Preliminary Study to Investigate Possible Cyto-Genotoxic and Oxidative Effects of Few-Layer Graphene in Human Bronchial Cells
Abstract
:1. Introduction
2. Results
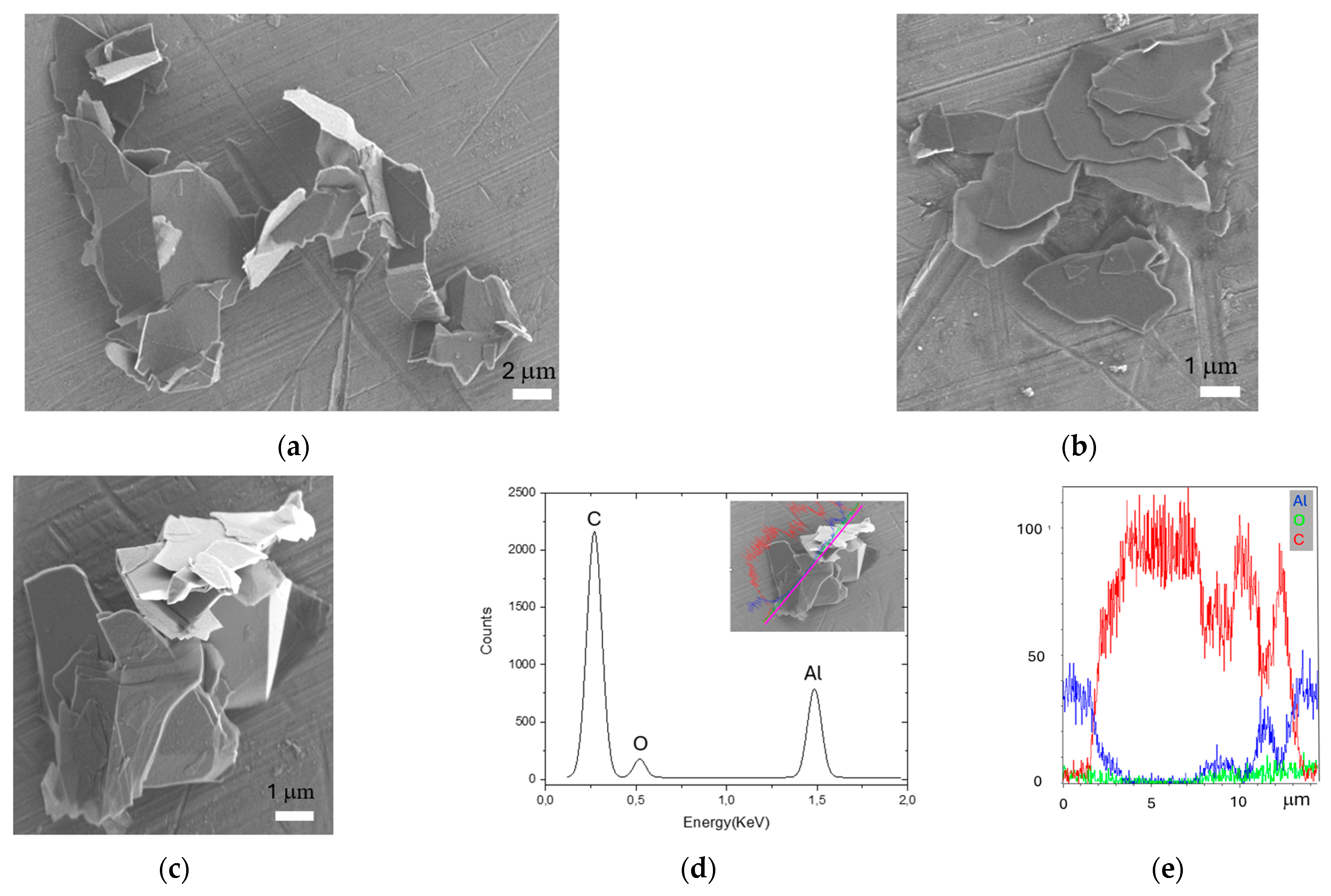
2.1. FLG Characterization
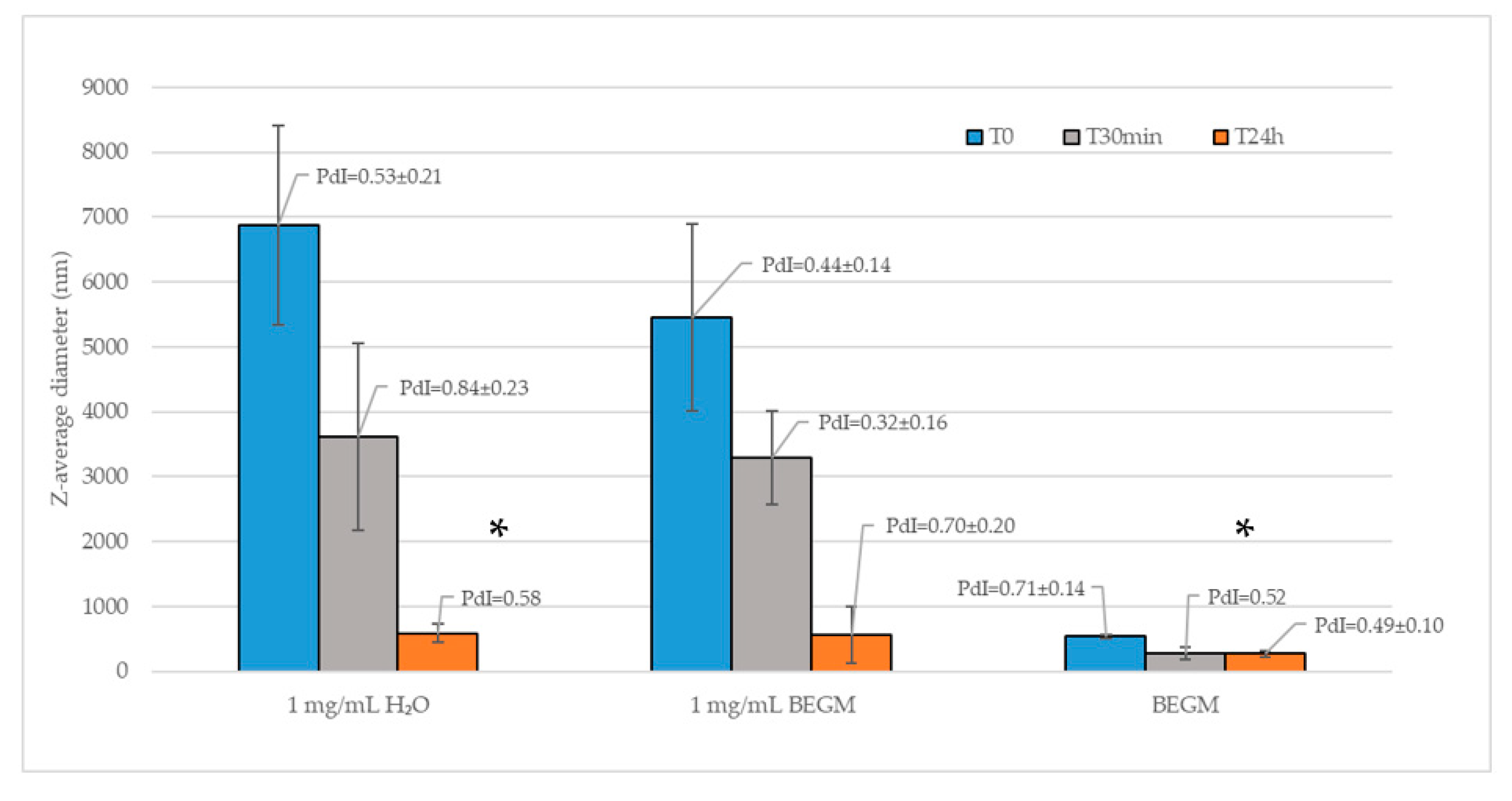
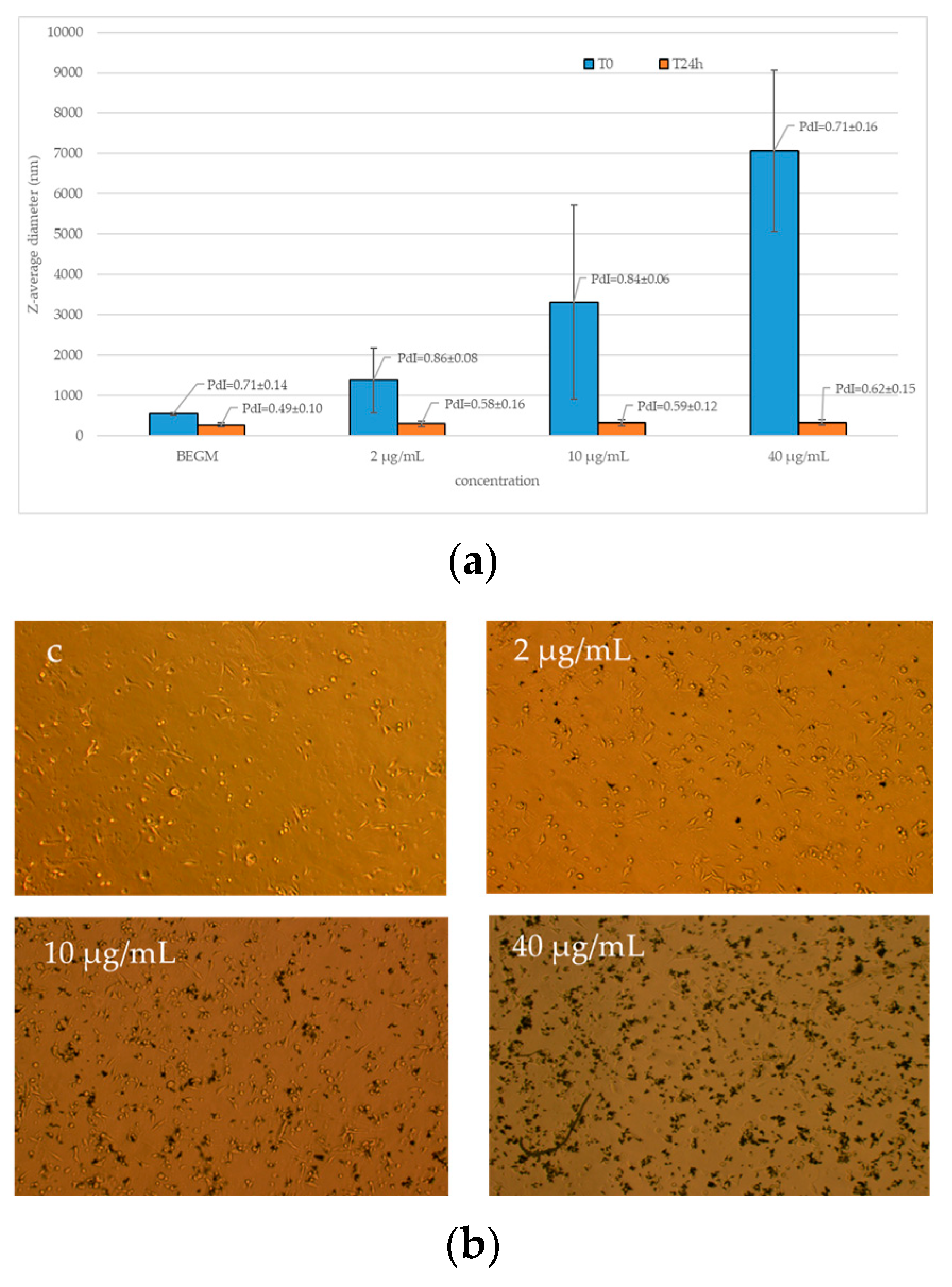
2.2. FLG Dispersion
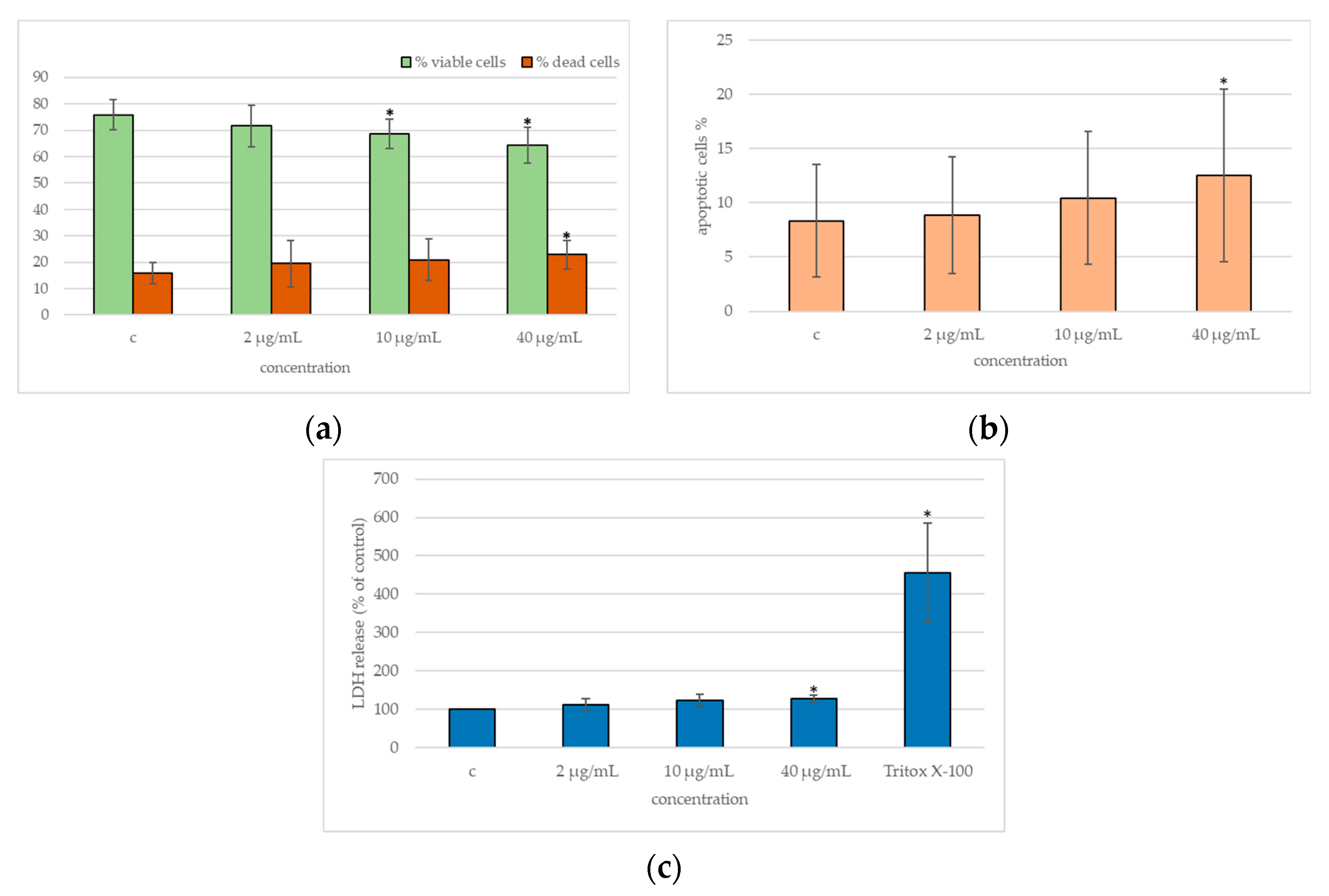
2.3. Cytotoxicity
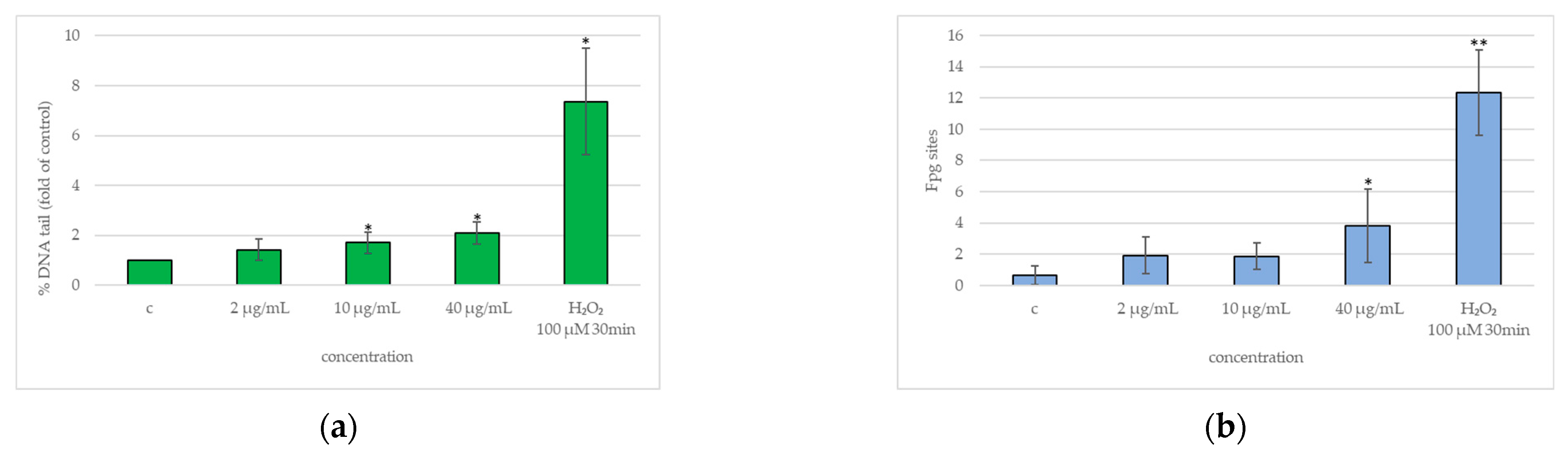
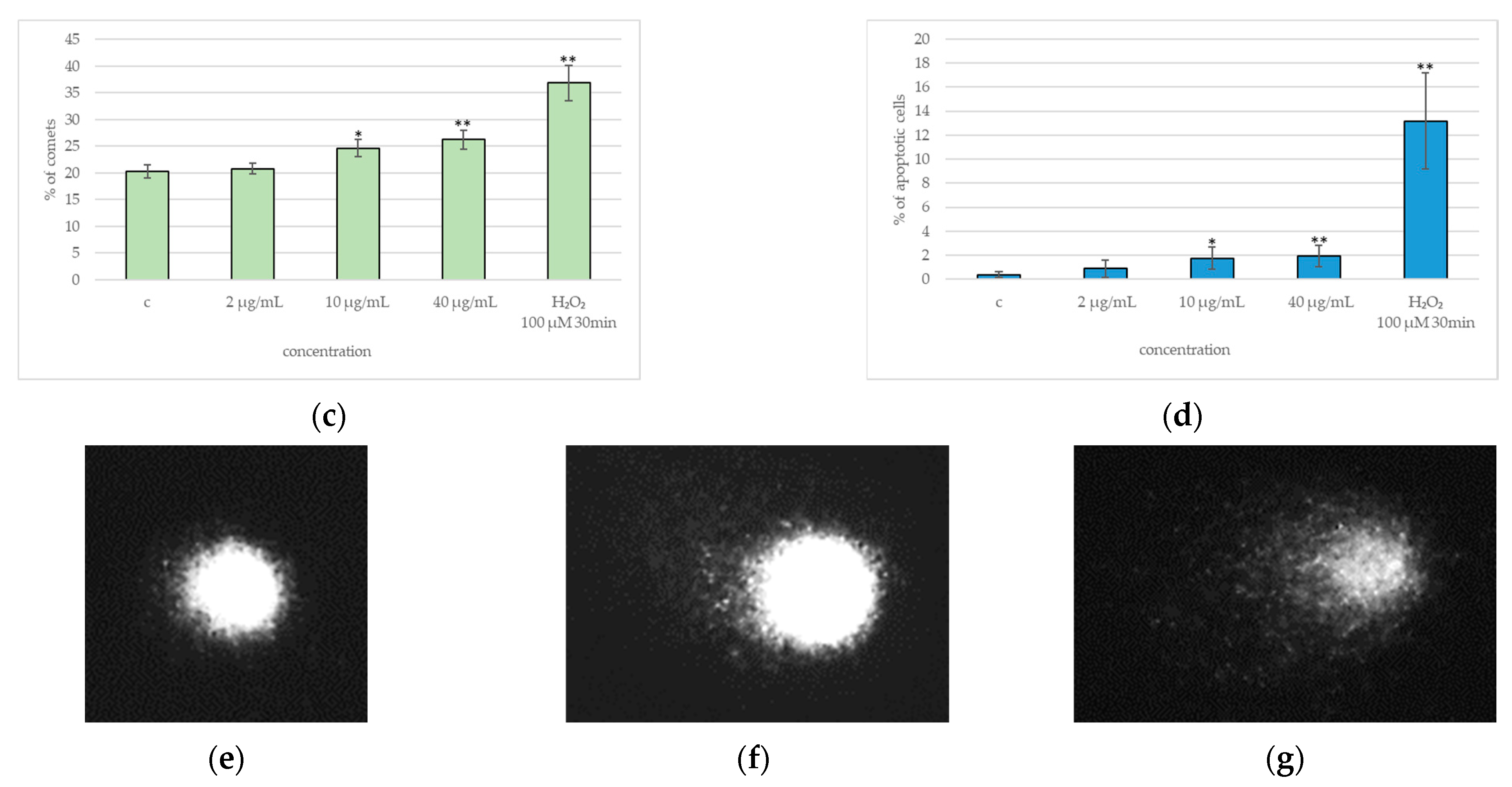
2.4. DNA Damage
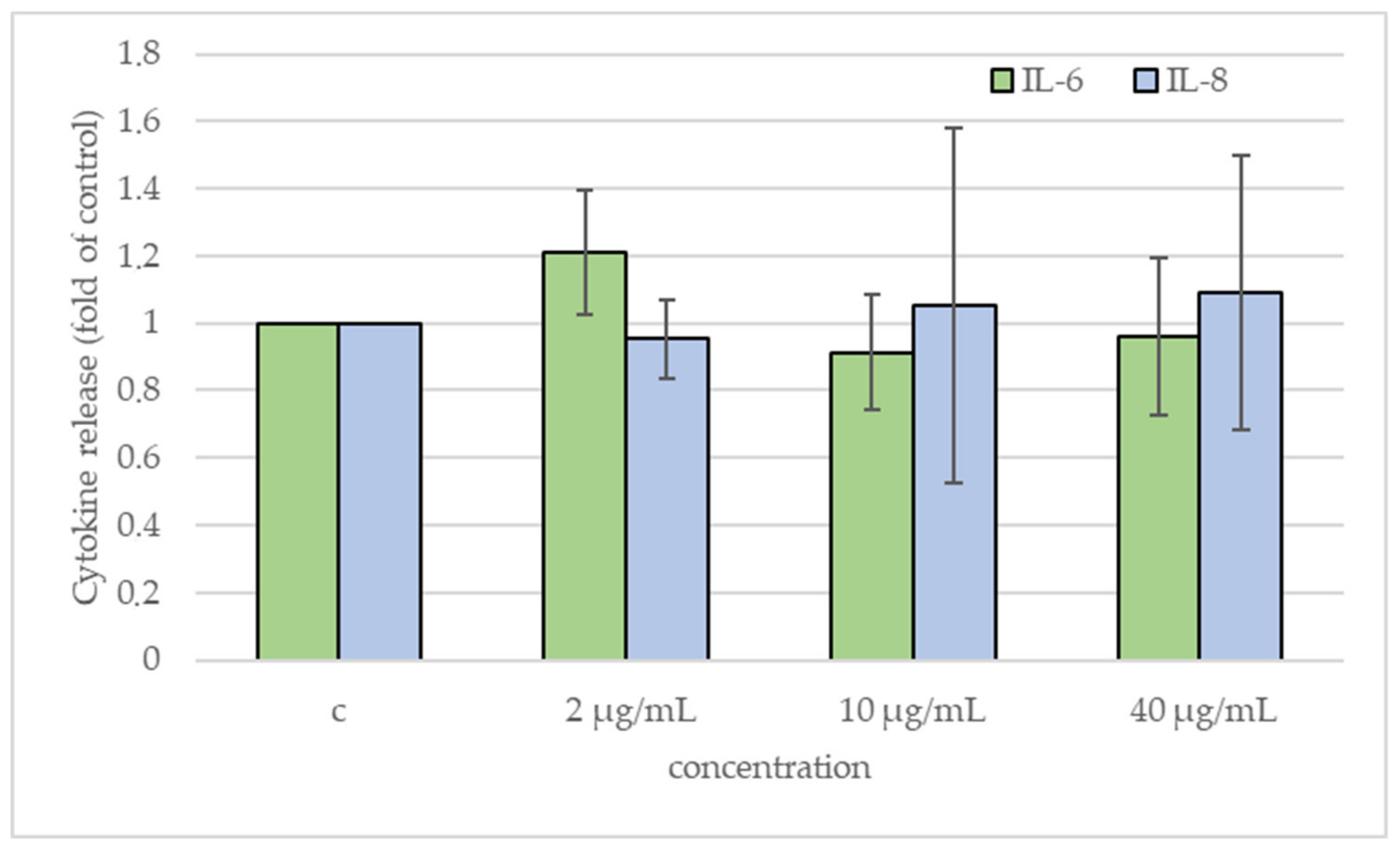
2.5. Cytokines Release
3. Discussion
4. Materials and Methods
4.1. Characterization
4.2. Cell Culture
4.3. Exposure
4.4. Viability and Apoptosis
4.5. Membrane Damage
4.6. Genotoxic/Oxidative DNA Damage
4.7. Cytokines
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradley, D. Is graphene safe? Mater. Today 2012, 15, 230. [Google Scholar] [CrossRef]
- Ristic, B.; Harhaji-Trajkovic, L.; Bosnjak, M.; Dakic, I.; Mijatovic, S.; Trajkovic, V. Modulation of Cancer Cell Autophagic Responses by Graphene-Based Nanomaterials: Molecular Mechanisms and Therapeutic Implications. Cancers 2021, 13, 4145. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Garraus, A.; Passerino, C.; Vales, G.; Carlin, M.; Suhonen, S.; Tubaro, A.; Gómez, J.; Pelin, M.; Catalán, J. Impact of physico-chemical properties on the toxicological potential of reduced graphene oxide in human bronchial epithelial cells. Nanotoxicology 2023, 17, 471–495. [Google Scholar] [CrossRef] [PubMed]
- Molés, G.; Connolly, M.; Valdehita, A.; Pulido-Reyes, G.; Fernandez-Cruz, M.L.; Flahaut, E.; Navas, J.M. Testing the Aquatic Toxicity of 2D Few-Layer Graphene Inks Using Rainbow Trout (Oncorhynchus mykiss): In Vivo and In Vitro Approaches to Support an SSbD Assessment. Toxics 2024, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, L.; Zhao, J.; Zhang, Y.; Xue, X. Graphene oxide had adverse effects on sperm motility and morphology through oxidative stress. Toxicol. Vitr. 2023, 92, 105653. [Google Scholar] [CrossRef]
- Cebadero-Domínguez, Ó.; Diez-Quijada, L.; López, S.; Sánchez-Ballester, S.; Puerto, M.; Cameán, A.M.; Jos, A. Impact of Gastrointestinal Digestion In Vitro Procedure on the Characterization and Cytotoxicity of Reduced Graphene Oxide. Nanomaterials 2023, 13, 2285. [Google Scholar] [CrossRef]
- Frontiñan-Rubio, J.; García-Carpintero, S.; González, V.J.; Vázquez, E.; Durán-Prado, M. Assessment of genotoxicity induced by subchronic exposure to graphene in HaCaT human skin cell line. Nanotoxicology 2023, 17, 42–61. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, H.Y.; Wang, J.Q.; Wu, G.D.; Wang, L. Graphene Oxide and Reduced Graphene Oxide Exhibit Cardiotoxicity Through the Regulation of Lipid Peroxidation, Oxidative Stress, and Mitochondrial Dysfunction. Front. Cell Dev. Biol. 2021, 9, 616888. [Google Scholar] [CrossRef]
- Ou, L.; Lv, X.; Wu, Z.; Xia, W.; Huang, Y.; Chen, L.; Sun, W.; Qi, Y.; Yang, M.; Qi, L. Oxygen content-related DNA damage of graphene oxide on human retinal pigment epithelium cells. J. Mater. Sci. Mater. Med. 2021, 32, 20. [Google Scholar] [CrossRef]
- May, S.; Hirsch, C.; Rippl, A.; Burkle, A.; Wick, P. Assessing Genotoxicity of Ten Different Engineered Nanomaterials by the Novel Semi-Automated FADU Assay and the Alkaline Comet Assay. Nanomaterials 2022, 12, 220. [Google Scholar] [CrossRef]
- Xiaoli, F.; Yaqing, Z.; Ruhui, L.; Xuan, L.; Aijie, C.; Yanli, Z.; Chen, H.; Lili, C.; Longquan, S. Graphene oxide disrupted mitochondrial homeostasis through inducing intracellular redox deviation and autophagy-lysosomal network dysfunction in SH-SY5Y cells. J. Hazard. Mater. 2021, 416, 126158. [Google Scholar] [CrossRef] [PubMed]
- Creutzenberg, O.; Oliveira, H.; Farcal, L.; Schaudien, D.; Mendes, A.; Menezes, A.C.; Tischler, T.; Burla, S.; Ziemann, C. PLATOX: Integrated In Vitro/In Vivo Approach for Screening of Adverse Lung Effects of Graphene-Related 2D Nanomaterials. Nanomaterials 2022, 12, 1254. [Google Scholar] [CrossRef] [PubMed]
- Malkova, A.; Svadlakova, T.; Singh, A.; Kolackova, M.; Vankova, R.; Borsky, P.; Holmannova, D.; Karas, A.; Borska, L.; Fiala, Z. In Vitro Assessment of the Genotoxic Potential of Pristine Graphene Platelets. Nanomaterials 2021, 11, 2210. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.; Figueira, E.; Silva, M.S.S.; Sá, C.; Marques, P. Effects of graphene oxide nanosheets in the polychaete Hediste diversicolor: Behavioural, physiological and biochemical responses. Environ. Pollut. 2022, 299, 118869. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yang, Y.; Zhao, L.; Chen, J.; Duan, G.; Yang, Z.; Zhou, R. Graphene oxide toxicity in W(1118) flies. Sci. Total Environ. 2022, 805, 150302. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Duo, L. Biochemical toxicity, lysosomal membrane stability and DNA damage induced by graphene oxide in earthworms. Environ. Pollut. 2021, 269, 116225. [Google Scholar] [CrossRef]
- Demir, E. Mechanisms and biological impacts of graphene and multi-walled carbon nanotubes on Drosophila melanogaster: Oxidative stress, genotoxic damage, phenotypic variations, locomotor behavior, parasitoid resistance, and cellular immune response. J. Appl. Toxicol. 2022, 42, 450–474. [Google Scholar] [CrossRef]
- Burgum, M.J.; Clift, M.J.D.; Evans, S.J.; Hondow, N.; Miller, M.; Lopez, S.B.; Williams, A.; Tarat, A.; Jenkins, G.J.; Doak, S.H. In Vitro Primary-Indirect Genotoxicity in Bronchial Epithelial Cells Promoted by Industrially Relevant Few-Layer Graphene. Small 2021, 17, e2002551. [Google Scholar] [CrossRef]
- Dias, D.; Vale, A.C.; Cunha, E.P.F.; C Paiva, M.; Reis, R.L.; Vaquette, C.; Alves, N.M. 3D-printed cryomilled poly(epsilon-caprolactone)/graphene composite scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 961–972. [Google Scholar] [CrossRef]
- Ajaykumar, A.P.; Nikhila, K.; Sabira, O.; Jayaraj, K.N.; Varma, S.R.; Rasheed, V.A.; Binitha, V.S.; Sreeja, K.; Ramakrishnan, R.M.; Babu, A. A bio-inspired approach for the synthesis of few-layer graphene using beetle defensive gland extract. RSC Adv. 2024, 14, 5729–5739. [Google Scholar] [CrossRef]
- Sallustrau, A.; Keck, M.; Barbe, P.; Georgin, D.; Fresneau, N.; Campidelli, S.; Pibaleau, B.; Pinault, M.; Mayne-L’Hermite, M.; Granotier-Beckers, C.; et al. One-year post-exposure assessment of 14C-few-layer graphene biodistribution in mice: Single versus repeated intratracheal administration. Nanoscale 2023, 15, 17621–17632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, Z.; Zhao, J.; Li, Q.; Zhang, W.; Lu, C. Exfoliation/dispersion of low-temperature expandable graphite in nanocellulose matrix by wet co-milling. Carbohydr. Polym. 2017, 157, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Frontinan-Rubio, J.; Gonzalez, V.J.; Vazquez, E.; Duran-Prado, M. Rapid and efficient testing of the toxicity of graphene-related materials in primary human lung cells. Sci. Rep. 2022, 12, 7664. [Google Scholar] [CrossRef] [PubMed]
- Burgum, M.J.; Clift, M.J.D.; Evans, S.J.; Hondow, N.; Tarat, A.; Jenkins, G.J.; Doak, S.H. Few-layer graphene induces both primary and secondary genotoxicity in epithelial barrier models in vitro. J. Nanobiotechnol. 2021, 19, 24. [Google Scholar] [CrossRef]
- Pelin, M.; Lin, H.; Gazzi, A.; Sosa, S.; Ponti, C.; Ortega, A.; Zurutuza, A.; Vazquez, E.; Prato, M.; Tubaro, A.; et al. Partial Reversibility of the Cytotoxic Effect Induced by Graphene-Based Materials in Skin Keratinocytes. Nanomaterials 2020, 10, 1602. [Google Scholar] [CrossRef]
- Fusco, L.; Garrido, M.; Martin, C.; Sosa, S.; Ponti, C.; Centeno, A.; Alonso, B.; Zurutuza, A.; Vazquez, E.; Tubaro, A.; et al. Skin irritation potential of graphene-based materials using a non-animal test. Nanoscale 2020, 12, 610–622. [Google Scholar] [CrossRef]
- Malanagahalli, S.; Murera, D.; Martin, C.; Lin, H.; Wadier, N.; Dumortier, H.; Vazquez, E.; Bianco, A. Few Layer Graphene Does Not Affect Cellular Homeostasis of Mouse Macrophages. Nanomaterials 2020, 10, 228. [Google Scholar] [CrossRef]
- Lin, H.; Ji, D.K.; Lucherelli, M.A.; Reina, G.; Ippolito, S.; Samori, P.; Bianco, A. Comparative Effects of Graphene and Molybdenum Disulfide on Human Macrophage Toxicity. Small 2020, 16, e2002194. [Google Scholar] [CrossRef]
- Ursini, C.L.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Del Frate, V.; Folesani, G.; Galetti, M.; Poli, D.; Buresti, G.; Di Cristo, L.; et al. Occupational exposure to graphene and silica nanoparticles. Part II: Pilot study to identify a panel of sensitive biomarkers of genotoxic, oxidative and inflammatory effects on suitable biological matrices. Nanotoxicology 2021, 15, 223–237. [Google Scholar] [CrossRef]
- Cavallo, D.; Ursini, C.L.; Fresegna, A.M.; Ciervo, A.; Boccuni, F.; Ferrante, R.; Tombolini, F.; Maiello, R.; Chiarella, P.; Buresti, G.; et al. A follow-up study on workers involved in the graphene production process after the introduction of exposure mitigation measures: Evaluation of genotoxic and oxidative effects. Nanotoxicology 2022, 16, 776–790. [Google Scholar] [CrossRef]
- Bellagamba, I.; Boccuni, F.; Ferrante, R.; Tombolini, F.; Marra, F.; Sarto, M.S.; Iavicoli, S. Workers’ Exposure Assessment during the Production of Graphene Nanoplatelets in R&D Laboratory. Nanomaterials 2020, 10, 1520. [Google Scholar] [CrossRef] [PubMed]
- Boccuni, F.; Ferrante, R.; Tombolini, F.; Natale, C.; Gordiani, A.; Sabella, S.; Iavicoli, S. Occupational exposure to graphene and silica nanoparticles. Part I: Workplace measurements and samplings. Nanotoxicology 2020, 14, 1280–1300. [Google Scholar] [CrossRef] [PubMed]
- Tombolini, F.; Boccuni, F.; Ferrante, R.; Natale, C.; Marasco, L.; Mantero, E.; Del Rio Castillo, A.E.; Leoncino, L.; Pellegrini, V.; Sabella, S.; et al. An integrated and multi-technique approach to characterize airborne graphene flakes in the workplace during production phases. Nanoscale 2021, 13, 3841–3852. [Google Scholar] [CrossRef] [PubMed]
- Ursini, C.L.; Cavallo, D.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Buresti, G.; Casciardi, S.; Bellucci, S.; Iavicoli, S. Differences in cytotoxic, genotoxic, and inflammatory response of bronchial and alveolar human lung epithelial cells to pristine and COOH-functionalized multiwalled carbon nanotubes. Biomed Res. Int. 2014, 2014, 359506. [Google Scholar] [CrossRef]
- Ursini, C.L.; Cavallo, D.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Tassone, P.; Buresti, G.; Casciardi, S.; Iavicoli, S. Evaluation of cytotoxic, genotoxic and inflammatory response in human alveolar and bronchial epithelial cells exposed to titanium dioxide nanoparticles. J. Appl. Toxicol. 2014, 34, 1209–1219. [Google Scholar] [CrossRef]
- Ursini, C.L.; Maiello, R.; Ciervo, A.; Fresegna, A.M.; Buresti, G.; Superti, F.; Marchetti, M.; Iavicoli, S.; Cavallo, D. Evaluation of uptake, cytotoxicity and inflammatory effects in respiratory cells exposed to pristine and -OH and -COOH functionalized multi-wall carbon nanotubes. J. Appl. Toxicol. 2016, 36, 394–403. [Google Scholar] [CrossRef]
- Fresegna, A.M.; Ursini, C.L.; Ciervo, A.; Maiello, R.; Casciardi, S.; Iavicoli, S.; Cavallo, D. Assessment of the Influence of Crystalline Form on Cyto-Genotoxic and Inflammatory Effects Induced by TiO2 Nanoparticles on Human Bronchial and Alveolar Cells. Nanomaterials 2021, 11, 253. [Google Scholar] [CrossRef]
- Cavallo, D.; Ciervo, A.; Fresegna, A.M.; Maiello, R.; Tassone, P.; Buresti, G.; Casciardi, S.; Iavicoli, S.; Ursini, C.L. Investigation on cobalt-oxide nanoparticles cyto-genotoxicity and inflammatory response in two types of respiratory cells. J. Appl. Toxicol. 2015, 35, 1102–1113. [Google Scholar] [CrossRef]
- Domenech, J.; Rodriguez-Garraus, A.; Lopez de Cerain, A.; Azqueta, A.; Catalan, J. Genotoxicity of Graphene-Based Materials. Nanomaterials 2022, 12, 1795. [Google Scholar] [CrossRef]
- Kaur, I.; Ellis, L.J.; Romer, I.; Tantra, R.; Carriere, M.; Allard, S.; Mayne-L’Hermite, M.; Minelli, C.; Unger, W.; Potthoff, A.; et al. Dispersion of Nanomaterials in Aqueous Media: Towards Protocol Optimization. J. Vis. Exp. 2017, 130, 56074. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, H.; von dem Bussche, A.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gao, H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Natl. Acad. Sci. USA 2013, 110, 12295–12300. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Chen, J.; Li, P.; Wu, Y.; Zhang, G.; Sang, B.; Li, R.; Shi, Y.; Cui, X.; Zhou, T. Respiratory Toxicology of Graphene-Based Nanomaterials: A Review. Toxics 2024, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Sosa, S.; Prato, M.; Tubaro, A. Occupational exposure to graphene based nanomaterials: Risk assessment. Nanoscale 2018, 10, 15894–15903. [Google Scholar] [CrossRef] [PubMed]
- Del Rio Castillo, A.E.; Pellegrini, V.; Ansaldo, A.; Ricciardella, F.; Sun, H.; Marasco, L.; Buha, J.; Dang, Z.; Gagliani, L.; Lago, E.; et al. High-yield production of 2D crystals by wet-jet milling. Mater. Horiz. 2018, 5, 890–904. [Google Scholar] [CrossRef]
- Cavallo, D.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Chiarella, P.; Buresti, G.; Del Frate, V.; Di Basilio, M.; Iavicoli, S.; Ursini, C.L. Evaluation of Systemic Genotoxic/Oxidative and Proinflammatory Effects in Workers of a Titanium Dioxide Production Plant. Biomed Res. Int. 2023, 13, 7066090. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fresegna, A.M.; Ciervo, A.; Ursini, C.L.; Maiello, R.; Tombolini, F.; Del Frate, V.; Gentile, M.; Cavallo, D. Preliminary Study to Investigate Possible Cyto-Genotoxic and Oxidative Effects of Few-Layer Graphene in Human Bronchial Cells. Int. J. Mol. Sci. 2024, 25, 13515. https://doi.org/10.3390/ijms252413515
Fresegna AM, Ciervo A, Ursini CL, Maiello R, Tombolini F, Del Frate V, Gentile M, Cavallo D. Preliminary Study to Investigate Possible Cyto-Genotoxic and Oxidative Effects of Few-Layer Graphene in Human Bronchial Cells. International Journal of Molecular Sciences. 2024; 25(24):13515. https://doi.org/10.3390/ijms252413515
Chicago/Turabian StyleFresegna, Anna Maria, Aureliano Ciervo, Cinzia Lucia Ursini, Raffaele Maiello, Francesca Tombolini, Valentina Del Frate, Marco Gentile, and Delia Cavallo. 2024. "Preliminary Study to Investigate Possible Cyto-Genotoxic and Oxidative Effects of Few-Layer Graphene in Human Bronchial Cells" International Journal of Molecular Sciences 25, no. 24: 13515. https://doi.org/10.3390/ijms252413515
APA StyleFresegna, A. M., Ciervo, A., Ursini, C. L., Maiello, R., Tombolini, F., Del Frate, V., Gentile, M., & Cavallo, D. (2024). Preliminary Study to Investigate Possible Cyto-Genotoxic and Oxidative Effects of Few-Layer Graphene in Human Bronchial Cells. International Journal of Molecular Sciences, 25(24), 13515. https://doi.org/10.3390/ijms252413515




