Association of Neuroblastoma (NB) SH-SY5Y Cells with Antibodies of Parasitic Origin (Anti-Acanthamoeba and Anti-Toxocara canis)
Abstract
:1. Introduction
2. Results
2.1. Recognition of Anti-Acanthamoeba and Anti-Toxocara canis Antibodies on the Total Proteins of SH-5S5Y
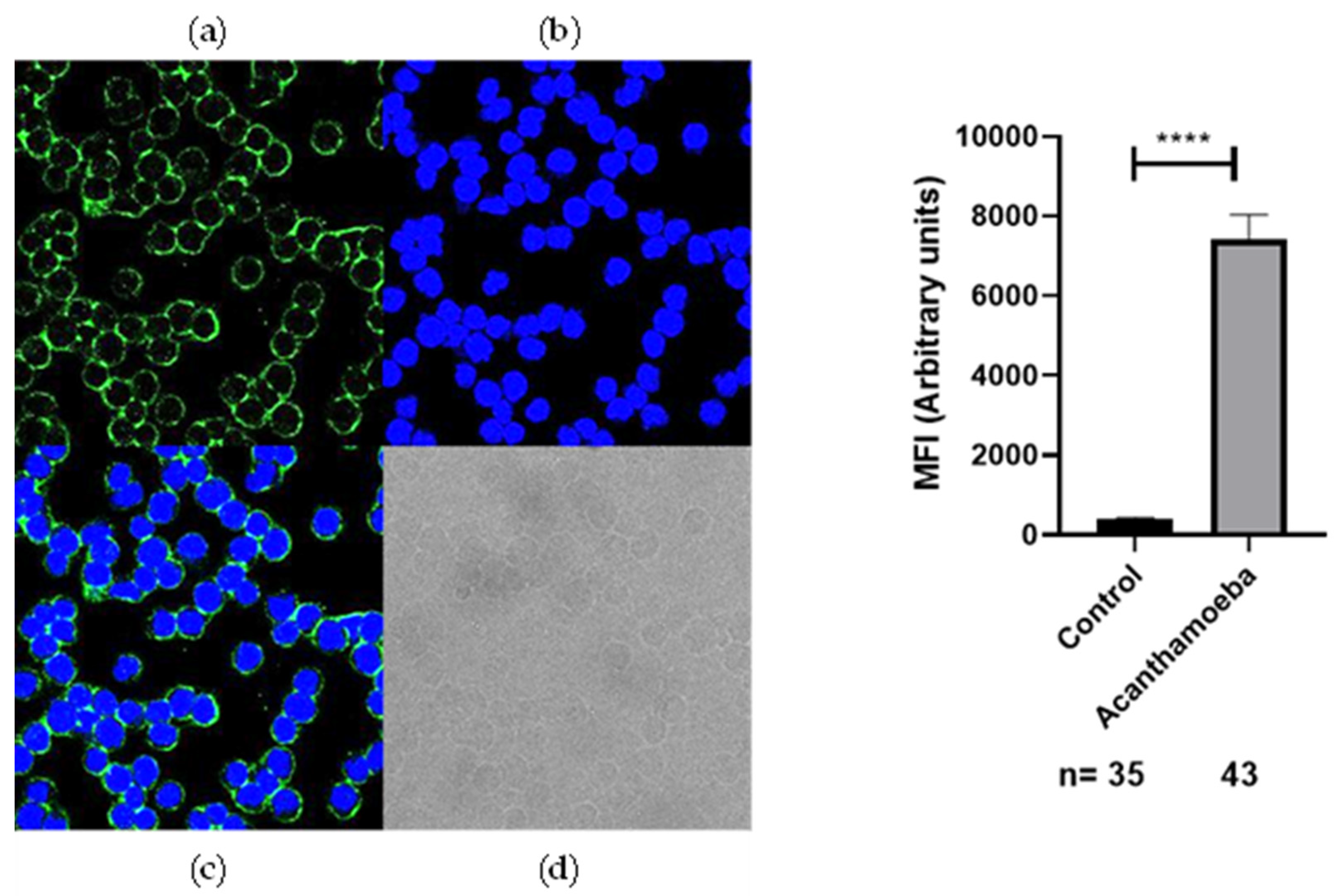
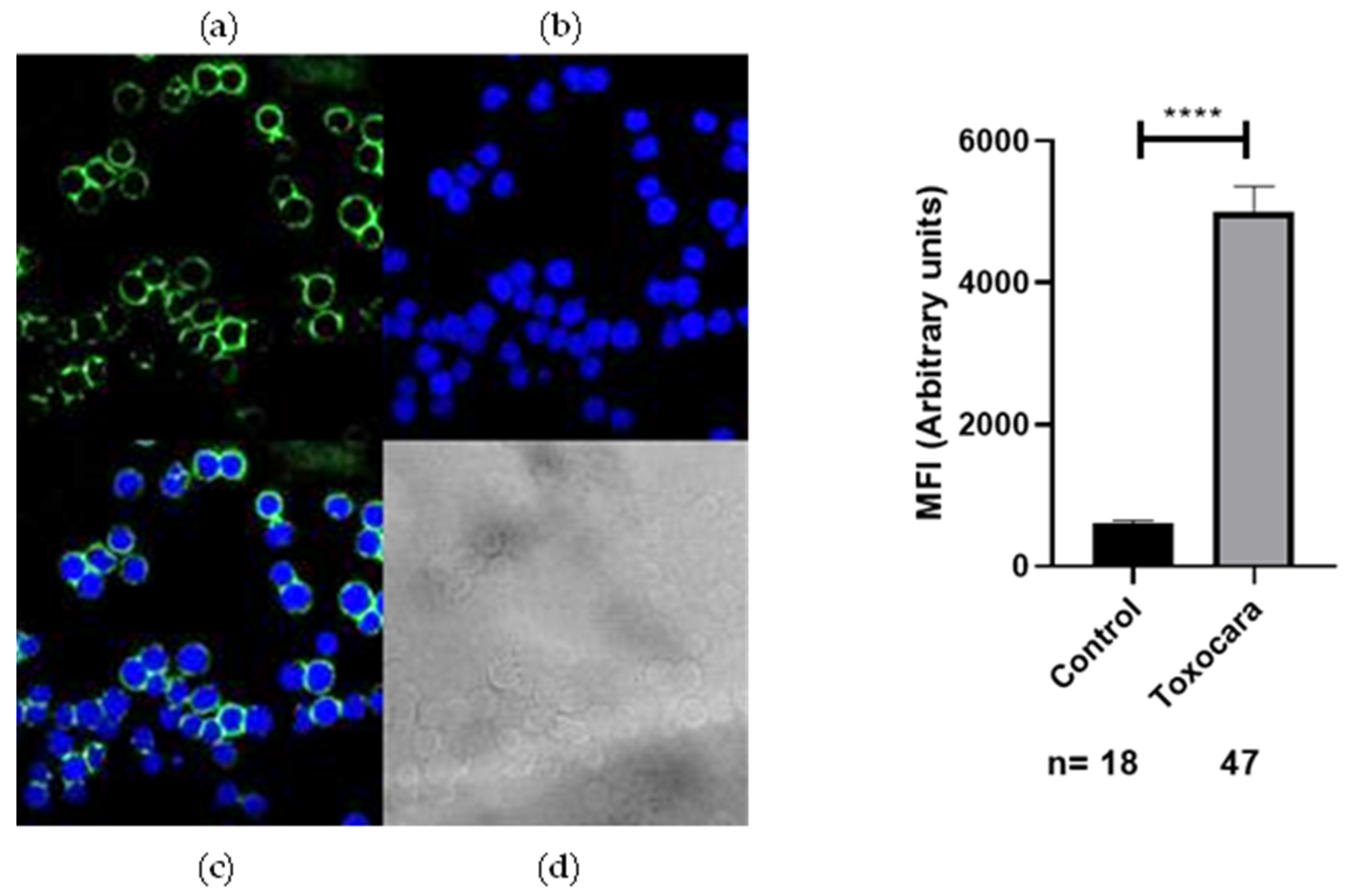
2.2. Recognition of Anti-Acanthamoeba and Anti-Toxocara canis Antibodies on the Membrane of SH-5S5Y
3. Discussion
4. Materials and Methods
4.1. Cell Line of SH-SY5Y and Obtaining the Antigen
4.2. Acanthamoeba Trophozoites Culture and Obtaining the Antigen
4.3. Toxocara canis Eggs Culture and Obtaining the Antigen
4.4. Generation of Anti-Acanthamoeba and Anti-Toxocara canis In Vivo with New Zeeland Rabbits
4.5. Purification of Anti-Acanthamoeba and Anti-Toxocara canis Antibodies by Affinity Chromatography with Protein A/G
4.6. Identification of SH-SY5Y Proteins Recognized by Anti-Acanthamoeba and Anti-Toxocara canis Antibodies by Western Blotting
4.7. Determination of Anti-Acantamoeba and Anti-Toxocara canis Antibodies Recognition with Neuroblastom Cells by Immunofluorescence in Confocal Microscopy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, K.M.; Shah, N.R.; Chukkapalli, S.; King, S.; Grant, C.N.; Brown, E.G.; Avanzini, S.; Lal, D.R.; Sarnacki, S.; Newman, E.A. Modern surgical strategies in pediatric neuroblastoma: Evolving approaches and treatment principles. Pediatr. Blood Cancer, 2024; e31317Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Rados, M.; Landegger, A.; Schmutzler, L.; Rabidou, K.; Taschner-Mandl, S.; Fetahu, I.S. Natural killer cells in neuroblastoma: Immunological insights and therapeutic perspectives. Cancer Metastasis Rev. 2024; Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Esser, R.; Müller, T.; Stefes, D.; Kloess, S.; Seidel, D.; Gillies, S.D.; Aperlo-Iffland, C.; Huston, J.S.; Uherek, C.; Schönfeld, K.; et al. NK cells engineered to express a GD2-specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J. Cell. Mol. Med. 2012, 16, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Raiser, P.; Schleiermacher, G.; Gambart, M.; Dumont, B.; Defachelles, A.S.; Thebaud, E.; Tandonnet, J.; Pasqualini, C.; Proust, S.; Entz-Werle, N.; et al. Chemo-immunotherapy with dinutuximab beta in patients with relapsed/progressive high-risk neuroblastoma: Does chemotherapy backbone matter? Eur. J. Cancer 2024, 202, 114001. [Google Scholar] [CrossRef] [PubMed]
- Walter, N.S.; Bhattacharyya, S. Mining parasites for their potential as novel therapeutic agents against cancer. Med. Oncol. 2024, 41, 211. [Google Scholar] [CrossRef]
- Eissa, M.M.; Salem, A.E.; El Skhawy, N. Parasites revive hope for cancer therapy. Eur. J. Med. Res. 2024, 29, 489. [Google Scholar] [CrossRef]
- Xin, W.; Li, S.; Li, Z.; Zheng-Quan, L.; Jiang, L.; Lin-Xi, Z. Impact of Toxoplasma gondii on the proliferation and apoptosis of tumor cell lines. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi (CJPPD) 2012, 30, 17. [Google Scholar]
- Pyo, K.-H.; Jung, B.-K.; Chai, J.-Y.; Shin, E.-H. Suppressed CD31 expression in sarcoma-180 tumors after injection with Toxoplasma gondii lysate antigen in BALB/c mice. Korean J. Parasitol. 2010, 48, 171–174. [Google Scholar] [CrossRef]
- Darani, H.Y.; Shirzad, H.; Mansoori, F.; Zabardast, N.; Mahmoodzadeh, M. Effects of Toxoplasma gondii and Toxocara canis antigens on WEHI-164 fibrosarcoma growth in a mouse model. Korean J. Parasitol. 2009, 47, 175–177. [Google Scholar] [CrossRef]
- Hunter, C.A.; Yu, D.; Gee, M.; Ngo, C.V.; Sevignani, C.; Goldschmidt, M.; Golovkina, T.V.; Evans, S.; Lee, W.F.; Thomas-Tikhonenko, A. Cutting edge: Systemic inhibition of angiogenesis underlies resistance to tumors during acute toxoplasmosis. J. Immunol. 2001, 166, 5878–5881. [Google Scholar] [CrossRef]
- Boghozian, R.; Saei, A.; Mirzaei, R.; Jamali, A.; Vaziri, B.; Razavi, A.; Hadjati, J. Identification of Toxoplasma gondii protein fractions induce immune response against melanoma in mice. Apmis 2015, 123, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Fox, B.A.; Sanders, K.L.; Rommereim, L.M.; Guevara, R.B.; Bzik, D.J. Secretion of rhoptry and dense granule effector proteins by nonreplicating Toxoplasma gondii uracil auxotrophs controls the development of anti-tumor immunity. PLOS Genet. 2016, 12, e1006189. [Google Scholar] [CrossRef] [PubMed]
- Choo, J.-D.; Lee, J.-S.; Kang, J.-S.; Lee, H.-S.; Yeom, J.-Y.; Lee, Y.-H. Inhibitory effects of Toxoplasma antigen on proliferation and invasion of human glioma cells. J. Korean Neurosurg. Soc. 2005, 37, 129–136. [Google Scholar]
- Nguyen, Y.; Zhao, X.; Ewald, S.; Harris, T.; Zong, H. Harness the immune-modulatory activities of Toxoplasma gondii to improve lymphocyte infiltration into brain tumors. Cancer Immunol. Res. 2022, 10, 40. [Google Scholar] [CrossRef]
- Pyo, K.-H.; Jung, B.-K.; Xin, C.-F.; Lee, Y.-W.; Chai, J.-Y.; Shin, E.-H. Prominent IL-12 production and tumor reduction in athymic nude mice after Toxoplasma gondii lysate antigen treatment. Korean J. Parasitol. 2014, 52, 605–612. [Google Scholar] [CrossRef]
- Luo, Q.; Sun, L.; Tian, Q.-Q.; Ren, H.; Liu, H.; Yang, C.-J.; Li, X. Effect of culture supernatant of Toxoplasma gondii on the proliferation and apoptosis of BGC-823 cells. Chin. J. Parasitol. Parasit. Dis. 2014, 32, 123–127. [Google Scholar]
- Wang, G.; Gao, M. Influence of Toxoplasma gondii on in vitro proliferation and apoptosis of hepatoma carcinoma H7402 cell. Asian Pac. J. Trop. Med. 2016, 9, 63–66. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, N.; Sun, L.; Luo, Q.; An, F. Apoptosis of human leukemia K562 cell in vitro induced by Toxoplasma gondii. Chin. J. Parasitol. Parasit. Dis. 2007, 25, 185–188. [Google Scholar]
- Wang, X.; Fu, B.; Yang, S.; Wu, X.; Cui, G.; Zhao, Y.; Yu, Y.; Liu, X.; Deng, H.; Chen, Q.; et al. Trichinella spiralis—A potential anti-tumor agent. Vet. Parasitol. 2009, 159, 249–252. [Google Scholar] [CrossRef]
- Eissa, M.M.; Ismail, C.A.; El-Azzouni, M.Z.; Ghazy, A.A.; Hadi, M.A. Immuno-therapeutic potential of Schistosoma mansoni and Trichinella spiralis antigens in a murine model of colon cancer. Investig. New Drugs 2019, 37, 47–56. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Jo, J.-O.; Cho, M.-K.; Yu, H.-S.; Leem, S.-H.; Song, K.S.; Ock, M.S.; Cha, H.-J. Trichinella spiralis infection reduces tumor growth and metastasis of B16-F10 melanoma cells. Vet. Parasitol. 2013, 196, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Tsocheva-Gaytandzhieva, N.; Toshkova, R.; Gardeva, E.; Yossifova, L.; Petkova, S.; Nanev, V. Antiproliferative activity against tumour cells of biologically active substances isolated from livers of healthy and Trichinella spiralis infected rats. Proc. Bulg. Acad. Sci. 2016, 69, 1443–1448. [Google Scholar]
- Li, X.; Zhang, G.; Zhang, X. Effect of Trichinella on growth of human colorectal carcinoma HCT-8 cells in BALB/c mice. Chin. J. Biol. 2008, 4, 285–287. [Google Scholar]
- Luo, J.; Yu, L.; Xie, G.; Li, D.; Su, M.; Zhao, X.; Du, L. Study on the mitochondrial apoptosis pathways of small cell lung cancer H446 cells induced by Trichinella spiralis muscle larvae ESPs. Parasitology 2017, 144, 793–800. [Google Scholar] [CrossRef]
- Sanders, K.L.; Fox, B.A.; Bzik, D.J. Attenuated Toxoplasma gondii stimulates immunity to pancreatic cancer by manipulation of myeloid cell populations. Cancer Immunol. Res. 2015, 3, 891–901. [Google Scholar] [CrossRef]
- Qiao, J.-C.; Zhang, H.; Jiao, Y.-M.; Yang, Y.-T.; Dong, J.-J.; Wang, Z.-Z.; Li, H.-Y.; Meng, L.-W.; Yang, X.-D.; Tao, Z.-Y.; et al. Anti-tumor effect of Plasmodium yoelii infection on melanoma in mice. Chin. J. Schistosomiasis Control 2017, 29, 315. [Google Scholar] [CrossRef]
- Tao, Z.; Ding, W.; Cheng, Z.; Feng, Y.; Kang, Z.; Qiu, R.; Zhao, S.; Hu, W.; Zhou, F.; Wu, D.; et al. Preclinical study of Plasmodium immunotherapy combined with radiotherapy for solid tumors. Cells 2022, 11, 3600. [Google Scholar] [CrossRef]
- Yao, X.; Cao, Y.; Lu, L.; Xu, Y.; Chen, H.; Liu, C.; Chen, D.; Wang, K.; Xu, J.; Fang, R.; et al. Plasmodium infection suppresses colon cancer growth by inhibiting proliferation and promoting apoptosis associated with disrupting mitochondrial biogenesis and mitophagy in mice. Parasites Vectors 2022, 15, 192. [Google Scholar] [CrossRef]
- Adah, D.; Yang, Y.; Liu, Q.; Gadidasu, K.; Tao, Z.; Yu, S.; Dai, L.; Li, X.; Zhao, S.; Qin, L.; et al. Plasmodium infection inhibits the expansion and activation of MDSCs and Tregs in the tumor microenvironment in a murine Lewis lung cancer model. Cell Commun. Signal. 2019, 17, 32. [Google Scholar] [CrossRef]
- Pan, J.; Ma, M.; Qin, L.; Kang, Z.; Adah, D.; Tao, Z.; Li, X.; Dai, L.; Zhao, S.; Chen, X.; et al. Plasmodium infection inhibits triple negative 4T1 breast cancer potentially through induction of CD8+ T cell-mediated anti-tumor responses in mice. Biomed. Pharmacother. 2021, 138, 111406. [Google Scholar] [CrossRef]
- Wang, B.; Li, Q.; Wang, J.; Zhao, S.; Nashun, B.; Qin, L.; Chen, X. Plasmodium infection inhibits tumor angiogenesis through effects on tumor-associated macrophages in a murine implanted hepatoma model. Cell Commun. Signal. 2020, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.-Z.; Fang, Z.-M.; Zhang, Q.; Zhan, Y.; Zhang, Y.; Jiang, W.-F.; Hou, X.; Li, Y.-L.; Wang, T. Plasmodium yoelii infection inhibits murine leukaemia WEHI-3 cell proliferation in vivo by promoting immune responses. Infect. Dis. Poverty 2018, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Sheklakova, L.; Kallinikova, V.; Karpenko, L. Genetic heterogeneity of Trypanosoma cruzi and its direct anti-cancer effect in cultured human tumor cells. Bull. Exp. Biol. Med. 2003, 135, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Guzmán, L.; Lobos-González, L.; Rosas, C.; Vallejos, G.; Falcón, C.; Sosoniuk, E.; Coddou, F.; Leyton, L.; Lemus, D.; Quest, A.F.G.; et al. Human survivin and Trypanosoma cruzi calreticulin act in synergy against a murine melanoma in vivo. PLoS ONE 2014, 9, e95457. [Google Scholar] [CrossRef]
- Eligio García, L.; Crisóstomo Vázquez, M.D.P.; Maravelez Acosta, V.A.; Soria Guerrero, M.; Cortés Campos, A.; Jiménez Cardoso, E. Trypanosoma cruzi Antigenic Proteins Shared with Acute Lymphoblastic Leukemia and Neuroblastoma. Pharmaceuticals 2022, 15, 1421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ubillos, L.; Freire, T.; Berriel, E.; Chiribao, M.L.; Chiale, C.; Festari, M.F.; Medeiros, A.; Mazal, D.; Rondán, M.; Bollati-Fogolín, M.; et al. Trypanosoma cruzi extracts elicit protective immune response against chemical-ly induced colon and mammary cancers. Int. J. Cancer 2016, 138, 1719–1731. [Google Scholar] [CrossRef]
- Freire, T.; Landeira, M.; Giacomini, C.; Festari, M.F.; Pittini, Á.; Cardozo, V.; Brosque, A.; Monin, L.; da Costa, V.; Faral-Tello, P.; et al. Trypanosoma cruzi-derived molecules induce anti-tumour protection by favouring both innate and adaptive immune responses. Int. J. Mol. Sci. 2022, 23, 15032. [Google Scholar] [CrossRef]
- Barati, N.; Tanzadehpanah, H.; Asl, S.S.; Khazaei, S.; Motavallihaghi, S. Anticancer Activity of Antigen B from hydatid cyst fluid of Echinococcus granulosus on melanoma cancer cell line. Chemother. Open Access 2022, 11, 1–7. [Google Scholar]
- Ranasinghe, S.L.; Boyle, G.M.; Fischer, K.; Potriquet, J.; Mulvenna, J.P.; McManus, D.P. Kunitz type protease inhibitor EgKI-1 from the canine tapeworm Echinococcus granulosus as a promising therapeutic against breast cancer. PLoS ONE 2018, 13, e0200433. [Google Scholar] [CrossRef]
- Motavallihaghi, S.; Tanzadehpanah, H.; Soleimani Asl, S.; Shojaeian, A.; Yousefimashouf, M.; Barati, N. In vitro anti-cancer activity of hydatid cyst fluid on colon cancer cell line (C26). Egypt. J. Med. Hum. Genet. 2023, 24, 15. [Google Scholar]
- Doğan, S.; Çakir, M.; Kartal, A.; Öztaş, H.; Oltulu, P. Can Echinococcus granulosus infestation prevent pancreatic cancer? An in vivo experimental study. Asian Pac. J. Cancer Prev. 2023, 24, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Karadayi, S.; Arslan, S.; Sumer, Z.; Turan, M.; Sumer, H.; Karadayi, K. Does hydatid disease have protective effects against lung cancer? Mol. Biol. Rep. 2013, 40, 4701–4704. [Google Scholar] [CrossRef] [PubMed]
- Yousofi Darani, H.; Soozangar, N.; Khorami, S.; Taji, F.; Yousofi, M.; Shirzad, H. Hydatid cyst protoscolices induce cell death in WEHI-164 fibrosarcoma cells and inhibit the proliferation of baby hamster kidney fibroblasts in vitro. J. Parasitol. Res. 2012, 2012, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Asouli, A.; Sadr, S.; Mohebalian, H.; Borji, H. Anti-tumor effect of protoscolex hydatid cyst somatic antigen on inhibition cell growth of K562. Acta Parasitol. 2023, 68, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhao, L.; Brittingham, A.; Bai, Q.; Wakefield, M.R.; Fang, Y. Trichomonas vaginalis inhibits HeLa cell growth through modulation of critical molecules for cell proliferation and apoptosis. Anticancer Res. 2018, 38, 5079–5086. [Google Scholar] [CrossRef]
- Zhu, Z.; Davidson, K.T.; Brittingham, A.; Wakefield, M.R.; Bai, Q.; Xiao, H.; Fang, Y. Trichomonas vaginalis: A possible foe to prostate cancer. Med. Oncol. 2016, 33, 115. [Google Scholar] [CrossRef]
- Salvador-Membreve, D.M.C.; Jacinto, S.D.; Rivera, W.L. Trichomonas vaginalis induces cytopathic effect on human lung alveolar basal carcinoma epithelial cell line A549. Exp. Parasitol. 2014, 147, 33–40. [Google Scholar] [CrossRef]
- Pereira, F.E.L.; Raso, P.; Coelho, P.M.Z. Evolution of sarcoma 180 (ascitic tumor) in mice infected with Schistosoma mansoni. Rev. Soc. Bras. Med. Trop. 1986, 19, 39–42. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Acanthamoeba is an evolutionary ancestor of macrophages: A myth or reality? Exp. Parasitol. 2012, 130, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Castellani, A. An amoeba found in cultures of a yeast preliminary note-third note-an amoeba growing in cultures of a yeast second note-fourth note. J. Trop. Med. Hyg. 1930, 33, 160. [Google Scholar]
- Pinto, L.F.; Andriolo, B.N.G.; Hofling-Lima, A.L.; Freitas, D. The role of Acanthamoeba spp. in biofilm communities: A systematic review. Parasitol. Res. 2021, 120, 2717–2729. [Google Scholar] [CrossRef] [PubMed]
- Mungroo, M.R.; Siddiqui, R.; Khan, N.A. War of the microbial world: Acanthamoeba spp. interactions with microorganisms. Folia Microbiol. 2021, 66, 689–699. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, N.A.; Siddiqui, R. Depredadores vs. alienígenas: Interacciones de bacterias con Acanthamoeba. Parasitología 2014, 141, 869–874. [Google Scholar] [CrossRef]
- Pidherney, M.S.; Alizadeh, H.; Stewart, G.L.; McCulley, J.P.; Niederkorn, J.Y. In vitro and in vivo tumoricidal properties of a pathogenic/free-living amoeba. Cancer Lett. 1993, 72, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, H.; Pidherney, M.S.; McCulley, J.P.; Niederkorn, J.Y. Apoptosis as a mechanism of cytolysis of tumor cells by a pathogenic free-living amoeba. Infect. Immun. 1994, 62, 1298–1303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qian, M.; Zhang, X.H.; Mei, B.; Zhang, P.; Yan, Z.; Yu, M. [Cytotoxic effect of Acanthamoeba trophozoite on HeLa cells]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2001, 19, 37–40. (In Chinese) [Google Scholar] [PubMed]
- Chusattayanond, A.D.; Boonsilp, S.; Kasisit, J.; Boonmee, A.; Warit, S. Thai Acanthamoeba isolate (T4) induced apoptotic death in neuroblastoma cells via the Bax-mediated pathway. Parasitol. Int. 2010, 59, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Zeybek, Z.; Kebabcı, Ç.; Bugan Gül, İ.; Akbaş, F. Çevresel Acanthamoeba Suşlarının Hücresiz Sıvılarının Metastatik Hücre Hatlarının Canlılığı Üzerindeki İnhibisyon Etkisi [Inhibition Effect of Cell-Free Supernatants of Environmental Acanthamoeba Strains on the Viability of Metastatic Cell-Lines]. Mikrobiyoloji Bul. 2023, 57, 283–292. (In Turkish) [Google Scholar] [CrossRef] [PubMed]
- Archelli, S.; Leonora, K. Toxocara y toxocariosis. Acta Bioquím. Clín. Latinoam En Linea 2008, 42, 379–384. [Google Scholar]
- Magnaval, J.F.; Glickman, L.T.; Dorchies, P.; Morassin, B. Highlights of human toxocariasis. Korean J. Parasitol. 2001, 39, 1. [Google Scholar] [CrossRef]
- Bonilla-Aldana, D.K.; Morales-Garcia, L.V.; Ulloque Badaracco, J.R.; Mosquera-Rojas, M.D.; Alarcón-Braga, E.A.; Hernandez-Bustamante, E.A.; Al-Kassab-Córdova, A.; Benites-Zapata, V.A.; Rodriguez-Morales, A.J.; Delgado, O. Prevalence of Toxocara eggs in Latin American parks: A systematic review and meta-analysis. Infez. Med. 2023, 31, 329–349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strube, C.; Heuer, L.; Janecek, E. Toxocara spp. infections in paratenic hosts. Vet. Parasitol. 2013, 193, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Rassier, G.L.; Borsuk, S.; Pappen, F.; Scaini, C.J.; Gallina, T.; Villela, M.M.; Farias, N.A.d.R.; Benavides, M.V.; Berne, M.E.A. Toxocara spp. seroprevalence in sheep from southern Brazil. Parasitol. Res. 2013, 112, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Bahadory, S.; Sadraei, J.; Zibaei, M.; Pirestani, M.; Dalimi, A. In vitro anti-gastrointestinal cancer activity of Toxocara canis-derived peptide: Analyzing the expression level of factors related to cell proliferation and tumor growth. Front. Pharmacol. 2022, 13, 878724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Botelho, M.C.; Soares, R.; Vale, N.; Ribeiro, R.; Camilo, V.; Almeida, R.; Medeiros, R.; Gomes, P.; Machado, J.C.; da Costa, J.M.C. Schistosoma haematobium: Identificación de nuevas moléculas estrogénicas con actividad antagonista del estradiol y capacidad para inactivar el receptor de estrógeno en células de mamíferos. Exp. Parasitol. 2010, 126, 526–535. [Google Scholar] [CrossRef]
- Machicado, C.; Marcos, L.A. Carcinogenesis associated with parasites other than Schistosoma, Opisthorchis and Clonorchis: A systematic review. Int. J. Cancer 2016, 138, 2915–2921. [Google Scholar] [CrossRef]
- Maizels, R.M. Toxocara canis: Molecular basis of immune recognition and evasion. Vet. Parasitol. 2013, 193, 365–374. [Google Scholar] [CrossRef]
- Ruiz-Manzano, R.A.; Palacios-Arreola, M.I.; Hernández-Cervantes, R.; Del Río-Araiza, V.H.; Nava-Castro, K.E.; Ostoa-Saloma, P.; Muñoz-Cruz, S.; Morales-Montor, J. Potential Novel Risk Factor for Breast Cancer: Toxocara canis Infection Increases Tumor Size Due to Modulation of the Tumor Immune Microenvironment. Front. Oncol. 2020, 10, 736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aragón-Franco, R.; Ruiz-Manzano, R.A.; Nava-Castro, K.E.; Del Rìo Araiza, V.H.; Garay-Canales, C.A.; Pérez-Torres, A.; Chacón-Salinas, R.; Girón-Pérez, M.I.; Morales-Montor, J. Convergence between helminths and breast cancer: Intratumoral injection of the excretory/secretory antigens of the human parasite Toxocara canis (EST) increase lung macro and micro metastasis. Front. Immunol. 2024, 15, 1332933. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kazemi, F.; Moradi-Sardareh, H.; Arjmand, R.; Tavalla, M.; Amari, A.; Cheraghian, B. Toxocara Canis Increases the Potential of Breast Cancer by Reducing the Expression of the P53 Protein. Curr. Mol. Med. 2024, 24, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Manzano, R.A.; Hernández-Cervantes, R.; Del Río-Araiza, V.H.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Morales-Montor, J. Immune response to chronic Toxocara canis infection in a mice model. Parasite Immunol. 2019, 41, e12672. [Google Scholar] [CrossRef] [PubMed]
- Maravelez Acosta, V.A.; Crisóstomo Vázquez, M.D.P.; Eligio García, L.; Franco Sandoval, L.O.; Castro Pérez, D.; Patiño López, G.; Medina Contreras, O.; Jiménez Cardoso, E. Tumor-Suppressive Cross-Linking of Anti-T. cruzi Antibodies in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2024, 25, 8307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zenina, A.V.; Kravtsov, E.G.; Tsetsegsaikhan, B.; Yashina, N.V.; Dalin, M.V.; Karpenko, L.P.; Sheklakova, L.A.; Kallinikova, V.D. The study of immunological component in antitumor effect of Trypanosoma cruzi. Bull. Exp. Biol. Med. 2008, 145, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Alejandra Morillo, D.; Elena González, B.; Sulbarán Larrarte, A.J.; Ibarra, A.; Álvarez, C.; Colmenares, M.V.; Bonfante-Cabarcas, R.A. La infección por Trypanosoma cruzi disminuye el desarrollo del melanoma maligno e incrementa la supervivencia en ratones C57BL/6 [Trypanosoma cruzi infection decreases malignant melanoma development and increases survival in C57BL/6 mice]. Investig. Clin. 2014, 55, 227–237. [Google Scholar]

| Parasite | Antitumor Effect |
|---|---|
| Toxoplasma gondii | Breast cancer, prostate cancer DU-145 cells and lung cancer cells A549 [7], murine sarcoma 180 cells [8], fibrosarcoma WEHI-164 cells [9], human melanoma B16-F10 cells [10], mouse melanoma [11], ovarian cancer A2780 and resistant A2780-CP cells [12], human glioma U373MG and U87MG cells [13], medulloblastoma Shh-subtype cells [14], colorectal carcinoma CT26 cells [15], human gastric cancer BGC-823 cells [16], hepatocellular carcinoma H7402 cells [17] and human chronic myeloid leukemia K562 cells [18]. |
| Trichinella spiralis | Murin sarcoma cells 180, hepatoma H22 and H7402 cells murine forestomach carcinoma MFC cells and human chronic myeloid leukemia K562 cells [19], human osteosarcoma MG-63 cells [20], melanoma B16-F10 cells [21], human cervical carcinoma HeLa and T24 cells, human transitional cell bladder carcinoma, IV grade [22], HCT-8 human colorectal carcinoma [23], lung cancer H446 SCLC cells [24] and breast cancer [25]. |
| Plasmodium yoelii | Human melanoma B16-F10 cells [26], mouse glioma GL261 cells and lung cancer LLC cells [27], colon cancer cells and murin melanoma B16-F10 cells [28]. Murine Lewis lung cancer cells [29], murine triple-negative breast cancer [30], hepatoma cells [31] and murine WEHI-3 leukemia cells [32]. |
| Trypanosoma cruzi | Breast cancer cell [33], murine melanoma B16-F10 cells [34], human neuroblastoma SH.5S5Y cells and human leukemia SUPB15 cells [35], colon and mammary rat cancer cells and human colon cancer cells [36] and murine lung cancer [37]. |
| Echinococcus granulosus | Human melanoma cancer A375 cells [38], breast cancer MDA-MB-231, MCF-7 and T47D cells [39], mouse colon cancer C26 cells [40], pancreas cancer induced in a rat [41], lung cancer HCL-H209/Anl cells [42], murine fibrosarcoma WEHI-164 cells [43] and cronic myeloid leukemia K562 cells [44]. |
| Trichomonas vaginalis | Human cervical carcinoma HeLa cells [45], prostate cancer PC-3 and DU145 cells [46] and human lung alveolar basal carcinoma epithelial A549 cells [47]. |
| Schistosoma mansoni | Murine sarcoma 180 cells [48], murine fibrosarcoma WEHI-164 cells [43] and DMH-induced colon carcinogenesis [20]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maravelez Acosta, V.A.; Garcia, M.d.L.C.; Patiño López, G.; Crisóstomo Vázquez, M.d.P.; Franco Sandoval, L.O.; Eligio García, L. Association of Neuroblastoma (NB) SH-SY5Y Cells with Antibodies of Parasitic Origin (Anti-Acanthamoeba and Anti-Toxocara canis). Int. J. Mol. Sci. 2024, 25, 13577. https://doi.org/10.3390/ijms252413577
Maravelez Acosta VA, Garcia MdLC, Patiño López G, Crisóstomo Vázquez MdP, Franco Sandoval LO, Eligio García L. Association of Neuroblastoma (NB) SH-SY5Y Cells with Antibodies of Parasitic Origin (Anti-Acanthamoeba and Anti-Toxocara canis). International Journal of Molecular Sciences. 2024; 25(24):13577. https://doi.org/10.3390/ijms252413577
Chicago/Turabian StyleMaravelez Acosta, Víctor Alberto, Maria de Lourdes Caballero Garcia, Genaro Patiño López, María del Pilar Crisóstomo Vázquez, Luz Ofelia Franco Sandoval, and Leticia Eligio García. 2024. "Association of Neuroblastoma (NB) SH-SY5Y Cells with Antibodies of Parasitic Origin (Anti-Acanthamoeba and Anti-Toxocara canis)" International Journal of Molecular Sciences 25, no. 24: 13577. https://doi.org/10.3390/ijms252413577
APA StyleMaravelez Acosta, V. A., Garcia, M. d. L. C., Patiño López, G., Crisóstomo Vázquez, M. d. P., Franco Sandoval, L. O., & Eligio García, L. (2024). Association of Neuroblastoma (NB) SH-SY5Y Cells with Antibodies of Parasitic Origin (Anti-Acanthamoeba and Anti-Toxocara canis). International Journal of Molecular Sciences, 25(24), 13577. https://doi.org/10.3390/ijms252413577









