Androgen and Estrogen β Receptor Expression Enhances Efficacy of Antihormonal Treatments in Triple-Negative Breast Cancer Cell Lines
Abstract
1. Introduction
2. Results
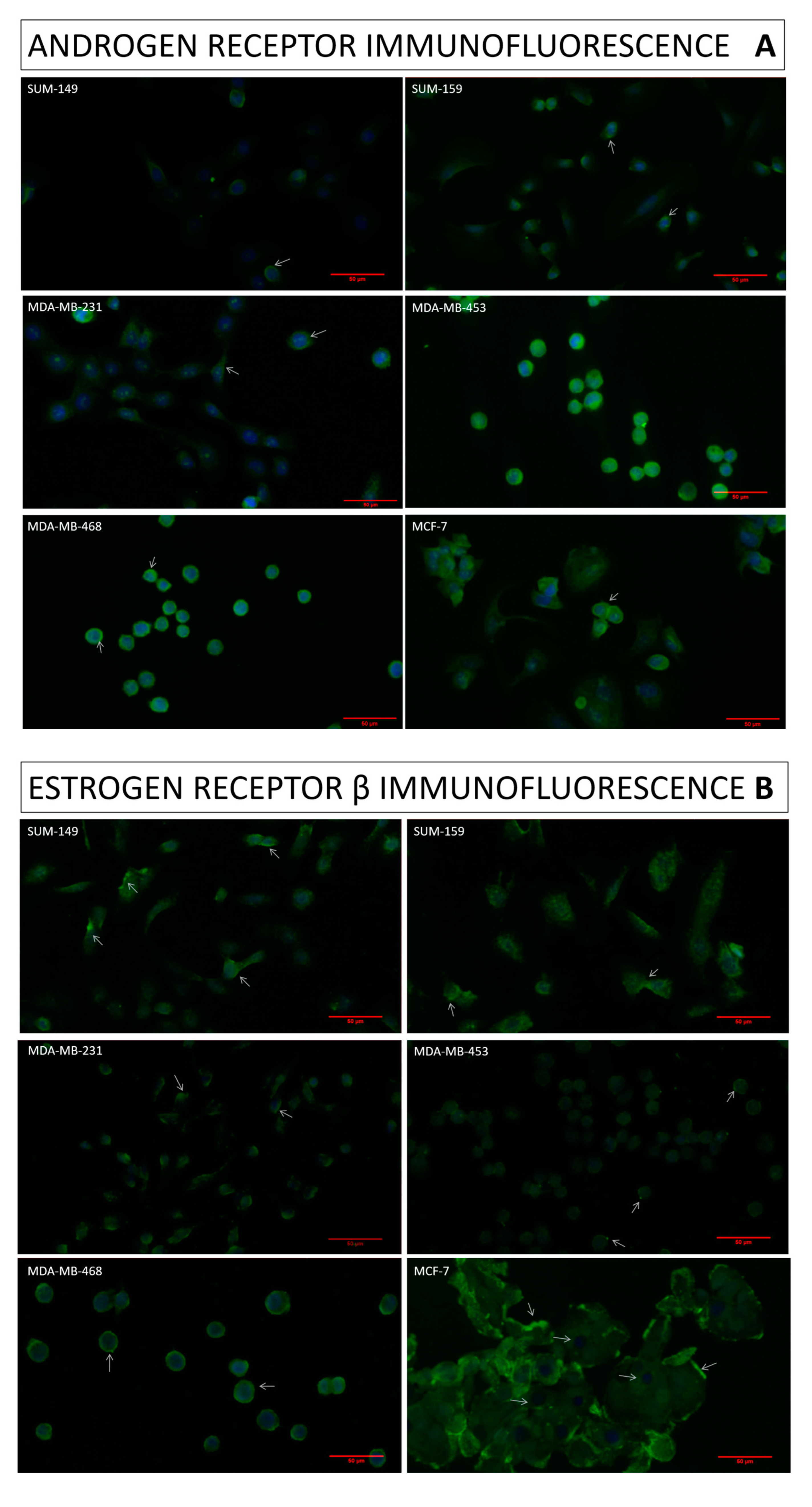
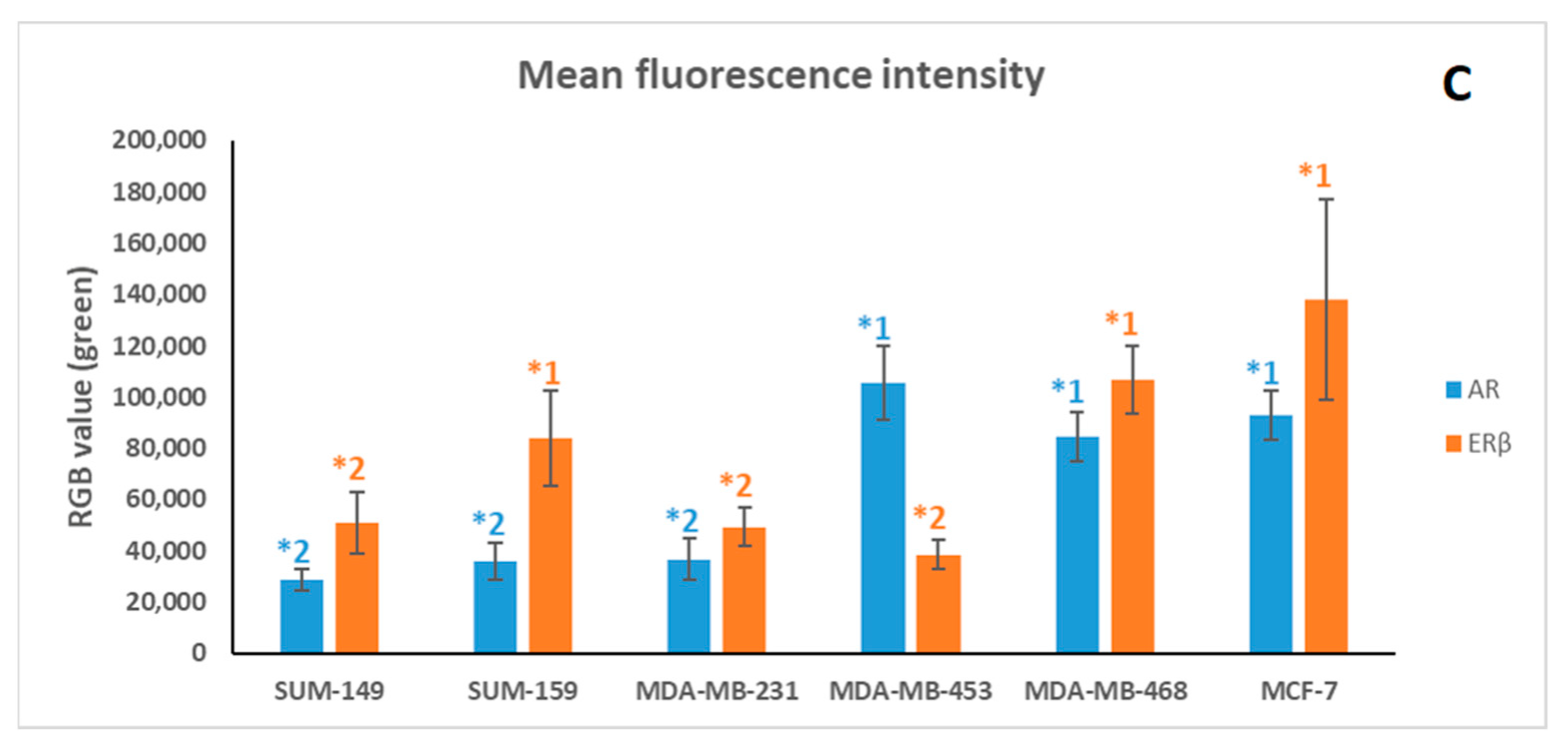
2.1. Immunofluorescence Assay
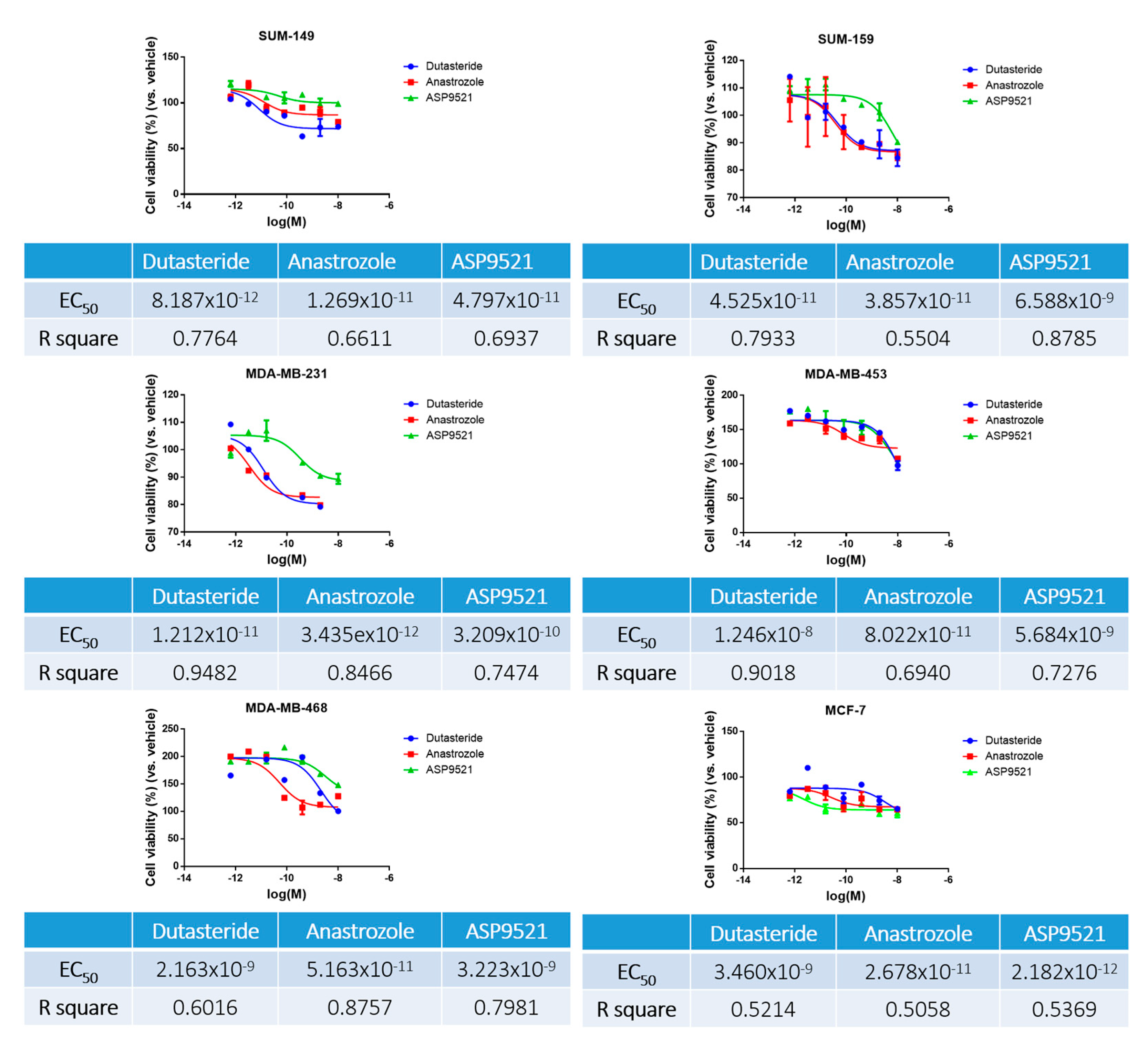
2.2. Sensitivity Assay
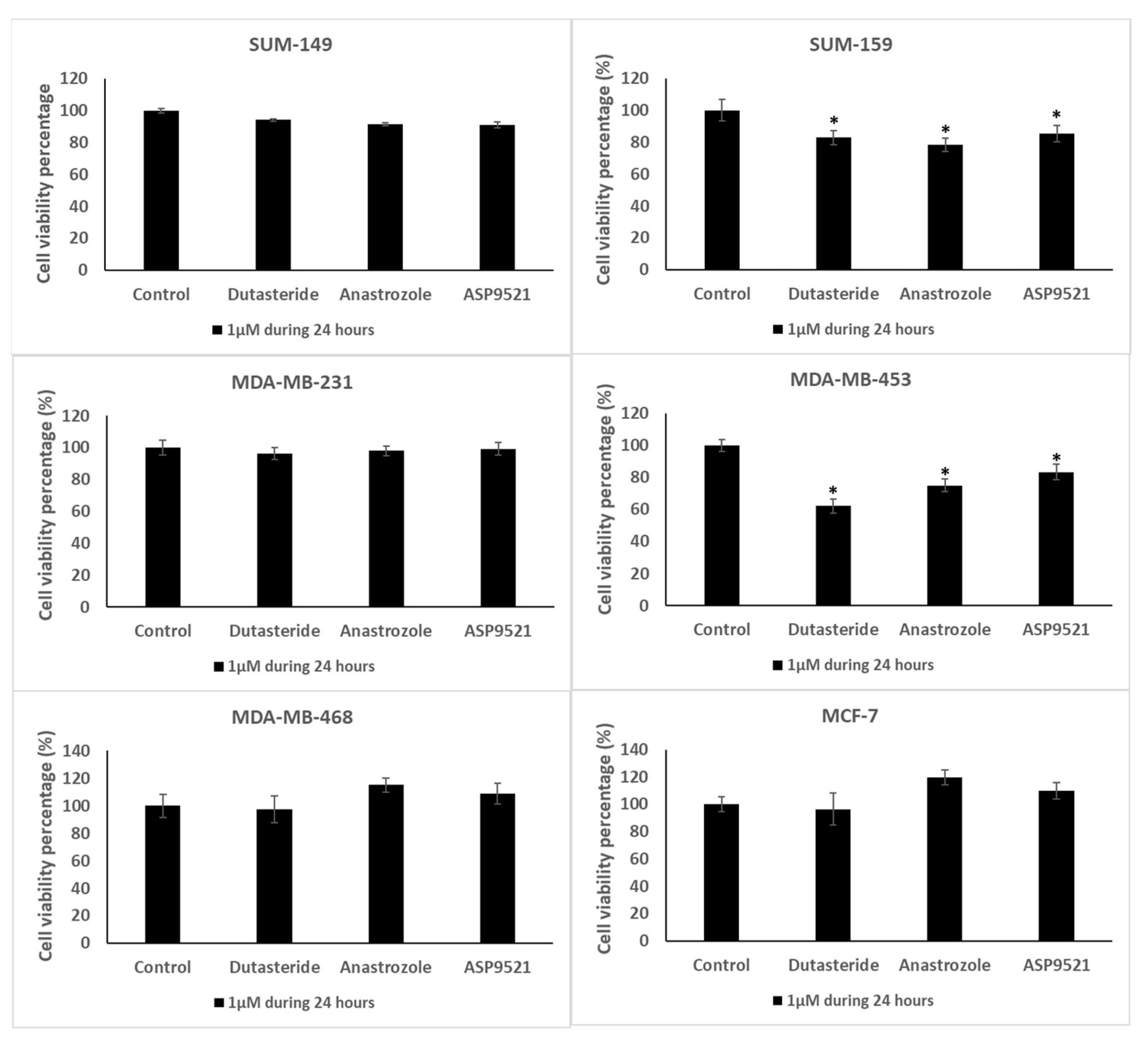
2.3. Cell Viability Assay
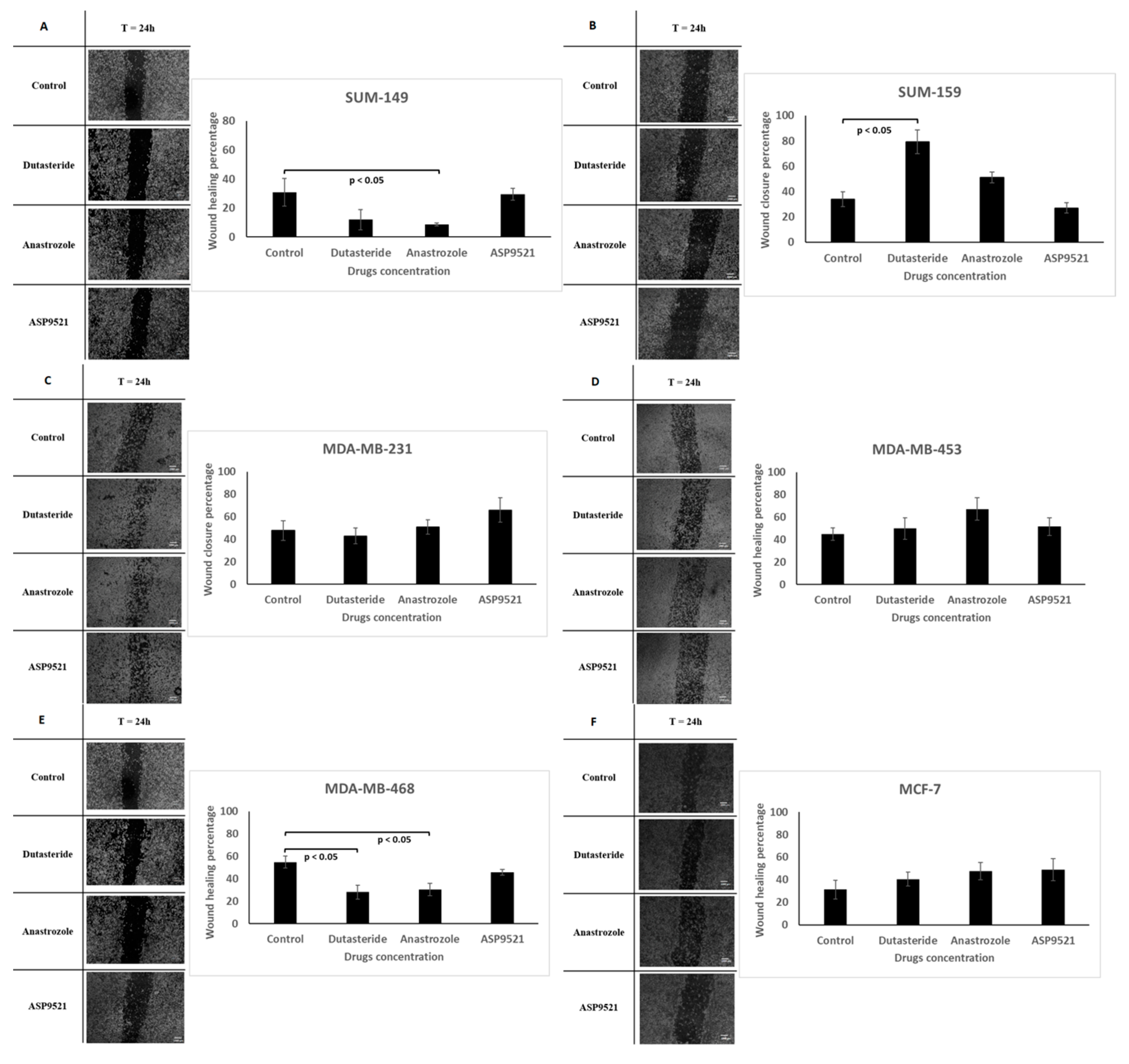
2.4. Wound Healing Assay
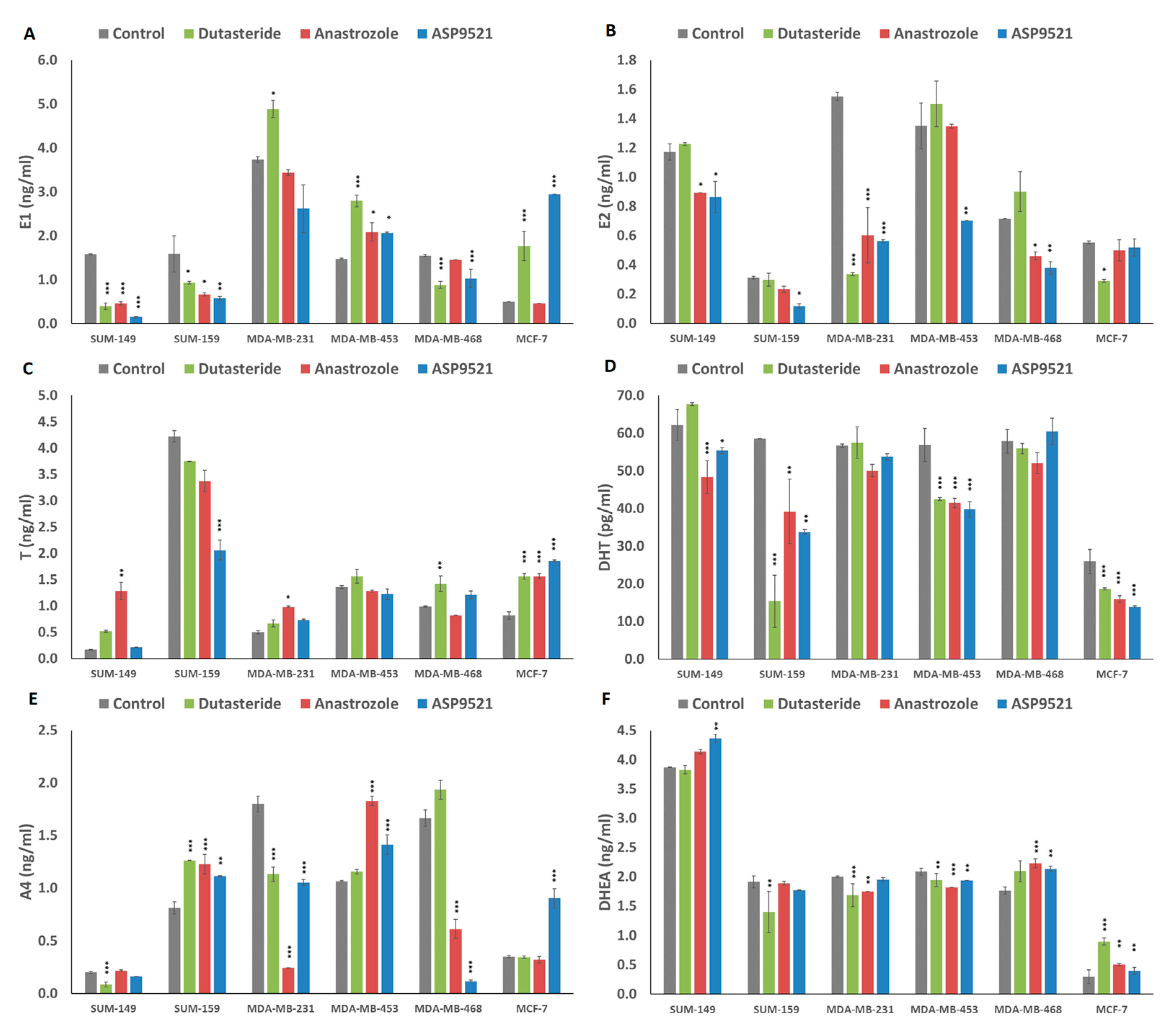
2.5. Hormonal Levels in Culture Media
3. Discussion
4. Materials and Methods
4.1. Cell Line Culture
4.2. Treatments
4.3. Immunofluorescence
4.4. Sensitivity Assay
4.5. Cell Viability Assay
4.6. Wound Healing Assay
4.7. Steroid Determinations in Culture Media
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Barnard, M.E.; Boeke, C.E.; Tamimi, R.M. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim. Biophys. Acta 2015, 1856, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Adams, S.; Cimino-Mathews, A.; Disis, M.L.; Gatti-Mays, M.E.; Ho, A.Y.; Kalinsky, K.; McArthur, H.L.; Mittendorf, E.A.; Nanda, R.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of breast cancer. J. Immunother. Cancer 2021, 9, e002597. [Google Scholar] [CrossRef] [PubMed]
- Burguin, A.; Dioro, C.; Durocher, F. Breast cancer treatments: Updates and new challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.M.; Smith, K.L.; Stearns, V. Management of hormone receptor-positive, HER2-negative early breast cancer. Semin. Oncol. 2021, 47, 187–200. [Google Scholar] [CrossRef]
- Dobrolecki, L.E.; Airhart, S.D.; Alferez, D.G.; Aparicio, S.; Behbod, F.; Bentures-Alj, M.; Brisken, C.; Bult, C.J.; Cai, S.; Clarke, R.B.; et al. Patient-derived Xenograft (PDX) models in basic and translational breast cancer research. Cancer Metastasis Rev. 2017, 35, 547–573. [Google Scholar] [CrossRef]
- Diaz-Gil, L.; Brasó-Maristany, F.; Locatelli, C.; Centa, A.; Győrffy, B.; Ocaña, A.; Prat, A.; Pandiella, A. Modelling hypersensitivity to trastuzumab defines biomarkers of response in HER2 positive breast cancer. J. Exp. Clin. Cancer Res. 2021, 40, 313. [Google Scholar] [CrossRef]
- McGhan, L.J.; McCullough, A.E.; Protheroe, C.A.; Dueck, A.C.; Lee, J.J.; Nunez-Nateras, R.; Castle, E.P.; Gray, R.J.; Wasif, N.; Goetz, M.P.; et al. Androgen receptor-positive triple negative breast cancer: A unique breast cancer subtype. Breast Oncol. 2014, 21, 361–367. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Pietenpol, J.A. Clinical implications of molecular heterogeneity in triple negative breast cancer. Breast 2015, 24, S36–S40. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Brumec, M.; Sobočan, M.; Takač, I.; Arko, D. Clinical implications of androgen-positive triple-negative breast cancer. Cancers 2021, 13, 1642. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Pietenpol, J.A. Identification and use of biomarkers in treatment strategies for triple negative breast cancer subtypes. J. Pathol. 2013, 232, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Jhonson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of triple-negative breast cancer molecular subtypes: Implications for neoadjuvant chemotherapy selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef] [PubMed]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular subtypes and local-regional control of breast cancer. Surg. Oncol. Clin. 2018, 27, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, Y.; Xu, K.; Li, L.; Huang, J.; Qju, F. Androgen receptor in breast cancer: From bench to bedside. Front. Endocrinol. 2020, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Honma, N.; Matsuda, Y.; Mikami, T. Carcinogenesis of triple-negative breast cancer and sex steroid hormones. Cancer 2021, 13, 2588. [Google Scholar] [CrossRef]
- Luo, X.; Shi, Y.-X.; Li, Z.-M.; Jiang, W.-Q. Expression and clinical significance of androgen receptor in triple negative breast cancer. Chin. J. Cancer 2010, 29, 585–590. [Google Scholar] [CrossRef]
- Barton, V.N.; D’Amato, N.C.; Gordon, M.A.; Lind, H.T.; Spoelstra, N.S.; Babbs, B.L.; Heinz, R.E.; Elias, A.; Jedlicka, P.; Jacobsen, B.M.; et al. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol. Cancer Ther. 2015, 14, 769–778. [Google Scholar] [CrossRef]
- Hickey, T.E.; Robinson, J.L.L.; Carroll, J.S.; Tilley, W.D. Minireview: The androgen receptor in breast tissues: Growth inhibitor, tumor suppressor, oncogene? Mol. Endocrinol. 2012, 26, 1252–1267. [Google Scholar] [CrossRef]
- Yan, S.; Dey, P.; Ziegler, Y.; Jiao, X.; Kim, S.H.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Contrasting activities of estrogen receptor beta isoforms in triple negative breast cancer. Breast Cancer Res. Treat. 2021, 185, 281–292. [Google Scholar] [CrossRef]
- Bleach, R.; Mcllroy, M. The divergent function of androgen receptor in breast cancer; analysis of steroid mediators and tumor intracrinology. Front. Endocrinol. 2018, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.M.; Sasano, H. The intracrinology of breast cancer. J. Steroid Biochem. Mol. Biol. 2015, 145, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Caceres, S.; Monsalve, B.; Alonso-Diez, A.; Crespo, B.; Illera, M.J.; de Andres, P.J.; Silvan, G.; Illera, J.C. Blocking estrogen synthesis leads to different hormonal responses in canine and human triple negative inflammatory breast cancer. Cancers 2021, 13, 4967. [Google Scholar] [CrossRef] [PubMed]
- Crespo, B.; Caceres, S.; Silvan, G.; Illera, M.J.; Illera, J.C. The inhibition of steroid hormones determines the fate of IPC-366 tumor cells, highlighting the crucial role of androgen production in tumor processes. Res. Vet. Sci. 2023, 161, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mostaghel, E.A.; Geng, L.; Holcomb, I.; Coleman, I.M.; Lucas, J.; True, L.D.; Nelson, P.S. Variability in the androgen response of prostate epithelium to 5-alpha reductase inhibition: Implications for prostate cancer chemoprevention. Cancer Res. 2010, 70, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II trial of bicalutamide in patients with androgen receptor–positive, estrogen receptor–negative metastatic breast cancer. Clin. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef] [PubMed]
- Amory, J.K.; Anawalt, B.D.; Matsumoto, A.M.; Page, S.T.; Bremner, W.J.; Wang, C.; Swerdloff, R.S.; Clark, R.V. The effect of 5alpha-reductase inhibition with dutasteride and finasteride on bone mineral density, serum lipoproteins, hemoglobin, prostate specific antigen and sexual function in healthy young men. J. Urol. 2008, 179, 2333–2338. [Google Scholar] [CrossRef]
- Azuma, T.; Matayoshi, Y.; Sato, Y.; Nagase, Y. Effect of dutasteride on castration-resistant prostate cancer. Mol. Clin. Oncol. 2017, 8, 133–136. [Google Scholar] [CrossRef]
- Burstein, H.J.; Prestrud, A.A.; Seidenfeld, J.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.E.; Giordano, S.H.; Hudis, C.A.; Malin, J.; et al. American Society of Clinical Oncology Clinical Practice Guideline: Update on adjuvant endocrine therapy for women with hormone receptor–positive breast cancer. J. Clin. Oncol. 2010, 28, 3784–3796. [Google Scholar] [CrossRef]
- Ingle, J.N.; Kalari, K.R.; Buzdar, A.U.; Robson, M.E.; Goetz, M.P.; Desta, Z.; Barmna, P.; Dudenkov, T.T.; Northfelt, D.W.; Perez, E.A.; et al. Estrogens and their precursors in postmenopausal women with early breast cancer receiving anastrozole. Steroids 2015, 99 Pt A, 32–38. [Google Scholar] [CrossRef]
- Penning, T.M. AKR1C3 (type 5 17β-hydroxysteroid dehydrogenase/prostaglandin F synthase): Roles in malignancy and endocrine disorders. Mol. Cell Endocrinol. 2019, 489, 82–91. [Google Scholar] [CrossRef]
- Teply, B.A.; Antonarakis, E.S. Novel mechanism-based therapeutics for androgen axis blockade in castration-resistant prostate cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 279–290. [Google Scholar] [CrossRef]
- Shastry, M.; Yardley, D.A. Updates in the treatment of basal/triple-negative breast cancer. Curr. Opin. Obstet. Gynecol. 2013, 25, 40–48. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Kumar, D.N.; Dehari, D.; Patil, R.; Singh, S.; Kumar, D.; Agrawal, A.K. Endorsement of TNBC Biomarkers in Precision Therapy by Nanotechnology. Cancers 2023, 15, 2661. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Treeck, O.; Schüler-Toprak, S.; Ortmann, O. Estrogen actions in triple-negative breast cancer. Cells 2020, 9, 2358. [Google Scholar] [CrossRef]
- Xu, T.; Ma, D.; Chen, S.; Tang, R.; Yang, J.; Meng, C.; Feng, Y.; Liu, L.; Wang, J.; Luo, H.; et al. High GPER expression in triple-negative breast cancer is linked to pro-metastatic pathways and predicts poor patient outcomes. NPJ Breast Cancer 2022, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.G.; Iturriaga, M.P.; Cruz, P. Hormonal carcinogenesis in canine mammary cancer: Molecular mechanism of estradiol involved in malignant progression. Animals 2021, 11, 608. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.R.; Bernales, S.; Jacobsen, B.M.; Cittelly, D.M.; Howe, E.N.; D’Amato, N.C.; Spoelstra, N.S.; Edgerton, S.M.; Jean, A.; Guerrero, J.; et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014, 16, R7. [Google Scholar] [CrossRef] [PubMed]
- Honma, N.; Horii, R.; Iwase, T.; Saji, S.; Younes, M.; Takubo, K.; Matsuura, M.; Ito, Y.; Akiyama, F.; Sakamoto, G. Clinical Importance of estrogen receptor-β evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J. Clin. Oncol. 2008, 26, 3727–3734. [Google Scholar] [CrossRef] [PubMed]
- Aleskandarany, M.A.; Rakha, E.A.; Ahmed, M.A.; Powe, D.G.; Ellis, I.O.; Green, A.R. Clinicopathologic and molecular significance of phospho-Akt expression in early invasive breast cancer. Breast Cancer Res. Treat. 2010, 127, 407–416. [Google Scholar] [CrossRef]
- Rajarajan, S.; Snijesh, V.P.; Anupama, C.E.; Nair, M.G.; Mavatkar, A.D.; Naidu, C.M.; Patil, S.; Nimbalkar, V.P.; Alezander, A.; Pillai, M.; et al. An androgen receptor regulated gene score is associated with epithelial to mesenchymal transition features in triple negative breast cancers. Trans. Oncol. 2023, 37, 101761. [Google Scholar] [CrossRef]
- Dong, S.; Yousefi, H.; Savage, I.V.; Okpechi, S.C.; Wright, M.K.; Matossian, M.D.; Collins-Burow, B.M.; Burow, M.E.; Alahari, S.K. Ceritinib is a novel triple negative breast cancer therapeutic agent. Mol. Cancer 2022, 21, 138. [Google Scholar] [CrossRef]
- Hamilton, N.; Marquez-Garban, D.; Mah, V.; Fernando, G.; Elshimali, Y.; Garban, H.; Elashoff, D.; Vadgama, J.; Goodglick, L.; Pietras, R. Biologic roles of estrogen receptor-β and insulin-like growth factor-2 in triple-negative breast cancer. Biomed. Res. Int. 2015, 2015, 925703. [Google Scholar] [CrossRef]
- Božović, A.; Mandušić, V.; Todorović, L.; Krajnović, M. Estrogen receptor beta: The promising biomarker and potential target in metastases. Int. J. Mol. Sci. 2021, 22, 1656. [Google Scholar] [CrossRef]
- Mukhopadhyay, U.K.; Oturkar, C.C.; Adams, C.; Wickramasekera, N.; Bansal, S.; Medisetty, R.; Miller, A.; Swetzig, W.M.; Silwal-Pandit, L.; Børresen-Dale, A.-L.; et al. TP53 status as a determinant of pro- vs anti-tumorigenic effects of estrogen receptor-beta in breast cancer. J. Natl. Cancer Inst. 2019, 111, 1202–1215. [Google Scholar] [CrossRef]
- Anestis, A.; Sarantis, P.; Theocharis, S.; Zoi, I.; Tryfonopoulos, D.; Korogiannos, A.; Koumarianou, A.; Xingi, E.; Thomaidou, D.; Kontos, M.; et al. Estrogen receptor beta increases sensitivity to enzalutamide in androgen receptor-positive triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Tang, L.; Xu, Y.; Sun, Q.; Yang, F.; Guan, X. ERβ1 inhibits metastasis of androgen receptor-positive triple-negative breast cancer by suppressing ZEB1. J. Exp. Clin. Cancer Res. 2017, 36, 75. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.M.; Green, A.R.; Karthik, S.; Alizadeh, Y.; Hughes, T.A.; Harkins, L.; Ellis, I.O.; Robertson, J.F.; Paish, E.C.; Saunders, P.T.K.; et al. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin. Cancer Res. 2008, 14, 5228–5235. [Google Scholar] [CrossRef] [PubMed]
- Bates, G.J.; Fox, S.B.; Han, C.; Launchbury, R.; Leek, R.D.; Harris, A.L.; Banham, A.H. Expression of the forkhead transcription factor FOXP1 is associated with that of estrogen receptorβ in primary invasive breast carcinomas. Breast Cancer Res. Treat. 2007, 111, 453–459. [Google Scholar] [CrossRef]
- Alonso-Diez, A.; Caceres, S.; Peña, L.; Crespo, B.; Illera, J.C. Anti-angiogenic treatments interact with steroid secretion in inflammatory breast cancer triple negative cell lines. Cancers 2021, 13, 3668. [Google Scholar] [CrossRef]
- Regenthal, R.; Voskanian, M.; Baumann, F.; Teichert, J.; Brätter, C.; Aigner, A.; Abraham, G. Pharmacokinetic evaluation of a transdermal anastrozole-in-adhesive formulation. Drug Des. Devel. Ther. 2018, 12, 3653–3664. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M. Aldo-Keto Reductase (AKR) 1C3 inhibitors: A patent review. Expert. Opin. Ther. Pat. 2017, 27, 1329–1340. [Google Scholar] [CrossRef]
- Valko-Rokytovská, M.; Očenáš, P.; Salayová, A.; Kostecká, Z. Breast Cancer: Targeting of steroid hormones in cancerogenesis and diagnostics. Int. J. Mol. Sci. 2021, 22, 5878. [Google Scholar] [CrossRef]
- Augimeri, G.; Camera, G.L.; Gelsomino, L.; Giordano, C.; Panza, S.; Sisci, D.; Morelli, C.; Györffy, B.; Bonofigli, D.; Andò, S.; et al. Evidence for enhanced exosome production in aromatase inhibitor-resistant breast cancer cells. Int. J. Mol. Sci. 2020, 21, 5841. [Google Scholar] [CrossRef]
- Austin, D.; Hamilton, N.; Elshimali, Y.; Pietras, R.; Wu, Y.; Vadgama, J. Estrogen receptor-beta is a potential target for triple negative breast cancer treatment. Oncotarget 2018, 9, 33912–33930. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, L.; Arlt, W.; Storbeck, K.H. Intracrine androgen biosynthesis, metabolism and action revisited. Mol. Cell Endocrinol. 2018, 465, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ellsworth, K.A.; Moon, I.; Pelleymounter, L.L.; Eckloff, B.W.; Martin, Y.N.; Fridley, B.L.; Jenkins, G.D.; Batzler, A.; Suman, V.J.; et al. Functional genetic polymorphisms in the aromatase gene CYP19 vary the response of breast cancer patients to neoadjuvant therapy with aromatase inhibitors. Cancer Res. 2010, 70, 319–328. [Google Scholar] [CrossRef]
- Bernard, L.; Legay, C.; Adriaenssens, E.; Mougel, A.; Ricort, J.-M. Estradiol regulates the insulin-like growth factor-I (IGF-I) signalling pathway: A crucial role of phosphatidylinositol 3-kinase (PI 3-kinase) in estrogens requirement for growth of MCF-7 human breast carcinoma cells. Biochem. Biophys. Res. Commun. 2006, 350, 916–921. [Google Scholar] [CrossRef]
- Jamrozik, M.; Piska, K.; Bucki, A.; Koczurkiewicz-Adamczyk, P.; Sapa, M.; Władyka, B.; Pekala, E.; Kołaczkowski, M. In silico and in vitro assessment of carbonyl reductase 1 inhibition using ASP9521—A potent aldo-keto reductase 1C3 inhibitor with the potential to support anticancer therapy using anthracycline antibiotics. Molecules 2023, 28, 3767. [Google Scholar] [CrossRef]
- Kim, S.-J.; Seo, I.; Kim, M.H.; Park, J.-W.; Kim, S.; Park, W.-J. Ceramide synthase 4 overexpression exerts oncogenic properties in breast cancer. Lipids Health Dis. 2023, 22, 183. [Google Scholar] [CrossRef] [PubMed]
- Caceres, S.; Peña, L.; Silvan, G.; Illera, M.J.; Woodward, W.A.; Reuben, J.M.; Illera, J.C. Steroid tumor environment in male and female mice model of canine and human inflammatory breast cancer. Biomed. Res. Int. 2016, 2016, 8909878. [Google Scholar] [CrossRef] [PubMed]
| Hormone | Abbreviation | Antibody Code | Dilution |
|---|---|---|---|
| Estrone | E1 | R522-2 | 1/12,000 |
| 17β-estradiol | E2 | C6-E91 | 1/4000 |
| Testosterone | T | R156 | 1/8000 |
| Androstenedione | A4 | C9111 | 1/5000 |
| Dihydrotestosterone | DHT | DE5761 | |
| Dihydroepiandrostenedione | DHEA | DEH3344 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo, B.; Illera, J.C.; Silvan, G.; Lopez-Plaza, P.; Herrera de la Muela, M.; de la Puente Yagüe, M.; Diaz del Arco, C.; Illera, M.J.; Caceres, S. Androgen and Estrogen β Receptor Expression Enhances Efficacy of Antihormonal Treatments in Triple-Negative Breast Cancer Cell Lines. Int. J. Mol. Sci. 2024, 25, 1471. https://doi.org/10.3390/ijms25031471
Crespo B, Illera JC, Silvan G, Lopez-Plaza P, Herrera de la Muela M, de la Puente Yagüe M, Diaz del Arco C, Illera MJ, Caceres S. Androgen and Estrogen β Receptor Expression Enhances Efficacy of Antihormonal Treatments in Triple-Negative Breast Cancer Cell Lines. International Journal of Molecular Sciences. 2024; 25(3):1471. https://doi.org/10.3390/ijms25031471
Chicago/Turabian StyleCrespo, Belen, Juan Carlos Illera, Gema Silvan, Paula Lopez-Plaza, María Herrera de la Muela, Miriam de la Puente Yagüe, Cristina Diaz del Arco, Maria Jose Illera, and Sara Caceres. 2024. "Androgen and Estrogen β Receptor Expression Enhances Efficacy of Antihormonal Treatments in Triple-Negative Breast Cancer Cell Lines" International Journal of Molecular Sciences 25, no. 3: 1471. https://doi.org/10.3390/ijms25031471
APA StyleCrespo, B., Illera, J. C., Silvan, G., Lopez-Plaza, P., Herrera de la Muela, M., de la Puente Yagüe, M., Diaz del Arco, C., Illera, M. J., & Caceres, S. (2024). Androgen and Estrogen β Receptor Expression Enhances Efficacy of Antihormonal Treatments in Triple-Negative Breast Cancer Cell Lines. International Journal of Molecular Sciences, 25(3), 1471. https://doi.org/10.3390/ijms25031471







