Non-Invasive Nanometer Resolution Assessment of Cell–Soft Hydrogel System Mechanical Properties by Scanning Ion Conductance Microscopy
Abstract
1. Introduction
2. Results
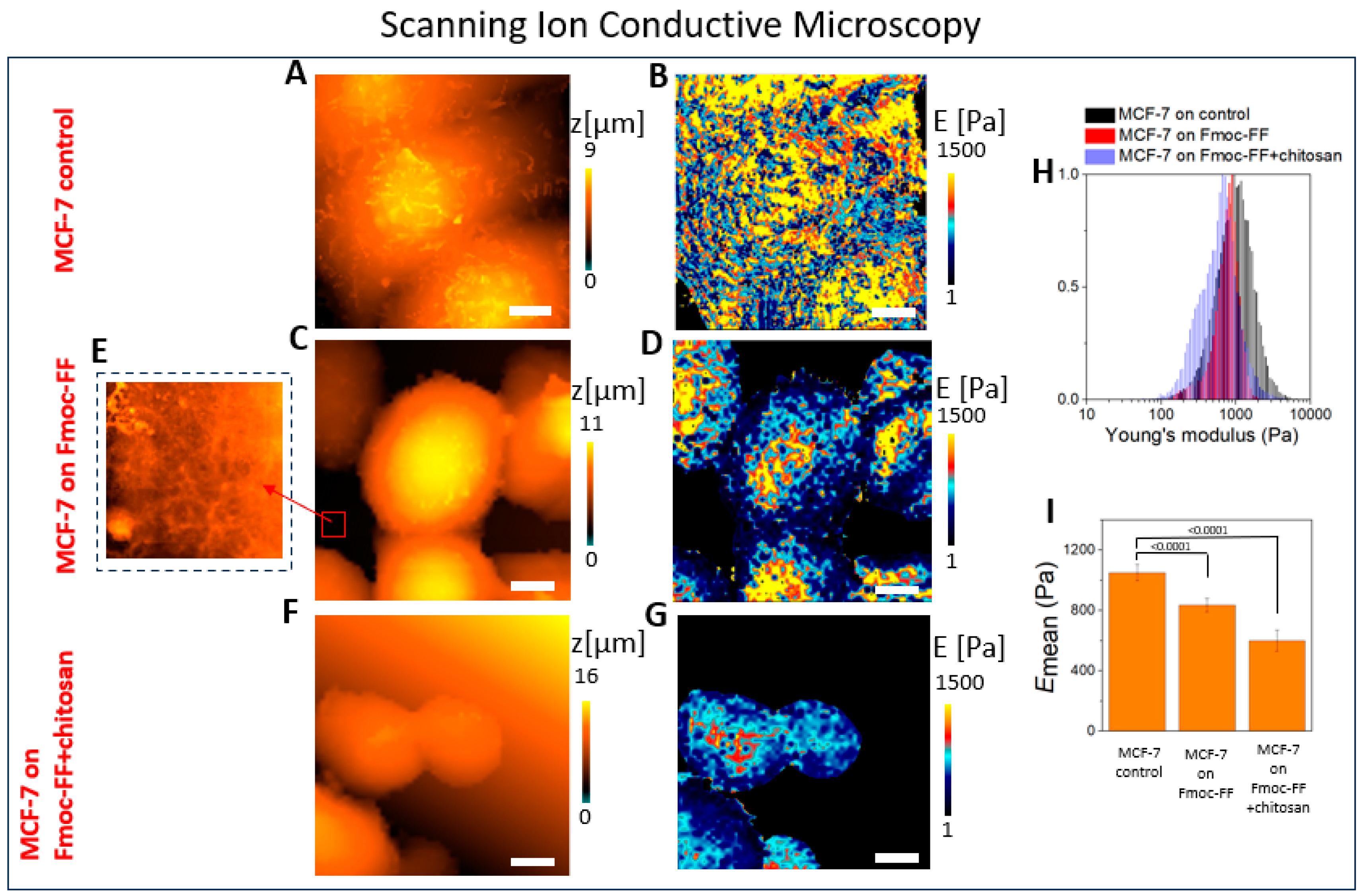
2.1. The Mechanical Properties of the Cell–Hydrogel System Assessed Using SICM
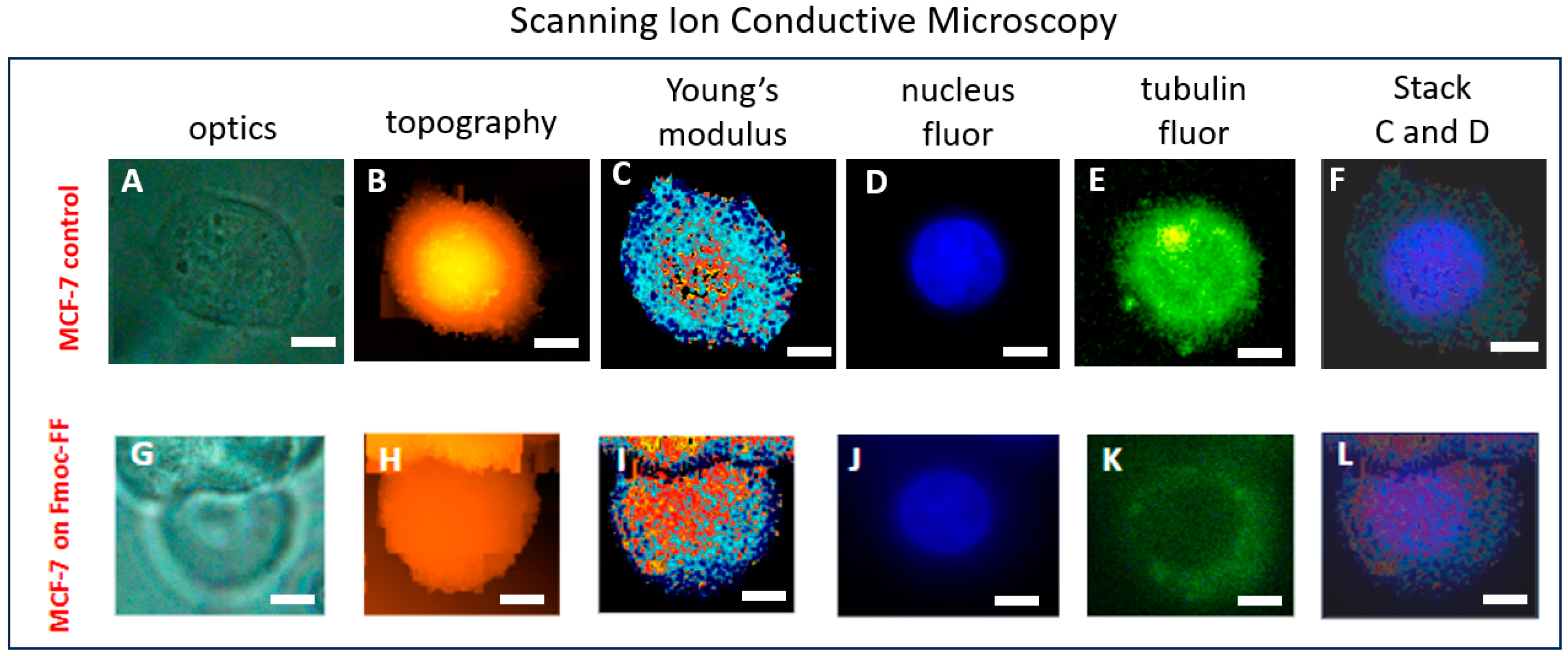
2.2. The Correlation of MCF-7 Young’s Modulus Distribution with Fluorescence Imaging
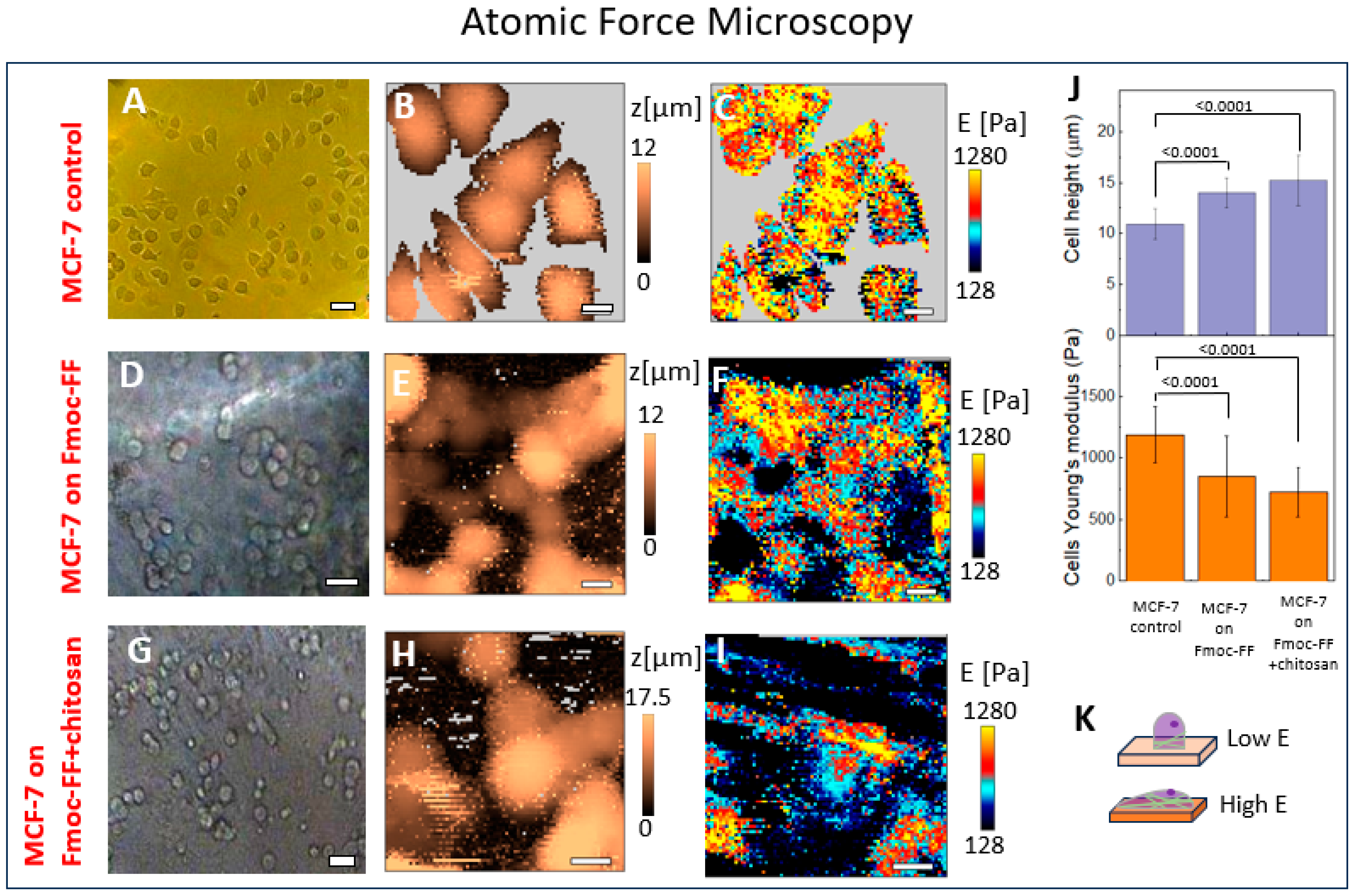
2.3. The Mechanical Properties of the Cell–Hydrogel System Assessed Using AFM
2.4. The Swelling Determination of Soft Fmoc-FF Hydrogel
2.5. Biocompatibility of Soft Hydrogels
2.6. Comparison of Fmoc-FF Hydrogel Biocompatibility Prepared by pH-Switch and Solvent-Switch Methods
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.1.1. Reagents
4.1.2. Hydrogels
4.1.3. Cell Culturing
4.2. Scanning Ion Conductance Microscopy (SICM) Measurements
4.3. Fluorescence Study of Stained Cells. Confocal Measurements
4.4. Atomic Force Microscopy Measurements
4.5. Swelling and Degradation of Fmoc-FF Hydrogel
4.6. MTT Test
4.7. Live/Dead Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Tang, M.; Zhao, J.; Chai, R.; Kang, J. Looking into the Future: Toward Advanced 3D Biomaterials for Stem-Cell-Based Regenerative Medicine. Adv. Mater. 2018, 30, e1705388. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, H.; Liu, S.; Pu, L.; Hu, X.; Ding, J.; Xu, G.; Xu, W.; Xiang, S.; Yuan, Z. A review of protein hydrogels: Protein assembly mechanisms, properties, and biological applications. Colloids Surf. B Biointerfaces 2022, 220, 112973. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Borro, B.C.; Nordström, R.; Malmsten, M. Microgels and hydrogels as delivery systems for antimicrobial peptides. Colloids Surf. B Biointerfaces 2020, 187, 110835. [Google Scholar] [CrossRef]
- Lou, J.; Mooney, D.J. Chemical strategies to engineer hydrogels for cell culture. Nat. Rev. Chem. 2022, 6, 726–744. [Google Scholar] [CrossRef]
- Stowers, R.S. Advances in Extracellular Matrix-Mimetic Hydrogels to Guide Stem Cell Fate. Cells Tissues Organs 2021, 211, 703–720. [Google Scholar] [CrossRef]
- Tayler, I.M.; Stowers, R.S. Engineering hydrogels for personalized disease modeling and regenerative medicine. Acta Biomater. 2021, 132, 4–22. [Google Scholar] [CrossRef]
- Halperin-Sternfeld, M.; Pokhojaev, A.; Ghosh, M.; Rachmiel, D.; Kannan, R.; Grinberg, I.; Asher, M.; Aviv, M.; Ma, P.X.; Binderman, I.; et al. Immunomodulatory fibrous hyaluronic acid-Fmoc-diphenylalanine-based hydrogel induces bone regeneration. J. Clin. Periodontol. 2023, 50, 200–219. [Google Scholar] [CrossRef]
- Wang, T.-W.; Spector, M. Development of hyaluronic acid-based scaffolds for brain tissue engineering. Acta Biomater. 2009, 5, 2371–2384. [Google Scholar] [CrossRef]
- Van Nieuwenhove, I.; Tytgat, L.; Ryx, M.; Blondeel, P.; Stillaert, F.; Thienpont, H.; Ottevaere, H.; Dubruel, P.; Van Vlierberghe, S. Soft tissue fillers for adipose tissue regeneration: From hydrogel development toward clinical applications. Acta Biomater. 2017, 63, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Patnaik, S.; Guo, X.; Li, Z.; Lo, W.; Butler, R.; Claude, A.; Liu, Z.; Zhang, G.; Liao, J.; et al. Cardiac differentiation of cardiosphere-derived cells in scaffolds mimicking morphology of the cardiac extracellular matrix. Acta Biomater. 2014, 10, 3449–3462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.-W.; Li, J.-G.; Sun, X.-D. Preparation and mechanical property of a novel 3D porous magnesium scaffold for bone tissue engineering. Mater. Sci. Eng. C 2014, 42, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Liu, M.; Chen, G.; Xu, Z.; Lv, Y. Demineralized Bone Scaffolds with Tunable Matrix Stiffness for Efficient Bone Integration. ACS Appl. Mater. Interfaces 2018, 10, 27669–27680. [Google Scholar] [CrossRef]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Sun, B. The mechanics of fibrillar collagen extracellular matrix. Cell Rep. Phys. Sci. 2021, 2, 100515. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/chitosan/hyaluronic acid–based injectable hydrogels for tissue engineering applications–design, physicochemical and biological characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef]
- Halper, J.; Kjaer, M. Basic components of connective tissues and extracellular matrix: Elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. In Progress in Heritable Soft Connective Tissue Diseases; Springer: Dordrecht, The Netherlands, 2014; pp. 31–47. [Google Scholar]
- Nelson, D.W.; Gilbert, R.J. Extracellular matrix-mimetic hydrogels for treating neural tissue injury: A focus on fibrin, hyaluronic acid, and elastin-like polypeptide hydrogels. Adv. Healthcare Mater. 2021, 10, 2101329. [Google Scholar] [CrossRef]
- Basavalingappa, V.; Guterman, T.; Tang, Y.; Nir, S.; Lei, J.; Chakraborty, P.; Schnaider, L.; Reches, M.; Wei, G.; Gazit, E. Expanding the Functional Scope of the Fmoc-Diphenylalanine Hydrogelator by Introducing a Rigidifying and Chemically Active Urea Backbone Modification. Adv. Sci. 2019, 6, 1900218. [Google Scholar] [CrossRef]
- Diaferia, C.; Ghosh, M.; Sibillano, T.; Gallo, E.; Stornaiuolo, M.; Giannini, C.; Morelli, G.; Adler-Abramovich, L.; Accardo, A. Fmoc-FF and hexapeptide-based multicomponent hydrogels as scaffold materials. Soft Matter 2018, 15, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Ligorio, C.; McAvan, B.; Hodson, N.W.; Allan, C.; Richardson, S.M.; Hoyland, J.A.; Bella, J. Hydroxyapatite-decorated Fmoc-hydrogel as a bone-mimicking substrate for osteoclast differentiation and culture. Acta Biomater. 2021, 138, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, T.N.; Kolmogorov, V.S.; Timoshenko, R.V.; Vaneev, A.N.; Cohen-Gerassi, D.; Osminkina, L.A.; Gorelkin, P.V.; Erofeev, A.S.; Sysoev, N.N.; Adler-Abramovich, L.; et al. Sensing Cells-Peptide Hydrogel Interaction In Situ via Scanning Ion Conductance Microscopy. Cells 2022, 11, 4137. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, T.N.; Cohen-Gerassi, D.; Arnon, Z.A.; Efremov, Y.; Timashev, P.; Adler-Abramovich, L.; Shirshin, E.A. Tunable Self-Assembled Peptide Hydrogel Sensor for Pharma Cold Supply Chain. ACS Appl. Mater. Interfaces 2022, 14, 55392–55401. [Google Scholar] [CrossRef]
- Ramaswamy, Y.; Roohani, I.; No, Y.J.; Madafiglio, G.; Chang, F.; Zhao, F.; Lu, Z.; Zreiqat, H. Nature-inspired topographies on hydroxyapatite surfaces regulate stem cells behaviour. Bioact. Mater. 2020, 6, 1107–1117. [Google Scholar] [CrossRef]
- Farzanian, K.; Ghahremaninezhad, A. On the Effect of Chemical Composition on the Desorption of Superabsorbent Hydrogels in Contact with a Porous Cementitious Material. Gels 2018, 4, 70. [Google Scholar] [CrossRef]
- Yu, S.; Liu, D.; Wang, T.; Lee, Y.Z.; Wong, J.C.N.; Song, X. Micropatterning of polymer substrates for cell culture. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1525–1533. [Google Scholar] [CrossRef]
- Wang, T.; Lai, J.H.; Yang, F. Effects of Hydrogel Stiffness and Extracellular Compositions on Modulating Cartilage Regeneration by Mixed Populations of Stem Cells and Chondrocytes In Vivo. Tissue Eng. Part A 2016, 22, 1348–1356. [Google Scholar] [CrossRef]
- Lomboni, D.J.; Steeves, A.; Schock, S.; Bonetti, L.; De Nardo, L.; Variola, F. Compounded topographical and physicochemical cueing by micro-engineered chitosan substrates on rat dorsal root ganglion neurons and human mesenchymal stem cells. Soft Matter 2021, 17, 5284–5302. [Google Scholar] [CrossRef]
- Trkov, S.; Eng, G.; Di Liddo, R.; Parnigotto, P.P.; Vunjak-Novakovic, G. Micropatterned three-dimensional hydrogel system to study human endothelial-mesenchymal stem cell interactions. J. Tissue Eng. Regen. Med. 2009, 4, 205–215. [Google Scholar] [CrossRef]
- Xue, B.; Tang, D.; Wu, X.; Xu, Z.; Gu, J.; Han, Y.; Zhu, Z.; Qin, M.; Zou, X.; Wang, W.; et al. Engineering hydrogels with homogeneous mechanical properties for controlling stem cell lineage specification. Proc. Natl. Acad. Sci. USA 2021, 118, e2110961118. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Soman, P.; Zhu, W.; Nikkhah, M.; Camci-Unal, G.; Chen, S.; Khademhosseini, A. Structural reinforcement of cell-laden hydrogels with microfabricated three dimensional scaffolds. Biomater. Sci. 2013, 2, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Zimny, M.; Walenko, K.; Wrobel, E.; Mrowka, P.; Mikulska, A.; Przybylski, J. Effect of substrate stiffness on the osteogenic differentiation of bone marrow stem cells and bone-derived cells. Cell Biol. Int. 2013, 37, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, C.; Wei, D.; Chen, S.; Xiao, Z.; Zhu, H.; Luo, H.; Sun, J.; Fan, H. Biomimetic mineralized microenvironment stiffness regulated BMSCs osteogenic differentiation through cytoskeleton mediated mechanical signaling transduction. Mater. Sci. Eng. C 2020, 119, 111613. [Google Scholar] [CrossRef] [PubMed]
- Magazzù, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: A Comprehensive Review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef]
- Dao, M.; Lim, C.; Suresh, S. Mechanics of the human red blood cell deformed by optical tweezers. J. Mech. Phys. Solids 2003, 51, 2259–2280. [Google Scholar] [CrossRef]
- Agrawal, R.; Smart, T.; Nobre-Cardoso, J.; Richards, C.; Bhatnagar, R.; Tufail, A.; Shima, D.; Jones, P.H.; Pavesio, C. Assessment of red blood cell deformability in type 2 diabetes mellitus and diabetic retinopathy by dual optical tweezers stretching technique. Sci. Rep. 2016, 6, srep15873. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.Y.; Hwang, M.J. Magnetic Resonance Elastography for the Detection and Classification of Prostate Cancer. Cancers 2024, 16, 3494. [Google Scholar] [CrossRef]
- Kolmogorov, V.S.; Erofeev, A.S.; Woodcock, E.; Efremov, Y.M.; Iakovlev, A.P.; Savin, N.A.; Alova, A.V.; Lavrushkina, S.V.; Kireev, I.I.; Prelovskaya, A.O.; et al. Mapping mechanical properties of living cells at nanoscale using intrinsic nanopipette–sample force interactions. Nanoscale 2021, 13, 6558–6568. [Google Scholar] [CrossRef]
- Rheinlaender, J.; Schäffer, T.E. Mapping the mechanical stiffness of live cells with the scanning ion conductance microscope. Soft Matter 2013, 9, 3230–3236. [Google Scholar] [CrossRef]
- Seifert, J.; Rheinlaender, J.; von Eysmondt, H.; Schäffer, T.E. Mechanics of migrating platelets investigated with scanning ion conductance microscopy. Nanoscale 2022, 14, 8192–8199. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Masola, V.; Karamanos, K.-A.; Franchi, L.; Kyriakopoulou, K.; Onisto, M.; Cappadone, C. Basement Membrane, Collagen, and Fibronectin: Physical Interactions with Cancer Cells. In The Extracellular Matrix and the Tumor Microenvironment; Springer: Cham, Switzerland, 2022; pp. 247–277. [Google Scholar]
- Gaeta, I.M.; Meenderink, L.M.; Postema, M.M.; Cencer, C.S.; Tyska, M.J. Direct visualization of epithelial microvilli biogenesis. Curr. Biol. 2021, 31, 2561–2575.e6. [Google Scholar] [CrossRef] [PubMed]
- Boido, M.; Ghibaudi, M.; Gentile, P.; Favaro, E.; Fusaro, R.; Tonda-Turo, C. Chitosan-based hydrogel to support the paracrine activity of mesenchymal stem cells in spinal cord injury treatment. Sci. Rep. 2019, 9, 6402. [Google Scholar] [CrossRef] [PubMed]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Ji, C.; Khademhosseini, A.; Dehghani, F. Enhancing cell penetration and proliferation in chitosan hydrogels for tissue engineering applications. Biomaterials 2011, 32, 9719–9729. [Google Scholar] [CrossRef]
- Ferreira, D.; Conceição, D.; Calhelha, R.; Sousa, T.; Socoteanu, R.; Ferreira, I.; Ferreira, L.V. Porphyrin dye into biopolymeric chitosan films for localized photodynamic therapy of cancer. Carbohydr. Polym. 2016, 151, 160–171. [Google Scholar] [CrossRef]
- He, K.; Wang, X. Rapid prototyping of tubular polyurethane and cell/hydrogel constructs. J. Bioact. Compat. Polym. 2011, 26, 363–374. [Google Scholar] [CrossRef]
- Adams, D.J.; Mullen, L.M.; Berta, M.; Chen, L.; Frith, W.J. Relationship between molecular structure, gelation behaviour and gel properties of Fmoc-dipeptides. Soft Matter 2010, 6, 1971–1980. [Google Scholar] [CrossRef]
- Taira, N.; Nashimoto, Y.; Ino, K.; Ida, H.; Imaizumi, T.; Kumatani, A.; Takahashi, Y.; Shiku, H. Micropipet-Based Navigation in a Microvascular Model for Imaging Endothelial Cell Topography Using Scanning Ion Conductance Microscopy. Anal. Chem. 2021, 93, 4902–4908. [Google Scholar] [CrossRef]
- Schierbaum, N.; Rheinlaender, J.; Schäffer, T.E. Combined atomic force microscopy (AFM) and traction force microscopy (TFM) reveals a correlation between viscoelastic material properties and contractile prestress of living cells. Soft Matter 2019, 15, 1721–1729. [Google Scholar] [CrossRef]
- Rheinlaender, J.; Dimitracopoulos, A.; Wallmeyer, B.; Kronenberg, N.M.; Chalut, K.J.; Gather, M.C.; Betz, T.; Charras, G.; Franze, K. Cortical cell stiffness is independent of substrate mechanics. Nat. Mater. 2020, 19, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Hogrebe, N.J.; Gooch, K.J. Direct influence of culture dimensionality on human mesenchymal stem cell differentiation at various matrix stiffnesses using a fibrous self-assembling peptide hydrogel. J. Biomed. Mater. Res. Part A 2016, 104, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Vernerey, F.J.; Sridhar, S.L.; Muralidharan, A.; Bryant, S.J. Mechanics of 3D cell–hydrogel interactions: Experiments, models, and mechanisms. Chem. Rev. 2021, 121, 11085–11148. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, A.M.; Kloxin, C.J.; Bowman, C.N.; Anseth, K.S. Mechanical Properties of Cellularly Responsive Hydrogels and Their Experimental Determination. Adv. Mater. 2010, 22, 3484–3494. [Google Scholar] [CrossRef]
- Gil-Redondo, J.C.; Weber, A.; Zbiral, B.; Vivanco, M.D.; Toca-Herrera, J.L. Substrate stiffness modulates the viscoelastic properties of MCF-7 cells. J. Mech. Behav. Biomed. Mater. 2022, 125, 104979. [Google Scholar] [CrossRef]
- Darnell, M.C.; Sun, J.-Y.; Mehta, M.; Johnson, C.; Arany, P.R.; Suo, Z.; Mooney, D.J. Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials 2013, 34, 8042–8048. [Google Scholar] [CrossRef]
- Raeburn, J.; Pont, G.; Chen, L.; Cesbron, Y.; Lévy, R.; Adams, D.J. Fmoc-diphenylalanine hydrogels: Understanding the variability in reported mechanical properties. Soft Matter 2011, 8, 1168–1174. [Google Scholar] [CrossRef]
- Chowdhury, T.K. Fabrication of extremely fine glass micropipette electrodes. J. Phys. E Sci. Instrum. 1969, 2, 1087–1090. [Google Scholar] [CrossRef]
- Clarke, R.W.; Zhukov, A.; Richards, O.; Johnson, N.; Ostanin, V.; Klenerman, D. Pipette–Surface Interaction: Current Enhancement and Intrinsic Force. J. Am. Chem. Soc. 2012, 135, 322–329. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Wang, W.-H.; Hardy, S.D.; Geahlen, R.L.; Raman, A. Measuring nanoscale viscoelastic parameters of cells directly from AFM force-displacement curves. Sci. Rep. 2017, 7, 1541. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Velay-Lizancos, M.; Weaver, C.J.; Athamneh, A.I.; Zavattieri, P.D.; Suter, D.M.; Raman, A. Anisotropy vs isotropy in living cell indentation with AFM. Sci. Rep. 2019, 9, 5757. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.R.; Charras, G.T. Experimental validation of atomic force microscopy-based cell elasticity measurements. Nanotechnology 2011, 22, 345102. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Yang, Y.; El Haj, A.J.; Then, K.Y.; Liu, K.-K. Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. J. R. Soc. Interface 2005, 2, 455–463. [Google Scholar] [CrossRef]
- Kulkarni, S.G.; Pérez-Domínguez, S.; Radmacher, M. Influence of cantilever tip geometry and contact model on AFM elasticity measurement of cells. J. Mol. Recognit. 2023, 36, e3018. [Google Scholar] [CrossRef] [PubMed]
- Efremov, Y.M.; Shpichka, A.I.; Kotova, S.L.; Timashev, P.S. Viscoelastic mapping of cells based on fast force volume and PeakForce Tapping. Soft Matter 2019, 15, 5455–5463. [Google Scholar] [CrossRef]
- Sneddon, I.N. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int. J. Eng. Sci. 1965, 3, 47–57. [Google Scholar] [CrossRef]
- Rico, F.; Roca-Cusachs, P.; Gavara, N.; Farré, R.; Rotger, M.; Navajas, D. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys. Rev. E 2005, 72, 021914. [Google Scholar] [CrossRef]
- Das, M.P.; R., S.P.; Prasad, K.; Jv, V.; M, R. EXTRACTION AND CHARACTERIZATION OF GELATIN: A FUNCTIONAL BIOPOLYMER. Int. J. Pharm. Pharm. Sci. 2017, 9, 239–242. [Google Scholar] [CrossRef]
- Hanani, Z.; Roos, Y.; Kerry, J.; Kerry, J. Fourier Transform Infrared (FTIR) spectroscopic analysis of biodegradable gelatin films immersed in water. In Proceedings of the 11th International Congress on Engineering and Food, ICEF11, Athens, Greece, 22–26 May 2011; pp. 22–26. [Google Scholar]
- Lohrasbi, S.; Mirzaei, E.; Karimizade, A.; Takallu, S.; Rezaei, A. Collagen/cellulose nanofiber hydrogel scaffold: Physical, mechanical and cell biocompatibility properties. Cellulose 2019, 27, 927–940. [Google Scholar] [CrossRef]
- Clarke, R.W.; Novak, P.; Zhukov, A.; Tyler, E.J.; Cano-Jaimez, M.; Drews, A.; Richards, O.; Volynski, K.; Bishop, C.; Klenerman, D. Low Stress Ion Conductance Microscopy of Sub-Cellular Stiffness. Soft Matter 2016, 12, 7953–7958. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Kamal, M.S. Effect of Water/Decane Ratios and Salt on the Stability, Rheology, and Interfacial Tension of Water/Decane Emulsions. Energy Fuels 2019, 33, 8456–8462. [Google Scholar] [CrossRef]
| SICM | AFM | AFM, CoCS Model | ||
|---|---|---|---|---|
| MCF-7 on Petri dish, E, Pa | 1050 ± 55 | 1190 ± 230 | - | |
| MCF-7 on Fmoc-FF hydrogel, E, Pa | MCF-7 | 835 ± 45 | 980 ± 240 | 1060 ± 250 |
| Fmoc-FF | 300 ± 30 | 500 ± 170 | - | |
| MCF-7 on Fmoc-FF+chitosan hydrogel, E, Pa | MCF-7 | 600 ± 70 | 860 ± 220 | 930 ± 230 |
| Fmoc-FF+chitosan | 270 ± 60 | 450 ± 140 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhonova, T.N.; Barkovaya, A.V.; Efremov, Y.M.; Mamed-Nabizade, V.V.; Kolmogorov, V.S.; Timashev, P.S.; Sysoev, N.N.; Fadeev, V.V.; Gorelkin, P.V.; Adler-Abramovich, L.; et al. Non-Invasive Nanometer Resolution Assessment of Cell–Soft Hydrogel System Mechanical Properties by Scanning Ion Conductance Microscopy. Int. J. Mol. Sci. 2024, 25, 13479. https://doi.org/10.3390/ijms252413479
Tikhonova TN, Barkovaya AV, Efremov YM, Mamed-Nabizade VV, Kolmogorov VS, Timashev PS, Sysoev NN, Fadeev VV, Gorelkin PV, Adler-Abramovich L, et al. Non-Invasive Nanometer Resolution Assessment of Cell–Soft Hydrogel System Mechanical Properties by Scanning Ion Conductance Microscopy. International Journal of Molecular Sciences. 2024; 25(24):13479. https://doi.org/10.3390/ijms252413479
Chicago/Turabian StyleTikhonova, Tatiana N., Anastasia V. Barkovaya, Yuri M. Efremov, Vugara V. Mamed-Nabizade, Vasilii S. Kolmogorov, Peter S. Timashev, Nikolay N. Sysoev, Victor V. Fadeev, Petr V. Gorelkin, Lihi Adler-Abramovich, and et al. 2024. "Non-Invasive Nanometer Resolution Assessment of Cell–Soft Hydrogel System Mechanical Properties by Scanning Ion Conductance Microscopy" International Journal of Molecular Sciences 25, no. 24: 13479. https://doi.org/10.3390/ijms252413479
APA StyleTikhonova, T. N., Barkovaya, A. V., Efremov, Y. M., Mamed-Nabizade, V. V., Kolmogorov, V. S., Timashev, P. S., Sysoev, N. N., Fadeev, V. V., Gorelkin, P. V., Adler-Abramovich, L., Erofeev, A. S., & Shirshin, E. A. (2024). Non-Invasive Nanometer Resolution Assessment of Cell–Soft Hydrogel System Mechanical Properties by Scanning Ion Conductance Microscopy. International Journal of Molecular Sciences, 25(24), 13479. https://doi.org/10.3390/ijms252413479






