Mitochondrial Dysfunction, Oxidative Stress, and Inter-Organ Miscommunications in T2D Progression
Abstract
1. Introduction
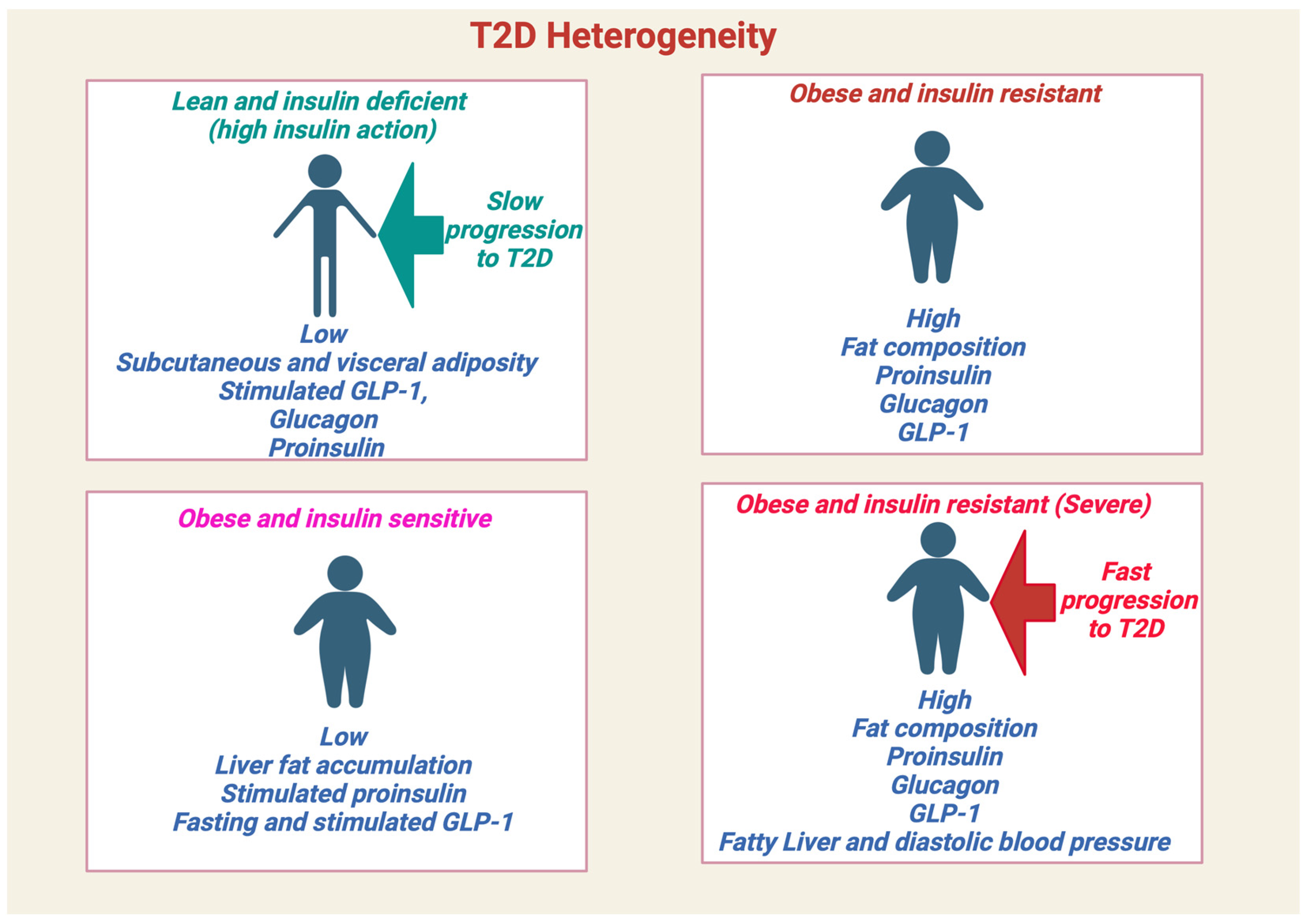
1.1. Prediabetes and Type 2 Diabetes
1.2. Oxidative Stress
2. Physiological ROS Production and Glucose Metabolism in Health
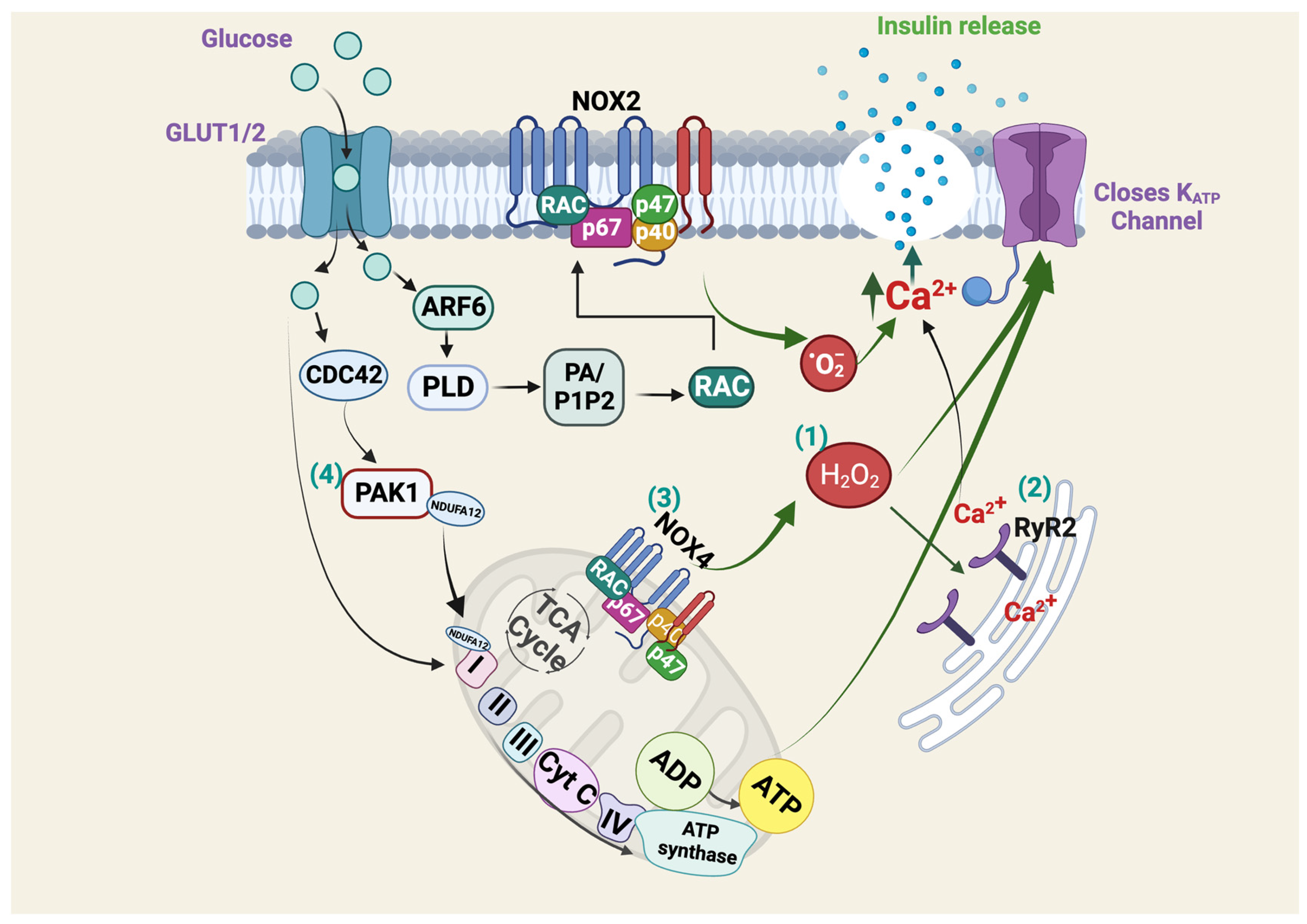
2.1. Glucose Sensing, Insulin Secretion and Islet β-Cells
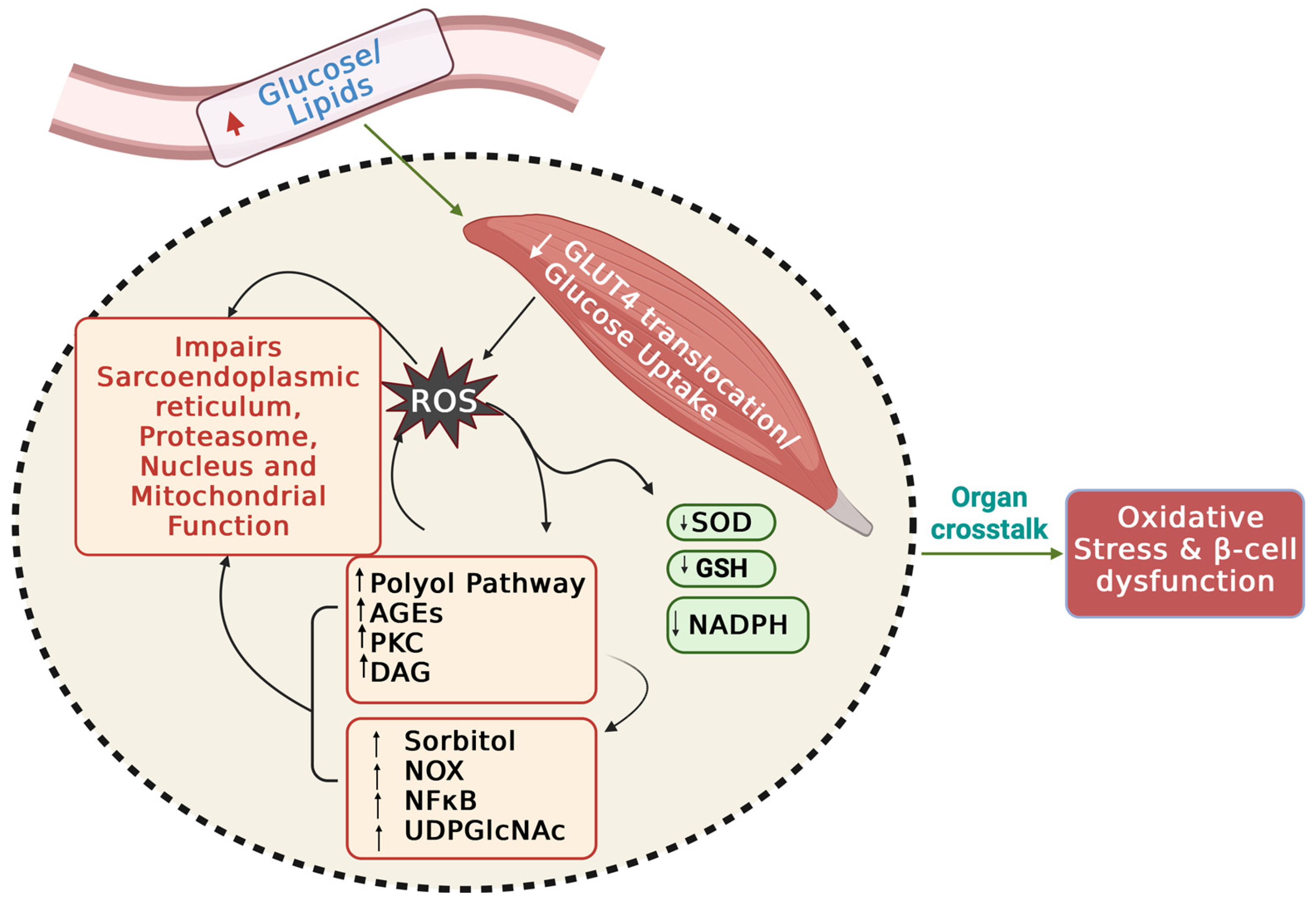
2.2. Glucose Uptake and Skeletal Muscle Function
3. Impaired Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Insulin Resistance
3.1. Impaired Mitochondrial Dynamics in Skeletal Muscle IR
3.2. Molecular Pathways Involved in Mitophagy: A Novel Role for STX4?
3.3. Mitophagy in Skeletal Muscle IR: Helpful or Harmful?
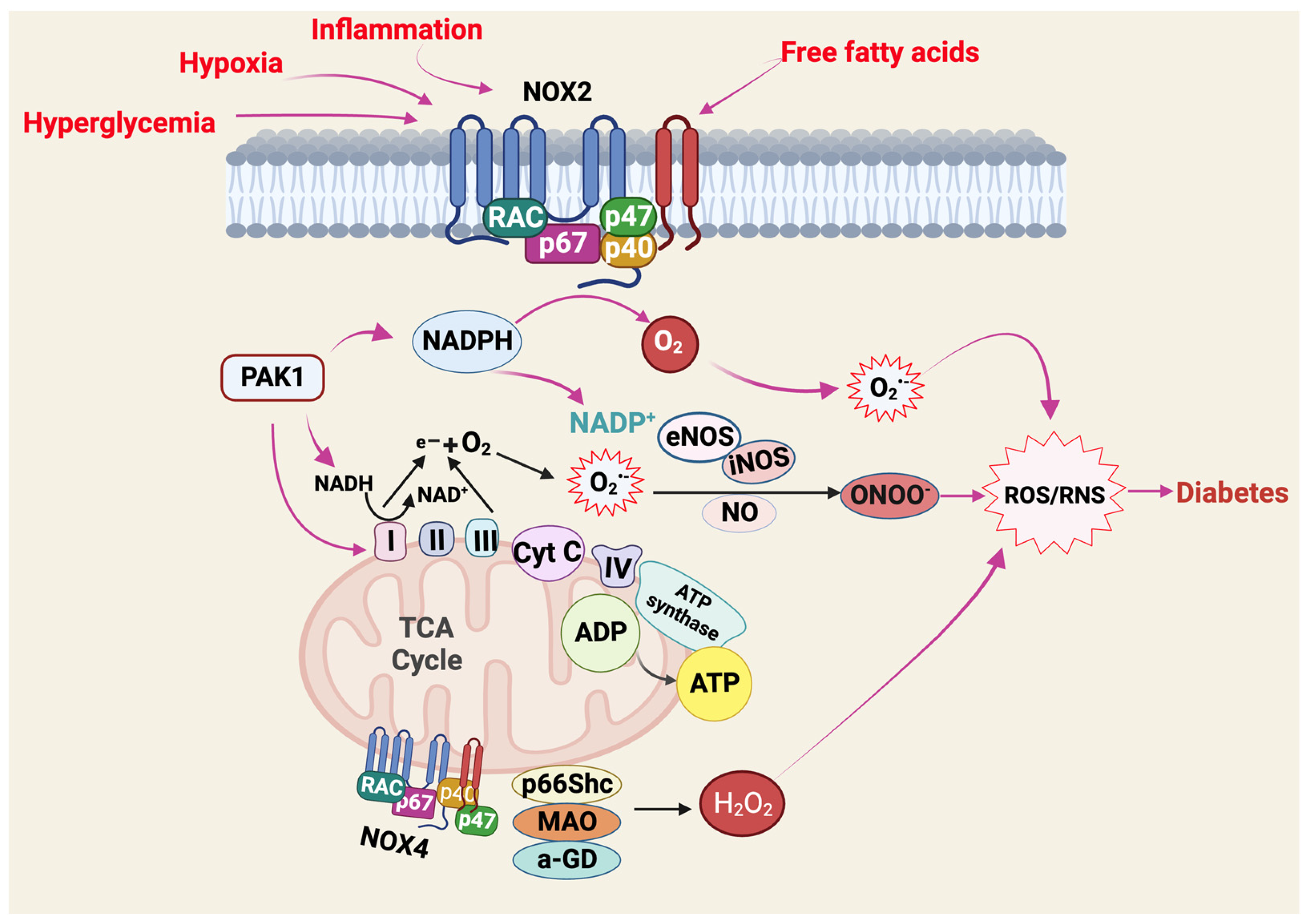
4. ROS-Related Molecular Pathways Underlying IR and T2D
5. Mitochondrial Dysfunction and Islet β-Cell Dysfunction/Failure
5.1. Abnormal Mitochondrial Function in PAK1-Deficient β-Cells
5.2. Abnormal Islet β-Cell Mitochondrial Morphology and Dynamics
5.3. Mitophagy and Elevating Islet β-Cell Dysfunction
5.4. Mitochondrial-Endoplasmic Reticulum Miscommunication in Islet β-Cell Dysfunction
6. Inter-Organ Miscommunications and Oxidative Stress in Insulin Resistance
6.1. Skeletal Muscle-Derived EVs-Islet β-Cell Crosstalk
6.2. Adipose-Islet β-Cell Crosstalk and Mitochondrial Transfer
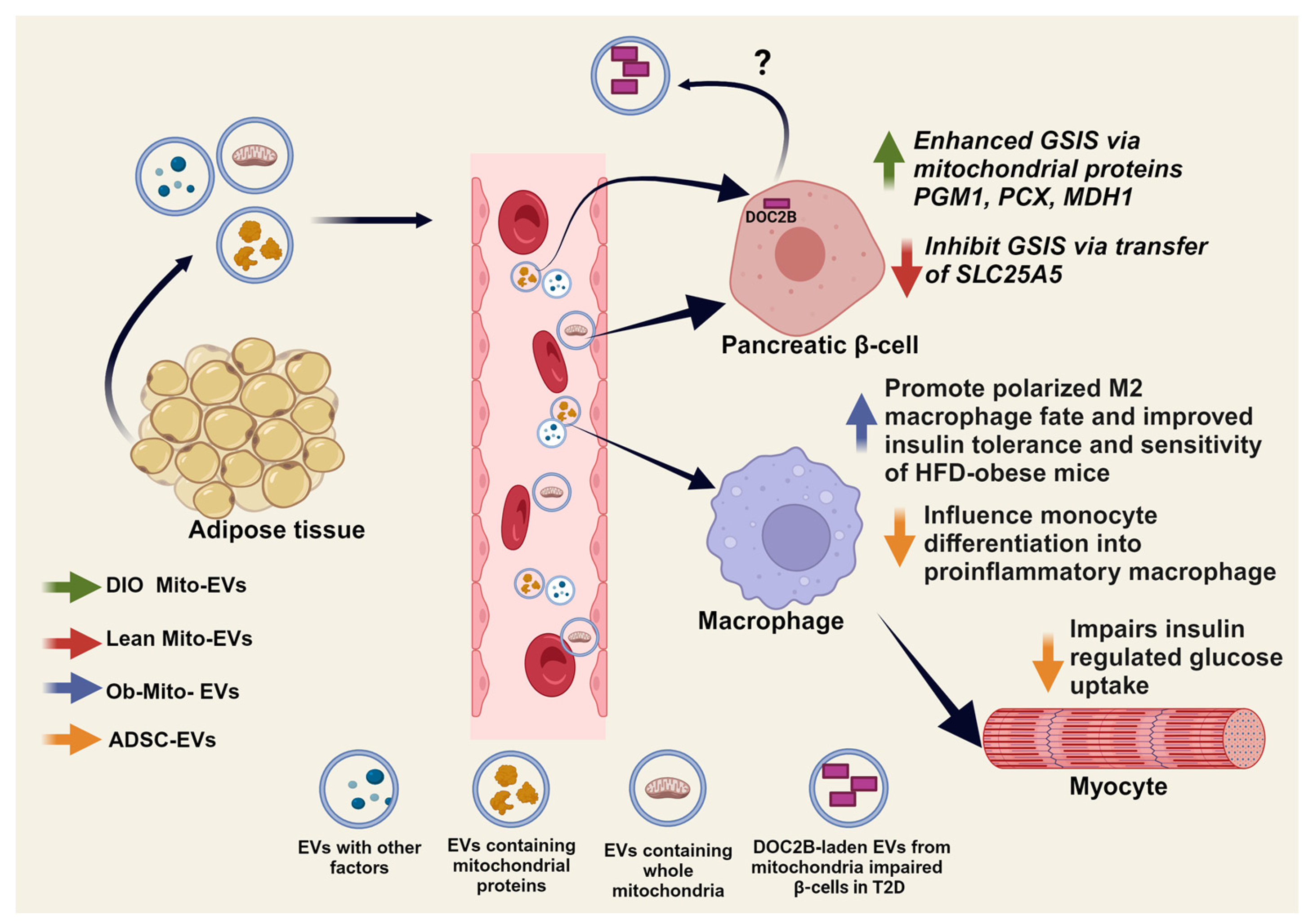
6.3. Adipose-Immune Cell Crosstalk and Mitochondrial Transfer
7. Antioxidant Interventions for Prediabetes and T2D
8. Perspectives: Mitochondria and ROS as Therapeutic Targets for Prediabetes
Author Contributions
Funding
Conflicts of Interest
References
- Cefalu, W.T.; Andersen, D.K.; Arreaza-Rubín, G.; Pin, C.L.; Sato, S.; Verchere, C.B.; Woo, M.; Rosenblum, N.D. Heterogeneity of Diabetes: β-Cells, Phenotypes, and Precision Medicine: Proceedings of an International Symposium of the Canadian Institutes of Health Research’s Institute of Nutrition, Metabolism and Diabetes and the U.S. National Institutes of Health’s National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes Care 2022, 45, 3–22. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47, S20–S42. [Google Scholar] [CrossRef]
- Wesolowska-Andersen, A.; Brorsson, C.A.; Bizzotto, R.; Mari, A.; Tura, A.; Koivula, R.; Mahajan, A.; Vinuela, A.; Tajes, J.F.; Sharma, S.; et al. Four groups of type 2 diabetes contribute to the etiological and clinical heterogeneity in newly diagnosed individuals: An IMI DIRECT study. Cell Rep. Med. 2022, 3, 100477. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Tojjar, D.; Yamada, S.; Toda, K.; Patel, C.J.; Butte, A.J. Ethnic differences in the relationship between insulin sensitivity and insulin response: A systematic review and meta-analysis. Diabetes Care 2013, 36, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Vicks, W.S.; Lo, J.C.; Guo, L.; Rana, J.S.; Zhang, S.; Ramalingam, N.D.; Gordon, N.P. Prevalence of prediabetes and diabetes vary by ethnicity among U.S. Asian adults at healthy weight, overweight, and obesity ranges: An electronic health record study. BMC Public Health 2022, 22, 1954. [Google Scholar] [CrossRef] [PubMed]
- Staimez, L.R.; Weber, M.B.; Ranjani, H.; Ali, M.K.; Echouffo-Tcheugui, J.B.; Phillips, L.S.; Mohan, V.; Narayan, K.M. Evidence of reduced beta-cell function in Asian Indians with mild dysglycemia. Diabetes Care 2013, 36, 2772–2778. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Narayan, K.M.V.; Chan, J.C.N.; Jha, P.; Shah, B.R. Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations. Nat. Rev. Endocrinol. 2022, 18, 413–432. [Google Scholar] [CrossRef]
- Kanaya, A.M.; Herrington, D.; Vittinghoff, E.; Ewing, S.K.; Liu, K.; Blaha, M.J.; Dave, S.S.; Qureshi, F.; Kandula, N.R. Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: The MASALA and MESA studies. Diabetes Care 2014, 37, 1621–1628. [Google Scholar] [CrossRef]
- Gopaul, N.K.; Manraj, M.D.; Hebe, A.; Lee Kwai Yan, S.; Johnston, A.; Carrier, M.J.; Anggard, E.E. Oxidative stress could precede endothelial dysfunction and insulin resistance in Indian Mauritians with impaired glucose metabolism. Diabetologia 2001, 44, 706–712. [Google Scholar] [CrossRef]
- Urakawa, H.; Katsuki, A.; Sumida, Y.; Gabazza, E.C.; Murashima, S.; Morioka, K.; Maruyama, N.; Kitagawa, N.; Tanaka, T.; Hori, Y.; et al. Oxidative stress is associated with adiposity and insulin resistance in men. J. Clin. Endocrinol. Metab. 2003, 88, 4673–4676. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Hudish, L.I.; Reusch, J.E.; Sussel, L. Beta Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J. Clin. Investig. 2019, 129, 4001–4008. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef]
- Dimova, R.; Chakarova, N.; Grozeva, G.; Kirilov, G.; Tankova, T. The relationship between glucose variability and insulin sensitivity and oxidative stress in subjects with prediabetes. Diabetes Res. Clin. Pract. 2019, 158, 107911. [Google Scholar] [CrossRef] [PubMed]
- Samocha-Bonet, D.; Heilbronn, L.K.; Lichtenberg, D.; Campbell, L.V. Does skeletal muscle oxidative stress initiate insulin resistance in genetically predisposed individuals? Trends Endocrinol. Metab. 2010, 21, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabres, M.; Capo, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaset-Ismail, A.; Ciechanowicz, A.K.; Bujko, K.; Ratajczak, J.; Kucia, M.; Ratajczak, M.Z. The Nox2-ROS-Nlrp3 Inflammasome Signaling Stimulates in the Hematopoietic Stem/Progenitor Cells Lipogenesis to Facilitate Membrane Lipid Raft Formation. Stem Cell Rev. Rep. 2023, 19, 92–103. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Rolling, L.; Sikora, V.; Chen, Z.; Gurwin, J.; Barabell, C.; Lin, J.; Duan, C. ROS signaling-induced mitochondrial Sgk1 expression regulates epithelial cell renewal. Proc. Natl. Acad. Sci. USA 2023, 120, e2216310120. [Google Scholar] [CrossRef]
- Pi, J.; Bai, Y.; Zhang, Q.; Wong, V.; Floering, L.M.; Daniel, K.; Reece, J.M.; Deeney, J.T.; Andersen, M.E.; Corkey, B.E.; et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 2007, 56, 1783–1791. [Google Scholar] [CrossRef]
- Plecita-Hlavata, L.; Jaburek, M.; Holendova, B.; Tauber, J.; Pavluch, V.; Berkova, Z.; Cahova, M.; Schroder, K.; Brandes, R.P.; Siemen, D.; et al. Glucose-Stimulated Insulin Secretion Fundamentally Requires H2O2 Signaling by NADPH Oxidase 4. Diabetes 2020, 69, 1341–1354. [Google Scholar] [CrossRef]
- Merry, T.L.; Steinberg, G.R.; Lynch, G.S.; McConell, G.K. Skeletal muscle glucose uptake during contraction is regulated by nitric oxide and ROS independently of AMPK. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E577–E585. [Google Scholar] [CrossRef]
- Henriquez-Olguin, C.; Knudsen, J.R.; Raun, S.H.; Li, Z.; Dalbram, E.; Treebak, J.T.; Sylow, L.; Holmdahl, R.; Richter, E.A.; Jaimovich, E.; et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019, 10, 4623. [Google Scholar] [CrossRef]
- Xirouchaki, C.E.; Jia, Y.; McGrath, M.J.; Greatorex, S.; Tran, M.; Merry, T.L.; Hong, D.; Eramo, M.J.; Broome, S.C.; Woodhead, J.S.T.; et al. Skeletal muscle NOX4 is required for adaptive responses that prevent insulin resistance. Sci. Adv. 2021, 7, eabl4988. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Nazarewicz, R.R.; Bikineyeva, A.; Hilenski, L.; Lassegue, B.; Griendling, K.K.; Harrison, D.G.; Dikalova, A.E. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid. Redox Signal. 2014, 20, 281–294. [Google Scholar] [CrossRef]
- Kroller-Schon, S.; Steven, S.; Kossmann, S.; Scholz, A.; Daub, S.; Oelze, M.; Xia, N.; Hausding, M.; Mikhed, Y.; Zinssius, E.; et al. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid. Redox Signal. 2014, 20, 247–266. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Harrison, D.E.; Ashcroft, S.J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature 1984, 312, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.L.; Hales, C.N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature 1984, 311, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.E.; Newgard, C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 2021, 22, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Camargo, L.L.; Harvey, A.P.; Rios, F.J.; Tsiropoulou, S.; Da Silva, R.N.O.; Cao, Z.; Graham, D.; McMaster, C.; Burchmore, R.J.; Hartley, R.C.; et al. Vascular Nox (NADPH Oxidase) Compartmentalization, Protein Hyperoxidation, and Endoplasmic Reticulum Stress Response in Hypertension. Hypertension 2018, 72, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Block, K.; Gorin, Y.; Abboud, H.E. Subcellular localization of Nox4 and regulation in diabetes. Proc. Natl. Acad. Sci. USA 2009, 106, 14385–14390. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, K.; Nayak, B.K.; Friedrichs, W.E.; Kaushik, D.; Rodriguez, R.; Block, K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat. Commun. 2017, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nuevo, A.; Torres-Sanchez, A.; Duran, J.M.; De Guirior, C.; Martinez-Zamora, M.A.; Boke, E. Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I. Nature 2022, 607, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Suman, S.; Kallakury, B.V.; Fornace, A.J., Jr. Exposure to heavy ion radiation induces persistent oxidative stress in mouse intestine. PLoS ONE 2012, 7, e42224. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, C.D.; Roy, N.; Turner-Brannen, E.; Schultz, A.; Vaghasiya, J.; Ravandi, A.; Halayko, A.J.; West, A.R. Oxidized phosphatidylcholines induce multiple functional defects in airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 321, L703–L717. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Brun, T.; Cnop, M.; Cunha, D.A.; Eizirik, D.L.; Maechler, P. Transient oxidative stress damages mitochondrial machinery inducing persistent beta-cell dysfunction. J. Biol. Chem. 2009, 284, 23602–23612. [Google Scholar] [CrossRef]
- Urbano, F.; Bugliani, M.; Filippello, A.; Scamporrino, A.; Di Mauro, S.; Di Pino, A.; Scicali, R.; Noto, D.; Rabuazzo, A.M.; Averna, M.; et al. Atorvastatin but Not Pravastatin Impairs Mitochondrial Function in Human Pancreatic Islets and Rat beta-Cells. Direct Effect of Oxidative Stress. Sci. Rep. 2017, 7, 11863. [Google Scholar] [CrossRef]
- Vanhorebeek, I.; De Vos, R.; Mesotten, D.; Wouters, P.J.; De Wolf-Peeters, C.; Van den Berghe, G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet 2005, 365, 53–59. [Google Scholar] [CrossRef]
- Kelley, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef]
- Vincent, A.M.; Brownlee, M.; Russell, J.W. Oxidative stress and programmed cell death in diabetic neuropathy. Ann. N. Y. Acad. Sci. 2002, 959, 368–383. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Erdos, M.R.; Huyghe, J.R.; Welch, R.P.; Beck, A.T.; Wolford, B.N.; Chines, P.S.; Didion, J.P.; Narisu, N.; Stringham, H.M.; et al. The genetic regulatory signature of type 2 diabetes in human skeletal muscle. Nat. Commun. 2016, 7, 11764. [Google Scholar] [CrossRef] [PubMed]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef]
- Patti, M.E.; Butte, A.J.; Crunkhorn, S.; Cusi, K.; Berria, R.; Kashyap, S.; Miyazaki, Y.; Kohane, I.; Costello, M.; Saccone, R.; et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA 2003, 100, 8466–8471. [Google Scholar] [CrossRef] [PubMed]
- La Sala, L.; Mrakic-Sposta, S.; Tagliabue, E.; Prattichizzo, F.; Micheloni, S.; Sangalli, E.; Specchia, C.; Uccellatore, A.C.; Lupini, S.; Spinetti, G.; et al. Circulating microRNA-21 is an early predictor of ROS-mediated damage in subjects with high risk of developing diabetes and in drug-naive T2D. Cardiovasc. Diabetol. 2019, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.; Rountree, A.; Kernan, K.; Van Yserloo, B.; Zhang, H.; Reed, B.J.; Osborne, W.; Wang, W.; Sweet, I.R. Real-time imaging of intracellular hydrogen peroxide in pancreatic islets. Biochem. J. 2016, 473, 4443–4456. [Google Scholar] [CrossRef] [PubMed]
- Llanos, P.; Contreras-Ferrat, A.; Barrientos, G.; Valencia, M.; Mears, D.; Hidalgo, C. Glucose-Dependent Insulin Secretion in Pancreatic beta-Cell Islets from Male Rats Requires Ca2+ Release via ROS-Stimulated Ryanodine Receptors. PLoS ONE 2015, 10, e0129238. [Google Scholar] [CrossRef]
- Syed, I.; Kyathanahalli, C.N.; Jayaram, B.; Govind, S.; Rhodes, C.J.; Kowluru, R.A.; Kowluru, A. Increased phagocyte-like NADPH oxidase and ROS generation in type 2 diabetic ZDF rat and human islets: Role of Rac1-JNK1/2 signaling pathway in mitochondrial dysregulation in the diabetic islet. Diabetes 2011, 60, 2843–2852. [Google Scholar] [CrossRef]
- Jayaram, B.; Kowluru, A. Phagocytic NADPH oxidase links ARNO-Arf6 signaling pathway in glucose-stimulated insulin secretion from the pancreatic beta-cell. Cell Physiol. Biochem. 2012, 30, 1351–1362. [Google Scholar] [CrossRef]
- Leloup, C.; Tourrel-Cuzin, C.; Magnan, C.; Karaca, M.; Castel, J.; Carneiro, L.; Colombani, A.L.; Ktorza, A.; Casteilla, L.; Penicaud, L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 2009, 58, 673–681. [Google Scholar] [CrossRef]
- Affourtit, C.; Brand, M.D. Uncoupling protein-2 contributes significantly to high mitochondrial proton leak in INS-1E insulinoma cells and attenuates glucose-stimulated insulin secretion. Biochem. J. 2008, 409, 199–204. [Google Scholar] [CrossRef]
- Affourtit, C.; Jastroch, M.; Brand, M.D. Uncoupling protein-2 attenuates glucose-stimulated insulin secretion in INS-1E insulinoma cells by lowering mitochondrial reactive oxygen species. Free Radic. Biol. Med. 2011, 50, 609–616. [Google Scholar] [CrossRef]
- Martyn, K.D.; Frederick, L.M.; von Loehneysen, K.; Dinauer, M.C.; Knaus, U.G. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell. Signal. 2006, 18, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Elksnis, A.; Cen, J.; Wikström, P.; Carlsson, P.O.; Welsh, N. Pharmacological Inhibition of NOX4 Improves Mitochondrial Function and Survival in Human Beta-Cells. Biomedicines 2021, 9, 1865. [Google Scholar] [CrossRef] [PubMed]
- Stancill, J.S.; Hansen, P.A.; Mathison, A.J.; Schmidt, E.E.; Corbett, J.A. Deletion of Thioredoxin Reductase Disrupts Redox Homeostasis and Impairs β-Cell Function. Function 2022, 3, zqac034. [Google Scholar] [CrossRef] [PubMed]
- Stancill, J.S.; Broniowska, K.A.; Oleson, B.J.; Naatz, A.; Corbett, J.A. Pancreatic beta-cells detoxify H2O2 through the peroxiredoxin/thioredoxin antioxidant system. J. Biol. Chem. 2019, 294, 4843–4853. [Google Scholar] [CrossRef] [PubMed]
- Baumel-Alterzon, S.; Katz, L.S.; Brill, G.; Jean-Pierre, C.; Li, Y.; Tse, I.; Biswal, S.; Garcia-Ocana, A.; Scott, D.K. Nrf2 Regulates beta-Cell Mass by Suppressing beta-Cell Death and Promoting beta-Cell Proliferation. Diabetes 2022, 71, 989–1011. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Sato, Y.; Felig, P.; Wahren, J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J. Clin. Investig. 1981, 68, 1468–1474. [Google Scholar] [CrossRef]
- Baron, A.D.; Brechtel, G.; Wallace, P.; Edelman, S.V. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am. J. Physiol. 1988, 255, E769–E774. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar] [CrossRef]
- Higaki, Y.; Mikami, T.; Fujii, N.; Hirshman, M.F.; Koyama, K.; Seino, T.; Tanaka, K.; Goodyear, L.J. Oxidative stress stimulates skeletal muscle glucose uptake through a phosphatidylinositol 3-kinase-dependent pathway. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E889–E897. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Ishihara, T.; Ichimura, A.; Ishihara, N. Mitochondrial dynamics define muscle fiber type by modulating cellular metabolic pathways. Cell Rep. 2023, 42, 112434. [Google Scholar] [CrossRef]
- Jani, S.; Da Eira, D.; Hadday, I.; Bikopoulos, G.; Mohasses, A.; de Pinho, R.A.; Ceddia, R.B. Distinct mechanisms involving diacylglycerol, ceramides, and inflammation underlie insulin resistance in oxidative and glycolytic muscles from high fat-fed rats. Sci. Rep. 2021, 11, 19160. [Google Scholar] [CrossRef] [PubMed]
- Makinen, S.; Nguyen, Y.H.; Skrobuk, P.; Koistinen, H.A. Palmitate and oleate exert differential effects on insulin signalling and glucose uptake in human skeletal muscle cells. Endocr. Connect. 2017, 6, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Zanetti, M.; Gortan Cappellari, G.; Semolic, A.; Boschelle, M.; Codarin, E.; Pirulli, A.; Cattin, L.; Guarnieri, G. Fatty acids acutely enhance insulin-induced oxidative stress and cause insulin resistance by increasing mitochondrial reactive oxygen species (ROS) generation and nuclear factor-kappaB inhibitor (IkappaB)-nuclear factor-kappaB (NFkappaB) activation in rat muscle, in the absence of mitochondrial dysfunction. Diabetologia 2012, 55, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Sheu, S.S.; Robotham, J.L.; Yoon, Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc. Res. 2008, 79, 341–351. [Google Scholar] [CrossRef]
- Frank, S.; Gaume, B.; Bergmann-Leitner, E.S.; Leitner, W.W.; Robert, E.G.; Catez, F.; Smith, C.L.; Youle, R.J. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 2001, 1, 515–525. [Google Scholar] [CrossRef]
- Gundersen, A.E.; Kugler, B.A.; McDonald, P.M.; Veraksa, A.; Houmard, J.A.; Zou, K. Altered mitochondrial network morphology and regulatory proteins in mitochondrial quality control in myotubes from severely obese humans with or without type 2 diabetes. Appl. Physiol. Nutr. Metab. 2020, 45, 283–293. [Google Scholar] [CrossRef]
- Espinosa, A.; Campos, C.; Diaz-Vegas, A.; Galgani, J.E.; Juretic, N.; Osorio-Fuentealba, C.; Bucarey, J.L.; Tapia, G.; Valenzuela, R.; Contreras-Ferrat, A.; et al. Insulin-dependent H2O2 production is higher in muscle fibers of mice fed with a high-fat diet. Int. J. Mol. Sci. 2013, 14, 15740–15754. [Google Scholar] [CrossRef]
- Dos Santos, J.M.; de Oliveira, D.S.; Moreli, M.L.; Benite-Ribeiro, S.A. The role of mitochondrial DNA damage at skeletal muscle oxidative stress on the development of type 2 diabetes. Mol. Cell. Biochem. 2018, 449, 251–255. [Google Scholar] [CrossRef]
- Fink, L.N.; Costford, S.R.; Lee, Y.S.; Jensen, T.E.; Bilan, P.J.; Oberbach, A.; Bluher, M.; Olefsky, J.M.; Sams, A.; Klip, A. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity 2014, 22, 747–757. [Google Scholar] [CrossRef]
- Shabani, M.; Sadeghi, A.; Hosseini, H.; Teimouri, M.; Babaei Khorzoughi, R.; Pasalar, P.; Meshkani, R. Resveratrol alleviates obesity-induced skeletal muscle inflammation via decreasing M1 macrophage polarization and increasing the regulatory T cell population. Sci. Rep. 2020, 10, 3791. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, M.; Parker, B.L.; Jensen, T.E.; Raun, S.H.; Pham, P.; Han, X.; James, D.E.; Richter, E.A.; Sylow, L. Quantitative proteomic characterization of cellular pathways associated with altered insulin sensitivity in skeletal muscle following high-fat diet feeding and exercise training. Sci. Rep. 2018, 8, 10723. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.E.; Hwang, J.; Zhou, C.; Veluthakal, R.; McCown, E.M.; Hamilton, A.; Oh, E.; Dai, W.; Fueger, P.T.; Jiang, L.; et al. Enrichment of the exocytosis protein STX4 in skeletal muscle remediates peripheral insulin resistance and alters mitochondrial dynamics via Drp1. Nat. Commun. 2022, 13, 424. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Luscombe-Marsh, N.; Naumovski, N.; Abeywardena, M.; O’Callaghan, N. Palmitic Acid, but Not Lauric Acid, Induces Metabolic Inflammation, Mitochondrial Fragmentation, and a Drop in Mitochondrial Membrane Potential in Human Primary Myotubes. Front. Nutr. 2021, 8, 663838. [Google Scholar] [CrossRef] [PubMed]
- Jheng, H.F.; Tsai, P.J.; Guo, S.M.; Kuo, L.H.; Chang, C.S.; Su, I.J.; Chang, C.R.; Tsai, Y.S. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell. Biol. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Lambertucci, R.H.; Hirabara, S.M.; Silveira Ldos, R.; Levada-Pires, A.C.; Curi, R.; Pithon-Curi, T.C. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J. Cell. Physiol. 2008, 216, 796–804. [Google Scholar] [CrossRef]
- Livingston, M.J.; Wang, J.; Zhou, J.; Wu, G.; Ganley, I.G.; Hill, J.A.; Yin, X.M.; Dong, Z. Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys. Autophagy 2019, 15, 2142–2162. [Google Scholar] [CrossRef]
- Feng, X.T.; Wang, T.Z.; Leng, J.; Chen, Y.; Liu, J.B.; Liu, Y.; Wang, W.J. Palmitate contributes to insulin resistance through downregulation of the Src-mediated phosphorylation of Akt in C2C12 myotubes. Biosci. Biotechnol. Biochem. 2012, 76, 1356–1361. [Google Scholar] [CrossRef]
- Burman, J.L.; Pickles, S.; Wang, C.; Sekine, S.; Vargas, J.N.S.; Zhang, Z.; Youle, A.M.; Nezich, C.L.; Wu, X.; Hammer, J.A.; et al. Mitochondrial fission facilitates the selective mitophagy of protein aggregates. J. Cell Biol. 2017, 216, 3231–3247. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Elorza, A.; Molina, A.J.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef]
- Oh, S.J.; Park, K.; Sonn, S.K.; Oh, G.T.; Lee, M.S. Pancreatic beta-cell mitophagy as an adaptive response to metabolic stress and the underlying mechanism that involves lysosomal Ca2+ release. Exp. Mol. Med. 2023, 55, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Holmstrom, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Ordureau, A.; Heo, J.M.; Duda, D.M.; Paulo, J.A.; Olszewski, J.L.; Yanishevski, D.; Rinehart, J.; Schulman, B.A.; Harper, J.W. Defining roles of PARKIN and ubiquitin phosphorylation by PINK1 in mitochondrial quality control using a ubiquitin replacement strategy. Proc. Natl. Acad. Sci. USA 2015, 112, 6637–6642. [Google Scholar] [CrossRef] [PubMed]
- Gladkova, C.; Maslen, S.L.; Skehel, J.M.; Komander, D. Mechanism of parkin activation by PINK1. Nature 2018, 559, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Kane, L.A.; Lazarou, M.; Fogel, A.I.; Li, Y.; Yamano, K.; Sarraf, S.A.; Banerjee, S.; Youle, R.J. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J. Cell Biol. 2014, 205, 143–153. [Google Scholar] [CrossRef]
- Okatsu, K.; Oka, T.; Iguchi, M.; Imamura, K.; Kosako, H.; Tani, N.; Kimura, M.; Go, E.; Koyano, F.; Funayama, M.; et al. PINK1 autophosphorylation upon membrane potential dissipation is essential for Parkin recruitment to damaged mitochondria. Nat. Commun. 2012, 3, 1016. [Google Scholar] [CrossRef]
- Gao, A.; Jiang, J.; Xie, F.; Chen, L. Bnip3 in mitophagy: Novel insights and potential therapeutic target for diseases of secondary mitochondrial dysfunction. Clin. Chim. Acta 2020, 506, 72–83. [Google Scholar] [CrossRef]
- Tong, B.; Zhang, Z.; Li, X.; Liu, J.; Wang, H.; Song, L.; Feng, J.; Dai, Z.; Xu, Y. FUNDC1 modulates mitochondrial defects and pancreatic beta-cell dysfunction under lipotoxicity. Biochem. Biophys. Res. Commun. 2023, 672, 54–64. [Google Scholar] [CrossRef]
- Okatsu, K.; Kimura, M.; Oka, T.; Tanaka, K.; Matsuda, N. Unconventional PINK1 localization to the outer membrane of depolarized mitochondria drives Parkin recruitment. J. Cell Sci. 2015, 128, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.W.; Grenier, K.; Aguileta, M.A.; Muise, S.; Farazifard, R.; Haque, M.E.; McBride, H.M.; Park, D.S.; Fon, E.A. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep. 2012, 13, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Craessaerts, K.; De Strooper, B.; Morais, V.A. PINK1 kinase catalytic activity is regulated by phosphorylation on serines 228 and 402. J. Biol. Chem. 2015, 290, 2798–2811. [Google Scholar] [CrossRef]
- Wauer, T.; Swatek, K.N.; Wagstaff, J.L.; Gladkova, C.; Pruneda, J.N.; Michel, M.A.; Gersch, M.; Johnson, C.M.; Freund, S.M.; Komander, D. Ubiquitin Ser65 phosphorylation affects ubiquitin structure, chain assembly and hydrolysis. EMBO J. 2015, 34, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Holzbaur, E.L. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc. Natl. Acad. Sci. USA 2014, 111, E4439–E4448. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Holzbaur, E.L. Temporal dynamics of PARK2/parkin and OPTN/optineurin recruitment during the mitophagy of damaged mitochondria. Autophagy 2015, 11, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Ma, K.; Zhou, C.; Ding, P.; Zhu, Y.; Chen, Q.; Xia, B. Structural basis for the phosphorylation of FUNDC1 LIR as a molecular switch of mitophagy. Autophagy 2016, 12, 2363–2373. [Google Scholar] [CrossRef]
- Wirth, M.; Zhang, W.; Razi, M.; Nyoni, L.; Joshi, D.; O’Reilly, N.; Johansen, T.; Tooze, S.A.; Mouilleron, S. Molecular determinants regulating selective binding of autophagy adapters and receptors to ATG8 proteins. Nat. Commun. 2019, 10, 2055. [Google Scholar] [CrossRef]
- Wei, Y.; Chiang, W.C.; Sumpter, R., Jr.; Mishra, P.; Levine, B. Prohibitin 2 Is an Inner Mitochondrial Membrane Mitophagy Receptor. Cell 2017, 168, 224–238.e10. [Google Scholar] [CrossRef]
- da Silva Rosa, S.C.; Martens, M.D.; Field, J.T.; Nguyen, L.; Kereliuk, S.M.; Hai, Y.; Chapman, D.; Diehl-Jones, W.; Aliani, M.; West, A.R.; et al. BNIP3L/Nix-induced mitochondrial fission, mitophagy, and impaired myocyte glucose uptake are abrogated by PRKA/PKA phosphorylation. Autophagy 2021, 17, 2257–2272. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Li, W.; Chen, H.; Du, L.; Liu, D.; Wang, X.; Xu, T.; Liu, L.; Chen, Q. Deficiency of mitophagy receptor FUNDC1 impairs mitochondrial quality and aggravates dietary-induced obesity and metabolic syndrome. Autophagy 2019, 15, 1882–1898. [Google Scholar] [CrossRef]
- Fu, T.; Xu, Z.; Liu, L.; Guo, Q.; Wu, H.; Liang, X.; Zhou, D.; Xiao, L.; Liu, L.; Liu, Y.; et al. Mitophagy Directs Muscle-Adipose Crosstalk to Alleviate Dietary Obesity. Cell Rep. 2018, 23, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Gong, L.; Chen, L.; Xu, M.; Abou-Hamdan, H.; Tang, M.; Desaubry, L.; Song, Z. PHB2 (prohibitin 2) promotes PINK1-PRKN/Parkin-dependent mitophagy by the PARL-PGAM5-PINK1 axis. Autophagy 2020, 16, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Spurlin, B.A.; Park, S.Y.; Nevins, A.K.; Kim, J.K.; Thurmond, D.C. Syntaxin 4 transgenic mice exhibit enhanced insulin-mediated glucose uptake in skeletal muscle. Diabetes 2004, 53, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Coker, K.J.; Kim, J.K.; Mora, S.; Thurmond, D.C.; Davis, A.C.; Yang, B.; Williamson, R.A.; Shulman, G.I.; Pessin, J.E. Syntaxin 4 heterozygous knockout mice develop muscle insulin resistance. J. Clin. Investig. 2001, 107, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, Y.; Hoshijima, M.; Seo, K.; Bedja, D.; Sysa-Shah, P.; Andrabi, S.A.; Chen, W.; Höke, A.; Dawson, V.L.; Dawson, T.M.; et al. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014, 33, 2798–2813. [Google Scholar] [CrossRef] [PubMed]
- Pernaute, B.; Pérez-Montero, S.; Sánchez Nieto, J.M.; Di Gregorio, A.; Lima, A.; Lawlor, K.; Bowling, S.; Liccardi, G.; Tomás, A.; Meier, P.; et al. DRP1 levels determine the apoptotic threshold during embryonic differentiation through a mitophagy-dependent mechanism. Dev. Cell 2022, 57, 1316–1330.e7. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, D.P.; Praharaj, P.P.; Behera, B.P.; Patra, S.; Patil, S.; Patro, B.S.; Bhutia, S.K. The inner mitochondrial membrane fission protein MTP18 serves as a mitophagy receptor to prevent apoptosis in oral cancer. J. Cell Sci. 2023, 136, jcs259986. [Google Scholar] [CrossRef]
- Ehrlicher, S.E.; Stierwalt, H.D.; Newsom, S.A.; Robinson, M.M. Short-Term High-Fat Feeding Does Not Alter Mitochondrial Lipid Respiratory Capacity but Triggers Mitophagy Response in Skeletal Muscle of Mice. Front. Endocrinol. 2021, 12, 651211. [Google Scholar] [CrossRef]
- Lee, H.; Ha, T.Y.; Jung, C.H.; Nirmala, F.S.; Park, S.Y.; Huh, Y.H.; Ahn, J. Mitochondrial dysfunction in skeletal muscle contributes to the development of acute insulin resistance in mice. J. Cachexia Sarcopenia Muscle 2021, 12, 1925–1939. [Google Scholar] [CrossRef]
- Yeo, D.; Zhang, T.; Liu, T.; Zhang, Y.; Kang, C.; Ji, L.L. Protective effects of extra virgin olive oil and exercise training on rat skeletal muscle against high-fat diet feeding. J. Nutr. Biochem. 2022, 100, 108902. [Google Scholar] [CrossRef]
- Hyatt, J.K.; de Cabo, R.; Mattison, J.A. Resveratrol Blunts Mitochondrial Loss in Slow and Mixed Skeletal Muscle Phenotypes of Non-Human Primates following a Long-Term High Fat/Sugar Diet. J. Diet. Suppl. 2023, 20, 563–581. [Google Scholar] [CrossRef]
- Bhansali, S.; Bhansali, A.; Walia, R.; Saikia, U.N.; Dhawan, V. Alterations in Mitochondrial Oxidative Stress and Mitophagy in Subjects with Prediabetes and Type 2 Diabetes Mellitus. Front. Endocrinol. 2017, 8, 347. [Google Scholar] [CrossRef]
- Ohman, T.; Teppo, J.; Datta, N.; Makinen, S.; Varjosalo, M.; Koistinen, H.A. Skeletal muscle proteomes reveal downregulation of mitochondrial proteins in transition from prediabetes into type 2 diabetes. iScience 2021, 24, 102712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lee, J.H.; Shin, H.E.; Kwak, S.E.; Bae, J.H.; Tang, L.; Song, W. The Effects of Exercise and Restriction of Sugar-Sweetened Beverages on Muscle Function and Autophagy Regulation in High-Fat High-Sucrose-Fed Obesity Mice. Diabetes Metab. J. 2021, 45, 773–786. [Google Scholar] [CrossRef]
- Yeon, M.H.; Seo, E.; Lee, J.H.; Jun, H.S. Bavachin and Corylifol A Improve Muscle Atrophy by Enhancing Mitochondria Quality Control in Type 2 Diabetic Mice. Antioxidants 2023, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yi, L.; Song, S.; Wang, L.; Liang, Q.; Wang, Y.; Wu, Y.; Gao, Q. Puerarin attenuates palmitate-induced mitochondrial dysfunction, impaired mitophagy and inflammation in L6 myotubes. Life Sci. 2018, 206, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Mughal, W.; Nguyen, L.; Pustylnik, S.; da Silva Rosa, S.C.; Piotrowski, S.; Chapman, D.; Du, M.; Alli, N.S.; Grigull, J.; Halayko, A.J.; et al. A conserved MADS-box phosphorylation motif regulates differentiation and mitochondrial function in skeletal, cardiac, and smooth muscle cells. Cell Death Dis. 2015, 6, e1944. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.A.; Mehndiratta, M.; Madhu, S.V.; Kar, R.; Puri, D. PINK1 and oxidative stress in lean and obese patients with type 2 diabetes mellitus. J. Diabetes Complicat. 2023, 37, 108542. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.E.; Tunduguru, R.; Ahn, M.; Salunkhe, V.A.; Veluthakal, R.; Hwang, J.; Bhattacharya, S.; McCown, E.M.; Garcia, P.A.; Zhou, C.; et al. Changes in Skeletal Muscle PAK1 Levels Regulate Tissue Crosstalk to Impact Whole Body Glucose Homeostasis. Front. Endocrinol. 2022, 13, 821849. [Google Scholar] [CrossRef]
- Balakrishnan, R.G.P.; Veluthakal, R.; Huss, J.M.; Hoolachan, J.M.; Thurmond, D.C. Toward Ameliorating Insulin Resistance: Targeting a Novel PAK1 Signaling Pathway Required for Skeletal Muscle Mitochondrial Function. Antioxidants 2023, 12, 1658. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Oh, E.; McCown, E.M.; Wang, X.; Veluthakal, R.; Thurmond, D.C. A requirement for PAK1 to support mitochondrial function and maintain cellular redox balance via electron transport chain proteins to prevent β-cell apoptosis. Metabolism 2021, 115, 154431. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; LeRoith, D.; Stasinopoulos, M.; Zelenko, Z.; Shiloach, J. Polyol accumulation in muscle and liver in a mouse model of type 2 diabetes. J. Diabetes Complicat. 2016, 30, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ma, Y.; Wang, X.; Zhang, Y.; Zhu, L.; Shi, S.; Pan, S.; Liu, Z. Advanced glycation end products induce skeletal muscle atrophy and insulin resistance via activating ROS-mediated ER stress PERK/FOXO1 signaling. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E279–E287. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.E.; Prasad, C.; Vijayagopal, P.; Juma, S.; Imrhan, V. Advanced Glycation End Products, Inflammation, and Chronic Metabolic Diseases: Links in a Chain? Crit. Rev. Food Sci. Nutr. 2016, 56, 989–998. [Google Scholar] [CrossRef]
- Wu, Y.; Song, P.; Xu, J.; Zhang, M.; Zou, M.H. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J. Biol. Chem. 2007, 282, 9777–9788. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Ho, E.C.; Lam, K.S.; Chung, S.K. Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 2003, 14, S233–S236. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J. Pathogenesis of chronic hyperglycemia: From reductive stress to oxidative stress. J. Diabetes Res. 2014, 2014, 137919. [Google Scholar] [CrossRef]
- Andrich, D.E.; Melbouci, L.; Ou, Y.; Auclair, N.; Mercier, J.; Grenier, J.C.; Lira, F.S.; Barreiro, L.B.; Danialou, G.; Comtois, A.S.; et al. A Short-Term High-Fat Diet Alters Glutathione Levels and IL-6 Gene Expression in Oxidative Skeletal Muscles of Young Rats. Front. Physiol. 2019, 10, 372. [Google Scholar] [CrossRef]
- Sondergard, S.D.; Cintin, I.; Kuhlman, A.B.; Morville, T.H.; Bergmann, M.L.; Kjaer, L.K.; Poulsen, H.E.; Giustarini, D.; Rossi, R.; Dela, F.; et al. The effects of 3 weeks of oral glutathione supplementation on whole body insulin sensitivity in obese males with and without type 2 diabetes: A randomized trial. Appl. Physiol. Nutr. Metab. 2021, 46, 1133–1142. [Google Scholar] [CrossRef]
- Patti, M.E.; Virkamaki, A.; Landaker, E.J.; Kahn, C.R.; Yki-Jarvinen, H. Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance of early postreceptor insulin signaling events in skeletal muscle. Diabetes 1999, 48, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Buse, M.G.; Robinson, K.A.; Marshall, B.A.; Mueckler, M. Differential effects of GLUT1 or GLUT4 overexpression on hexosamine biosynthesis by muscles of transgenic mice. J. Biol. Chem. 1996, 271, 23197–23202. [Google Scholar] [CrossRef] [PubMed]
- Habegger, K.M.; Penque, B.A.; Sealls, W.; Tackett, L.; Bell, L.N.; Blue, E.K.; Gallagher, P.J.; Sturek, M.; Alloosh, M.A.; Steinberg, H.O.; et al. Fat-induced membrane cholesterol accrual provokes cortical filamentous actin destabilisation and glucose transport dysfunction in skeletal muscle. Diabetologia 2012, 55, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Penque, B.A.; Hoggatt, A.M.; Herring, B.P.; Elmendorf, J.S. Hexosamine biosynthesis impairs insulin action via a cholesterolgenic response. Mol. Endocrinol. 2013, 27, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Bhonagiri, P.; Pattar, G.R.; Habegger, K.M.; McCarthy, A.M.; Tackett, L.; Elmendorf, J.S. Evidence coupling increased hexosamine biosynthesis pathway activity to membrane cholesterol toxicity and cortical filamentous actin derangement contributing to cellular insulin resistance. Endocrinology 2011, 152, 3373–3384. [Google Scholar] [CrossRef] [PubMed]
- Haga, Y.; Ishii, K.; Suzuki, T. N-glycosylation is critical for the stability and intracellular trafficking of glucose transporter GLUT4. J. Biol. Chem. 2011, 286, 31320–31327. [Google Scholar] [CrossRef] [PubMed]
- Yki-Jarvinen, H.; Daniels, M.C.; Virkamaki, A.; Makimattila, S.; DeFronzo, R.A.; McClain, D. Increased glutamine:fructose-6-phosphate amidotransferase activity in skeletal muscle of patients with NIDDM. Diabetes 1996, 45, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Sandhya, N.; Sampathkumar, R.; Farooq, S.; Mohan, V.; Balasubramanyam, M. Glutamine fructose-6-phosphate amidotransferase (GFAT) gene expression and activity in patients with type 2 diabetes: Inter-relationships with hyperglycaemia and oxidative stress. Clin. Biochem. 2007, 40, 952–957. [Google Scholar] [CrossRef]
- Coughlan, M.T.; Thorburn, D.R.; Penfold, S.A.; Laskowski, A.; Harcourt, B.E.; Sourris, K.C.; Tan, A.L.; Fukami, K.; Thallas-Bonke, V.; Nawroth, P.P.; et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J. Am. Soc. Nephrol. 2009, 20, 742–752. [Google Scholar] [CrossRef]
- Xing, Y.; Pan, S.; Zhu, L.; Cui, Q.; Tang, Z.; Liu, Z.; Liu, F. Advanced Glycation End Products Induce Atherosclerosis via RAGE/TLR4 Signaling Mediated-M1 Macrophage Polarization-Dependent Vascular Smooth Muscle Cell Phenotypic Conversion. Oxid. Med. Cell Longev. 2022, 2022, 9763377. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, Z.W.; Li, H.M.; Yan, X.F.; Feng, B. AGE/RAGE-Induced EMP Release via the NOX-Derived ROS Pathway. J. Diabetes Res. 2018, 2018, 6823058. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.; Beulens, J.W.; van Dieren, S.; Scheijen, J.L.; van der Daphne, A.; Spijkerman, A.M.; van der Schouw, Y.T.; Stehouwer, C.D.; Schalkwijk, C.G. Plasma advanced glycation end products are associated with incident cardiovascular events in individuals with type 2 diabetes: A case-cohort study with a median follow-up of 10 years (EPIC-NL). Diabetes 2015, 64, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Cassese, A.; Esposito, I.; Fiory, F.; Barbagallo, A.P.; Paturzo, F.; Mirra, P.; Ulianich, L.; Giacco, F.; Iadicicco, C.; Lombardi, A.; et al. In skeletal muscle advanced glycation end products (AGEs) inhibit insulin action and induce the formation of multimolecular complexes including the receptor for AGEs. J. Biol. Chem. 2008, 283, 36088–36099. [Google Scholar] [CrossRef] [PubMed]
- Chiappalupi, S.; Sorci, G.; Vukasinovic, A.; Salvadori, L.; Sagheddu, R.; Coletti, D.; Renga, G.; Romani, L.; Donato, R.; Riuzzi, F. Targeting RAGE prevents muscle wasting and prolongs survival in cancer cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Kuroda, A.; Ishizu, M.; Ohishi, M.; Takashi, Y.; Otsuka, Y.; Taniguchi, S.; Tamaki, M.; Kurahashi, K.; Yoshida, S.; et al. Association of accumulated advanced glycation end-products with a high prevalence of sarcopenia and dynapenia in patients with type 2 diabetes. J. Diabetes Investig. 2019, 10, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.C. Glucolipotoxicity, beta-Cells, and Diabetes: The Emperor Has No Clothes. Diabetes 2020, 69, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.; Jonsson, J.; Ofori, J.K.; Volkov, P.; Perfilyev, A.; Dekker Nitert, M.; Eliasson, L.; Ling, C.; Bacos, K. Glucolipotoxicity Alters Insulin Secretion via Epigenetic Changes in Human Islets. Diabetes 2019, 68, 1965–1974. [Google Scholar] [CrossRef] [PubMed]
- Brun, T.; Li, N.; Jourdain, A.A.; Gaudet, P.; Duhamel, D.; Meyer, J.; Bosco, D.; Maechler, P. Diabetogenic milieus induce specific changes in mitochondrial transcriptome and differentiation of human pancreatic islets. Hum. Mol. Genet. 2015, 24, 5270–5284. [Google Scholar] [CrossRef]
- Tiedge, M.; Lortz, S.; Drinkgern, J.; Lenzen, S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997, 46, 1733–1742. [Google Scholar] [CrossRef]
- Lenzen, S.; Drinkgern, J.; Tiedge, M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 1996, 20, 463–466. [Google Scholar] [CrossRef]
- Argaev-Frenkel, L.; Rosenzweig, T. Redox Balance in Type 2 Diabetes: Therapeutic Potential and the Challenge of Antioxidant-Based Therapy. Antioxidants 2023, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Stancill, J.S.; Corbett, J.A. Hydrogen peroxide detoxification through the peroxiredoxin/thioredoxin antioxidant system: A look at the pancreatic beta-cell oxidant defense. Vitam. Horm. 2023, 121, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Zengi, A.; Ercan, G.; Caglayan, O.; Tamsel, S.; Karadeniz, M.; Simsir, I.; Harman, E.; Kahraman, C.; Orman, M.; Cetinkalp, S.; et al. Increased oxidative DNA damage in lean normoglycemic offspring of type 2 diabetic patients. Exp. Clin. Endocrinol. Diabetes 2011, 119, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Iwasaki, N.; Nishimura, C.; Doi, K.; Noiri, E.; Nakamura, S.; Takizawa, M.; Ogata, M.; Fujimaki, R.; Grarup, N.; et al. Identification of KCNJ15 as a susceptibility gene in Asian patients with type 2 diabetes mellitus. Am. J. Hum. Genet. 2010, 86, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Oh, E.; Clapp, D.W.; Chernoff, J.; Thurmond, D.C. Inhibition or ablation of p21-activated kinase (PAK1) disrupts glucose homeostatic mechanisms in vivo. J. Biol. Chem. 2011, 286, 41359–41367. [Google Scholar] [CrossRef] [PubMed]
- Yoder, S.M.; Dineen, S.L.; Wang, Z.; Thurmond, D.C. YES, a Src family kinase, is a proximal glucose-specific activator of cell division cycle control protein 42 (Cdc42) in pancreatic islet β cells. J. Biol. Chem. 2014, 289, 11476–11487. [Google Scholar] [CrossRef]
- Janssen, R.J.; Distelmaier, F.; Smeets, R.; Wijnhoven, T.; Østergaard, E.; Jaspers, N.G.; Raams, A.; Kemp, S.; Rodenburg, R.J.; Willems, P.H.; et al. Contiguous gene deletion of ELOVL7, ERCC8 and NDUFAF2 in a patient with a fatal multisystem disorder. Hum. Mol. Genet. 2009, 18, 3365–3374. [Google Scholar] [CrossRef]
- Anello, M.; Lupi, R.; Spampinato, D.; Piro, S.; Masini, M.; Boggi, U.; Del Prato, S.; Rabuazzo, A.M.; Purrello, F.; Marchetti, P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia 2005, 48, 282–289. [Google Scholar] [CrossRef]
- Maechler, P.; Wollheim, C.B. Mitochondrial function in normal and diabetic beta-cells. Nature 2001, 414, 807–812. [Google Scholar] [CrossRef]
- Deng, S.; Vatamaniuk, M.; Huang, X.; Doliba, N.; Lian, M.M.; Frank, A.; Velidedeoglu, E.; Desai, N.M.; Koeberlein, B.; Wolf, B.; et al. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes 2004, 53, 624–632. [Google Scholar] [CrossRef]
- Weksler-Zangen, S. Is Type 2 Diabetes a Primary Mitochondrial Disorder? Cells 2022, 11, 1617. [Google Scholar] [CrossRef]
- Mulder, H. Transcribing beta-cell mitochondria in health and disease. Mol. Metab. 2017, 6, 1040–1051. [Google Scholar] [CrossRef]
- Molina, A.J.; Wikstrom, J.D.; Stiles, L.; Las, G.; Mohamed, H.; Elorza, A.; Walzer, G.; Twig, G.; Katz, S.; Corkey, B.E.; et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes 2009, 58, 2303–2315. [Google Scholar] [CrossRef]
- Dlaskova, A.; Spacek, T.; Santorova, J.; Plecita-Hlavata, L.; Berkova, Z.; Saudek, F.; Lessard, M.; Bewersdorf, J.; Jezek, P. 4Pi microscopy reveals an impaired three-dimensional mitochondrial network of pancreatic islet beta-cells, an experimental model of type-2 diabetes. Biochim. Biophys. Acta 2010, 1797, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, M.; Zhou, Y.T.; Levi, M.; Unger, R.H. Fatty acid-induced beta cell apoptosis: A link between obesity and diabetes. Proc. Natl. Acad. Sci. USA 1998, 95, 2498–2502. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Mellado-Gil, J.M.; Bahn, Y.J.; Pathy, S.M.; Zhang, Y.E.; Rane, S.G. Protection from beta-cell apoptosis by inhibition of TGF-beta/Smad3 signaling. Cell Death Dis. 2020, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Weksler-Zangen, S.; Raz, I.; Lenzen, S.; Jorns, A.; Ehrenfeld, S.; Amir, G.; Oprescu, A.; Yagil, Y.; Yagil, C.; Zangen, D.H.; et al. Impaired glucose-stimulated insulin secretion is coupled with exocrine pancreatic lesions in the Cohen diabetic rat. Diabetes 2008, 57, 279–287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aharon-Hananel, G.; Romero-Afrima, L.; Saada, A.; Mantzur, C.; Raz, I.; Weksler-Zangen, S. Cytochrome c Oxidase Activity as a Metabolic Regulator in Pancreatic Beta-Cells. Cells 2022, 11, 929. [Google Scholar] [CrossRef] [PubMed]
- Weksler-Zangen, S.; Aharon-Hananel, G.; Mantzur, C.; Aouizerat, T.; Gurgul-Convey, E.; Raz, I.; Saada, A. IL-1beta hampers glucose-stimulated insulin secretion in Cohen diabetic rat islets through mitochondrial cytochrome c oxidase inhibition by nitric oxide. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E648–E657. [Google Scholar] [CrossRef] [PubMed]
- Sidarala, V.; Pearson, G.L.; Parekh, V.S.; Thompson, B.; Christen, L.; Gingerich, M.A.; Zhu, J.; Stromer, T.; Ren, J.; Reck, E.C.; et al. Mitophagy protects beta cells from inflammatory damage in diabetes. JCI Insight 2020, 5, e141138. [Google Scholar] [CrossRef]
- Guo, T.; Liu, T.; Sun, Y.; Liu, X.; Xiong, R.; Li, H.; Li, Z.; Zhang, Z.; Tian, Z.; Tian, Y. Sonodynamic therapy inhibits palmitate-induced beta cell dysfunction via PINK1/Parkin-dependent mitophagy. Cell Death Dis. 2019, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, Z.; Yang, Y.; Yang, Y.; Huang, T.; Hong, Y.; Zhang, J.; Chen, Q.; Zhao, T.; Xiao, Z.; et al. Selenium Nanodots (SENDs) as Antioxidants and Antioxidant-Prodrugs to Rescue Islet beta Cells in Type 2 Diabetes Mellitus by Restoring Mitophagy and Alleviating Endoplasmic Reticulum Stress. Adv. Sci. 2023, 10, e2300880. [Google Scholar] [CrossRef] [PubMed]
- Rieusset, J. The role of endoplasmic reticulum-mitochondria contact sites in the control of glucose homeostasis: An update. Cell Death Dis. 2018, 9, 388. [Google Scholar] [CrossRef]
- Thivolet, C.; Vial, G.; Cassel, R.; Rieusset, J.; Madec, A.M. Reduction of endoplasmic reticulum-mitochondria interactions in beta cells from patients with type 2 diabetes. PLoS ONE 2017, 12, e0182027. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Cruz, M.S.; Simmen, T. Over Six Decades of Discovery and Characterization of the Architecture at Mitochondria-Associated Membranes (MAMs). Adv. Exp. Med. Biol. 2017, 997, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Csordas, G.; Varnai, P.; Golenar, T.; Roy, S.; Purkins, G.; Schneider, T.G.; Balla, T.; Hajnoczky, G. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol. Cell 2010, 39, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhou, R.; Zhang, C.; He, S.; Su, Z. Mitochondria-Associated Endoplasmic Reticulum Membranes in the Pathogenesis of Type 2 Diabetes Mellitus. Front. Cell Dev. Biol. 2020, 8, 571554. [Google Scholar] [CrossRef]
- Lupi, R.; Dotta, F.; Marselli, L.; Del Guerra, S.; Masini, M.; Santangelo, C.; Patane, G.; Boggi, U.; Piro, S.; Anello, M.; et al. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: Evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 2002, 51, 1437–1442. [Google Scholar] [CrossRef]
- Veluthakal, R.; Palanivel, R.; Zhao, Y.; McDonald, P.; Gruber, S.; Kowluru, A. Ceramide induces mitochondrial abnormalities in insulin-secreting INS-1 cells: Potential mechanisms underlying ceramide-mediated metabolic dysfunction of the beta cell. Apoptosis 2005, 10, 841–850. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Zhang, Z.; Zhang, X.; Zhu, Y.; Zhang, C.; Bi, Y. Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance. Cell Metab. 2022, 34, 1264–1279.e8. [Google Scholar] [CrossRef]
- Chidester, S.; Livinski, A.A.; Fish, A.F.; Joseph, P.V. The Role of Extracellular Vesicles in β-Cell Function and Viability: A Scoping Review. Front. Endocrinol. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, X.; Zhang, F.L.; Jin, H.; Yan, X.L.; Huang, S.; Guo, Z.N.; Yang, Y. Clinical Potential of Extracellular Vesicles in Type 2 Diabetes. Front. Endocrinol. 2020, 11, 596811. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Sudo, Y.; Makino, T.; Kimura, S.; Tomita, K.; Noguchi, M.; Sakurai, H.; Shimizu, M.; Takahashi, Y.; Sato, R.; et al. Skeletal muscle releases extracellular vesicles with distinct protein and microRNA signatures that function in the muscle microenvironment. PNAS Nexus 2022, 1, pgac173. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar] [CrossRef] [PubMed]
- Jalabert, A.; Vial, G.; Guay, C.; Wiklander, O.P.; Nordin, J.Z.; Aswad, H.; Forterre, A.; Meugnier, E.; Pesenti, S.; Regazzi, R.; et al. Exosome-like vesicles released from lipid-induced insulin-resistant muscles modulate gene expression and proliferation of beta recipient cells in mice. Diabetologia 2016, 59, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Aswad, H.; Forterre, A.; Wiklander, O.P.; Vial, G.; Danty-Berger, E.; Jalabert, A.; Lamaziere, A.; Meugnier, E.; Pesenti, S.; Ott, C.; et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia 2014, 57, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Carson, B.P. The Potential Role of Contraction-Induced Myokines in the Regulation of Metabolic Function for the Prevention and Treatment of Type 2 Diabetes. Front. Endocrinol. 2017, 8, 97. [Google Scholar] [CrossRef]
- Chen, X.; Wang, K.; Chen, J.; Guo, J.; Yin, Y.; Cai, X.; Guo, X.; Wang, G.; Yang, R.; Zhu, L.; et al. In vitro evidence suggests that miR-133a-mediated regulation of uncoupling protein 2 (UCP2) is an indispensable step in myogenic differentiation. J. Biol. Chem. 2009, 284, 5362–5369. [Google Scholar] [CrossRef]
- Robson-Doucette, C.A.; Sultan, S.; Allister, E.M.; Wikstrom, J.D.; Koshkin, V.; Bhattacharjee, A.; Prentice, K.J.; Sereda, S.B.; Shirihai, O.S.; Wheeler, M.B. Beta-cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion. Diabetes 2011, 60, 2710–2719. [Google Scholar] [CrossRef]
- Lisi, V.; Senesi, G.; Balbi, C. Converging protective pathways: Exploring the linkage between physical exercise, extracellular vesicles and oxidative stress. Free Radic. Biol. Med. 2023, 208, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Eguchi, A.; Tamai, Y.; Fukuda, S.; Tempaku, M.; Izuoka, K.; Iwasa, M.; Takei, Y.; Togashi, K. Protein Composition of Circulating Extracellular Vesicles Immediately Changed by Particular Short Time of High-Intensity Interval Training Exercise. Front. Physiol. 2021, 12, 693007. [Google Scholar] [CrossRef] [PubMed]
- Plomgaard, P.; Halban, P.A.; Bouzakri, K. Bimodal impact of skeletal muscle on pancreatic beta-cell function in health and disease. Diabetes Obes. Metab. 2012, 14 (Suppl. 3), 78–84. [Google Scholar] [CrossRef] [PubMed]
- Natalicchio, A.; Marrano, N.; Biondi, G.; Spagnuolo, R.; Labarbuta, R.; Porreca, I.; Cignarelli, A.; Bugliani, M.; Marchetti, P.; Perrini, S.; et al. The Myokine Irisin Is Released in Response to Saturated Fatty Acids and Promotes Pancreatic beta-Cell Survival and Insulin Secretion. Diabetes 2017, 66, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Morinaga, H.; Kim, J.J.; Lagakos, W.; Taylor, S.; Keshwani, M.; Perkins, G.; Dong, H.; Kayali, A.G.; Sweet, I.R.; et al. The fractalkine/CX3CR1 system regulates beta cell function and insulin secretion. Cell 2013, 153, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Riopel, M.; Seo, J.B.; Bandyopadhyay, G.K.; Li, P.; Wollam, J.; Chung, H.; Jung, S.R.; Murphy, A.; Wilson, M.; de Jong, R.; et al. Chronic fractalkine administration improves glucose tolerance and pancreatic endocrine function. J. Clin. Investig. 2018, 128, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Fred, R.G.; Bang-Berthelsen, C.H.; Mandrup-Poulsen, T.; Grunnet, L.G.; Welsh, N. High glucose suppresses human islet insulin biosynthesis by inducing miR-133a leading to decreased polypyrimidine tract binding protein-expression. PLoS ONE 2010, 5, e10843. [Google Scholar] [CrossRef] [PubMed]
- Bouzakri, K.; Plomgaard, P.; Berney, T.; Donath, M.Y.; Pedersen, B.K.; Halban, P.A. Bimodal effect on pancreatic beta-cells of secretory products from normal or insulin-resistant human skeletal muscle. Diabetes 2011, 60, 1111–1121. [Google Scholar] [CrossRef]
- Javeed, N.; Her, T.K.; Brown, M.R.; Vanderboom, P.; Rakshit, K.; Egan, A.M.; Vella, A.; Lanza, I.; Matveyenko, A.V. Pro-inflammatory beta cell small extracellular vesicles induce beta cell failure through activation of the CXCL10/CXCR3 axis in diabetes. Cell Rep. 2021, 36, 109613. [Google Scholar] [CrossRef]
- Clement, E.; Lazar, I.; Attane, C.; Carrie, L.; Dauvillier, S.; Ducoux-Petit, M.; Esteve, D.; Menneteau, T.; Moutahir, M.; Le Gonidec, S.; et al. Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. EMBO J. 2020, 39, e102525. [Google Scholar] [CrossRef]
- Crewe, C.; Funcke, J.B.; Li, S.; Joffin, N.; Gliniak, C.M.; Ghaben, A.L.; An, Y.A.; Sadek, H.A.; Gordillo, R.; Akgul, Y.; et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 2021, 33, 1853–1868.e11. [Google Scholar] [CrossRef] [PubMed]
- Rosina, M.; Ceci, V.; Turchi, R.; Chuan, L.; Borcherding, N.; Sciarretta, F.; Sanchez-Diaz, M.; Tortolici, F.; Karlinsey, K.; Chiurchiu, V.; et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 2022, 34, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Rackham, C.L.; Hubber, E.L.; Czajka, A.; Malik, A.N.; King, A.J.F.; Jones, P.M. Optimizing beta cell function through mesenchymal stromal cell-mediated mitochondria transfer. Stem Cells 2020, 38, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes from Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; Chen, S.; Bu, D.; Gliniak, C.M.; Wernstedt Asterholm, I.; Yu, X.X.; Joffin, N.; de Souza, C.O.; Funcke, J.B.; Oh, D.Y.; et al. Deficient Caveolin-1 Synthesis in Adipocytes Stimulates Systemic Insulin-Independent Glucose Uptake via Extracellular Vesicles. Diabetes 2022, 71, 2496–2512. [Google Scholar] [CrossRef] [PubMed]
- Kulaj, K.; Harger, A.; Bauer, M.; Caliskan, O.S.; Gupta, T.K.; Chiang, D.M.; Milbank, E.; Reber, J.; Karlas, A.; Kotzbeck, P.; et al. Adipocyte-derived extracellular vesicles increase insulin secretion through transport of insulinotropic protein cargo. Nat. Commun. 2023, 14, 709. [Google Scholar] [CrossRef] [PubMed]
- Adiga, D.; Bhat, S.; Shukla, V.; Shah, H.V.; Kuthethur, R.; Chakrabarty, S.; Kabekkodu, S.P. Double C-2 like domain beta (DOC2B) induces calcium dependent oxidative stress to promote lipotoxicity and mitochondrial dysfunction for its tumor suppressive function. Free Radic. Biol. Med. 2023, 201, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Esparza, D.; Oh, E.; Ghaeli, I.; Jovanovic-Talisman, T.; Thurmond, D.C. 1770-P: Islet ß-Cells Shed DOC2B-Laden EVs via Sorting Signals Present in DOC2B Protein. Diabetes 2023, 72, 1770. [Google Scholar] [CrossRef]
- He, F.; Huang, Y.; Song, Z.; Zhou, H.J.; Zhang, H.; Perry, R.J.; Shulman, G.I.; Min, W. Mitophagy-mediated adipose inflammation contributes to type 2 diabetes with hepatic insulin resistance. J. Exp. Med. 2021, 218, e20201416. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, F.; Li, C.; Mao, J.; Wang, Y.; Zhou, X.; Xie, H.; Wen, C. Application of Single Extracellular Vesicle Analysis Techniques. Int. J. Nanomed. 2023, 18, 5365–5376. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Wilen, C.B.; Moley, J.R.; Li, Y.; Zou, W.; Malvin, N.P.; Rowen, M.N.; Saunders, B.T.; Ma, H.; Mack, M.R.; et al. Intercellular Mitochondria Transfer to Macrophages Regulates White Adipose Tissue Homeostasis and Is Impaired in Obesity. Cell Metab. 2021, 33, 270–282.e278. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.B.; Poliakov, A.; Hardy, R.W.; Clements, R.; Liu, C.; Liu, Y.; Wang, J.; Xiang, X.; Zhang, S.; Zhuang, X.; et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 2009, 58, 2498–2505. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Rostamirad, A.; Seyyedebrahimi, S.; Meshkani, R. Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NF-kB pathway and ROS production. Inflammopharmacology 2018, 26, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, A.; Moradi, S.; Askari, G.; Iraj, B.; Sathyapalan, T.; Guest, P.C.; Bagherniya, M.; Sahebkar, A. Effect of Curcumin on Glycemic Control in Patients with Type 2 Diabetes: A Systematic Review of Randomized Clinical Trials. Adv. Exp. Med. Biol. 2021, 1291, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.; Monteiro-Alfredo, T.; Henriques, R.; Ribeiro, C.F.; Seica, R.; Cruz, T.; Cabral, C.; Fernandes, R.; Piedade, F.; Robalo, M.P.; et al. Improvement of Glycaemia and Endothelial Function by a New Low-Dose Curcuminoid in an Animal Model of Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 5652. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Vlavcheski, F.; Giacca, A.; Tsiani, E. Attenuation of Free Fatty Acid (FFA)-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol is Linked to Activation of AMPK and Inhibition of mTOR and p70 S6K. Int. J. Mol. Sci. 2020, 21, 4900. [Google Scholar] [CrossRef] [PubMed]
- Vlavcheski, F.; Den Hartogh, D.J.; Giacca, A.; Tsiani, E. Amelioration of High-Insulin-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol Is Linked to Activation of AMPK and Restoration of GLUT4 Translocation. Nutrients 2020, 12, 914. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhao, H.; Dong, L.; Zhen, Y.F.; Xing, H.Y.; Ma, H.J.; Song, G.Y. Resveratrol ameliorates high-fat diet-induced insulin resistance and fatty acid oxidation via ATM-AMPK axis in skeletal muscle. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9117–9125. [Google Scholar] [CrossRef]
- Szkudelska, K.; Deniziak, M.; Hertig, I.; Wojciechowicz, T.; Tyczewska, M.; Jaroszewska, M.; Szkudelski, T. Effects of Resveratrol in Goto-Kakizaki Rat, a Model of Type 2 Diabetes. Nutrients 2019, 11, 2488. [Google Scholar] [CrossRef]
- Seyyedebrahimi, S.; Khodabandehloo, H.; Nasli Esfahani, E.; Meshkani, R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018, 55, 341–353. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M. Resveratrol supplementation and type 2 diabetes: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4465–4480. [Google Scholar] [CrossRef]
- Jeyaraman, M.M.; Al-Yousif, N.S.H.; Singh Mann, A.; Dolinsky, V.W.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 1, CD011919. [Google Scholar] [CrossRef]
- Zeraattalab-Motlagh, S.; Jayedi, A.; Shab-Bidar, S. The effects of resveratrol supplementation in patients with type 2 diabetes, metabolic syndrome, and nonalcoholic fatty liver disease: An umbrella review of meta-analyses of randomized controlled trials. Am. J. Clin. Nutr. 2021, 114, 1675–1685. [Google Scholar] [CrossRef]
- Mason, S.A.; Parker, L.; van der Pligt, P.; Wadley, G.D. Vitamin C supplementation for diabetes management: A comprehensive narrative review. Free Radic. Biol. Med. 2023, 194, 255–283. [Google Scholar] [CrossRef]
- Boonthongkaew, C.; Tong-Un, T.; Kanpetta, Y.; Chaungchot, N.; Leelayuwat, C.; Leelayuwat, N. Vitamin C supplementation improves blood pressure and oxidative stress after acute exercise in patients with poorly controlled type 2 diabetes mellitus: A randomized, placebo-controlled, cross-over study. Chin. J. Physiol. 2021, 64, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Park, S. A Causal Relationship between Vitamin C Intake with Hyperglycemia and Metabolic Syndrome Risk: A Two-Sample Mendelian Randomization Study. Antioxidants 2022, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Keske, M.A.; Wadley, G.D. Effects of Vitamin C Supplementation on Glycemic Control and Cardiovascular Risk Factors in People with Type 2 Diabetes: A GRADE-Assessed Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Care 2021, 44, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Benetti, E.; Mastrocola, R.; Chiazza, F.; Nigro, D.; D’Antona, G.; Bordano, V.; Fantozzi, R.; Aragno, M.; Collino, M.; Minetto, M.A. Effects of vitamin D on insulin resistance and myosteatosis in diet-induced obese mice. PLoS ONE 2018, 13, e0189707. [Google Scholar] [CrossRef] [PubMed]
- Chang, E. 1,25-Dihydroxyvitamin D Decreases Tertiary Butyl-Hydrogen Peroxide-Induced Oxidative Stress and Increases AMPK/SIRT1 Activation in C2C12 Muscle Cells. Molecules 2019, 24, 3903. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, M.A.; Daneshzad, E.; Fung, T.T.; Zahidi, F.; Muhammadi, M.; Bellissimo, N.; Azadbakht, L. What is the impact of vitamin D supplementation on glycemic control in people with type-2 diabetes: A systematic review and meta-analysis of randomized controlled trails. BMC Endocr. Disord. 2023, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Hands, J.M.; Patrick, R.; Frame, L.A. Vitamin D and Risk for Type 2 Diabetes in People with Prediabetes. Ann. Intern. Med. 2023, 176, eL230201. [Google Scholar] [CrossRef] [PubMed]
- Cojic, M.; Kocic, R.; Klisic, A.; Kocic, G. The Effects of Vitamin D Supplementation on Metabolic and Oxidative Stress Markers in Patients with Type 2 Diabetes: A 6-Month Follow Up Randomized Controlled Study. Front. Endocrinol. 2021, 12, 610893. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.C.; Wu, Y.G.; Huang, W.G.; Fan, X.J.; Chen, X.H.; Zhou, B.; Lin, Z.J.; Feng, X.L. Effect of vitamin D on oxidative stress and serum inflammatory factors in the patients with type 2 diabetes. J. Clin. Lab. Anal. 2022, 36, e24430. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Ryul Shim, S.; Jamali, N.; Hassanzadeh-Rostami, Z.; Soltani, S.; Sasani, N.; Mohsenpour, M.A.; Firoozi, D.; Basirat, R.; Hosseini, R.; et al. Comparison of nutritional supplements for glycemic control in type 2 diabetes: A systematic review and network meta-analysis of randomized trials. Diabetes Res. Clin. Pract. 2022, 191, 110037. [Google Scholar] [CrossRef] [PubMed]
- Whytock, K.L.; Pino, M.F.; Sun, Y.; Yu, G.; De Carvalho, F.G.; Yeo, R.X.; Vega, R.B.; Parmar, G.; Divoux, A.; Kapoor, N.; et al. Comprehensive interrogation of human skeletal muscle reveals a dissociation between insulin resistance and mitochondrial capacity. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E291–E302. [Google Scholar] [CrossRef]
- Wikstrom, J.D.; Katzman, S.M.; Mohamed, H.; Twig, G.; Graf, S.A.; Heart, E.; Molina, A.J.; Corkey, B.E.; de Vargas, L.M.; Danial, N.N.; et al. beta-Cell mitochondria exhibit membrane potential heterogeneity that can be altered by stimulatory or toxic fuel levels. Diabetes 2007, 56, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Herman, W.H.; Ratner, R.E. Metformin Should Be Used to Treat Prediabetes in Selected Individuals. Diabetes Care 2020, 43, 1988–1990. [Google Scholar] [CrossRef]
- de Marañón, A.M.; Canet, F.; Abad-Jiménez, Z.; Jover, A.; Morillas, C.; Rocha, M.; Victor, V.M. Does Metformin Modulate Mitochondrial Dynamics and Function in Type 2 Diabetic Patients? Antioxid. Redox Signal. 2021, 35, 377–385. [Google Scholar] [CrossRef]
- de Marañón, A.M.; Díaz-Pozo, P.; Canet, F.; Díaz-Morales, N.; Abad-Jiménez, Z.; López-Domènech, S.; Vezza, T.; Apostolova, N.; Morillas, C.; Rocha, M.; et al. Metformin modulates mitochondrial function and mitophagy in peripheral blood mononuclear cells from type 2 diabetic patients. Redox Biol. 2022, 53, 102342. [Google Scholar] [CrossRef]
- Li, A.; Zhang, S.; Li, J.; Liu, K.; Huang, F.; Liu, B. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol. Cell Endocrinol. 2016, 434, 36–47. [Google Scholar] [CrossRef]
- Marshall, S.M. 60 years of metformin use: A glance at the past and a look to the future. Diabetologia 2017, 60, 1561–1565. [Google Scholar] [CrossRef]


| Myokines or miRNA from Skeletal Muscle EVs | Relevance with Oxidative Stress (ROS), Insulin Resistance (IR) or Type 2 Diabetes (T2D) | Effect on Pancreatic β-Cells | Ref. |
|---|---|---|---|
| Follistatin | Reduces ROS in T2D | Enhances GSIS and β-cell proliferation | [194] |
| Irisin | Reduces inflammation-mediated oxidative stress | Enhances insulin production, GSIS and β-cell proliferation | [195] |
| CX3CL1 | Reduces ROS | Enhances GSIS | [196,197] |
| miR-133a | Expression increased during oxidative stress | Reduces GSIS | [198] |
| CXCL10 | Enhanced by oxidative stress or IR | Enhances apoptosis and reduces β-cell proliferation | [199] |
| Cell Source | Mitochondrial Cargo within Mito-EVs | Relevance to ROS, IR, and T2D | Effects on Recipient Cells | Ref. |
|---|---|---|---|---|
| β-cells | PHGDH, ALDH9A1, ADSL, and DNML1 | Increase inflammation-mediated oxidative stress | Horizontal transfer of EVs promoted immune cell recruitment to islets insulin-positive β-cells | [200] |
| Adipocytes | Fatty acid oxidation enzymes ECHA and HCDH | Tumorigenicity exacerbated in obesity | Adipocyte-EVs promoted melanoma tumor cell progression via transfer of fatty acids under obesity conditions in vivo and ex vivo | [201] |
| Adipocytes | HSP60, VDAC1, COXIV | Promote ROS under obesity | Induce mitochondrial dysfunction of the host network and free radical production in cardiomyocytes. A beneficial effect that protects the heart from ischemia reperfusion injury | [202] |
| Adipocytes | mtDNA, AMP, ATP, that are MitoTracker Green (MTG) positive, without changes to PDHβ | Increased ROS | Adapt EVs as a quality control to remove damaged mitochondria | [203] |
| Adipose tissue MSCs and adipocytes | PGM1, PCX, MDH1 | Insulin secretion under obesity-induced IR/T2D | Promote GSIS in β-cells | [204] |
| Adipocytes | HSP70 and HSP90 | Promote IR in myocytes | Influence monocyte differentiation into pro-inflammatory macrophages, which directly act on myocytes to impair insulin-stimulated glucose uptake and insulin signaling in vitro and in vivo | [205] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veluthakal, R.; Esparza, D.; Hoolachan, J.M.; Balakrishnan, R.; Ahn, M.; Oh, E.; Jayasena, C.S.; Thurmond, D.C. Mitochondrial Dysfunction, Oxidative Stress, and Inter-Organ Miscommunications in T2D Progression. Int. J. Mol. Sci. 2024, 25, 1504. https://doi.org/10.3390/ijms25031504
Veluthakal R, Esparza D, Hoolachan JM, Balakrishnan R, Ahn M, Oh E, Jayasena CS, Thurmond DC. Mitochondrial Dysfunction, Oxidative Stress, and Inter-Organ Miscommunications in T2D Progression. International Journal of Molecular Sciences. 2024; 25(3):1504. https://doi.org/10.3390/ijms25031504
Chicago/Turabian StyleVeluthakal, Rajakrishnan, Diana Esparza, Joseph M. Hoolachan, Rekha Balakrishnan, Miwon Ahn, Eunjin Oh, Chathurani S. Jayasena, and Debbie C. Thurmond. 2024. "Mitochondrial Dysfunction, Oxidative Stress, and Inter-Organ Miscommunications in T2D Progression" International Journal of Molecular Sciences 25, no. 3: 1504. https://doi.org/10.3390/ijms25031504
APA StyleVeluthakal, R., Esparza, D., Hoolachan, J. M., Balakrishnan, R., Ahn, M., Oh, E., Jayasena, C. S., & Thurmond, D. C. (2024). Mitochondrial Dysfunction, Oxidative Stress, and Inter-Organ Miscommunications in T2D Progression. International Journal of Molecular Sciences, 25(3), 1504. https://doi.org/10.3390/ijms25031504







