How the ‘Aerobic/Anaerobic Glycolysis’ Meme Formed a ‘Habit of Mind’ Which Impedes Progress in the Field of Brain Energy Metabolism
Abstract
:1. Who’s Who of Muscle Carbohydrate Metabolism
2. Habit of Mind Fuels Resistance to and Disregard of Findings That May Debunk the Dogma
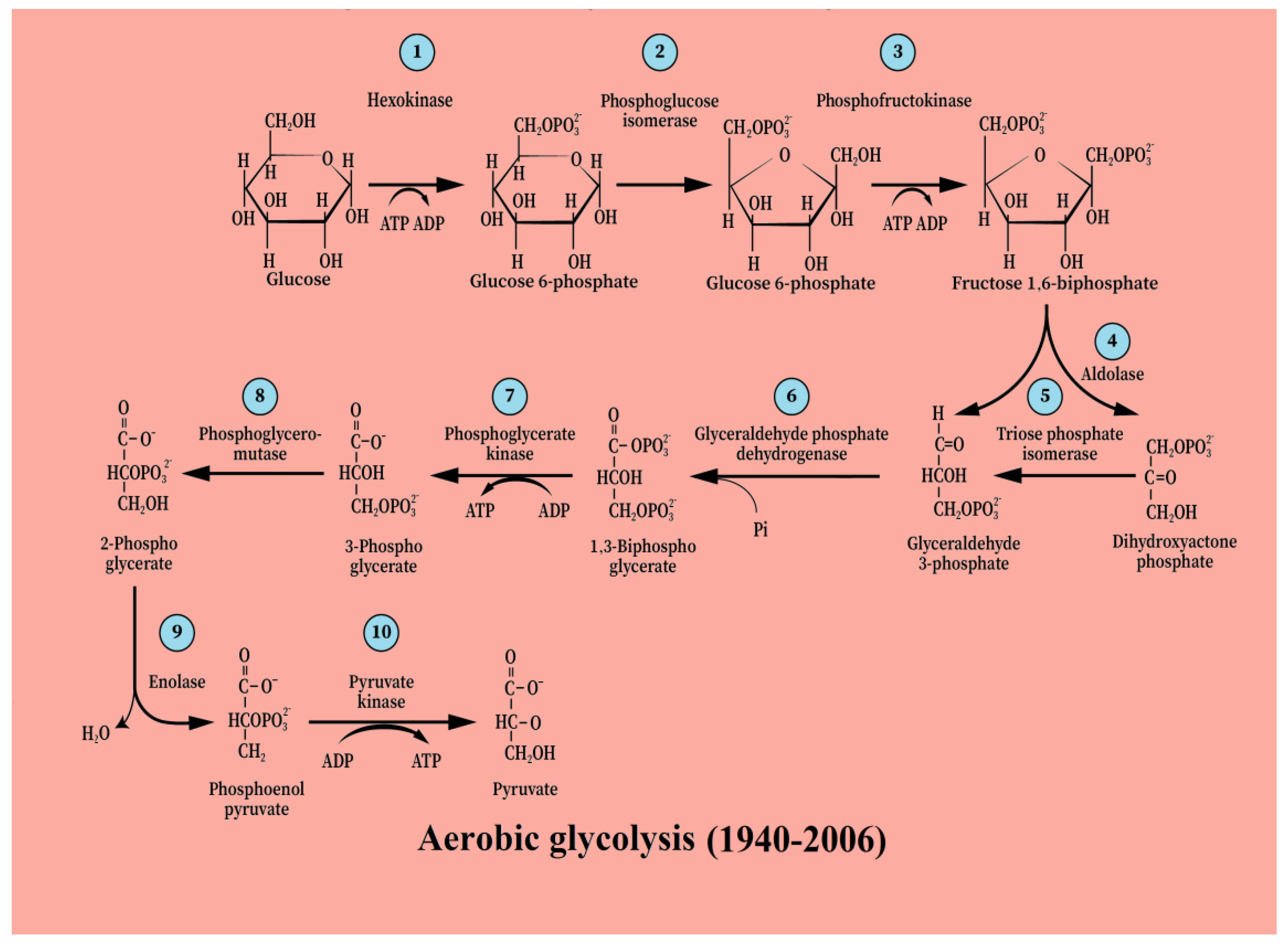
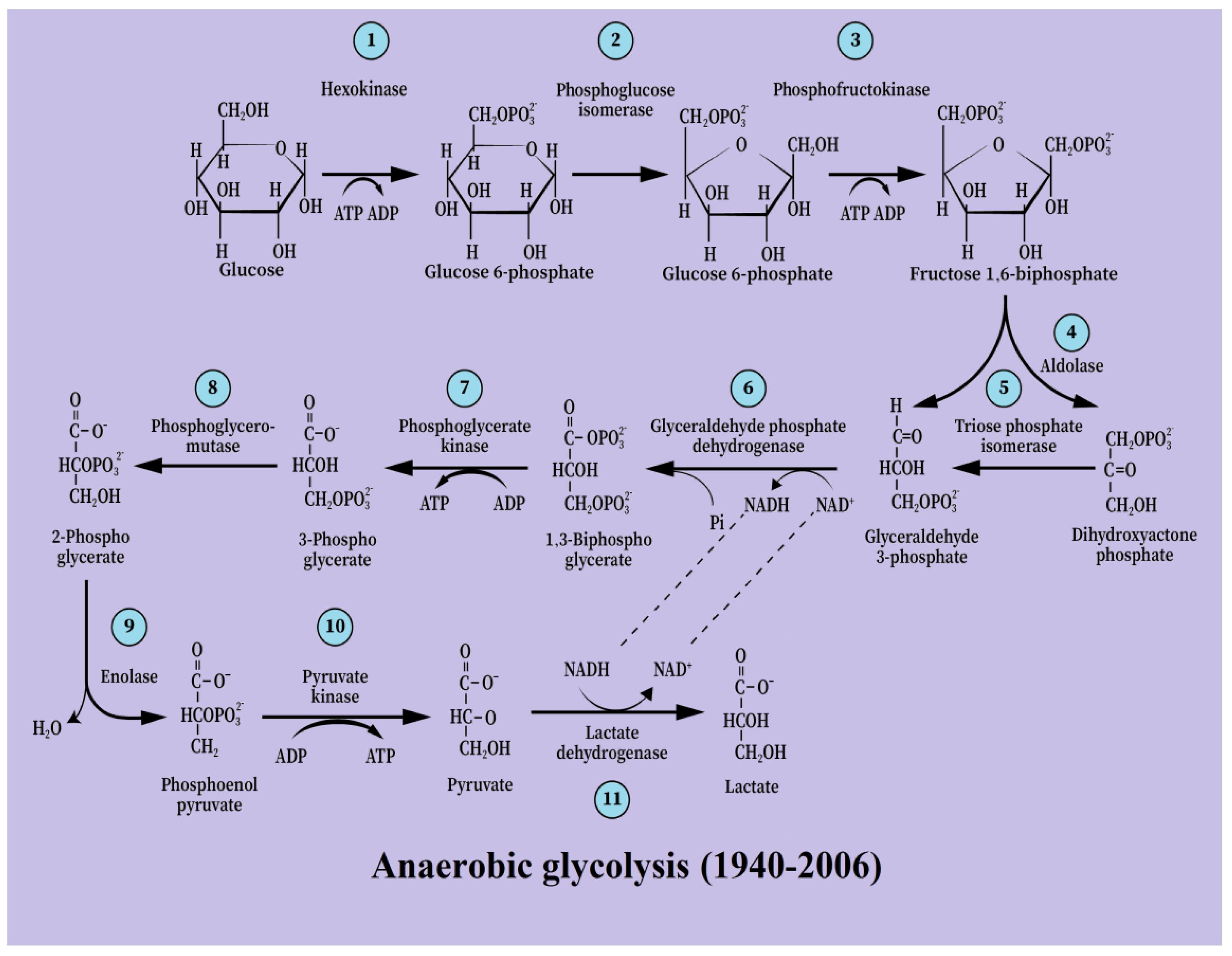
3. From [Anaerobic Glycolysis → Lactate] to [Aerobic Glycolysis → Lactate]
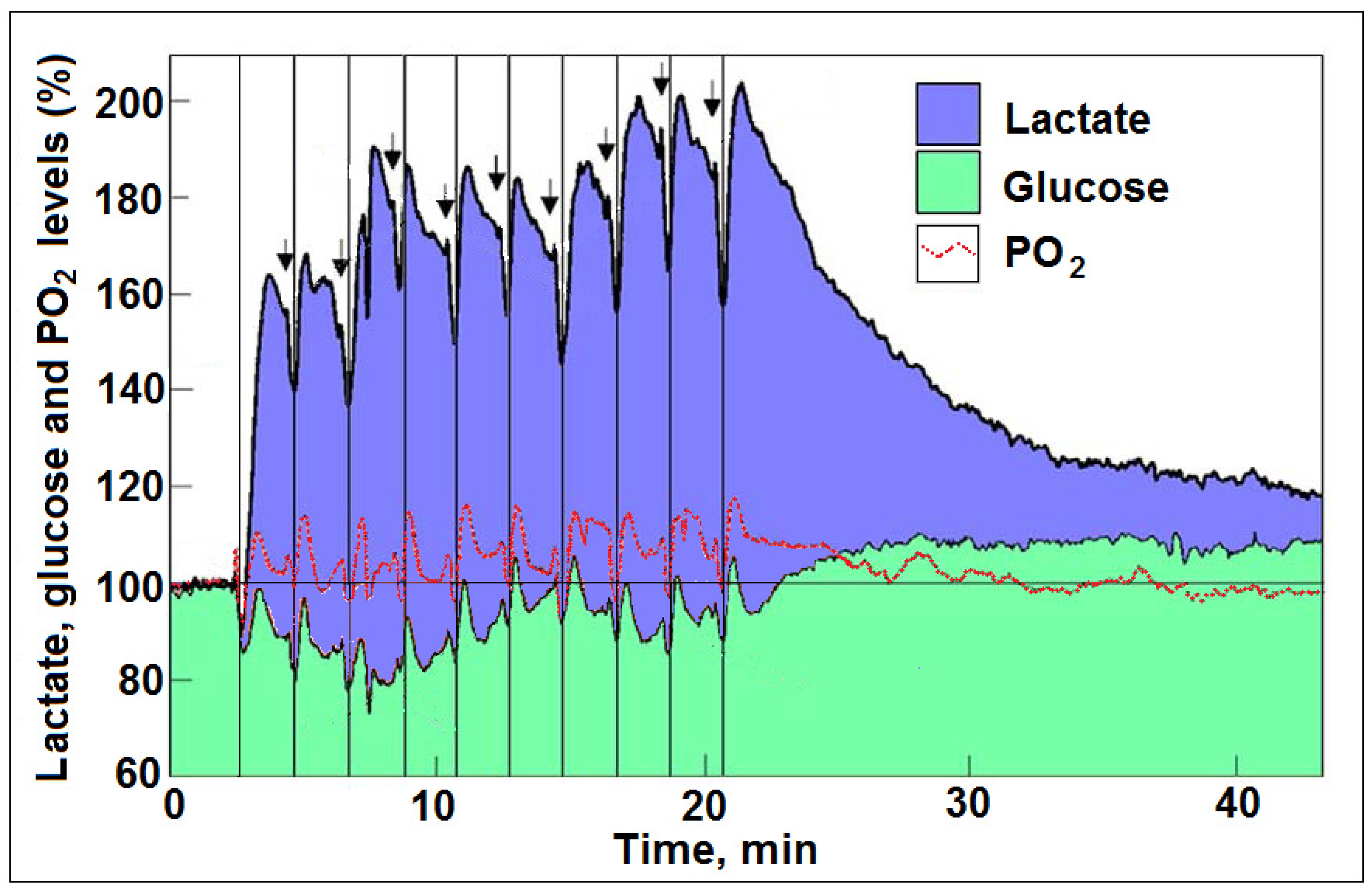
4. New Hypothesis to Circumvent a Flawed Dogma?
5. Glycolysis Always Ends with Lactate, the Aerobic Substrate of the Mitochondrial OXPHOS
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Krebs, H.A.; Johnson, W.A. The role of citric acid in intermediary metabolism in animal tissue. Enzymologia 1937, 4, 148–156. [Google Scholar]
- Holmes, B.E.; Holmes, E.G. Contributions to the study of brain metabolism. I. Carbohydrate metabolism. Preliminary paper. Biochem. J. 1925, 19, 492–499. [Google Scholar] [CrossRef]
- Holmes, E.G.; Holmes, B.E. Contributions to the study of brain metabolism. II. Carbohydrate metabolism. Biochem. J. 1925, 19, 836–839. [Google Scholar] [CrossRef]
- Holmes, E.G.; Holmes, B.E. Contributions to the study of brain metabolism. III. Carbohydrate metabolism relationship of glycogen and lactic acid. Biochem. J. 1926, 20, 1196–1203. [Google Scholar] [CrossRef]
- Holmes, E.G.; Holmes, B.E. Contributions to the study of brain metabolism. IV. Carbohydrate metabolism of the brain tissue of depancreatised cats. Biochem. J. 1927, 21, 412–418. [Google Scholar] [CrossRef]
- Ashford, C.A.; Holmes, E.G. Contributions to the study of brain metabolism. V. Role of phosphates in lactic acid production. Biochem. J. 1929, 23, 748–759. [Google Scholar] [CrossRef]
- Holmes, E.G. Oxidations in central and peripheral nervous tissue. Biochem. J. 1930, 24, 914–925. [Google Scholar] [CrossRef]
- Holmes, E.G.; Ashford, C.A. (Lactic acid oxidation in brain with reference to the “Meyerfof cycle”. Biochem. J. 1930, 24, 1119–1127. [Google Scholar] [CrossRef]
- Ashford, C.A.; Holmes, E.G. Further observations on the oxidation of lactic acid by brain tissue. Biochem. J. 1931, 25, 2028–2049. [Google Scholar] [CrossRef]
- Holmes, E.G. The metabolism of brain and nerve. Ann. Rev. Biochem. 1932, 1, 487–506. [Google Scholar] [CrossRef]
- Holmes, E.G. The relation between carbohydrate metabolism and the function of the grey matter of the central nervous system. Biochem. J. 1933, 27, 523–536. [Google Scholar] [PubMed]
- Schurr, A. Lactate: The ultimate cerebral oxidative energy substrate? J. Cereb. Blood Flow Metab. 2006, 26, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Margolis, H. Paradigms and Barriers: How Habits of Mind Govern Scientific Beliefs; The University of Chicago Press, Ltd.: London, UK, 1993. [Google Scholar]
- Schurr, A. Cerebral glycolysis: A century of persistent misunderstanding and misconception. Front. Neurosci. 2014, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Lactate: Glycolytic product and oxidative substrate during sustained exercise in mammals—‘The lactate shuttle’. In Comparative Physiology and Biochemistry—Currecnt Topics and Trends Vol A. Respiration-Metabolism-Circulation; Gilles, R., Ed.; Springer: Berlin, Germany, 1985; pp. 208–218. [Google Scholar]
- Brooks, G.A.; Dubouchaud, H.; Brown, M.; Sicurello, J.P.; Butz, C.E. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc. Natl. Acad. Sci. USA 1999, 96, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Mammalian fuel utilization during sustained exercise. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 120, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Intra- and extra-cellular lactate shuttles. Med. Sci. Sports Exerc. 2000, 32, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; West, C.A.; Rigor, B.M. Lactate-Supported Synaptic Function in the Rat Hippocampal Slice Preparation. Science 1988, 240, 1326–1328. [Google Scholar] [CrossRef]
- Schurr, A.; Payne, R.S.; Miller, J.J.; Rigor, B.M. Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: An in vitro study. Brain Res. 1997, 744, 105–111. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate shuttle—Between but not within cells? J. Physiol. 2002, 541, 333. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate shuttles in nature. Biochem. Soc. Trans. 2002, 30, 258–264. [Google Scholar] [CrossRef]
- Sahlin, K.; Fernstrom, M.; Tonkonogi, M. No evidence of an intracellular lactate shuttle in rat skeletal muscle. J. Physiol. 2002, 541, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.N.; van Hall, G.; Rasmussen, U.F. Lactate dehydrogenase is not a mitochondrial enzyme in human and mouse vastus lateralis muscle. J. Physiol. 2002, 541, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Rehncrona, S.; Rosen, I.; Seisö, B.K. Brain lactic acidosis and ischemic cell-damage. 1. Biochemistry and neurophysiology. J. Cereb. Blood Flow Metab. 1981, 1, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Kalimo, H.; Rehncrona, S.; Soderfeldt, B.; Olsson, Y.; Siejö, B.K. Brain lactic acidosis and ischemic cell-damage. 2. Histopathology. J. Cereb. Blood Flow Metab. 1981, 1, 313–327. [Google Scholar] [CrossRef]
- Siesjö, B.K. Cell-damage in the brain—A speculative synthesis. J. Cereb. Blood Flow Metab. 1981, 1, 155–185. [Google Scholar] [CrossRef]
- Lluis, C. Lactate dehydrogenase associated with the mitochondrial fraction and with a mitochondrial inhibitor--II. Enzyme in-teraction with a mitochondrial inhibitor. Int. J. Biochem. 1984, 16, 1005–1013. [Google Scholar] [CrossRef]
- Lluis, C. Lactate dehydrogenase binding to the mitochondrial fraction and to a mitochondrial inhibitor as a function of the isoenzymatic composition. Int. J. Biochem. 1985, 17, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Kline, E.S.; Brandt, R.B.; Laux, J.E.; Spainhour, S.E.; Higgins, E.S.; Rogers, K.S.; Tinsley, S.B.; Waters, M. G Localization of l-lactate dehydrogenase in mitochondri. Arch. Biochem. Biophys. 1986, 246, 673–680. [Google Scholar] [CrossRef]
- Brandt, R.B.; Laux, J.E.; Spainhour, S.E.; Kline, E.S. Lactate dehydrogenase in rat mitochondria. Arch. Biochem. Biophys. 1987, 259, 412–422. [Google Scholar] [CrossRef]
- Schurr, A.; Dong, W.; Reid, K.H.; West, C.A.; Rigor, B.M. Lactic acidosis and recovery of neuronal function following cerebral hypoxia in vitro. Brain Res. 1988, 438, 311–314. [Google Scholar] [CrossRef]
- Tombaugh, G.C.; Sapolsky, R.M. Mild acidosis protects hippocampal neurons from injury induced by oxygen and glucose deprivation. Brain Res. 1990, 506, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.E.; Yamaguchi, S. Nervous system effects of cardiac arrest in monkeys. Preservation of vision. Arch. Neurol. 1977, 34, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; Payne, R.S.; Tseng, M.T.; Miller, J.J.; Rigor, B.M. The glucose paradox in cerebral ischemia. New insights. Ann. N. Y. Acad. Sci. 1999, 893, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; Payne, R.S.; Miller, J.J.; Tseng, M.T. Preischemic hyperglycemia-aggravated damage: Evidence that lactate utilization is beneficial and glucose-induced corticosterone release is detrimental. J. Neurosci. Res. 2001, 66, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.D.; Sokoloff, L. Ciculation and energy metabolism of the brain (Chap. 31). In Basic Neurochemistry, 5th ed.; Siegle, G.J., Agranoff, B.W., Albers, R.W., Molinoff, P.B., Eds.; Raven Press: New York, NY, USA, 1994; pp. 645–680. [Google Scholar]
- Chih, C.-P.; Lipton, P.; Roberts, E.L., Jr. Do active cerebral neurons really use lactate rather than glucose? Trends Neurosci. 2001, 24, 573–578. [Google Scholar] [CrossRef]
- Schurr, A.; Gozal, E. Aerobic production and utilization of lactate satisfy increased energy demands upon neuronal activation in hippocampal slices and provide neuroprotection against oxidative stress. Front. Pharmacol. 2012, 2, 96. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain Lactate Metabolism: The Discoveries and the Controversies. J. Cereb. Blood Flow Metab. 2012, 32, 1107–1138. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef]
- Schurr, A. Glycolysis Paradigm Shift Dictates a Reevaluation of Glucose and Oxygen Metabolic Rates of Activated Neural Tissue. Front. Neurosci. 2018, 12, 700. [Google Scholar] [CrossRef]
- Bak, L.K.; Schousboe, A. Misconceptions regarding basic thermodynamics and enzyme kinetics have led to erroneous conclusions regarding the metabolic importance of lactate dehydrogenase isoenzyme expression. J. Neurosci. Res. 2017, 95, 2098–2102. [Google Scholar] [CrossRef] [PubMed]
- Atlante, A.; de Bari, L.; Bobba, A.; Marra, E.; Passarella, S. Transport and metabolism of L-lactate occur in mitochondria from cerebellar granule cells and are modified in cells undergoing low potassium dependent apoptosis. Biochim. Biophys. Acta 2007, 1767, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; de Bari, L.; Valenti, D.; Pizzuto, R.; Paventi, G.; Altane, A. Miochondria and l-lactate metabolism. FEBS Lett. 2008, 582, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.A. Lactate oxidation at the mitochondria: A lactate-malate-aspartate shuttle at work. Front. Neurosci. 2014, 8, 366. [Google Scholar] [CrossRef] [PubMed]
- Rogatzki, M.J.; Ferguson, B.; Goodwin, M.L.; Gladden, L.B. Lactate is always the end product of glycolysis. Front. Neurosci. 2015, 9, 22. [Google Scholar] [CrossRef]
- Altinok, O.; Poggio, J.L.; Stein, D.E.; Bowne, W.B.; Shieh, A.C.; Snyder, N.W.; Orynbayeva, Z. Malate-aspartate shuttle promotes l-lactate oxidation in mitochondria. J. Cell. Physiol. 2020, 235, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Curl, C.C.; Leija, R.G.; Osmond, A.D.; Duong, J.J.; Arevalo, J.A. Tracing the lactate shuttle to the mito-chondrial reticulum. Exp. Mol. Med. 2022, 54, 1332–1347. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Xu, L.; Wang, A.; Zou, Y.; Li, T.; Huang, L.; Chen, W.; Liu, S.; Jiang, K.; et al. Ultrasensitive sensors reveal the spatiotemporal landscape of lactate metabolism in physiology and disease. Cell Metab. 2022, 34, 1–12. [Google Scholar] [CrossRef]
- John, S.; Calmettes, G.; Xu, S.; Ribalet, B. Real-time resolution studies of the regulation of pyruvate-dependent lactate metabolism by hexokinases in single cells. PLoS ONE 2023, 18, e0286660. [Google Scholar] [CrossRef]
- Passarella, S.; Paventi, G.; Pizzuto, R. The mitochondrial L-lactate dehydrogenase affair. Front. Neurosci. 2014, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Oldford, C.; Mailloux, R. Lactate dehydrogenase supports lactate oxidation in mitochondria isolated from different mouse tissues. Redox Biol. 2020, 28, 101339. [Google Scholar] [CrossRef] [PubMed]
- Bergersen, L.H. Lactate transport and signaling in the brain: Potential therapeutic targets and roles in body–brain interaction. J. Cereb. Blood Flow Metab. 2015, 35, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; Passarella, S. Aerobic Glycolysis: A DeOxymoron of (Neuro)Biology. Metabolites 2022, 12, 72. [Google Scholar] [CrossRef]
- Dawkins, R. The Selfish Gene; Oxford University Press: Oxford, UK, 1976; pp. 245–260. [Google Scholar]
- Warburg, O.; Posener, K.; Negelein, E. Ueber den stoffwechsel der tumoren. Biochem. Z. 1924, 152, 319–344. [Google Scholar]
- Warburg, O. On the origin of cancer cells. Science 1956, 24, 309–314. [Google Scholar] [CrossRef]
- Fox, P.T.; Raichle, M.E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. USA 1986, 83, 1140–1144. [Google Scholar] [CrossRef]
- Fox, P.T.; Raichle, M.E.; Mintun, M.A.; Dence, C. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988, 241, 462–464. [Google Scholar] [CrossRef]
- Wiback, S.J.; Palsson, B.O. Extreme pathway analysis of human red blood cell metabolism. Biophys. J. 2000, 83, 808–818. [Google Scholar] [CrossRef]
- Hillman, E.M.C. Coupling mechanism and significance of the BOLD signal: A status report. Annu. Rev. Neurosci. 2014, 37, 161–181. [Google Scholar] [CrossRef]
- Hu, Y.; Wilson, G.S. Rapid changes in local extracellular rat brain glucose observed with an in vivo glucose sensor. J. Neurochem. 1997, 68, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wilson, G.S. A temporary local energy pool coupled to neuronal activity: Fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J. Neurochem. 1997, 69, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Theriault, J.E.; Shaffer, C.; Dienel, G.A.; Sander, C.Y.; Hooker, J.M.; Dickerson, B.C.; Barrett, L.F.; Quigley, K.S. A func-tional account of stimulation-based aerobic glycolysis and its role in interpreting BOLD signal intensity increases in neuroim-aging experiments. Neurosci. Behav. Rev. 2023, 153, 105373. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A. From rags to riches: Lactate ascension as a pivotal metabolite in neuroenergetics. Front. Neurosci. 2023, 17, 1145358. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schurr, A. How the ‘Aerobic/Anaerobic Glycolysis’ Meme Formed a ‘Habit of Mind’ Which Impedes Progress in the Field of Brain Energy Metabolism. Int. J. Mol. Sci. 2024, 25, 1433. https://doi.org/10.3390/ijms25031433
Schurr A. How the ‘Aerobic/Anaerobic Glycolysis’ Meme Formed a ‘Habit of Mind’ Which Impedes Progress in the Field of Brain Energy Metabolism. International Journal of Molecular Sciences. 2024; 25(3):1433. https://doi.org/10.3390/ijms25031433
Chicago/Turabian StyleSchurr, Avital. 2024. "How the ‘Aerobic/Anaerobic Glycolysis’ Meme Formed a ‘Habit of Mind’ Which Impedes Progress in the Field of Brain Energy Metabolism" International Journal of Molecular Sciences 25, no. 3: 1433. https://doi.org/10.3390/ijms25031433
APA StyleSchurr, A. (2024). How the ‘Aerobic/Anaerobic Glycolysis’ Meme Formed a ‘Habit of Mind’ Which Impedes Progress in the Field of Brain Energy Metabolism. International Journal of Molecular Sciences, 25(3), 1433. https://doi.org/10.3390/ijms25031433





