Sodium Butyrate Inhibits the Expression of Thymidylate Synthase and Induces Cell Death in Colorectal Cancer Cells
Abstract
1. Introduction
2. Results
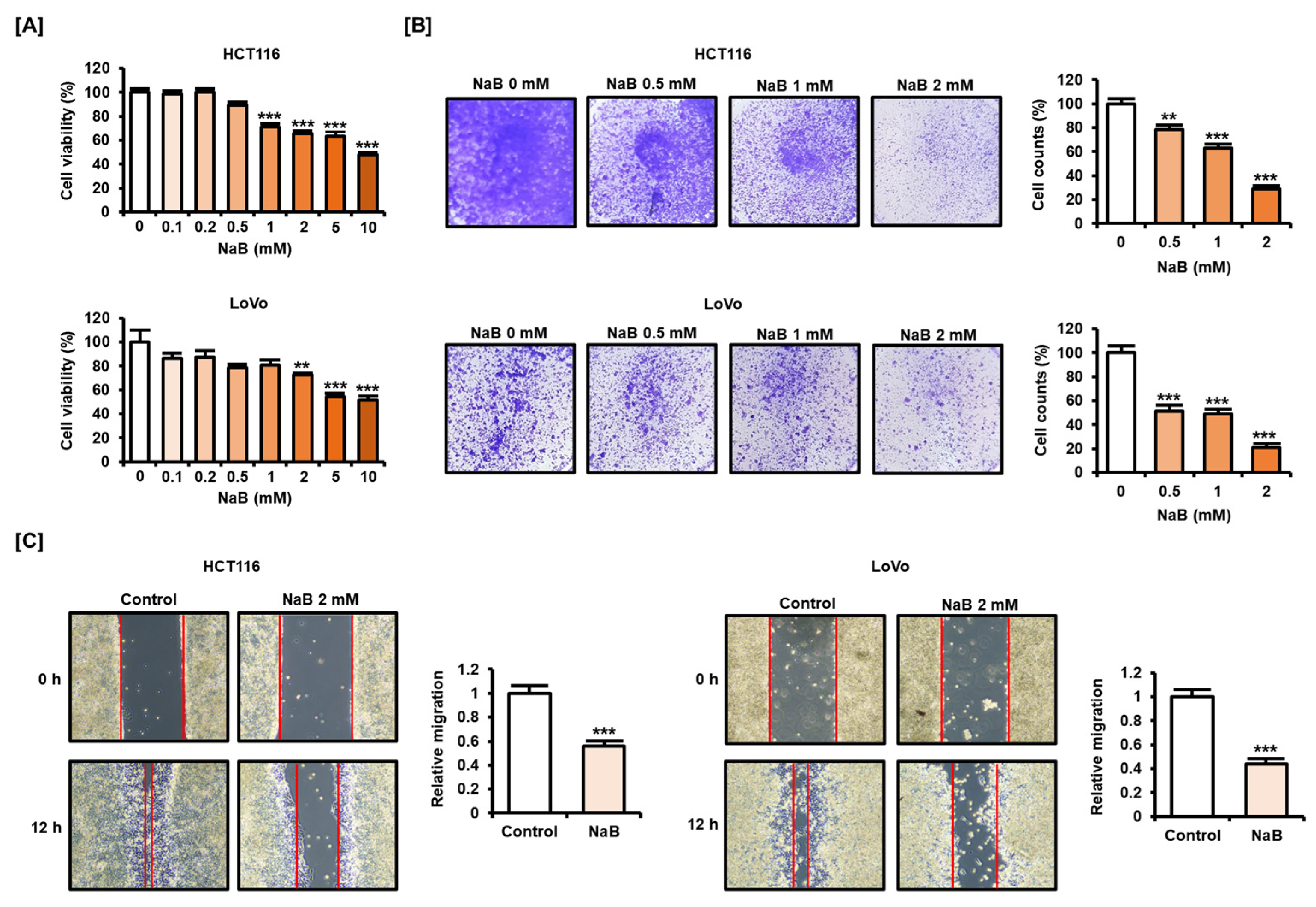
2.1. NaB Impaired CRC Cell Viability and Migration
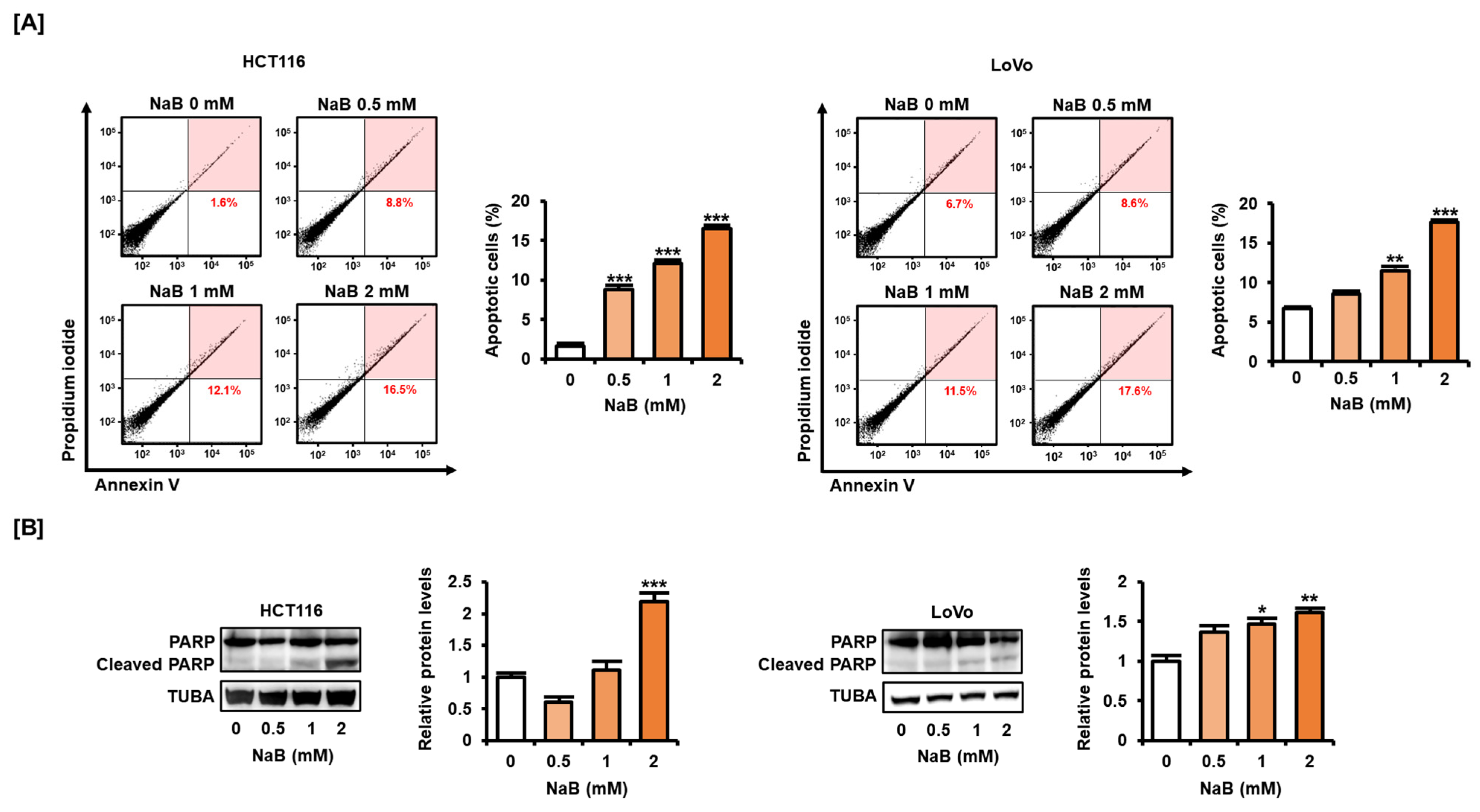
2.2. NaB Induced CRC Cell Apoptosis
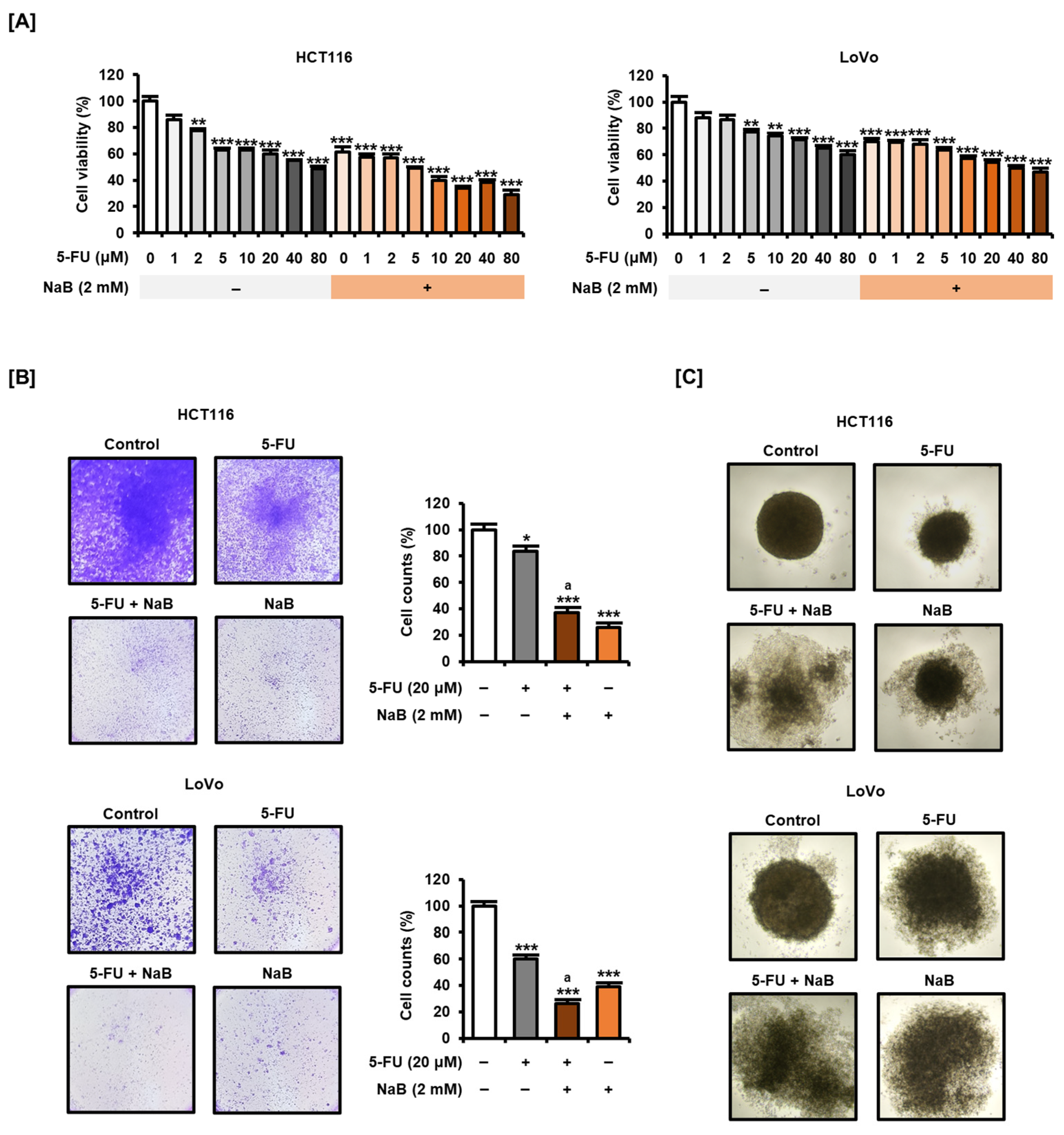
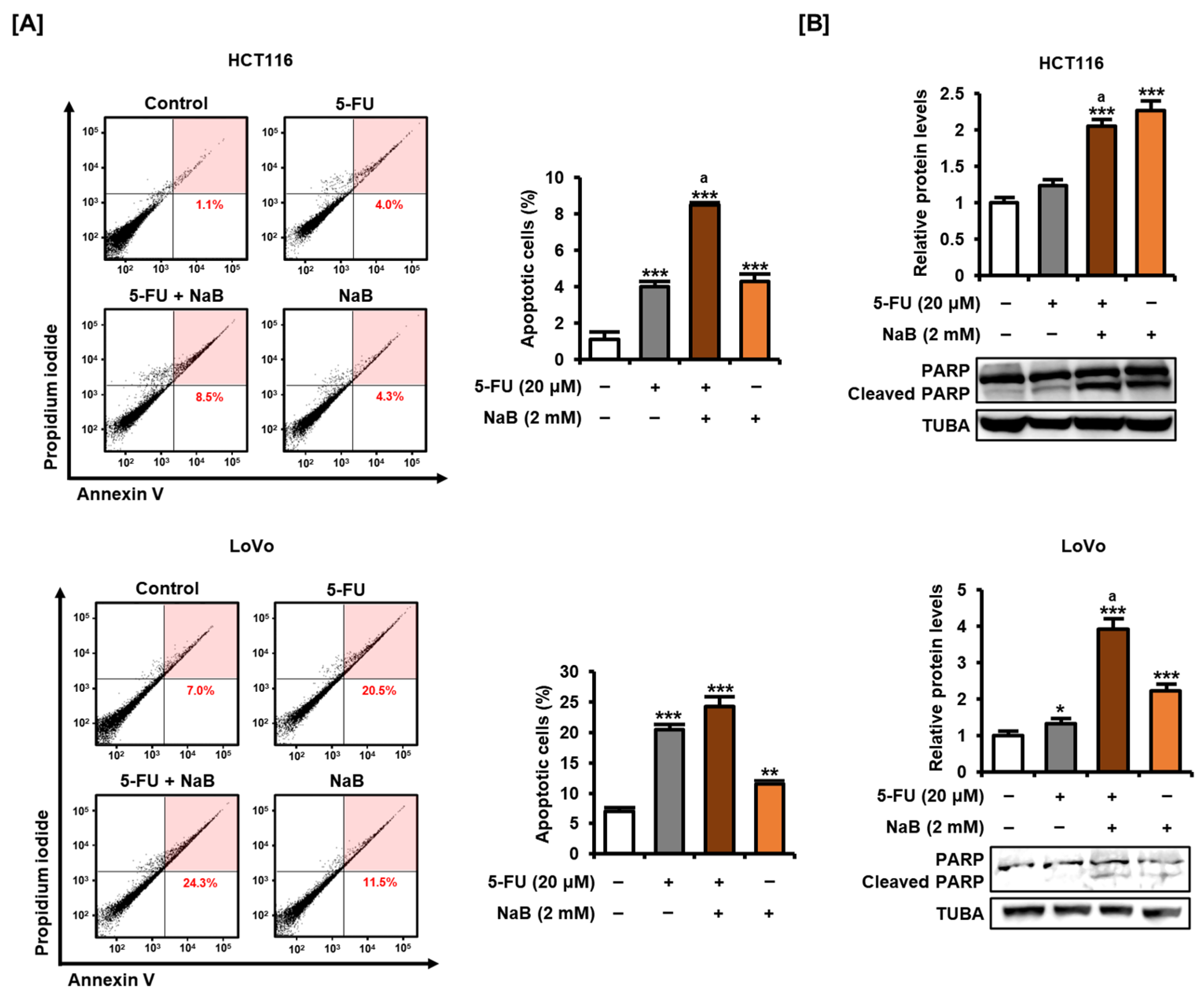
2.3. NaB in Combination with 5-FU Enhanced CRC Cell Growth Suppression and Apoptosis Induction
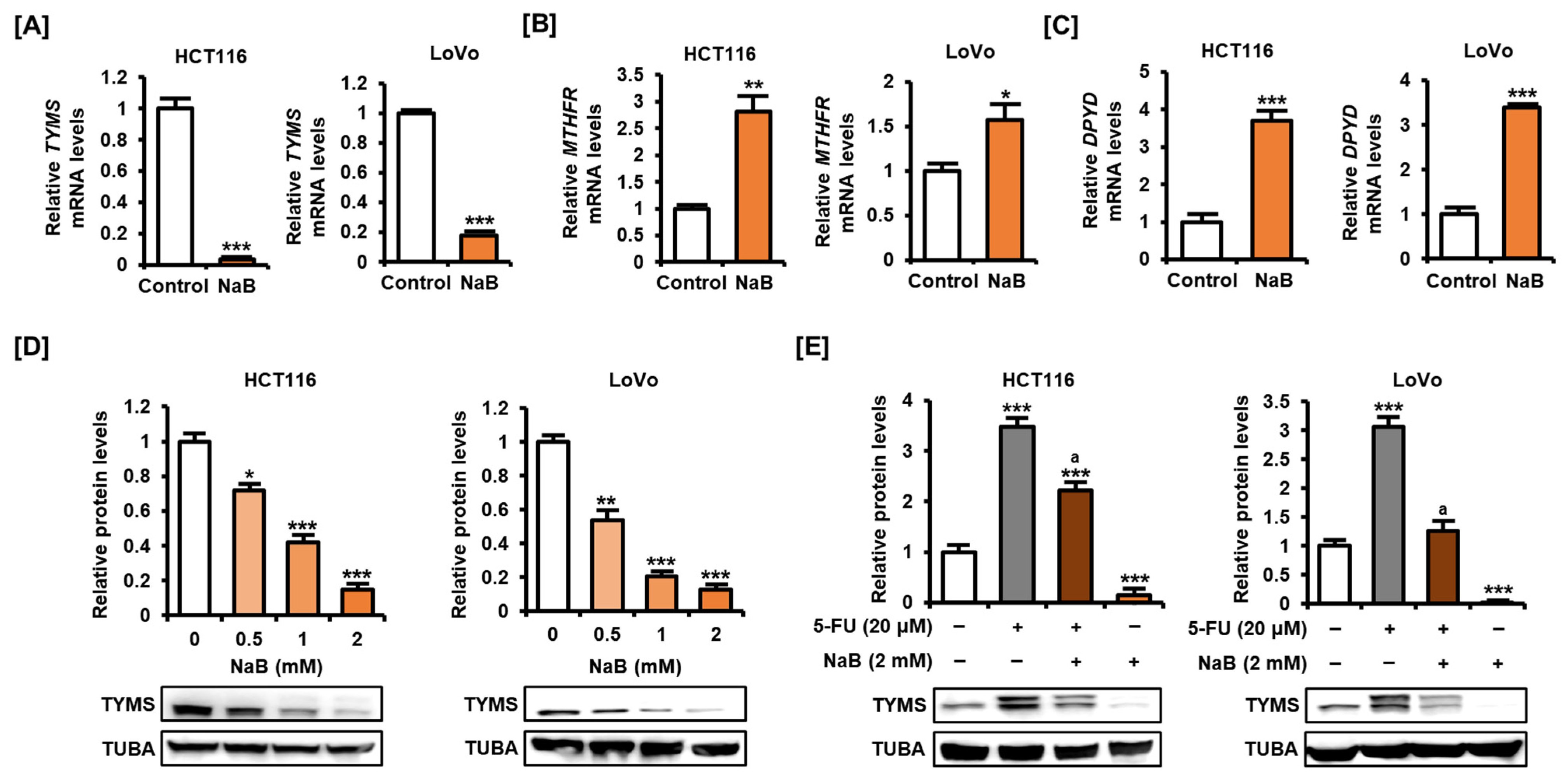
2.4. NaB Decreased TYMS Expression in CRC Cells
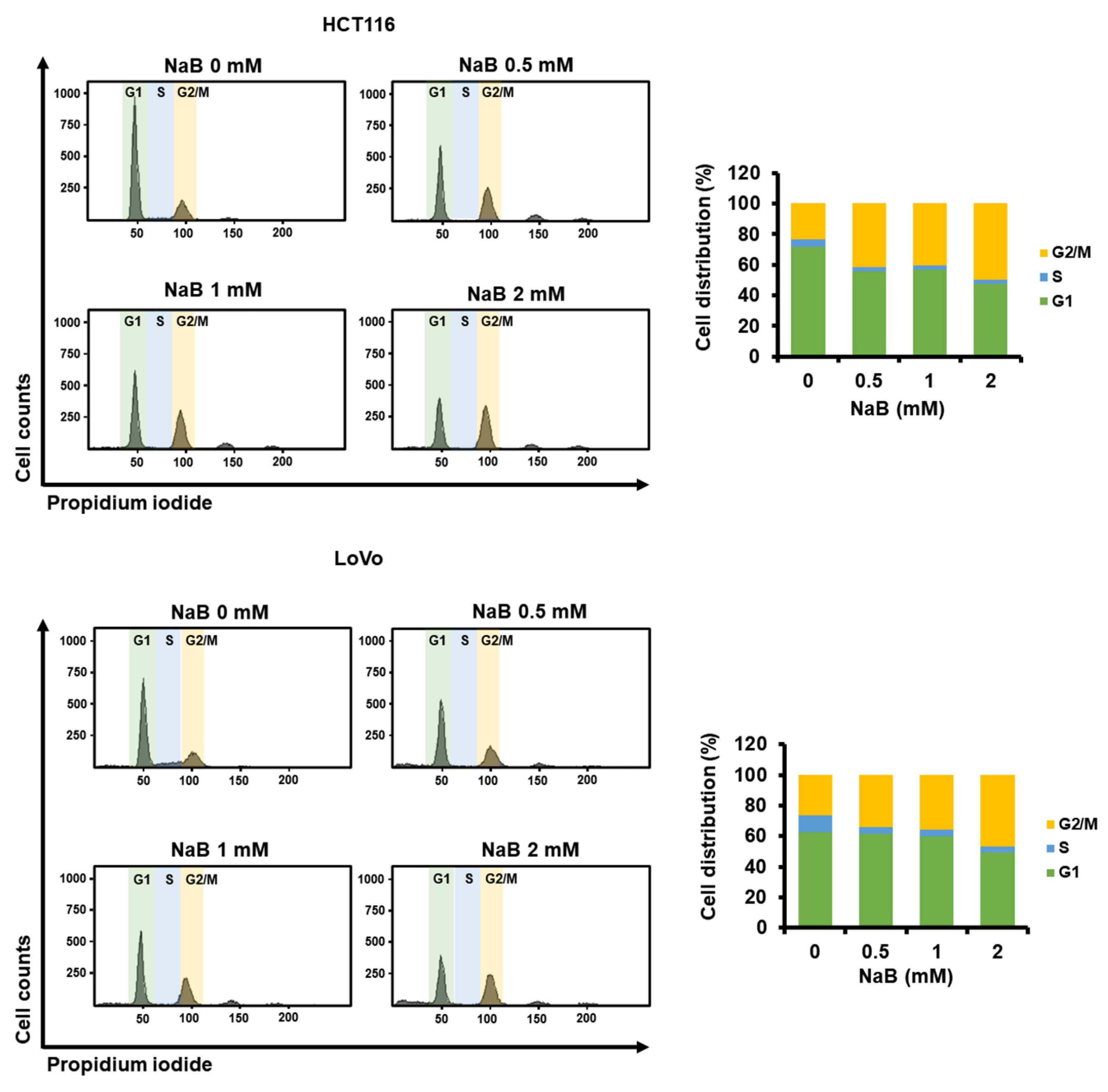
2.5. The Higher Concentration of NaB Further Shifted the Cell Cycle Stage Distribution of CRC Cells toward G2/M
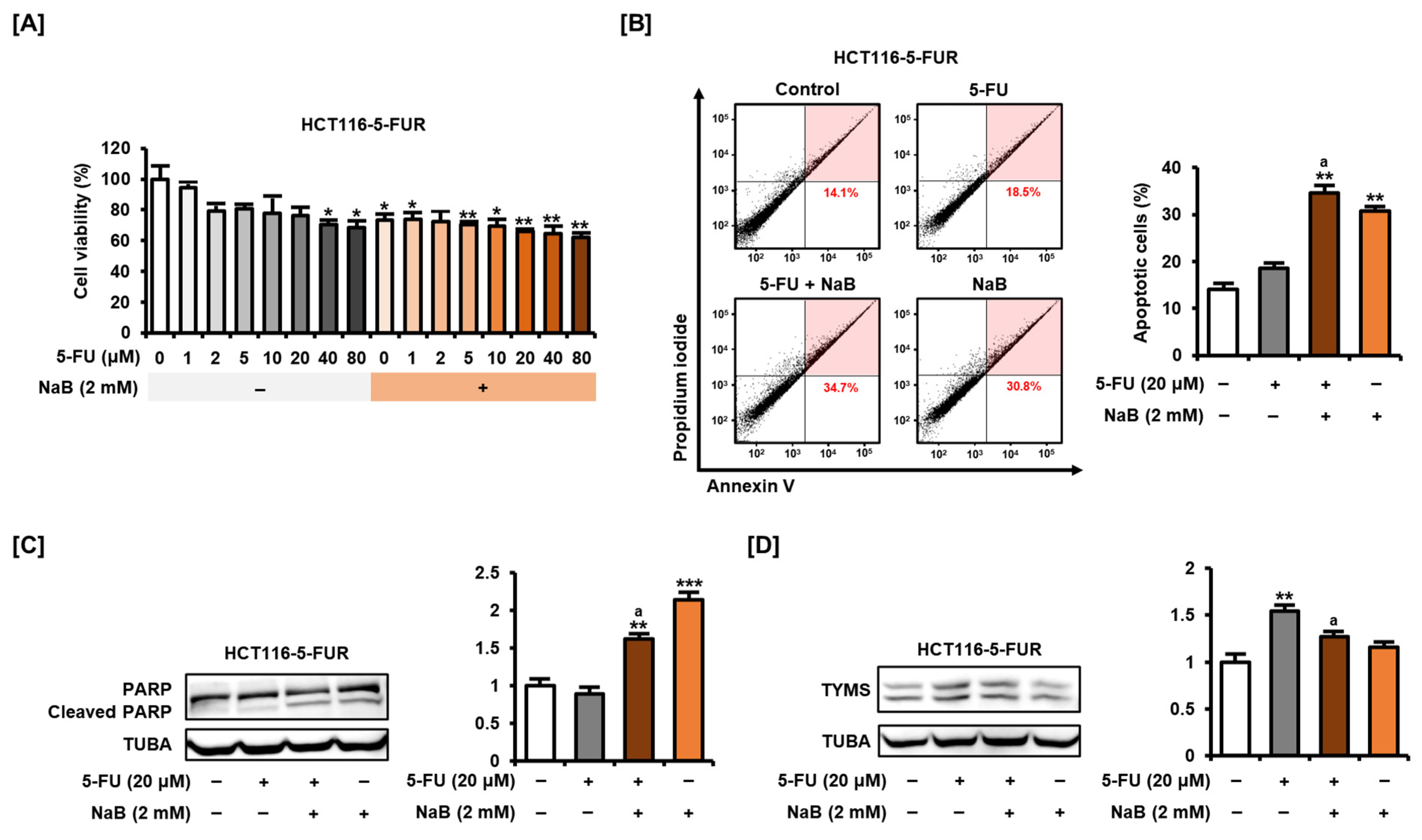
2.6. NaB Alone and in Combination with 5-FU Enhanced 5-FU Resistant CRC Cell Growth Suppression and Apoptosis Induction
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability Test
4.3. Crystal Violet Staining
4.4. Migration Assay
4.5. Annexin V and PI Staining
4.6. Western Blot Analysis
4.7. Spheroid Culture
4.8. Quantitative Polymerase Chain Reaction (qPCR)
4.9. Cell Cycle Assay
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef]
- Yuan, X.; Xue, J.; Tan, Y.; Yang, Q.; Qin, Z.; Bao, X.; Li, S.; Pan, L.; Jiang, Z.; Wang, Y.; et al. Albuca Bracteate Polysaccharides Synergistically Enhance the Anti-Tumor Efficacy of 5-Fluorouracil Against Colorectal Cancer by Modulating β-Catenin Signaling and Intestinal Flora. Front. Pharmacol. 2021, 12, 736627. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Copur, S.; Aiba, K.; Drake, J.C.; Allegra, C.J.; Chu, E. Thymidylate synthase gene amplification in human colon cancer cell lines resistant to 5-fluorouracil. Biochem. Pharmacol. 1995, 49, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Soong, R.; Shah, N.; Salto-Tellez, M.; Tai, B.C.; Soo, R.A.; Han, H.C.; Ng, S.S.; Tan, W.L.; Zeps, N.; Joseph, D.; et al. Prognostic significance of thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase protein expression in colorectal cancer patients treated with or without 5-fluorouracil-based chemotherapy. Ann. Oncol. 2008, 19, 915–919. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K.; Singh, H.; Thareja, S.; Kumar, P. Regulation of thymidylate synthase: An approach to overcome 5-FU resistance in colorectal cancer. Med. Oncol. 2022, 40, 3. [Google Scholar] [CrossRef] [PubMed]
- Varghese, V.; Magnani, L.; Harada-Shoji, N.; Mauri, F.; Szydlo, R.M.; Yao, S.; Lam, E.W.; Kenny, L.M. FOXM1 modulates 5-FU resistance in colorectal cancer through regulating TYMS expression. Sci. Rep. 2019, 9, 1505. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Yue, X.; Wen, S.; Long-Kun, D.; Man, Y.; Chang, S.; Min, Z.; Shuang-Yu, L.; Xin, Q.; Jie, M.; Liang, W. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. 2022, 23, 19. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Cummings, J.H. Colonic absorption: The importance of short chain fatty acids in man. Scand. J. Gastroenterol. Suppl. 1984, 93, 89–99. [Google Scholar]
- Perrin, P.; Pierre, F.; Patry, Y.; Champ, M.; Berreur, M.; Pradal, G.; Bornet, F.; Meflah, K.; Menanteau, J. Only fibres promoting a stable butyrate producing colonic ecosystem decrease the rate of aberrant crypt foci in rats. Gut 2001, 48, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fang, D.; Zhang, H.; Xue, J.; Wangchuk, D.; Du, J.; Jiang, L. Sodium Butyrate Selectively Kills Cancer Cells and Inhibits Migration in Colorectal Cancer by Targeting Thioredoxin-1. OncoTargets Ther. 2020, 13, 4691–4704. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Li, Z.; Mao, L.; Chen, S.; Sun, S. Sodium butyrate induces autophagy in colorectal cancer cells through LKB1/AMPK signaling. J. Physiol. Biochem. 2019, 75, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Bras-Goncalves, R.A.; Pocard, M.; Formento, J.L.; Poirson-Bichat, F.; de Pinieux, G.; Pandrea, I.; Arvelo, F.; Ronco, G.; Villa, P.; Coquelle, A.; et al. Synergistic efficacy of 3n-butyrate and 5-fluorouracil in human colorectal cancer xenografts via modulation of DNA synthesis. Gastroenterology 2001, 120, 874–888. [Google Scholar] [CrossRef]
- Whitacre, C.M.; Zborowska, E.; Willson, J.K.; Berger, N.A. Detection of poly(ADP-ribose) polymerase cleavage in response to treatment with topoisomerase I inhibitors: A potential surrogate end point to assess treatment effectiveness. Clin. Cancer Res. 1999, 5, 665–672. [Google Scholar]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef]
- Zeng, H.; Lazarova, D.L.; Bordonaro, M. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J. Gastrointest. Oncol. 2014, 6, 41–51. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Roediger, W.E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 1980, 21, 793–798. [Google Scholar] [CrossRef]
- Ferreira, T.M.; Leonel, A.J.; Melo, M.A.; Santos, R.R.; Cara, D.C.; Cardoso, V.N.; Correia, M.I.; Alvarez-Leite, J.I. Oral supplementation of butyrate reduces mucositis and intestinal permeability associated with 5-Fluorouracil administration. Lipids 2012, 47, 669–678. [Google Scholar] [CrossRef]
- Tailor, D.; Hahm, E.R.; Kale, R.K.; Singh, S.V.; Singh, R.P. Sodium butyrate induces DRP1-mediated mitochondrial fusion and apoptosis in human colorectal cancer cells. Mitochondrion 2014, 16, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Shuwen, H.; Yangyanqiu, W.; Jian, C.; Boyang, H.; Gong, C.; Jing, Z. Synergistic effect of sodium butyrate and oxaliplatin on colorectal cancer. Transl. Oncol. 2023, 27, 101598. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Park, J.W.; Lee, J.Y.; Kwon, T.K. Sodium butyrate sensitizes TRAIL-mediated apoptosis by induction of transcription from the DR5 gene promoter through Sp1 sites in colon cancer cells. Carcinogenesis 2004, 25, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yi, M.; Zha, L.; Chen, S.; Li, Z.; Li, C.; Gong, M.; Deng, H.; Chu, X.; Chen, J.; et al. Sodium Butyrate Induces Endoplasmic Reticulum Stress and Autophagy in Colorectal Cells: Implications for Apoptosis. PLoS ONE 2016, 11, e0147218. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, J.; Hwang, S.; Choi, J.; Song, G.; Lim, W. Apigenin enhances apoptosis induction by 5-fluorouracil through regulation of thymidylate synthase in colorectal cancer cells. Redox Biol. 2021, 47, 102144. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; An, J.; Ha, E.M. Lactobacillus plantarum-derived metabolites sensitize the tumor-suppressive effects of butyrate by regulating the functional expression of SMCT1 in 5-FU-resistant colorectal cancer cells. J. Microbiol. 2022, 60, 100–117. [Google Scholar] [CrossRef] [PubMed]
- De Mattia, E.; Toffoli, G. C677T and A1298C MTHFR polymorphisms, a challenge for antifolate and fluoropyrimidine-based therapy personalisation. Eur. J. Cancer 2009, 45, 1333–1351. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I. Folate and carcinogenesis: Evidence, mechanisms, and implications. J. Nutr. Biochem. 1999, 10, 66–88. [Google Scholar] [CrossRef] [PubMed]
- Van Kuilenburg, A.B.; de Abreu, R.A.; van Gennip, A.H. Pharmacogenetic and clinical aspects of dihydropyrimidine dehydrogenase deficiency. Ann. Clin. Biochem. 2003, 40, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Salonga, D.; Danenberg, K.D.; Johnson, M.; Metzger, R.; Groshen, S.; Tsao-Wei, D.D.; Lenz, H.J.; Leichman, C.G.; Leichman, L.; Diasio, R.B.; et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin. Cancer Res. 2000, 6, 1322–1327. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.; Yang, C. Sodium Butyrate Inhibits the Expression of Thymidylate Synthase and Induces Cell Death in Colorectal Cancer Cells. Int. J. Mol. Sci. 2024, 25, 1572. https://doi.org/10.3390/ijms25031572
Kim N, Yang C. Sodium Butyrate Inhibits the Expression of Thymidylate Synthase and Induces Cell Death in Colorectal Cancer Cells. International Journal of Molecular Sciences. 2024; 25(3):1572. https://doi.org/10.3390/ijms25031572
Chicago/Turabian StyleKim, Nayeon, and Changwon Yang. 2024. "Sodium Butyrate Inhibits the Expression of Thymidylate Synthase and Induces Cell Death in Colorectal Cancer Cells" International Journal of Molecular Sciences 25, no. 3: 1572. https://doi.org/10.3390/ijms25031572
APA StyleKim, N., & Yang, C. (2024). Sodium Butyrate Inhibits the Expression of Thymidylate Synthase and Induces Cell Death in Colorectal Cancer Cells. International Journal of Molecular Sciences, 25(3), 1572. https://doi.org/10.3390/ijms25031572





