Irisin: A Multifaceted Hormone Bridging Exercise and Disease Pathophysiology
Abstract
:1. Introduction
2. Irisin, Metabolic Diseases, and Insulin Resistance
3. Irisin and Muscle/Bone Homeostasis
3.1. Autophagy as an Additional Mechanism in Irisin Signaling
3.2. Irisin Signaling, Chronic Inflammation, and Insulin in Bone and Muscle Homeostasis
4. Irisin, Neurodegeneration, and Alzheimer’s Disease
5. Main Limitations in Findings
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin Is Expressed and Produced by Human Muscle and Adipose Tissue in Association with Obesity and Insulin Resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef] [PubMed]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/Irisin Is Not Only a Myokine but Also an Adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef] [PubMed]
- Timmons, J.A.; Baar, K.; Davidsen, P.K.; Atherton, P.J. Is Irisin a Human Exercise Gene? Nature 2012, 488, E9–E10. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and Irisin in Humans: I. Predictors of Circulating Concentrations in Serum and Plasma and II. MRNA Expression and Circulating Concentrations in Response to Weight Loss and Exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Eren, M.N.; Yilmaz, M.; Sahin, I.; Kalayci, M.; Sarman, E.; Kaya, N.; Yilmaz, O.F.; et al. Irisin: A Potentially Candidate Marker for Myocardial Infarction. Peptides 2014, 55, 85–91. [Google Scholar] [CrossRef]
- Shulman, G.I.; Rothman, D.L.; Jue, T.; Stein, P.; DeFronzo, R.A.; Shulman, R.G. Quantitation of Muscle Glycogen Synthesis in Normal Subjects and Subjects with Non-Insulin-Dependent Diabetes by 13C Nuclear Magnetic Resonance Spectroscopy. N. Engl. J. Med. 1990, 322, 223–228. [Google Scholar] [CrossRef]
- Sylow, L.; Tokarz, V.L.; Richter, E.A.; Klip, A. The Many Actions of Insulin in Skeletal Muscle, the Paramount Tissue Determining Glycemia. Cell Metab. 2021, 33, 758–780. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32 (Suppl. S2), S157. [Google Scholar] [CrossRef]
- Krssak, M.; Roden, M. The Role of Lipid Accumulation in Liver and Muscle for Insulin Resistance and Type 2 Diabetes Mellitus in Humans. Rev. Endocr. Metab. Disord. 2004, 5, 127–134. [Google Scholar] [CrossRef]
- Itani, S.I.; Ruderman, N.B.; Schmieder, F.; Boden, G. Lipid-Induced Insulin Resistance in Human Muscle Is Associated with Changes in Diacylglycerol, Protein Kinase C, and IkappaB-Alpha. Diabetes 2002, 51, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Jani, S.; Da Eira, D.; Hadday, I.; Bikopoulos, G.; Mohasses, A.; de Pinho, R.A.; Ceddia, R.B. Distinct Mechanisms Involving Diacylglycerol, Ceramides, and Inflammation Underlie Insulin Resistance in Oxidative and Glycolytic Muscles from High Fat-Fed Rats. Sci. Rep. 2021, 11, 19160. [Google Scholar] [CrossRef] [PubMed]
- Kolczynska, K.; Loza-Valdes, A.; Hawro, I.; Sumara, G. Diacylglycerol-Evoked Activation of PKC and PKD Isoforms in Regulation of Glucose and Lipid Metabolism: A Review. Lipids Heal. Dis. 2020, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Lebed, B.; Schatz, M.; Homko, C.; Lemieux, S. Effects of Acute Changes of Plasma Free Fatty Acids on Intramyocellular Fat Content and Insulin Resistance in Healthy Subjects. Diabetes 2001, 50, 1612–1617. [Google Scholar] [CrossRef]
- Khajebishak, Y.; Faghfouri, A.H.; Soleimani, A.; Ilaei, S.; Peyrovi, S.; Madani, S.; Payahoo, L. The Potential Relationship Between Serum Irisin Concentration with Inflammatory Cytokines, Oxidative Stress Biomarkers, Glycemic Indices and Lipid Profiles in Obese Patients with Type 2 Diabetes Mellitus: A Pilot Study. J. ASEAN Fed. Endocr. Soc. 2023, 38, 45–51. [Google Scholar] [CrossRef]
- Yosaee, S.; Basirat, R.; Hamidi, A.; Esteghamati, A.; Khodadost, M.; Shidfar, F.; Bitarafan, V.; Djafarian, K. Serum Irisin Levels in Metabolically Healthy versus Metabolically Unhealthy Obesity: A Case-Control Study. Med. J. Islam. Repub. Iran 2020, 34, 46. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The Anti-Inflammatory Effects of Exercise: Mechanisms and Implications for the Prevention and Treatment of Disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ando, D.; Takamatsu, K.; Goto, K. Resistance Exercise Induces a Greater Irisin Response than Endurance Exercise. Metabolism 2015, 64, 1042–1050. [Google Scholar] [CrossRef]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating Irisin in Relation to Insulin Resistance and the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899. [Google Scholar] [CrossRef]
- Sesti, G.; Andreozzi, F.; Fiorentino, T.V.; Mannino, G.C.; Sciacqua, A.; Marini, M.A.; Perticone, F. High Circulating Irisin Levels Are Associated with Insulin Resistance and Vascular Atherosclerosis in a Cohort of Nondiabetic Adult Subjects. Acta Diabetol. 2014, 51, 705–713. [Google Scholar] [CrossRef]
- Kelley, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of Mitochondria in Human Skeletal Muscle in Type 2 Diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Shen, Y.M.; Ni, C.; Ye, J.; Xin, Y.; Zhang, W.; Ren, Y.Z. Irisin Reverses Insulin Resistance in C2C12 Cells via the P38-MAPK-PGC-1α Pathway. Peptides 2019, 119, 170120. [Google Scholar] [CrossRef] [PubMed]
- Schubert, K.M.; Scheid, M.P.; Duronio, V. Ceramide Inhibits Protein Kinase B/Akt by Promoting Dephosphorylation of Serine 473. J. Biol. Chem. 2000, 275, 13330–13335. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Somwar, R.; Bilan, P.J.; Liu, Z.; Jin, J.; Woodgett, J.R.; Klip, A. Protein Kinase B/Akt Participates in GLUT4 Translocation by Insulin in L6 Myoblasts. Mol. Cell. Biol. 1999, 19, 4008–4018. [Google Scholar] [CrossRef]
- Rabiee, F.; Lachinani, L.; Ghaedi, S.; Nasr-Esfahani, M.H.; Megraw, T.L.; Ghaedi, K. New Insights into the Cellular Activities of Fndc5/Irisin and Its Signaling Pathways. Cell Biosci. 2020, 10, 51. [Google Scholar] [CrossRef]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-Induced Irisin Secretion Is Independent of Age or Fitness Level and Increased Irisin May Directly Modulate Muscle Metabolism Through AMPK Activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwivedi, U.N.; Kakkar, P. AMP-Activated Protein Kinase: An Energy Sensor and Survival Mechanism in the Reinstatement of Metabolic Homeostasis. Exp. Cell Res. 2023, 428, 113614. [Google Scholar] [CrossRef]
- Yun, C.L.; Zierath, J.R. AMP-Activated Protein Kinase Signaling in Metabolic Regulation. J. Clin. Investig. 2006, 116, 1776–1783. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.O.; Kim, N.; Kim, J.K.; Kim, H.I.; Lee, Y.W.; Kim, S.J.; Choi, J.I.; Oh, Y.; Kim, J.H.; et al. Irisin, a Novel Myokine, Regulates Glucose Uptake in Skeletal Muscle Cells via AMPK. Mol. Endocrinol. 2015, 29, 873–881. [Google Scholar] [CrossRef]
- Chen, S.Q.; Ding, L.N.; Zeng, N.X.; Liu, H.M.; Zheng, S.H.; Xu, J.W.; Li, R.M. Icariin Induces Irisin/FNDC5 Expression in C2C12 Cells via the AMPK Pathway. Biomed. Pharmacother. 2019, 115, 108930. [Google Scholar] [CrossRef]
- Natalicchio, A.; Marrano, N.; Biondi, G.; Spagnuolo, R.; Labarbuta, R.; Porreca, I.; Cignarelli, A.; Bugliani, M.; Marchetti, P.; Perrini, S.; et al. The Myokine Irisin Is Released in Response to Saturated Fatty Acids and Promotes Pancreatic β-Cell Survival and Insulin Secretion. Diabetes 2017, 66, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Hardy, O.T.; Czech, M.P.; Corvera, S. What Causes the Insulin Resistance Underlying Obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef] [PubMed]
- Michael, M.D.; Kulkarni, R.N.; Postic, C.; Previs, S.F.; Shulman, G.I.; Magnuson, M.A.; Kahn, C.R. Loss of Insulin Signaling in Hepatocytes Leads to Severe Insulin Resistance and Progressive Hepatic Dysfunction. Mol. Cell 2000, 6, 87–97. [Google Scholar] [CrossRef]
- Boden, G.; Duan, X.; Homko, C.; Molina, E.J.; Song, W.; Perez, O.; Cheung, P.; Merali, S. Increase in Endoplasmic Reticulum Stress-Related Proteins and Genes in Adipose Tissue of Obese, Insulin-Resistant Individuals. Diabetes 2008, 57, 2438–2444. [Google Scholar] [CrossRef]
- Fernandes-da-Silva, A.; Miranda, C.S.; Santana-Oliveira, D.A.; Oliveira-Cordeiro, B.; Rangel-Azevedo, C.; Silva-Veiga, F.M.; Martins, F.F.; Souza-Mello, V. Endoplasmic Reticulum Stress as the Basis of Obesity and Metabolic Diseases: Focus on Adipose Tissue, Liver, and Pancreas. Eur. J. Nutr. 2021, 60, 2949–2960. [Google Scholar] [CrossRef]
- Cnop, M.; Vidal, J.; Hull, R.L.; Utzschneider, K.M.; Carr, D.B.; Schraw, T.; Scherer, P.E.; Boyko, E.J.; Fujimoto, W.Y.; Kahn, S.E. Progressive Loss Of-Cell Function Leads to Worsening Glucose Tolerance in First-Degree Relatives of Subjects with Type 2 Diabetes. Diabetes Care 2007, 30, 677–682. [Google Scholar] [CrossRef]
- Weyer, C.; Bogardus, C.; Mott, D.M.; Pratley, R.E. The Natural History of Insulin Secretory Dysfunction and Insulin Resistance in the Pathogenesis of Type 2 Diabetes Mellitus. J. Clin. Investig. 1999, 104, 787–794. [Google Scholar] [CrossRef]
- Titchenell, P.M.; Lazar, M.A.; Birnbaum, M.J. Unraveling the Regulation of Hepatic Metabolism by Insulin. Trends Endocrinol. Metab. 2017, 28, 497–505. [Google Scholar] [CrossRef]
- Liu, T.Y.; Shi, C.X.; Gao, R.; Sun, H.J.; Xiong, X.Q.; Ding, L.; Chen, Q.; Li, Y.H.; Wang, J.J.; Kang, Y.M.; et al. Irisin Inhibits Hepatic Gluconeogenesis and Increases Glycogen Synthesis via the PI3K/Akt Pathway in Type 2 Diabetic Mice and Hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Diehl, J.A. Coordination of ER and Oxidative Stress Signaling: The PERK/Nrf2 Signaling Pathway. Int. J. Biochem. Cell Biol. 2006, 38, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Oakes, S.A.; Papa, F.R. The Role of Endoplasmic Reticulum Stress in Human Pathology. Annu. Rev. Pathol. 2015, 10, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.F.; Wang, M.Z.; Bi, J.B.; Zhang, J.; Zhang, L.; Liu, W.M.; Wei, S.S.; Lv, Y.; Wu, R.Q.; Wu, Z. Irisin Attenuates Intestinal Injury, Oxidative and Endoplasmic Reticulum Stress in Mice with L-Arginine-Induced Acute Pancreatitis. World J. Gastroenterol. 2019, 25, 6653–6667. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, Q.; Wang, Y.; Wei, S.; Yang, L.; Zhang, J.; Liu, C.; et al. Irisin Alleviates Liver Ischemia-Reperfusion Injury by Inhibiting Excessive Mitochondrial Fission, Promoting Mitochondrial Biogenesis and Decreasing Oxidative Stress. Redox Biol. 2019, 20, 296–306. [Google Scholar] [CrossRef]
- Nesci, S.; Rubattu, S. UCP2, a Member of the Mitochondrial Uncoupling Proteins: An Overview from Physiological to Pathological Roles. Biomedicines 2024, 12, 1307. [Google Scholar] [CrossRef]
- So, W.Y.; Leung, P.S. Irisin Ameliorates Hepatic Glucose/Lipid Metabolism and Enhances Cell Survival in Insulin-Resistant Human HepG2 Cells through Adenosine Monophosphate-Activated Protein Kinase Signaling. Int. J. Biochem. Cell Biol. 2016, 78, 237–247. [Google Scholar] [CrossRef]
- Serbest, S.; Tiftikçi, U.; Tosun, H.B.; Kısa, Ü. The Irisin Hormone Profile and Expression in Human Bone Tissue in the Bone Healing Process in Patients. Med. Sci. Monit. 2017, 23, 4278–4283. [Google Scholar] [CrossRef]
- Hirschfeld, H.P.; Kinsella, R.; Duque, G. Osteosarcopenia: Where Bone, Muscle, and Fat Collide. Osteoporos. Int. 2017, 28, 2781–2790. [Google Scholar] [CrossRef]
- Shimonty, A.; Bonewald, L.F.; Huot, J.R. Metabolic Health and Disease: A Role of Osteokines? Calcif. Tissue Int. 2023, 113, 21–38. [Google Scholar] [CrossRef]
- Das, D.K.; Graham, Z.A.; Cardozo, C.P. Myokines in Skeletal Muscle Physiology and Metabolism: Recent Advances and Future Perspectives. Acta Physiol. 2020, 228, e13367. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via AV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [PubMed]
- Mu, A.; Wales, T.E.; Zhou, H.; Draga-Coletă, S.V.; Gorgulla, C.; Blackmore, K.A.; Mittenbühler, M.J.; Kim, C.R.; Bogoslavski, D.; Zhang, Q.; et al. Irisin Acts through Its Integrin Receptor in a Two-Step Process Involving Extracellular Hsp90α. Mol. Cell 2023, 83, 1903–1920.e12. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin Binds to LRP5/6 and Antagonizes Canonical Wnt Signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef] [PubMed]
- Schoppet, M.; Preissner, K.T.; Hofbauer, L.C. RANK Ligand and Osteoprotegerin: Paracrine Regulators of Bone Metabolism and Vascular Function. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Oranger, A.; Mori, G.; Brunetti, G.; Colucci, S.; Cinti, S.; Grano, M. Irisin Enhances Osteoblast Differentiation In Vitro. Int. J. Endocrinol. 2014, 2014, 902186. [Google Scholar] [CrossRef]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin Prevents and Restores Bone Loss and Muscle Atrophy in Hind-Limb Suspended Mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Comite, M.D.; Mori, G.; et al. The Myokine Irisin Increases Cortical Bone Mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J. Bone Miner. Res. 2020, 35, 766–775. [Google Scholar] [CrossRef]
- Xiao, Y.; Xie, X.; Chen, Z.; Yin, G.; Kong, W.; Zhou, J. Advances in the Roles of ATF4 in Osteoporosis. Biomed. Pharmacother. 2023, 169, 115864. [Google Scholar] [CrossRef]
- Zhou, K.; Qiao, X.; Cai, Y.; Li, A.; Shan, D. Lower Circulating Irisin in Middle-Aged and Older Adults with Osteoporosis: A Systematic Review and Meta-Analysis. Menopause 2019, 26, 1302–1310. [Google Scholar] [CrossRef]
- Roomi, A.B.; Nori, W.; Hamed, R.M. Lower Serum Irisin Levels Are Associated with Increased Osteoporosis and Oxidative Stress in Postmenopausal. Reports Biochem. Mol. Biol. 2021, 10, 13–19. [Google Scholar] [CrossRef]
- Engin-Üstün, Y.; Çağlayan, E.K.; Göçmen, A.Y.; Polat, M.F. Postmenopausal Osteoporosis Is Associated with Serum Chemerin and Irisin but Not with Apolipoprotein M Levels. J. Menopausal Med. 2016, 22, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, X.; Wang, X.; Chen, T.; Tao, F.; Liu, C.; Tu, Q.; Shen, G.; Chen, J.J. Irisin Deficiency Disturbs Bone Metabolism. J. Cell. Physiol. 2021, 236, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Kishikawa, A.; Ogawa, S.; Shen, W.R.; Qi, J.; Noguchi, T.; Nara, Y.; Mizoguchi, I. TNF-α Directly Enhances Osteocyte RANKL Expression and Promotes Osteoclast Formation. Front. Immunol. 2019, 10, 2925. [Google Scholar] [CrossRef]
- Kang, Y.S.; Kim, J.C.; Kim, J.S.; Kim, S.H. Effects of Swimming Exercise on Serum Irisin and Bone FNDC5 in Rat Models of High-Fat Diet-Induced Osteoporosis. J. Sports Sci. Med. 2019, 18, 596. [Google Scholar]
- Westendorf, J.J.; Kahler, R.A.; Schroeder, T.M. Wnt Signaling in Osteoblasts and Bone Diseases. Gene 2004, 341, 19–39. [Google Scholar] [CrossRef]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical Stimulation of Bone in Vivo Reduces Osteocyte Expression of Sost/Sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef]
- Pesce, M.; Ballerini, P.; Paolucci, T.; Puca, I.; Farzaei, M.H.; Patruno, A. Irisin and Autophagy: First Update. Int. J. Mol. Sci. 2020, 21, 7587. [Google Scholar] [CrossRef]
- Chen, X.; Sun, K.; Zhao, S.; Geng, T.; Fan, X.; Sun, S.; Zheng, M.; Jin, Q. Irisin Promotes Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by Activating Autophagy via the Wnt//β-Catenin Signal Pathway. Cytokine 2020, 136, 155292. [Google Scholar] [CrossRef]
- Liu, T.Y.; Xiong, X.Q.; Ren, X.S.; Zhao, M.X.; Shi, C.X.; Wang, J.J.; Zhou, Y.B.; Zhang, F.; Han, Y.; Gao, X.Y.; et al. FNDC5 Alleviates Hepatosteatosis by Restoring AMPK/MTOR-Mediated Autophagy, Fatty Acid Oxidation, and Lipogenesis in Mice. Diabetes 2016, 65, 3262–3275. [Google Scholar] [CrossRef]
- Rabanal-Ruiz, Y.; Otten, E.G.; Korolchuk, V.I. MTORC1 as the Main Gateway to Autophagy. Essays Biochem. 2017, 61, 565. [Google Scholar] [CrossRef]
- Li, Y.; Su, J.; Sun, W.; Cai, L.; Deng, Z. AMP-Activated Protein Kinase Stimulates Osteoblast Differentiation and Mineralization through Autophagy Induction. Int. J. Mol. Med. 2018, 41, 2535–2544. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.Y.; Nie, Y.; Ma, Y.X.; Chen, Y.; Cheng, R.; Yinrg, W.Y.; Hu, Y.; Xu, W.M.; Xu, L.Z. Irisin Promotes Osteoblast Proliferation and Differentiation via Activating the MAP Kinase Signaling Pathways. Sci. Rep. 2016, 6, 18732. [Google Scholar] [CrossRef]
- Zhou, W.; Shi, Y.; Wang, H.; Chen, L.; Yu, C.; Zhang, X.; Yang, L.; Zhang, X.; Wu, A. Exercise-Induced FNDC5/Irisin Protects Nucleus Pulposus Cells against Senescence and Apoptosis by Activating Autophagy. Exp. Mol. Med. 2022, 54, 1038–1048. [Google Scholar] [CrossRef]
- Hardy, R.; Cooper, M.S. Bone Loss in Inflammatory Disorders. J. Endocrinol. 2009, 201, 309–320. [Google Scholar] [CrossRef]
- Abildgaard, J.; Tingstedt, J.; Zhao, Y.; Hartling, H.J.; Pedersen, A.T.; Lindegaard, B.; Nielsen, S.D. Increased Systemic Inflammation and Altered Distribution of T-Cell Subsets in Postmenopausal Women. PLoS ONE 2020, 15, e0235174. [Google Scholar] [CrossRef]
- Ashai, S.; Harvey, N.C. Rheumatoid Arthritis and Bone Health. Clin. Med. 2020, 20, 565. [Google Scholar] [CrossRef]
- van Bodegraven, A.A.; Bravenboer, N. Perspective on Skeletal Health in Inflammatory Bowel Disease. Osteoporos. Int. 2020, 31, 637–646. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Geng, Z.; Zhou, B.; Zhang, F.; Han, Y.; Zhou, Y.B.; Wang, J.J.; Gao, X.Y.; Chen, Q.; Li, Y.H.; et al. FNDC5 Attenuates Adipose Tissue Inflammation and Insulin Resistance via AMPK-Mediated Macrophage Polarization in Obesity. Metabolism 2018, 83, 31–41. [Google Scholar] [CrossRef]
- Li, Q.; Tan, Y.; Chen, S.; Xiao, X.; Zhang, M.; Wu, Q.; Dong, M. Irisin Alleviates LPS-Induced Liver Injury and Inflammation through Inhibition of NLRP3 Inflammasome and NF-ΚB Signaling. J. Recept. Signal Transduct. 2021, 41, 294–303. [Google Scholar] [CrossRef]
- Jiang, X.; Hu, Y.; Zhou, Y.; Chen, J.; Sun, C.; Chen, Z.; Jing, C.; Xu, L.; Liu, F.; Ni, W.; et al. Irisin Protects Female Mice with LPS-Induced Endometritis through the AMPK/NF-ΚB Pathway. Iran. J. Basic Med. Sci. 2021, 24, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.; Hwang, H.R.; Park, H.J.; Kwon, A.; Qadir, A.S.; Ko, S.H.; Woo, K.M.; Ryoo, H.M.; Kim, G.S.; Baek, J.H. TNF-α Upregulates Sclerostin Expression in Obese Mice Fed a High-Fat Diet. J. Cell. Physiol. 2014, 229, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Alles, N.; Soysa, N.S.; Hayashi, J.; Khan, M.; Shimoda, A.; Shimokawa, H.; Ritzeler, O.; Akiyoshi, K.; Aoki, K.; Ohya, K. Suppression of NF-ΚB Increases Bone Formation and Ameliorates Osteopenia in Ovariectomized Mice. Endocrinology 2010, 151, 4626–4634. [Google Scholar] [CrossRef]
- Takami, K.; Okamoto, K.; Etani, Y.; Hirao, M.; Miyama, A.; Okamura, G.; Goshima, A.; Miura, T.; Kurihara, T.; Fukuda, Y.; et al. Anti–NF-ΚB Peptide Derived from Nuclear Acidic Protein Attenuates Ovariectomy-Induced Osteoporosis in Mice. JCI Insight 2023, 8, e171962. [Google Scholar] [CrossRef]
- Cacciatore, S.; Duque, G.; Marzetti, E. Osteosarcopenic Obesity: A Triple Threat for Older Adults? Eur. Geriatr. Med. 2023, 14, 1191–1193. [Google Scholar] [CrossRef]
- Guarnotta, V.; Prinzi, A.; Pitrone, M.; Pizzolanti, G.; Giordano, C. Circulating Irisin Levels as a Marker of Osteosarcopenic-Obesity in Cushing’s Disease. Diabetes Metab. Syndr. Obes. 2020, 13, 1565–1574. [Google Scholar] [CrossRef]
- Guo, M.; Yao, J.; Li, J.; Zhang, J.; Wang, D.; Zuo, H.; Zhang, Y.; Xu, B.; Zhong, Y.; Shen, F.; et al. Irisin Ameliorates Age-Associated Sarcopenia and Metabolic Dysfunction. J. Cachexia. Sarcopenia Muscle 2023, 14, 391–405. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, C. feng Causal Relationship between Insulin Resistance and Sarcopenia. Diabetol. Metab. Syndr. 2023, 15, 46. [Google Scholar] [CrossRef]
- Fu, Y.H.; Liu, W.J.; Lee, C.L.; Wang, J.S. Associations of Insulin Resistance and Insulin Secretion with Bone Mineral Density and Osteoporosis in a General Population. Front. Endocrinol. 2022, 13, 971960. [Google Scholar] [CrossRef]
- Fulzele, K.; Riddle, R.C.; DiGirolamo, D.J.; Cao, X.; Wan, C.; Chen, D.; Faugere, M.C.; Aja, S.; Hussain, M.A.; Brüning, J.C.; et al. Insulin Receptor Signaling in Osteoblasts Regulates Postnatal Bone Acquisition and Body Composition. Cell 2010, 142, 309–319. [Google Scholar] [CrossRef]
- Thrailkill, K.M.; Lumpkin, C.K.; Bunn, R.C.; Kemp, S.F.; Fowlkes, J.L. Is Insulin an Anabolic Agent in Bone? Dissecting the Diabetic Bone for Clues. Am. J. Physiol.-Endocrinol. Metab. 2005, 289, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Hussey, S.E.; Mcgee, S.L.; Garnham, A.; Mcconell, G.K.; Hargreaves, M. Exercise Increases Skeletal Muscle GLUT4 Gene Expression in Patients with Type 2 Diabetes. Diabetes Obes. Metab. 2012, 14, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Frey, J.L.; Wong, G.W.; Faugere, M.C.; Wolfgang, M.J.; Kim, J.K.; Riddle, R.C.; Clemens, T.L. Glucose Transporter-4 Facilitates Insulin-Stimulated Glucose Uptake in Osteoblasts. Endocrinology 2016, 157, 4094–4103. [Google Scholar] [CrossRef]
- Song, R.; Zhao, X.; Cao, R.; Liang, Y.; Zhang, D.Q.; Wang, R. Irisin Improves Insulin Resistance by Inhibiting Autophagy through the PI3K/Akt Pathway in H9c2 Cells. Gene 2021, 769, 145209. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Dzięgiel, P.; Nowińska, K. The Role of FNDC5/Irisin in Cardiovascular Disease. Cells 2024, 13, 277. [Google Scholar] [CrossRef]
- Harrison, D.G.; Coffman, T.M.; Wilcox, C.S. Pathophysiology of Hypertension: The Mosaic Theory and Beyond. Circ. Res. 2021, 128, 847–863. [Google Scholar] [CrossRef]
- Guzik, T.J.; Nosalski, R.; Maffia, P.; Drummond, G.R. Immune and Inflammatory Mechanisms in Hypertension. Nat. Rev. Cardiol. 2024, 21, 396–416. [Google Scholar] [CrossRef]
- Hirooka, Y. Sympathetic Activation in Hypertension: Importance of the Central Nervous System. Am. J. Hypertens. 2020, 33, 914–926. [Google Scholar] [CrossRef]
- Savić, B.; Murphy, D.; Japundžić-Žigon, N. The Paraventricular Nucleus of the Hypothalamus in Control of Blood Pressure and Blood Pressure Variability. Front. Physiol. 2022, 13, 858941. [Google Scholar] [CrossRef]
- Huo, C.J.; Yu, X.J.; Sun, Y.J.; Li, H.B.; Su, Q.; Bai, J.; Li, Y.; Liu, K.L.; Qi, J.; Zhou, S.W.; et al. Irisin Lowers Blood Pressure by Activating the Nrf2 Signaling Pathway in the Hypothalamic Paraventricular Nucleus of Spontaneously Hypertensive Rats. Toxicol. Appl. Pharmacol. 2020, 394, 114953. [Google Scholar] [CrossRef]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global Estimates on the Number of Persons across the Alzheimer’s Disease Continuum. Alzheimer’s Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s Disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Maggiore, A.; Latina, V.; D’Erme, M.; Amadoro, G.; Coccurello, R. Non-Canonical Pathways Associated to Amyloid Beta and Tau Protein Dyshomeostasis in Alzheimer’s Disease: A Narrative Review. Ageing Res. Rev. 2024, 102, 102578. [Google Scholar] [CrossRef]
- Hiebel, C.; Kromm, T.; Stark, M.; Behl, C. Cannabinoid Receptor 1 Modulates the Autophagic Flux Independent of MTOR- and BECLIN1-Complex. J. Neurochem. 2014, 131, 484–497. [Google Scholar] [CrossRef]
- Dun, S.L.; Lyu, R.M.; Chen, Y.H.; Chang, J.K.; Luo, J.J.; Dun, N.J. Irisin-Immunoreactivity in Neural and Non-Neural Cells of the Rodent. Neuroscience 2013, 240, 155–162. [Google Scholar] [CrossRef]
- Piya, M.K.; Harte, A.L.; Sivakumar, K.; Tripathi, G.; Voyias, P.D.; James, S.; Sabico, S.; Al-Daghri, N.M.; Saravanan, P.; Barber, T.M.; et al. The Identification of Irisin in Human Cerebrospinal Fluid: Influence of Adiposity, Metabolic Markers, and Gestational Diabetes. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E512–E518. [Google Scholar] [CrossRef]
- Albayrak, S.; Atci, I.B.; Kalayci, M.; Yilmaz, M.; Kuloglu, T.; Aydin, S.; Kom, M.; Ayden, O.; Aydin, S. Effect of Carnosine, Methylprednisolone and Their Combined Application on Irisin Levels in the Plasma and Brain of Rats with Acute Spinal Cord Injury. Neuropeptides 2015, 52, 47–54. [Google Scholar] [CrossRef]
- Wang, K.; Li, H.; Wang, H.; Wang, J.H.; Song, F.; Sun, Y. Irisin Exerts Neuroprotective Effects on Cultured Neurons by Regulating Astrocytes. Mediat. Inflamm. 2018, 2018, 9070341. [Google Scholar] [CrossRef]
- Kuwano, T.; Nakao, S.; Yamamoto, H.; Tsuneyoshi, M.; Yamamoto, T.; Kuwano, M.; Ono, M. Cyclooxygenase 2 Is a Key Enzyme for Inflammatory Cytokine-Induced Angiogenesis. FASEB J. 2004, 18, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Samad, T.A.; Moore, K.A.; Sapirstein, A.; Billet, S.; Allchorne, A.; Poole, S.; Bonventre, J.V.; Woolf, C.J. Interleukin-1β-Mediated Induction of Cox-2 in the CNS Contributes to Inflammatory Pain Hypersensitivity. Nature 2001, 410, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Linker, R.A.; Deng, J.; Kaltschmidt, C. Cyclooxygenase-2 Is a Neuronal Target Gene of NF-ΚB. BMC Mol. Biol. 2002, 3, 16. [Google Scholar] [CrossRef]
- Li, D.J.; Li, Y.H.; Yuan, H.B.; Qu, L.F.; Wang, P. The Novel Exercise-Induced Hormone Irisin Protects against Neuronal Injury via Activation of the Akt and ERK1/2 Signaling Pathways and Contributes to the Neuroprotection of Physical Exercise in Cerebral Ischemia. Metabolism 2017, 68, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D.; Decandia, D.; Giacovazzo, G.; Coccurello, R. Physical Exercise as Disease-Modifying Alternative against Alzheimer’s Disease: A Gut-Muscle-Brain Partnership. Int. J. Mol. Sci. 2023, 24, 14686. [Google Scholar] [CrossRef]
- Yu, Q.; Li, G.; Ding, Q.; Tao, L.; Li, J.; Sun, L.; Sun, X.; Yang, Y. Irisin Protects Brain against Ischemia/Reperfusion Injury through Suppressing TLR4/MyD88 Pathway. Cerebrovasc. Dis. 2020, 49, 346–354. [Google Scholar] [CrossRef]
- Streit, W.J.; Mrak, R.E.; Griffin, W.S.T. Microglia and Neuroinflammation: A Pathological Perspective. J. Neuroinflamm. 2004, 1, 14. [Google Scholar] [CrossRef]
- Lehnardt, S.; Massillon, L.; Follett, P.; Jensen, F.E.; Ratan, R.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. Activation of Innate Immunity in the CNS Triggers Neurodegeneration through a Toll-like Receptor 4-Dependent Pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 8514–8519. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Miron, J.; Picard, C.; Frappier, J.; Dea, D.; Théroux, L.; Poirier, J. TLR4 Gene Expression and Pro-Inflammatory Cytokines in Alzheimer’s Disease and in Response to Hippocampal Deafferentation in Rodents. J. Alzheimer’s Dis. 2018, 63, 1547–1556. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, W.; Zhang, M.; Tian, X.; Li, Y.; Lü, Y. Imbalance of Microglial TLR4/TREM2 in LPS-Treated APP/PS1 Transgenic Mice: A Potential Link Between Alzheimer’s Disease and Systemic Inflammation. Neurochem. Res. 2019, 44, 1138–1151. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Liu, J.; Kong, D.; Guo, X.; Li, J.; Long, Z.; Peng, J.; Wang, Z.; Wu, H.; Liu, P.; et al. Irisin Drives Macrophage Anti-Inflammatory Differentiation via JAK2-STAT6-Dependent Activation of PPARγ and Nrf2 Signaling. Free Radic. Biol. Med. 2023, 201, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Zille, M.; Farr, T.D.; Keep, R.F.; Römer, C.; Xi, G.; Boltze, J. Novel Targets, Treatments, and Advanced Models for Intracerebral Haemorrhage. eBioMedicine 2022, 76, 103880. [Google Scholar] [CrossRef] [PubMed]
- Welser-Alves, J.V.; Boroujerdi, A.; Tigges, U.; Milner, R. Microglia Use Multiple Mechanisms to Mediate Interactions with Vitronectin; Non-Essential Roles for the Highly-Expressed Avβ3 and Avβ5 Integrins. J. Neuroinflamm. 2011, 8, 157. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, M.; Tan, J.; Pei, X.; Lu, C.; Xin, Y.; Deng, S.; Zhao, F.; Gao, Y.; Gong, Y. Irisin Ameliorates Neuroinflammation and Neuronal Apoptosis through Integrin AVβ5/AMPK Signaling Pathway after Intracerebral Hemorrhage in Mice. J. Neuroinflamm. 2022, 19, 82. [Google Scholar] [CrossRef]
- Henkel, J.S.; Beers, D.R.; Zhao, W.; Appel, S.H. Microglia in ALS: The Good, the Bad, and the Resting. J. Neuroimmune Pharmacol. 2009, 4, 389–398. [Google Scholar] [CrossRef]
- Olson, J.K.; Miller, S.D. Microglia Initiate Central Nervous System Innate and Adaptive Immune Responses through Multiple TLRs. J. Immunol. 2004, 173, 3916–3924. [Google Scholar] [CrossRef]
- Bedolla, A.; Wegman, E.; Weed, M.; Paranjpe, A.; Alkhimovitch, A.; Ifergan, I.; McClain, L.; Luo, Y. Microglia-Derived TGF-Β1 Ligand Maintains Microglia Homeostasis via Autocrine Mechanism and Is Critical for Normal Cognitive Function in Adult Mouse Brain. bioRxiv 2023. [Google Scholar] [CrossRef]
- Nakajima, K.; Honda, S.; Tohyama, Y.; Imai, Y.; Kohsaka, S.; Kurihara, T. Neurotrophin Secretion from Cultured Microglia. J. Neurosci. Res. 2001, 65, 322–331. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Torres-Alemán, I. The Many Faces of Insulin-like Peptide Signalling in the Brain. Nat. Rev. Neurosci. 2012, 13, 225–239. [Google Scholar] [CrossRef]
- Alexaki, V.I.; Fodelianaki, G.; Neuwirth, A.; Mund, C.; Kourgiantaki, A.; Ieronimaki, E.; Lyroni, K.; Troullinaki, M.; Fujii, C.; Kanczkowski, W.; et al. DHEA Inhibits Acute Microglia-Mediated Inflammation through Activation of the TrkA-Akt1/2-CREB-Jmjd3 Pathway. Mol. Psychiatry 2017, 23, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Fodelianaki, G.; Lansing, F.; Bhattarai, P.; Troullinaki, M.; Zeballos, M.A.; Charalampopoulos, I.; Gravanis, A.; Mirtschink, P.; Chavakis, T.; Alexaki, V.I. Nerve Growth Factor Modulates LPS—Induced Microglial Glycolysis and Inflammatory Responses. Exp. Cell Res. 2019, 377, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Dicarlo, M.; Pignataro, P.; Zerlotin, R.; Suriano, C.; Zecca, C.; Dell’Abate, M.T.; Storlino, G.; Oranger, A.; Sanesi, L.; Mori, G.; et al. Short-Term Irisin Treatment Enhanced Neurotrophin Expression Differently in the Hippocampus and the Prefrontal Cortex of Young Mice. Int. J. Mol. Sci. 2023, 24, 9111. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Yamada, K.; Izawa, S.; Kito, T.; Sawada, H.; Chihara, T.; Aizu, N.; Iwata, D.; Nishii, K. FNDC5/Irisin Mediates the Protective Effects of Innovative Theta-Shaking Exercise on Mouse Memory. Heliyon 2024, 10, e29090. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and Synaptic Plasticity, Cognitive Function, and Dysfunction. Handb. Exp. Pharmacol. 2014, 220, 223–250. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R.; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.B. Microglia Promote Learning-Dependent Synapse Formation through Brain-Derived Neurotrophic Factor. Cell 2013, 155, 1596–1609. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.Y. Aquarobic Exercises Improve the Serum Blood Irisin and Brain-Derived Neurotrophic Factor Levels in Elderly Women. Exp. Gerontol. 2018, 104, 60–65. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Nigam, S.M.; Xu, S.; Kritikou, J.S.; Marosi, K.; Brodin, L.; Mattson, M.P. Exercise and BDNF Reduce Aβ Production by Enhancing α-Secretase Processing of APP. J. Neurochem. 2017, 142, 286–296. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-Linked FNDC5/Irisin Rescues Synaptic Plasticity and Memory Defects in Alzheimer’s Models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.Y.; Huang, X.; Bi, C.F.; Mao, L.L.; Peng, L.J.; Qian, H.R. PGC-1α or FNDC5 Is Involved in Modulating the Effects of Aβ1-42 Oligomers on Suppressing the Expression of BDNF, a Beneficial Factor for Inhibiting Neuronal Apoptosis, Aβ Deposition and Cognitive Decline of APP/PS1 Tg Mice. Front. Aging Neurosci. 2017, 9, 65. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D.; Giacovazzo, G.; Decandia, D.; Coccurello, R. Alzheimer’s Disease and Depression in the Elderly: A Trajectory Linking Gut Microbiota and Serotonin Signaling. Front. Psychiatry 2022, 13, 2605. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D.; Coccurello, R. Editorial: The Affective Side of Alzheimer’s Disease (AD): Neuropsychiatric Symptoms as Early Sentinel of Cognitive Decline and Pathogenetic Factors in Disease Progression. Front. Psychiatry 2023, 14, 1197763. [Google Scholar] [CrossRef]
- Phillips, H.S.; Hains, J.M.; Armanini, M.; Laramee, G.R.; Johnson, S.A.; Winslow, J.W. BDNF MRNA Is Decreased in the Hippocampus of Individuals with Alzheimer’s Disease. Neuron 1991, 7, 695–702. [Google Scholar] [CrossRef]
- Ginsberg, S.D.; Malek-Ahmadi, M.H.; Alldred, M.J.; Chen, Y.; Chen, K.; Chao, M.V.; Counts, S.E.; Mufson, E.J. Brain-Derived Neurotrophic Factor (BDNF) and TrkB Hippocampal Gene Expression Are Putative Predictors of Neuritic Plaque and Neurofibrillary Tangle Pathology. Neurobiol. Dis. 2019, 132, 104540. [Google Scholar] [CrossRef]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front. Cell. Neurosci. 2020, 14, 500839. [Google Scholar] [CrossRef]
- Coccurello, R. Anhedonia in Depression Symptomatology: Appetite Dysregulation and Defective Brain Reward Processing. Behav. Brain Res. 2019, 372, 112041. [Google Scholar] [CrossRef]
- Winter, C.; von Rumohr, A.; Mundt, A.; Petrus, D.; Klein, J.; Lee, T.; Morgenstern, R.; Kupsch, A.; Juckel, G. Lesions of Dopaminergic Neurons in the Substantia Nigra Pars Compacta and in the Ventral Tegmental Area Enhance Depressive-like Behavior in Rats. Behav. Brain Res. 2007, 184, 133–141. [Google Scholar] [CrossRef]
- Muir, J.; Lopez, J.; Bagot, R.C. Wiring the Depressed Brain: Optogenetic and Chemogenetic Circuit Interrogation in Animal Models of Depression. Neuropsychopharmacology 2019, 44, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, A.; Wermers, M.E.; Habermann, T.J.; Singh, B. Long-Term Efficacy and Tolerability of Adjunctive Aripiprazole for Major Depressive Disorder: Systematic Review and Meta-Analysis. Prim. Care Companion CNS Disord. 2021, 23, 34898. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Numakawa, T.; Ninomiya, M.; Yoon, H.S.; Kunugi, H. Cabergoline, a Dopamine Receptor Agonist, Has an Antidepressant-like Property and Enhances Brain-Derived Neurotrophic Factor Signaling. Psychopharmacology 2010, 211, 291–301. [Google Scholar] [CrossRef]
- Karege, F.; Perret, G.; Bondolfi, G.; Schwald, M.; Bertschy, G.; Aubry, J.M. Decreased Serum Brain-Derived Neurotrophic Factor Levels in Major Depressed Patients. Psychiatry Res. 2002, 109, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Brain-Derived Neurotrophic Factor as a Biomarker for Mood Disorders: An Historical Overview and Future Directions. Psychiatry Clin. Neurosci. 2010, 64, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Role of the Brain’s Reward Circuitry in Depression: Transcriptional Mechanisms. Int. Rev. Neurobiol. 2015, 124, 151–170. [Google Scholar]
- Nobili, A.; Latagliata, E.C.; Viscomi, M.T.; Cavallucci, V.; Cutuli, D.; Giacovazzo, G.; Krashia, P.; Rizzo, F.R.; Marino, R.; Federici, M.; et al. Dopamine Neuronal Loss Contributes to Memory and Reward Dysfunction in a Model of Alzheimer’s Disease. Nat. Commun. 2017, 8, 14727. [Google Scholar] [CrossRef]
- Bastioli, G.; Arnold, J.C.; Mancini, M.; Mar, A.C.; Gamallo-Lana, B.; Saadipour, K.; Chao, M.V.; Rice, M.E. Voluntary Exercise Boosts Striatal Dopamine Release: Evidence for the Necessary and Sufficient Role of BDNF. J. Neurosci. 2022, 42, 4725–4736. [Google Scholar] [CrossRef]
- Dinas, P.C.; Koutedakis, Y.; Flouris, A.D. Effects of Exercise and Physical Activity on Depression. Ir. J. Med. Sci. 2011, 180, 319–325. [Google Scholar] [CrossRef]
- Wang, S.; Pan, J. Irisin Ameliorates Depressive-like Behaviors in Rats by Regulating Energy Metabolism. Biochem. Biophys. Res. Commun. 2016, 474, 22–28. [Google Scholar] [CrossRef]
- Steiner, J.L.; Murphy, E.A.; McClellan, J.L.; Carmichael, M.D.; Davis, J.M. Exercise Training Increases Mitochondrial Biogenesis in the Brain. J. Appl. Physiol. 2011, 111, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Zsuga, J.; Tajti, G.; Papp, C.; Juhasz, B.; Gesztelyi, R. FNDC5/Irisin, a Molecular Target for Boosting Reward-Related Learning and Motivation. Med. Hypotheses 2016, 90, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Guillin, O.; Griffon, N.; Bezard, E.; Leriche, L.; Diaz, J.; Gross, C.; Sokoloff, P. Brain-Derived Neurotrophic Factor Controls Dopamine D3 Receptor Expression: Therapeutic Implications in Parkinson’s Disease. Eur. J. Pharmacol. 2003, 480, 89–95. [Google Scholar] [CrossRef]
- Murphy, A.; Nestor, L.J.; McGonigle, J.; Paterson, L.; Boyapati, V.; Ersche, K.D.; Flechais, R.; Kuchibatla, S.; Metastasio, A.; Orban, C.; et al. Acute D3 Antagonist GSK598809 Selectively Enhances Neural Response During Monetary Reward Anticipation in Drug and Alcohol Dependence. Neuropsychopharmacology 2017, 42, 1049–1057. [Google Scholar] [CrossRef]
- Yardimci, A.; Ertugrul, N.U.; Ozgen, A.; Ozbeg, G.; Ozdede, M.R.; Ercan, E.C.; Canpolat, S. Effects of Chronic Irisin Treatment on Brain Monoamine Levels in the Hypothalamic and Subcortical Nuclei of Adult Male and Female Rats: An HPLC-ECD Study. Neurosci. Lett. 2023, 806, 137245. [Google Scholar] [CrossRef]
- Watamura, N.; Kakiya, N.; Fujioka, R.; Kamano, N.; Takahashi, M.; Nilsson, P.; Saito, T.; Iwata, N.; Fujisawa, S.; Saido, T.C. The Dopaminergic System Promotes Neprilysin-Mediated Degradation of Amyloid-β in the Brain. Sci. Signal. 2024, 17, eadk1822. [Google Scholar] [CrossRef]
- Rofo, F.; Yilmaz, C.U.; Metzendorf, N.; Gustavsson, T.; Beretta, C.; Erlandsson, A.; Sehlin, D.; Syvänen, S.; Nilsson, P.; Hultqvist, G. Enhanced Neprilysin-Mediated Degradation of Hippocampal Aβ42 with a Somatostatin Peptide That Enters the Brain. Theranostics 2021, 11, 789–804. [Google Scholar] [CrossRef]
- Iwata, N.; Tsubuki, S.; Takaki, Y.; Shirotani, K.; Lu, B.; Gerard, N.P.; Gerard, C.; Hama, E.; Lee, H.J.; Saido, T.C. Metabolic Regulation of Brain Abeta by Neprilysin. Science 2001, 292, 1550–1552. [Google Scholar] [CrossRef]
- Ou-Yang, W.L.; Guo, B.; Xu, F.; Lin, X.; Li, F.X.Z.; Shan, S.K.; Wu, F.; Wang, Y.; Zheng, M.H.; Xu, Q.S.; et al. The Controversial Role of Irisin in Clinical Management of Coronary Heart Disease. Front. Endocrinol. 2021, 12, 678309. [Google Scholar] [CrossRef]
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisløff, U.; Tjønna, A.E.; Raastad, T.; et al. Evidence against a Beneficial Effect of Irisin in Humans. PLoS ONE 2013, 8, e73680. [Google Scholar] [CrossRef]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436–456. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Han, F.; Sun, X. The Relationship between Circulating Irisin Levels and Endothelial Function in Lean and Obese Subjects. Clin. Endocrinol. 2015, 83, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Esin, K.; Batirel, S.; Ülfer, G.; Yigit, P.; Sanlier, N. Association of Serum Irisin Levels with Body Composition, Metabolic Profile, Leptin, and Adiponectin Levels in Lean and Obese Children. Medicina 2023, 59, 1954. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Song, R.; Zhao, X.; Yang, C.; Feng, Y. Lower Circulating Irisin Levels in Type 2 Diabetes Mellitus Patients with Chronic Complications: A Meta-Analysis. Heliyon 2023, 9, e21859. [Google Scholar] [CrossRef]
- Conti, E.; Grana, D.; Stefanoni, G.; Corsini, A.; Botta, M.; Magni, P.; Aliprandi, A.; Lunetta, C.; Appollonio, I.; Ferrarese, C.; et al. Irisin and BDNF Serum Levels and Behavioral Disturbances in Alzheimer’s Disease. Neurol. Sci. 2019, 40, 1145–1150. [Google Scholar] [CrossRef]
- Kim, E.; Kim, H.; Jedrychowski, M.P.; Bakiasi, G.; Park, J.; Kruskop, J.; Choi, Y.; Kwak, S.S.; Quinti, L.; Kim, D.Y.; et al. Irisin Reduces Amyloid-β by Inducing the Release of Neprilysin from Astrocytes Following Downregulation of ERK-STAT3 Signaling. Neuron 2023, 111, 3619–3633.e8. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Martin, S. Insulin and Aging—A Disappointing Relationship. Front. Endocrinol. 2023, 14, 1261298. [Google Scholar] [CrossRef]
- Mosconi, L.; De Santi, S.; Li, J.; Tsui, W.H.; Li, Y.; Boppana, M.; Laska, E.; Rusinek, H.; de Leon, M.J. Hippocampal Hypometabolism Predicts Cognitive Decline from Normal Aging. Neurobiol. Aging 2008, 29, 676–692. [Google Scholar] [CrossRef]
- Öz, G.; Seaquist, E.R.; Kumar, A.; Criego, A.B.; Benedict, L.E.; Rao, J.P.; Henry, P.G.; Van De Moortele, P.F.; Gruetter, R. Human Brain Glycogen Content and Metabolism: Implications on Its Role in Brain Energy Metabolism. Am. J. Physiol.—Endocrinol. Metab. 2007, 292, E946–E951. [Google Scholar] [CrossRef]
- Chen, Y.; Joo, J.; Chu, J.M.T.; Chang, R.C.C.; Wong, G.T.C. Downregulation of the Glucose Transporter GLUT 1 in the Cerebral Microvasculature Contributes to Postoperative Neurocognitive Disorders in Aged Mice. J. Neuroinflamm. 2023, 20, 237. [Google Scholar] [CrossRef]
- Leão, L.L.; Tangen, G.; Barca, M.L.; Engedal, K.; Santos, S.H.S.; Machado, F.S.M.; de Paula, A.M.B.; Monteiro-Junior, R.S. Does Hyperglycemia Downregulate Glucose Transporters in the Brain? Med. Hypotheses 2020, 139, 109614. [Google Scholar] [CrossRef] [PubMed]
- Simpson, I.A.; Chundu, K.R.; Davies-Hill, T.; Honer, W.G.; Davies, P. Decreased Concentrations of GLUT1 and GLUT3 Glucose Transporters in the Brains of Patients with Alzheimer’s Disease. Ann. Neurol. 1994, 35, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.S.S.; Fernandes, C.S.; Vieira, M.N.N.; De Felice, F.G. Insulin Resistance in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 830. [Google Scholar] [CrossRef]
- Razani, E.; Pourbagheri-Sigaroodi, A.; Safaroghli-Azar, A.; Zoghi, A.; Shanaki-Bavarsad, M.; Bashash, D. The PI3K/Akt Signaling Axis in Alzheimer’s Disease: A Valuable Target to Stimulate or Suppress? Cell Stress Chaperones 2021, 26, 871. [Google Scholar] [CrossRef]
- Li, M.; Dai, F.; Du, X.; Yang, Q.; Zhang, X.; Chen, Y. Infusion of BDNF into the Nucleus Accumbens of Aged Rats Improves Cognition and Structural Synaptic Plasticity through PI3K-ILK-Akt Signaling. Behav. Brain Res. 2012, 231, 146–153. [Google Scholar] [CrossRef]
- Wu, Y.; Eisel, U.L.M. Microglia-Astrocyte Communication in Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 95, 785. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, D.; Singh, J. GLUT-1: An Effective Target to Deliver Brain-Derived Neurotrophic Factor Gene across the Blood Brain Barrier. ACS Chem. Neurosci. 2020, 11, 1620–1633. [Google Scholar] [CrossRef]
- Tambaro, S.; Mitra, S.; Gera, R.; Linderoth, B.; Wahlberg, L.U.; Darreh-Shori, T.; Behbahani, H.; Nilsson, P.; Eriksdotter, M. Feasibility and Therapeutical Potential of Local Intracerebral Encapsulated Cell Biodelivery of BDNF to AppNL−G−F Knock-in Alzheimer Mice. Alzheimer’s Res. Ther. 2023, 15, 137. [Google Scholar] [CrossRef]
- Alcalá-Barraza, S.R.; Lee, M.S.; Hanson, L.R.; McDonald, A.A.; Frey, W.H.; McLoon, L.K. Intranasal Delivery of Neurotrophic Factors BDNF, CNTF, EPO, and NT-4 to the CNS. J. Drug Target. 2010, 18, 179. [Google Scholar] [CrossRef]
- Talbot, K.; Wang, H.Y.; Kazi, H.; Han, L.Y.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S.; et al. Demonstrated Brain Insulin Resistance in Alzheimer’s Disease Patients Is Associated with IGF-1 Resistance, IRS-1 Dysregulation, and Cognitive Decline. J. Clin. Investig. 2012, 122, 1316–1338. [Google Scholar] [CrossRef]
- Miao, J.; Zhang, Y.; Su, C.; Zheng, Q.; Guo, J. Insulin-Like Growth Factor Signaling in Alzheimer’s Disease: Pathophysiology and Therapeutic Strategies. Mol. Neurobiol. 2024, 2024, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Chen, N.; Kang, X.; Hu, Y.; Shi, S. Irisin Alleviates FFA Induced β-Cell Insulin Resistance and Inflammatory Response through Activating PI3K/AKT/FOXO1 Signaling Pathway. Endocrine 2022, 75, 740–751. [Google Scholar] [CrossRef]
- Draznin, B. Molecular Mechanisms of Insulin Resistance: Serine Phosphorylation of Insulin Receptor Substrate-1 and Increased Expression of P85αThe Two Sides of a Coin. Diabetes 2006, 55, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Vliora, M.; Nintou, E.; Karligiotou, E.; Ioannou, L.G.; Grillo, E.; Mitola, S.; Flouris, A.D. Implication of Irisin in Different Types of Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 9971. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Kang, H.; Lin, C.Y.; Fan, Y. Unlocking the Therapeutic Potential of Irisin: Harnessing Its Function in Degenerative Disorders and Tissue Regeneration. Int. J. Mol. Sci. 2023, 24, 6551. [Google Scholar] [CrossRef]
- Munoz, I.Y.M.; Del Socorro Camarillo Romero, E.; De Jesus Garduno Garcia, J. Irisin a Novel Metabolic Biomarker: Present Knowledge and Future Directions. Int. J. Endocrinol. 2018, 2018, 7816806. [Google Scholar] [CrossRef]
- Corbett, A.; Pickett, J.; Burns, A.; Corcoran, J.; Dunnett, S.B.; Edison, P.; Hagan, J.J.; Holmes, C.; Jones, E.; Katona, C.; et al. Drug Repositioning for Alzheimer’s Disease. Nat. Rev. Drug Discov. 2012, 11, 833–846. [Google Scholar] [CrossRef]
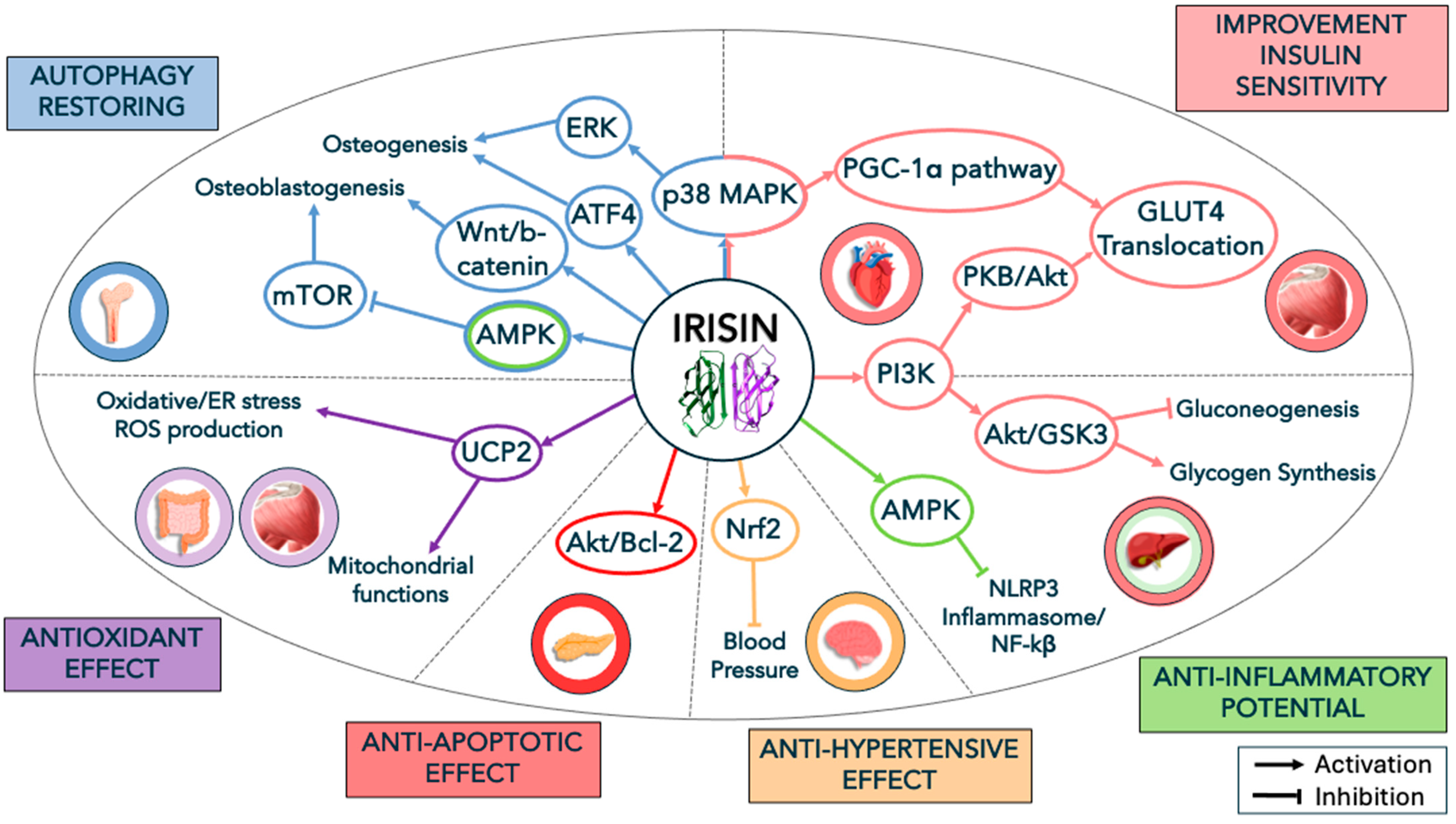
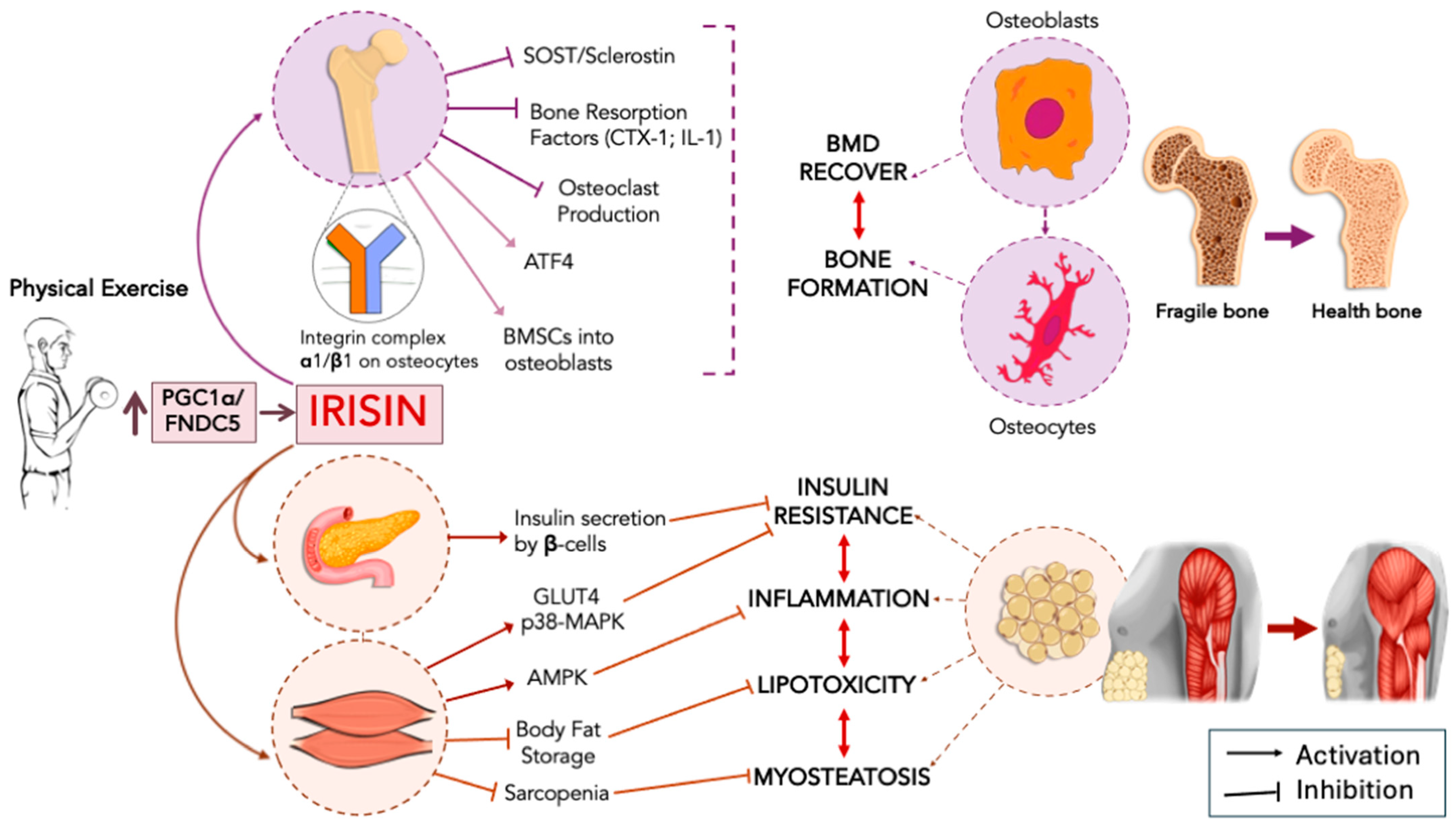

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paoletti, I.; Coccurello, R. Irisin: A Multifaceted Hormone Bridging Exercise and Disease Pathophysiology. Int. J. Mol. Sci. 2024, 25, 13480. https://doi.org/10.3390/ijms252413480
Paoletti I, Coccurello R. Irisin: A Multifaceted Hormone Bridging Exercise and Disease Pathophysiology. International Journal of Molecular Sciences. 2024; 25(24):13480. https://doi.org/10.3390/ijms252413480
Chicago/Turabian StylePaoletti, Ilaria, and Roberto Coccurello. 2024. "Irisin: A Multifaceted Hormone Bridging Exercise and Disease Pathophysiology" International Journal of Molecular Sciences 25, no. 24: 13480. https://doi.org/10.3390/ijms252413480
APA StylePaoletti, I., & Coccurello, R. (2024). Irisin: A Multifaceted Hormone Bridging Exercise and Disease Pathophysiology. International Journal of Molecular Sciences, 25(24), 13480. https://doi.org/10.3390/ijms252413480





