The Study of Ropivacaine Pharmacokinetics in a Clinical Setting: A Critical Scoping Review from the Perspective of Analytical Methodologies
Abstract
:1. Introduction
1.1. History and Clinical Use of Ropivacaine
1.2. Pharmaceutical Formulation and Administration
1.3. Indications and Contraindications
1.4. Special Situations
1.5. Adverse Effects
1.6. Risk Factors
1.7. LAST Management
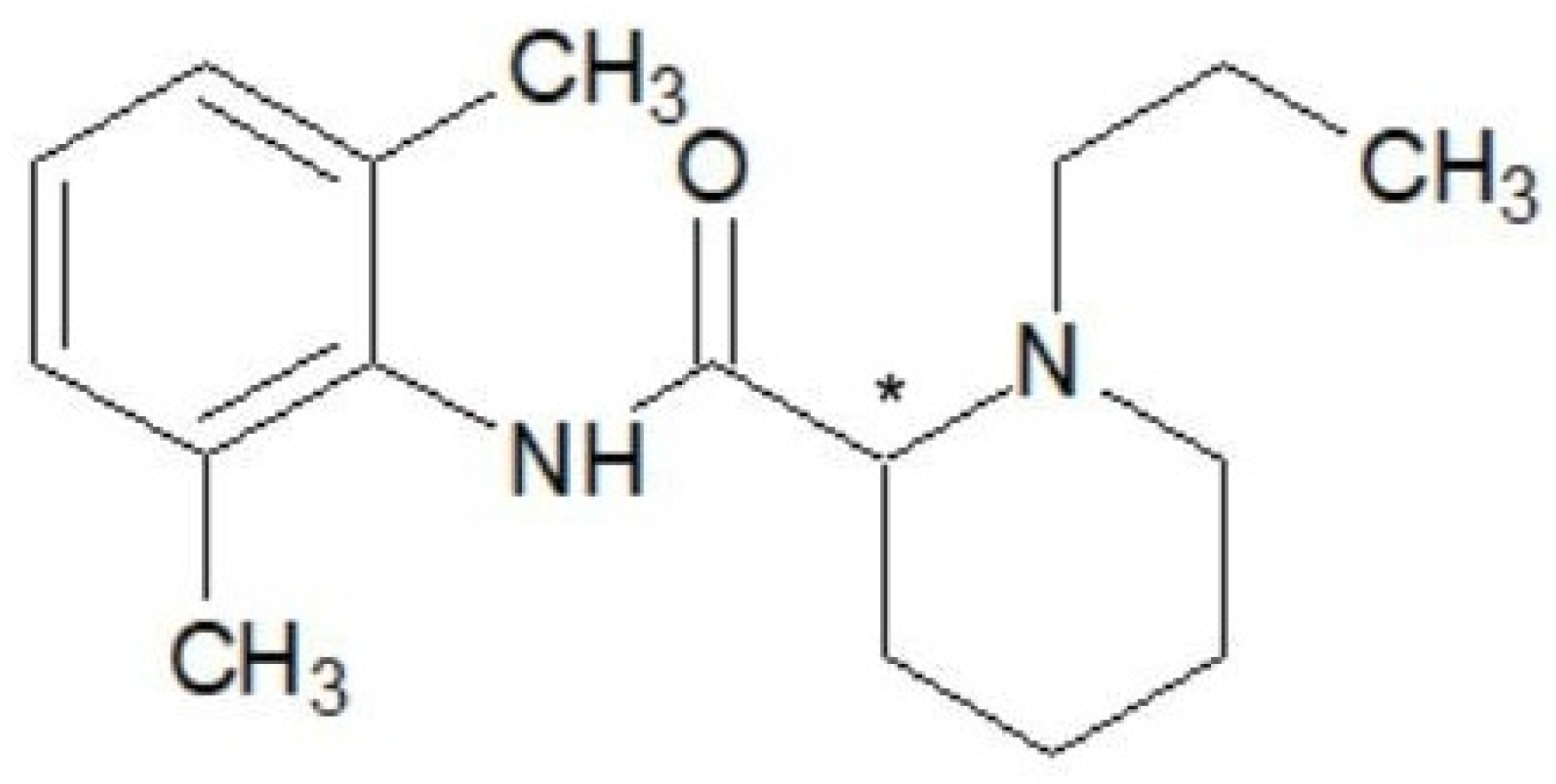
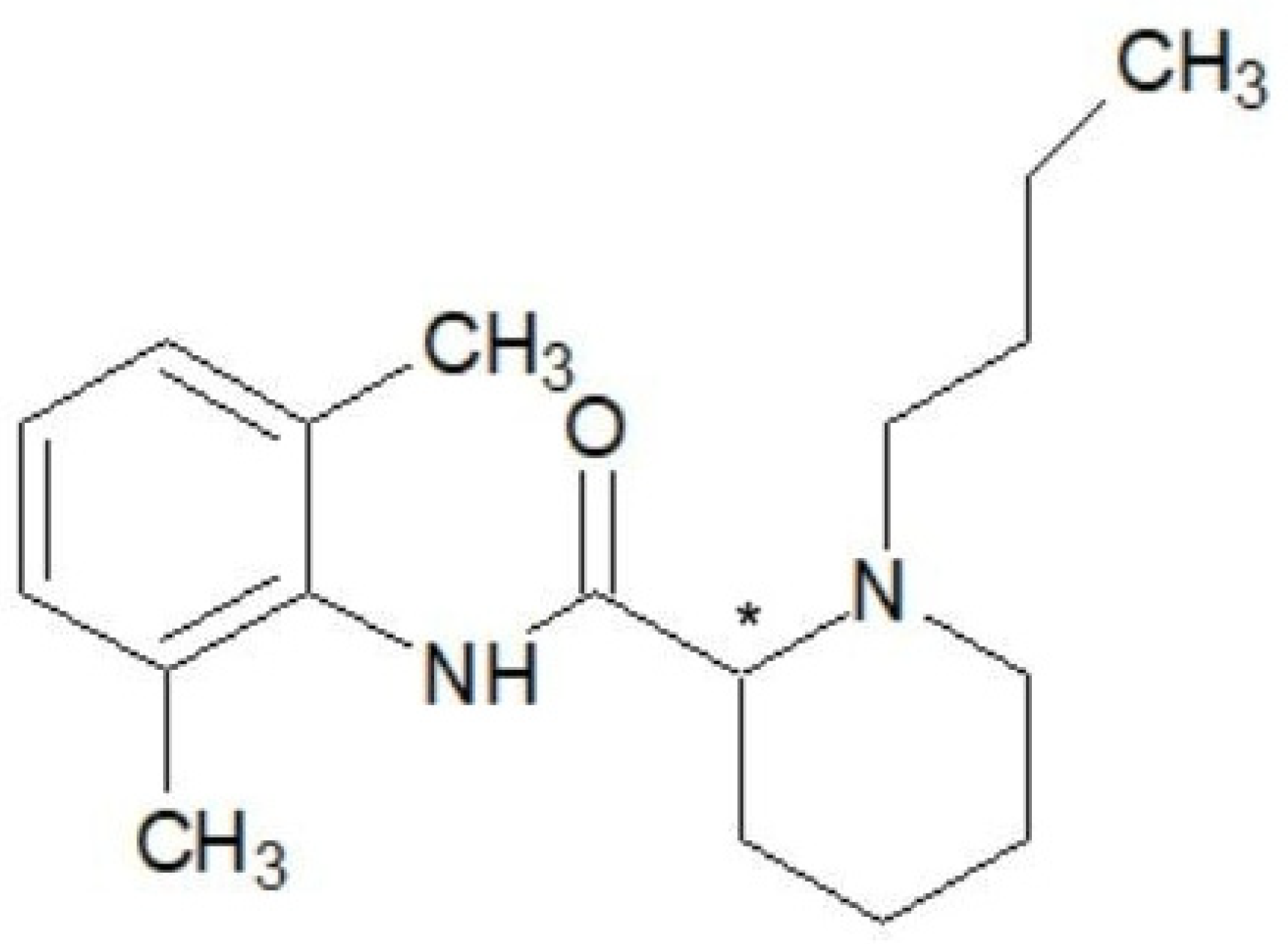
1.8. Pharmacological and Chemical Properties
1.9. Pharmacodynamics
1.10. Pharmacokinetics
1.11. Volume of Distribution
1.12. Biomonitoring and Pharmacokinetics
1.13. Bioanalytical Techniques and Methodologies
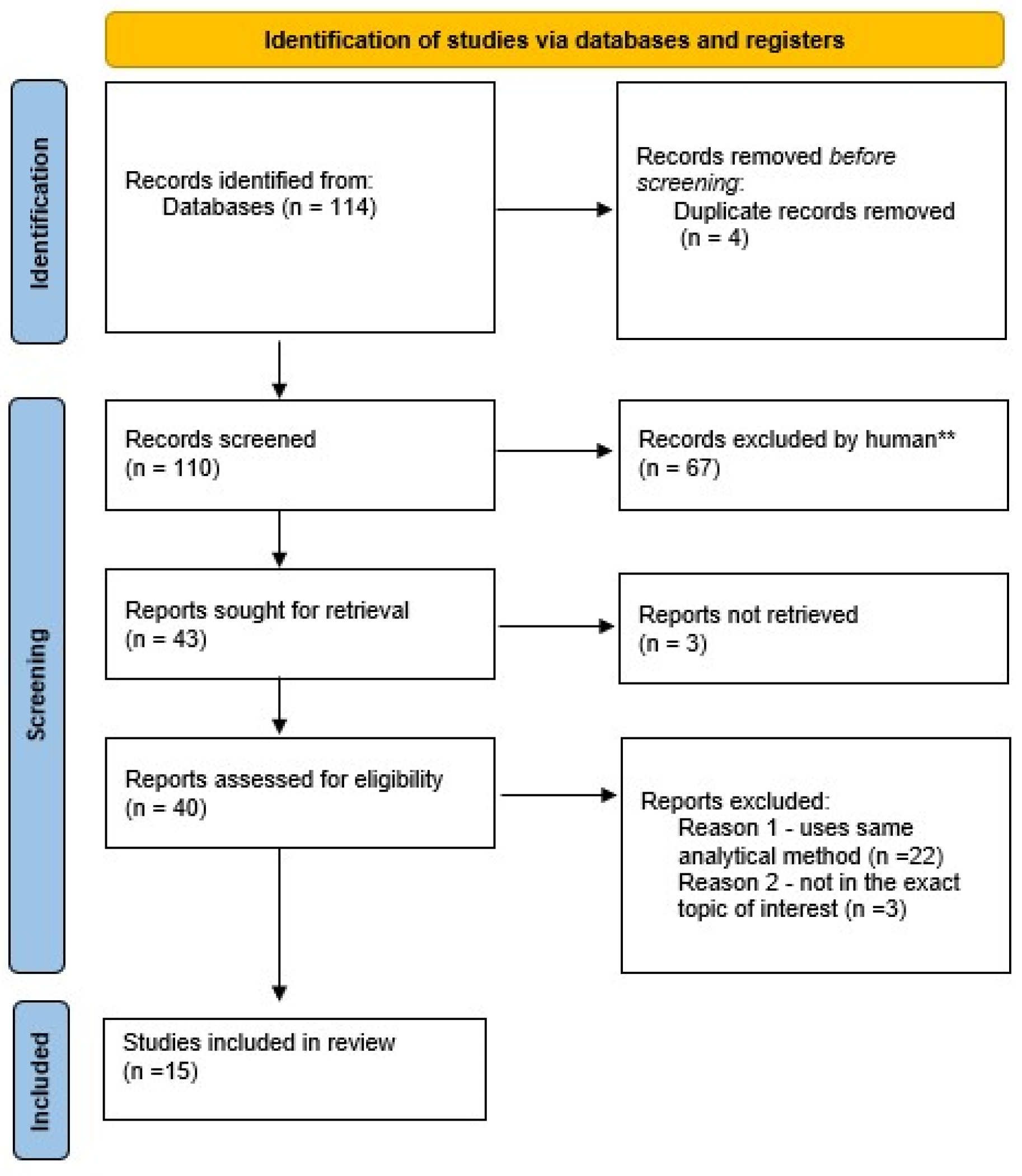
2. Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IASP Subcommittee on Taxonomy. Pain terms: A list with definitions and notes on usage. Recommended by the IASP Sub-committee on Taxonomy. Pain 1979, 6, 249–252. [Google Scholar]
- Ruetsch, Y.A.; Boni, T.; Borgeat, A. From Cocaine to Ropivacaine: The History of Local Anesthetic Drugs. Curr. Top. Med. Chem. 2001, 1, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.M. Miller’s Anesthesia: Pharmacology of local anesthetics used in ambulatory surgery. In Miller’s Anesthesia, 9th ed.; Elsevier: Philadelphia, PA, USA, 2020; pp. 888–920. [Google Scholar]
- Kirksey, M.A.; Haskins, S.C.; Cheng, J.; Liu, S.S. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: A systematic qualitative review. PLoS ONE 2015, 10, e0137312. [Google Scholar] [CrossRef] [PubMed]
- Graff, V.; Gabutti, L.; Treglia, G.; Pascale, M.; Anselmi, L.; Cafarotti, S.; La Regina, D.; Mongelli, F.; Saporito, A. Perioperative costs of local or regional anesthesia versus general anesthesia in the outpatient setting: A systematic review of recent literature. Braz. J. Anesthesiol. 2021, 73, 316–339. [Google Scholar] [CrossRef] [PubMed]
- Park, W.Y.; Thompson, J.S.; Lee, K.K. Effect of Epidural Anesthesia and Analgesia on Perioperative Outcome: A randomized, controlled Veterans Affairs cooperative study. Ann. Surg. 2001, 234, 560–569; discussion 569–571. [Google Scholar] [CrossRef]
- Jia, W.; Shen, J.; Wei, S.; Li, C.; Shi, J.; Zhao, L.; Jia, H. Ropivacaine inhibits the malignant behavior of lung cancer cells by regulating retinoblastoma-binding protein 4. PeerJ 2023, 11, e16471. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, M.; Xu, Z.; Li, W.; Dong, X.; Chen, Y.; Lin, B.; Li, M. Ropivacaine promotes apoptosis of hepatocellular carcinoma cells through damaging mitochondria and activating caspase-3 activity. Biol. Res. 2019, 52, 36. [Google Scholar] [CrossRef]
- Bátai, I.; Kerényi, M.; Falvai, J.; Szabó, G. Bacterial Growth in Ropivacaine Hydrochloride. Anesth. Analg. 2002, 94, 729–731. [Google Scholar] [CrossRef]
- Kampe, S.; Poetter, C.; Buzello, S.; Wenchel, H.-M.; Paul, M.; Kiencke, P.; Kasper, S.-M. Ropivacaine 0.1% with Sufentanil 1 microg/mL Inhibits In Vitro Growth of Pseudomonas Aeruginosa and Does Not Promote Multiplication of Staphylococcus Aureus. Anesth. Analg. 2003, 97, 409–411. [Google Scholar] [CrossRef]
- T3DB. Ropivacaine (T3D2741). Available online: http://www.t3db.ca/toxins/T3D2741 (accessed on 30 September 2024).
- Gitman, M.; Fettiplace, M.R.; Weinberg, G.L.; Neal, J.M.; Barrington, M.J. Local Anesthetic Systemic Toxicity: A Narrative Literature Review and Clinical Update on Prevention, Diagnosis, and Management. Plast. Reconstr. Surg. 2019, 144, 783–795. [Google Scholar] [CrossRef]
- Rubin, D.S.; Matsumoto, M.M.; Weinberg, G.; Roth, S. Local Anesthetic Systemic Toxicity in Total Joint Arthroplasty: Incidence and Risk Factors in the United States from the National Inpatient Sample 1998–2013. Reg. Anesth. Pain Med. 2018, 43, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Bern, S.; Weinberg, G. Local anesthetic toxicity and lipid resuscitation in pregnancy. Curr. Opin. Anaesthesiol. 2011, 24, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.M.; Barrington, M.J.; Fettiplace, M.R.; Gitman, M.; Memtsoudis, S.G.; Mörwald, E.E.; Rubin, D.S.; Weinberg, G. The Third American Society of Regional Anesthesia and Pain Medicine Practice Advisory on Local Anesthetic Systemic Toxicity: Executive Summary 2017. Reg. Anesth. Pain Med. 2018, 43, 113–123. [Google Scholar] [CrossRef]
- Heavner, J.E.; Dryden, C.F.; Sanghani, V.; Huemer, G.; Bessire, A.; Badgwell, J.M. Severe Hypoxia Enhances Central Nervous System and Cardiovascular Toxicity of Bupivacaine in Lightly Anesthetized Pigs. Anesthesiology 1992, 77, 142–147. [Google Scholar] [CrossRef]
- Rosen, M.A.; Thigpen, J.W.; Shnider, S.M.; Foutz, S.E.; Levinson, G.; Koike, M. Bupivacaine-induced cardiotoxicity in hypoxic and acidotic sheep. Anesth. Analg. 1985, 64, 1089–1096. [Google Scholar] [CrossRef]
- Ohmura, S.; Ohta, T.; Yamamoto, K.; Kobayashi, T. A Comparison of the Effects of Propofol and Sevoflurane on the Systemic Toxicity of Intravenous Bupivacaine in Rats. Anesth. Analg. 1999, 88, 155–159. [Google Scholar] [CrossRef]
- Weinberg, G.L. Treatment of Local Anesthetic Systemic Toxicity (LAST). Reg. Anesth. Pain Med. 2010, 35, 188–193. [Google Scholar] [CrossRef]
- PubChem. Ropivacaine—4 Chemical and Physical Properties. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ropivacaine#section=Chemical-and-Physical-Properties (accessed on 30 September 2024).
- Dossou, K.; Chiap, P.; Servais, A.-C.; Fillet, M.; Crommen, J. Development and validation of a LC method for the enantiomeric purity determination of S-ropivacaine in a pharmaceutical formulation using a recently commercialized cellulose-based chiral stationary phase and polar non-aqueous mobile phase. J. Pharm. Biomed. Anal. 2011, 54, 687–693. [Google Scholar] [CrossRef]
- Hansen, T.G. Ropivacaine: A pharmacological review. Expert Rev. Neurother. 2004, 4, 781–791. [Google Scholar] [CrossRef]
- Åberg, G. Toxicological and Local Anaesthetic Effects of Optically Active Isomers of Two Local Anaesthetic Compounds. Acta Pharmacol. Toxicol. 1972, 31, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Riff, C.; Le Caloch, A.; Dupouey, J.; Allanioux, L.; Leone, M.; Blin, O.; Bourgoin, A.; Guilhaumou, R. Local Anesthetic Plasma Concentrations as a Valuable Tool to Confirm the Diagnosis of Local Anesthetic Systemic Toxicity? A Report of 10 Years of Experience. Pharmaceutics 2022, 14, 708. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.M.; Abraham, I.; Eberbach, N.; Kunst, G.; Stowe, D.F.; Martin, E. Differences in Cardiotoxicity of Bupivacaine and Ropivacaine Are the Result of Physicochemical and Stereoselective Properties. Anesthesiology 2002, 96, 1427–1434. [Google Scholar] [CrossRef]
- Knudsen, K.; Suurküla, M.B.; Blomberg, S.; Sjövall, J.; Edvardsson, N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br. J. Anaesth. 1997, 78, 507–514. [Google Scholar] [CrossRef]
- Rosenberg, P.H.; Veering, B.T.; Urmey, W.F. Maximum recommended doses of local anesthetics: A multifactorial concept. Reg. Anesth. Pain Med. 2004, 29, 564–575; discussion 524. [Google Scholar] [CrossRef]
- Naropin. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020533s038lbl.pdf (accessed on 30 September 2024).
- Simpson, D.; Curran, M.P.; Oldfield, V.; Keating, G.M. Ropivacaine: A review of its use in regional anaesthesia and acute pain man-agement. Drugs 2005, 65, 2675–2717. [Google Scholar] [CrossRef]
- Tolonen, H.; Andersson, A.-M.; Holmboe, S.A.; Meltzer, H.M. Health information for human biomonitoring studies. Int. J. Hyg. Environ. Health 2022, 246, 114051. [Google Scholar] [CrossRef]
- Guidi, M.; Csajka, C.; Buclin, T. Parametric Approaches in Population Pharmacokinetics. J. Clin. Pharmacol. 2022, 62, 125–141. [Google Scholar] [CrossRef]
- Ingle, R.G.; Zeng, S.; Jiang, H.; Fang, W.-J. Current developments of bioanalytical sample preparation techniques in pharmaceuticals. J. Pharm. Anal. 2022, 12, 517–529. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Rifai, N.; Hsin, O.; Hope, T.; Sakamoto, M. Simultaneous Measurement of Plasma Ropivacaine and Bupivacaine Concentrations by HPLC with UV Detection. Ther. Drug Monit. 2001, 23, 182–186. [Google Scholar] [CrossRef]
- Yu, Z.; Abdel-Rehim, M.; Westerlund, D. Determination of amide-type local anaesthetics by direct injection of plasma in a column-switching high-performance liquid chromatographic system using a pre-column with a semipermeable surface. J. Chromatogr. B Biomed. Sci. Appl. 1994, 654, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Nakamura, T.; Inomata, S.; Honda, K. Simultaneous determination of three local anesthetic drugs from the pipecoloxylidide group in human serum by high-performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 834, 213–216. [Google Scholar] [CrossRef]
- Gaudreault, F.; Drolet, P.; Varin, F. High-Performance Liquid Chromatography Using UV Detection for the Simultaneous Quantification of Ropivacaine and Bupivacaine in Human Plasma. Ther. Drug Monit. 2009, 31, 753–757. [Google Scholar] [CrossRef]
- Mouzi, L.K.; Adams, O.; Cuff, G.; Lukasiewicz, E.; Champeil, E.; Atchabahian, A. Plasma concentrations of ropivacaine following ultrasound-guided or nerve-stimulator-guided femoral nerve block: A prospective randomised study. Anaesth. Crit. Care Pain Med. 2016, 35, 45–48. [Google Scholar] [CrossRef]
- Kawata, T.; Homma, M.; Kakiuchi, Y.; Inomata, S.; Miyabe, M.; Kobayashi, D.; Morimoto, Y.; Kohda, Y. Liquid Chromatographic Determination of Plasma Ropivacaine for Assessing Pharmacokinetics of the Viscous Preparation. Biol. Pharm. Bull. 2005, 28, 2271–2273. [Google Scholar] [CrossRef]
- Zhang, F.; Lv, C.; Yang, L.; Wang, S.; Zhang, M.; Guo, X. Pharmacokinetics of ropivacaine in elderly patients receiving fascia iliaca compartment block. Exp. Ther. Med. 2019, 18, 2648–2652. [Google Scholar] [CrossRef]
- Koehler, A.; Oertel, R.; Kirch, W. Simultaneous determination of bupivacaine, mepivacain, prilocaine and ropivacain in human serum by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2005, 1088, 126–130. [Google Scholar] [CrossRef]
- Mathieu, O.; Hillaire-Buys, D.; Dadure, C.; Barnay, F.; Mathieu-Daudé, J.C.; Bressolle, F. Liquid chromatography–electrospray mass spectrometry determination of free and total concentrations of ropivacaine in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 831, 91–98. [Google Scholar] [CrossRef]
- Sawaki, K.; Okubo, M.; Shimomiya, T.; Tukagoshi, E.; Sakai, T.; Yamazaki, T.; Kenmochi, M.; Miyao, M.; Kaneko, Y.; Ichinohe, T.; et al. Evaluation of high-performance liquid chromatography and mass spectrometry method for pharmacokinetic study of local anesthetic ropivacaine in plasma. Biomed. Res. 2009, 30, 319–324. [Google Scholar] [CrossRef]
- Breindahl, T.; Simonsen, O.; Andreasen, K. Column-switching HPLC–MS/MS analysis of ropivacaine in serum, ultrafiltrate and drainage blood for validating the safety of blood reinfusion. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 76–82. [Google Scholar] [CrossRef]
- Tonooka, K.; Naruki, N.; Honma, K.; Agei, K.; Okutsu, M.; Hosono, T.; Kunisue, Y.; Terada, M.; Tomobe, K.; Shinozuka, T. Sensitive liquid chromatography/tandem mass spectrometry method for the simultaneous determination of nine local anesthetic drugs. Forensic. Sci. Int. 2016, 265, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Fall, F.; Boigne, L.; Gromov, K.; Fabresse, N.; Grassin-Delyle, S. Validation according to European and American regulatory agencies guidelines of an LC-MS/MS method for the quantification of free and total ropivacaine in human plasma. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rehim, M.; Altun, Z.; Blomberg, L. Microextraction in packed syringe (MEPS) for liquid and gas chromatographic applications. Part II—Determination of ropivacaine and its metabolites in human plasma samples using MEPS with liquid chromatography/tandem mass spectrometry. J. Mass Spectrom. 2004, 39, 1488–1493. [Google Scholar] [CrossRef]
- Butiulca, M.; Farczadi, L.; Vari, C.E.; Imre, S.; Pui, M.; Lazar, A. LC-MS/MS assisted biomonitoring of ropivacaine and 3-OH-ropivacaine after plane block anesthesia for cardiac device implantation. Front. Mol. Biosci. 2023, 10, 1243103. [Google Scholar] [CrossRef]

| Method | Advantages | Disadvantages |
|---|---|---|
| HPLC-UV | high reproducibility, simpler sample preparation, lower cost, widely available | low selectivity, low sensitivity |
| (HP)LC-MS | high selectivity, high sensitivity, simpler sample preparation | lower reproducibility, higher cost, not widely available |
| HPLC-FLD | higher specificity | not widely available, complicated/costly sample preparation (derivatization) |
| GC(-MS) | higher specificity, higher sensitivity, widely available | complicated/costly sample preparation (derivatization) |
| ELISA | simple sample preparation, low cost | equipment widely available but kit not always available |
| Biological Matrix | Column | Analytical Separation and Detection Method | Sample Preparation Technique | Biomarker of Exposure | Internal Standard | Calibration Range | Author |
|---|---|---|---|---|---|---|---|
| plasma | LC8 DB column (5 μm particle size, 5.0 cm × 4.6 mm) | HPLC-UV | solid-phase extraction | ropivacaine, bupivacaine | pentycaine | 200–1000 ng/mL | Rifai et al. [34] |
| plasma | Hypercarb analytical column (size 100 × 3.2 mm) | HPLC-UV | solid-phase extraction | ropivacaine, bupivacaine | pentycaine | 1.6–120 μg/mL | Yu et al. [35] |
| human serum | Inertsil C18 reversed-phase column (150 mm × 4.6 mm, particle and 5 μm) | HPLC-UV | protein precipitation, solvent evaporation, resolubilization | mepivacaine, bupivacaine, ropivacaine | tetracaine | 2–1000 ng/mL | Tanaka et al. [36] |
| plasma | C8 reversed-phase column (size 150 × 4.6 mm, particle diameter 5 μm) | HPLC-UV | Liquid–liquid extraction | ropivacaine, bupivacaine | tetracaine | 4–1000 ng/mL | Gaudreault et al. [37] |
| plasma | C18 type reversed-phase column | HPLC-UV | Ethylether extraction, solvent evaporation | ropivacaine | bupivacaine | 50–5000 ng/mL | Mouzi et al. [38] |
| plasma | C18 column TSK-GEL with 4.6 × 150 mm size | HPLC-UV | Liquid–liquid extraction | ropivacaine | bupivacaine | 25–1000 ng/mL | Kawata et al. [39] |
| plasma | not specified | not specified | equilibrium dialysis | ropivacaine | not specified | not specified | Zhang et al. [40] |
| human serum | Synergy 4 μm Polar-RP column (150 mm × 2 mm) | LC-MS/MS | protein precipitation | bupivacaine, mepivacaine, prilocaine, ropivacaine | no internal standard | 1–200 ng/mL | Koehler et al. [41] |
| plasma | XD8 C8 column (5 μm particles, dimensions of 150 × 4.6 mm) | LC-MS/MS-ESI | protein precipitation | ropivacaine | etidocaine | 50–400 μg/L | Mathieu et al. [42] |
| plasma | Chemcosorb 7-ODS-H (250 × 4.6 mm) | LC-MS/MS | protein precipitation | ropivacaine | no internal standard | not specified | Sawaki et al. [43] |
| plasma, ultrafiltrate, drainage blood | Agilent Zorbax SB-Aq column (50 × 2.1 mm) | LC-MS/MS | sample dilution, protein precipitation, and ultrafiltration | ropivacaine | ropivacaine-D7 | 0.1–10 µg/mL | Breindahl et al. [44] |
| human serum | Mightysil-RP-18 GP II column (150 × 2 mm, particle size 5 μm) | LC-MS/MS-ESI | solid-phase extraction | procaine, mepivacaine, lidocaine, ropivacaine, oxybuprocaine, tetracaine, bupivacaine, T-caine and dibucaine | lidocaine | 10–100 ng/mL | Tonooka et al. [45] |
| human serum | reversed-phase column | LC-MS/MS-ESI | equilibrium dialysis | free and total ropivacaine | ropivacaine-D7 | 0.5–3000 ng/mL | Lamy et al. [46] |
| human serum | Optiguard C8 column (1 × 10 mm) | LC-MS/MS-ESI | solid-phase microextraction | ropivacaine, PPx, 3-OH-ropivacaine | ropivacaine-D7 | 2–2000 nM for all analytes | Abdel-Rehim et al. [47] |
| human serum | Gemini NX-18 (3 × 100 mm) | LC-MS/MS-ESI | protein precipitation | ropivacaine, 3-OH-ropivacaine | ropivacaine-D7 | 0.5–1000 ng/mL for ropivacaine, 1–1000 ng/mL for 3-OH-ropivacaine | Butiulca et al. [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butiulca, M.; Farczadi, L.; Vari, C.E.; Imre, S.; Azamfirei, L.; Lazar, A. The Study of Ropivacaine Pharmacokinetics in a Clinical Setting: A Critical Scoping Review from the Perspective of Analytical Methodologies. Int. J. Mol. Sci. 2024, 25, 13487. https://doi.org/10.3390/ijms252413487
Butiulca M, Farczadi L, Vari CE, Imre S, Azamfirei L, Lazar A. The Study of Ropivacaine Pharmacokinetics in a Clinical Setting: A Critical Scoping Review from the Perspective of Analytical Methodologies. International Journal of Molecular Sciences. 2024; 25(24):13487. https://doi.org/10.3390/ijms252413487
Chicago/Turabian StyleButiulca, Mihaela, Lenard Farczadi, Camil Eugen Vari, Silvia Imre, Leonard Azamfirei, and Alexandra Lazar. 2024. "The Study of Ropivacaine Pharmacokinetics in a Clinical Setting: A Critical Scoping Review from the Perspective of Analytical Methodologies" International Journal of Molecular Sciences 25, no. 24: 13487. https://doi.org/10.3390/ijms252413487
APA StyleButiulca, M., Farczadi, L., Vari, C. E., Imre, S., Azamfirei, L., & Lazar, A. (2024). The Study of Ropivacaine Pharmacokinetics in a Clinical Setting: A Critical Scoping Review from the Perspective of Analytical Methodologies. International Journal of Molecular Sciences, 25(24), 13487. https://doi.org/10.3390/ijms252413487






