Neurobiology of Stress-Induced Nicotine Relapse
Abstract
1. Introduction
2. Experimental Models of Stress-Induced Nicotine Relapse
2.1. Nicotine-Induced CPP and Nicotine Self-Administration
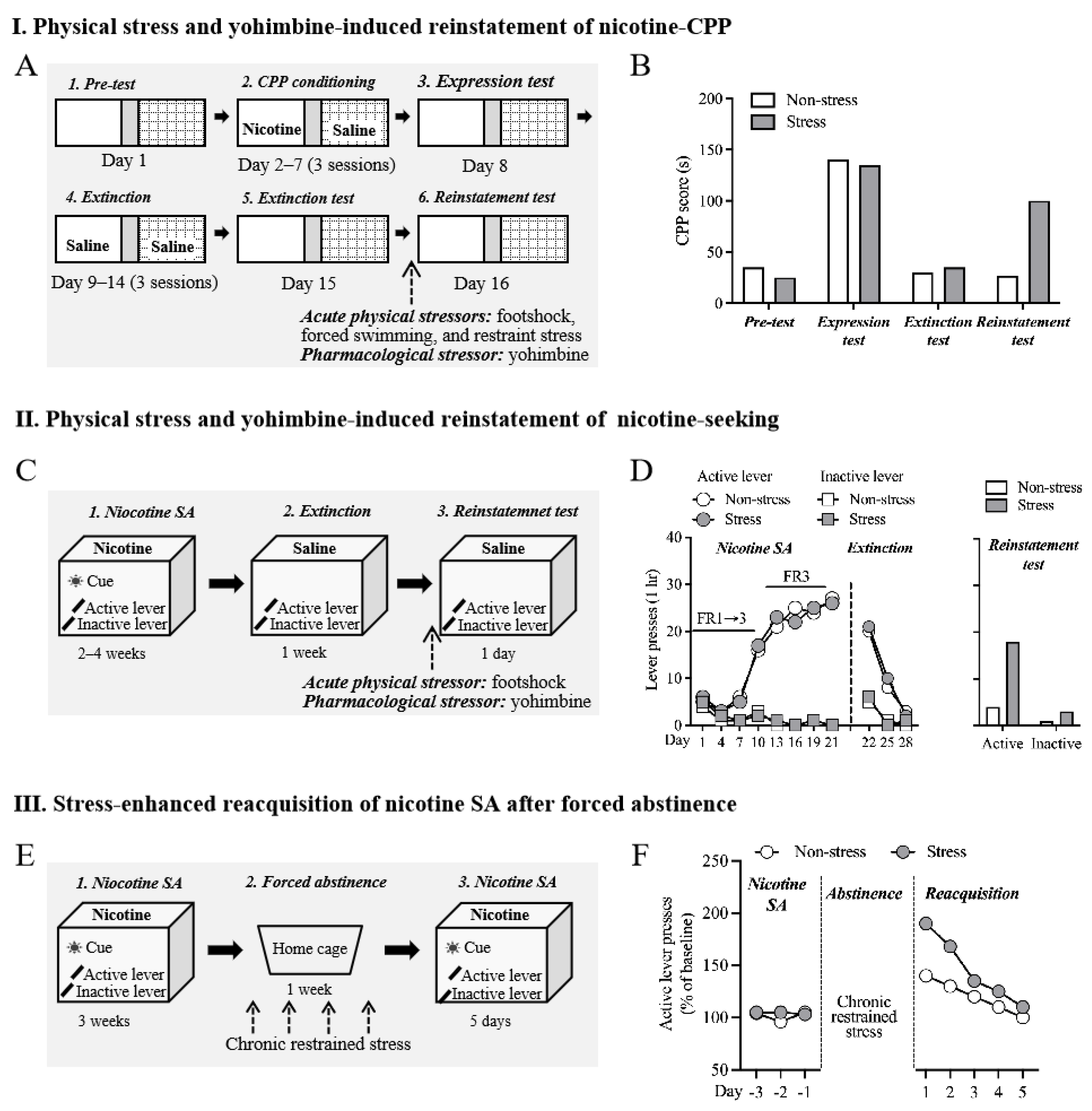
2.2. Physiological Stress-Induced Nicotine Relapse
2.3. Yohimbine-Induced Reinstatement of Nicotine-Seeking and Nicotine CPP
2.4. Stress-Enhanced Reacquisition after Extinction after Abstinence
3. Therapeutic Targets for Stress-Induced Nicotine Relapse
3.1. α3β4 nAChR
3.2. α2-Adrenergic Receptor
3.3. CB1 Receptor
3.4. Trace Amine-Associated Receptor 1 (TAAR1)
3.5. Neuropeptide Systems
3.6. CRF
3.7. Dynorphin and Kappa Opioid Receptor (KOR)
3.8. Other Peptide Systems
4. Factors That Influence the Effects of Stress on Nicotine Relapse
5. Social Stress-Induced Nicotine Relapse
6. Perspectives on Future Research Directions
6.1. The Brain System Actively Involved in Early Withdrawal
6.2. Stress-Induced Nicotine Relapse and Acute Withdrawal Symptoms
6.3. Stress Response May Predict Relapse
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, T.W.; Asman, K.; Gentzke, A.S.; Cullen, K.A.; Holder-Hayes, E.; Reyes-Guzman, C.; Jamal, A.; Neff, L.; King, B.A. Tobacco Product Use Among Adults—United States, 2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Gendall, P.; Hoek, J.; Stanley, J.; Jenkins, M.; Every-Palmer, S. Changes in Tobacco Use During the 2020 COVID-19 Lockdown in New Zealand. Nicotine Tob. Res. 2021, 23, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Shahab, L.; Dobbie, F.; Hiscock, R.; McNeill, A.; Bauld, L. Prevalence and Impact of Long-term Use of Nicotine Replacement Therapy in UK Stop-Smoking Services: Findings From the ELONS Study. Nicotine Tob. Res. 2017, 20, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Gorniak, B.; Yong, H.H.; Borland, R.; Cummings, K.M.; Thrasher, J.F.; McNeill, A.; Hyland, A.; Fong, G.T. Do post-quitting experiences predict smoking relapse among former smokers in Australia and the United Kingdom? Findings from the International Tobacco Control Surveys. Drug Alcohol Rev. 2022, 41, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Torres, O.V.; O’Dell, L.E. Stress is a principal factor that promotes tobacco use in females. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 260–268. [Google Scholar] [CrossRef]
- Bruijnzeel, A.W. Tobacco addiction and the dysregulation of brain stress systems. Neurosci. Biobehav. Rev. 2012, 36, 1418–1441. [Google Scholar] [CrossRef]
- Richards, J.M.; Stipelman, B.A.; Bornovalova, M.A.; Daughters, S.B.; Sinha, R.; Lejuez, C.W. Biological mechanisms underlying the relationship between stress and smoking: State of the science and directions for future work. Biol. Psychol. 2011, 88, 1–12. [Google Scholar] [CrossRef]
- Pomerleau, O.F.; Pomerleau, C.S. Research on stress and smoking: Progress and problems. Br. J. Addict. 1991, 86, 599–603. [Google Scholar] [CrossRef]
- Woodcock, E.A.; Stanley, J.A.; Diwadkar, V.A.; Khatib, D.; Greenwald, M.K. A neurobiological correlate of stress-induced nicotine-seeking behavior among cigarette smokers. Addict. Biol. 2020, 25, e12819. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, S.; Maier, S.U.; Lee, S.A.; Goldfarb, E.V.; Ahn, W.Y. Acute stress enhances memory and preference for smoking-related associations in smokers. Nicotine Tob. Res. 2023, ntad152. [Google Scholar] [CrossRef]
- Koob, G.F. Dynamics of neuronal circuits in addiction: Reward, antireward, and emotional memory. Pharmacopsychiatry 2009, 42 (Suppl. S1), S32–S41. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Vendruscolo, L. Theoretical Frameworks and Mechanistic Aspects of Alcohol Addiction: Alcohol Addiction as a Reward Deficit/Stress Surfeit Disorder; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Sharp, B.M. Basolateral amygdala, nicotinic cholinergic receptors, and nicotine: Pharmacological effects and addiction in animal models and humans. Eur. J. Neurosci. 2019, 50, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Ossip-Klein, D.J.; Bigelow, G.; Parker, S.R.; Curry, S.; Hall, S.; Kirkland, S. Classification and assessment of smoking behavior. Health Psychol. 1986, 5, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Oberleitner, L.M.S.; Moore, K.E.; Verplaetse, T.; Roberts, W.; McKee, S.A. Developing a laboratory model of smoking lapse targeting stress and brief nicotine deprivation. Exp. Clin. Psychopharmacol. 2018, 26, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Erb, S.; Shaham, Y.; Stewart, J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology 1996, 128, 408–412. [Google Scholar] [CrossRef]
- Shaham, Y.; Stewart, J. Stress reinstates heroin-seeking in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacology 1995, 119, 334–341. [Google Scholar] [CrossRef]
- Buczek, Y.; Le, A.D.; Wang, A.; Stewart, J.; Shaham, Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology 1999, 144, 183–188. [Google Scholar] [CrossRef]
- Jackson, A.; Bagdas, D.; Muldoon, P.P.; Lichtman, A.H.; Carroll, F.I.; Greenwald, M.; Miles, M.F.; Damaj, M.I. In vivo interactions between alpha7 nicotinic acetylcholine receptor and nuclear peroxisome proliferator-activated receptor-alpha: Implication for nicotine dependence. Neuropharmacology 2017, 118, 38–45. [Google Scholar] [CrossRef]
- Mantsch, J.R.; Baker, D.A.; Funk, D.; Le, A.D.; Shaham, Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology 2016, 41, 335–356. [Google Scholar] [CrossRef]
- Le, A.D.; Li, Z.; Funk, D.; Shram, M.; Li, T.K.; Shaham, Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J. Neurosci. 2006, 26, 1872–1879. [Google Scholar] [CrossRef]
- Fredriksson, I.; Venniro, M.; Reiner, D.J.; Chow, J.J.; Bossert, J.M.; Shaham, Y. Animal Models of Drug Relapse and Craving after Voluntary Abstinence: A Review. Pharmacol. Rev. 2021, 73, 1050–1083. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.A.; Jin, M.; Shaham, Y. Animal Models of (or for) Aggression Reward, Addiction, and Relapse: Behavior and Circuits. J. Neurosci. 2019, 39, 3996–4008. [Google Scholar] [CrossRef]
- Liu, J.; Totty, M.S.; Melissari, L.; Bayer, H.; Maren, S. Convergent Coding of Recent and Remote Fear Memory in the Basolateral Amygdala. Biol. Psychiatry 2022, 91, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, T.; Zhang, Y.; Gao, Y.; Sun, Z.; Li, W.; Cheng, H.; Gu, Y.; Abumaria, N. A neural circuit for regulating a behavioral switch in response to prolonged uncontrollability in mice. Neuron 2023, 111, 2727–2741.e7. [Google Scholar] [CrossRef] [PubMed]
- Chudoba, R.; Dabrowska, J. Distinct populations of corticotropin-releasing factor (CRF) neurons mediate divergent yet complementary defensive behaviors in response to a threat. Neuropharmacology 2023, 228, 109461. [Google Scholar] [CrossRef]
- Stretch, R.; Gerber, G.J. Drug-induced reinstatement of amphetamine self-administration behaviour in monkeys. Can. J. Psychol. 1973, 27, 168–177. [Google Scholar] [CrossRef]
- Shaham, Y.; Rajabi, H.; Stewart, J. Relapse to heroin-seeking in rats under opioid maintenance: The effects of stress, heroin priming, and withdrawal. J. Neurosci. 1996, 16, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Maldonado, R.; Berrendero, F. THC exposure during adolescence does not modify nicotine reinforcing effects and relapse in adult male mice. Psychopharmacology 2020, 237, 801–809. [Google Scholar] [CrossRef]
- Qi, X.; Yamada, H.; Corrie, L.W.; Ji, Y.; Bauzo, R.M.; Alexander, J.C.; Bruijnzeel, A.W. A critical role for the melanocortin 4 receptor in stress-induced relapse to nicotine seeking in rats. Addict. Biol. 2015, 20, 324–335. [Google Scholar] [CrossRef]
- Leao, R.M.; Cruz, F.C.; Planeta, C.S. Exposure to acute restraint stress reinstates nicotine-induced place preference in rats. Behav. Pharmacol. 2009, 20, 109–113. [Google Scholar] [CrossRef]
- Titomanlio, F.; Perfumi, M.; Mattioli, L. Rhodiola rosea L. extract and its active compound salidroside antagonized both induction and reinstatement of nicotine place preference in mice. Psychopharmacology 2014, 231, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Nygard, S.K.; Hourguettes, N.J.; Sobczak, G.G.; Carlezon, W.A.; Bruchas, M.R. Stress-Induced Reinstatement of Nicotine Preference Requires Dynorphin/Kappa Opioid Activity in the Basolateral Amygdala. J. Neurosci. 2016, 36, 9937–9948. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Shikano, K.; Kuroiwa, M.; Horikawa, M.; Ito, W.; Nishi, A.; Segi-Nishida, E.; Suzuki, H. Noradrenaline activation of hippocampal dopamine D(1) receptors promotes antidepressant effects. Proc. Natl. Acad. Sci. USA 2022, 119, e2117903119. [Google Scholar] [CrossRef] [PubMed]
- Bierwirth, P.; Stockhorst, U. Role of noradrenergic arousal for fear extinction processes in rodents and humans. Neurobiol. Learn. Mem. 2022, 194, 107660. [Google Scholar] [CrossRef] [PubMed]
- Varodayan, F.P.; Patel, R.R.; Matzeu, A.; Wolfe, S.A.; Curley, D.E.; Khom, S.; Gandhi, P.J.; Rodriguez, L.; Bajo, M.; D’Ambrosio, S.; et al. The Amygdala Noradrenergic System Is Compromised With Alcohol Use Disorder. Biol. Psychiatry 2022, 91, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Shepard, J.D.; Bossert, J.M.; Liu, S.Y.; Shaham, Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol. Psychiatry 2004, 55, 1082–1089. [Google Scholar] [CrossRef]
- Morgan, C.A., 3rd; Southwick, S.M.; Grillon, C.; Davis, M.; Krystal, J.H.; Charney, D.S. Yohimbine-facilitated acoustic startle reflex in humans. Psychopharmacology 1993, 110, 342–346. [Google Scholar] [CrossRef]
- Browne, R.G. Anxiolytics antagonize yohimbine’s discriminative stimulus properties. Psychopharmacology 1981, 74, 245–249. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E. Are the anxiogenic effects of yohimbine mediated by its action at benzodiazepine receptors? Neurosci. Lett. 1985, 55, 5–9. [Google Scholar] [CrossRef]
- Feltenstein, M.W.; Ghee, S.M.; See, R.E. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012, 121, 240–246. [Google Scholar] [CrossRef]
- Yamada, H.; Bruijnzeel, A.W. Stimulation of alpha2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropharmacology 2011, 60, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Zislis, G.; Desai, T.V.; Prado, M.; Shah, H.P.; Bruijnzeel, A.W. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology 2007, 53, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Chen, H.; Sharp, B.M. Amplified reacquisition of nicotine self-administration in rats by repeated stress during abstinence. Psychopharmacology 2014, 231, 3189–3195. [Google Scholar] [CrossRef]
- Yu, G.; Sharp, B.M. Basolateral amygdala and ventral hippocampus in stress-induced amplification of nicotine self-administration during reacquisition in rat. Psychopharmacology 2015, 232, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Biala, G.; Pekala, K.; Boguszewska-Czubara, A.; Michalak, A.; Kruk-Slomka, M.; Grot, K.; Budzynska, B. Behavioral and Biochemical Impact of Chronic Unpredictable Mild Stress on the Acquisition of Nicotine Conditioned Place Preference in Rats. Mol. Neurobiol. 2018, 55, 3270–3289. [Google Scholar] [CrossRef] [PubMed]
- Nic Dhonnchadha, B.A.; Szalay, J.J.; Achat-Mendes, C.; Platt, D.M.; Otto, M.W.; Spealman, R.D.; Kantak, K.M. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology 2010, 35, 357–367. [Google Scholar] [CrossRef]
- Achat-Mendes, C.; Nic Dhonnchadha, B.A.; Platt, D.M.; Kantak, K.M.; Spealman, R.D. Glycine transporter-1 inhibition preceding extinction training inhibits reacquisition of cocaine seeking. Neuropsychopharmacology 2012, 37, 2837–2845. [Google Scholar] [CrossRef]
- Sticht, M.; Mitsubata, J.; Tucci, M.; Leri, F. Reacquisition of heroin and cocaine place preference involves a memory consolidation process sensitive to systemic and intra-ventral tegmental area naloxone. Neurobiol. Learn. Mem. 2010, 93, 248–260. [Google Scholar] [CrossRef]
- Lu, L.; Xu, N.J.; Ge, X.; Yue, W.; Su, W.J.; Pei, G.; Ma, L. Reactivation of morphine conditioned place preference by drug priming: Role of environmental cues and sensitization. Psychopharmacology 2002, 159, 125–132. [Google Scholar] [CrossRef]
- Su, R.B.; Wang, W.P.; Lu, X.Q.; Wu, N.; Liu, Z.M.; Li, J. Agmatine blocks acquisition and re-acquisition of intravenous morphine self-administration in rats. Pharmacol. Biochem. Behav. 2009, 92, 676–682. [Google Scholar] [CrossRef]
- Evans, S.M.; Foltin, R.W.; Hicks, M.J.; Rosenberg, J.B.; De, B.P.; Janda, K.D.; Kaminsky, S.M.; Crystal, R.G. Efficacy of an adenovirus-based anti-cocaine vaccine to reduce cocaine self-administration and reacqusition using a choice procedure in rhesus macaques. Pharmacol. Biochem. Behav. 2016, 150–151, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Broomer, M.C.; Bouton, M.E. A comparison of renewal, spontaneous recovery, and reacquisition after punishment and extinction. Learn. Behav. 2023, 51, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Malagon, A.M.; Yasuda, D.; Belluzzi, J.D.; Leslie, F.M.; Zaveri, N.T. The alpha3beta4 nAChR partial agonist AT-1001 attenuates stress-induced reinstatement of nicotine seeking in a rat model of relapse and induces minimal withdrawal in dependent rats. Behav. Brain Res. 2017, 333, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Gueye, A.B.; Pryslawsky, Y.; Trigo, J.M.; Poulia, N.; Delis, F.; Antoniou, K.; Loureiro, M.; Laviolette, S.R.; Vemuri, K.; Makriyannis, A.; et al. The CB1 Neutral Antagonist AM4113 Retains the Therapeutic Efficacy of the Inverse Agonist Rimonabant for Nicotine Dependence and Weight Loss with Better Psychiatric Tolerability. Int. J. Neuropsychopharmacol. 2016, 19, pyw068. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Zabala, A.; Martin-Garcia, E.; de Lecea, L.; Maldonado, R.; Berrendero, F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J. Neurosci. 2010, 30, 2300–2310. [Google Scholar] [CrossRef] [PubMed]
- Bruijnzeel, A.W. kappa-Opioid receptor signaling and brain reward function. Brain Res. Rev. 2009, 62, 127–146. [Google Scholar] [CrossRef]
- Jackson, K.J.; McLaughlin, J.P.; Carroll, F.I.; Damaj, M.I. Effects of the kappa opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. Psychopharmacology 2013, 226, 763–768. [Google Scholar] [CrossRef]
- Grella, S.L.; Funk, D.; Coen, K.; Li, Z.; Le, A.D. Role of the kappa-opioid receptor system in stress-induced reinstatement of nicotine seeking in rats. Behav. Brain Res. 2014, 265, 188–197. [Google Scholar] [CrossRef]
- Hamidovic, A.; Khafaja, M.; Brandon, V.; Anderson, J.; Ray, G.; Allan, A.M.; Burge, M.R. Reduction of smoking urges with intranasal insulin: A randomized, crossover, placebo-controlled clinical trial. Mol. Psychiatry 2017, 22, 1413–1421. [Google Scholar] [CrossRef]
- Kearns, N.T.; Carl, E.; Stein, A.T.; Vujanovic, A.A.; Zvolensky, M.J.; Smits, J.A.J.; Powers, M.B. Posttraumatic stress disorder and cigarette smoking: A systematic review. Depress. Anxiety 2018, 35, 1056–1072. [Google Scholar] [CrossRef]
- Kotlyar, M.; Drone, D.; Thuras, P.; Hatsukami, D.K.; Brauer, L.; Adson, D.E.; al’Absi, M. Effect of stress and bupropion on craving, withdrawal symptoms, and mood in smokers. Nicotine Tob. Res. 2011, 13, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Sansone, L.; Milani, F.; Fabrizi, R.; Belli, M.; Cristina, M.; Zaga, V.; de Iure, A.; Cicconi, L.; Bonassi, S.; Russo, P. Nicotine: From Discovery to Biological Effects. Int. J. Mol. Sci. 2023, 24, 14570. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Picciotto, M.R. Nicotine addiction: More than just dopamine. Curr. Opin. Neurobiol. 2023, 83, 102797. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.A.; Kenny, P.J. Central and peripheral actions of nicotine that influence blood glucose homeostasis and the development of diabetes. Pharmacol. Res. 2023, 194, 106860. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, M.R.; Kenny, P.J. Mechanisms of Nicotine Addiction. Cold Spring Harb. Perspect. Med. 2021, 11, a039610. [Google Scholar] [CrossRef]
- Calabresi, P.; Lacey, M.G.; North, R.A. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br. J. Pharmacol. 1989, 98, 135–140. [Google Scholar] [CrossRef]
- Pidoplichko, V.I.; DeBiasi, M.; Williams, J.T.; Dani, J.A. Nicotine activates and desensitizes midbrain dopamine neurons. Nature 1997, 390, 401–404. [Google Scholar] [CrossRef]
- Le Houezec, J. Nicotine: Abused substance and therapeutic agent. J. Psychiatry Neurosci. 1998, 23, 95–108. [Google Scholar]
- Dani, J.A.; Ji, D.; Zhou, F.M. Synaptic plasticity and nicotine addiction. Neuron 2001, 31, 349–352. [Google Scholar] [CrossRef]
- Laviolette, S.R.; van der Kooy, D. The neurobiology of nicotine addiction: Bridging the gap from molecules to behaviour. Nat. Rev. Neurosci. 2004, 5, 55–65. [Google Scholar] [CrossRef]
- Dani, J.A.; De Biasi, M. Cellular mechanisms of nicotine addiction. Pharmacol. Biochem. Behav. 2001, 70, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Zoli, M.; Clementi, F. Brain nicotinic acetylcholine receptors: Native subtypes and their relevance. Trends Pharmacol. Sci. 2006, 27, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, G.G.; Schilstrom, B.; Hildebrand, B.E.; Panagis, G.; Grenhoff, J.; Svensson, T.H. Role of alpha7 nicotinic receptors in nicotine dependence and implications for psychiatric illness. Behav. Brain Res. 2000, 113, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.D.; Lu, Q.; Johnson, P.M.; Marks, M.J.; Kenny, P.J. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature 2011, 471, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Tapper, A.R.; McKinney, S.L.; Nashmi, R.; Schwarz, J.; Deshpande, P.; Labarca, C.; Whiteaker, P.; Marks, M.J.; Collins, A.C.; Lester, H.A. Nicotine activation of alpha4* receptors: Sufficient for reward, tolerance, and sensitization. Science 2004, 306, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, T.M.; Patzlaff, N.E.; Grady, S.R.; Heinemann, S.F.; Booker, T.K. alpha4beta2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. J. Neurosci. 2011, 31, 10891–10902. [Google Scholar] [CrossRef]
- Elayouby, K.S.; Ishikawa, M.; Dukes, A.J.; Smith, A.C.W.; Lu, Q.; Fowler, C.D.; Kenny, P.J. alpha3* Nicotinic Acetylcholine Receptors in the Habenula-Interpeduncular Nucleus Circuit Regulate Nicotine Intake. J. Neurosci. 2021, 41, 1779–1787. [Google Scholar] [CrossRef]
- Quick, M.W.; Ceballos, R.M.; Kasten, M.; McIntosh, J.M.; Lester, R.A. Alpha3beta4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology 1999, 38, 769–783. [Google Scholar] [CrossRef]
- Perry, D.C.; Xiao, Y.; Nguyen, H.N.; Musachio, J.L.; Davila-Garcia, M.I.; Kellar, K.J. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J. Neurochem. 2002, 82, 468–481. [Google Scholar] [CrossRef]
- Leslie, F.M.; Mojica, C.Y.; Reynaga, D.D. Nicotinic receptors in addiction pathways. Mol. Pharmacol. 2013, 83, 753–758. [Google Scholar] [CrossRef]
- Leri, F.; Flores, J.; Rodaros, D.; Stewart, J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J. Neurosci. 2002, 22, 5713–5718. [Google Scholar] [CrossRef] [PubMed]
- Forget, B.; Wertheim, C.; Mascia, P.; Pushparaj, A.; Goldberg, S.R.; Le Foll, B. Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology 2010, 35, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Erb, S.; Hitchcott, P.K.; Rajabi, H.; Mueller, D.; Shaham, Y.; Stewart, J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology 2000, 23, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.; Valverde, O.; Berrendero, F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006, 29, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Scherma, M.; Fadda, P.; Le Foll, B.; Forget, B.; Fratta, W.; Goldberg, S.R.; Tanda, G. The endocannabinoid system: A new molecular target for the treatment of tobacco addiction. CNS Neurol. Disord. Drug Targets 2008, 7, 468–481. [Google Scholar] [CrossRef]
- Berrendero, F.; Robledo, P.; Trigo, J.M.; Martin-Garcia, E.; Maldonado, R. Neurobiological mechanisms involved in nicotine dependence and reward: Participation of the endogenous opioid system. Neurosci. Biobehav. Rev. 2010, 35, 220–231. [Google Scholar] [CrossRef]
- He, Y.; Galaj, E.; Bi, G.H.; Wang, X.F.; Gardner, E.; Xi, Z.X. beta-Caryophyllene, a dietary terpenoid, inhibits nicotine taking and nicotine seeking in rodents. Br. J. Pharmacol. 2020, 177, 2058–2072. [Google Scholar] [CrossRef]
- Xi, Z.X.; He, Y.; Shen, H.; Bi, G.H.; Zhang, H.Y.; Soler-Cedeno, O.; Alton, H.; Yang, Y. GPR55 is expressed in glutamate neurons and functionally modulates nicotine taking and seeking in rats and mice. Res. Sq. 2023. [Google Scholar] [CrossRef]
- De Vries, T.J.; de Vries, W.; Janssen, M.C.; Schoffelmeer, A.N. Suppression of conditioned nicotine and sucrose seeking by the cannabinoid-1 receptor antagonist SR141716A. Behav. Brain Res. 2005, 161, 164–168. [Google Scholar] [CrossRef]
- Le Foll, B.; Goldberg, S.R. Rimonabant, a CB1 antagonist, blocks nicotine-conditioned place preferences. Neuroreport 2004, 15, 2139–2143. [Google Scholar] [CrossRef]
- Cohen, C.; Perrault, G.; Griebel, G.; Soubrie, P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: Reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716). Neuropsychopharmacology 2005, 30, 145–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Le Foll, B.; Forget, B.; Aubin, H.J.; Goldberg, S.R. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: Insights from pre-clinical and clinical studies. Addict. Biol. 2008, 13, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Castane, A.; Berrendero, F.; Maldonado, R. The role of the cannabinoid system in nicotine addiction. Pharmacol. Biochem. Behav. 2005, 81, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.; Perrault, G.; Voltz, C.; Steinberg, R.; Soubrie, P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav. Pharmacol. 2002, 13, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.; Le Foll, B. Novel therapeutic and drug development strategies for tobacco use disorder: Endocannabinoid modulation. Expert. Opin. Drug Discov. 2020, 15, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Carai, M.A.; Colombo, G.; Gessa, G.L. Rimonabant: The first therapeutically relevant cannabinoid antagonist. Life Sci. 2005, 77, 2339–2350. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.; Kodas, E.; Griebel, G. CB1 receptor antagonists for the treatment of nicotine addiction. Pharmacol. Biochem. Behav. 2005, 81, 387–395. [Google Scholar] [CrossRef]
- Simonnet, A.; Cador, M.; Caille, S. Nicotine reinforcement is reduced by cannabinoid CB1 receptor blockade in the ventral tegmental area. Addict. Biol. 2013, 18, 930–936. [Google Scholar] [CrossRef]
- Schindler, C.W.; Redhi, G.H.; Vemuri, K.; Makriyannis, A.; Le Foll, B.; Bergman, J.; Goldberg, S.R.; Justinova, Z. Blockade of Nicotine and Cannabinoid Reinforcement and Relapse by a Cannabinoid CB1-Receptor Neutral Antagonist AM4113 and Inverse Agonist Rimonabant in Squirrel Monkeys. Neuropsychopharmacology 2016, 41, 2283–2293. [Google Scholar] [CrossRef]
- Liu, J.; Wu, R.; Li, J.X. TAAR1 and Psychostimulant Addiction. Cell Mol. Neurobiol. 2020, 40, 229–238. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, R.; Chiellini, G.; Scanlan, T.S.; Grandy, D.K. Trace amine-associated receptors and their ligands. Br. J. Pharmacol. 2006, 149, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Borowsky, B.; Adham, N.; Jones, K.A.; Raddatz, R.; Artymyshyn, R.; Ogozalek, K.L.; Durkin, M.M.; Lakhlani, P.P.; Bonini, J.A.; Pathirana, S.; et al. Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 8966–8971. [Google Scholar] [CrossRef] [PubMed]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Revel, F.G.; Moreau, J.L.; Gainetdinov, R.R.; Bradaia, A.; Sotnikova, T.D.; Mory, R.; Durkin, S.; Zbinden, K.G.; Norcross, R.; Meyer, C.A.; et al. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc. Natl. Acad. Sci. USA 2011, 108, 8485–8490. [Google Scholar] [CrossRef]
- Bradaia, A.; Trube, G.; Stalder, H.; Norcross, R.D.; Ozmen, L.; Wettstein, J.G.; Pinard, A.; Buchy, D.; Gassmann, M.; Hoener, M.C.; et al. The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc. Natl. Acad. Sci. USA 2009, 106, 20081–20086. [Google Scholar] [CrossRef]
- Revel, F.G.; Moreau, J.L.; Gainetdinov, R.R.; Ferragud, A.; Velazquez-Sanchez, C.; Sotnikova, T.D.; Morairty, S.R.; Harmeier, A.; Groebke Zbinden, K.; Norcross, R.D.; et al. Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics. Biol. Psychiatry 2012, 72, 934–942. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.T.; Wang, H.; Niu, W.P.; Zhang, C.C.; Zhang, Y.; Wang, X.D.; Si, T.M.; Su, Y.A. Role of trace amine-associated receptor 1 in the medial prefrontal cortex in chronic social stress-induced cognitive deficits in mice. Pharmacol. Res. 2021, 167, 105571. [Google Scholar] [CrossRef]
- Liu, J.F.; Seaman, R., Jr.; Siemian, J.N.; Bhimani, R.; Johnson, B.; Zhang, Y.; Zhu, Q.; Hoener, M.C.; Park, J.; Dietz, D.M.; et al. Role of trace amine-associated receptor 1 in nicotine’s behavioral and neurochemical effects. Neuropsychopharmacology 2018, 43, 2435–2444. [Google Scholar] [CrossRef]
- Sukhanov, I.; Dorofeikova, M.; Dolgorukova, A.; Dorotenko, A.; Gainetdinov, R.R. Trace Amine-Associated Receptor 1 Modulates the Locomotor and Sensitization Effects of Nicotine. Front. Pharmacol. 2018, 9, 329. [Google Scholar] [CrossRef]
- Wu, R.; Liu, J.; Johnson, B.; Huang, Y.; Zhang, Y.; Li, J.X. Activation of trace amine-associated receptor 1 attenuates nicotine withdrawal-related effects. Addict. Biol. 2022, 27, e13075. [Google Scholar] [CrossRef] [PubMed]
- Matzeu, A.; Martin-Fardon, R. Understanding the Role of Orexin Neuropeptides in Drug Addiction: Preclinical Studies and Translational Value. Front. Behav. Neurosci. 2021, 15, 787595. [Google Scholar] [CrossRef] [PubMed]
- al’Absi, M.; DeAngelis, B.; Nakajima, M.; Hatsukami, D.; Allen, S. Early life adversity and appetite hormones: The effects of smoking status, nicotine withdrawal, and relapse on ghrelin and peptide YY during smoking cessation. Addict. Behav. 2021, 118, 106866. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, A.M.; al’Absi, M. Changes in circulating peptide YY and ghrelin are associated with early smoking relapse. Biol. Psychol. 2018, 131, 43–48. [Google Scholar] [CrossRef] [PubMed]
- al’Absi, M.; Lemieux, A.; Hodges, J.S.; Allen, S. Circulating orexin changes during withdrawal are associated with nicotine craving and risk for smoking relapse. Addict. Biol. 2019, 24, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Zabala, A.; Maldonado, R.; Berrendero, F. The hypocretin/orexin system: Implications for drug reward and relapse. Mol. Neurobiol. 2012, 45, 424–439. [Google Scholar] [CrossRef]
- Bruijnzeel, A.W. Neuropeptide systems and new treatments for nicotine addiction. Psychopharmacology 2017, 234, 1419–1437. [Google Scholar] [CrossRef]
- Kelly, E.A.; Fudge, J.L. The neuroanatomic complexity of the CRF and DA systems and their interface: What we still don’t know. Neurosci. Biobehav. Rev. 2018, 90, 247–259. [Google Scholar] [CrossRef]
- Grieder, T.E.; Herman, M.A.; Contet, C.; Tan, L.A.; Vargas-Perez, H.; Cohen, A.; Chwalek, M.; Maal-Bared, G.; Freiling, J.; Schlosburg, J.E.; et al. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat. Neurosci. 2014, 17, 1751–1758. [Google Scholar] [CrossRef]
- de Leon Reyes, N.S.; Sierra Diaz, P.; Nogueira, R.; Ruiz-Pino, A.; Nomura, Y.; de Solis, C.A.; Schulkin, J.; Asok, A.; Leroy, F. Corticotropin-releasing hormone signaling from prefrontal cortex to lateral septum suppresses interaction with familiar mice. Cell 2023, 186, 4152–4171.e31. [Google Scholar] [CrossRef]
- Hauger, R.L.; Grigoriadis, D.E.; Dallman, M.F.; Plotsky, P.M.; Vale, W.W.; Dautzenberg, F.M. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol. Rev. 2003, 55, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010, 1314, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Buck, C.L.; Cohen, A.; Edwards, S.; Park, P.E.; Schlosburg, J.E.; Schmeichel, B.; Vendruscolo, L.F.; Wade, C.L.; Whitfield, T.W., Jr. Addiction as a stress surfeit disorder. Neuropharmacology 2014, 76 Pt B, 370–382. [Google Scholar] [CrossRef]
- George, O.; Ghozland, S.; Azar, M.R.; Cottone, P.; Zorrilla, E.P.; Parsons, L.H.; O’Dell, L.E.; Richardson, H.N.; Koob, G.F. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc. Natl. Acad. Sci. USA 2007, 104, 17198–17203. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Treweek, J.; Edwards, S.; Leao, R.M.; Schulteis, G.; Koob, G.F.; George, O. Extended access to nicotine leads to a CRF1 receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addict. Biol. 2015, 20, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Bruijnzeel, A.W.; Prado, M.; Isaac, S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol. Psychiatry 2009, 66, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Carlezon, W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.; Koob, G.F. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology 2010, 210, 121–135. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Gorraez de la Mora, T.; de la Torre-Rendon, F.; Gould, E. Mixed medullary-papillary carcinoma of the thyroid: A previously unrecognized variant of thyroid carcinoma. Hum. Pathol. 1990, 21, 1151–1155. [Google Scholar] [CrossRef]
- Al-Hasani, R.; McCall, J.G.; Bruchas, M.R. Exposure to chronic mild stress prevents kappa opioid-mediated reinstatement of cocaine and nicotine place preference. Front. Pharmacol. 2013, 4, 96. [Google Scholar] [CrossRef]
- Muschamp, J.W.; Hollander, J.A.; Thompson, J.L.; Voren, G.; Hassinger, L.C.; Onvani, S.; Kamenecka, T.M.; Borgland, S.L.; Kenny, P.J.; Carlezon, W.A., Jr. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc. Natl. Acad. Sci. USA 2014, 111, E1648–E1655. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Fukatsu, N.; Rahaman, S.M.; Mukai, Y.; Izawa, S.; Ono, D.; Kilduff, T.S.; Yamanaka, A. Deficiency of orexin signaling during sleep is involved in abnormal REM sleep architecture in narcolepsy. Proc. Natl. Acad. Sci. USA 2023, 120, e2301951120. [Google Scholar] [CrossRef]
- Beckenstrom, A.C.; Coloma, P.M.; Dawson, G.R.; Finlayson, A.K.; Malik, A.; Post, A.; Steiner, M.A.; Potenza, M.N. Use of experimental medicine approaches for the development of novel psychiatric treatments based on orexin receptor modulation. Neurosci. Biobehav. Rev. 2023, 147, 105107. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, J.D.W.; Krupp, K.T.; Jacobs, B.M.; Onserio, B.O.; Meyerink, B.L.; Cain, J.T.; Ronan, P.J.; Renner, K.J.; DiLeone, R.J.; Summers, C.H. Orexin 1 Receptor Antagonism in the Basolateral Amygdala Shifts the Balance From Pro- to Antistress Signaling and Behavior. Biol. Psychiatry 2022, 91, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.A.; Lu, Q.; Cameron, M.D.; Kamenecka, T.M.; Kenny, P.J. Insular hypocretin transmission regulates nicotine reward. Proc. Natl. Acad. Sci. USA 2008, 105, 19480–19485. [Google Scholar] [CrossRef] [PubMed]
- Lengel, D.; Kenny, P.J. New medications development for smoking cessation. Addict. Neurosci. 2023, 7, 100103. [Google Scholar] [CrossRef] [PubMed]
- Mellentin, A.I.; Finn, S.W.; Skot, L.; Thaysen-Petersen, D.; Mistarz, N.; Fink-Jensen, A.; Nielsen, D.G. The effectiveness of oxytocin for treating substance use disorders:A systematic review of randomized placebo-controlled trials. Neurosci. Biobehav. Rev. 2023, 151, 105185. [Google Scholar] [CrossRef]
- Van Hedger, K.; Bershad, A.K.; Lee, R.; de Wit, H. Effects of Intranasal Oxytocin on Stress-Induced Cigarette Craving in Daily Smokers. Nicotine Tob. Res. 2020, 22, 89–95. [Google Scholar] [CrossRef]
- McReynolds, J.R.; Wolf, C.P.; Starck, D.M.; Mathy, J.C.; Schaps, R.; Krause, L.A.; Hillard, C.J.; Mantsch, J.R. Role of mesolimbic cannabinoid receptor 1 in stress-driven increases in cocaine self-administration in male rats. Neuropsychopharmacology 2023, 48, 1121–1132. [Google Scholar] [CrossRef]
- Panin, F.; Lintas, A.; Diana, M. Nicotine-induced increase of dopaminergic mesoaccumbal neuron activity is prevented by acute restraint stress. In vivo electrophysiology in rats. Eur. Neuropsychopharmacol. 2014, 24, 1175–1180. [Google Scholar] [CrossRef]
- Enrico, P.; Sirca, D.; Mereu, M.; Peana, A.T.; Mercante, B.; Diana, M. Acute restraint stress prevents nicotine-induced mesolimbic dopaminergic activation via a corticosterone-mediated mechanism: A microdialysis study in the rat. Drug Alcohol Depend. 2013, 127, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Bilkei-Gorzo, A.; Racz, I.; Michel, K.; Darvas, M.; Maldonado, R.; Zimmer, A. A common genetic predisposition to stress sensitivity and stress-induced nicotine craving. Biol. Psychiatry 2008, 63, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.L. Epigenetics of Stress, Addiction, and Resilience: Therapeutic Implications. Mol. Neurobiol. 2016, 53, 545–560. [Google Scholar] [CrossRef] [PubMed]
- al’Absi, M.; Nakajima, M.; Lemieux, A. Impact of early life adversity on the stress biobehavioral response during nicotine withdrawal. Psychoneuroendocrinology 2018, 98, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.M.; Calarco, C.A.; McKee, S.A.; Mineur, Y.S.; Picciotto, M.R. Variability in nicotine conditioned place preference and stress-induced reinstatement in mice: Effects of sex, initial chamber preference, and guanfacine. Genes Brain Behav. 2020, 19, e12601. [Google Scholar] [CrossRef] [PubMed]
- al’Absi, M.; Nakajima, M.; Allen, S.; Lemieux, A.; Hatsukami, D. Sex differences in hormonal responses to stress and smoking relapse: A prospective examination. Nicotine Tob. Res. 2015, 17, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Yararbas, G.; Keser, A.; Kanit, L.; Pogun, S. Nicotine-induced conditioned place preference in rats: Sex differences and the role of mGluR5 receptors. Neuropharmacology 2010, 58, 374–382. [Google Scholar] [CrossRef]
- Xu, J.; Azizian, A.; Monterosso, J.; Domier, C.P.; Brody, A.L.; Fong, T.W.; London, E.D. Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob. Res. 2008, 10, 1653–1661. [Google Scholar] [CrossRef]
- Bohadana, A.; Nilsson, F.; Rasmussen, T.; Martinet, Y. Gender differences in quit rates following smoking cessation with combination nicotine therapy: Influence of baseline smoking behavior. Nicotine Tob. Res. 2003, 5, 111–116. [Google Scholar] [CrossRef]
- Ward, K.D.; Klesges, R.C.; Zbikowski, S.M.; Bliss, R.E.; Garvey, A.J. Gender differences in the outcome of an unaided smoking cessation attempt. Addict. Behav. 1997, 22, 521–533. [Google Scholar] [CrossRef]
- Wetter, D.W.; Kenford, S.L.; Smith, S.S.; Fiore, M.C.; Jorenby, D.E.; Baker, T.B. Gender differences in smoking cessation. J. Consult. Clin. Psychol. 1999, 67, 555–562. [Google Scholar] [CrossRef] [PubMed]
- McKee, S.A.; Maciejewski, P.K.; Falba, T.; Mazure, C.M. Sex differences in the effects of stressful life events on changes in smoking status. Addiction 2003, 98, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zou, S.; Coen, K.; Funk, D.; Shram, M.J.; Le, A.D. Sex differences in yohimbine-induced increases in the reinforcing efficacy of nicotine in adolescent rats. Addict. Biol. 2014, 19, 156–164. [Google Scholar] [CrossRef] [PubMed]
- al’Absi, M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int. J. Psychophysiol. 2006, 59, 218–227. [Google Scholar] [CrossRef] [PubMed]
- al’Absi, M.; Nakajima, M.; Grabowski, J. Stress response dysregulation and stress-induced analgesia in nicotine dependent men and women. Biol. Psychol. 2013, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wardle, M.C.; Munafo, M.R.; de Wit, H. Effect of social stress during acute nicotine abstinence. Psychopharmacology 2011, 218, 39–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schepis, T.S.; Tapscott, B.E.; Krishnan-Sarin, S. Stress-related increases in risk taking and attentional failures predict earlier relapse to smoking in young adults: A pilot investigation. Exp. Clin. Psychopharmacol. 2016, 24, 110–119. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, C.W.; Im, H.B.; Jang, M. Patterns and predictors of smoking relapse among inpatient smoking intervention participants: A 1-year follow-up study in Korea. Epidemiol. Health 2021, 43, e2021043. [Google Scholar] [CrossRef]
- Megias-Robles, A.; Perea-Baena, J.M.; Fernandez-Berrocal, P. The protective role of emotional intelligence in smoking relapse during a 12-month follow-up smoking cessation intervention. PLoS ONE 2020, 15, e0234301. [Google Scholar] [CrossRef]
- Koob, G.F.; Caine, S.B.; Parsons, L.; Markou, A.; Weiss, F. Opponent process model and psychostimulant addiction. Pharmacol. Biochem. Behav. 1997, 57, 513–521. [Google Scholar] [CrossRef]
- Epping-Jordan, M.P.; Watkins, S.S.; Koob, G.F.; Markou, A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature 1998, 393, 76–79. [Google Scholar] [CrossRef]
- Markou, A.; Kosten, T.R.; Koob, G.F. Neurobiological similarities in depression and drug dependence: A self-medication hypothesis. Neuropsychopharmacology 1998, 18, 135–174. [Google Scholar] [CrossRef] [PubMed]
- Ashare, R.L.; Lerman, C.; Cao, W.; Falcone, M.; Bernardo, L.; Ruparel, K.; Hopson, R.; Gur, R.; Pruessner, J.C.; Loughead, J. Nicotine withdrawal alters neural responses to psychosocial stress. Psychopharmacology 2016, 233, 2459–2467. [Google Scholar] [CrossRef] [PubMed]
- Hogle, J.M.; Kaye, J.T.; Curtin, J.J. Nicotine withdrawal increases threat-induced anxiety but not fear: Neuroadaptation in human addiction. Biol. Psychiatry 2010, 68, 719–725. [Google Scholar] [CrossRef] [PubMed]
- al’Absi, M.; Hatsukami, D.; Davis, G.L. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology 2005, 181, 107–117. [Google Scholar] [CrossRef]
- al’Absi, M. Stress and Addiction: When a Robust Stress Response Indicates Resiliency. Psychosom. Med. 2018, 80, 2–16. [Google Scholar] [CrossRef]
- Shaw, D.; al’Absi, M. Attenuated beta endorphin response to acute stress is associated with smoking relapse. Pharmacol. Biochem. Behav. 2008, 90, 357–362. [Google Scholar] [CrossRef][Green Version]
| Target | Intervention | Stress Type | Nicotine Relapse-like Behavior | Effect | Reference |
|---|---|---|---|---|---|
| α3β4 nAChR | Partial agonist AT-1001 (s.c.) | Yohimbine | Reinstatement of nicotine-seeking | Reduced | [54] |
| α2-AR | Agonist clonidine (s.c.) | Footshock | Reinstatement of nicotine-seeking | Reduced | [43] |
| Agonists clonidine and dexmedetomidine (intra-CeA) | Reduced | [42] | |||
| β1/β2-AR | Antagonist propranolol (intra-CeA) | Footshock | Reinstatement of nicotine-seeking | No effect | [42] |
| α1-AR | Antagonist prazosin (intra-CeA) | Footshock | Reinstatement of nicotine-seeking | No effect | [42] |
| CB1R | Neutral antagonist AM4113 (i.p.) | Yohimbine | Reinstatement of nicotine-seeking | Reduced | [55] |
| CRFR | CRF1/2R antagonist D-Phe CRF(12–41) (i.c.v.) | Footshock | Reinstatement of nicotine-seeking | Reduced | [43] |
| CRF1R antagonist antalarmin (s.c.) | Reduced | [56] | |||
| CRF1R antagonist R2789/CRA0450 (i.c.v.) | Reduced | [57] | |||
| CRF2R antagonist Astressin 2B (i.c.v.) | No effect | [57] | |||
| KOR | Antagonist nor-BNI (s.c.) | Forced swimming | Reinstatement of nicotine CPP | Reduced | [58] |
| Nor-BNI (i.p.) | Footshock and yohimbine | Reduced | [33] | ||
| Nor-BNI (intra-BLA) | Yohimbine | Reduced | [33] | ||
| Nor-BNI (i.p.) | Yohimbine | Reinstatement of nicotine-seeking | Reduced | [59] | |
| KOR agonist U50,488 (i.p.) | NA | Induced | [59] | ||
| OX1R | Antagonist SB334867 (i.p.) | Footshock | Reinstatement of nicotine-seeking | No effect | [56] |
| Orexin A peptide (i.c.v.) | NA | Induced | [56] | ||
| MC4R | Antagonists HS014 and HS024 (i.c.v.) | Footshock | Reinstatement of nicotine-seeking | Reduced | [30] |
| NA | Oxytocin (intranasal) | Psychosocial stress | Nicotine craving (human) | No effect | [60] |
| NA | Insulin (Intranasal) | Psychosocial stress | Nicotine cravings (human) | Reduced | [61] |
| NA | Bupropion | Psychosocial and physical stress | Nicotine cravings (human) | Reduced | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chen, Y.; Dong, J.; Ge, J.; Liu, X.; Liu, J. Neurobiology of Stress-Induced Nicotine Relapse. Int. J. Mol. Sci. 2024, 25, 1482. https://doi.org/10.3390/ijms25031482
Wang X, Chen Y, Dong J, Ge J, Liu X, Liu J. Neurobiology of Stress-Induced Nicotine Relapse. International Journal of Molecular Sciences. 2024; 25(3):1482. https://doi.org/10.3390/ijms25031482
Chicago/Turabian StyleWang, Xinyu, Yun Chen, Jing Dong, Jing Ge, Xiaoliu Liu, and Jianfeng Liu. 2024. "Neurobiology of Stress-Induced Nicotine Relapse" International Journal of Molecular Sciences 25, no. 3: 1482. https://doi.org/10.3390/ijms25031482
APA StyleWang, X., Chen, Y., Dong, J., Ge, J., Liu, X., & Liu, J. (2024). Neurobiology of Stress-Induced Nicotine Relapse. International Journal of Molecular Sciences, 25(3), 1482. https://doi.org/10.3390/ijms25031482




