Amylin, Another Important Neuroendocrine Hormone for the Treatment of Diabesity
Abstract
1. Introduction
2. Amylin
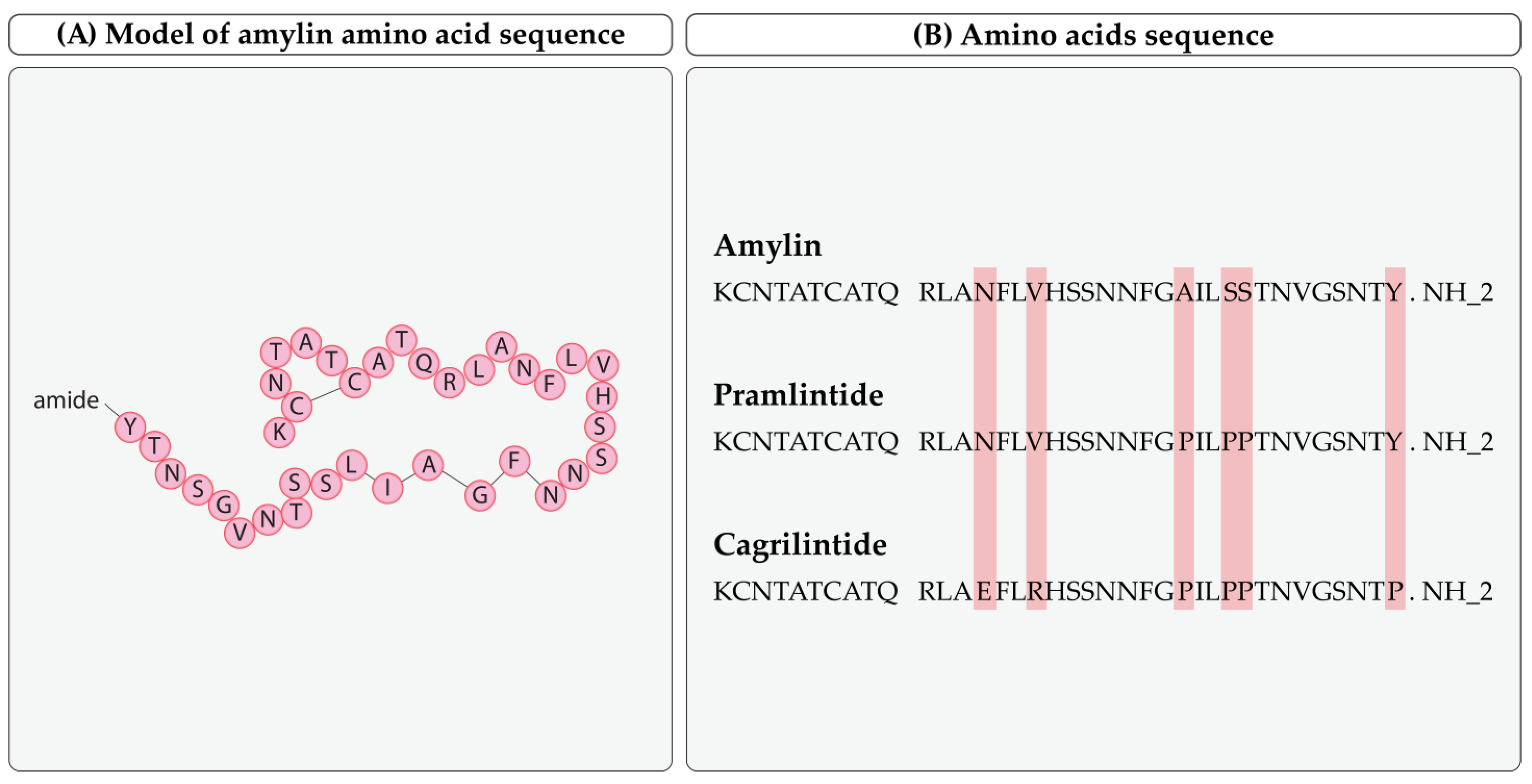
2.1. Biochemical Structure
2.2. Amylin Synthesis and Metabolism
2.3. Physiology and Neuroendocrine Effects of Amylin
2.4. Role of Amylin in the Pathogenesis of Diseases
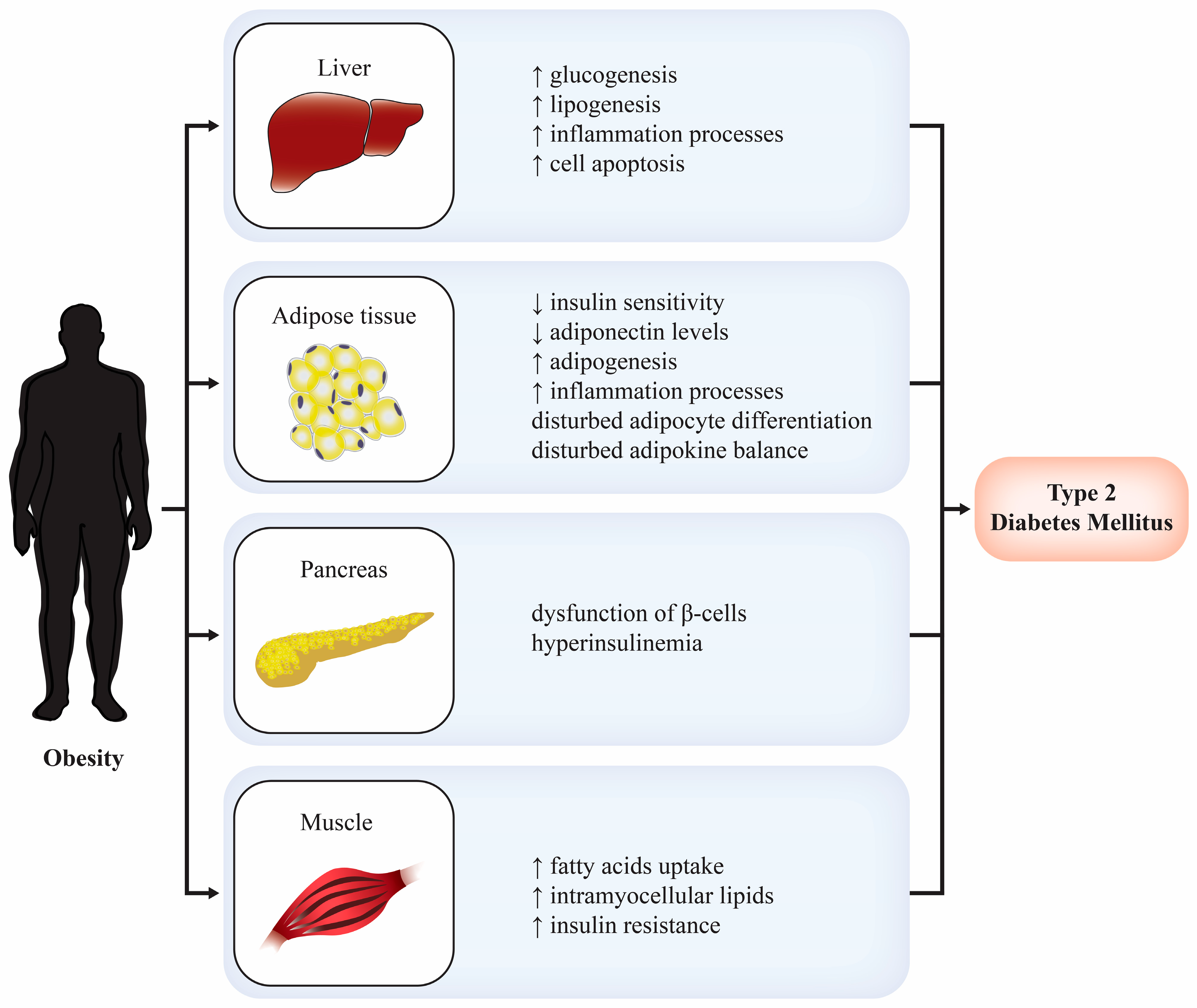
Amylin’s Role in Diabetes and Obesity
2.5. Amylin Receptor
3. Amyline Analogs
3.1. Pramlintide
3.1.1. Pharmacokinetics
3.1.2. Administration and Dosage
3.1.3. Clinical Use
3.1.4. Adverse Effects
3.1.5. Contraindications and Carcinogenicity
| Authors | Research Design (Primary Goal, Duration) | Number of Patients | Results | Main Conclusions |
|---|---|---|---|---|
| Karl D et al., 2007 [66] | Open-label study. Safety and efficacy of pramlintide therapy. Duration: 2 years. | 166 insulin-treated patients with T2DM | The change in HBA1c from baseline was −0.56%. Pramlintide therapy notably reduced weight (−2.8 kg) and postprandial glucose excursions. | Pramlintide initiation and mealtime insulin reduction led to weight loss. It also improved postprandial glucose excursions and HBA1c. |
| Pencek R et al., 2010 [67] | Open-label, multicenter, observational study. Primary goal was to assess the risk of insulin-induced severe hypoglycemia after pramlintide initiation. Duration: 6 months | 1297 patients with T1DM and T2DM with inadequate glycaemic control | After 3 months, the incidence of patient-ascertained severe hypoglycemia (PASH) was 2.8% in patients with T2DM and 4.8% in patients with T1DM. | The possibility of insulin-induced severe hypoglycemia after pramlintide therapy initiation is low in patients with T2DM or T1DM. |
| Riddle M et al., 2007 [68] | A randomized, double-blind, placebo-controlled, multicenter study. Safety of adding pramlinitide to insulin glargine therapy. Duration: one year. | 212 patients with T2DM using insulin glargine in addition to pramlintide or placebo | Reductions in HBA1c (−0.70% against −0.36%) and postprandial glucose increase were more significant in pramlintide-treated patients. Pramlinitide-treated patients experienced weight loss (−1.6 kg), while placebo gained weight (+0.7 kg). | Pramlintide improved HBA1c and postprandial glucose with weight reduction in T2DM patients. |
| Peyrot M et al., 2010 [69] | A randomized, open-label, parallel-group, multicenter study. The effectiveness of basal insulin regimens with rapid-acting insulin or pramlintide. Duration: 9 months. | 112 patients with T2DM and basal insulin therapy in addition to pramlintide or rapid-acting insulin | Total diabetes distress in pramlintide patients improved significantly. On the other hand, patients with rapid-acting insulin did not. The perception of hypoglycemia was improved only in pramlintide patients. | Adding pramlintide to basal insulin treatment improved life quality and satisfaction compared with rapid-acting insulin analogs. |
| Whitehouse et al., 2002 [70] | A double-blinded clinical trial with parallel assignment. Effects of pramlintide on HBA1c and weight. Duration: 52 weeks. | 480 patients with T1DM | HBA1c was lower in patients with pramlintide (−0.39%) in comparison with placebo (−0.12%). The patients with pramlinitide had a weight loss (−0.5%) in contrast to placebo patients with weight gain (+1.0%). | Pramlintide has a positive effect on HBA1c and weight loss compared to placebo. |
| Ratner et al., 2000 [62] | The study was double-blinded with parallel assignment. Effects of pramlinitide on weight and HBA1c. Duration: 52 weeks. | 538 patients with insulin-treated T2DM | The patients with pramlintide had a weight loss (−0.3% to −1.3% depending on the dosage); on the contrary, placebo patients had a weight gain (+1.0%). HBA1c was lower in patients with pramlintide (−0.3% to −6.0% depending on the dosage) in comparison with placebo (−0.2%). | Pramlintide has a positive effect on weight loss and HBA1c compared with placebo. |
| Hollander et al., 2003 [63] | The study was double-blinded with parallel assignment. Pramlintide effects on HBA1c and weight. Duration: 52 weeks. | 498 patients with T2DM | HBA1c was lower in patients with pramlinitide (−0.35% to −0.62% depending on the dosage) than a placebo (−0.22%). The patients with pramlintide had a weight loss (−0.5% to −1.2% depending on the dosage); on the contrary, placebo patients had a weight gain (+0.7%). | Pramlintide has a positive effect on HBA1c and weight loss compared to placebo. |
| Gottlieb et al., 1999 [71] | The study was double-blinded with parallel assignment. Effects of pramlintide on HBA1c and weight. Duration: 26 weeks. | 499 patients with T2DM | The patients with pramlintide had a weight loss (−0.8% to −1.4% depending on the dosage); on the contrary, placebo patients had a weight gain (+0.1%). HBA1c was lower in patients with pramlinitide (−0.3% to −0.4% depending on the dosage) in comparison with placebo (−0.1%). | Pramlintide has a positive effect on HBA1c and weight loss compared to the placebo. |
3.1.6. Cardiovascular Safety
3.1.7. Pramlintide and T1DM
3.1.8. Pramlintide and T2DM
3.2. Cagrilintide
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and Type 2 Diabetes Mellitus: Connections in Epidemiology, Pathogenesis, and Treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef] [PubMed]
- Gæde, P.; Vedel, P.; Larsen, N.; Jensen, G.V.H.; Parving, H.-H.; Pedersen, O. Multifactorial Intervention and Cardiovascular Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2003, 348, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Paschou, S.A.; Papadopoulou-Marketou, N.; Chrousos, G.P.; Kanaka-Gantenbein, C. On Type 1 Diabetes Mellitus Pathogenesis. Endocr. Connect. 2018, 7, R38–R46. [Google Scholar] [CrossRef] [PubMed]
- Michaelidou, M.; Pappachan, J.M.; Jeeyavudeen, M.S. Management of Diabesity: Current Concepts. World J. Diabetes 2023, 14, 396–411. [Google Scholar] [CrossRef] [PubMed]
- Sims, E.A.; Danforth, E.; Horton, E.S.; Bray, G.A.; Glennon, J.A.; Salans, L.B. Endocrine and Metabolic Effects of Experimental Obesity in Man. Recent. Prog. Horm. Res. 1973, 29, 457–496. [Google Scholar] [CrossRef]
- Lahiri, S.W. Personalizing Type 2 Diabetes Management: Use of a Patient-Centered Approach to Individualizing A1C Goals and Pharmacological Regimens. Clin. Diabetes 2017, 35, 321–328. [Google Scholar] [CrossRef]
- Bany Bakar, R.; Reimann, F.; Gribble, F.M. The Intestine as an Endocrine Organ and the Role of Gut Hormones in Metabolic Regulation. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 784–796. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Function and Mechanisms of Enteroendocrine Cells and Gut Hormones in Metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef]
- Ludvik, B.; Kautzky-Willer, A.; Prager, R.; Thomaseth, K.; Pacini, G. Amylin: History and Overview. Diabet. Med. 1997, 14 (Suppl. 2), S9–S13. [Google Scholar] [CrossRef]
- Rink, T.J.; Beaumont, K.; Koda, J.; Young, A. Structure and Biology of Amylin. Trends Pharmacol. Sci. 1993, 14, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Bower, R.L.; Hay, D.L. Amylin Structure–Function Relationships and Receptor Pharmacology: Implications for Amylin Mimetic Drug Development. Br. J. Pharmacol. 2016, 173, 1883–1898. [Google Scholar] [CrossRef] [PubMed]
- PubChem Amylin (1–13) (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/102601526 (accessed on 28 December 2023).
- Cao, J.; Belousoff, M.; Gerrard, E.; Danev, R.; Fletcher, M.; Maso, E.; Schreuder, H.; Lorenz, K.; Evers, A.; Tiwari, G.; et al. Structural Insight into Selectivity of Amylin and Calcitonin Receptor Agonists. Nat. Chem. Biol. 2023, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.T.R.; Icart, L.P. Amyloidogenicity of Peptides Targeting Diabetes and Obesity. Colloids Surf. B Biointerfaces 2022, 209, 112157. [Google Scholar] [CrossRef]
- Alghrably, M.; Czaban, I.; Jaremko, Ł.; Jaremko, M. Interaction of Amylin Species with Transition Metals and Membranes. J. Inorg. Biochem. 2019, 191, 69–76. [Google Scholar] [CrossRef]
- Yang, F. Amylin in Vasodilation, Energy Expenditure and Inflammation. Front. Biosci. 2014, 19, 936. [Google Scholar] [CrossRef]
- Boyle, C.N.; Zheng, Y.; Lutz, T.A. Mediators of Amylin Action in Metabolic Control. J. Clin. Med. 2022, 11, 2207. [Google Scholar] [CrossRef]
- Lutz, T.A. Creating the Amylin Story. Appetite 2022, 172, 105965. [Google Scholar] [CrossRef]
- Lutz, T. Therapeutic Potential of an Old Friend—The Dichotomy of Amylin in Physiology and Pathophysiology. In Proceedings of the Endocrine Abstracts, Harrogate, UK, 14–16 November 2022; Bioscientifica: Bristol, UK, 2022; Volume 86. [Google Scholar]
- Höppener, J.W.M.; Ahrén, B.; Lips, C.J.M. Islet Amyloid and Type 2 Diabetes Mellitus. N. Engl. J. Med. 2000, 343, 411–419. [Google Scholar] [CrossRef]
- Pittner, R.A.; Albrandt, K.; Beaumont, K.; Gaeta, L.S.L.; Koda, J.E.; Moore, C.X.; Rittenhouse, J.; Rink, T.J. Molecular Physiology of Amylin. J. Cell. Biochem. 1994, 55, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.L.; Chen, S.; Lutz, T.A.; Parkes, D.G.; Roth, J.D. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol. Rev. 2015, 67, 564–600. [Google Scholar] [CrossRef]
- Scherbaum, W.A. The Role of Amylin in the Physiology of Glycemic Control. Exp. Clin. Endocrinol. Diabetes 2009, 106, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Gedulin, B.R.; Rink, T.J.; Young, A.A. Dose-Response for Glucagonostatic Effect of Amylin in Rats. Metabolism 1997, 46, 67–70. [Google Scholar] [CrossRef]
- Lutz, T.A.; Meyer, U. Amylin at the Interface between Metabolic and Neurodegenerative Disorders. Front. Neurosci. 2015, 9, 216. [Google Scholar] [CrossRef]
- Abegg, K.; Hermann, A.; Boyle, C.N.; Bouret, S.G.; Lutz, T.A.; Riediger, T. Involvement of Amylin and Leptin in the Development of Projections from the Area Postrema to the Nucleus of the Solitary Tract. Front. Endocrinol. 2017, 8, 324. [Google Scholar] [CrossRef] [PubMed]
- Reidelberger, R.D.; Kelsey, L.; Heimann, D. Effects of Amylin-Related Peptides on Food Intake, Meal Patterns, and Gastric Emptying in Rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2002, 282, R1395–R1404. [Google Scholar] [CrossRef] [PubMed]
- Mollet, A.; Gilg, S.; Riediger, T.; Lutz, T.A. Infusion of the Amylin Antagonist AC 187 into the Area Postrema Increases Food Intake in Rats. Physiol. Behav. 2004, 81, 149–155. [Google Scholar] [CrossRef]
- Trevaskis, J.L.; Wittmer, C.; Athanacio, J.; Griffin, P.S.; Parkes, D.G.; Roth, J.D. Amylin/Leptin Synergy Is Absent in Extreme Obesity and Not Restored by Calorie Restriction-induced Weight Loss in Rats. Obes. Sci. Pract. 2016, 2, 385–391. [Google Scholar] [CrossRef]
- Isaksson, B.; Wang, F.; Permert, J.; Olsson, M.; Fruin, B.; Herrington, M.K.; Enochsson, L.; Erlanson-Albertsson, C.; Arnelo, U. Chronically Administered Islet Amyloid Polypeptide in Rats Serves as an Adiposity Inhibitor and Regulates Energy Homeostasis. Pancreatology 2005, 5, 29–36. [Google Scholar] [CrossRef]
- Wielinga, P.Y.; Löwenstein, C.; Muff, S.; Munz, M.; Woods, S.C.; Lutz, T.A. Central Amylin Acts as an Adiposity Signal to Control Body Weight and Energy Expenditure. Physiol. Behav. 2010, 101, 45–52. [Google Scholar] [CrossRef]
- Ling, W.; Huang, Y.-M.; Qiao, Y.-C.; Zhang, X.-X.; Zhao, H.-L. Human Amylin: From Pathology to Physiology and Pharmacology. Curr. Protein Pept. Sci. 2019, 20, 944–957. [Google Scholar] [CrossRef]
- Zhu, H.; Tao, Q.; Ang, T.F.A.; Massaro, J.; Gan, Q.; Salim, S.; Zhu, R.; Kolachalama, V.B.; Zhang, X.; Devine, S.; et al. Association of Plasma Amylin Concentration With Alzheimer Disease and Brain Structure in Older Adults. JAMA Netw. Open 2019, 2, e199826. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, A.; Alcarraz-Vizán, G.; Fernández, M.; Fernández-Santiago, R.; Ezquerra, M.; Cámara, A.; Serrano, M.; Novials, A.; Muñoz, E.; Valldeoriola, F.; et al. Peripheral Insulin and Amylin Levels in Parkinson’s Disease. Park. Relat. Disord. 2020, 79, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Delamarre, A.; Rigalleau, V.; Meissner, W.G. Insulin Resistance, Diabetes and Parkinson’s Disease: The Match Continues. Park. Relat. Disord. 2020, 80, 199–200. [Google Scholar] [CrossRef]
- Hieronymus, L.; Griffin, S. Role of Amylin in Type 1 and Type 2 Diabetes. Diabetes Educ. 2015, 41, 47S–56S. [Google Scholar] [CrossRef]
- Ludvik, B.; Lell, B.; Hartter, E.; Schnack, C.; Prager, R. Decrease of Stimulated Amylin Release Precedes Impairment of Insulin Secretion in Type II Diabetes. Diabetes 1991, 40, 1615–1619. [Google Scholar] [CrossRef]
- Enoki, S.; Mitsukawa, T.; Takemura, J.; Nakazato, M.; Aburaya, J.; Toshimori, H.; Matsukara, S. Plasma Islet Amyloid Polypeptide Levels in Obesity, Impaired Glucose Tolerance and Non-Insulin-Dependent Diabetes Mellitus. Diabetes Res. Clin. Pract. 1992, 15, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Perry, K.J.; Quiza, M.; Myers, D.E.; Morfis, M.; Christopoulos, G.; Sexton, P.M. Characterization of Amylin and Calcitonin Receptor Binding in the Mouse α-Thyroid-Stimulating Hormone Thyrotroph Cell Line*. Endocrinology 1997, 138, 3486–3496. [Google Scholar] [CrossRef]
- Christopoulos, G.; Perry, K.J.; Morfis, M.; Tilakaratne, N.; Gao, Y.; Fraser, N.J.; Main, M.J.; Foord, S.M.; Sexton, P.M. Multiple Amylin Receptors Arise from Receptor Activity-Modifying Protein Interaction with the Calcitonin Receptor Gene Product. Mol. Pharmacol. 1999, 56, 235–242. [Google Scholar] [CrossRef]
- Muff, R.; Bühlmann, N.; Fischer, J.A.; Born, W. An Amylin Receptor Is Revealed Following Co-Transfection of a Calcitonin Receptor with Receptor Activity Modifying Proteins-1 or -3. Endocrinology 1999, 140, 2924–2927. [Google Scholar] [CrossRef]
- Tilakaratne, N.; Christopoulos, G.; Zumpe, E.T.; Foord, S.M.; Sexton, P.M. Amylin Receptor Phenotypes Derived from Human Calcitonin Receptor/RAMP Coexpression Exhibit Pharmacological Differences Dependent on Receptor Isoform and Host Cell Environment. J. Pharmacol. Exp. Ther. 2000, 294, 61–72. [Google Scholar]
- Gorn, A.H.; Lin, H.Y.; Yamin, M.; Auron, P.E.; Flannery, M.R.; Tapp, D.R.; Manning, C.A.; Lodish, H.F.; Krane, S.M.; Goldring, S.R. Cloning, Characterization, and Expression of a Human Calcitonin Receptor from an Ovarian Carcinoma Cell Line. J. Clin. Investig. 1992, 90, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Kuestner, R.E.; Elrod, R.D.; Grant, F.J.; Hagen, F.S.; Kuijper, J.L.; Matthewes, S.L.; O’Hara, P.J.; Sheppard, P.O.; Stroop, S.D.; Thompson, D.L. Cloning and Characterization of an Abundant Subtype of the Human Calcitonin Receptor. Mol. Pharmacol. 1994, 46, 246–255. [Google Scholar] [PubMed]
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. RAMPs Regulate the Transport and Ligand Specificity of the Calcitonin-Receptor-like Receptor. Nature 1998, 393, 333–339. [Google Scholar] [CrossRef]
- Bühlmann, N.; Leuthäuser, K.; Muff, R.; Fischer, J.A.; Born, W. A Receptor Activity Modifying Protein (RAMP)2-Dependent Adrenomedullin Receptor Is a Calcitonin Gene-Related Peptide Receptor When Coexpressed with Human RAMP1. Endocrinology 1999, 140, 2883–2890. [Google Scholar] [CrossRef]
- Fraser, N.J.; Wise, A.; Brown, J.; McLatchie, L.M.; Main, M.J.; Foord, S.M. The Amino Terminus of Receptor Activity Modifying Proteins Is a Critical Determinant of Glycosylation State and Ligand Binding of Calcitonin Receptor-like Receptor. Mol. Pharmacol. 1999, 55, 1054–1059. [Google Scholar] [CrossRef]
- Just, R.; Simms, J.; Furness, S.G.B.; Christopoulos, A.; Sexton, P.M. Understanding Amylin Receptors. In The Calcitonin Gene-related Peptide Family; Hay, D.L., Dickerson, I.M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 41–57. ISBN 978-90-481-2908-9. [Google Scholar]
- Despa, F.; DeCarli, C. Amylin: What Might Be Its Role in Alzheimer’s Disease and How Could This Affect Therapy? Expert Rev. Proteom. 2013, 10, 403–405. [Google Scholar] [CrossRef]
- Boyle, C.N.; Lutz, T.A.; Le Foll, C. Amylin—Its Role in the Homeostatic and Hedonic Control of Eating and Recent Developments of Amylin Analogs to Treat Obesity. Mol. Metab. 2017, 8, 203–210. [Google Scholar] [CrossRef]
- Fu, W.; Patel, A.; Jhamandas, J.H. Amylin Receptor: A Common Pathophysiological Target in Alzheimer’s Disease and Diabetes Mellitus. Front. Aging Neurosci. 2013, 5, 42. [Google Scholar] [CrossRef]
- Soudy, R.; Kimura, R.; Patel, A.; Fu, W.; Kaur, K.; Westaway, D.; Yang, J.; Jhamandas, J. Short Amylin Receptor Antagonist Peptides Improve Memory Deficits in Alzheimer’s Disease Mouse Model. Sci. Rep. 2019, 9, 10942. [Google Scholar] [CrossRef]
- Mietlicki-Baase, E.G.; Rupprecht, L.E.; Olivos, D.R.; Zimmer, D.J.; Alter, M.D.; Pierce, R.C.; Schmidt, H.D.; Hayes, M.R. Amylin Receptor Signaling in the Ventral Tegmental Area Is Physiologically Relevant for the Control of Food Intake. Neuropsychopharmacology 2013, 38, 1685–1697. [Google Scholar] [CrossRef]
- Walker, C.S.; Eftekhari, S.; Bower, R.L.; Wilderman, A.; Insel, P.A.; Edvinsson, L.; Waldvogel, H.J.; Jamaluddin, M.A.; Russo, A.F.; Hay, D.L. A Second Trigeminal CGRP Receptor: Function and Expression of the AMY1 Receptor. Ann. Clin. Transl. Neurol. 2015, 2, 595–608. [Google Scholar] [CrossRef]
- Hoogwerf, B. Pramlintide, the Synthetic Analogue of Amylin: Physiology, Pathophysiology, and Effects on Glycemic Control, Body Weight, and Selected Biomarkers of Vascular Risk. Vasc. Health Risk Manag. 2008, 4, 355–362. [Google Scholar] [CrossRef] [PubMed]
- McQueen, J. Pramlintide Acetate. Am. J. Health-Syst. Pharm. 2005, 62, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Katzung, B.G. (Ed.) A Lange medical book. In Basic & Clinical Pharmacology, 14th ed.; McGraw-Hill Education: New York, NY, USA, 2018; ISBN 978-1-259-64115-2. [Google Scholar]
- DeMarsilis, A.; Reddy, N.; Boutari, C.; Filippaios, A.; Sternthal, E.; Katsiki, N.; Mantzoros, C. Pharmacotherapy of Type 2 Diabetes: An Update and Future Directions. Metabolism 2022, 137, 155332. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- Ratner, R.E.; Want, L.L.; Fineman, M.S.; Velte, M.J.; Ruggles, J.A.; Gottlieb, A.; Weyer, C.; Kolterman, O.G. Adjunctive Therapy with the Amylin Analogue Pramlintide Leads to a Combined Improvement in Glycemic and Weight Control in Insulin-Treated Subjects with Type 2 Diabetes. Diabetes Technol. Ther. 2002, 4, 51–61. [Google Scholar] [CrossRef]
- Hollander, P.A.; Levy, P.; Fineman, M.S.; Maggs, D.G.; Shen, L.Z.; Strobel, S.A.; Weyer, C.; Kolterman, O.G. Pramlintide as an Adjunct to Insulin Therapy Improves Long-Term Glycemic and Weight Control in Patients with Type 2 Diabetes. Diabetes Care 2003, 26, 784–790. [Google Scholar] [CrossRef] [PubMed]
- SYMLIN® (Pramlintide Acetate) Injection. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021332s006lbl.pdf (accessed on 28 December 2023).
- Drug Approval Package: Symlin (Pramlintide Acetate) NDA #021332. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/21-332_Symlin.cfm (accessed on 28 December 2023).
- Karl, D.; Philis-Tsimikas, A.; Darsow, T.; Lorenzi, G.; Kellmeyer, T.; Lutz, K.; Wang, Y.; Frias, J.P. Pramlintide as An Adjunct to Insulin in Patients with Type 2 Diabetes in A Clinical Practice Setting Reduced A1C, Postprandial Glucose Excursions, And Weight. Diabetes Technol. Ther. 2007, 9, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Pencek, R.; Roddy, T.; Peters, Y.; De Young, M.B.; Herrmann, K.; Meller, L.; Nguyen, H.; Chen, S.; Lutz, K. Safety of Pramlintide Added to Mealtime Insulin in Patients with Type 1 or Type 2 Diabetes: A Large Observational Study. Diabetes Obes. Metab. 2010, 12, 548–551. [Google Scholar] [CrossRef]
- Riddle, M.; Frias, J.; Zhang, B.; Maier, H.; Brown, C.; Lutz, K.; Kolterman, O. Pramlintide Improved Glycemic Control and Reduced Weight in Patients with Type 2 Diabetes Using Basal Insulin. Diabetes Care 2007, 30, 2794–2799. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, M.; Rubin, R.R.; Polonsky, W.H.; Jennie Best, H. Patient Reported Outcomes in Adults with Type 2 Diabetes on Basal Insulin Randomized to Addition of Mealtime Pramlintide or Rapid-Acting Insulin Analogs. Curr. Med. Res. Opin. 2010, 26, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, F.; Kruger, D.F.; Fineman, M.; Shen, L.; Ruggles, J.A.; Maggs, D.G.; Weyer, C.; Kolterman, O.G. A Randomized Study and Open-Label Extension Evaluating the Long-Term Efficacy of Pramlintide as an Adjunct to Insulin Therapy in Type 1 Diabetes. Diabetes Care 2002, 25, 724–730. [Google Scholar] [CrossRef]
- Gottlieb, A.; Fineman, M.; Bahner, A. Pramlintide Therapy in Addition to Insulin in Type 2 Diabetes: Effect on Metabolic Control after 6 Months. Diabetologia 1999, 42, A232. [Google Scholar]
- Al-Keilani, M.; Alsmadi, D.; Darweesh, R.; Alzoubi, K. Pramlintide, an Antidiabetic, Is Antineoplastic in Colorectal Cancer and Synergizes with Conventional Chemotherapy. Clin. Pharmacol. Adv. Appl. 2018, 10, 23–29. [Google Scholar] [CrossRef]
- Venkatanarayan, A.; Raulji, P.; Norton, W.; Chakravarti, D.; Coarfa, C.; Su, X.; Sandur, S.K.; Ramirez, M.S.; Lee, J.; Kingsley, C.V.; et al. IAPP-Driven Metabolic Reprogramming Induces Regression of P53-Deficient Tumours in Vivo. Nature 2015, 517, 626–630. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Z.; Flores, E.R.; Kleinerman, E.S. Pramlintide: A Novel Therapeutic Approach for Osteosarcoma through Metabolic Reprogramming. Cancers 2022, 14, 4310. [Google Scholar] [CrossRef]
- Herrmann, K.; Zhou, M.; Wang, A.; De Bruin, T.W.A. Cardiovascular Safety Assessment of Pramlintide in Type 2 Diabetes: Results from a Pooled Analysis of Five Clinical Trials. Clin. Diabetes Endocrinol. 2016, 2, 12. [Google Scholar] [CrossRef]
- ORIGIN Trial Investigators; Gerstein, H.C.; Bosch, J.; Dagenais, G.R.; Díaz, R.; Jung, H.; Maggioni, A.P.; Pogue, J.; Probstfield, J.; Ramachandran, A.; et al. Basal Insulin and Cardiovascular and Other Outcomes in Dysglycemia. N. Engl. J. Med. 2012, 367, 319–328. [Google Scholar] [CrossRef]
- Edelman, S.; Garg, S.; Frias, J.; Maggs, D.; Wang, Y.; Zhang, B.; Strobel, S.; Lutz, K.; Kolterman, O. A Double-Blind, Placebo-Controlled Trial Assessing Pramlintide Treatment in the Setting of Intensive Insulin Therapy in Type 1 Diabetes. Diabetes Care 2006, 29, 2189–2195. [Google Scholar] [CrossRef]
- Ratner, R.E.; Dickey, R.; Fineman, M.; Maggs, D.G.; Shen, L.; Strobel, S.A.; Weyer, C.; Kolterman, O.G. Amylin Replacement with Pramlintide as an Adjunct to Insulin Therapy Improves Long-term Glycaemic and Weight Control in Type 1 Diabetes Mellitus: A 1-year, Randomized Controlled Trial. Diabet. Med. 2004, 21, 1204–1212. [Google Scholar] [CrossRef]
- Weinzimer, S.A.; Sherr, J.L.; Cengiz, E.; Kim, G.; Ruiz, J.L.; Carria, L.; Voskanyan, G.; Roy, A.; Tamborlane, W.V. Effect of Pramlintide on Prandial Glycemic Excursions during Closed-Loop Control in Adolescents and Young Adults with Type 1 Diabetes. Diabetes Care 2012, 35, 1994–1999. [Google Scholar] [CrossRef]
- Andersen, G.; Meiffren, G.; Famulla, S.; Heise, T.; Ranson, A.; Seroussi, C.; Eloy, R.; Gaudier, M.; Charvet, R.; Chan, Y.; et al. ADO09, a Co-formulation of the Amylin Analogue Pramlintide and the Insulin Analogue A21G, Lowers Postprandial Blood Glucose versus Insulin Lispro in Type 1 Diabetes. Diabetes Obes. Metab. 2021, 23, 961–970. [Google Scholar] [CrossRef]
- Larsen, A.T.; Mohamed, K.E.; Sonne, N.; Bredtoft, E.; Andersen, F.; Karsdal, M.; Henriksen, K. Does Receptor Balance Matter?—Comparing the Efficacies of the Dual Amylin and Calcitonin Receptor Agonists Cagrilintide and KBP-336 on Metabolic Parameters in Preclinical Models. Biomed. Pharmacother. 2022, 156, 113842. [Google Scholar] [CrossRef]
- Bailey, C.J.; Flatt, P.R.; Conlon, J.M. An Update on Peptide-Based Therapies for Type 2 Diabetes and Obesity. Peptides 2023, 161, 170939. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Deenadayalan, S.; Erichsen, L.; Knop, F.K.; Lingvay, I.; Macura, S.; Mathieu, C.; Pedersen, S.D.; Davies, M. Efficacy and Safety of Co-Administered Once-Weekly Cagrilintide 2·4 Mg with Once-Weekly Semaglutide 2·4 Mg in Type 2 Diabetes: A Multicentre, Randomised, Double-Blind, Active-Controlled, Phase 2 Trial. Lancet 2023, 402, 720–730. [Google Scholar] [CrossRef]
- Novo Nordisk A/S. Efficacy and Safety of Co-Administered Cagrilintide and Semaglutide (CagriSema) s.c. in Doses 2.4/2.4 Mg and 1.0/1.0 Mg Once Weekly versus Semaglutide 2.4 Mg and 1.0 Mg, Cagrilintide 2.4 Mg and Placebo in Participants with Type 2 Diabetes Inadequately Controlled on Metformin with or without an SGLT2 Inhibitor. 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT06065540 (accessed on 18 January 2024).
- Novo Nordisk A/S. Efficacy and Safety of Cagrilintide s.c. 2.4 Mg in Combination with Semaglutide s.c. 2.4 Mg (CagriSema s.c. 2.4 Mg/2.4 Mg) Once-Weekly in Participants with Overweight or Obesity and Type 2 Diabetes. 2023. Available online: https://clinicaltrials.gov/study/NCT05394519?term=NCT05394519&rank=1 (accessed on 18 January 2024).
- Novo Nordisk A/S. Efficacy and Safety of Co-Administered Cagrilintide and Semaglutide (CagriSema 2.4 Mg/2.4 Mg) Once Weekly versus Semaglutide 2.4 Mg, Cagrilintide 2.4 Mg and Placebo in People with Chronic Kidney Disease and Type 2 Diabetes Living with Overweight or Obesity. 2023. Available online: https://clinicaltrials.gov/study/NCT06131372?term=NCT06131372&rank=1 (accessed on 18 January 2024).
- Novo Nordisk A/S. Efficacy and Safety of Cagrilintide s.c. 2.4 Milligram (Mg) in Combination with Semaglutide Subcutaneous (s.c). 2.4 Mg (CagriSema s.c. 2.4 Mg/2.4 Mg) Once-Weekly in Participants with Overweight or Obesity. 2023. Available online: https://clinicaltrials.gov/study/NCT05567796?term=NCT05567796&rank=1 (accessed on 18 January 2023).
- Lau, D.C.W.; Erichsen, L.; Francisco, A.M.; Satylganova, A.; le Roux, C.W.; McGowan, B.; Pedersen, S.D.; Pietiläinen, K.H.; Rubino, D.; Batterham, R.L. Once-Weekly Cagrilintide for Weight Management in People with Overweight and Obesity: A Multicentre, Randomised, Double-Blind, Placebo-Controlled and Active-Controlled, Dose-Finding Phase 2 Trial. Lancet 2021, 398, 2160–2172. [Google Scholar] [CrossRef] [PubMed]
- Enebo, L.B.; Berthelsen, K.K.; Kankam, M.; Lund, M.T.; Rubino, D.M.; Satylganova, A.; Lau, D.C.W. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Concomitant Administration of Multiple Doses of Cagrilintide with Semaglutide 2·4 Mg for Weight Management: A Randomised, Controlled, Phase 1b Trial. Lancet 2021, 397, 1736–1748. [Google Scholar] [CrossRef] [PubMed]

| Authors | Research Design (Duration, Comparator) | Number of Patients | Results | Main Findings |
|---|---|---|---|---|
| Frias J P et al., 2023 [83] | Multicenter, double-blinded randomized study. The safety and effect of cagrilintide and semaglutide combination in T2DM patients. Duration: one year. | Patients were divided into three groups: 31 with CagriSema, 31 with semaglutide, and 30 with cagrilintide | CagriSema had a significant HbA1c reduction compared with cagrilintide (−1.3%) but not with semaglutide (−0.4%). CagriSema had better results regarding the change in body weight (−15.6%) in comparison with cagrilintide –(8.1%) and semaglutide (−5.1%). | CagriSema therapy led to better glycaemic control in T2DM patients. CagriSema had better results in HBA1c reduction than cagrilinitide but not semaglutide. CagriSema had the best results regarding weight loss. |
| NCT06065540 Study start: 2023 [84] | The study is randomized with parallel assignment. The effect of CagriSema, cagrilintide, semaglutide, and placebo on blood glucose and body weight) in T2DM patients treated with metformin. | Estimated enrollment of 2700 participants | Not available | Ongoing study |
| NCT05394519 Study start: 2023 [85] | The study is randomized with parallel assignment. The effect of CagriSema on weight loss in patients with excess body weight and T2DM. | Estimated enrollment of 1200 participants | Not available | Ongoing study |
| NCT06131372 Study start: 2024 [86] | Randomized with parallel assignment. Comparison between CagriSema, cagrilintide, semaglutide, and placebo regarding the renal damage in people with chronic kidney disease, T2DM, and excess body weight. | Estimated enrollment of 618 participants | Not available | Ongoing study |
| NCT05567796 Study start: 2022 [87] | The study is randomized with parallel assignment. Safety and effectiveness of CagriSema in patients with excess body weight. | Estimated enrollment of 3400 participants | Not available | Ongoing study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eržen, S.; Tonin, G.; Jurišić Eržen, D.; Klen, J. Amylin, Another Important Neuroendocrine Hormone for the Treatment of Diabesity. Int. J. Mol. Sci. 2024, 25, 1517. https://doi.org/10.3390/ijms25031517
Eržen S, Tonin G, Jurišić Eržen D, Klen J. Amylin, Another Important Neuroendocrine Hormone for the Treatment of Diabesity. International Journal of Molecular Sciences. 2024; 25(3):1517. https://doi.org/10.3390/ijms25031517
Chicago/Turabian StyleEržen, Stjepan, Gašper Tonin, Dubravka Jurišić Eržen, and Jasna Klen. 2024. "Amylin, Another Important Neuroendocrine Hormone for the Treatment of Diabesity" International Journal of Molecular Sciences 25, no. 3: 1517. https://doi.org/10.3390/ijms25031517
APA StyleEržen, S., Tonin, G., Jurišić Eržen, D., & Klen, J. (2024). Amylin, Another Important Neuroendocrine Hormone for the Treatment of Diabesity. International Journal of Molecular Sciences, 25(3), 1517. https://doi.org/10.3390/ijms25031517






