Genome-Wide Comparative Analysis of SRCR Gene Superfamily in Invertebrates Reveals Massive and Independent Gene Expansions in the Sponge and Sea Urchin
Abstract
:1. Introduction
2. Results
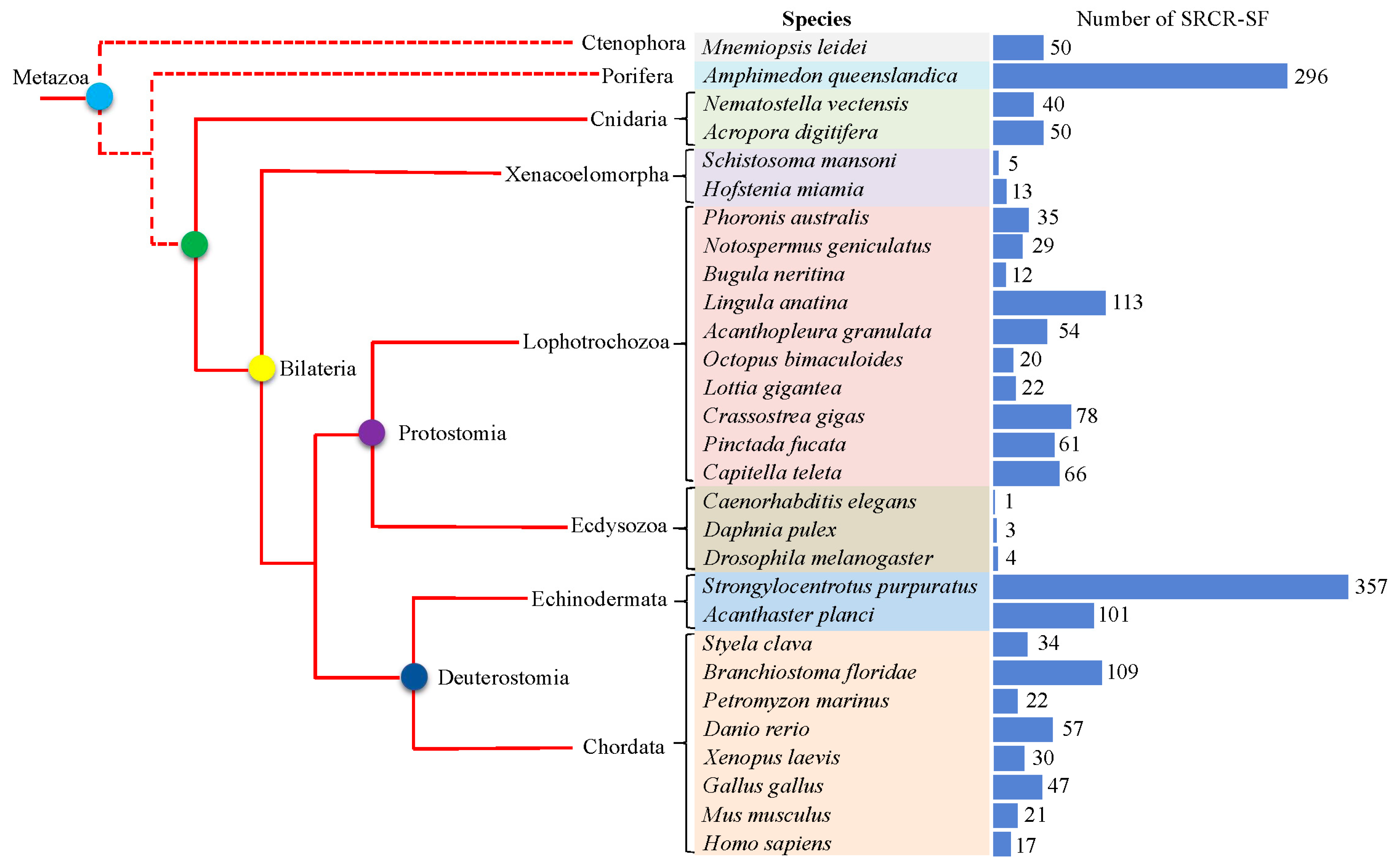
2.1. Identification of SRCR-SFs in Metazoans
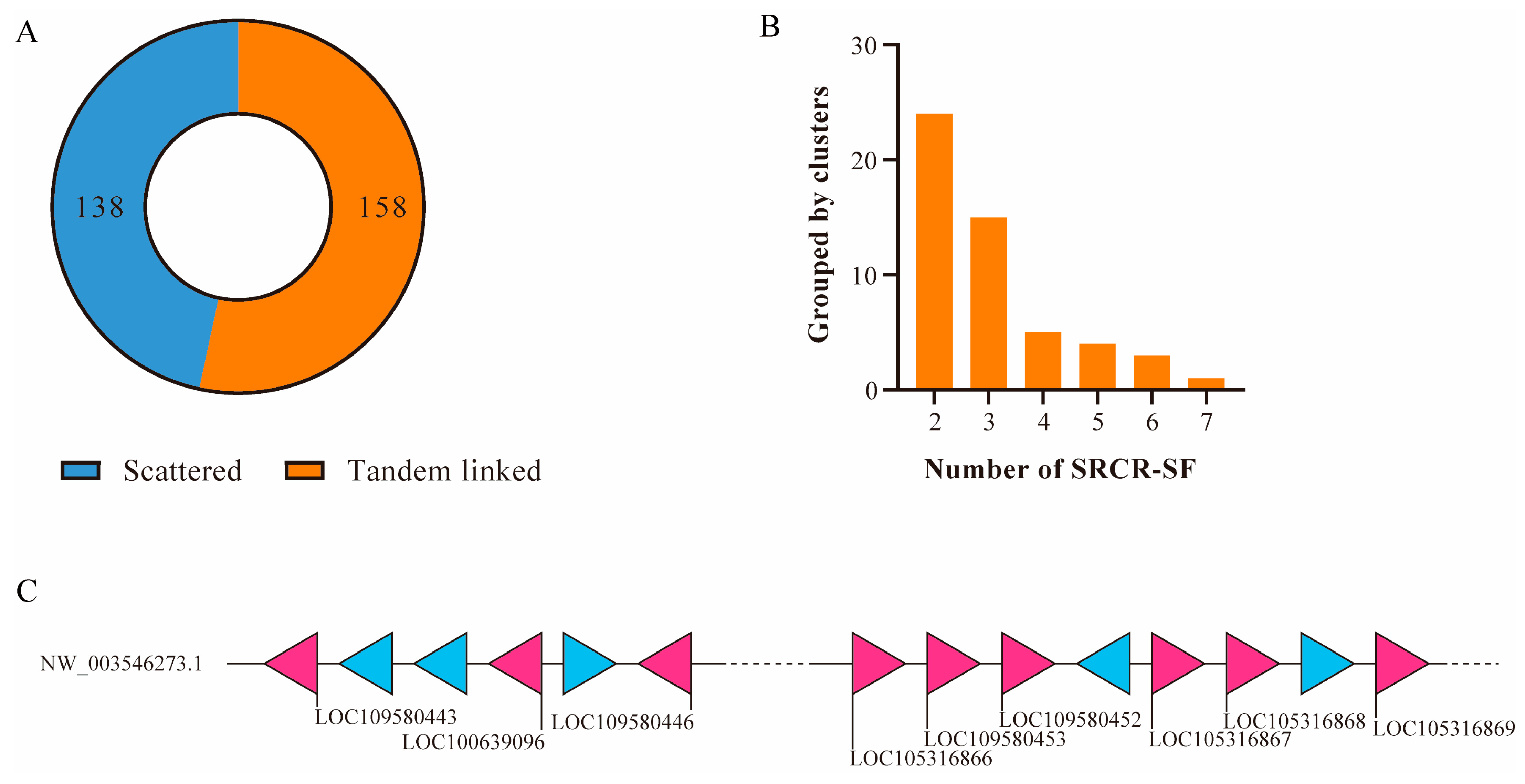
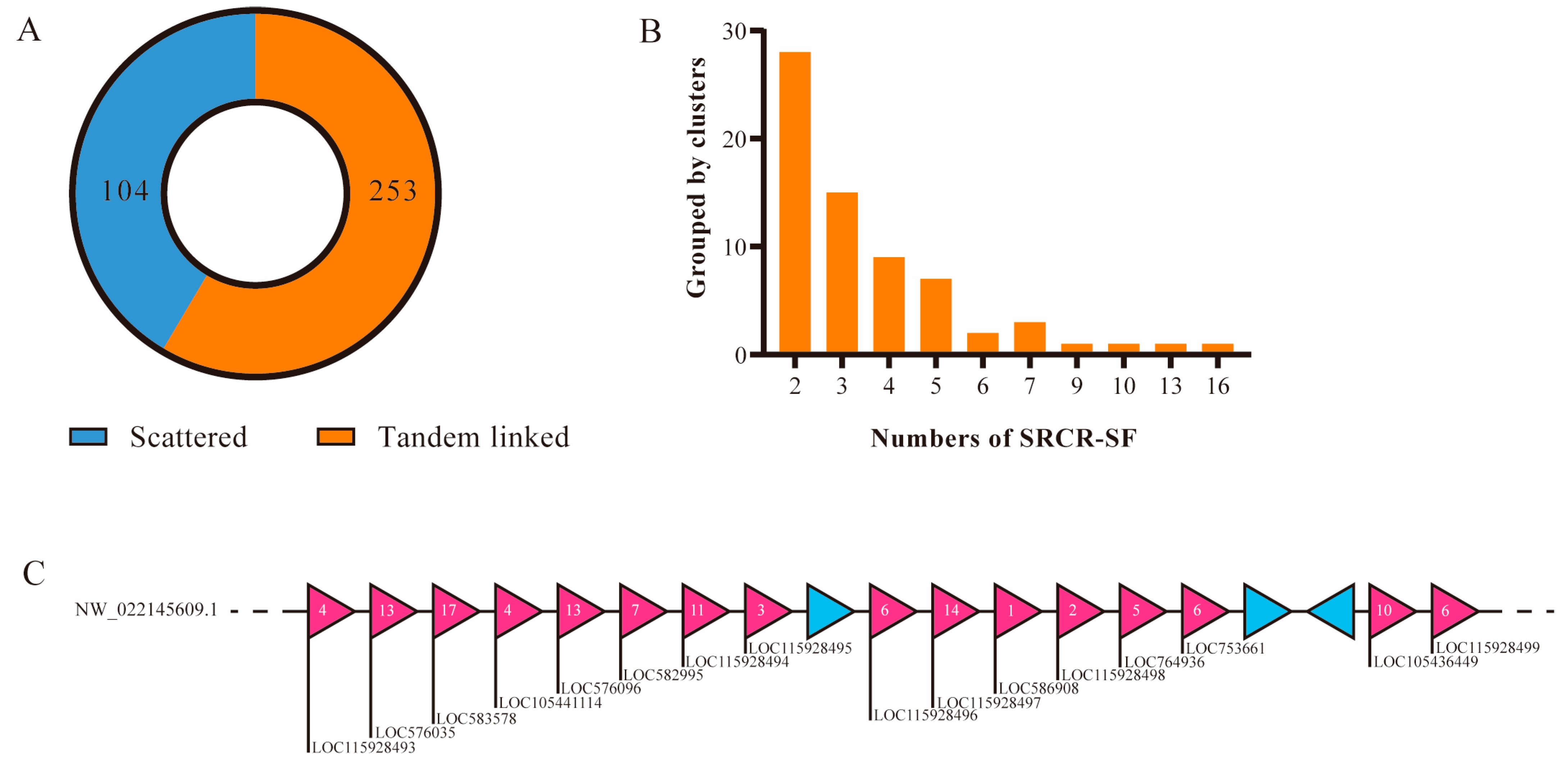
2.2. Domain Architectures and Genomic Distribution of SRCR-SFs
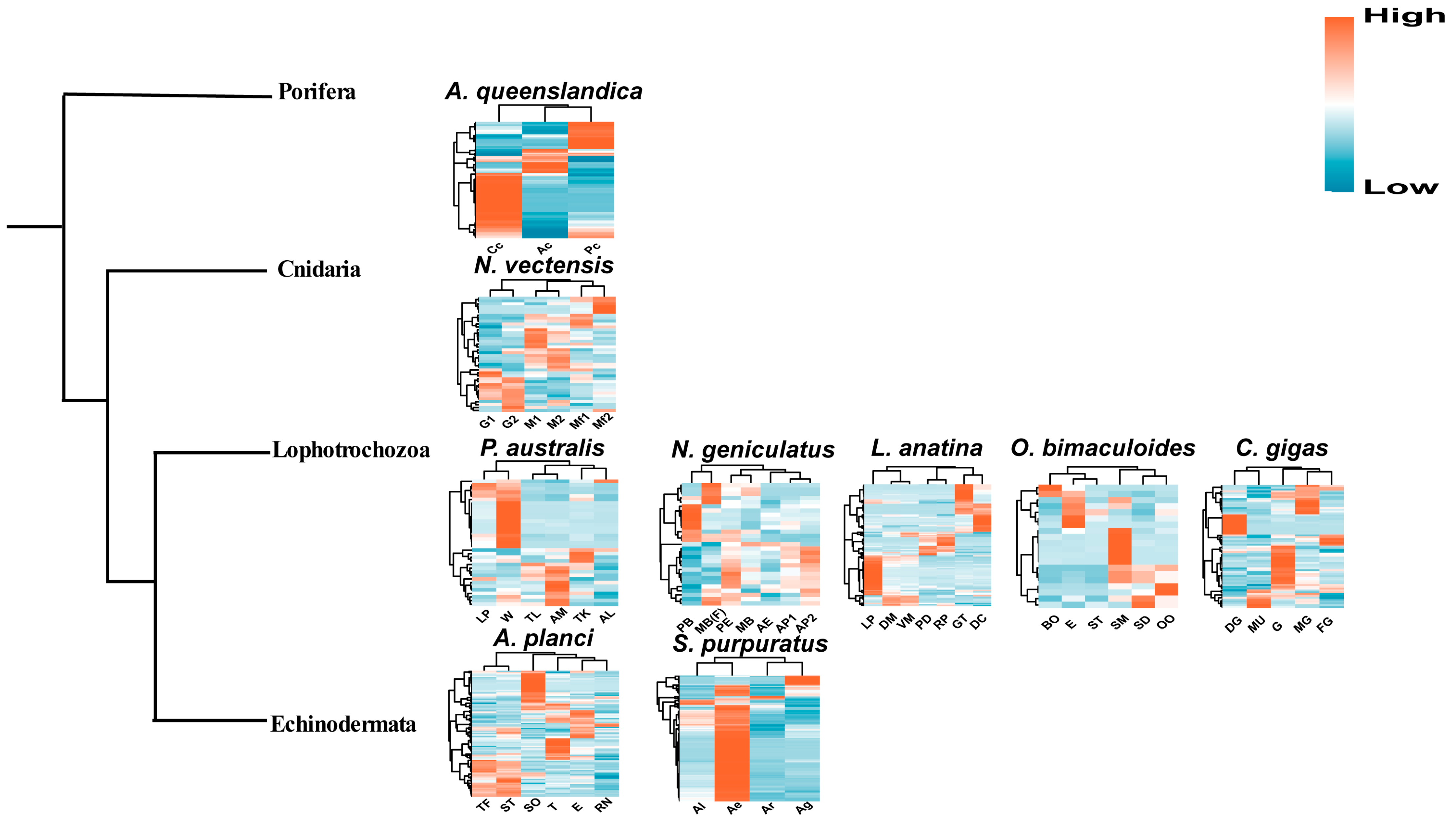
2.3. Expression Profiles of Metazoan SRCR-SFs
2.4. Extensive Expansion of SRCR-SFs in the Sponge
2.5. Extensive Expansion of SRCR-SFs in Sea Urchin
2.6. Comparative Analysis of SRCR-SF Distribution in the Sponge and Sea Urchin
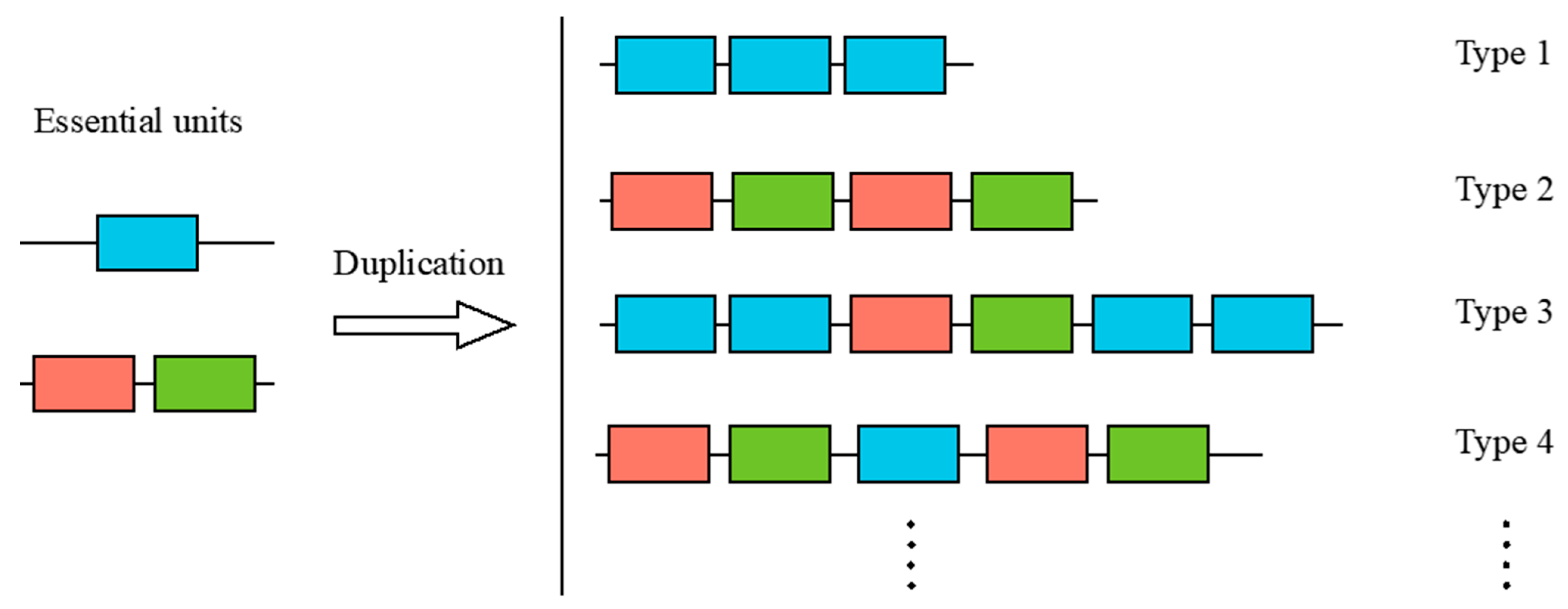
2.7. Comparative Analysis of SRCR-SF Structures in the Sponge and Sea Urchin
3. Discussion
4. Materials and Methods
4.1. Data Collection
4.2. Identification and Domain Annotation of SRCR-SFs
4.3. Localization and Tandem Repeat Identification of SRCR-SF Genes
4.4. SRCR Domain Structure and Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pechinskii, S.V.; Kuregyan, A.G. The Impact of Carotenoids on Immunity. Pharm. Chem. J. 2014, 47, 509–513. [Google Scholar] [CrossRef]
- Hibino, T.; Loza-Coll, M.; Messier, C.; Majeske, A.J.; Cohen, A.H.; Terwilliger, D.P.; Buckley, K.M.; Brockton, V.; Nair, S.V.; Berney, K.; et al. The immune gene repertoire encoded in the purple sea urchin genome. Dev. Biol. 2006, 300, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Peiris, T.H.; Hoyer, K.K.; Oviedo, N.J. Innate immune system and tissue regeneration in planarians: An area ripe for exploration. Semin. Immunol. 2014, 26, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Sousa, H.; Hinzmann, M. Review: Antibacterial components of the Bivalve’s immune system and the potential of freshwater bivalves as a source of new antibacterial compounds. Fish Shellfish Immunol. 2020, 98, 971–980. [Google Scholar] [CrossRef]
- Kurtz, J. Memory in the innate and adaptive immune systems. Microbes Infect. 2004, 6, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Weaver, C. Janeway’s Immunobiology; W.W. Norton: New York, NY, USA, 2016. [Google Scholar]
- Prigot-Maurice, C.; Beltran-Bech, S.; Braquart-Varnier, C. Why and how do protective symbionts impact immune priming with pathogens in invertebrates? Dev. Comp. Immunol. 2022, 126, 104245. [Google Scholar] [CrossRef]
- Verma, M.; Michalec, L.; Sripada, A.; McKay, J.; Sirohi, K.; Dyjack, N.T.; Gorska, M.M.; Seibold, M.; Martin, R.J.; Alam, R. Innate Lymphoid Cells (ILCs) Generate Memory for Pathogen-Associated Molecular Patterns (PAMPs) of Allergens, Which Contributes to Asthma. J. Allergy Clin. Immunol. 2019, 143, AB199. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Shekarian, T.; Valsesia-Wittmann, S.; Brody, J.; Michallet, M.C.; Depil, S.; Caux, C.; Marabelle, A. Pattern recognition receptors: Immune targets to enhance cancer immunotherapy. Ann. Oncol. 2017, 28, 1756–1766. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Q.; Wang, T.; Xu, J.; Li, T.; Liu, Q.; Yao, Q.; Wang, P. Pattern Recognition Receptors (PRRs) in Macrophages Possess Prognosis and Immunotherapy Potential for Melanoma. Front. Immunol. 2021, 12, 765615. [Google Scholar] [CrossRef]
- Taghavi, M.; Khosravi, A.; Mortaz, E.; Nikaein, D.; Athari, S.S. Role of pathogen-associated molecular patterns (PAMPS) in immune responses to fungal infections. Eur. J. Pharmacol. 2017, 808, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54 Pt 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.; Ashkenas, J.; Rees, D.J.; Kingsley, D.M.; Copeland, N.G.; Jenkins, N.A.; Krieger, M. An ancient, highly conserved family of cysteine-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc. Natl. Acad. Sci. USA 1990, 87, 8810–8814. [Google Scholar] [CrossRef]
- Bowdish, D.M.; Gordon, S. Conserved domains of the class A scavenger receptors: Evolution and function. Immunol. Rev. 2009, 227, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Nielsen, O.; Fenger, C.; Madsen, J.; Hansen, S.; Tornoe, I.; Eggleton, P.; Reid, K.B.; Holmskov, U. The scavenger receptor, cysteine-rich domain-containing molecule gp-340 is differentially regulated in epithelial cell lines by phorbol ester. Clin. Exp. Immunol. 2002, 130, 449–458. [Google Scholar] [CrossRef]
- Hohenester, E.; Sasaki, T.; Timpl, R. Crystal structure of a scavenger receptor cysteine-rich domain sheds light on an ancient superfamily. Nat. Struct. Biol. 1999, 6, 228–232. [Google Scholar] [CrossRef]
- Sarrias, M.R.; Grønlund, J.; Padilla, O.; Madsen, J.; Holmskov, U.; Lozano, F. The Scavenger Receptor Cysteine-Rich (SRCR) domain: An ancient and highly conserved protein module of the innate immune system. Crit. Rev. Immunol. 2004, 24, 1–37. [Google Scholar] [CrossRef]
- Casadó-Llombart, S.; Velasco-de Andrés, M.; Català, C.; Leyton-Pereira, A.; Gutiérrez-Cózar, R.; Suárez, B.; Armiger, N.; Carreras, E.; Esteller, M.; Ricart, E.; et al. Experimental and genetic evidence for the impact of CD5 and CD6 expression and variation in inflammatory bowel disease. Front. Immunol. 2022, 13, 966184. [Google Scholar] [CrossRef]
- Qiu, R.; Sun, B.-G.; Li, J.; Liu, X.; Sun, L. Identification and characterization of a cell surface scavenger receptor cysteine-rich protein of Sciaenops ocellatus: Bacterial interaction and its dependence on the conserved structural features of the SRCR domain. Fish Shellfish Immunol. 2013, 34, 810–818. [Google Scholar] [CrossRef]
- Reichhardt, M.P.; Loimaranta, V.; Lea, S.M.; Johnson, S. Structures of SALSA/DMBT1 SRCR domains reveal the conserved ligand-binding mechanism of the ancient SRCR fold. Life Sci. Alliance 2020, 3, e201900502. [Google Scholar] [CrossRef]
- Kanno, S.; Hirano, S.; Sakamoto, T.; Furuyama, A.; Takase, H.; Kato, H.; Fukuta, M.; Aoki, Y. Scavenger receptor MARCO contributes to cellular internalization of exosomes by dynamin-dependent endocytosis and macropinocytosis. Sci. Rep. 2020, 10, 21795. [Google Scholar] [CrossRef]
- Whitley, K.V.; Freitas, C.M.T.; Moreno, C.; Haynie, C.; Bennett, J.; Hancock, J.C.; Cox, T.D.; Pickett, B.E.; Weber, K.S. CD5 Deficiency Alters Helper T Cell Metabolic Function and Shifts the Systemic Metabolome. Biomedicines 2022, 10, 704. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Huang, C.; Wang, X.J.; Xin, D.E.; Wang, L.S.; Zou, Q.C.; Zhang, Y.S.; Tan, M.D.; Wang, Y.M.; Zhao, T.C.; et al. Lysyl Oxidase 3 Is a Dual-Specificity Enzyme Involved in STAT3 Deacetylation and Deacetylimination Modulation. Mol. Cell 2017, 65, 296–309. [Google Scholar] [CrossRef]
- Pahler, S.; Blumbach, B.; Müller, I.; Müller, W.E.G. Putative multiadhesive protein from the marine sponge Geodia cydonium: Cloning of the cDNA encoding a fibronectin-, an SRCR-, and a complement control protein module. J. Exp. Zool. 1998, 282, 332–343. [Google Scholar] [CrossRef]
- Gonçalves, C.M.; Castro, M.A.A.; Henriques, T.; Oliveira, M.I.; Pinheiro, H.C.; Oliveira, C.; Sreenu, V.B.; Evans, E.J.; Davis, S.J.; Moreira, A.; et al. Molecular cloning and analysis of SSc5D, a new member of the scavenger receptor cysteine-rich superfamily. Mol. Immunol. 2009, 46, 2585–2596. [Google Scholar] [CrossRef]
- Solé, R. Revisiting Leigh Van Valen’s “A New Evolutionary Law” (1973). Biol. Theory 2022, 17, 120–125. [Google Scholar] [CrossRef]
- Martínez, V.G.; Moestrup, S.K.; Holmskov, U.; Mollenhauer, J.; Lozano, F. The conserved scavenger receptor cysteine-rich superfamily in therapy and diagnosis. Pharmacol. Rev. 2011, 63, 967–1000. [Google Scholar] [CrossRef]
- Sarukhan, A.; Martinez-Florensa, M.; Escoda-Ferran, C.; Carrasco, E.; Carreras, E.; Lozano, F. Pattern Recognition by CD6: A Scavenger-Like Lymphocyte Receptor. Curr. Drug Targets 2016, 17, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Rast, J.P.; Smith, L.C.; Loza-Coll, M.; Hibino, T.; Litman, G.W. Genomic Insights into the Immune System of the Sea Urchin. Science 2006, 314, 952–956. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Guo, X.; Litman, G.W.; Dishaw, L.J.; Zhang, G. Massive expansion and functional divergence of innate immune genes in a protostome. Sci. Rep. 2015, 5, 8693. [Google Scholar] [CrossRef]
- Huang, S.; Yuan, S.; Guo, L.; Yu, Y.; Li, J.; Wu, T.; Liu, T.; Yang, M.; Wu, K.; Liu, H.; et al. Genomic analysis of the immune gene repertoire of amphioxus reveals extraordinary innate complexity and diversity. Genome Res. 2008, 18, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, J.; Qiu, L.; Wang, L.; Zhang, H.; Wang, M.; Vinu, S.S.; Song, L. A novel scavenger receptor-cysteine-rich (SRCR) domain containing scavenger receptor identified from mollusk mediated PAMP recognition and binding. Dev. Comp. Immunol. 2011, 35, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.J.; Eddy, S.R. nhmmer: DNA homology search with profile HMMs. Bioinformatics 2013, 29, 2487–2489. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2017, 46, D493–D496. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Jeppe, H.; Konstantinos, D.T.; Mads Damgaard, P.; José Juan Almagro, A.; Paolo, M.; Henrik, N.; Anders, K.; Ole, W. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv 2022. [Google Scholar] [CrossRef]
- Harris, R.M.; Hofmann, H.A. Seeing is believing: Dynamic evolution of gene families. Proc. Natl. Acad. Sci. USA 2015, 112, 1252–1253. [Google Scholar] [CrossRef]
- Cho, S.-J.; Vallès, Y.; Giani, V.C., Jr.; Seaver, E.C.; Weisblat, D.A. Evolutionary Dynamics of the wnt Gene Family: A Lophotrochozoan Perspective. Mol. Biol. Evol. 2010, 27, 1645–1658. [Google Scholar] [CrossRef]
- Li, Y.; Xue, Y.; Peng, Z.; Zhang, L. Immune diversity in lophotrochozoans, with a focus on recognition and effector systems. Comput. Struct. Biotechnol. J. 2023, 21, 2262–2275. [Google Scholar] [CrossRef]
- Canciani, A.; Catucci, G.; Forneris, F. Structural characterization of the third scavenger receptor cysteine-rich domain of murine neurotrypsin. Protein Sci. 2019, 28, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, N.; Song, W.; Yin, G.; Qin, Y.; Yan, Y.; Hu, Y. Soybean (Glycine max) expansin gene superfamily origins: Segmental and tandem duplication events followed by divergent selection among subfamilies. BMC Plant Biol. 2014, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, Z.; Yan, X.; Yu, T.; Huang, G.; Yan, Q.; Pontarotti, P.A.; Zhao, H.; Li, J.; Yang, P.; et al. Decelerated genome evolution in modern vertebrates revealed by analysis of multiple lancelet genomes. Nat. Commun. 2014, 5, 5896. [Google Scholar] [CrossRef] [PubMed]
- Resnick, D.; Chatterton, J.E.; Schwartz, K.; Slayter, H.; Krieger, M. Structures of class A macrophage scavenger receptors. Electron microscopic study of flexible, multidomain, fibrous proteins and determination of the disulfide bond pattern of the scavenger receptor cysteine-rich domain. J. Biol. Chem. 1996, 271, 26924–26930. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Yan, X.; Chen, S.; Wu, C.; Huang, H.; Shi, Y.; Huang, G.; Dong, M.; Xu, A.; et al. Broad distribution, high diversity and ancient origin of the ApeC-containing proteins. Mol. Phylogenet. Evol. 2021, 155, 107009. [Google Scholar] [CrossRef] [PubMed]
- Yuen, B.; Bayes, J.M.; Degnan, S.M. The Characterization of Sponge NLRs Provides Insight into the Origin and Evolution of This Innate Immune Gene Family in Animals. Mol. Biol. Evol. 2013, 31, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Buckley, K.M.; Rast, J.P. Diversity of animal immune receptors and the origins of recognition complexity in the deuterostomes. Dev. Comp. Immunol. 2015, 49, 179–189. [Google Scholar] [CrossRef]
- Vogel, C.; Teichmann, S.A.; Pereira-Leal, J. The relationship between domain duplication and recombination. J. Mol. Biol. 2005, 346, 355–365. [Google Scholar] [CrossRef]
- de Souza, S.J. Domain shuffling and the increasing complexity of biological networks. Bioessays 2012, 34, 655–657. [Google Scholar] [CrossRef]
- Magadum, S.; Banerjee, U.; Murugan, P.; Gangapur, D.; Ravikesavan, R. Gene duplication as a major force in evolution. J. Genet. 2013, 92, 155–161. [Google Scholar] [CrossRef]
- Yao, S.; Chan, J.; Xu, Y.; Wu, S.; Zhang, L. Divergences of the RLR Gene Families across Lophotrochozoans: Domain Grafting, Exon-Intron Structure, Expression, and Positive Selection. Int. J. Mol. Sci. 2022, 23, 3415. [Google Scholar] [CrossRef]
- Oren, M.; Barela Hudgell, M.A.; D’Allura, B.; Agronin, J.; Gross, A.; Podini, D.; Smith, L.C. Short tandem repeats, segmental duplications, gene deletion, and genomic instability in a rapidly diversified immune gene family. BMC Genom. 2016, 17, 900. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-L.; Shiao, Y.-J.; Hou, S.-J.; Yang, C.-N.; Chen, Y.-J.; Lin, C.-H.; Shie, F.-S.; Tsay, H.-J. Cysteine-rich domain of scavenger receptor AI modulates the efficacy of surface targeting and mediates oligomeric Aβ internalization. J. Biomed. Sci. 2013, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, L.M. Immune proteins in brain development and synaptic plasticity. Neuron 2009, 64, 93–109. [Google Scholar] [CrossRef] [PubMed]
- End, C.; Bikker, F.; Renner, M.; Bergmann, G.; Lyer, S.; Blaich, S.; Hudler, M.; Helmke, B.; Gassler, N.; Autschbach, F.; et al. DMBT1 functions as pattern-recognition molecule for poly-sulfated and poly-phosphorylated ligands. Eur. J. Immunol. 2009, 39, 833–842. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Pees, B.; Yang, W.; Zárate-Potes, A.; Schulenburg, H.; Dierking, K. High Innate Immune Specificity through Diversified C-Type Lectin-Like Domain Proteins in Invertebrates. J. Innate Immun. 2015, 8, 129–142. [Google Scholar] [CrossRef]
- Turvey, S.E.; Broide, D.H. Innate immunity. J. Allergy Clin. Immunol. 2010, 125, S24–S32. [Google Scholar] [CrossRef]
- Chen, Y.; Sankala, M.; Ojala, J.R.; Sun, Y.; Tuuttila, A.; Isenman, D.E.; Tryggvason, K.; Pikkarainen, T. A phage display screen and binding studies with acetylated low density lipoprotein provide evidence for the importance of the scavenger receptor cysteine-rich (SRCR) domain in the ligand-binding function of MARCO. J. Biol. Chem. 2006, 281, 12767–12775. [Google Scholar] [CrossRef]
- Hogg, P.J. Contribution of allosteric disulfide bonds to regulation of hemostasis. J. Thromb. Haemost. 2009, 7 (Suppl. S1), 13–16. [Google Scholar] [CrossRef]
- Pijning, A.E.; Hogg, P. Classification of Protein Disulphide Bonds. Methods Mol. Biol. 2019, 1967, 1–8. [Google Scholar] [CrossRef]
- Butera, D.; Passam, F.; Ju, L.; Cook, K.M.; Woon, H.; Aponte-Santamaría, C.; Gardiner, E.; Davis, A.K.; Murphy, D.A.; Bronowska, A.; et al. Autoregulation of von Willebrand factor function by a disulfide bond switch. Sci. Adv. 2018, 4, eaaq1477. [Google Scholar] [CrossRef] [PubMed]
- Ojala, J.R.; Pikkarainen, T.; Tuuttila, A.; Sandalova, T.; Tryggvason, K. Crystal structure of the cysteine-rich domain of scavenger receptor MARCO reveals the presence of a basic and an acidic cluster that both contribute to ligand recognition. J. Biol. Chem. 2007, 282, 16654–16666. [Google Scholar] [CrossRef] [PubMed]
- Rodamilans, B.; Muñoz, I.G.; Bragado-Nilsson, E.; Sarrias, M.R.; Padilla, O.; Blanco, F.J.; Lozano, F.; Montoya, G. Crystal structure of the third extracellular domain of CD5 reveals the fold of a group B scavenger cysteine-rich receptor domain. J. Biol. Chem. 2007, 282, 12669–12677. [Google Scholar] [CrossRef]
- Timmerman, L.A.; Clipstone, N.A.; Ho, S.N.; Northrop, J.P.; Crabtree, G.R. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature 1996, 383, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Garza-Garcia, A.; Esposito, D.; Rieping, W.; Harris, R.; Briggs, C.; Brown, M.H.; Driscoll, P.C. Three-dimensional solution structure and conformational plasticity of the N-terminal scavenger receptor cysteine-rich domain of human CD5. J. Mol. Biol. 2008, 378, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Telfer, J.C.; Hsu, H.; Tyner, M.D.; Le Page, L. Assessment of Scavenger Receptor Cysteine-Rich Domain Binding to Bacteria. Methods Mol. Biol. 2022, 2421, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Somoza, J.R.; Ho, J.D.; Luong, C.; Ghate, M.; Sprengeler, P.A.; Mortara, K.; Shrader, W.D.; Sperandio, D.; Chan, H.; McGrath, M.E.; et al. The structure of the extracellular region of human hepsin reveals a serine protease domain and a novel scavenger receptor cysteine-rich (SRCR) domain. Structure 2003, 11, 1123–1131. [Google Scholar] [CrossRef]
- Zambounis, A.; Elias, M.; Sterck, L.; Maumus, F.; Gachon, C.M.M. Highly Dynamic Exon Shuffling in Candidate Pathogen Receptors…What if Brown Algae Were Capable of Adaptive Immunity? Mol. Biol. Evol. 2011, 29, 1263–1276. [Google Scholar] [CrossRef]
- Kambris, Z.; Hoffmann, J.A.; Imler, J.L.; Capovilla, M. Tissue and stage-specific expression of the Tolls in Drosophila embryos. Gene Expr. Patterns 2002, 2, 311–317. [Google Scholar] [CrossRef]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Bourexis, D.; Brister, J.R.; Canese, K.; Comeau, D.C.; Funk, K.; Kim, S.; Klimke, W.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2021, 49, D10–D17. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Kolde, R.; Kolde, M.R. Package ‘pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A user-friendly online tool for drawing genetic maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2014, 31, 1296–1297. [Google Scholar] [CrossRef]
- Shen, W.; Le, S.; Li, Y.; Hu, F. SeqKit: A Cross-Platform and Ultrafast Toolkit for FASTA/Q File Manipulation. PLoS ONE 2016, 11, e0163962. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]



| Species | A. queenslandica | P. australis | C. gigas | S. purpuratus | A. planci | D. rerio | G. gallus |
|---|---|---|---|---|---|---|---|
| EGF_CA (PF07645) | CUB (PF00431) | TSP 1 (PF00090) | EGF-like (PF00008) | Sushi repeat (PF00084) | Trypsin (PF00089) | Trypsin (PF00089) | |
| cEGF (PF12662) | Kringle (PF00051) | LDL A (PF00057) | Sushi repeat (PF00084) | EGF-like (PF00008) | Collagen (PF01391) | LDL-A (PF00057) | |
| Domain types | FXa_inhibition (PF14670) | WSC (PF01822) | EGF-like (PF00008) | F5/8 type C (PF00754) | LDL-A (PF00057) | Ig_3 (PF13927) | Collagen (PF01391) |
| PK_Tyr_Ser-Thr (PF07714) | LDL-A (PF00057) | MAM (PF00629) | WSC (PF01822) | F5/8 type C (PF00754) | Lysyl oxidase (PF01186) | CUB (PF00431) | |
| Astacin (PF01400) | PKD (PF00801) | EGF_CA (PF07645) | CUB (PF00431) | 7tm_2 (PF00002) | Kringle (PF00051) | Lysyl oxidase (PF01186) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Z.; Zhang, W.; Fu, H.; Li, Y.; Zhang, C.; Li, J.; Chan, J.; Zhang, L. Genome-Wide Comparative Analysis of SRCR Gene Superfamily in Invertebrates Reveals Massive and Independent Gene Expansions in the Sponge and Sea Urchin. Int. J. Mol. Sci. 2024, 25, 1515. https://doi.org/10.3390/ijms25031515
Peng Z, Zhang W, Fu H, Li Y, Zhang C, Li J, Chan J, Zhang L. Genome-Wide Comparative Analysis of SRCR Gene Superfamily in Invertebrates Reveals Massive and Independent Gene Expansions in the Sponge and Sea Urchin. International Journal of Molecular Sciences. 2024; 25(3):1515. https://doi.org/10.3390/ijms25031515
Chicago/Turabian StylePeng, Zhangjie, Wei Zhang, Hailun Fu, Yuzhu Li, Chunyu Zhang, Jie Li, Jiulin Chan, and Linlin Zhang. 2024. "Genome-Wide Comparative Analysis of SRCR Gene Superfamily in Invertebrates Reveals Massive and Independent Gene Expansions in the Sponge and Sea Urchin" International Journal of Molecular Sciences 25, no. 3: 1515. https://doi.org/10.3390/ijms25031515
APA StylePeng, Z., Zhang, W., Fu, H., Li, Y., Zhang, C., Li, J., Chan, J., & Zhang, L. (2024). Genome-Wide Comparative Analysis of SRCR Gene Superfamily in Invertebrates Reveals Massive and Independent Gene Expansions in the Sponge and Sea Urchin. International Journal of Molecular Sciences, 25(3), 1515. https://doi.org/10.3390/ijms25031515







