The Multifaced Role of Collagen in Cancer Development and Progression
Abstract
1. Introduction
2. Collagen: Role and Structure
3. Biosynthesis
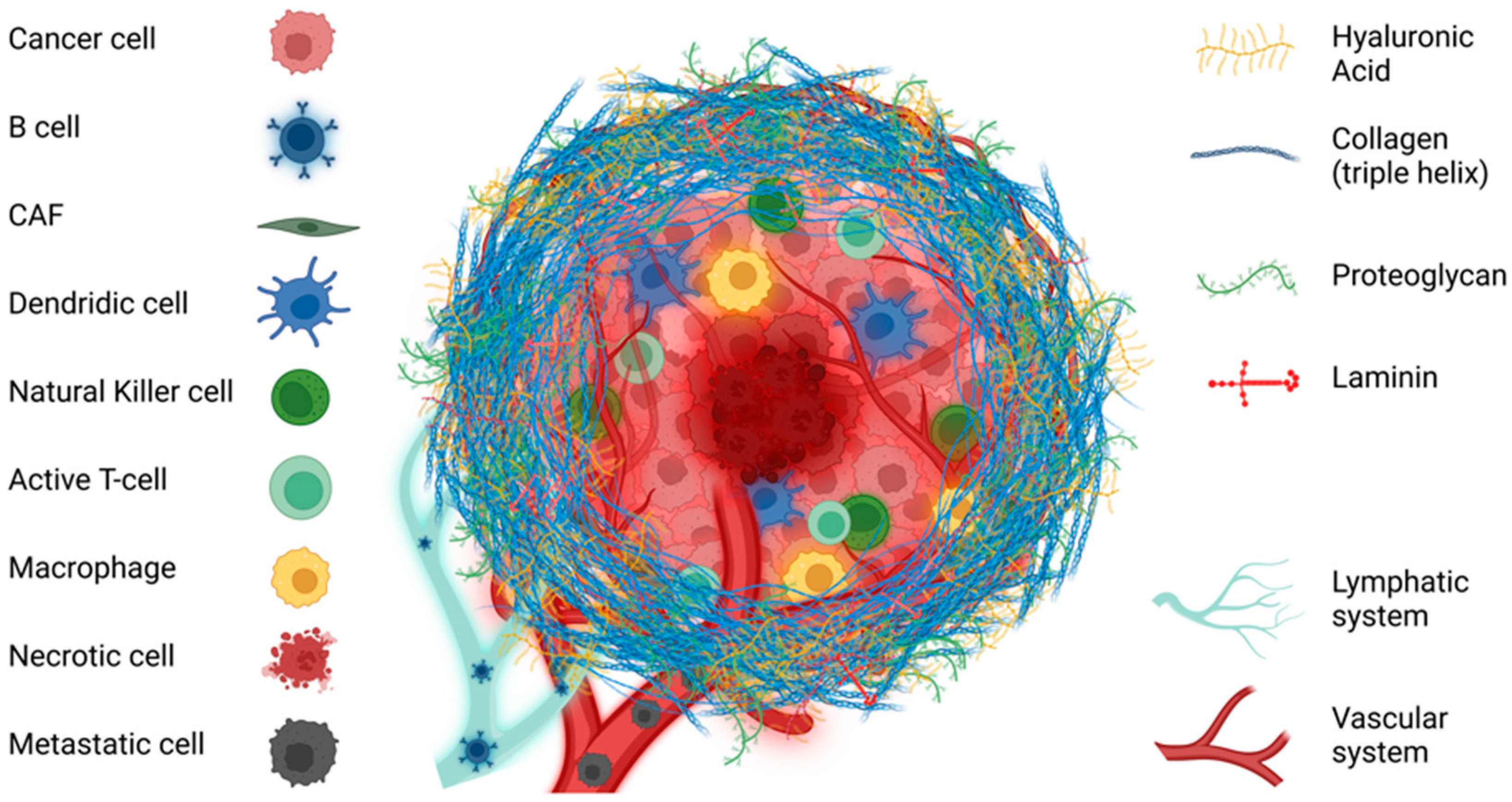
4. Tumoral Extracellular Matrix
5. Collagens in Solid Tumors
5.1. Collagen and Cancer Cell Proliferation
5.2. Collagen and Tumor Metabolism
5.3. Collagen and Apoptosis
5.4. Collagen and Tumor Cell Invasion
5.5. Collagen and Metastasis
5.6. Collagen and Tumor Angiogenesis
5.7. Collagen and Chemoresistance
5.8. Collagen and Immunomodulation
6. Mechanotransduction Mediated by Collagen in Cancer
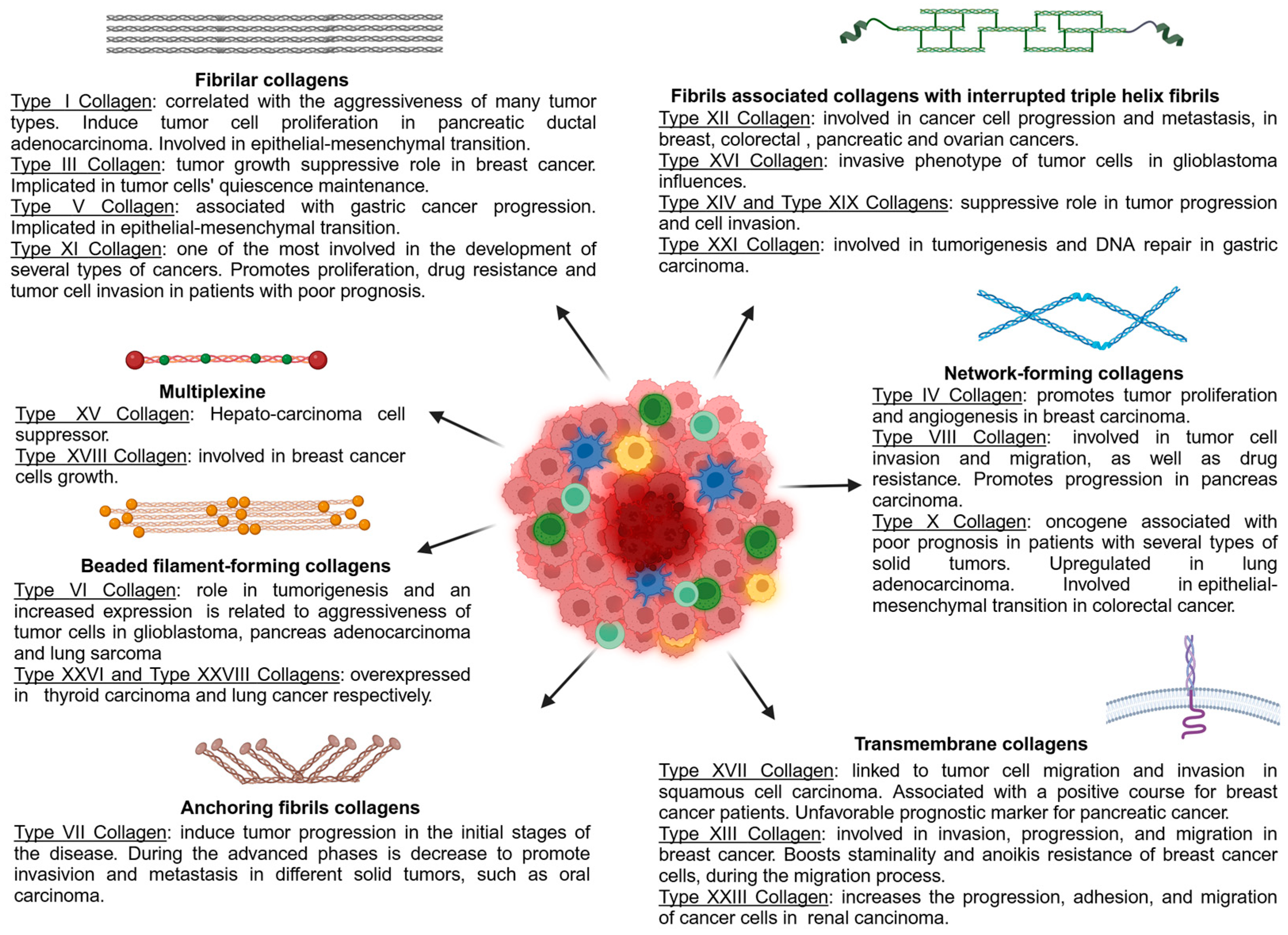
7. Collagen Classification
7.1. Fibrillar Collagens
7.2. Fibrils-Associated Collagens with Interrupted Triple Helices (FACITs)
7.3. Network-Forming Collagens
7.4. Beaded Filament-Forming Collagens
7.5. Anchoring Fibrils Collagens
7.6. Transmembrane Collagens
7.7. Multiplexine
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Holwerda, A.M.; van Loon, L.J.C. The Impact of Collagen Protein Ingestion on Musculoskeletal Connective Tissue Remodeling: A Narrative Review. Nutr. Rev. 2022, 80, 1497–1514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sioud, M. Tumor-Associated Macrophage Subsets: Shaping Polarization and Targeting. Int. J. Mol. Sci. 2023, 24, 7493. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular Matrix Remodeling in Tumor Progression and Immune Escape: From Mechanisms to Treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.; Topping, R.S.; Decker, S.J. Src Family Tyrosine Kinases Regulate Adhesion-Dependent Tyrosine Phosphorylation of 5′-Inositol Phosphatase SHIP2 during Cell Attachment and Spreading on Collagen I. J. Cell Sci. 2002, 115, 3807–3815. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular Matrix Structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Sun, B. The Mechanics of Fibrillar Collagen Extracellular Matrix. Cell Rep. Phys. Sci. 2021, 2, 100515. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Piersma, B.; Hayward, M.-K.; Weaver, V.M. Fibrosis and Cancer: A Strained Relationship. Biochim. Biophys. Acta—Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned Collagen Is a Prognostic Signature for Survival in Human Breast Carcinoma. Am. J. Pathol. 2011, 178, 1221–1232. [Google Scholar] [CrossRef]
- Sarwar, M.; Sykes, P.H.; Chitcholtan, K.; Evans, J.J. Collagen I Dysregulation Is Pivotal for Ovarian Cancer Progression. Tissue Cell 2022, 74, 101704. [Google Scholar] [CrossRef]
- Nelson, M.S.; Liu, Y.; Wilson, H.M.; Li, B.; Rosado-Mendez, I.M.; Rogers, J.D.; Block, W.F.; Eliceiri, K.W. Multiscale Label-Free Imaging of Fibrillar Collagen in the Tumor Microenvironment; Springer: Berlin/Heidelberg, Germany, 2023; pp. 187–235. [Google Scholar]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The Role of Collagen in Cancer: From Bench to Bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Pang, M.; Hou, X.; Yuan, S.; Sun, L. PLOD2 in Cancer Research. Biomed. Pharmacother. 2017, 90, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Poonja, S.; Forero Pinto, A.; Lloyd, M.C.; Damaghi, M.; Rejniak, K.A. Dynamics of Fibril Collagen Remodeling by Tumor Cells: A Model of Tumor-Associated Collagen Signatures. Cells 2023, 12, 2688. [Google Scholar] [CrossRef] [PubMed]
- Necula, L.; Matei, L.; Dragu, D.; Pitica, I.; Neagu, A.; Bleotu, C.; Diaconu, C.C.; Chivu-Economescu, M. Collagen Family as Promising Biomarkers and Therapeutic Targets in Cancer. Int. J. Mol. Sci. 2022, 23, 12415. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70. [Google Scholar] [CrossRef]
- Zhang, M.; Serna-Salas, S.; Damba, T.; Borghesan, M.; Demaria, M.; Moshage, H. Hepatic Stellate Cell Senescence in Liver Fibrosis: Characteristics, Mechanisms and Perspectives. Mech. Ageing Dev. 2021, 199, 111572. [Google Scholar] [CrossRef]
- Goldmann, T.; Zissel, G.; Watz, H.; Drömann, D.; Reck, M.; Kugler, C.; Rabe, K.F.; Marwitz, S. Human Alveolar Epithelial Cells Type II Are Capable of TGFβ-Dependent Epithelial-Mesenchymal-Transition and Collagen-Synthesis. Respir. Res. 2018, 19, 138. [Google Scholar] [CrossRef]
- van den Akker, J.; Tuna, B.G.; Pistea, A.; Sleutel, A.J.J.; Bakker, E.N.T.P.; van Bavel, E. Vascular Smooth Muscle Cells Remodel Collagen Matrices by Long-Distance Action and Anisotropic Interaction. Med. Biol. Eng. Comput. 2012, 50, 701–715. [Google Scholar] [CrossRef]
- Zunich, S.M.; Valdovinos, M.; Douglas, T.; Walterhouse, D.; Iannaccone, P.; Lamm, M.L.G. Osteoblast-Secreted Collagen Upregulates Paracrine Sonic Hedgehog Signaling by Prostate Cancer Cells and Enhances Osteoblast Differentiation. Mol. Cancer 2012, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- RHEE, S.; GRINNELL, F. Fibroblast Mechanics in 3D Collagen Matrices. Adv. Drug Deliv. Rev. 2007, 59, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Sobierajska, K.; Ciszewski, W.M.; Sacewicz-Hofman, I.; Niewiarowska, J. Endothelial Cells in the Tumor Microenvironment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 71–86. [Google Scholar]
- Varma, S.; Orgel, J.P.R.O.; Schieber, J.D. Nanomechanics of Type I Collagen. Biophys. J. 2016, 111, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef]
- Silver, F.H.; Kelkar, N.; Deshmukh, T. Molecular Basis for Mechanical Properties of ECMs: Proposed Role of Fibrillar Collagen and Proteoglycans in Tissue Biomechanics. Biomolecules 2021, 11, 1018. [Google Scholar] [CrossRef]
- Costa, S.; Ragusa, M.A.; Lo Buglio, G.; Scilabra, S.D.; Nicosia, A. The Repertoire of Tissue Inhibitors of Metalloproteases: Evolution, Regulation of Extracellular Matrix Proteolysis, Engineering and Therapeutic Challenges. Life 2022, 12, 1145. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Siadat, S.M.; Ruberti, J.W. Mechanochemistry of Collagen. Acta Biomater. 2023, 163, 50–62. [Google Scholar] [CrossRef]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a Double-Edged Sword in Tumor Progression. Tumor Biol. 2014, 35, 2871–2882. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for Bone Tissue Regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Cram, D.J. The Design of Molecular Hosts, Guests, and Their Complexes. Science 1988, 240, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Sricholpech, M. Lysine Post-Translational Modifications of Collagen. Essays Biochem. 2012, 52, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Vallet, S.D.; Ricard-Blum, S. Lysyl Oxidases: From Enzyme Activity to Extracellular Matrix Cross-Links. Essays Biochem. 2019, 63, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Zhang, Q.; An, Z.-Y.; Jiang, W.; Jin, W.-L.; He, X.-Y. Collagen Code in Tumor Microenvironment: Functions, Molecular Mechanisms, and Therapeutic Implications. Biomed. Pharmacother. 2023, 166, 115390. [Google Scholar] [CrossRef]
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the Microenvironment in Solid Tumors. Cancer Treat. Rev. 2018, 65, 22–32. [Google Scholar] [CrossRef]
- Xing, F. Cancer Associated Fibroblasts (CAFs) in Tumor Microenvironment. Front. Biosci. 2010, 15, 166. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and Therapeutic Relevance of Cancer-Associated Fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Hu, D.; Li, Z.; Zheng, B.; Lin, X.; Pan, Y.; Gong, P.; Zhuo, W.; Hu, Y.; Chen, C.; Chen, L.; et al. Cancer-associated Fibroblasts in Breast Cancer: Challenges and Opportunities. Cancer Commun. 2022, 42, 401–434. [Google Scholar] [CrossRef]
- Tian, H.; Shi, H.; Yu, J.; Ge, S.; Ruan, J. Biophysics Role and Biomimetic Culture Systems of ECM Stiffness in Cancer EMT. Glob. Chall. 2022, 6, 2100094. [Google Scholar] [CrossRef]
- Paszek, M.J.; Weaver, V.M. The Tension Mounts: Mechanics Meets Morphogenesis and Malignancy. J. Mammary Gland Biol. Neoplasia 2004, 9, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting Extracellular Matrix Stiffness and Mechanotransducers to Improve Cancer Therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Yan, L.; Zou, C.; Wang, K.; Chen, M.; Xu, B.; Zhou, Z.; Zhang, D. Integrins Regulate Stemness in Solid Tumor: An Emerging Therapeutic Target. J. Hematol. Oncol. 2021, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, S.; Guo, J.; Zhou, L.; You, L.; Zhang, T.; Zhao, Y. Insights into the Distinct Roles of MMP-11 in Tumor Biology and Future Therapeutics (Review). Int. J. Oncol. 2016, 48, 1783–1793. [Google Scholar] [CrossRef]
- Wall, S.J.; Werner, E.; Werb, Z.; DeClerck, Y.A. Discoidin Domain Receptor 2 Mediates Tumor Cell Cycle Arrest Induced by Fibrillar Collagen. J. Biol. Chem. 2005, 280, 40187–40194. [Google Scholar] [CrossRef]
- Di Martino, J.S.; Nobre, A.R.; Mondal, C.; Taha, I.; Farias, E.F.; Fertig, E.J.; Naba, A.; Aguirre-Ghiso, J.A.; Bravo-Cordero, J.J. A Tumor-Derived Type III Collagen-Rich ECM Niche Regulates Tumor Cell Dormancy. Nat. Cancer 2021, 3, 90–107. [Google Scholar] [CrossRef]
- Koohestani, F.; Braundmeier, A.G.; Mahdian, A.; Seo, J.; Bi, J.; Nowak, R.A. Extracellular Matrix Collagen Alters Cell Proliferation and Cell Cycle Progression of Human Uterine Leiomyoma Smooth Muscle Cells. PLoS ONE 2013, 8, e75844. [Google Scholar] [CrossRef]
- Deka, J.; Wiedemann, N.; Anderle, P.; Murphy-Seiler, F.; Bultinck, J.; Eyckerman, S.; Stehle, J.-C.; André, S.; Vilain, N.; Zilian, O.; et al. Bcl9/Bcl9l Are Critical for Wnt-Mediated Regulation of Stem Cell Traits in Colon Epithelium and Adenocarcinomas. Cancer Res. 2010, 70, 6619–6628. [Google Scholar] [CrossRef]
- Hou, S.; Isaji, T.; Hang, Q.; Im, S.; Fukuda, T.; Gu, J. Distinct Effects of Β1 Integrin on Cell Proliferation and Cellular Signaling in MDA-MB-231 Breast Cancer Cells. Sci. Rep. 2016, 6, 18430. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Mah, E.J.; Lefebvre, A.E.Y.T.; McGahey, G.E.; Yee, A.F.; Digman, M.A. Collagen Density Modulates Triple-Negative Breast Cancer Cell Metabolism through Adhesion-Mediated Contractility. Sci. Rep. 2018, 8, 17094. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Giannitti, G.; Marchesi, S.; Limonta, P. The PI3K/Akt Pathway and Glucose Metabolism: A Dangerous Liaison in Cancer. Int. J. Biol. Sci. 2024, 20, 3113–3125. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hou, Y.; Yuan, J.; Tang, S.; Zhang, H.; Zhu, Q.; Du, Y.; Zhou, M.; Wen, S.; Xu, L.; et al. Twist Promotes Reprogramming of Glucose Metabolism in Breast Cancer Cells through PI3K/AKT and P53 Signaling Pathways. Oncotarget 2015, 6, 25755–25769. [Google Scholar] [CrossRef] [PubMed]
- Mahout, M.; Schwartz, L.; Attal, R.; Bakkar, A.; Peres, S. Metabolic Modelling Links Warburg Effect to Collagen Formation, Angiogenesis and Inflammation in the Tumoral Stroma. PLoS ONE 2024, 19, e0313962. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhou, R.; Cai, J.; Yang, N.; Wen, Z.; Zhang, Z.; Sun, H.; Huang, G.; Guan, Y.; Huang, N.; et al. Matrix Stiffness Triggers Lipid Metabolic Cross-Talk between Tumor and Stromal Cells to Mediate Bevacizumab Resistance in Colorectal Cancer Liver Metastases. Cancer Res. 2023, 83, 3577–3592. [Google Scholar] [CrossRef]
- Cai, J.; Chen, T.; Jiang, Z.; Yan, J.; Ye, Z.; Ruan, Y.; Tao, L.; Shen, Z.; Liang, X.; Wang, Y.; et al. Bulk and Single-Cell Transcriptome Profiling Reveal Extracellular Matrix Mechanical Regulation of Lipid Metabolism Reprograming through YAP/TEAD4/ACADL Axis in Hepatocellular Carcinoma. Int. J. Biol. Sci. 2023, 19, 2114–2131. [Google Scholar] [CrossRef]
- Morana, O.; Wood, W.; Gregory, C.D. The Apoptosis Paradox in Cancer. Int. J. Mol. Sci. 2022, 23, 1328. [Google Scholar] [CrossRef]
- Lv, Y.; Shan, Y.; Song, L.; Zhao, Y.; Lai, R.; Su, J.; Zhang, X. Type I Collagen Promotes Tumor Progression of Integrin Β1 Positive Gastric Cancer through a BCL9L/β-Catenin Signaling Pathway. Aging 2021, 13, 19064–19076. [Google Scholar] [CrossRef]
- Saby, C.; Rammal, H.; Magnien, K.; Buache, E.; Brassart-Pasco, S.; Van-Gulick, L.; Jeannesson, P.; Maquoi, E.; Morjani, H. Age-Related Modifications of Type I Collagen Impair DDR1-Induced Apoptosis in Non-Invasive Breast Carcinoma Cells. Cell Adh. Migr. 2018, 12, 335–347. [Google Scholar] [CrossRef]
- Maquoi, E.; Assent, D.; Detilleux, J.; Pequeux, C.; Foidart, J.-M.; Noël, A. MT1-MMP Protects Breast Carcinoma Cells against Type I Collagen-Induced Apoptosis. Oncogene 2012, 31, 480–493. [Google Scholar] [CrossRef]
- Tu, H.; Li, J.; Lin, L.; Wang, L. COL11A1 Was Involved in Cell Proliferation, Apoptosis and Migration in Non-Small Cell Lung Cancer Cells. J. Investig. Surg. 2021, 34, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, P.; Xie, L.; Sugimoto, H.; Colorado, P.; Sund, M.; Holthaus, K.; Sudhakar, A.; Salo, T.; Kalluri, R. Characterization of the Anti-Angiogenic Properties of Arresten, an A1β1 Integrin-Dependent Collagen-Derived Tumor Suppressor. Exp. Cell Res. 2008, 314, 3292–3305. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, J.; Chen, B.; Wang, K.; Tang, Y.; Liang, X. Plasticity of Cancer Cell Invasion: Patterns and Mechanisms. Transl. Oncol. 2021, 14, 100899. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhang, A.; Shi, F.; Chang, F.; Mei, J.; Liu, Y.; Zhu, Y. Integrin Avβ3–Associated DAAM1 Is Essential for Collagen-Induced Invadopodia Extension and Cell Haptotaxis in Breast Cancer Cells. J. Biol. Chem. 2018, 293, 10172–10185. [Google Scholar] [CrossRef]
- Artym, V.V.; Swatkoski, S.; Matsumoto, K.; Campbell, C.B.; Petrie, R.J.; Dimitriadis, E.K.; Li, X.; Mueller, S.C.; Bugge, T.H.; Gucek, M.; et al. Dense Fibrillar Collagen Is a Potent Inducer of Invadopodia via a Specific Signaling Network. J. Cell Biol. 2015, 208, 331–350. [Google Scholar] [CrossRef]
- Han, B.; Guan, X.; Ma, M.; Liang, B.; Ren, L.; Liu, Y.; Du, Y.; Jiang, S.-H.; Song, D. Stiffened Tumor Microenvironment Enhances Perineural Invasion in Breast Cancer via Integrin Signaling. Cell. Oncol. 2024, 47, 867–882. [Google Scholar] [CrossRef]
- Lee, C.S.; Siprashvili, Z.; Mah, A.; Bencomo, T.; Elcavage, L.E.; Che, Y.; Shenoy, R.M.; Aasi, S.Z.; Khavari, P.A. Mutant Collagen COL11A1 Enhances Cancerous Invasion. Oncogene 2021, 40, 6299–6307. [Google Scholar] [CrossRef]
- Koorman, T.; Jansen, K.A.; Khalil, A.; Haughton, P.D.; Visser, D.; Rätze, M.A.K.; Haakma, W.E.; Sakalauskaitè, G.; van Diest, P.J.; de Rooij, J.; et al. Spatial Collagen Stiffening Promotes Collective Breast Cancer Cell Invasion by Reinforcing Extracellular Matrix Alignment. Oncogene 2022, 41, 2458–2469. [Google Scholar] [CrossRef]
- Budden, T.; Gaudy-Marqueste, C.; Porter, A.; Kay, E.; Gurung, S.; Earnshaw, C.H.; Roeck, K.; Craig, S.; Traves, V.; Krutmann, J.; et al. Ultraviolet Light-Induced Collagen Degradation Inhibits Melanoma Invasion. Nat. Commun. 2021, 12, 2742. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Lamar, J.M.; Carr, S.A.; Hynes, R.O. Extracellular Matrix Signatures of Human Mammary Carcinoma Identify Novel Metastasis Promoters. eLife 2014, 3, e01308. [Google Scholar] [CrossRef]
- Sahai, E.; Wyckoff, J.; Philippar, U.; Segall, J.E.; Gertler, F.; Condeelis, J. Simultaneous Imaging of GFP, CFP and Collagen in Tumors in Vivousing Multiphoton Microscopy. BMC Biotechnol. 2005, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, Z.; Liu, W.; Fu, M.; Jiang, W.; Xu, S.; Wang, G.; Chen, F.; Lu, J.; Chen, H.; et al. Predicting Postoperative Peritoneal Metastasis in Gastric Cancer with Serosal Invasion Using a Collagen Nomogram. Nat. Commun. 2021, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Kang, Y.; Cheng, C.-C.; Li, X.; Dai, B.; Katz, M.H.; Men, T.; Kim, M.P.; Koay, E.A.; Huang, H.; et al. DDR1-Induced Neutrophil Extracellular Traps Drive Pancreatic Cancer Metastasis. JCI Insight 2021, 6, e146133. [Google Scholar] [CrossRef] [PubMed]
- Akinjiyan, F.A.; Ibitoye, Z.; Zhao, P.; Shriver, L.P.; Patti, G.J.; Longmore, G.D.; Fuh, K.C. DDR2-Regulated Arginase Activity in Ovarian Cancer-Associated Fibroblasts Promotes Collagen Production and Tumor Progression. Oncogene 2024, 43, 189–201. [Google Scholar] [CrossRef]
- Song, M.; Schnettler, E.; Venkatachalam, A.; Wang, Y.; Feldman, L.; Argenta, P.; Rodriguez-Rodriguez, L.; Ramakrishnan, S. Increased Expression of Collagen Prolyl Hydroxylases in Ovarian Cancer Is Associated with Cancer Growth and Metastasis. Am. Cancer Res. 2023, 13, 6051. [Google Scholar]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The Role of Hypoxia in Cancer Progression, Angiogenesis, Metastasis, and Resistance to Therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Lin, K.; Zhao, Y.; Tang, Y.; Chen, Y.; Lin, M.; He, L. Collagen I-Induced VCAN/ERK Signaling and PARP1/ZEB1-Mediated Metastasis Facilitate OSBPL2 Defect to Promote Colorectal Cancer Progression. Cell Death Dis. 2024, 15, 85. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Yang, X.; Lu, W.; Chen, Y.; Lin, Y.; Wang, J.; Lin, S.; Yun, J.-P. H3K27 Acetylation Activated-COL6A1 Promotes Osteosarcoma Lung Metastasis by Repressing STAT1 and Activating Pulmonary Cancer-Associated Fibroblasts. Theranostics 2021, 11, 1473–1492. [Google Scholar] [CrossRef]
- Edgar, L.T.; Underwood, C.J.; Guilkey, J.E.; Hoying, J.B.; Weiss, J.A. Extracellular Matrix Density Regulates the Rate of Neovessel Growth and Branching in Sprouting Angiogenesis. PLoS ONE 2014, 9, e85178. [Google Scholar] [CrossRef]
- Balcioglu, H.E.; van de Water, B.; Danen, E.H.J. Tumor-Induced Remote ECM Network Orientation Steers Angiogenesis. Sci. Rep. 2016, 6, 22580. [Google Scholar] [CrossRef]
- Chen, M.-C.; Hsu, W.-L.; Hwang, P.-A.; Chou, T.-C. Low Molecular Weight Fucoidan Inhibits Tumor Angiogenesis through Downregulation of HIF-1/VEGF Signaling under Hypoxia. Mar. Drugs 2015, 13, 4436–4451. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, A.; Tarnawski, A.S. Critical Role of Hypoxia Sensor-HIF-1α in VEGF Gene Activation. Implications for Angiogenesis and Tissue Injury Healing. Curr. Med. Chem. 2012, 19, 90–97. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Wu, W.; Wang, X.; Fang, L.; Adam, V.; Nepovimova, E.; Wu, Q.; Kuca, K. The Role of Hypoxia-inducible Factor 1 in Tumor Immune Evasion. Med. Res. Rev. 2021, 41, 1622–1643. [Google Scholar] [CrossRef] [PubMed]
- Oudart, J.-B.; Villemin, M.; Brassart, B.; Sellier, C.; Terryn, C.; Dupont-Deshorgue, A.; Monboisse, J.C.; Maquart, F.-X.; Ramont, L.; Brassart-Pasco, S. F4, a Collagen XIX-Derived Peptide, Inhibits Tumor Angiogenesis through Avβ3 and A5β1 Integrin Interaction. Cell Adh. Migr. 2021, 15, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Mundel, T.M.; Kalluri, R. Type IV Collagen-Derived Angiogenesis Inhibitors. Microvasc. Res. 2007, 74, 85–89. [Google Scholar] [CrossRef]
- Ramos, A.; Sadeghi, S.; Tabatabaeian, H. Battling Chemoresistance in Cancer: Root Causes and Strategies to Uproot Them. Int. J. Mol. Sci. 2021, 22, 9451. [Google Scholar] [CrossRef]
- Lin, J.; Zou, B.; Li, H.; Wang, J.; Li, S.; Cao, J.; Xie, D.; Wang, F. Collagen XVII Promotes Dormancy of Colorectal Cancer Cells by Activating MTORC2 Signaling. Cell Signal. 2024, 120, 111234. [Google Scholar] [CrossRef]
- Saatci, O.; Kaymak, A.; Raza, U.; Ersan, P.G.; Akbulut, O.; Banister, C.E.; Sikirzhytski, V.; Tokat, U.M.; Aykut, G.; Ansari, S.A.; et al. Targeting Lysyl Oxidase (LOX) Overcomes Chemotherapy Resistance in Triple Negative Breast Cancer. Nat. Commun. 2020, 11, 2416. [Google Scholar] [CrossRef]
- Sharom, F.J. ABC Multidrug Transporters: Structure, Function and Role in Chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef]
- Baltes, F.; Pfeifer, V.; Silbermann, K.; Caspers, J.; Wantoch von Rekowski, K.; Schlesinger, M.; Bendas, G. B1-Integrin Binding to Collagen Type 1 Transmits Breast Cancer Cells into Chemoresistance by Activating ABC Efflux Transporters. Biochim. Biophys. Acta—Mol. Cell Res. 2020, 1867, 118663. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Huang, Y.-F.; Wu, P.-Y.; Chang, T.-H.; Huang, S.-C.; Chou, C.-Y. The Downregulation of MiR-509-3p Expression by Collagen Type XI Alpha 1-Regulated Hypermethylation Facilitates Cancer Progression and Chemoresistance via the DNA Methyltransferase 1/Small Ubiquitin-like Modifier-3 Axis in Ovarian Cancer Cells. J. Ovarian. Res. 2023, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Collado, J.; Boland, L.; Ahrendsen, J.T.; Miska, J.; Lee-Chang, C. Understanding the Glioblastoma Tumor Microenvironment: Leveraging the Extracellular Matrix to Increase Immunotherapy Efficacy. Front. Immunol. 2024, 15, 1336476. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.H.; Rodriguez, B.L.; Diao, L.; Chen, L.; Wang, J.; Byers, L.A.; Wei, Y.; Chapman, H.A.; Yamauchi, M.; Behrens, C.; et al. Collagen Promotes Anti-PD-1/PD-L1 Resistance in Cancer through LAIR1-Dependent CD8+ T Cell Exhaustion. Nat. Commun. 2020, 11, 4520. [Google Scholar] [CrossRef] [PubMed]
- Rømer, A.M.A.; Thorseth, M.-L.; Madsen, D.H. Immune Modulatory Properties of Collagen in Cancer. Front. Immunol. 2021, 12, 791453. [Google Scholar] [CrossRef]
- Salmon, H.; Franciszkiewicz, K.; Damotte, D.; Dieu-Nosjean, M.-C.; Validire, P.; Trautmann, A.; Mami-Chouaib, F.; Donnadieu, E. Matrix Architecture Defines the Preferential Localization and Migration of T Cells into the Stroma of Human Lung Tumors. J. Clin. Investig. 2012, 122, 899–910. [Google Scholar] [CrossRef]
- Ohno, S.; Tachibana, M.; Fujii, T.; Ueda, S.; Kubota, H.; Nagasue, N. Role of Stromal Collagen in Immunomodulation and Prognosis of Advanced Gastric Carcinoma. Int. J. Cancer 2002, 97, 770–774. [Google Scholar] [CrossRef]
- Kuczek, D.E.; Larsen, A.M.H.; Thorseth, M.-L.; Carretta, M.; Kalvisa, A.; Siersbæk, M.S.; Simões, A.M.C.; Roslind, A.; Engelholm, L.H.; Noessner, E.; et al. Collagen Density Regulates the Activity of Tumor-Infiltrating T Cells. J. Immunother. Cancer 2019, 7, 68. [Google Scholar] [CrossRef]
- Sun, X.; Wu, B.; Chiang, H.-C.; Deng, H.; Zhang, X.; Xiong, W.; Liu, J.; Rozeboom, A.M.; Harris, B.T.; Blommaert, E.; et al. Tumour DDR1 Promotes Collagen Fibre Alignment to Instigate Immune Exclusion. Nature 2021, 599, 673–678. [Google Scholar] [CrossRef]
- Liu, J.; Chiang, H.-C.; Xiong, W.; Laurent, V.; Griffiths, S.C.; Dülfer, J.; Deng, H.; Sun, X.; Yin, Y.W.; Li, W.; et al. A Highly Selective Humanized DDR1 MAb Reverses Immune Exclusion by Disrupting Collagen Fiber Alignment in Breast Cancer. J. Immunother. Cancer 2023, 11, e006720. [Google Scholar] [CrossRef]
- Tripathi, S.; Najem, H.; Dussold, C.; Pacheco, S.; Miska, J.; McCortney, K.; Steffens, A.; Walshon, J.; Winkowski, D.; Cloney, M.; et al. Cancer-Associated Fibroblast–Secreted Collagen Is Associated with Immune Inhibitor Receptor LAIR1 in Gliomas. J. Clin. Investig. 2024, 134, e176613. [Google Scholar] [CrossRef]
- Rodriguez, B.L.; Huang, J.; Gibson, L.; Fradette, J.J.; Chen, H.-I.H.; Koyano, K.; Cortez, C.; Li, B.; Ho, C.; Ashique, A.M.; et al. Antitumor Activity of a Novel LAIR1 Antagonist in Combination with Anti-PD1 to Treat Collagen-Rich Solid Tumors. Mol. Cancer Ther. 2024, 23, 1144–1158. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Gui, X.; Deng, M.; Chen, H.; Chen, Y.; Liu, X.; Ku, Z.; Tan, L.; Huang, R.; He, Y.; et al. Blocking LAIR1 Signaling in Immune Cells Inhibits Tumor Development. Front. Immunol. 2022, 13, 996026. [Google Scholar] [CrossRef] [PubMed]
- Vijver, S.V.; Singh, A.; Mommers-Elshof, E.T.A.M.; Meeldijk, J.; Copeland, R.; Boon, L.; Langermann, S.; Flies, D.; Meyaard, L.; Ramos, M.I.P. Collagen Fragments Produced in Cancer Mediate T Cell Suppression Through Leukocyte-Associated Immunoglobulin-Like Receptor 1. Front. Immunol. 2021, 12, 733561. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Boluda, A.; Vaquero, J.; Vimeux, L.; Guilbert, T.; Barrin, S.; Kantari-Mimoun, C.; Ponzo, M.; Renault, G.; Deptula, P.; Pogoda, K.; et al. Tumor Stiffening Reversion Through Collagen Crosslinking Inhibition Improves T Cell Migration and Anti-PD-1 Treatment. eLife 2021, 10, e58688. [Google Scholar] [CrossRef]
- Trackman, P.C. Lysyl Oxidase Isoforms and Potential Therapeutic Opportunities for Fibrosis and Cancer. Expert Opin. Ther. Targets 2016, 20, 935–945. [Google Scholar] [CrossRef]
- Li, Z.; Mo, F.; Guo, K.; Ren, S.; Wang, Y.; Chen, Y.; Schwartz, P.B.; Richmond, N.; Liu, F.; Ronnekleiv-Kelly, S.M.; et al. Nanodrug-Bacteria Conjugates-Mediated Oncogenic Collagen Depletion Enhances Immune Checkpoint Blockade Therapy against Pancreatic Cancer. Med 2024, 5, 348–367.e7. [Google Scholar] [CrossRef]
- Fukuda, Y.; Kim, S.-H.; Bustos, M.A.; Cho, S.-N.; Roszik, J.; Burks, J.K.; Kim, H.; Hoon, D.S.B.; Grimm, E.A.; Ekmekcioglu, S. Inhibition of Microsomal Prostaglandin E2 Synthase Reduces Collagen Deposition in Melanoma Tumors and May Improve Immunotherapy Efficacy by Reducing T-Cell Exhaustion. Cancer Res. Commun. 2023, 3, 1397–1408. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Yang, X.; Jiao, W.; Shen, C.; Zhao, X.; Wang, Y. High Expression of COL6A1 Predicts Poor Prognosis and Response to Immunotherapy in Bladder Cancer. Cell Cycle 2023, 22, 610–618. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, S.; Tavormina, J.; Tampe, D.; Zeisberg, M.; Wang, H.; Mahadevan, K.K.; Wu, C.-J.; Sugimoto, H.; Chang, C.-C.; et al. Oncogenic Collagen I Homotrimers from Cancer Cells Bind to A3β1 Integrin and Impact Tumor Microbiome and Immunity to Promote Pancreatic Cancer. Cancer Cell 2022, 40, 818–834.e9. [Google Scholar] [CrossRef]
- Saad, E.E.; Michel, R.; Borahay, M.A. Immunosuppressive Tumor Microenvironment and Uterine Fibroids: Role in Collagen Synthesis. Cytokine Growth Factor Rev. 2024, 75, 93–100. [Google Scholar] [CrossRef]
- Saad, E.E.; Michel, R.; Borahay, M.A. Senescence-Associated Secretory Phenotype (SASP) and Uterine Fibroids: Association with PD-L1 Activation and Collagen Deposition. Ageing Res. Rev. 2024, 97, 102314. [Google Scholar] [CrossRef] [PubMed]
- Tohme, S.; Yazdani, H.O.; Al-Khafaji, A.B.; Chidi, A.P.; Loughran, P.; Mowen, K.; Wang, Y.; Simmons, R.L.; Huang, H.; Tsung, A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016, 76, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage Plasticity and Polarization: In Vivo Veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.-R.; Li, J.-H.; Zhang, R.; Chen, R.-X.; Wang, Y.-H. M2-Polarized Tumor-Associated Macrophages Facilitated Migration and Epithelial-Mesenchymal Transition of HCC Cells via the TLR4/STAT3 Signaling Pathway. World. J. Surg. Oncol. 2018, 16, 9. [Google Scholar] [CrossRef]
- Santoni, M.; Massari, F.; Amantini, C.; Nabissi, M.; Maines, F.; Burattini, L.; Berardi, R.; Santoni, G.; Montironi, R.; Tortora, G.; et al. Emerging Role of Tumor-Associated Macrophages as Therapeutic Targets in Patients with Metastatic Renal Cell Carcinoma. Cancer Immunol. Immunother. 2013, 62, 1757–1768. [Google Scholar] [CrossRef]
- Qiu, S.; Deng, L.; Liao, X.; Nie, L.; Qi, F.; Jin, K.; Tu, X.; Zheng, X.; Li, J.; Liu, L.; et al. Tumor-associated Macrophages Promote Bladder Tumor Growth through PI3K/AKT Signal Induced by Collagen. Cancer Sci. 2019, 110, 2110–2118. [Google Scholar] [CrossRef]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular Mechanotransduction in Health and Diseases: From Molecular Mechanism to Therapeutic Targets. Signal. Transduct. Target Ther. 2023, 8, 282. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and Extracellular Matrix Homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Haller, S.J.; Dudley, A.T. Extracellular Mechanotransduction. J. Gen. Physiol. 2022, 154, e202113026. [Google Scholar] [CrossRef]
- Mousavizadeh, R.; Hojabrpour, P.; Eltit, F.; McDonald, P.C.; Dedhar, S.; McCormack, R.G.; Duronio, V.; Jafarnejad, S.M.; Scott, A. Β1 Integrin, ILK and MTOR Regulate Collagen Synthesis in Mechanically Loaded Tendon Cells. Sci. Rep. 2020, 10, 12644. [Google Scholar] [CrossRef] [PubMed]
- Romani, P.; Valcarcel-Jimenez, L.; Frezza, C.; Dupont, S. Crosstalk between Mechanotransduction and Metabolism. Nat. Rev. Mol. Cell. Biol. 2021, 22, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Li, K.; Huang, M.; Liang, C.; Siemann, D.; Wu, L.; Tan, Y.; Tang, X. Biophysics in Tumor Growth and Progression: From Single Mechano-Sensitive Molecules to Mechanomedicine. Oncogene 2023, 42, 3457–3490. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lieu, Z.Z.; Wolfenson, H.; Hameed, F.M.; Bershadsky, A.D.; Sheetz, M.P. Mechanosensing Controlled Directly by Tyrosine Kinases. Nano Lett. 2016, 16, 5951–5961. [Google Scholar] [CrossRef]
- Fattet, L.; Jung, H.-Y.; Matsumoto, M.W.; Aubol, B.E.; Kumar, A.; Adams, J.A.; Chen, A.C.; Sah, R.L.; Engler, A.J.; Pasquale, E.B.; et al. Matrix Rigidity Controls Epithelial-Mesenchymal Plasticity and Tumor Metastasis via a Mechanoresponsive EPHA2/LYN Complex. Dev. Cell 2020, 54, 302–316.e7. [Google Scholar] [CrossRef]
- Khalil, A.A.; Smits, D.; Haughton, P.D.; Koorman, T.; Jansen, K.A.; Verhagen, M.P.; van der Net, M.; van Zwieten, K.; Enserink, L.; Jansen, L.; et al. A YAP-Centered Mechanotransduction Loop Drives Collective Breast Cancer Cell Invasion. Nat. Commun. 2024, 15, 4866. [Google Scholar] [CrossRef]
- Wu, T.; Xiong, S.; Chen, M.; Tam, B.T.; Chen, W.; Dong, K.; Ma, Z.; Wang, Z.; Ouyang, G. Matrix Stiffening Facilitates the Collective Invasion of Breast Cancer through the Periostin-Integrin Mechanotransduction Pathway. Matrix Biol. 2023, 121, 22–40. [Google Scholar] [CrossRef]
- Shiu, J.-Y.; Aires, L.; Lin, Z.; Vogel, V. Nanopillar Force Measurements Reveal Actin-Cap-Mediated YAP Mechanotransduction. Nat. Cell Biol. 2018, 20, 262–271. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.-L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410.e14. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chang, J.K.; Dominguez, A.A.; Lee, H.; Nam, S.; Chang, J.; Varma, S.; Qi, L.S.; West, R.B.; Chaudhuri, O. YAP-Independent Mechanotransduction Drives Breast Cancer Progression. Nat. Commun. 2019, 10, 1848. [Google Scholar] [CrossRef]
- Miyahara, Y.; Takano, S.; Sogawa, K.; Tomizawa, S.; Furukawa, K.; Takayashiki, T.; Kuboki, S.; Ohtsuka, M. Prosaposin, Tumor-secreted Protein, Promotes Pancreatic Cancer Progression by Decreasing Tumor-infiltrating Lymphocytes. Cancer Sci. 2022, 113, 2548–2559. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sun, L.; Zou, W.; Xu, J.; Liu, H.; Wang, W.; Yun, X.; Gu, J. Prosaposin, a Regulator of Estrogen Receptor Alpha, Promotes Breast Cancer Growth. Cancer Sci. 2012, 103, 1820–1825. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, J.; Luo, P.; Gao, H.; Ma, Y.; Chen, Y.-S.; Li, L.; Zou, D.; Zhang, Y.; Jing, Z. Prosaposin Promotes the Proliferation and Tumorigenesis of Glioma through Toll-like Receptor 4 (TLR4)-Mediated NF-ΚB Signaling Pathway. EBioMedicine 2018, 37, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Inman, D.R.; Li, W.-J.; Ponik, S.M.; Keely, P.J. Mechano-Signal Transduction in Mesenchymal Stem Cells Induces Prosaposin Secretion to Drive the Proliferation of Breast Cancer Cells. Cancer Res. 2017, 77, 6179–6189. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.; Wu, Y.; Danaoui, Y.; Ghosh, G. Engineering Microenvironments towards Harnessing Pro-Angiogenic Potential of Mesenchymal Stem Cells. Mater. Sci. Eng. C 2019, 102, 75–84. [Google Scholar] [CrossRef]
- Dong, Y.; Xie, X.; Wang, Z.; Hu, C.; Zheng, Q.; Wang, Y.; Chen, R.; Xue, T.; Chen, J.; Gao, D.; et al. Increasing Matrix Stiffness Upregulates Vascular Endothelial Growth Factor Expression in Hepatocellular Carcinoma Cells Mediated by Integrin Β1. Biochem. Biophys. Res. Commun. 2014, 444, 427–432. [Google Scholar] [CrossRef]
- Thorlacius-Ussing, J.; Jensen, C.; Nissen, N.I.; Cox, T.R.; Kalluri, R.; Karsdal, M.; Willumsen, N. The Collagen Landscape in Cancer: Profiling Collagens in Tumors and in Circulation Reveals Novel Markers of Cancer-associated Fibroblast Subtypes. J. Pathol. 2024, 262, 22–36. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- van der Rest, M.; Dublet, B.; Champliaud, M.F. Fibril-Associated Collagens. Biomaterials 1990, 11, 28–31. [Google Scholar]
- Song, K.; Yu, Z.; Zu, X.; Li, G.; Hu, Z.; Xue, Y. Collagen Remodeling along Cancer Progression Providing a Novel Opportunity for Cancer Diagnosis and Treatment. Int. J. Mol. Sci. 2022, 23, 10509. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Zhang, H.; Wang, J.; Hua, H.; Jiang, Y. The Role of Network-forming Collagens in Cancer Progression. Int. J. Cancer 2022, 151, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.J.; Noble, F.; Ward, M.; Bullock, M.; Drifka, C.; Mellone, M.; Manousopoulou, A.; Johnston, H.E.; Hayden, A.; Thirdborough, S.; et al. A Subset of Myofibroblastic Cancer-Associated Fibroblasts Regulate Collagen Fiber Elongation, Which Is Prognostic in Multiple Cancers. Oncotarget 2016, 7, 6159–6174. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Diop-Frimpong, B.; Munn, L.L.; Jain, R.K. Diffusion Anisotropy in Collagen Gels and Tumors: The Effect of Fiber Network Orientation. Biophys. J. 2010, 99, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Brisson, B.K.; Mauldin, E.A.; Lei, W.; Vogel, L.K.; Power, A.M.; Lo, A.; Dopkin, D.; Khanna, C.; Wells, R.G.; Puré, E.; et al. Type III Collagen Directs Stromal Organization and Limits Metastasis in a Murine Model of Breast Cancer. Am. J. Pathol. 2015, 185, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhou, T.; Guo, L.; Si, J. Collagen Type I Regulates β-Catenin Tyrosine Phosphorylation and Nuclear Translocation to Promote Migration and Proliferation of Gastric Carcinoma Cells. Oncol. Rep. 2010, 23, 1247–1255. [Google Scholar] [CrossRef][Green Version]
- Mendonsa, A.M.; Na, T.-Y.; Gumbiner, B.M. E-Cadherin in Contact Inhibition and Cancer. Oncogene 2018, 37, 4769–4780. [Google Scholar] [CrossRef]
- Su, H.; Karin, M. Multifaceted Collagen-DDR1 Signaling in Cancer. Trends Cell Biol. 2024, 34, 406–415. [Google Scholar] [CrossRef]
- Kothiwale, S.; Borza, C.M.; Lowe, E.W.; Pozzi, A.; Meiler, J. Discoidin Domain Receptor 1 (DDR1) Kinase as Target for Structure-Based Drug Discovery. Drug Discov. Today 2015, 20, 255–261. [Google Scholar] [CrossRef]
- Su, H.; Yang, F.; Fu, R.; Trinh, B.; Sun, N.; Liu, J.; Kumar, A.; Baglieri, J.; Siruno, J.; Le, M.; et al. Collagenolysis-Dependent DDR1 Signalling Dictates Pancreatic Cancer Outcome. Nature 2022, 610, 366–372. [Google Scholar] [CrossRef]
- Chen, T.; You, Y.; Jiang, H.; Wang, Z.Z. Epithelial–Mesenchymal Transition (EMT): A Biological Process in the Development, Stem Cell Differentiation, and Tumorigenesis. J. Cell Physiol. 2017, 232, 3261–3272. [Google Scholar] [CrossRef]
- Medici, D.; Nawshad, A. Type I Collagen Promotes Epithelial–Mesenchymal Transition through ILK-Dependent Activation of NF-ΚB and LEF-1. Matrix Biol. 2010, 29, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-S.; Dunleavey, J.M.; Szot, C.; Yang, L.; Hilton, M.B.; Morris, K.; Seaman, S.; Feng, Y.; Lutz, E.M.; Koogle, R.; et al. Cancer Cell Survival Depends on Collagen Uptake into Tumor-Associated Stroma. Nat. Commun. 2022, 13, 7078. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Hamberger, F.; Ravichandra, A.; Miller, M.; Nair, A.; Affo, S.; Filliol, A.; Chin, L.; Savage, T.M.; Yin, D.; et al. Tumor Restriction by Type I Collagen Opposes Tumor-Promoting Effects of Cancer-Associated Fibroblasts. J. Clin. Investig. 2021, 131, e146987. [Google Scholar] [CrossRef] [PubMed]
- Brisson, B.K.; Stewart, D.C.; Burgwin, C.; Chenoweth, D.; Wells, R.G.; Adams, S.L.; Volk, S.W. Cysteine-Rich Domain of Type III Collagen N-Propeptide Inhibits Fibroblast Activation by Attenuating TGFβ Signaling. Matrix Biol. 2022, 109, 19–33. [Google Scholar] [CrossRef]
- Ramirez, M.; Rajaram, S.; Steininger, R.J.; Osipchuk, D.; Roth, M.A.; Morinishi, L.S.; Evans, L.; Ji, W.; Hsu, C.-H.; Thurley, K.; et al. Diverse Drug-Resistance Mechanisms Can Emerge from Drug-Tolerant Cancer Persister Cells. Nat. Commun. 2016, 7, 10690. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Wang, T.; Yang, T.; Yang, X.; Guo, K.; Hu, L.; Ming, J. Recombinant Humanized Collagen Type III with High Antitumor Activity Inhibits Breast Cancer Cells Autophagy, Proliferation, and Migration through DDR1. Int. J. Biol. Macromol. 2023, 243, 125130. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Guo, X.; Bu, X.; Yuan, L.; Ji, L. Collagen Type V Alpha 2 Promotes the Development of Gastric Cancer via M2 Macrophage Polarization. Chin. J. Physiol. 2023, 66, 93. [Google Scholar] [CrossRef]
- Lee, B.Y.; Timpson, P.; Horvath, L.G.; Daly, R.J. FAK Signaling in Human Cancer as a Target for Therapeutics. Pharmacol. Ther. 2015, 146, 132–149. [Google Scholar] [CrossRef]
- Jin, Y.; Song, X.; Sun, X.; Ding, Y. Up-Regulation of Collagen Type V Alpha 2 (COL5A2) Promotes Malignant Phenotypes in Gastric Cancer Cell via Inducing Epithelial–Mesenchymal Transition (EMT). Open Med. 2023, 18, 20220593. [Google Scholar] [CrossRef]
- Arolt, C.; Meyer, M.; Hoffmann, F.; Wagener-Ryczek, S.; Schwarz, D.; Nachtsheim, L.; Beutner, D.; Odenthal, M.; Guntinas-Lichius, O.; Buettner, R.; et al. Expression Profiling of Extracellular Matrix Genes Reveals Global and Entity-Specific Characteristics in Adenoid Cystic, Mucoepidermoid and Salivary Duct Carcinomas. Cancers 2020, 12, 2466. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, R.E.; Seebach, J.; Field, L.A.; Heckman, C.; Kane, J.; Hooke, J.A.; Love, B.; Shriver, C.D. A Gene Expression Signature That Defines Breast Cancer Metastases. Clin. Exp. Metastasis 2009, 26, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Galván, J.A.; García-Martínez, J.; Vázquez-Villa, F.; García-Ocaña, M.; García-Pravia, C.; Menéndez-Rodríguez, P.; González-del Rey, C.; Barneo-Serra, L.; de los Toyos, J.R. Validation of COL11A1/Procollagen 11A1 Expression in TGF-Β1-Activated Immortalised Human Mesenchymal Cells and in Stromal Cells of Human Colon Adenocarcinoma. BMC Cancer 2014, 14, 867. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Singh, A.; Phukan, R.; Purkayastha, J.; Kataki, A.; Mahanta, J.; Saxena, S.; Kapur, S. Genome-Wide Analysis of Chromosomal Alterations in Patients with Esophageal Squamous Cell Carcinoma Exposed to Tobacco and Betel Quid from High-Risk Area in India. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2010, 696, 130–138. [Google Scholar] [CrossRef]
- Hao, S.; Lv, J.; Yang, Q.; Wang, A.; Li, Z.; Guo, Y.; Zhang, G. Identification of Key Genes and Circular RNAs in Human Gastric Cancer. Med. Sci. Monit. 2019, 25, 2488–2504. [Google Scholar] [CrossRef]
- Nizri, E.; Bar-David, S.; Aizic, A.; Sternbach, N.; Lahat, G.; Wolf, I.; Klausner, J. Desmoplasia in Lymph Node Metastasis of Pancreatic Adenocarcinoma Reveals Activation of Cancer-Associated Fibroblasts Pattern and T-Helper 2 Immune Cell Infiltration. Pancreas 2019, 48, 367–373. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Rada, M.; Heiserman, J.P.; Cha, J.; Sage, J.; Zhou, B.; Yang, W.; Hu, Y.; Korgaonkar, C.; Hanos, C.T.; et al. Inhibition of Collagen XI Alpha 1-Induced Fatty Acid Oxidation Triggers Apoptotic Cell Death in Cisplatin-Resistant Ovarian Cancer. Cell Death Dis. 2020, 11, 258. [Google Scholar] [CrossRef]
- Vázquez-Villa, F.; García-Ocaña, M.; Galván, J.A.; García-Martínez, J.; García-Pravia, C.; Menéndez-Rodríguez, P.; Rey, C.G.; Barneo-Serra, L.; de los Toyos, J.R. COL11A1/(pro)Collagen 11A1 Expression Is a Remarkable Biomarker of Human Invasive Carcinoma-Associated Stromal Cells and Carcinoma Progression. Tumor Biol. 2015, 36, 2213–2222. [Google Scholar] [CrossRef]
- Jia, D.; Liu, Z.; Deng, N.; Tan, T.Z.; Huang, R.Y.-J.; Taylor-Harding, B.; Cheon, D.-J.; Lawrenson, K.; Wiedemeyer, W.R.; Walts, A.E.; et al. A COL11A1-Correlated Pan-Cancer Gene Signature of Activated Fibroblasts for the Prioritization of Therapeutic Targets. Cancer Lett. 2016, 382, 203–214. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Chou, C.-Y. Collagen XI Alpha 1 Chain, a Novel Therapeutic Target for Cancer Treatment. Front. Oncol. 2022, 12, 925165. [Google Scholar] [CrossRef]
- Rada, M.; Nallanthighal, S.; Cha, J.; Ryan, K.; Sage, J.; Eldred, C.; Ullo, M.; Orsulic, S.; Cheon, D.-J. Inhibitor of Apoptosis Proteins (IAPs) Mediate Collagen Type XI Alpha 1-Driven Cisplatin Resistance in Ovarian Cancer. Oncogene 2018, 37, 4809–4820. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Chang, T.-H.; Huang, Y.-F.; Chen, C.-C.; Chou, C.-Y. COL11A1 Confers Chemoresistance on Ovarian Cancer Cells through the Activation of Akt/c/EBPβ Pathway and PDK1 Stabilization. Oncotarget 2015, 6, 23748–23763. [Google Scholar] [CrossRef] [PubMed]
- La, T.; Liu, G.Z.; Farrelly, M.; Cole, N.; Feng, Y.C.; Zhang, Y.Y.; Sherwin, S.K.; Yari, H.; Tabatabaee, H.; Yan, X.G.; et al. A P53-Responsive MiRNA Network Promotes Cancer Cell Quiescence. Cancer Res. 2018, 78, 6666–6679. [Google Scholar] [CrossRef] [PubMed]
- Zvackova, I.; Matalova, E.; Lesot, H. Regulators of Collagen Fibrillogenesis during Molar Development in the Mouse. Front. Physiol. 2017, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Vizzini, A.; Pergolizzi, M.; Vazzana, M.; Salerno, G.; Di Sano, C.; Macaluso, P.; Arizza, V.; Parrinello, D.; Cammarata, M.; Parrinello, N. FACIT Collagen (1α-Chain) Is Expressed by Hemocytes and Epidermis during the Inflammatory Response of the Ascidian Ciona Intestinalis. Dev. Comp. Immunol. 2008, 32, 682–692. [Google Scholar] [CrossRef]
- Coulson-Thomas, V.J.; Coulson-Thomas, Y.M.; Gesteira, T.F.; de Paula, C.A.A.; Mader, A.M.; Waisberg, J.; Pinhal, M.A.; Friedl, A.; Toma, L.; Nader, H.B. Colorectal Cancer Desmoplastic Reaction Up-Regulates Collagen Synthesis and Restricts Cancer Cell Invasion. Cell Tissue Res. 2011, 346, 223–236. [Google Scholar] [CrossRef]
- Chivu-Economescu, M.; Necula, L.G.; Matei, L.; Dragu, D.; Bleotu, C.; Sorop, A.; Herlea, V.; Dima, S.; Popescu, I.; Diaconu, C.C. Collagen Family and Other Matrix Remodeling Proteins Identified by Bioinformatics Analysis as Hub Genes Involved in Gastric Cancer Progression and Prognosis. Int. J. Mol. Sci. 2022, 23, 3214. [Google Scholar] [CrossRef]
- Papanicolaou, M.; Parker, A.L.; Yam, M.; Filipe, E.C.; Wu, S.Z.; Chitty, J.L.; Wyllie, K.; Tran, E.; Mok, E.; Nadalini, A.; et al. Temporal Profiling of the Breast Tumour Microenvironment Reveals Collagen XII as a Driver of Metastasis. Nat. Commun. 2022, 13, 4587. [Google Scholar] [CrossRef]
- Yan, Y.; Liang, Q.; Liu, Y.; Zhou, S.; Xu, Z. COL12A1 as a Prognostic Biomarker Links Immunotherapy Response in Breast Cancer. Endocr. Relat. Cancer 2023, 30, e230012. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Y. Integrated Bioinformatics Analysis of Expression and Gene Regulation Network of COL12A1 in Colorectal Cancer. Cancer Med. 2020, 9, 4743–4755. [Google Scholar] [CrossRef]
- Yao, W.; Yao, Y.; He, W.; Zhao, C.; Liu, D.; Wang, G.; Wang, Z. PABPC1 Promotes Cell Proliferation and Metastasis in Pancreatic Adenocarcinoma by Regulating COL12A1 Expression. Immun. Inflamm. Dis. 2023, 11, e919. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Nielsen, S.H.; Mortensen, J.H.; Kjeldsen, J.; Klinge, L.G.; Krag, A.; Harling, H.; Jørgensen, L.N.; Karsdal, M.A.; Willumsen, N. Serum Type XVI Collagen Is Associated with Colorectal Cancer and Ulcerative Colitis Indicating a Pathological Role in Gastrointestinal Disorders. Cancer Med. 2018, 7, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Ratzinger, S.; Grässel, S.; Dowejko, A.; Reichert, T.E.; Bauer, R.J. Induction of Type XVI Collagen Expression Facilitates Proliferation of Oral Cancer Cells. Matrix Biol. 2011, 30, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Ratzinger, S.; Wales, L.; Bosserhoff, A.; Senner, V.; Grifka, J.; Grässel, S. Inhibition of Collagen XVI Expression Reduces Glioma Cell Invasiveness. Cell. Physiol. Biochem. 2011, 27, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Pach, E.; Kümper, M.; Fromme, J.E.; Zamek, J.; Metzen, F.; Koch, M.; Mauch, C.; Zigrino, P. Extracellular Matrix Remodeling by Fibroblast-MMP14 Regulates Melanoma Growth. Int. J. Mol. Sci. 2021, 22, 12276. [Google Scholar] [CrossRef]
- Oudart, J.-B.; Doué, M.; Vautrin, A.; Brassart, B.; Sellier, C.; Dupont-Deshorgue, A.; Monboisse, J.-C.; Maquart, F.-X.; Brassart-Pasco, S.; Ramont, L. The Anti-Tumor NC1 Domain of Collagen XIX Inhibits the FAK/PI3K/Akt/MTOR Signaling Pathway through Avβ3 Integrin Interaction. Oncotarget 2016, 7, 1516–1528. [Google Scholar] [CrossRef]
- Thorlacius-Ussing, J.; Jensen, C.; Madsen, E.A.; Nissen, N.I.; Manon-Jensen, T.; Chen, I.M.; Johansen, J.S.; Diab, H.M.H.; Jørgensen, L.N.; Karsdal, M.A.; et al. Type XX Collagen Is Elevated in Circulation of Patients with Solid Tumors. Int. J. Mol. Sci. 2022, 23, 4144. [Google Scholar] [CrossRef]
- Qin, L.H.; Zhu, X.J.; Zhang, L.Y.; Chen, J.Q.; Jin, G.Y.; Xiang, L.J. Identification of Hub Genes and Pathways in the Development of Gastric Cancer by Gene Co-expression Network Analysis. J. Biol. Regul. Homeost. Agents. 2021, 35, 35–44. [Google Scholar] [CrossRef]
- Knupp, C.; Squire, J.M. Molecular Packing in Network-Forming Collagens; Elsevier: Amsterdam, the Netherlands, 2005; pp. 375–403. [Google Scholar]
- Egeblad, M.; Rasch, M.G.; Weaver, V.M. Dynamic Interplay between the Collagen Scaffold and Tumor Evolution. Curr. Opin. Cell Biol. 2010, 22, 697–706. [Google Scholar] [CrossRef]
- Liang, Y.; Lv, Z.; Huang, G.; Qin, J.; Li, H.; Nong, F.; Wen, B. Prognostic Significance of Abnormal Matrix Collagen Remodeling in Colorectal Cancer Based on Histologic and Bioinformatics Analysis. Oncol. Rep. 2020, 44, 1671–1685. [Google Scholar] [CrossRef]
- Friedl, P.; Alexander, S. Cancer Invasion and the Microenvironment: Plasticity and Reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, P.A.; Bourne, J.W.; Cigler, T.; Vincent, C.T. A New Paradigm for Mechanobiological Mechanisms in Tumor Metastasis. Semin. Cancer Biol. 2012, 22, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV Is Essential for Basement Membrane Stability but Dispensable for Initiation of Its Assembly during Early Development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Bignon, M.; Pichol-Thievend, C.; Hardouin, J.; Malbouyres, M.; Bréchot, N.; Nasciutti, L.; Barret, A.; Teillon, J.; Guillon, E.; Etienne, E.; et al. Lysyl Oxidase-like Protein-2 Regulates Sprouting Angiogenesis and Type IV Collagen Assembly in the Endothelial Basement Membrane. Blood 2011, 118, 3979–3989. [Google Scholar] [CrossRef]
- Večurkovská, I.; Stupák, M.; Kaťuchová, J.; Roškovičová, V.; Mašlanková, J. Comparative Analysis of Matrix Metalloproteinases by Zymography in Patients With Colorectal Carcinoma. Physiol. Res. 2023, 72, S593–S596. [Google Scholar] [CrossRef]
- Kalluri, R. Basement Membranes: Structure, Assembly and Role in Tumour Angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef]
- Chamani, R.; Saberi, O.; Fathinejad, F. An Arresten-Derived Anti-Angiogenic Peptide Triggers Apoptotic Cell Death in Endothelial Cells. Mol. Biol. Rep. 2024, 51, 513. [Google Scholar] [CrossRef]
- Chamani, R.; Taleqani, M.H.; Imanpour, A.; Khatami, M. New Insights into Short Peptides Derived from the Collagen NC1 A1, A2, and A3 (IV) Domains: An Experimental and MD Simulations Study. Biochim. Biophys. Acta—Proteins Proteom. 2022, 1870, 140769. [Google Scholar] [CrossRef]
- Vautrin-Glabik, A.; Devy, J.; Bour, C.; Baud, S.; Choulier, L.; Hoarau, A.; Dupont-Deshorgue, A.; Sellier, C.; Brassart, B.; Oudart, J.-B.; et al. Angiogenesis Inhibition by a Short 13 Amino Acid Peptide Sequence of Tetrastatin, the A4(IV) NC1 Domain of Collagen IV. Front. Cell Dev. Biol. 2020, 8, 775. [Google Scholar] [CrossRef]
- Aikio, M.; Alahuhta, I.; Nurmenniemi, S.; Suojanen, J.; Palovuori, R.; Teppo, S.; Sorsa, T.; López-Otín, C.; Pihlajaniemi, T.; Salo, T.; et al. Arresten, a Collagen-Derived Angiogenesis Inhibitor, Suppresses Invasion of Squamous Cell Carcinoma. PLoS ONE 2012, 7, e51044. [Google Scholar] [CrossRef] [PubMed]
- Brassart-Pasco, S.; Brézillon, S.; Brassart, B.; Ramont, L.; Oudart, J.-B.; Monboisse, J.C. Tumor Microenvironment: Extracellular Matrix Alterations Influence Tumor Progression. Front. Oncol. 2020, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Assadian, S.; El-Assaad, W.; Wang, X.Q.D.; Gannon, P.O.; Barrès, V.; Latour, M.; Mes-Masson, A.-M.; Saad, F.; Sado, Y.; Dostie, J.; et al. P53 Inhibits Angiogenesis by Inducing the Production of Arresten. Cancer Res. 2012, 72, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Kamphaus, G.D.; Colorado, P.C.; Panka, D.J.; Hopfer, H.; Ramchandran, R.; Torre, A.; Maeshima, Y.; Mier, J.W.; Sukhatme, V.P.; Kalluri, R. Canstatin, a Novel Matrix-Derived Inhibitor of Angiogenesis and Tumor Growth. J. Biol. Chem. 2000, 275, 1209–1215. [Google Scholar] [CrossRef]
- Panka, D.J.; Mier, J.W. Canstatin Inhibits Akt Activation and Induces Fas-Dependent Apoptosis in Endothelial Cells. J. Biol. Chem. 2003, 278, 37632–37636. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Li, P.; Gao, Y.H.; Tian, B.G.; Wang, D.Y.; Zheng, Y.; Liu, L.Y.; Zhang, Z.Y.; Huang, S.S.; Wen, M.; et al. Type IV Collagen-Derived Angiogenesis Inhibitor: Canstatin Low Expressing in Brain-Invasive Meningiomas Using Liquid Chromatography–Mass Spectrometry (LC-MS/MS). J. Neurooncol. 2023, 161, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Guo, Z.; Zhang, J.; Yang, Y.; Liu, C.; Zhang, L.; Gu, Z.; Li, Y.; Ding, Z.; Shi, G. Recombinant Human Arresten and Canstatin Inhibit Angiogenic Behaviors of HUVECs via Inhibiting the PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 8995. [Google Scholar] [CrossRef]
- Guo, J.; Ma, X.; Liu, D.; Wang, F.; Xia, J.; Zhang, B.; Zhao, P.; Zhong, F.; Chen, L.; Long, Q.; et al. A Distinct Subset of Urothelial Cells with Enhanced EMT Features Promotes Chemotherapy Resistance and Cancer Recurrence by Increasing COL4A1-ITGB1 Mediated Angiogenesis. Drug Resist. Updates 2024, 76, 101116. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Zhu, Y.; Qiao, Y.; Gao, Y.; Chen, J.; Ge, G. Type IV Collagen α 5 Chain Promotes Luminal Breast Cancer Progression through c-Myc-Driven Glycolysis. J. Mol. Cell Biol. 2023, 14, mjac068. [Google Scholar] [CrossRef]
- Jansson, M.; Lindberg, J.; Rask, G.; Svensson, J.; Billing, O.; Nazemroaya, A.; Berglund, A.; Wärnberg, F.; Sund, M. Prognostic Value of Stromal Type IV Collagen Expression in Small Invasive Breast Cancers. Front. Mol. Biosci. 2022, 9, 904526. [Google Scholar] [CrossRef]
- Xia, Y.; Jiang, H.; Chen, J.; Xu, F.; Zhang, G.; Zhang, D. Low Dose Taxol Ameliorated Renal Fibrosis in Mice with Diabetic Kidney Disease by Downregulation of HIPK2. Life Sci. 2023, 320, 121540. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Sagara, A.; Tajima, K.; Miura, S.; Inaba, K.; Ando, Y.; Oku, T.; Murakami, T.; Kato, Y.; Yumoto, T. COL8A1 Facilitates the Growth of Triple-Negative Breast Cancer via FAK/Src Activation. Breast Cancer Res. Treat. 2022, 194, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Bruckner-Tuderman, L.; Höpfner, B.; Hammami-Hauasli, N. Biology of Anchoring Fibrils: Lessons from Dystrophic Epidermolysis Bullosa. Matrix Biol. 1999, 18, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wei, Y.; Liu, H.; Gong, Y.; Zhou, Y.; Yang, H.; Tang, L. Analysis of Collagen Type X Alpha 1 (COL10A1) Expression and Prognostic Significance in Gastric Cancer Based on Bioinformatics. Bioengineered 2021, 12, 127–137. [Google Scholar] [CrossRef]
- Huang, H.; Li, T.; Ye, G.; Zhao, L.; Zhang, Z.; Mo, D.; Wang, Y.; Zhang, C.; Deng, H.; Li, G.; et al. High Expression of COL10A1 Is Associated with Poor Prognosis in Colorectal Cancer. Onco. Targets Ther. 2018, 11, 1571–1581. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Chen, S.; Li, K.; Wan, S.; Yang, L. COL10A1 as a Prognostic Biomarker in Association with Immune Infiltration in Prostate Cancer. Curr. Cancer Drug Targets 2024, 24, 340–353. [Google Scholar] [CrossRef]
- Xu, S.; Liu, D.; Qin, Z.; Liang, Z.; Xie, H.; Yi, B.; Wang, K.; Lin, G.; Liu, R.; Yang, K.; et al. Experimental Validation and Pan-Cancer Analysis Identified COL10A1 as a Novel Oncogene and Potential Therapeutic Target in Prostate Cancer. Aging 2023, 15, 15134–15160. [Google Scholar] [CrossRef]
- Liang, Y.; Xia, W.; Zhang, T.; Chen, B.; Wang, H.; Song, X.; Zhang, Z.; Xu, L.; Dong, G.; Jiang, F. Upregulated Collagen COL10A1 Remodels the Extracellular Matrix and Promotes Malignant Progression in Lung Adenocarcinoma. Front. Oncol. 2020, 10, 573534. [Google Scholar] [CrossRef]
- Bruns, R.R. Beaded Filaments and Long-Spacing Fibrils: Relation to Type VI Collagen. J. Ultrastruct. Res. 1984, 89, 136–145. [Google Scholar] [CrossRef]
- Gara, S.K.; Grumati, P.; Urciuolo, A.; Bonaldo, P.; Kobbe, B.; Koch, M.; Paulsson, M.; Wagener, R. Three Novel Collagen VI Chains with High Homology to the A3 Chain. J. Biol. Chem. 2008, 283, 10658–10670. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Gu, S.; Zhao, X. A Pan-Cancer Analysis of Collagen VI Family on Prognosis, Tumor Microenvironment, and Its Potential Therapeutic Effect. BMC Bioinform. 2022, 23, 390. [Google Scholar] [CrossRef] [PubMed]
- Wishart, A.L.; Conner, S.J.; Guarin, J.R.; Fatherree, J.P.; Peng, Y.; McGinn, R.A.; Crews, R.; Naber, S.P.; Hunter, M.; Greenberg, A.S.; et al. Decellularized Extracellular Matrix Scaffolds Identify Full-Length Collagen VI as a Driver of Breast Cancer Cell Invasion in Obesity and Metastasis. Sci. Adv. 2020, 6, eabc3175. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Byun, Y.J.; Zheng, C.-M.; Moon, S.; Jo, S.J.; Kang, H.W.; Kim, W.T.; Choi, Y.H.; Moon, S.-K.; Kim, W.-J.; et al. COL6A1 Expression as a Potential Prognostic Biomarker for Risk Stratification of T1 High Grade Bladder Cancer: Unveiling the Aggressive Nature of a Distinct Non-Muscle Invasive Subtype. Investig. Clin. Urol. 2024, 65, 94. [Google Scholar] [CrossRef] [PubMed]
- Cescon, M.; Rampazzo, E.; Bresolin, S.; Da Ros, F.; Manfreda, L.; Cani, A.; Della Puppa, A.; Braghetta, P.; Bonaldo, P.; Persano, L. Collagen VI Sustains Cell Stemness and Chemotherapy Resistance in Glioblastoma. Cell. Mol. Life Sci. 2023, 80, 233. [Google Scholar] [CrossRef]
- Papalazarou, V.; Drew, J.; Juin, A.; Spence, H.J.; Whitelaw, J.; Nixon, C.; Salmeron-Sanchez, M.; Machesky, L.M. Collagen VI Expression Is Negatively Mechanosensitive in Pancreatic Cancer Cells and Supports the Metastatic Niche. J. Cell Sci. 2022, 135, jcs259978. [Google Scholar] [CrossRef]
- Liu, Y.; Murazzi, I.; Fuller, A.M.; Pan, H.; Irizarry-Negron, V.M.; Devine, A.; Katti, R.; Skuli, N.; Ciotti, G.E.; Pak, K.; et al. Sarcoma Cells Secrete Hypoxia-Modified Collagen VI to Weaken the Lung Endothelial Barrier and Promote Metastasis. Cancer Res. 2024, 84, 977–993. [Google Scholar] [CrossRef]
- Luo, Y.; Ye, Y.; Zhang, Y.; Chen, L.; Qu, X.; Yi, N.; Ran, J.; Chen, Y. New Insights into COL26A1 in Thyroid Carcinoma: Prognostic Prediction, Functional Characterization, Immunological Drug Target and CeRNA Network. Transl. Cancer Res. 2023, 12, 3384–3408. [Google Scholar] [CrossRef]
- Reese-Petersen, A.L.; Willumsen, N.; Palau, P.; Nunez, J.; Sun, S.; Jensen, T.M.; Kamall, B.; Karsdal, M.A.; Genovese, F. Evaluation of a Novel Biomarker of Type XXVIII Collagen Formation, PRO-C28, in Samples from Cancer and Heart Failure with Preserved Ejection Fraction Patients. J. Pharm. Biomed. Anal. 2021, 204, 114272. [Google Scholar] [CrossRef]
- Sakai, L.Y.; Keene, D.R.; Morris, N.P.; Burgeson, R.E. Type VII Collagen Is a Major Structural Component of Anchoring Fibrils. J. Cell Biol. 1986, 103, 1577–1586. [Google Scholar] [CrossRef]
- Gelse, K. Collagens—Structure, Function, and Biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Oh, S.E.; Oh, M.Y.; An, J.Y.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Choi, M.-G.; Kim, K.-M. Prognostic Value of Highly Expressed Type VII Collagen (COL7A1) in Patients With Gastric Cancer. Pathol. Oncol. Res. 2021, 27, 1609860. [Google Scholar] [CrossRef] [PubMed]
- You, W.-K.; Bonaldo, P.; Stallcup, W.B. Collagen VI Ablation Retards Brain Tumor Progression Due to Deficits in Assembly of the Vascular Basal Lamina. Am. J. Pathol. 2012, 180, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Poomsawat, S.; Kariya, A.; Nimmanon, T.; Kosanwat, T.; Juengsomjit, R.; Sirima, S. Diagnostic Potential of Type VII Collagen during Oral Carcinogenesis. J. Appl. Oral. Sci. 2023, 31, e20220486. [Google Scholar] [CrossRef] [PubMed]
- Franzke, C.-W.; Bruckner, P.; Bruckner-Tuderman, L. Collagenous Transmembrane Proteins: Recent Insights into Biology and Pathology. J. Biol. Chem. 2005, 280, 4005–4008. [Google Scholar] [CrossRef]
- Jones, V.A.; Patel, P.M.; Gibson, F.T.; Cordova, A.; Amber, K.T. The Role of Collagen XVII in Cancer: Squamous Cell Carcinoma and Beyond. Front. Oncol. 2020, 10, 352. [Google Scholar] [CrossRef]
- Krenacs, T.; Kiszner, G.; Stelkovics, E.; Balla, P.; Teleki, I.; Nemeth, I.; Varga, E.; Korom, I.; Barbai, T.; Plotar, V.; et al. Collagen XVII Is Expressed in Malignant but Not in Benign Melanocytic Tumors and It Can Mediate Antibody Induced Melanoma Apoptosis. Histochem. Cell Biol. 2012, 138, 653–667. [Google Scholar] [CrossRef]
- Kashiwagi, R.; Funayama, R.; Aoki, S.; Matsui, A.; Klein, S.; Sato, Y.; Suzuki, T.; Murakami, K.; Inoue, K.; Iseki, M.; et al. Collagen XVII Regulates Tumor Growth in Pancreatic Cancer through Interaction with the Tumor Microenvironment. Cancer Sci. 2023, 114, 4286–4298. [Google Scholar] [CrossRef]
- Tong, D.; Tanaka, M.; Eguchi, H.; Okazaki, Y.; Muramatsu, M.; Arai, T. COL17A1 Germline Variant p.Ser1029Ala and Mucosal Malignant Melanoma: An Autopsy Study. Mol. Clin. Oncol. 2021, 16, 32. [Google Scholar] [CrossRef]
- Lothong, M.; Sakares, W.; Rojsitthisak, P.; Tanikawa, C.; Matsuda, K.; Yodsurang, V. Collagen XVII Inhibits Breast Cancer Cell Proliferation and Growth through Deactivation of the AKT/MTOR Signaling Pathway. PLoS ONE 2021, 16, e0255179. [Google Scholar] [CrossRef]
- Ohta, Y.; Fujii, M.; Takahashi, S.; Takano, A.; Nanki, K.; Matano, M.; Hanyu, H.; Saito, M.; Shimokawa, M.; Nishikori, S.; et al. Cell–Matrix Interface Regulates Dormancy in Human Colon Cancer Stem Cells. Nature 2022, 608, 784–794. [Google Scholar] [CrossRef]
- Crespo-Bravo, M.; Thorlacius-Ussing, J.; Nissen, N.I.; Pedersen, R.S.; Boisen, M.K.; Liljefors, M.; Johansen, A.Z.; Johansen, J.S.; Karsdal, M.A.; Willumsen, N. Levels of Type XVII Collagen (BP180) Ectodomain Are Elevated in Circulation from Patients with Multiple Cancer Types and Is Prognostic for Patients with Metastatic Colorectal Cancer. BMC Cancer 2023, 23, 949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fredericks, T.; Xiong, G.; Qi, Y.; Rychahou, P.G.; Li, J.-D.; Pihlajaniemi, T.; Xu, W.; Xu, R. Membrane Associated Collagen XIII Promotes Cancer Metastasis and Enhances Anoikis Resistance. Breast Cancer Res. 2018, 20, 116. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, X.; Ou, Y.; Zou, L.; Zhang, D.; Yang, Q.; Qin, Y.; Du, X.; Li, W.; Yuan, Z.; et al. Anoikis Resistance–Protagonists of Breast Cancer Cells Survive and Metastasize after ECM Detachment. Cell Commun. Signal. 2023, 21, 190. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, W.; Ouyang, J.; Wang, J.; Xie, Z. Identification of Anoikis-Related Subgroups and Prognosis Model in Liver Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 2862. [Google Scholar] [CrossRef]
- Xu, F.; Chang, K.; Ma, J.; Qu, Y.; Xie, H.; Dai, B.; Gan, H.; Zhang, H.; Shi, G.; Zhu, Y.; et al. The Oncogenic Role of COL23A1 in Clear Cell Renal Cell Carcinoma. Sci. Rep. 2017, 7, 9846. [Google Scholar] [CrossRef]
- Keller, C.R.; Ruud, K.F.; Martinez, S.R.; Li, W. Identification of the Collagen Types Essential for Mammalian Breast Acinar Structures. Gels 2022, 8, 837. [Google Scholar] [CrossRef]
- Oh, S.P.; Kamagata, Y.; Muragaki, Y.; Timmons, S.; Ooshima, A.; Olsen, B.R. Isolation and Sequencing of CDNAs for Proteins with Multiple Domains of Gly-Xaa-Yaa Repeats Identify a Distinct Family of Collagenous Proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 4229–4233. [Google Scholar] [CrossRef]
- Lakshmanachetty, S.; Koster, M.I. Emerging Roles for Collagen XV and XVIII in Cancer Progression. Exp. Dermatol. 2016, 25, 346–347. [Google Scholar] [CrossRef]
- Mutolo, M.J.; Morris, K.J.; Leir, S.-H.; Caffrey, T.C.; Lewandowska, M.A.; Hollingsworth, M.A.; Harris, A. Tumor Suppression by Collagen XV Is Independent of the Restin Domain. Matrix Biol. 2012, 31, 285–289. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, M.; Xia, Y. COL15A1 Interacts with P4HB to Regulate the Growth and Malignancy of HepG2.2.15 Cells. Biochem. Biophys. Res. Commun. 2023, 681, 20–28. [Google Scholar] [CrossRef]
- Yao, T.; Hu, W.; Chen, J.; Shen, L.; Yu, Y.; Tang, Z.; Zang, G.; Zhang, Y.; Chen, X. Collagen XV Mediated the Epithelial-Mesenchymal Transition to Inhibit Hepatocellular Carcinoma Metastasis. J. Gastrointest. Oncol. 2022, 13, 2472–2484. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Shou, Y.; Xu, W.; Huang, Z.; Xu, J.; Chen, K.; Liu, J.; Liu, D.; Liang, H.; et al. Restoring the Epigenetically Silenced LncRNA COL18A1-AS1 Represses CcRCC Progression by Lipid Browning via MiR-1286/KLF12 Axis. Cell Death Dis. 2022, 13, 578. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, R.; Izzi, V.; Peltoketo, H.; Rask, G.; Kauppila, S.; Väisänen, M.-R.; Ruotsalainen, H.; Martínez-Nieto, G.; Karppinen, S.-M.; Väisänen, T.; et al. Targeting Collagen XVIII Improves the Efficiency of ErbB Inhibitors in Breast Cancer Models. J. Clin. Investig. 2023, 133, e159181. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Liu, L.; Zhao, L.; Hinz, U.; Luo, Y.; An, X.; Gladkich, J.; de la Torre, C.; Huang, Z.; Schrapel, D.; et al. Tumor and Stroma COL8A1 Secretion Induces Autocrine and Paracrine Progression Signaling in Pancreatic Ductal Adenocarcinoma. Matrix Biol. 2022, 114, 84–107. [Google Scholar] [CrossRef] [PubMed]
- Vaday, G.G.; Lider, O. Extracellular Matrix Moieties, Cytokines, and Enzymes: Dynamic Effects on Immune Cell Behavior and Inflammation. J. Leukoc. Biol. 2000, 67, 149–159. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Z.; Zhu, A.; Xiong, X.; Zhang, J.; Xu, J.; Sy, M.; Li, C. Targeting Type I Collagen for Cancer Treatment. Int. J. Cancer 2022, 151, 665–683. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Chen, L.-Q.; Sun, W.; Du, H.-H.; Dong, S.; Ahmed, A.M.Q.; Cao, D.; Cui, J.-H.; Zhang, Y.; Cao, Q.-R. Collagenase IV and Clusterin-Modified Polycaprolactone-Polyethylene Glycol Nanoparticles for Penetrating Dense Tumor Tissues. Theranostics 2021, 11, 906–924. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Q.; Wang, Y.; Xiang, L.; Feng, J.; Zhou, Z.; Fu, Q.; Zhang, L. Collagenase-Loaded PH-Sensitive Nanocarriers Efficiently Remodeled Tumor Stroma Matrixes and Improved the Enrichment of Nanomedicines. Nanoscale 2021, 13, 9402–9414. [Google Scholar] [CrossRef]
- García-Olmo, D.; Villarejo Campos, P.; Barambio, J.; Gomez-Heras, S.G.; Vega-Clemente, L.; Olmedillas-Lopez, S.; Guadalajara, H.; Garcia-Arranz, M. Intraperitoneal Collagenase as a Novel Therapeutic Approach in an Experimental Model of Colorectal Peritoneal Carcinomatosis. Sci. Rep. 2021, 11, 503. [Google Scholar] [CrossRef]
| Collagen Family | Collagen Type | Tumor | Roles | References |
|---|---|---|---|---|
| Fibrillar Collagen | Type I | Triple-negative breast carcinoma, pancreatic ductal adenocarcinoma, and gastric carcinoma | Increased metastasis, proliferation, and invasion. | [139,146,147,148,149,150,151,152,153,154,155] |
| Type II * | Not reported | Not reported | Not reported | |
| Type III | Breast cancer | Reduction in invasiveness, tumor growth suppression; increased cell quiescence; inhibition of autophagy | [48,146,155,156,157,158,159] | |
| Type V | Gastric cancer | Increased proliferation and epithelial–mesenchymal transition | [160,161,162] | |
| Type XI | Papillary thyroid carcinoma, breast cancer, colorectal carcinoma, esophageal cancer, gastric cancer, pancreatic cancer, lung cancer, and ovarian cancer | Promotion of proliferation, drug resistance, and tumor invasiveness | [63,163,164,165,166,167,168,169,170,171,172,173,174] | |
| Type XXVII | Melanoma cells | Promotion of tumor cells quiescence | [175] | |
| Type XXIV * | Not reported | Not reported | Not reported | |
| FACITs | Type XII | Breast cancer, colorectal cancer, pancreatic adenocarcinoma, and ovarian cancer | Promotion of tumor progression and metastasis | [179,180,181,182,183] |
| Type XVI | Colorectal cancer (CRC), glioblastoma, and oral squamous cell carcinoma | Increased invasiveness | [184,185,186] | |
| Type XIV | Melanoma | Reduction in malignancy | [187] | |
| Type XIX | Melanoma | Inhibition of tumor cell migration and invasion | [188] | |
| Type XX | Pancreatic ductal adenocarcinoma | Not reported | [189] | |
| Type XXII | Pancreatic ductal adenocarcinoma | Not reported | [189] | |
| Type XXI | Gastric carcinoma | Increased tumor progression | [190] | |
| Type IX * | Not reported | Not reported | Not reported | |
| Network-forming collagen | Type IV | Breast carcinoma, colorectal tumor, hepatocellular carcinoma | Promotion of tumor proliferation, durotaxis, angiogenesis, and metastasis. Reduction in angiogenesis | [192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214] |
| Type VIII | Breast and pancreatic ductal adenocarcinoma | Promotion of tumor cell invasion and migration, as well as drug resistance | [149,215,257] | |
| Type X | Breast cancer, colorectal cancer, gastric cancer, and lung adenocarcinoma | Promotion of tumor cell proliferation, migration, and invasion | [218,219,220,221] | |
| Beaded filament-forming collagen | Type VI | Glioblastoma, ovarian cancer, and pancreatic ductal adenocarcinoma | Promotion of metastasis, angiogenesis, and tumor progression | [224,225,226,227,228,229] |
| Type XXVI | Thyroid carcinoma | Not reported | [230] | |
| Type XXVIII | Lung cancer | Not reported | [231] | |
| Anchoring fibril | Type VII | Oral squamous cell carcinoma and gastric carcinoma | Promotion of tumor progression; block invasiveness | [232,233,234,235,236] |
| Transmembrane collagen | Type XVII | Squamous cell carcinoma; breast cancer, colorectal cancer | Promotion of cell migration and invasion. Block tumor progression | [238,239,240,241,242,243,244,245,246] |
| Type XIII | Breast, clear cell renal cell carcinoma, and prostate cancer | Tumor progression, invasion, resistance to anoikis, and tumor metastasis | [245,246,247] | |
| Type XXIII | Clear cell renal cell carcinoma | Promotion of tumor progression, adhesion, and migration | [248] | |
| Type XXV | Breast cancer | Not reported | [249] | |
| Multiplexin | Type XV | Cervical cancer; hepato-carcinoma | Reduction in growth, invasion, and migration | [251,252,253,254] |
| Type XVIII | Clear cell renal carcinoma, breast cancer | Increased proliferation and migration | [252,256,257] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Buglio, G.; Lo Cicero, A.; Campora, S.; Ghersi, G. The Multifaced Role of Collagen in Cancer Development and Progression. Int. J. Mol. Sci. 2024, 25, 13523. https://doi.org/10.3390/ijms252413523
Lo Buglio G, Lo Cicero A, Campora S, Ghersi G. The Multifaced Role of Collagen in Cancer Development and Progression. International Journal of Molecular Sciences. 2024; 25(24):13523. https://doi.org/10.3390/ijms252413523
Chicago/Turabian StyleLo Buglio, Gabriele, Alessandra Lo Cicero, Simona Campora, and Giulio Ghersi. 2024. "The Multifaced Role of Collagen in Cancer Development and Progression" International Journal of Molecular Sciences 25, no. 24: 13523. https://doi.org/10.3390/ijms252413523
APA StyleLo Buglio, G., Lo Cicero, A., Campora, S., & Ghersi, G. (2024). The Multifaced Role of Collagen in Cancer Development and Progression. International Journal of Molecular Sciences, 25(24), 13523. https://doi.org/10.3390/ijms252413523






