Evolution and Dynamic Transcriptome of Key Genes of Photoperiodic Flowering Pathway in Water Spinach (Ipomoea aquatica)
Abstract
:1. Introduction
2. Results
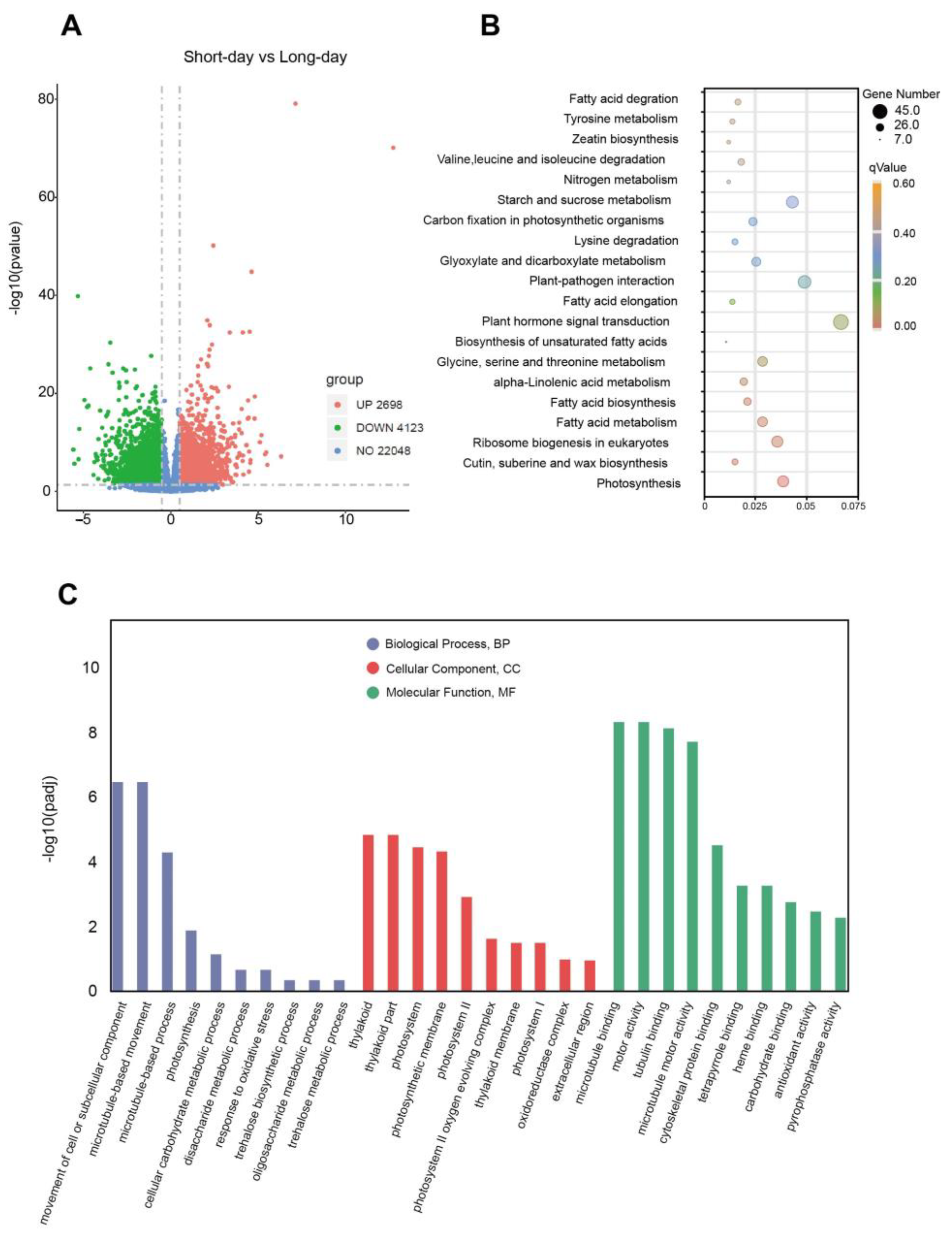
2.1. RNA-Seq Data Statistics
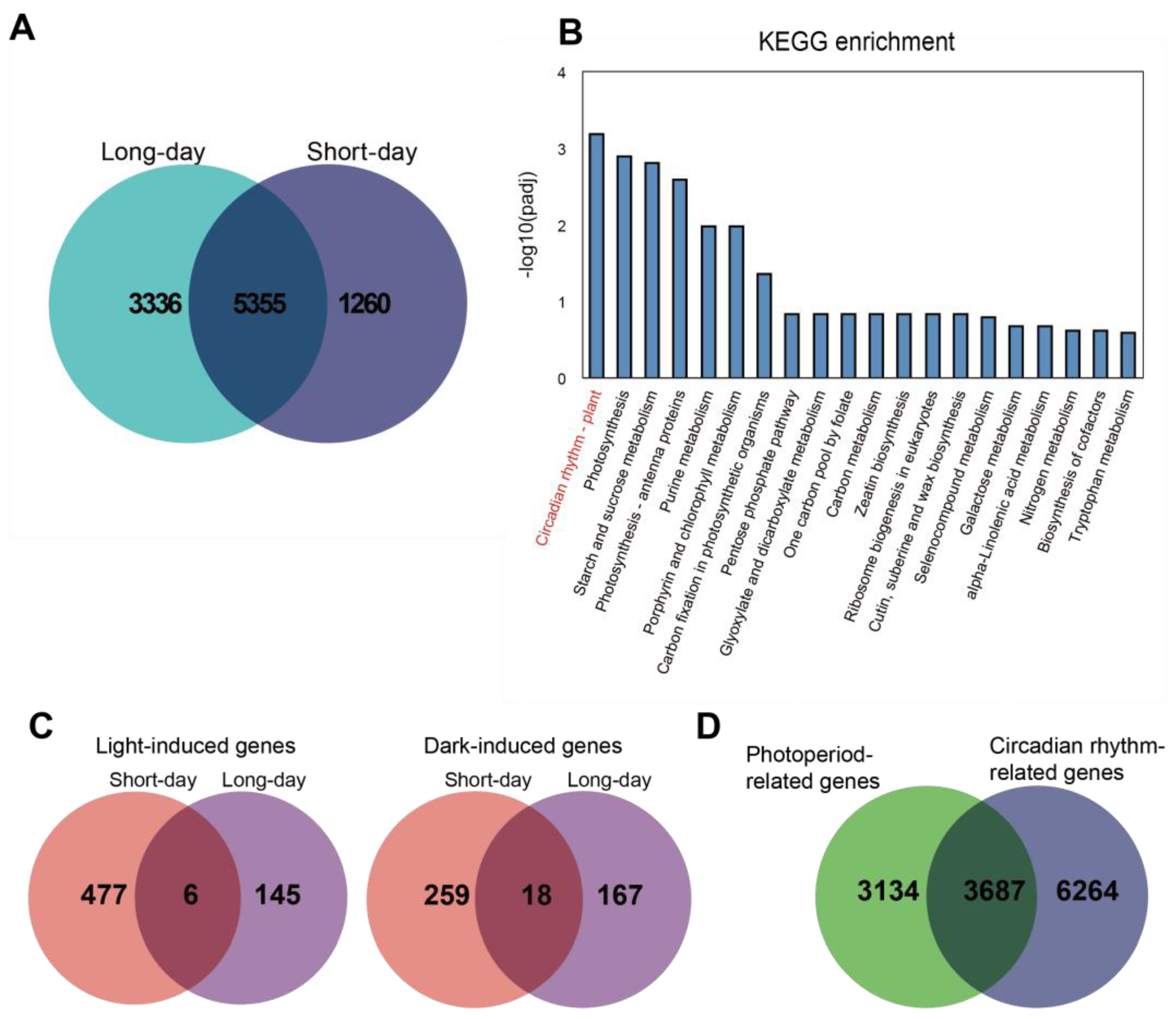
2.2. Analysis of Photoperiod-Related Genes and Circadian-Rhythm-Related Genes
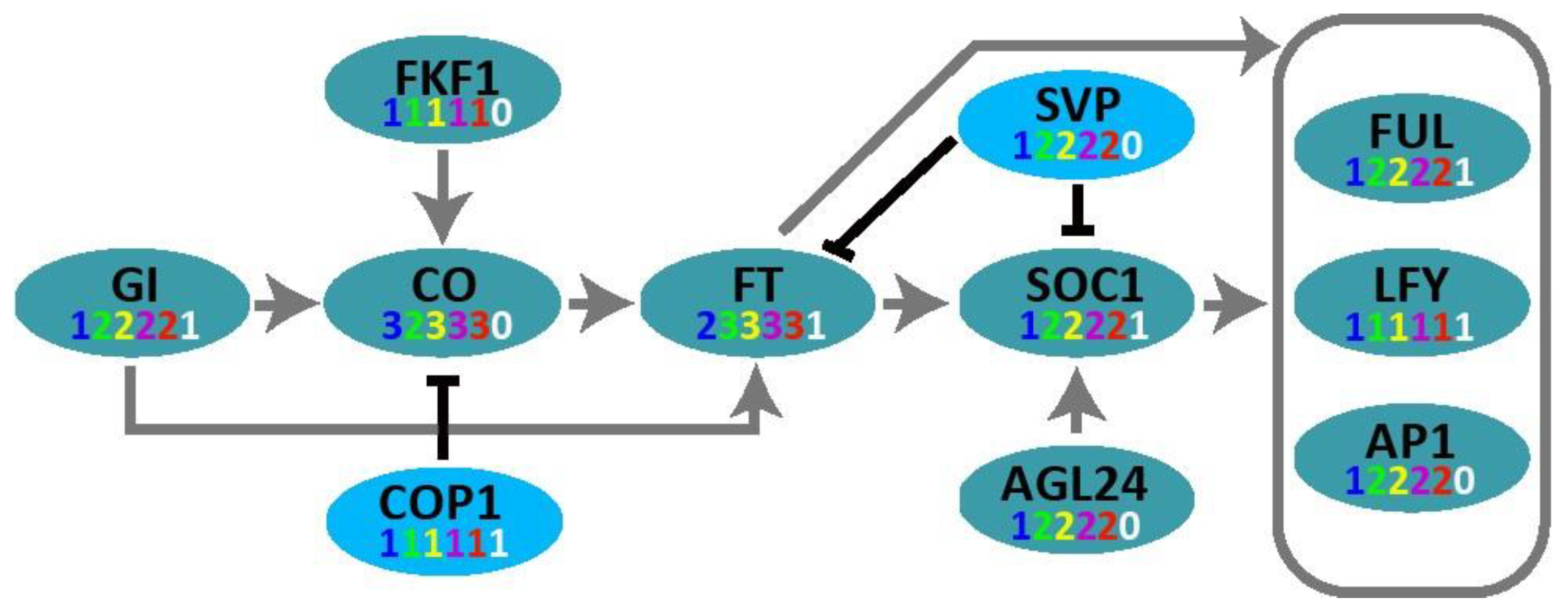
2.3. Molecular Network of Core-Clock Oscillator
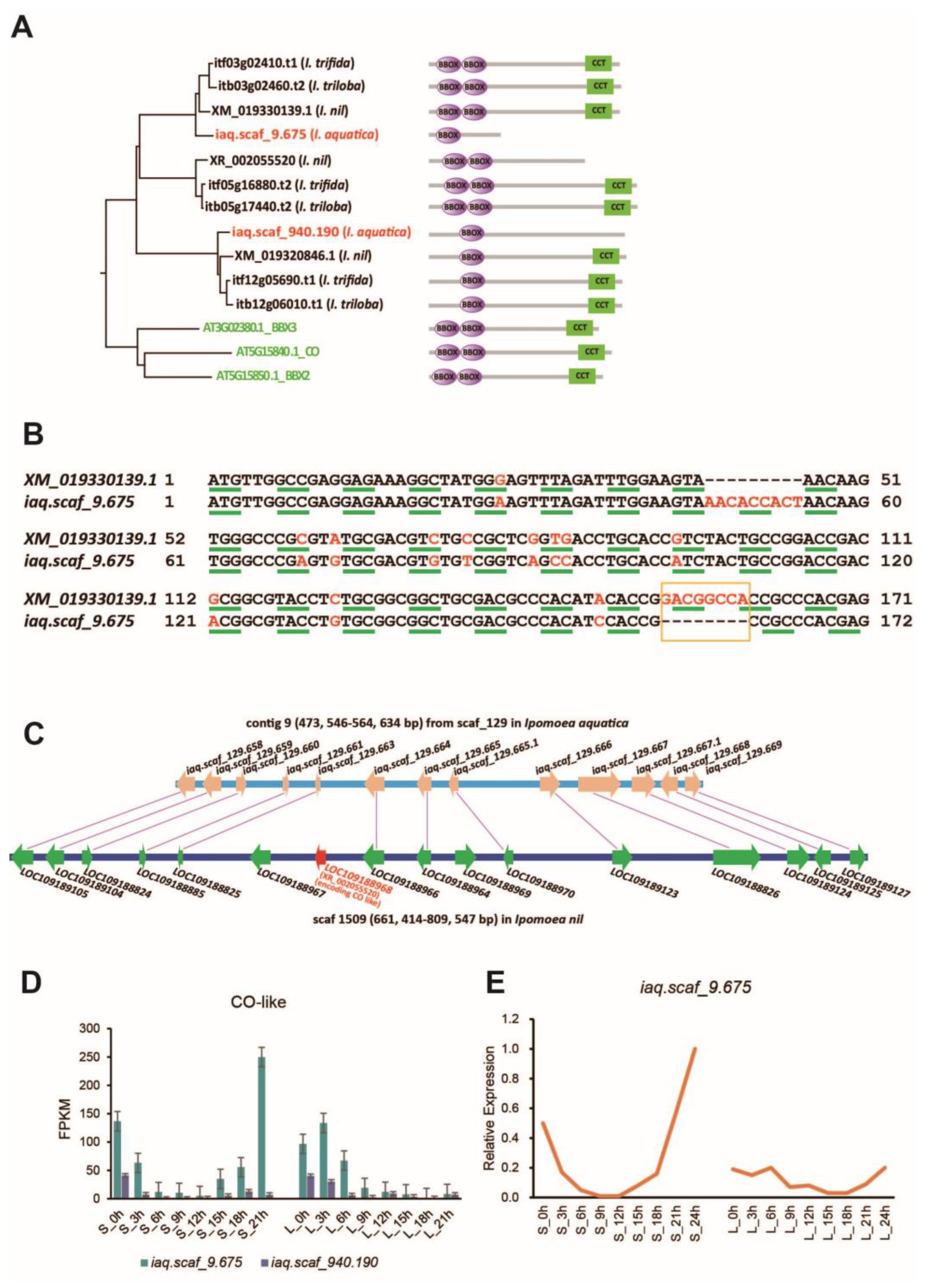
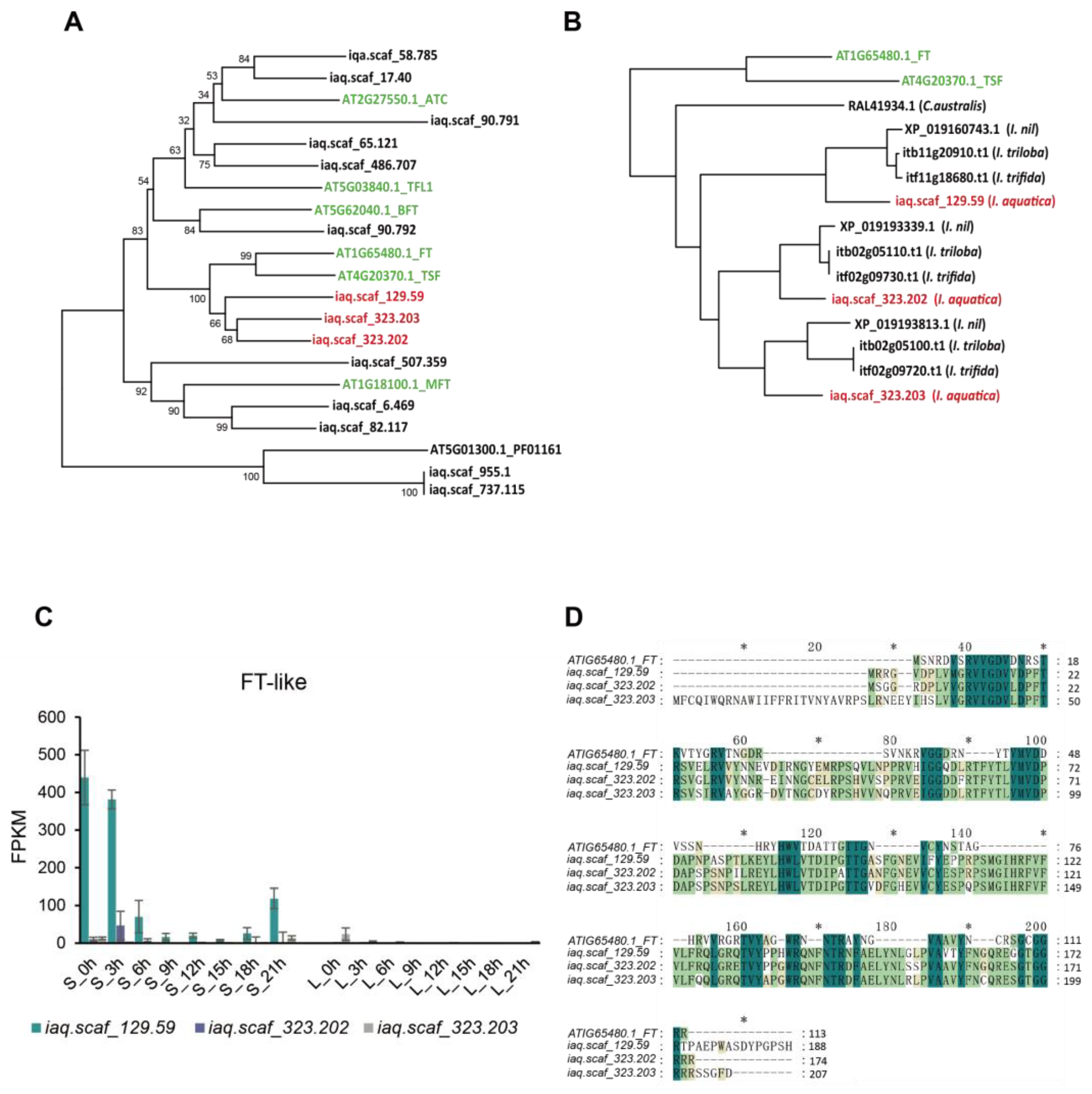
2.4. Analysis of Photoperiod-Mediated Flowering-Related Genes
2.5. Validation of RNA-Seq Data by qRT-PCR
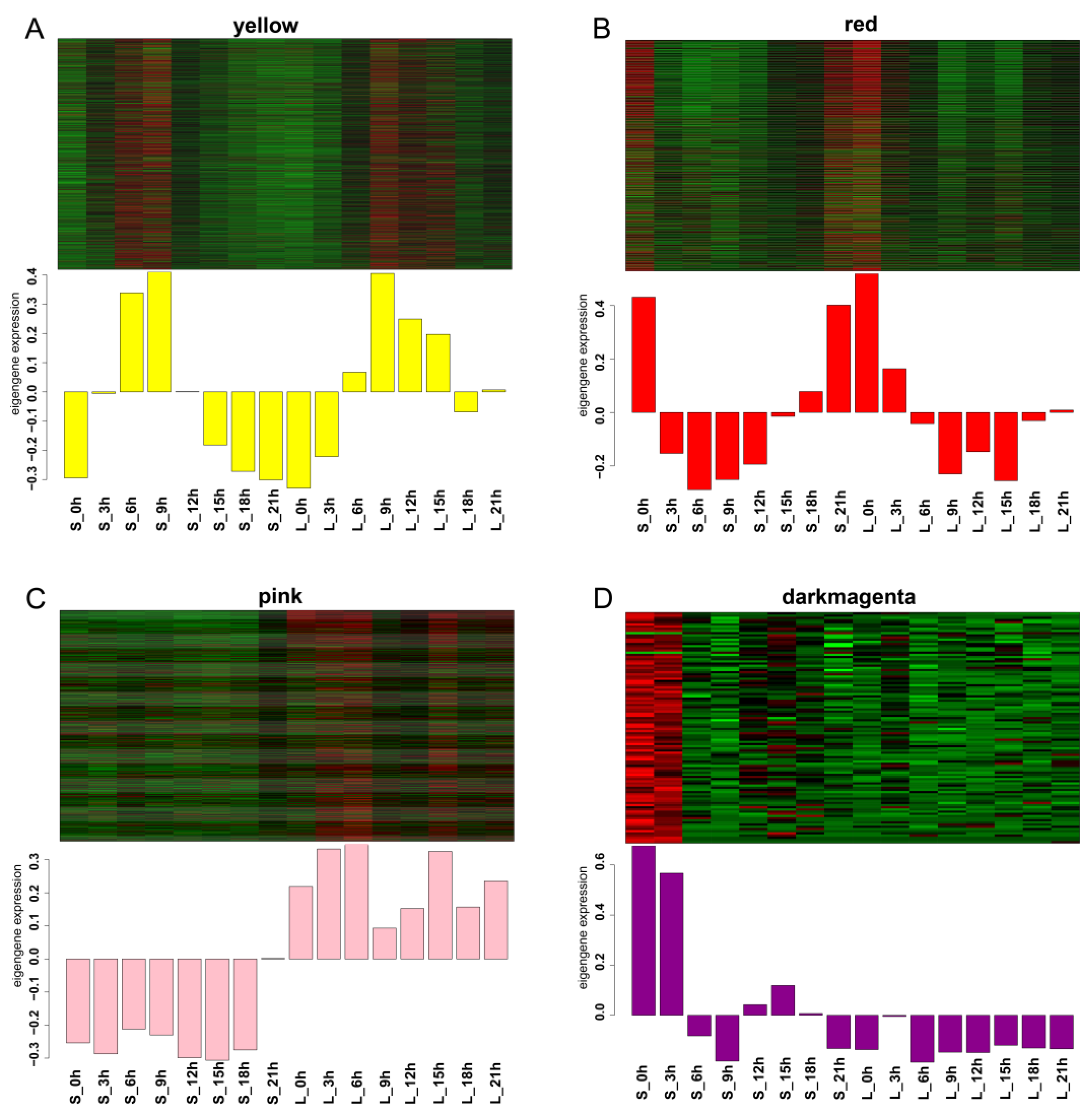
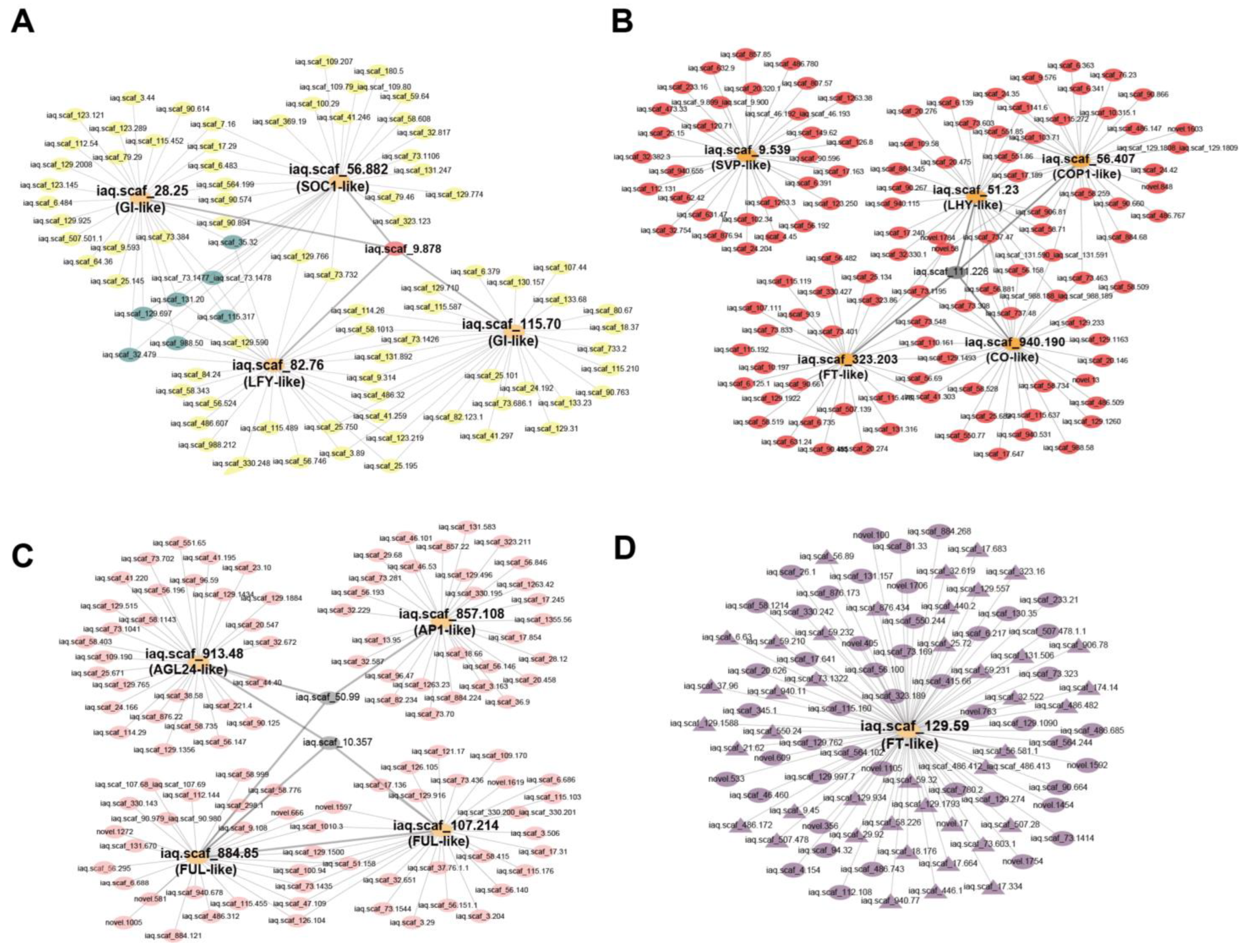
2.6. WGCNA Co-Expression Network Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sampling
4.2. RNA Quantification and Qualification
4.3. Differential Expression Gene Analysis
4.4. GO and KEGG Enrichment Analysis
4.5. Weighted Gene Co-Expression Network Analysis (WGCNA)
4.6. Sequence Alignment and Phylogenetic Tree Construction
4.7. Real-Time Quantitative PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malalavidhane, T.S.; Wickramasinghe, S.M.; Jansz, E.R. Oral hypoglycaemic activity of Ipomoea aquatica. J. Ethnopharmacol. 2000, 72, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Umar, K.J.; Hassan, L.G.; Dangoggo, S.M. Nutritional Composition of Water Spinach (Ipomoea aquatica Forsk.) Leaves. J. Appl. Sci. 2007, 7, 803–809. [Google Scholar] [CrossRef]
- Furukawa, K.; Fujita, M. Advanced Treatment and Food Production by Hydroponic Type Wastewater Treatment Plant. Water Sci. Technol. 1993, 28, 219–228. [Google Scholar] [CrossRef]
- Shim, J.S.; Imaizumi, T. Circadian clock and photoperiodic response in Arabidopsis: From seasonal flowering to redox homeostasis. Biochemistry 2015, 54, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Gil, K.E.; Park, M.J.; Lee, H.J. Alternative splicing provides a proactive mechanism for the diurnal CONSTANS dynamics in Arabidopsis photoperiodic flowering. Plant J. 2017, 89, 128–140. [Google Scholar] [CrossRef]
- Cho, L.H.; Yoon, J.; An, G. The control of flowering time by environmental factors. Plant J. 2017, 90, 708–719. [Google Scholar] [CrossRef]
- Srikanth, A.; Schmid, M. Regulation of flowering time:all roads lead to Rome. Cell Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef]
- Quiroz, S.; Yustis, J.C.; Chávez-Hernández, E.C. Beyond the Genetic Pathways, Flowering Regulation Complexity in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 5716. [Google Scholar] [CrossRef]
- Yusuke, N.; Shogo, I.; Norihito, N. Genetic Linkages of the Circadian Clock-Associated Genes, TOC1, CCA1 and LHY, in the Photoperiodic Control of Flowering Time in Arabidopsis thaliana. Plant Cell Physiol. 2007, 7, 925–937. [Google Scholar]
- Laubinger, S.; Marchal, V.; Le Gourrierec, J. Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. Development 2006, 133, 3213–3222. [Google Scholar] [CrossRef]
- D’Aloia, M.; Tamseddak, K.; Bonhomme, D. Gene activation cascade triggered by a single photoperiodic cycle inducing flowering in Sinapis alba. Plant J. 2009, 59, 962–973. [Google Scholar] [CrossRef]
- Lin, C. Photoreceptors and Regulation of Flowering Time. Plant Physiol. 2000, 123, 39–50. [Google Scholar] [CrossRef]
- Farré, E.M.; Weise, S.E. The interactions between the circadian clock and primary metabolism. Curr. Opin. Plant Biol. 2012, 15, 293–300. [Google Scholar] [CrossRef]
- Endo, M.; Araki, T.; Nagatani, A. Tissue-specific regulation of flowering by photoreceptors. Cell. Mol. Life Sci. 2016, 73, 829–839. [Google Scholar] [CrossRef]
- Nakamichi, N. The Transcriptional Network in the Arabidopsis Circadian Clock System. Genes 2020, 11, 1284. [Google Scholar] [CrossRef]
- Fujiwara, S.; Oda, A.; Yoshida, R. Circadian Clock Proteins LHY and CCA1 Regulate SVP Protein Accumulation to Control Flowering in Arabidopsis. Plant Cell. 2008, 20, 2960–2971. [Google Scholar] [CrossRef] [PubMed]
- Atamian, H.S.; Harmer, S.L. Circadian regulation of hormone signaling and plant physiology. Plant Mol. Biol. 2016, 91, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Hernando, C.E.; Romanowski, A.; Yanovsky, M.J. Transcriptional and post-transcriptional control of the plant circadian gene regulatory network. Biochimica et biophysica acta. Gene Regul. Mech. 2017, 1860, 84–94. [Google Scholar]
- Shim, J.S.; Kubota, A.; Imaizumi, T. Circadian Clock and Photoperiodic Flowering in Arabidopsis: CONSTANS Is a Hub for Signal Integration. Plant Physiol. 2017, 173, 5–15. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Wheatley, K.; Hanzawa, Y. LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev Cell. 2002, 2, 629–641. [Google Scholar] [CrossRef]
- Schaffer, R.; Ramsay, N.; Samach, A. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell 1998, 93, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.R.; Davis, S.J.; Bastow, R.M. The ELF4 gene controls circadian rhythms and flowering time in Arabidopsis thaliana. Nature 2002, 419, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhao, H.; Gan, S. Arabidopsis ELF4-like proteins EFL1 and EFL3 influence flowering time. Gene 2019, 700, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Gendron, J.M.; Pruneda-Paz, J.L.; Doherty, C.J. Arabidopsis circadian clock protein, TOC1, is a DNA-binding transcription factor. Proc. Nat. Acad. Sci. USA 2012, 109, 3167–3172. [Google Scholar] [CrossRef] [PubMed]
- Alabadí, D.; Oyama, T.; Yanovsky, M. Reciprocal Regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian. Clock. Sci. 2001, 293, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Doyle, M.R.; Amasino, R.M. A Complex Genetic Interaction between Arabidopsis thaliana TOC1 and CCA1/LHY in Driving the Circadian Clock and in Output Regulation. Genetics 2007, 176, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Song, H.R. Light-regulated translation mediates gated induction of the Arabidopsis clock protein LHY. EMBO J. 2014, 22, 935–944. [Google Scholar] [CrossRef]
- Anwer, M.U.; Davis, A.; Davis, S.J. Photoperiod sensing of the circadian clock is controlled by EARLY FLOWERING 3 and GIGANTEA. Plant J. 2020, 101, 1397–1410. [Google Scholar] [CrossRef]
- Suárez-López, P.; Wheatley, K.; Robson, F. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 2001, 410, 1116–1120. [Google Scholar] [CrossRef]
- Samach, A.; Onouchi, H.; Gold, S.E. Distinct Roles of CONSTANS Target Genes in Reproductive Development of Arabidopsis. Science 2000, 288, 1613–1616. [Google Scholar] [CrossRef]
- Yoo, S.K.; Chung, K.S.; Kim, J. CONSTANS Activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to Promote Flowering in Arabidopsis. Plant Physiol. 2005, 139, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Gangappa, S.N.; Botto, J.F. The BBX family of plant transcription factors. Trends Plant Sci. 2014, 19, 460–470. [Google Scholar] [CrossRef]
- Wu, F.; Price, B.W.; Haider, W. Functional and evolutionary characterization of the CONSTANS gene family in short-day photoperiodic flowering in soybean. PLoS ONE 2014, 9, e85754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dong, J.; Wang, Z. Mungbean VrCOL1 regulates flowering time under short-day conditions in Arabidopsis. Plant Cell Tissue Organ Cult. 2022, 148, 599–608. [Google Scholar] [CrossRef]
- Fornara, F.; Panigrahi, K.C.; Gissot, L. Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Dev. Cell. 2009, 17, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Schultz, T.F.; Harmon, F.G. FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 2005, 309, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Goralogia, G.S.; Liu, T.K.; Zhao, L. CYCLING DOF FACTOR 1 represses transcription through the TOPLESS co-repressor to control photoperiodic flowering in Arabidopsis. Plant J. 2017, 92, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Tran, H.G.; Swartz, T.E. FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature 2003, 426, 302–306. [Google Scholar] [CrossRef]
- Sawa, M.; Nusinow, D.A.; Kay, S.A. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 2007, 318, 261–265. [Google Scholar] [CrossRef]
- Song, Y.H.; Estrada, D.A.; Johnson, R.S. Distinct roles of FKF1, Gigantea, and Zeitlupe proteins in the regulation of Constans stability in Arabidopsis photoperiodic flowering. Proc. Nat. Acad. Sci. USA 2014, 111, 17672–17677. [Google Scholar] [CrossRef]
- Ito, S.; Song, Y.H.; Josephson-Day, A.R. FLOWERING BHLH transcriptional activators control expression of the photoperiodic flowering regulator CONSTANS in Arabidopsis. Proc. Nat. Acad. Sci. USA 2012, 109, 3582–3587. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, X.; Li, K. Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 2008, 322, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Sarid-Krebs, L.; Panigrahi, K.C.; Fornara, F. Phosphorylation of CONSTANS and its COP1-dependent degradation during photoperiodic flowering of Arabidopsis. Plant J. 2015, 84, 451–463. [Google Scholar] [CrossRef]
- Ordoñez-Herrera, N.; Trimborn, L.; Menje, M. The Transcription Factor COL12 Is a Substrate of the COP1/SPA E3 Ligase and Regulates Flowering Time and Plant Architecture. Plant Physiol. 2018, 176, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Ponnu, J.; Hoecker, U. Illuminating the COP1/SPA Ubiquitin Ligase: Fresh Insights into Its Structure and Functions during Plant Photomorphogenesis. Front. Plant Sci. 2021, 12, 662793. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Smith, R.W.; To, B.J. FKF1 conveys timing information for CONSTANS stabilization in photoperiodic flowering. Science 2012, 336, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Shen, Y.; Chang, H.C. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytol. 2010, 187, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Kobayashi, Y.; Goto, K. TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol. 2005, 46, 1175–1189. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Kardailsky, I.; Lee, J.S. Acceleration of flowering by overexpression of MFT (MOTHER OF FT AND TFL1). Mol. Cells 2004, 17, 95–101. [Google Scholar] [CrossRef]
- Liu, L.J.; Zhang, Y.C.; Li, Q.H. COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell. 2008, 20, 292–306. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Ma, D. CIB1 and CO interact to mediate CRY2-dependent regulation of flowering. EMBO Rep. 2018, 19, e45762. [Google Scholar] [CrossRef] [PubMed]
- Sawa, M.; Kay, S.A. GIGANTEA directly activates Flowering Locus T in Arabidopsis thaliana. Proc. Nat. Acad. Sci. USA 2011, 108, 11698–11703. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.; Shen, L. A Repressor Complex Governs the Integration of Flowering Signals in Arabidopsis. Dev. Cell. 2008, 15, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Collani, S.; Neumann, M.; Yant, L. FT Modulates Genome-Wide DNA-Binding of the bZIP Transcription Factor FD. Plant Physiol. 2019, 180, 367–380. [Google Scholar] [CrossRef]
- Abe, M.; Kosaka, S.; Shibuta, M. Transient activity of the florigen complex during the floral transition in Arabidopsis thaliana. Development 2019, 146, 171504. [Google Scholar] [CrossRef]
- Bouché, F.; Lobet, G.; Tocquin, P. FLOR-ID: An interactive database of flowering-time gene networks in Arabidopsis thaliana. Nucleic Acids Res. 2015, 44, D1167–D1171. [Google Scholar] [CrossRef]
- Teper-Bamnolker, P.; Samach, A. The flowering integrator FT regulates SEPALLATA3 and FRUITFULL accumulation in Arabidopsis leaves. Plant Cell. 2005, 17, 2661–2675. [Google Scholar] [CrossRef]
- Lau, O.S.; Huang, X.; Charron, J.B. Interaction of Arabidopsis DET1 with CCA1 and LHY in mediating transcriptional repression in the plant circadian clock. Mol. Cell. 2011, 43, 703–712. [Google Scholar] [CrossRef]
- Huang, W.; Pérez-García, P.; Pokhilko, A. Mapping the core of the Arabidopsis circadian clock defines the network structure of the oscillator. Science 2012, 336, 75–79. [Google Scholar] [CrossRef]
- Nakamichi, N.; Kita, M.; Niinuma, K. Arabidopsis clock-associated pseudo-response regulators PRR9, PRR7 and PRR5 coordinately and positively regulate flowering time through the canonical CONSTANS-dependent photoperiodic pathway. Plant Cell Physiol. 2007, 48, 822–832. [Google Scholar] [CrossRef]
- Green, R.M.; Tobin, E.M. Loss of the circadian clock-associated protein 1 in Arabidopsis results in altered clock-regulated gene expression. Proc. Nat. Acad. Sci. USA 1999, 96, 4176–4179. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Won, J.H.; Choi, Y.R. Brassinosteroids Regulate Circadian Oscillation via the BES1/TPL-CCA1/LHY Module in Arabidopsis thaliana. Science 2020, 23, 101528. [Google Scholar]
- Xu, G.; Jiang, Z.; Wang, H. The central circadian clock proteins CCA1 and LHY regulate iron homeostasis in Arabidopsis. J. Integr. Plant Biol. 2019, 61, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.T. Distinct Responses to Light in Plants. Plants 2020, 9, 894. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.M.; von Korff, M.; Davis, S.J. Connections between circadian clocks and carbon metabolism reveal species-specific effects on growth control. J. Exp. Bot. 2014, 65, 2915–2923. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Nakagawa, M.; Kamada, H. Circadian clock components in Arabidopsis III. LHY/CCA1/GI in regulating the floral integrator genes LFY/SOC1/FT to control flowering time and shoot architecture. Plant Biotechnol. 2005, 22, 327–331. [Google Scholar] [CrossRef]
- Fowler, S.; Lee, K.; Onouchi, H. GIGANTEA: A circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J. 1999, 18, 4679–4688. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, 1–12. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]



| Gene ID | Module | TF Family |
|---|---|---|
| iaq.scaf_90.574 | yellow | BTB |
| iaq.scaf_605.27 | yellow | Hist_deacetyl |
| iaq.scaf_20.597 | yellow | Pribosyltran |
| iaq.scaf_90.957 | yellow | RRM_1 |
| iaq.scaf_32.479 | yellow | 2-Hacid_dh_C |
| iaq.scaf_9.878 | yellow | G6PD_C |
| iaq.scaf_115.452 | yellow | Pkinase |
| iaq.scaf_107.111 | red | Pkinase_Tyr |
| iaq.scaf_507.139 | red | DHBP_synthase |
| iaq.scaf_129.1922 | red | Auxin_resp |
| iaq.scaf_330.427 | red | Aminotran_1_2 |
| iaq.scaf_90.203 | red | FAD_binding_8 |
| iaq.scaf_10.197 | red | START |
| iaq.scaf_73.1195 | red | Actin |
| iaq.scaf_47.109 | pink | Actin |
| iaq.scaf_129.318 | pink | ATP-synt_D |
| iaq.scaf_330.200_iaq.scaf_330.201 | pink | JmjC |
| iaq.scaf_940.73 | pink | HMG_box |
| iaq.scaf_41.108 | pink | Aminotran_1_2 |
| iaq.scaf_486.312 | pink | 3_5_exonuc |
| iaq.scaf_131.436 | pink | Lipase_GDSL |
| iaq.scaf_20.568 | pink | Kinesin |
| iaq.scaf_36.9 | pink | FAD_binding_3 |
| iaq.scaf_59.231 | dark magenta | Cu_bind_like |
| iaq.scaf_59.232 | dark magenta | Cu_bind_like |
| iaq.scaf_25.72 | dark magenta | p450 |
| iaq.scaf_17.334 | dark magenta | p450 |
| iaq.scaf_446.1 | dark magenta | p450 |
| iaq.scaf_6.63 | dark magenta | p450 |
| iaq.scaf_32.522 | dark magenta | p450 |
| iaq.scaf_129.934 | dark magenta | Pkinase_Tyr |
| iaq.scaf_59.210 | dark magenta | LRRNT_2 |
| iaq.scaf_56.581.1 | dark magenta | p450 |
| iaq.scaf_29.92 | dark magenta | Sugar_tr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Hao, Y.; Altaf, M.A.; Shu, H.; Cheng, S.; Wang, Z.; Zhu, G. Evolution and Dynamic Transcriptome of Key Genes of Photoperiodic Flowering Pathway in Water Spinach (Ipomoea aquatica). Int. J. Mol. Sci. 2024, 25, 1420. https://doi.org/10.3390/ijms25031420
Wang X, Hao Y, Altaf MA, Shu H, Cheng S, Wang Z, Zhu G. Evolution and Dynamic Transcriptome of Key Genes of Photoperiodic Flowering Pathway in Water Spinach (Ipomoea aquatica). International Journal of Molecular Sciences. 2024; 25(3):1420. https://doi.org/10.3390/ijms25031420
Chicago/Turabian StyleWang, Xin, Yuanyuan Hao, Muhammad Ahsan Altaf, Huangying Shu, Shanhan Cheng, Zhiwei Wang, and Guopeng Zhu. 2024. "Evolution and Dynamic Transcriptome of Key Genes of Photoperiodic Flowering Pathway in Water Spinach (Ipomoea aquatica)" International Journal of Molecular Sciences 25, no. 3: 1420. https://doi.org/10.3390/ijms25031420








