The Potency of Cytotoxic Mechanisms of Local Anesthetics in Human Chondrocyte Cells
Abstract
:1. Introduction
2. Results
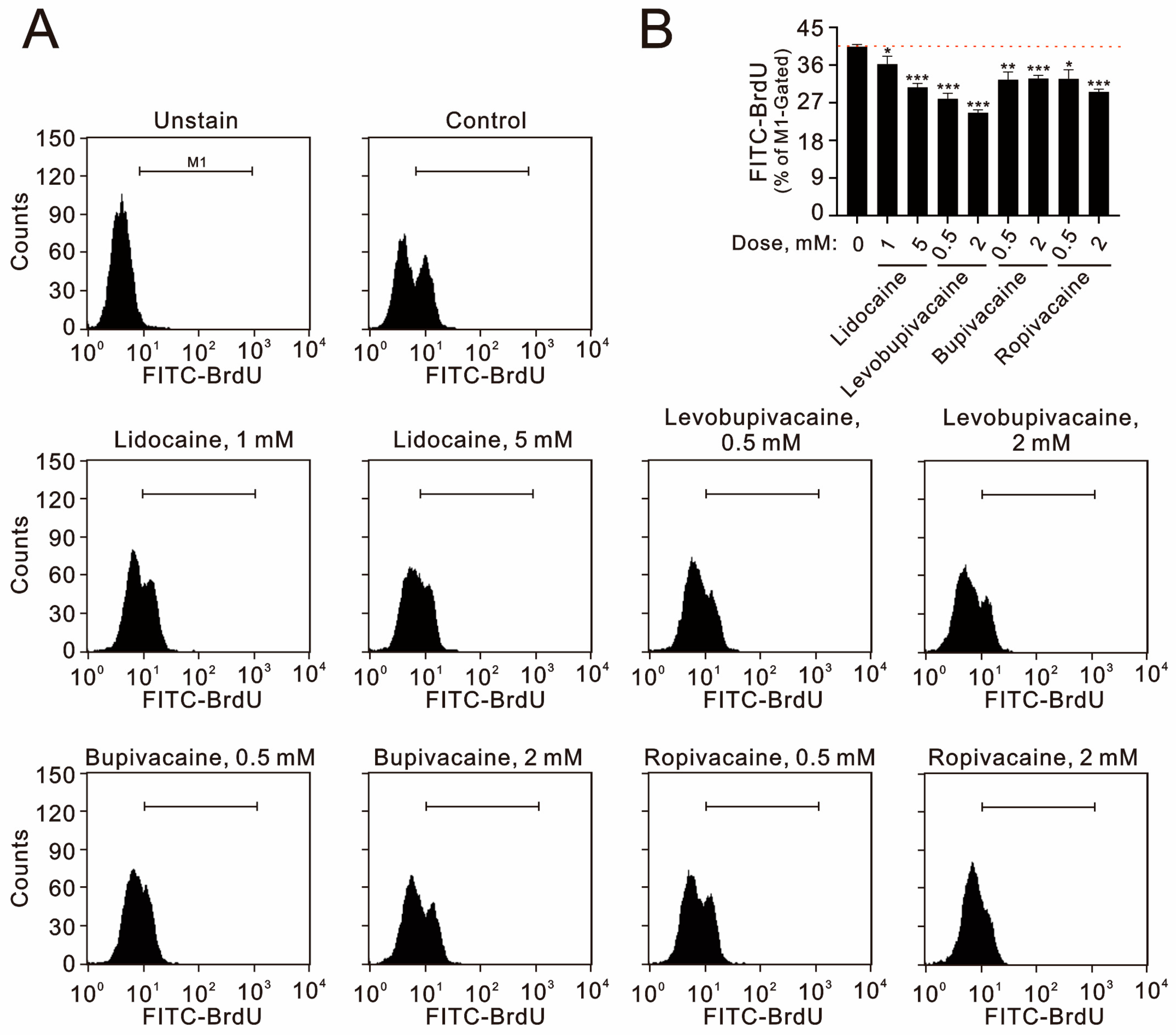
2.1. The Cytotoxic Pathways of Lidocaine, Levobupivacaine, Bupivacaine, and Ropivacaine Were Investigated in Human TC28a2 Chondrocyte Cells
2.2. Local Anesthetics Can Induce the Production of Reactive Oxygen Species (ROS), Alter Mitochondrial Membrane Potential, and Stimulate Autophagy in TC28a2 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Metabolic Activity Analysis
4.3. Fluorescence-Activated Cell Sorting (FACS) for Flow Cytometry Analyses of Cell Cycle Profiles, Apoptosis, Cellular Proliferation, Cytosolic and Mitochondrial ROS, and Mitochondrial Membrane Potential
4.4. Flow Cytometric Quantification of Acidic Vesicular Organelles
4.5. Statistical Analysis
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scholz, A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br. J. Anaesth. 2002, 89, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; McLeod, G. Basic pharmacology of local anaesthetics. BJA Educ. 2020, 20, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Yagiela, J.A. Local Anesthetics: A Century of Progress. Anesth. Prog. 2020, 67, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, V.F.; Anguita-Ortiz, N.; Faraji, S.; Nogueira, J.J. Voltage-Gated Ion Channels: Structure, Pharmacology and Photopharmacology. Chemphyschem 2024, 25, e202400162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhou, M.; Lu, P.; Wang, Y.; Zeng, R.; Liu, L.; Zhu, S.; Kong, L.; Zhang, J. Local anesthetic delivery systems for the management of postoperative pain. Acta Biomater. 2024, 181, 1–18. [Google Scholar] [CrossRef]
- Wolfe, R.C.; Spillars, A. Local Anesthetic Systemic Toxicity: Reviewing Updates From the American Society of Regional Anesthesia and Pain Medicine Practice Advisory. J. Perianesth. Nurs. 2018, 33, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Gulihar, A.; Robati, S.; Twaij, H.; Salih, A.; Taylor, G.J. Articular cartilage and local anaesthetic: A systematic review of the current literature. J. Orthop. 2015, 12, S200–S210. [Google Scholar] [CrossRef] [PubMed]
- Caballero, M.; Kobayashi, Y.; Gottschalk, A.W. Local Anesthetic Use in Musculoskeletal Injections. Ochsner. J. 2022, 22, 200–203. [Google Scholar] [CrossRef]
- Paladini, G.; Di Carlo, S.; Musella, G.; Petrucci, E.; Scimia, P.; Ambrosoli, A.; Cofini, V.; Fusco, P. Continuous Wound Infiltration of Local Anesthetics in Postoperative Pain Management: Safety, Efficacy and Current Perspectives. J. Pain Res. 2020, 13, 285–294. [Google Scholar] [CrossRef]
- Banks, A. Innovations in postoperative pain management: Continuous infusion of local anesthetics. AORN J. 2007, 85, 904–914; quiz 915–918. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, P.C.; Steinwachs, M.; Angele, P. Single-dose local anesthetics exhibit a type-, dose-, and time-dependent chondrotoxic effect on chondrocytes and cartilage: A systematic review of the current literature. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 819–830. [Google Scholar] [CrossRef]
- Dragoo, J.L.; Braun, H.J.; Kim, H.J.; Phan, H.D.; Golish, S.R. The in vitro chondrotoxicity of single-dose local anesthetics. Am. J. Sports Med. 2012, 40, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Korner, J.; Albani, S.; Sudha Bhagavath Eswaran, V.; Roehl, A.B.; Rossetti, G.; Lampert, A. Sodium Channels and Local Anesthetics-Old Friends With New Perspectives. Front. Pharmacol. 2022, 13, 837088. [Google Scholar] [CrossRef]
- Bennett, P.N.; Aarons, L.J.; Bending, M.R.; Steiner, J.A.; Rowland, M. Pharmacokinetics of lidocaine and its deethylated metabolite: Dose and time dependency studies in man. J. Pharmacokinet. Biopharm. 1982, 10, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Steverink, J.G.; Piluso, S.; Malda, J.; Verlaan, J.J. Comparison of in vitro and in vivo Toxicity of Bupivacaine in Musculoskeletal Applications. Front. Pain Res. 2021, 2, 723883. [Google Scholar] [CrossRef]
- Owen, M.D.; Dean, L.S. Ropivacaine. Expert Opin. Pharmacother. 2000, 1, 325–336. [Google Scholar] [CrossRef]
- Webb, S.T.; Ghosh, S. Intra-articular bupivacaine: Potentially chondrotoxic? Br. J. Anaesth. 2009, 102, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, J.; Walsh, P.; Mulhall, K.J. The chondrotoxicity of local anaesthetics: Any clinical impact? Jt. Bone Spine 2011, 78, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Breu, A.; Rosenmeier, K.; Kujat, R.; Angele, P.; Zink, W. The cytotoxicity of bupivacaine, ropivacaine, and mepivacaine on human chondrocytes and cartilage. Anesth. Analg. 2013, 117, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Sorbino, A.; Manocchio, N.; Pirri, N.; Devito, A.; Foti, C.; Migliore, A. Chondrotoxicity of Intra-Articular Injection Treatment: A Scoping Review. Int. J. Mol. Sci. 2024, 25, 7010. [Google Scholar] [CrossRef]
- Long, B.; Chavez, S.; Gottlieb, M.; Montrief, T.; Brady, W.J. Local anesthetic systemic toxicity: A narrative review for emergency clinicians. Am. J. Emerg. Med. 2022, 59, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Yang, C.; Piao, E.; Shi, J.; Zhang, J. Mechanisms of chondrocyte regulated cell death in osteoarthritis: Focus on ROS-triggered ferroptosis, parthanatos, and oxeiptosis. Biochem. Biophys. Res. Commun. 2024, 705, 149733. [Google Scholar] [CrossRef]
- Kubrova, E.; Su, M.; Galeano-Garces, C.; Galvan, M.L.; Jerez, S.; Dietz, A.B.; Smith, J.; Qu, W.; van Wijnen, A.J. Differences in Cytotoxicity of Lidocaine, Ropivacaine, and Bupivacaine on the Viability and Metabolic Activity of Human Adipose-Derived Mesenchymal Stem Cells. Am. J. Phys. Med. Rehabil. 2021, 100, 82–91. [Google Scholar] [CrossRef]
- Mwale, C.; Sunaga, T.; Wang, Y.; Bwalya, E.C.; Wijekoon, H.M.S.; Kim, S.; Okumura, M. In vitro chondrotoxicity of bupivacaine, levobupivacaine and ropivacaine and their effects on caspase activity in cultured canine articular chondrocytes. J. Vet. Med. Sci. 2023, 85, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Liu, S.T.; Huang, S.M.; Wu, Z.F. Apoptosis, Proliferation, and Autophagy Are Involved in Local Anesthetic-Induced Cytotoxicity of Human Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 15455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, M.; Yao, W.; Wan, L. Cytotoxicity of Local Anesthetics on Bone, Joint, and Muscle Tissues: A Narrative Review of the Current Literature. J. Pain Res. 2023, 16, 611–621. [Google Scholar] [CrossRef]
- Grishko, V.; Xu, M.; Wilson, G.; Pearsall, A.W. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J. Bone Jt. Surg. Am. 2010, 92, 609–618. [Google Scholar] [CrossRef]
- Albright, G.A. Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology 1979, 51, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.H.; Markham, A. Levobupivacaine: A review of its pharmacology and use as a local anaesthetic. Drugs 2000, 59, 551–579. [Google Scholar] [CrossRef] [PubMed]
- Gristwood, R.W.; Greaves, J.L. Levobupivacaine: A new safer long acting local anaesthetic agent. Expert Opin. Investig. Drugs 1999, 8, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Armstrong, R.; Urrego, D.; Qazzaz, M.; Pehar, M.; Armstrong, J.N.; Shutt, T.; Syed, N. The mitochondrial division inhibitor Mdivi-1 rescues mammalian neurons from anesthetic-induced cytotoxicity. Mol. Brain 2016, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Irwin, W.; Fontaine, E.; Agnolucci, L.; Penzo, D.; Betto, R.; Bortolotto, S.; Reggiani, C.; Salviati, G.; Bernardi, P. Bupivacaine myotoxicity is mediated by mitochondria. J. Biol. Chem. 2002, 277, 12221–12227. [Google Scholar] [CrossRef] [PubMed]
- Topor, B.; Oldman, M.; Nicholls, B. Best practices for safety and quality in peripheral regional anaesthesia. BJA Educ. 2020, 20, 341–347. [Google Scholar] [CrossRef]
- Schnabel, A.; Meyer-Friessem, C.H.; Zahn, P.K.; Pogatzki-Zahn, E.M. Ultrasound compared with nerve stimulation guidance for peripheral nerve catheter placement: A meta-analysis of randomized controlled trials. Br. J. Anaesth. 2013, 111, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.A.; Ashworth, H.; Nagdev, A. Ultrasound-Guided Nerve Blocks. Emerg. Med. Clin. N. Am. 2024, 42, 905–926. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.P.; Huang, T.H.; Liu, S.T.; Huang, S.M.; Chen, Y.C.; Wu, C.C. Glucosamine and Silibinin Alter Cartilage Homeostasis through Glycosylation and Cellular Stresses in Human Chondrocyte Cells. Int. J. Mol. Sci. 2024, 25, 4905. [Google Scholar] [CrossRef]
- Wu, T.M.; Liu, S.T.; Chen, S.Y.; Chen, G.S.; Wu, C.C.; Huang, S.M. Mechanisms and Applications of the Anti-cancer Effect of Pharmacological Ascorbic Acid in Cervical Cancer Cells. Front. Oncol. 2020, 10, 1483. [Google Scholar] [CrossRef]
- Fan, H.L.; Liu, S.T.; Chang, Y.L.; Chiu, Y.L.; Huang, S.M.; Chen, T.W. In Vitro Cell Density Determines the Sensitivity of Hepatocarcinoma Cells to Ascorbate. Front. Oncol. 2022, 12, 843742. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-L.; Liu, S.-T.; Wu, C.-C.; Chen, Y.-C.; Huang, S.-M. The Potency of Cytotoxic Mechanisms of Local Anesthetics in Human Chondrocyte Cells. Int. J. Mol. Sci. 2024, 25, 13474. https://doi.org/10.3390/ijms252413474
Chen J-L, Liu S-T, Wu C-C, Chen Y-C, Huang S-M. The Potency of Cytotoxic Mechanisms of Local Anesthetics in Human Chondrocyte Cells. International Journal of Molecular Sciences. 2024; 25(24):13474. https://doi.org/10.3390/ijms252413474
Chicago/Turabian StyleChen, Jia-Lin, Shu-Ting Liu, Chia-Chun Wu, Yi-Chou Chen, and Shih-Ming Huang. 2024. "The Potency of Cytotoxic Mechanisms of Local Anesthetics in Human Chondrocyte Cells" International Journal of Molecular Sciences 25, no. 24: 13474. https://doi.org/10.3390/ijms252413474
APA StyleChen, J.-L., Liu, S.-T., Wu, C.-C., Chen, Y.-C., & Huang, S.-M. (2024). The Potency of Cytotoxic Mechanisms of Local Anesthetics in Human Chondrocyte Cells. International Journal of Molecular Sciences, 25(24), 13474. https://doi.org/10.3390/ijms252413474





