The Therapeutic Mechanisms of Mesenchymal Stem Cells in MS—A Review Focusing on Neuroprotective Properties
Abstract
1. Introduction
1.1. Multiple Sclerosis
1.2. Mesenchymal Stem Cells
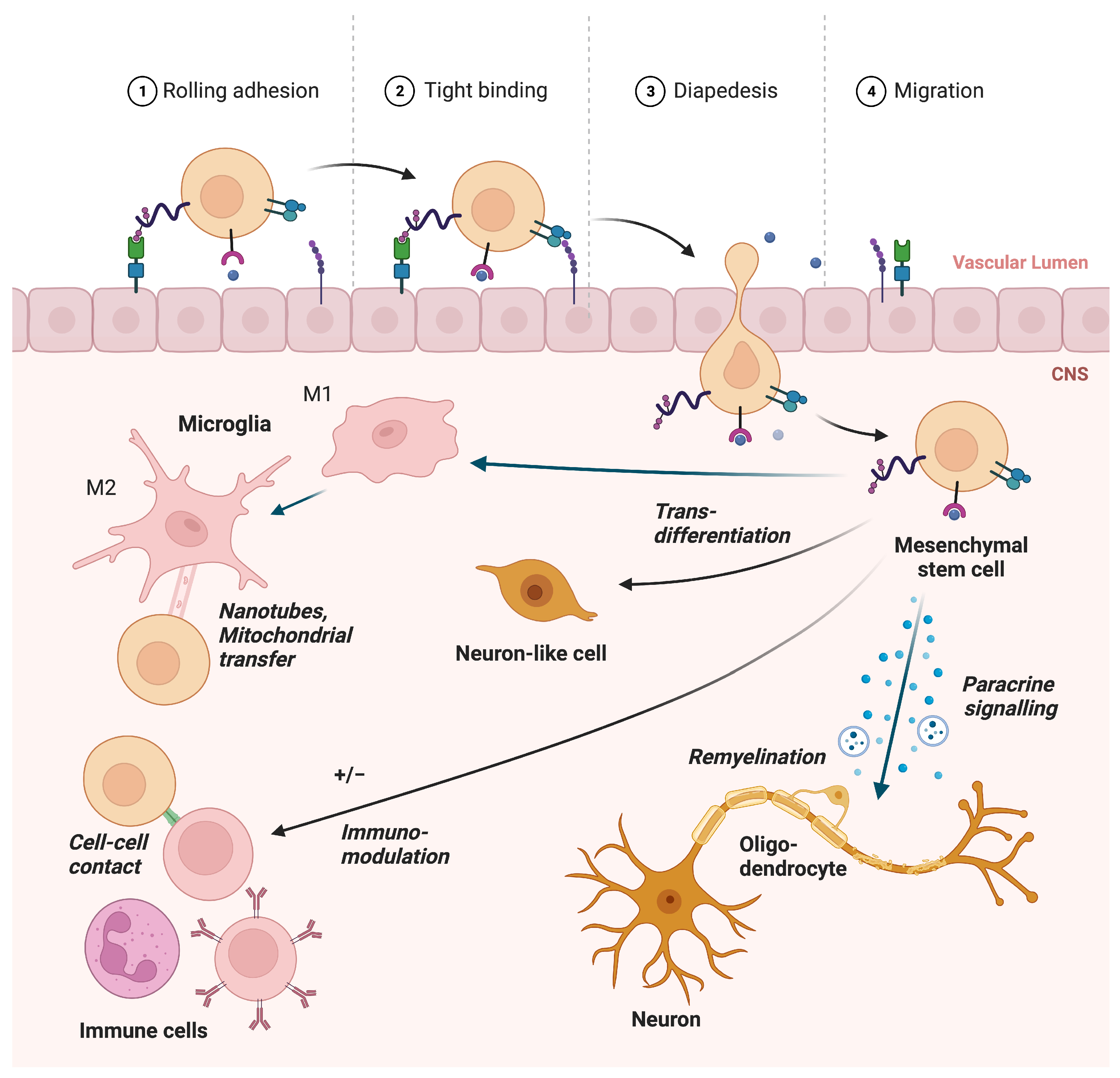
2. Mechanisms
2.1. Paracrine Function
2.2. Remyelination
2.3. Immunomodulation of the Adaptive Immune System in MS
2.4. Immunomodulation of Microglia in MS
2.5. Migration
2.6. Horizontal Information Transfer and Alleviation of Ferroptosis
2.7. Transdifferentiation
2.8. Priming of MSCs
3. Discussion
| First, Author, Country and Year | Condition and Important Inclusion Criteria | Timing of MSC Treatment after Debut of Condition | Design and Blinding | Follow-Up Time | Type of MSC & Administration | N Patients | N Controls | Main Results Safety | Main Results Efficacy |
|---|---|---|---|---|---|---|---|---|---|
| Controlled Studies | |||||||||
| Li, China 2014 [136] | RRMS/SPMS EDSS 4–8 Treatment failure NR | ≥2 years | +Randomized −Placebo −Blinded | 12 months | Allogeneic MSCs from UC in combination with methylprednisolone IV × 3 | 13 | 10 | No SAR | Lower EDSS and relapse rate in MSC group |
| Llufriu, Spain 2014 [56] | RRMS EDSS 3–6.5 Treatment failure | 2–10 years | +Randomized +Placebo +Double blinded +Cross-over | 6–12 months | Autologous MSCs from BM IV × 1 | 9 | 9 | No SAR | Trend lower rate of CEL |
| Lublin, USA 2014 [137] | RRMS/SPMS EDSS not specified Treatment failure | ≥2 years | +Randomized +Placebo +Double blinded | 6–12 months | Allogeneic, placenta-derived MSCs IV × 1 | 12 (6 low dose, 6 high dose) | 4 | One anaphylactoid reaction and one superficial thrombophlebitis | Not assessed between groups |
| Meng, China 2018 [138] | MS type and EDSS not specified Treatment failure | NS | −Randomized −Placebo −Blinded | 1–3 years | Allogeneic MSCs from UC IV × 7 | 2 | 1 | No SAR | Not assessed between groups |
| Fernandez, Spain 2018 [139] | SPMS EDSS 5.5–9 Treatment failure | NS | +Randomized +Placebo +Double blinded | 12 months | Autologous adipose-derived MSCs IV × 1 | 23 (11 low dose, 12 high dose) | 11 | No SAR | No significant effect |
| Petrou, Israel 2020 [131] | SPMS/PPMS EDSS 3–6.5 Treatment failure | ≥3 years | +Randomized +Placebo +Double blinded +Cross-over | 6–12 months | Autologous MSCs from BM IL and IV × 1–2 | 16 IT & 16 IV | 16 | No SAR | Fewer patients with treatment failure and more patients with NEDA in MSC group |
| Uccelli, Italy 2021 [128] | RRMS/SPMS/PPMS EDSS 2.5–6.5 Treatment failure NR | 2–15 years | +Randomized +Placebo +Double blinded +Cross-over | 24–48 weeks | Autologous MSCs from BM IV × 1 | 144 | 144 | No SAR | No significant effect |
| Uncontrolled studies | |||||||||
| Bonab, Iran 2007 [140] | Type MS NS EDSS ≤ 6 Treatment failure | NS | - | 12 months | Autologous MSCs from BM IT × 1–2 | 10 | - | Two iatrogenic meningitis | One EDSS improvement, stabile in four and worsening in five |
| Yamout, Lebanon 2010 [7] | MS type NS EDSS 4–7.5 Treatment failure | NS | - | 12 months | Autologous MSCs from BM IT × 1 | 10 | - | One transient encephalopathy with seizures | Five EDSS improvement, stabile in one and worsening in one |
| Bonab, Iran 2012 [8] | SPMS/PPMS EDSS 3.5–7 Treatment failure | 2–15 years | - | 12 months | Autologous MSCs from BM IT × 1 | 25 | - | No SAR reported | Four EDSS improvement, stabile in 12 and worsening in six |
| Connick, UK 2012 [9] | MS type not specified EDSS 2–6.5 Treatment failure NR | NS | - | 6 months | Autologous MSCs from BM IV × 1 | 10 | - | No SAR reported | Improved visual acuity and VEP |
| Odinak, Russia 2012 [10] | MS type and EDSS NS Treatment failure | NS | - | 12 months | Autologous MSCs from BM IV × 4–8 | 8 | - | No SAR reported | Six EDSS improvement, stabile in one and worsening in one |
| Harris, USA 2016 [11] | SPMS/PPMS EDSS ≥ 3 Treatment failure NS | NS | - | Mean 7.4 years | Autologous MSCs from BM (differentiated in neural direction) IT × 2–5 | 6 | - | No SAR reported | Four EDSS stable, worsening in two |
| Dahbour, Jordan 2017 [141] | MS type & EDSS NS Treatment failure | NS | - | 12 months | Autologous MSCs from BM IT × 2 | 10 | - | No SAR reported | Two EDSS improvement, stabile in four and worsening in four |
| Cohen, USA 2018 [142] | RRMS/SPMS EDSS 3–6.5 Treatment failure NR | NS | - | 6 months | Autologous MSCs from BM IV × 1 | 24 | - | No SAR reported | 17 EDSS improvement, stabile in 8 |
| Harris, USA 2018 [127] | SPMS/PPMS EDSS ≥ 3 Treatment failure NS | NS | - | 12 months | Autologous MSCs from BM (differentiated in neural direction) IT × 3 | 20 | - | No SAR reported | Eight EDSS improvement, stabile in ten and worsening in two |
| Riordan, Panama 2018 [143] | MS type NS EDSS 2–7 Treatment failure NR | NS | - | 12 months | Allogeneic MSCs from umbilical cord IV × 7 | 20 | - | No SAR reported | Mean reduction of 0.68 in EDSS score |
| Sahraian, Iran 2018 [144] | RRMS/SPMS EDSS ≤ 5.5 Treatment failure | 2–15 years | - | 2 years | Autologous MSCs from BM IT × 1–2 | 6 | - | One EDSS improvement, stabile in two and worsening in three | |
| Iacobeus, Sweden 2019 [145] | RRMS/SPMS/PPMS EDSS 3–7 Treatment failure | 2–20 years | - | 48 weeks | Autologous MSCs from BM IV × 1 | 7 | - | No SAR reported | No significant changes in EDSS, one patient discontinued due to relapse |
| Cohen, USA 2023 [135] | PPMS EDSS 3–6.5 Treatment failure NR | NS | - | 28 weeks | Pre-modified MSCs from BM IT × 3 | 18 | - | Two arachnoiditis (one patient discontinued) | 3 EDSS improvement, rates stable/worsening not reported |
| Center (NCT-Number) | Important Inclusion Criteria | Timing of MSC Treatment | Design and Blinding | Primary Endpoint | Follow-Up Time | Type of MSC & Administration | N Patients | Estimated Study Completion |
|---|---|---|---|---|---|---|---|---|
| Bergen, Norway (NCT0474966) | SPMS/PPMS EDSS4-7 Treatment failure NR | 2–15 years | +Randomized +Placebo +Double blinded +Cross-over | Neurophysiological parameters | 6 months | Autologous BM-derived MSCs IT × 1 | 18 | 2025 |
| St.John Antigua and Barbuda (NCT05003388) | MS | NS | −Randomized −Placebo −Blinded | Safety | 4 years | Allogeneic MSCs from umbilical cord IV × 1 | 15 | 2025 |
| Texas, United States (NCT04956744) | RRMS EDSS 3–6.5 Treatment failure | >6 months | +Randomized +Placebo +Double blinded | Quality of life | 1 year | Autologous adipose-derived MSCs IV × 6 | 30 | 2023 |
| Atlanta, United States (NCT04956744) | MS Treatment failure | NS | −Randomized −Placebo −Blinded | Safety | 60 months | Allogeneic embryonic MSCs IV × 1 | 30 | 2027 |
| Taichung, Taiwan (NCT05532943) | RRMS/SPMS EDSS 2–6.5 Treatment failure | 2–15 years | −Randomized −Placebo −Blinded (second part with control group) | First, part safety, second part efficacy | 1 year | Allogeneic umbilical cord MSCs IV and IT | 41 | 2026 |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSC | Mesenchymal Stem Cell |
| CNS | Central Nervous System |
| MS | Multiple Sclerosis |
| NGF | Nerve Growth Factor |
| BDNF | Brain-Derived Neurotrophic Factor |
| HGF | Hepatocyte Growth Factor |
| VEGF | Vascular Endothelial Growth Factor |
| OPC | Oligodendrocyte Precursor Cell |
| BM | Bone Marrow |
| SHED | Stem cells from Human Exfoliated Deciduous teeth |
| PD-1 | Programmed Death-1 |
| PD-L1 | Programmed Death-Ligand 1 |
| RRMS | Relapsing-Remitting Multiple Sclerosis |
| PPMS | Primary Progressive Multiple Sclerosis |
| SPMS | Secondary Progressive Multiple Sclerosis |
| EPC | Endothelial Progenitor Cell |
| MSC-NP | MSC-derived Neural Progenitor |
| EGF | Epidermal Growth Factor |
| bFGF | basic Fibroblast Growth Factor |
| TNT | Tunneling Nanotube |
| EV | Extracellular Vesicle |
| ROS | Reactive Oxygen Species |
References
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; Rocca, N.L.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Popescu, V.; Wuerfel, J.; Hellwig, K.; Iacobaeus, E.; Jensen, M.B.; García-Domínguez, J.M.; Sousa, L.; Rossi, N.D.; Hupperts, R.; et al. Smouldering multiple sclerosis: The ‘real MS’. Ther. Adv. Neurol. Disord. 2022, 15, 175628642110667. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Blanc, K.L.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, Y.; Liu, J.; Lan, Z.; Tang, X.; Hu, Z. The Efficacy of Mesenchymal Stem Cell Therapies in Rodent Models of Multiple Sclerosis: An Updated Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 711362. [Google Scholar] [CrossRef]
- Yamout, B.; Hourani, R.; Salti, H.; Barada, W.; El-Hajj, T.; Al-Kutoubi, A.; Herlopian, A.; Baz, E.K.; Mahfouz, R.; Khalil-Hamdan, R.; et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: A pilot study. J. Neuroimmunol. 2010, 227, 185–189. [Google Scholar] [CrossRef]
- Bonab, M.M.; Sahraian, M.A.; Aghsaie, A.; Karvigh, S.A.; Hosseinian, S.M.; Nikbin, B.; Lotfi, J.; Khorramnia, S.; Motamed, M.R.; Togha, M.; et al. Autologous Mesenchymal Stem Cell Therapy in Progressive Multiple Sclerosis: An Open Label Study. Curr. Stem Cell Res. Ther. 2012, 7, 407–414. [Google Scholar] [CrossRef]
- Connick, P.; Kolappan, M.; Crawley, C.; Webber, D.J.; Patani, R.; Michell, A.W.; Du, M.Q.; Luan, S.L.; Altmann, D.R.; Thompson, A.J.; et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012, 11, 150–156. [Google Scholar] [CrossRef]
- Odinak, M.M.; Bisaga, G.N.; Novitskii, A.V.; Tyrenko, V.V.; Fominykh, M.S.; Bilibina, A.A.; Kruglyakov, P.V.; Polyntsev, D.G. Transplantation of Mesenchymal Stem Cells in Multiple Sclerosis. Neurosci. Behav. Physiol. 2012, 42, 516–520. [Google Scholar] [CrossRef]
- Harris, V.K.; Vyshkina, T.; Sadiq, S.A. Clinical safety of intrathecal administration of mesenchymal stromal cell–derived neural progenitors in multiple sclerosis. Cytotherapy 2016, 18, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Katakowski, M.; Li, Y.; Lu, D.; Wang, L.; Zhang, L.; Chen, J.; Xu, Y.; Gautam, S.; Mahmood, A.; et al. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: Growth factor production. J. Neurosci. Res. 2002, 69, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Crigler, L.; Robey, R.C.; Asawachaicharn, A.; Gaupp, D.; Phinney, D.G. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp. Neurol. 2006, 198, 54–64. [Google Scholar] [CrossRef]
- Martins, L.F.; Costa, R.O.; Pedro, J.R.; Aguiar, P.; Serra, S.C.; Teixeira, F.G.; Sousa, N.; Salgado, A.J.; Almeida, R.D. Mesenchymal stem cells secretome-induced axonal outgrowth is mediated by BDNF. Sci. Rep. 2017, 7, 4153. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Zou, M.; Zhang, J.; Tian, Y.; Wu, F.; Gao, B.; Piao, F. NGF mediates protection of mesenchymal stem cells-conditioned medium against 2, 5-hexanedione-induced apoptosis of VSC4.1 cells via Akt/Bad pathway. Mol. Cell. Biochem. 2020, 469, 53–64. [Google Scholar] [CrossRef]
- Rivera, F.J.; Couillard-Despres, S.; Pedre, X.; Ploetz, S.; Caioni, M.; Lois, C.; Bogdahn, U.; Aigner, L. Mesenchymal Stem Cells Instruct Oligodendrogenic Fate Decision on Adult Neural Stem Cells. Stem Cells 2006, 24, 2209–2219. [Google Scholar] [CrossRef]
- Rivera, F.J.; Kandasamy, M.; Couillard-Despres, S.; Caioni, M.; Sanchez, R.; Huber, C.; Weidner, N.; Bogdahn, U.; Aigner, L. Oligodendrogenesis of adult neural progenitors: Differential effects of ciliary neurotrophic factor and mesenchymal stem cell derived factors. J. Neurochem. 2008, 107, 832–843. [Google Scholar] [CrossRef]
- Zappia, E.; Casazza, S.; Pedemonte, E.; Benvenuto, F.; Bonanni, I.; Gerdoni, E.; Giunti, D.; Ceravolo, A.; Cazzanti, F.; Frassoni, F.; et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005, 106, 1755–1761. [Google Scholar] [CrossRef]
- Agrelo, I.S.; Schira-Heinen, J.; Beyer, F.; Groh, J.; Bütermann, C.; Estrada, V.; Poschmann, G.; Bribian, A.; Jadasz, J.J.; Lopez-Mascaraque, L.; et al. Secretome Analysis of Mesenchymal Stem Cell Factors Fostering Oligodendroglial Differentiation of Neural Stem Cells In Vivo. Int. J. Mol. Sci. 2020, 21, 4350. [Google Scholar] [CrossRef]
- Pinho, A.G.; Cibrão, J.R.; Silva, N.A.; Monteiro, S.; Salgado, A.J. Cell Secretome: Basic Insights and Therapeutic Opportunities for CNS Disorders. Pharmaceuticals 2020, 13, 31. [Google Scholar] [CrossRef]
- Shimojima, C.; Takeuchi, H.; Jin, S.; Parajuli, B.; Hattori, H.; Suzumura, A.; Hibi, H.; Ueda, M.; Yamamoto, A. Conditioned Medium from the Stem Cells of Human Exfoliated Deciduous Teeth Ameliorates Experimental Autoimmune Encephalomyelitis. J. Immunol. 2016, 196, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.K.; Wollowitz, J.; Greenwald, J.; Carlson, A.L.; Sadiq, S.A. Mesenchymal stem cell-neural progenitors are enriched in cell signaling molecules implicated in their therapeutic effect in multiple sclerosis. PLoS ONE 2023, 18, e0290069. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Caplan, A.; Lennon, D.; Miller, R.H. Human Mesenchymal Stem Cells Signals Regulate Neural Stem Cell Fate. Neurochem. Res. 2006, 32, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Lennon, D.P.; Eaton, V.; Maier, K.; Caplan, A.I.; Miller, S.D.; Miller, R.H. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia 2009, 57, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef]
- Jakl, V.; Ehmele, M.; Winkelmann, M.; Ehrenberg, S.; Eiseler, T.; Friemert, B.; Rojewski, M.T.; Schrezenmeier, H. A novel approach for large-scale manufacturing of small extracellular vesicles from bone marrow-derived mesenchymal stromal cells using a hollow fiber bioreactor. Front. Bioeng. Biotechnol. 2023, 11, 1107055. [Google Scholar] [CrossRef]
- Giovannelli, L.; Bari, E.; Jommi, C.; Tartara, F.; Armocida, D.; Garbossa, D.; Cofano, F.; Torre, M.L.; Segale, L. Mesenchymal stem cell secretome and extracellular vesicles for neurodegenerative diseases: Risk-benefit profile and next steps for the market access. Bioact. Mater. 2023, 29, 16–35. [Google Scholar] [CrossRef]
- Li, Z.; Liu, F.; He, X.; Yang, X.; Shan, F.; Feng, J. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int. Immunopharmacol. 2019, 67, 268–280. [Google Scholar] [CrossRef]
- Fathollahi, A.; Hashemi, S.M.; Haji Molla Hoseini, M.; Tavakoli, S.; Farahani, E.; Yeganeh, F. Intranasal administration of small extracellular vesicles derived from mesenchymal stem cells ameliorated the experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2021, 90, 107207. [Google Scholar] [CrossRef]
- Stidworthy, M.F.; Genoud, S.; Suter, U.; Mantei, N.; Franklin, R.J.M. Quantifying the Early Stages of Remyelination Following Cuprizone-induced Demyelination. Brain Pathol. 2003, 13, 329–339. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; ffrench Constant, C. Regenerating CNS myelin — from mechanisms to experimental medicines. Nat. Rev. Neurosci. 2017, 18, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Hu, X.; Zhu, C.; Wang, D.; Zheng, X.; Liu, Q. Overexpression of CNTF in Mesenchymal Stem Cells reduces demyelination and induces clinical recovery in experimental autoimmune encephalomyelitis mice. J. Neuroimmunol. 2009, 206, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Lennon, D.P.; Caplan, A.I.; DeChant, A.; Hecker, J.; Kranso, J.; Zaremba, A.; Miller, R.H. Hepatocyte growth factor mediates mesenchymal stem cell—Induced recovery in multiple sclerosis models. Nat. Neurosci. 2012, 15, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Brodie, C.; Li, Y.; Zheng, X.; Roberts, C.; Lu, M.; Gao, Q.; Borneman, J.; Savant-Bhonsale, S.; Elias, S.B.; et al. Bone marrow stromal cell therapy reduces proNGF and p75 expression in mice with experimental autoimmune encephalomyelitis. J. Neurol. Sci. 2009, 279, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Harris, V.K.; Zigelbaum, A.; Goossens, A.M.; Lu, A.; Rosenthal, H.; Sadiq, S.A. Mesenchymal Stem Cells Enhance the Engraftment and Myelinating Ability of Allogeneic Oligodendrocyte Progenitors in Dysmyelinated Mice. Stem Cells Dev. 2011, 20, 2065–2076. [Google Scholar] [CrossRef]
- Rivera, F.J.; Aigner, L. Adult mesenchymal stem cell therapy for myelin repair in Multiple Sclerosis. Biol. Res. 2012, 45, 257–268. [Google Scholar] [CrossRef]
- Maciak, K.; Dziedzic, A.; Saluk, J. Remyelination in multiple sclerosis from the miRNA perspective. Front. Mol. Neurosci. 2023, 16, 1199313. [Google Scholar] [CrossRef]
- Fan, J.; Han, Y.; Sun, H.; Sun, S.; Wang, Y.; Guo, R.; Guo, J.; Tian, X.; Wang, J.; Wang, J. Mesenchymal stem cell-derived exosomal microRNA-367–3p alleviates experimental autoimmune encephalomyelitis via inhibition of microglial ferroptosis by targeting EZH2. Biomed. Pharmacother. 2023, 162, 114593. [Google Scholar] [CrossRef]
- Xiao, Y.; Geng, F.; Wang, G.; Li, X.; Zhu, J.; Zhu, W. Bone marrow—Derived mesenchymal stem cells—Derived exosomes prevent oligodendrocyte apoptosis through exosomal miR-134 by targeting caspase-8. J. Cell. Biochem. 2018, 120, 2109–2118. [Google Scholar] [CrossRef]
- Shiri, E.; Pasbakhsh, P.; Borhani-Haghighi, M.; Alizadeh, Z.; Nekoonam, S.; Mojaverrostami, S.; Mahabadi, V.P.; Mehdi, A.; Zibara, K.; Kashani, I.R. Mesenchymal Stem Cells Ameliorate Cuprizone-Induced Demyelination by Targeting Oxidative Stress and Mitochondrial Dysfunction. Cell. Mol. Neurobiol. 2020, 41, 1467–1481. [Google Scholar] [CrossRef]
- Kråkenes, T.; Wergeland, S.; Al-Sharabi, N.; Mohamed-Ahmed, S.; Fromreide, S.; Costea, D.E.; Mustafa, K.; Bø, L.; Kvistad, C.E. The neuroprotective potential of mesenchymal stem cells from bone marrow and human exfoliated deciduous teeth in a murine model of demyelination. PLoS ONE 2023, 18, e0293908. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.D.; Smith, M.D.; Kirby, L.A.; Baxi, E.G.; Whartenby, K.A. Disparate Effects of Mesenchymal Stem Cells in Experimental Autoimmune Encephalomyelitis and Cuprizone-Induced Demyelination. PLoS ONE 2015, 10, e0139008. [Google Scholar] [CrossRef]
- Nessler, J.; Bénardais, K.; Gudi, V.; Hoffmann, A.; Tejedor, L.S.; Janßen, S.; Prajeeth, C.K.; Baumgärtner, W.; Kavelaars, A.; Heijnen, C.J.; et al. Effects of Murine and Human Bone Marrow-Derived Mesenchymal Stem Cells on Cuprizone Induced Demyelination. PLoS ONE 2013, 8, e69795. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; McMenamin, P.G.; Dando, S.J. CNS infection and immune privilege. Nat. Rev. Neurosci. 2018, 19, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Rustenhoven, J.; Kipnis, J. Brain borders at the central stage of neuroimmunology. Nature 2022, 612, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Kolabas, Z.I.; Kuemmerle, L.B.; Perneczky, R.; Förstera, B.; Ulukaya, S.; Ali, M.; Kapoor, S.; Bartos, L.M.; Büttner, M.; Caliskan, O.S.; et al. Distinct molecular profiles of skull bone marrow in health and neurological disorders. Cell 2023, 186, 3706–3725.e29. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Queralt, M.; Fledrich, R.; Stassart, R.M. Schwann cell functions in peripheral nerve development and repair. Neurobiol. Dis. 2023, 176, 105952. [Google Scholar] [CrossRef]
- Clements, M.P.; Byrne, E.; Camarillo Guerrero, L.F.; Cattin, A.L.; Zakka, L.; Ashraf, A.; Burden, J.J.; Khadayate, S.; Lloyd, A.C.; Marguerat, S.; et al. The Wound Microenvironment Reprograms Schwann Cells to Invasive Mesenchymal-like Cells to Drive Peripheral Nerve Regeneration. Neuron 2017, 96, 98–114.e7. [Google Scholar] [CrossRef]
- Li, J.; Yawno, T.; Sutherland, A.; Loose, J.; Nitsos, I.; Bischof, R.; Castillo-Melendez, M.; McDonald, C.A.; Wong, F.Y.; Jenkin, G.; et al. Preterm white matter brain injury is prevented by early administration of umbilical cord blood cells. Exp. Neurol. 2016, 283, 179–187. [Google Scholar] [CrossRef]
- Matzinger, P. The Danger Model: A Renewed Sense of Self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef]
- Tomchuck, S.L.; Zwezdaryk, K.J.; Coffelt, S.B.; Waterman, R.S.; Danka, E.S.; Scandurro, A.B. Toll-like Receptors on Human Mesenchymal Stem Cells Drive Their Migration and Immunomodulating Responses. Stem Cells 2007, 26, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2019, 53, e12712. [Google Scholar] [CrossRef] [PubMed]
- Haghmorad, D.; Khaleghian, A.; Eslami, M.; Sadeghnejad, A.; Tarahomi, M.; Yousefi, B. Bone marrow mesenchymal stem cells to ameliorate experimental autoimmune encephalomyelitis via modifying expression patterns of miRNAs. Mol. Biol. Rep. 2023, 50, 9971–9984. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, S.; Pène, J.; Torcy-Moquet, G.; Jorgensen, C.; Yssel, H. Mesenchymal Stem Cells Inhibit Human Th17 Cell Differentiation and Function and Induce a T Regulatory Cell Phenotype. J. Immunol. 2010, 185, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Llufriu, S.; Sepúlveda, M.; Blanco, Y.; Marín, P.; Moreno, B.; Berenguer, J.; Gabilondo, I.; Martínez-Heras, E.; Sola-Valls, N.; Arnaiz, J.A.; et al. Randomized Placebo-Controlled Phase II Trial of Autologous Mesenchymal Stem Cells in Multiple Sclerosis. PLoS ONE 2014, 9, e113936. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Deng, Y.; Liang, J.; Huang, F.; Qiu, W.; Zhang, M.; Long, Y.; Hu, X.; Lu, Z.; Liu, W.; et al. Mesenchymal stromal cells attenuate multiple sclerosis via IDO-dependent increasing the suppressive proportion of CD5+ IL-10+ B cells. Am. J. Transl. Res. 2019, 11, 5673–5688. [Google Scholar]
- Karussis, D.; Karageorgiou, C.; Vaknin-Dembinsky, A.; Gowda-Kurkalli, B.; Gomori, J.M.; Kassis, I.; Bulte, J.W.M.; Petrou, P.; Ben-Hur, T.; Abramsky, O.; et al. Safety and Immunological Effects of Mesenchymal Stem Cell Transplantation in Patients with Multiple Sclerosis and Amyotrophic Lateral Sclerosis. Arch. Neurol. 2010, 67, 1187–1194. [Google Scholar] [CrossRef]
- Anderson, P.; Gonzalez-Rey, E.; O’Valle, F.; Martin, F.; Oliver, F.J.; Delgado, M. Allogeneic Adipose-Derived Mesenchymal Stromal Cells Ameliorate Experimental Autoimmune Encephalomyelitis by Regulating Self-Reactive T Cell Responses and Dendritic Cell Function. Stem Cells Int. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Huang, S.; Zhang, Z.; Yuan, X.; Feng, X.; Lu, L.; Sun, L. Serum IFN-γ Predicts the Therapeutic Effect of Mesenchymal Stem Cells Transplantation in Systemic Lupus Erythematosus Patients. Stem Cells Transl. Med. 2017, 6, 1777–1785. [Google Scholar] [CrossRef]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a Pro-Inflammatory MSC1 or an Immunosuppressive MSC2 Phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Heldring, N.; Kadri, N.; Le Blanc, K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells 2016, 35, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System during Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Jian, C.; Liao, Y.; Huang, Q.; Wu, Y.; Liu, X.; Zou, D.; Wu, Y. The role of microglia in multiple sclerosis. Neuropsychiatr. Dis. Treat. 2017, 13, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Wachowicz, B. Advances in Antioxidative Therapy of Multiple Sclerosis. Curr. Med. Chem. 2013, 20, 4720–4730. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 00198. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; Nardo, D.D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-Based Network Analysis Reveals a Spectrum Model of Human Macrophage Activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef]
- Sousa, C.; Golebiewska, A.; Poovathingal, S.K.; Kaoma, T.; Pires-Afonso, Y.; Martina, S.; Coowar, D.; Azuaje, F.; Skupin, A.; Balling, R.; et al. Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep. 2018, 19, e46171. [Google Scholar] [CrossRef]
- Olah, M.; Menon, V.; Habib, N.; Taga, M.F.; Ma, Y.; Yung, C.J.; Cimpean, M.; Khairallah, A.; Coronas-Samano, G.; Sankowski, R.; et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 2020, 11, 6129. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Yan, K.; Chen, F.; Huang, W.; Lv, B.; Sun, C.; Xu, L.; Li, F.; Jiang, X. Mesenchymal stem cells inhibit lipopolysaccharide-induced inflammatory responses of BV2 microglial cells through TSG-6. J. Neuroinflammation 2014, 11, 135. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, R.; Wang, Y.; Huang, W.; Hu, B.; Zhu, G.; Zhang, R.; Li, F.; Han, J.; Li, Y. Mesenchymal stem cells enhance microglia M2 polarization and attenuate neuroinflammation through TSG-6. Brain Res. 2019, 1724, 146422. [Google Scholar] [CrossRef] [PubMed]
- Barati, S.; Kashani, I.R.; Tahmasebi, F.; Mehrabi, S.; Joghataei, M.T. Effect of mesenchymal stem cells on glial cells population in cuprizone induced demyelination model. Neuropeptides 2019, 75, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Neubrand, V.E.; Pedreño, M.; Caro, M.; Forte-Lago, I.; Delgado, M.; Gonzalez-Rey, E. Mesenchymal stem cells induce the ramification of microglia via the small RhoGTPases Cdc42 and Rac1. Glia 2014, 62, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Sackstein, R.; Merzaban, J.S.; Cain, D.W.; Dagia, N.M.; Spencer, J.A.; Lin, C.P.; Wohlgemuth, R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 2008, 14, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Rüster, B.; Göttig, S.; Ludwig, R.J.; Bistrian, R.; Müller, S.; Seifried, E.; Gille, J.; Henschler, R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 2006, 108, 3938–3944. [Google Scholar] [CrossRef] [PubMed]
- Suila, H.; Hirvonen, T.; Kotovuori, A.; Ritamo, I.; Kerkelä, E.; Anderson, H.; Natunen, S.; Tuimala, J.; Laitinen, S.; Nystedt, J.; et al. Human Umbilical Cord Blood-Derived Mesenchymal Stromal Cells Display a Novel Interaction between P-Selectin and Galectin-1. Scand. J. Immunol. 2014, 80, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.M.; Lawrence, M.B.; Shang, H.; Katz, A.J.; Peirce, S.M. Agent-Based Model of Therapeutic Adipose-Derived Stromal Cell Trafficking during Ischemia Predicts Ability To Roll on P-Selectin. PLoS Comput. Biol. 2009, 5, e1000294. [Google Scholar] [CrossRef] [PubMed]
- Conaty, P.; Sherman, L.S.; Naaldijk, Y.; Ulrich, H.; Stolzing, A.; Rameshwar, P. Methods of Mesenchymal Stem Cell Homing to the Blood—Brain Barrier. In Methods in Molecular Biology; Springer: New York, NY, USA, 2018; pp. 81–91. [Google Scholar] [CrossRef]
- François, S.; Bensidhoum, M.; Mouiseddine, M.; Mazurier, C.; Allenet, B.; Semont, A.; Frick, J.; Saché, A.; Bouchet, S.; Thierry, D.; et al. Local Irradiation Not Only Induces Homing of Human Mesenchymal Stem Cells at Exposed Sites but Promotes Their Widespread Engraftment to Multiple Organs: A Study of Their Quantitative Distribution after Irradiation Damage. Stem Cells 2006, 24, 1020–1029. [Google Scholar] [CrossRef]
- Wynn, R.F.; Hart, C.A.; Corradi-Perini, C.; O’Neill, L.; Evans, C.A.; Wraith, J.E.; Fairbairn, L.J.; Bellantuono, I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 2004, 104, 2643–2645. [Google Scholar] [CrossRef]
- Bobis-Wozowicz, S.; Miekus, K.; Wybieralska, E.; Jarocha, D.; Zawisz, A.; Madeja, Z.; Majka, M. Genetically modified adipose tissue—derived mesenchymal stem cells overexpressing CXCR4 display increased motility, invasiveness, and homing to bone marrow of NOD/SCID mice. Exp. Hematol. 2011, 39, 686–696.e4. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhou, F.; He, D.; Zhang, L.; Shen, J. Overexpression of CXCR7 promotes mesenchymal stem cells to repair phosgene-induced acute lung injury in rats. Biomed. Pharmacother. 2019, 109, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Segers, V.F.M.; Van Riet, I.; Andries, L.J.; Lemmens, K.; Demolder, M.J.; De Becker, A.J.M.L.; Kockx, M.M.; De Keulenaer, G.W. Mesenchymal stem cell adhesion to cardiac microvascular endothelium: Activators and mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1370–H1377. [Google Scholar] [CrossRef] [PubMed]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F.; et al. Role for Interferon-γ in the Immunomodulatory Activity of Human Bone Marrow Mesenchymal Stem Cells. Stem Cells 2005, 24, 386–398. [Google Scholar] [CrossRef]
- Krampera, M.; Pasini, A.; Rigo, A.; Scupoli, M.T.; Tecchio, C.; Malpeli, G.; Scarpa, A.; Dazzi, F.; Pizzolo, G.; Vinante, F. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: Inducing cell expansion and reversibly preventing multilineage differentiation. Blood 2005, 106, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.; Egea, V.; Karow, M.; Kolb, H.; Jochum, M.; Neth, P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: Differential regulation by inflammatory cytokines. Blood 2007, 109, 4055–4063. [Google Scholar] [CrossRef] [PubMed]
- De Becker, A.; Van Hummelen, P.; Bakkus, M.; Vande Broek, I.; De Wever, J.; De Waele, M.; Van Riet, I. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica 2007, 92, 440–449. [Google Scholar] [CrossRef]
- Ponte, A.L.; Marais, E.; Gallay, N.; Langonné, A.; Delorme, B.; Hérault, O.; Charbord, P.; Domenech, J. The In Vitro Migration Capacity of Human Bone Marrow Mesenchymal Stem Cells: Comparison of Chemokine and Growth Factor Chemotactic Activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [CrossRef]
- Bayo, J.; Real, A.; Fiore, E.J.; Malvicini, M.; Sganga, L.; Bolontrade, M.; Andriani, O.; Bizama, C.; Fresno, C.; Podhajcer, O.; et al. IL-8,GRO and MCP-1 produced by hepatocellular carcinoma microenvironment determine the migratory capacity of human bone marrow-derived mesenchymal stromal cells without affecting tumor aggressiveness. Oncotarget 2016, 8, 80235–80248. [Google Scholar] [CrossRef]
- Rafei, M.; Campeau, P.M.; Aguilar-Mahecha, A.; Buchanan, M.; Williams, P.; Birman, E.; Yuan, S.; Young, Y.K.; Boivin, M.N.; Forner, K.; et al. Mesenchymal Stromal Cells Ameliorate Experimental Autoimmune Encephalomyelitis by Inhibiting CD4 Th17 T Cells in a CC Chemokine Ligand 2-Dependent Manner. J. Immunol. 2009, 182, 5994–6002. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Jia, T.; Mendez-Ferrer, S.; Hohl, T.; Serbina, N.; Lipuma, L.; Leiner, I.; Li, M.; Frenette, P.; Pamer, E. Bone Marrow Mesenchymal Stem and Progenitor Cells Induce Monocyte Emigration in Response to Circulating Toll-like Receptor Ligands. Immunity 2011, 34, 590–601. [Google Scholar] [CrossRef]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S. Pulmonary Passage is a Major Obstacle for Intravenous Stem Cell Delivery: The Pulmonary First-Pass Effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Tarasiuk, O.; Ballarini, E.; Donzelli, E.; Rodriguez-Menendez, V.; Bossi, M.; Cavaletti, G.; Scuteri, A. Making Connections: Mesenchymal Stem Cells Manifold Ways to Interact with Neurons. Int. J. Mol. Sci. 2022, 23, 5791. [Google Scholar] [CrossRef] [PubMed]
- Khattar, K.E.; Safi, J.; Rodriguez, A.M.; Vignais, M.L. Intercellular Communication in the Brain through Tunneling Nanotubes. Cancers 2022, 14, 1207. [Google Scholar] [CrossRef] [PubMed]
- Polyakova, N.; Kalashnikova, M.; Belyavsky, A. Non-Classical Intercellular Communications: Basic Mechanisms and Roles in Biology and Medicine. Int. J. Mol. Sci. 2023, 24, 6455. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.L.; Eng, S.P.; Hafez, P.; Karim, N.A.; Law, J.X.; Ng, M.H. Mesenchymal Stromal Cell Mitochondrial Transfer as a Cell Rescue Strategy in Regenerative Medicine: A Review of Evidence in Preclinical Models. Stem Cells Transl. Med. 2022, 11, 814–827. [Google Scholar] [CrossRef]
- Jackson, M.V.; Morrison, T.J.; Doherty, D.F.; McAuley, D.F.; Matthay, M.A.; Kissenpfennig, A.; O’Kane, C.M.; Krasnodembskaya, A.D. Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells 2016, 34, 2210–2223. [Google Scholar] [CrossRef]
- Liu, K.; Ji, K.; Guo, L.; Wu, W.; Lu, H.; Shan, P.; Yan, C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia—Reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 2014, 92, 10–18. [Google Scholar] [CrossRef]
- Liu, K.; Guo, L.; Zhou, Z.; Pan, M.; Yan, C. Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc. Res. 2019, 123, 74–80. [Google Scholar] [CrossRef]
- Nakhle, J.; Khattar, K.; Özkan, T.; Boughlita, A.; Moussa, D.A.; Darlix, A.; Lorcy, F.; Rigau, V.; Bauchet, L.; Gerbal-Chaloin, S.; et al. Mitochondria Transfer from Mesenchymal Stem Cells Confers Chemoresistance to Glioblastoma Stem Cells through Metabolic Rewiring. Cancer Res. Commun. 2023, 3, 1041–1056. [Google Scholar] [CrossRef] [PubMed]
- Boukelmoune, N.; Chiu, G.S.; Kavelaars, A.; Heijnen, C.J. Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin. Acta Neuropathol. Commun. 2018, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.; Ziabreva, I.; Reeve, A.K.; Krishnan, K.J.; Reynolds, R.; Howell, O.; Lassmann, H.; Turnbull, D.M.; Mahad, D.J. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann. Neurol. 2010, 69, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Zierfuss, B.; Wang, Z.; Jackson, A.N.; Moezzi, D.; Yong, V. Iron in multiple sclerosis—Neuropathology, immunology, and real-world considerations. Mult. Scler. Relat. Disord. 2023, 78, 104934. [Google Scholar] [CrossRef]
- Khattar, N.; Triebswetter, C.; Kiely, M.; Ferrucci, L.; Resnick, S.M.; Spencer, R.G.; Bouhrara, M. Investigation of the association between cerebral iron content and myelin content in normative aging using quantitative magnetic resonance neuroimaging. NeuroImage 2021, 239, 118267. [Google Scholar] [CrossRef]
- Weber, C.E.; Wittayer, M.; Kraemer, M.; Dabringhaus, A.; Bail, K.; Platten, M.; Schirmer, L.; Gass, A.; Eisele, P. Long-term dynamics of multiple sclerosis iron rim lesions. Mult. Scler. Relat. Disord. 2022, 57, 103340. [Google Scholar] [CrossRef]
- Van San, E.; Debruyne, A.C.; Veeckmans, G.; Tyurina, Y.Y.; Tyurin, V.A.; Zheng, H.; Choi, S.M.; Augustyns, K.; van Loo, G.; Michalke, B.; et al. Ferroptosis contributes to multiple sclerosis and its pharmacological targeting suppresses experimental disease progression. Cell Death Differ. 2023, 30, 2092–2103. [Google Scholar] [CrossRef]
- Luoqian, J.; Yang, W.; Ding, X.; Tuo, Q.Z.; Xiang, Z.; Zheng, Z.; Guo, Y.J.; Li, L.; Guan, P.; Ayton, S.; et al. Ferroptosis promotes T-cell activation-induced neurodegeneration in multiple sclerosis. Cell. Mol. Immunol. 2022, 19, 913–924. [Google Scholar] [CrossRef]
- Jhelum, P.; Santos-Nogueira, E.; Teo, W.; Haumont, A.; Lenoël, I.; Stys, P.K.; David, S. Ferroptosis Mediates Cuprizone-Induced Loss of Oligodendrocytes and Demyelination. J. Neurosci. 2020, 40, 9327–9341. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Pang, M.; Wang, Y.; Wang, X.; Lin, Y.; Lv, Y.; Xie, Z.; Hou, J.; Du, C.; Qiu, Y.; et al. Mesenchymal stem cell attenuates spinal cord injury by inhibiting mitochondrial quality control-associated neuronal ferroptosis. Redox Biol. 2023, 67, 102871. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.K.; Zelic, M.; Han, Y.; Teeple, E.; Chen, L.; Sadeghi, M.; Shankara, S.; Guo, L.; Li, C.; Pontarelli, F.; et al. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci. 2022, 26, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Bueno, C.; Martínez-Morga, M.; García-Bernal, D.; Moraleda, J.M.; Martínez, S. Differentiation of human adult-derived stem cells towards a neural lineage involves a dedifferentiation event prior to differentiation to neural phenotypes. Sci. Rep. 2021, 11, 12034. [Google Scholar] [CrossRef]
- Bae, K.S.; Park, J.B.; Kim, H.S.; Kim, D.S.; Park, D.J.; Kang, S.J. Neuron-like Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells. Yonsei Med. J. 2011, 52, 401. [Google Scholar] [CrossRef]
- Hernández, R.; Jiménez-Luna, C.; Perales-Adán, J.; Perazzoli, G.; Melguizo, C.; Prados, J. Differentiation of Human Mesenchymal Stem Cells towards Neuronal Lineage: Clinical Trials in Nervous System Disorders. Biomol. Ther. 2020, 28, 34–44. [Google Scholar] [CrossRef]
- Ren, C.; Yin, P.; Ren, N.; Wang, Z.; Wang, J.; Zhang, C.; Ge, W.; Geng, D.; Wang, X. Cerebrospinal fluid-stem cell interactions may pave the path for cell-based therapy in neurological diseases. Stem Cell Res. Ther. 2018, 9, 66. [Google Scholar] [CrossRef]
- Gordon, D.; Pavlovska, G.; Uney, J.B.; Wraith, D.C.; Scolding, N.J. Human Mesenchymal Stem Cells Infiltrate the Spinal Cord, Reduce Demyelination, and Localize to White Matter Lesions in Experimental Autoimmune Encephalomyelitis. J. Neuropathol. Exp. Neurol. 2010, 69, 1087–1095. [Google Scholar] [CrossRef]
- Yan, L.; Jiang, B.; Niu, Y.; Wang, H.; Li, E.; Yan, Y.; Sun, H.; Duan, Y.; Chang, S.; Chen, G.; et al. Intrathecal delivery of human ESC-derived mesenchymal stem cell spheres promotes recovery of a primate multiple sclerosis model. Cell Death Discov. 2018, 4, 89. [Google Scholar] [CrossRef]
- Schepici, G.; Gugliandolo, A.; Mazzon, E. Mesenchymal Stromal Cells Preconditioning: A New Strategy to Improve Neuroprotective Properties. Int. J. Mol. Sci. 2022, 23, 2088. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell. Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Thangavelu, S.R.; Diomede, F.; Bramanti, P.; Conti, P.; Trubiani, O.; Mazzon, E. Anti-inflammatory effects of hypoxia-preconditioned human periodontal ligament cell secretome in an experimental model of multiple sclerosis: A key role of IL-37. FASEB J. 2017, 31, 5592–5608. [Google Scholar] [CrossRef] [PubMed]
- Paquet, J.; Deschepper, M.; Moya, A.; Logeart-Avramoglou, D.; Boisson-Vidal, C.; Petite, H. Oxygen Tension Regulates Human Mesenchymal Stem Cell Paracrine Functions. Stem Cells Transl. Med. 2015, 4, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, X.; Liu, L.; Han, C.; Xie, Z.; Liu, X.; Xu, Y.; Li, F.; Bi, J.; Zheng, C. Transplantation of IFN-γ Primed hUCMSCs Significantly Improved Outcomes of Experimental Autoimmune Encephalomyelitis in a Mouse Model. Neurochem. Res. 2020, 45, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.K.; Yan, Q.J.; Vyshkina, T.; Sahabi, S.; Liu, X.; Sadiq, S.A. Clinical and pathological effects of intrathecal injection of mesenchymal stem cell-derived neural progenitors in an experimental model of multiple sclerosis. J. Neurol. Sci. 2012, 313, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.K.; Stark, J.; Vyshkina, T.; Blackshear, L.; Joo, G.; Stefanova, V.; Sara, G.; Sadiq, S.A. Phase I Trial of Intrathecal Mesenchymal Stem Cell-derived Neural Progenitors in Progressive Multiple Sclerosis. eBioMedicine 2018, 29, 23–30. [Google Scholar] [CrossRef]
- Uccelli, A.; Laroni, A.; Ali, R.; Battaglia, M.A.; Blinkenberg, M.; Brundin, L.; Clanet, M.; Fernandez, O.; Marriott, J.; Muraro, P.; et al. Safety, tolerability, and activity of mesenchymal stem cells versus placebo in multiple sclerosis (MESEMS): A phase 2, randomised, double-blind crossover trial. Lancet Neurol. 2021, 20, 917–929. [Google Scholar] [CrossRef]
- Antebi, B.; Asher, A.M.; Rodriguez, L.A.; Moore, R.K.; Mohammadipoor, A.; Cancio, L.C. Cryopreserved mesenchymal stem cells regain functional potency following a 24-h acclimation period. J. Transl. Med. 2019, 17, 297. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Xue, H.; Liu, L.; Zhu, J.; Jin, T. Autologous Mesenchymal Stem Cell Transplantation in Multiple Sclerosis: A Meta-Analysis. Stem Cells Int. 2019, 2019, 8536785. [Google Scholar] [CrossRef]
- Petrou, P.; Kassis, I.; Levin, N.; Paul, F.; Backner, Y.; Benoliel, T.; Oertel, F.C.; Scheel, M.; Hallimi, M.; Yaghmour, N.; et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain 2020, 143, 3574–3588. [Google Scholar] [CrossRef]
- Petrou, P.; Kassis, I.; Ginzberg, A.; Hallimi, M.; Karussis, D. Effects of Mesenchymal Stem Cell Transplantation on Cerebrospinal Fluid Biomarkers in Progressive Multiple Sclerosis. Stem Cells Transl. Med. 2022, 11, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Petrou, P.; Kassis, I.; Ginzberg, A.; Halimi, M.; Yaghmour, N.; Abramsky, O.; Karussis, D. Long-Term Clinical and Immunological Effects of Repeated Mesenchymal Stem Cell Injections in Patients with Progressive Forms of Multiple Sclerosis. Front. Neurol. 2021, 12, 639315. [Google Scholar] [CrossRef] [PubMed]
- Singer, W.; Dietz, A.B.; Zeller, A.D.; Gehrking, T.L.; Schmelzer, J.D.; Schmeichel, A.M.; Gehrking, J.A.; Suarez, M.D.; Sletten, D.M.; Pacheco, K.V.M.; et al. Intrathecal administration of autologous mesenchymal stem cells in multiple system atrophy. Neurology 2019, 93, e77–e87. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Lublin, F.D.; Lock, C.; Pelletier, D.; Chitnis, T.; Mehra, M.; Gothelf, Y.; Aricha, R.; Lindborg, S.; Lebovits, C.; et al. Evaluation of neurotrophic factor secreting mesenchymal stem cells in progressive multiple sclerosis. Mult. Scler. J. 2022, 29, 92–106. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, D.J.; Geng, T.; Chen, L.; Huang, H.; Yin, H.L.; Zhang, Y.Z.; Lou, J.Y.; Cao, B.; Wang, Y.L. The Potential of Human Umbilical Cord-Derived Mesenchymal Stem Cells as a Novel Cellular Therapy for Multiple Sclerosis. Cell Transplant. 2014, 23, 113–122. [Google Scholar] [CrossRef]
- Lublin, F.D.; Bowen, J.D.; Huddlestone, J.; Kremenchutzky, M.; Carpenter, A.; Corboy, J.R.; Freedman, M.S.; Krupp, L.; Paulo, C.; Hariri, R.J.; et al. Human placenta-derived cells (PDA-001) for the treatment of adults with multiple sclerosis: A randomized, placebo-controlled, multiple-dose study. Mult. Scler. Relat. Disord. 2014, 3, 696–704. [Google Scholar] [CrossRef]
- Meng, M.; Liu, Y.; Wang, W.; Wei, C.; Liu, F.; Du, Z.; Xie, Y.; Tang, W.; Hou, Z.; Li, Q. Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis. Am. J. Transl. Res. 2018, 10, 212–223. [Google Scholar]
- Fernández, O.; Izquierdo, G.; Fernández, V.; Leyva, L.; Reyes, V.; Guerrero, M.; León, A.; Arnaiz, C.; Navarro, G.; Páramo, M.D.; et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE 2018, 13, e0195891. [Google Scholar] [CrossRef]
- Mohyeddin Bonab, M.; Yazdanbakhsh, S.; Lotfi, J.; Alimoghaddom, K.; Talebian, F.; Hooshmand, F.; Ghavamzadeh, A.; Nikbin, B. Does Mesenchymal Stem Cell Therapy Help Multiple Sclerosis Patients? Report of a Pilot Study. Iran. J. Immunol. 2007, 4, 50–57. Available online: https://iji.sums.ac.ir/article_17180_585bec78febcf89c5381a673e3e957e4.pdf (accessed on 8 December 2023).
- Dahbour, S.; Jamali, F.; Alhattab, D.; Al-Radaideh, A.; Ababneh, O.; Al-Ryalat, N.; Al-Bdour, M.; Hourani, B.; Msallam, M.; Rasheed, M.; et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci. Ther. 2017, 23, 866–874. [Google Scholar] [CrossRef]
- Cohen, J.A.; Imrey, P.B.; Planchon, S.M.; Bermel, R.A.; Fisher, E.; Fox, R.J.; Bar-Or, A.; Sharp, S.L.; Skaramagas, T.T.; Jagodnik, P.; et al. Pilot trial of intravenous autologous culture-expanded mesenchymal stem cell transplantation in multiple sclerosis. Mult. Scler. J. 2017, 24, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Riordan, N.H.; Morales, I.; Fernández, G.; Allen, N.; Fearnot, N.E.; Leckrone, M.E.; Markovich, D.J.; Mansfield, D.; Avila, D.; Patel, A.N.; et al. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J. Transl. Med. 2018, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Sahraian, M.A.; Mohyeddin Bonab, M.; Baghbanian, S.M.; Owji, M.; Naser Moghadasi, A. Therapeutic Use of Intrathecal Mesenchymal Stem Cells in patients with Multiple Sclerosis: A Pilot Study with Booster Injection. Immunol. Investig. 2018, 48, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Iacobaeus, E.; Kadri, N.; Lefsihane, K.; Boberg, E.; Gavin, C.; Törnqvist Andrén, A.; Lilja, A.; Brundin, L.; Blanc, K.L. Short and Long Term Clinical and Immunologic Follow up after Bone Marrow Mesenchymal Stromal Cell Therapy in Progressive Multiple Sclerosis—A Phase I Study. J. Clin. Med. 2019, 8, 2102. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavasso, S.; Kråkenes, T.; Olsen, H.; Evjenth, E.C.; Ytterdal, M.; Haugsøen, J.B.; Kvistad, C.E. The Therapeutic Mechanisms of Mesenchymal Stem Cells in MS—A Review Focusing on Neuroprotective Properties. Int. J. Mol. Sci. 2024, 25, 1365. https://doi.org/10.3390/ijms25031365
Gavasso S, Kråkenes T, Olsen H, Evjenth EC, Ytterdal M, Haugsøen JB, Kvistad CE. The Therapeutic Mechanisms of Mesenchymal Stem Cells in MS—A Review Focusing on Neuroprotective Properties. International Journal of Molecular Sciences. 2024; 25(3):1365. https://doi.org/10.3390/ijms25031365
Chicago/Turabian StyleGavasso, Sonia, Torbjørn Kråkenes, Håkon Olsen, Elisabeth Claire Evjenth, Marie Ytterdal, Jonas Bull Haugsøen, and Christopher Elnan Kvistad. 2024. "The Therapeutic Mechanisms of Mesenchymal Stem Cells in MS—A Review Focusing on Neuroprotective Properties" International Journal of Molecular Sciences 25, no. 3: 1365. https://doi.org/10.3390/ijms25031365
APA StyleGavasso, S., Kråkenes, T., Olsen, H., Evjenth, E. C., Ytterdal, M., Haugsøen, J. B., & Kvistad, C. E. (2024). The Therapeutic Mechanisms of Mesenchymal Stem Cells in MS—A Review Focusing on Neuroprotective Properties. International Journal of Molecular Sciences, 25(3), 1365. https://doi.org/10.3390/ijms25031365





