sawalha, A.H.; Lu, Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021, 33, 155–162. [Google Scholar] [CrossRef]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.-S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1beta, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef]
- Tovo, P.A.; Garazzino, S.; Daprà, V.; Pruccoli, G.; Calvi, C.; Mignone, F.; Alliaudi, C.; Denina, M.; Scolfaro, C.; Zoppo, M.; et al. COVID-19 in children: Expressions of type I/II/III interferons, TRIM28, SETDB1, and endogenous retroviruses in mild and severe cases. Int. J. Mol. Sci. 2021, 22, 7481. [Google Scholar] [CrossRef] [PubMed]
- Ngara, M.; Siwo, G.H. Molecular relationships between SARS-CoV-2 Spike protein and LIFR, a pneumonia protective IL-6 family cytokine receptor. bioRxiv 2021. [Google Scholar] [CrossRef]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Gebrecherkos, T.; Challa, F.; Tasew, G.; Gessesse, Z.; Kiros, Y.; Gebreegziabxier, A.; Abdulkader, M.; Desta, A.A.; Atsbaha, A.H.; Tollera, G.; et al. Prognostic Value of C-Reactive Protein in SARS-CoV-2 Infection: A Simplified Biomarker of COVID-19 Severity in Notrhen Etiopia. Infect. Drug Resist. 2023, 16, 3019–3028. [Google Scholar] [CrossRef]
- Abdullah, A.J.; Arif, A.T.; Rahman, H.A.; Sofihussein, K.Q.; Hadi, J.M.; Aziz, J.M.A.; Tofiq, S.S.; Mustafa, A.M. Assessing serum C-reactive protein as a predictor of COVID-19 outcomes: A retrospective cross-sectional study. Ann. Med. Surg. 2023, 85, 3359–3363. [Google Scholar] [CrossRef]
- Lorkiewicz, P.; Waszkiewicz, N. Biomarkers of Post-COVID Depression. J. Clin. Med. 2021, 10, 4142. [Google Scholar] [CrossRef]
- Effendy, E.; Mardhiyah, S.A. COVID-19 survivors: The link between C-reactive protein and psychopathologies. IJID Reg. 2023, 8, S27–S30. [Google Scholar] [CrossRef]
- Garcia-Montojo, M.; Nath, A. HERV-W envelope expression in blood leukocytes as a marker of disease severity of COVID-19. EBioMedicine 2021, 67, 103363. [Google Scholar] [CrossRef]
- Brudek, T.; Christensen, T.; Aagaard, L.; Petersen, T.; Hansen, H.J.; Møller-Larsen, A. B cells and monocytes from patients with active multiple sclerosis exhibit increased surface expression of both HERV-H Env and HERV-W Env, accompanied by increased seroreactivity. Retrovirology 2009, 6, 104. [Google Scholar] [CrossRef]
- Giménez-Orenga, K.; Pierquin, J.; Brunel, J.; Charvet, B.; Martín-Martínez, E.; Perron, H.; Oltra, E. HERV-W ENV antigenemia and correlation of increased anti-SARS-CoV-2 immunoglobulin levels with post-COVID-19 symptoms. Front. Immunol. 2022, 13, 1020064. [Google Scholar] [CrossRef]
- Lee, E.E.; Song, K.H.; Hwang, W.; Ham, S.Y.; Jeong, H.; Kim, J.H.; Oh, H.S.; Kang, Y.M.; Lee, E.B.; Kim, N.J.; et al. Pattern of inflammatory immune response determines the clinical course and outcome of COVID19: Unbiased clustering analysis. Sci. Rep. 2021, 11, 8080. [Google Scholar] [CrossRef]
- Burke, M.J.; Del Rio, C. Long COVID has exposed medicine’s blind-spot. Lancet Infect. Dis. 2021, 21, 1062–1064. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Smith, B. Neurological issues during COVID-19: An overview. Neurosci. Lett. 2021, 742, 135533. [Google Scholar] [CrossRef]
- de Parseval, N.; Lazar, V.; Casella, J.-F.; Benit, L.; Heidmann, T. Survey of human genes of retroviral origin: Identification and transcriptome of the genes with coding capacity for complete envelope proteins. J. Virol. 2003, 77, 10414–10422. [Google Scholar] [CrossRef]
- Ansari, S.; Gupta, N.; Verma, R.; Singh, O.N.; Gupta, J.; Kumar, A.; Yadav, M.K.; Binayke, A.; Tiwari, M.; Periwal, N.; et al. Antiviral activity of the human endogenous retrovirus-R envelope protein against SARS-CoV-2. EMBO Rep. 2023, 24, e55900. [Google Scholar] [CrossRef]
- Bustamante Rivera, Y.Y.; Brütting, C.; Schmidt, C.; Volkmer, I.; Staege, M.S. Endogenous retrovirus 3–history, physiology, and pathology. Front. Microbiol. 2018, 8, 2691. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kanneganti, T.D. Innate immunity: The first line of defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Castro Dopico, X.; Ols, S.; Loré, K.; Karlsson Hedestam, G.B. Immunity to SARS-CoV-2 induced by infection or vaccination. J. Intern. Med. 2022, 291, 32–50. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets f or vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-kappaB pathway. eLife 2021, 10, e68563. [Google Scholar] [CrossRef]
- Sariol, A.; Perlman, S. SARS-CoV-2 takes its Toll. Nat. Immunol. 2021, 22, 801–802. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Zhang, L.; Bhushan, A.; Swanson, B.; Zhang, L.; Mamede, J.I.; Voigt, R.M.; Shaikh, M.; Engen, P.A.; Keshavarzian, A. The SARS-CoV-2 S1 Spike Protein Promotes MAPK and NF-kB Activation in Human Lung Cells and Inflammatory Cytokine Production in Human Lung and Intestinal Epithelial Cells. Microorganisms 2022, 10, 1996. [Google Scholar] [CrossRef]
- Theoharides, T.C. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Mol. Neurobiol. 2022, 59, 1850–1861. [Google Scholar] [CrossRef]
- Gudowska-Sawczuk, M.; Mroczko, B. The Role of Neuropilin-1 (NRP-1) in SARS-CoV-2 Infection: Review. J. Clin. Med. 2021, 10, 2772. [Google Scholar] [CrossRef]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 2020, 146, 105131. [Google Scholar] [CrossRef]
- Patterson, B.K.; Francisco, E.B.; Yogendra, R.; Long, E.; Pise, A.; Rodrigues, H.; Hall, E.; Herrera, M.; Parikh, P.; Guevara-Coto, J.; et al. Persistence of SARS-CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection. Front. Immunol. 2022, 12, 746021. [Google Scholar] [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-acute Coronavirus Disease 2019 Sequelae. Clin. Infect. Dis. 2023, 76, e487–e490. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, I.; Janovec, V.; Stranska, R.; Bendriss-Vermare, N. Cross talk between inhibitory immunoreceptor tyrosine-based activation motif-signaling and toll-like receptor pathways in macrophages and dendritic cells. Front. Immunol. 2017, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Charvet, B.; Brunel, J.; Pierquin, J.; Iampietro, M.; Decimo, D.; Queruel, N.; Lucas, A.; Encabo-Berzosa, M.d.M.; Arenaz, I.; Marmolejo, T.P.; et al. SARS-CoV-2 awakens ancient retroviral genes and the expression of proinflammatory HERV-W envelope protein in COVID-19 patients. iScience 2023, 26, 106604. [Google Scholar] [CrossRef]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef]
- Balestrieri, E.; Corinaldesi, E.; Fabi, M.; Cipriani, C.; Giudice, M.; Conti, A.; Minutolo, A.; Petrone, V.; Fanelli, M.; Miele, M.T.; et al. Preliminary Evidence of the Differential Expression of Human Endogenous Retroviruses in Kawasaki Disease and SARS-CoV-2-Associated Multisystem Inflammatory Syndrome in Children. Int. J. Mol. Sci. 2023, 24, 15086. [Google Scholar] [CrossRef]
- Grandi, N.; Erbì, M.C.; Scognamiglio, S.; Tramontano, E. Human Endogenous Retrovirus (HERV) Transcriptome Is Dynamically Modulated during SARS-CoV-2 Infection and Allows Discrimination of COVID-19 Clinical Stages. Microbiol. Spectr. 2023, 11, e0251622. [Google Scholar] [CrossRef]
- Plūme, J.; Galvanovskis, A.; Šmite, S.; Romanchikova, N.; Zayakin, P.; Linē, A. Early and strong antibody responses to SARS-CoV-2 predict disease severity in COVID-19 patients. J. Transl. Med. 2022, 20, 176. [Google Scholar] [CrossRef]
- Kempuraj, D.; Selvakumar, G.P.; Ahmed, M.E.; Raikwar, S.P.; Thangavel, R.; Khan, A.; Zaheer, S.A.; Iyer, S.S.; Burton, C.; James, D.; et al. COVID-19, Mast Cells, Cytokine Storm, Psychological Stress, and Neuroinflammation. Neuroscientist 2020, 26, 402–414. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Gychka, S.G. SARS-CoV-2 Spike Protein Elicits Cell Signaling in Human Host Cells: Implications for Possible Consequences of COVID-19 Vaccines. Vaccines 2021, 9, 36. [Google Scholar] [CrossRef]
- Danese, E.; Montagnana, M.; Salvagno, G.L.; Peserico, D.; Pighi, L.; De Nitto, S.; Henry, B.M.; Porru, S.; Lippi, G. Comprehensive assessment of humoral response after Pfizer BNT162b2 mRNA COVID-19 vaccination: A three-case series. Clin. Chem. Lab. Med. 2021, 59, 1585–1591. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Greaney, A.J.; Loes, A.N.; Gentles, L.E.; Crawford, K.H.D.; Starr, T.N.; Malone, K.D.; Chu, H.Y.; Bloom, J.D. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci. Transl. Med. 2021, 13, eabi9915. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, R.; Askari, N. A review of neurological side effects of COVID-19 vaccination. Eur. J. Med. Res. 2023, 28, 102. [Google Scholar] [CrossRef]
- Kyriakopoulos, A.M.; Nigh, G.; McCullough, P.A.; Olivier, M.D.; Seneff, S. Bell’s palsy or an aggressive infiltrating basaloid carcinoma post-mRNA vaccination for COVID-19? A case report and review of the literature. EXCLI J. 2023, 22, 992–1011. [Google Scholar] [CrossRef]
- Eens, S.; Van Hecke, M.; Favere, K.; Tousseyn, T.; Guns, P.J.; Roskams, T.; Heidbuchel, H. B-cell lymphoblastic lymphoma following intravenous BNT162b2 mRNA booster in a BALB/c mouse: A case report. Front. Oncol. 2023, 13, 1158124. [Google Scholar] [CrossRef]
- Cavanna, L.; Grassi, S.O.; Ruffini, L.; Michieletti, E.; Carella, E.; Palli, D.; Zangrandi, A.; Inzerilli, N.; Bernuzzi, P.; Di Nunzio, C.; et al. Non-Hodgkin Lymphoma Developed Shortly after mRNA COVID-19 Vaccination: Report of a Case and Review of the Literature. Medicina 2023, 59, 157. [Google Scholar] [CrossRef]
- Mizutani, M.; Mitsui, H.; Amano, T.; Ogawa, Y.; Deguchi, N.; Shimada, S.; Miwa, A.; Kawamura, T.; Ogido, Y. Two cases of axillary lymphadenopathy diagnosed as diffuse large B-cell lymphoma developed shortly after BNT162b2 COVID-19 vaccination. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e613–e615. [Google Scholar] [CrossRef]
- Wiseman, D.M.; Guetzkow, J.; Pantazatos, S.; Rose, J.; Seligmann, H. National Academies Committee on Review of Relevant Literature Regarding Adverse Events Associated with Vaccines 2023: Written material accompanying oral remarks. Available online: https://nationalcitizensinquiry.b-cdn.net/wp-content/uploads/2023/09/VT-3tt-Makis-Wiseman-06b-Turbo-Cancer-Paper16-Wiseman.pdf (accessed on 15 July 2024).
- Morrish, T.A.; Gilbert, N.; Myers, J.S.; Vincent, B.J.; Stamato, T.D.; Taccioli, G.E.; Batzer, M.A.; Moran, J.V. DNA repair mediated by endonuclease-independent LINE-1 retrotransposition. Nat. Genet. 2002, 31, 159–165. [Google Scholar] [CrossRef]
- Bray, S.; Turnbull, M.; Hebert, S.; Douville, R.N. Insight into the ERVK Integrase—Propensity for DNA Damage. Front. Microbiol. 2016, 7, 1941. [Google Scholar] [CrossRef]
- Martínez-Flores, D.; Zepeda-Cervantes, J.; Cruz-Reséndiz, A.; Aguirre-Sampieri, S.; Sampieri, A.; Vaca, L. SARS-CoV-2 Vaccines Based on the Spike Glycoprotein and Implications of New Viral Variants. Front. Immunol. 2021, 12, 701501. [Google Scholar]
- Kraljević Pavelić, S.; Pavelić, K. Open questions over the COVID-19 pandemic. Sci. Art Relig. 2022, 1, 210–220. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turčić, M.; Kraljević Pavelić, S.; Trivanović, D.; Pavelić, K. Interaction of HERVs with PAMPs in Dysregulation of Immune Response Cascade Upon SARS-CoV-2 Infections. Int. J. Mol. Sci. 2024, 25, 13360. https://doi.org/10.3390/ijms252413360
Turčić M, Kraljević Pavelić S, Trivanović D, Pavelić K. Interaction of HERVs with PAMPs in Dysregulation of Immune Response Cascade Upon SARS-CoV-2 Infections. International Journal of Molecular Sciences. 2024; 25(24):13360. https://doi.org/10.3390/ijms252413360
Chicago/Turabian StyleTurčić, Marijana, Sandra Kraljević Pavelić, Dragan Trivanović, and Krešimir Pavelić. 2024. "Interaction of HERVs with PAMPs in Dysregulation of Immune Response Cascade Upon SARS-CoV-2 Infections" International Journal of Molecular Sciences 25, no. 24: 13360. https://doi.org/10.3390/ijms252413360
APA StyleTurčić, M., Kraljević Pavelić, S., Trivanović, D., & Pavelić, K. (2024). Interaction of HERVs with PAMPs in Dysregulation of Immune Response Cascade Upon SARS-CoV-2 Infections. International Journal of Molecular Sciences, 25(24), 13360. https://doi.org/10.3390/ijms252413360








