Neuroimmune Activation and Microglia Reactivity in Female Rats Following Alcohol Dependence
Abstract
:1. Introduction
2. Results
2.1. Ethanol Binge Data
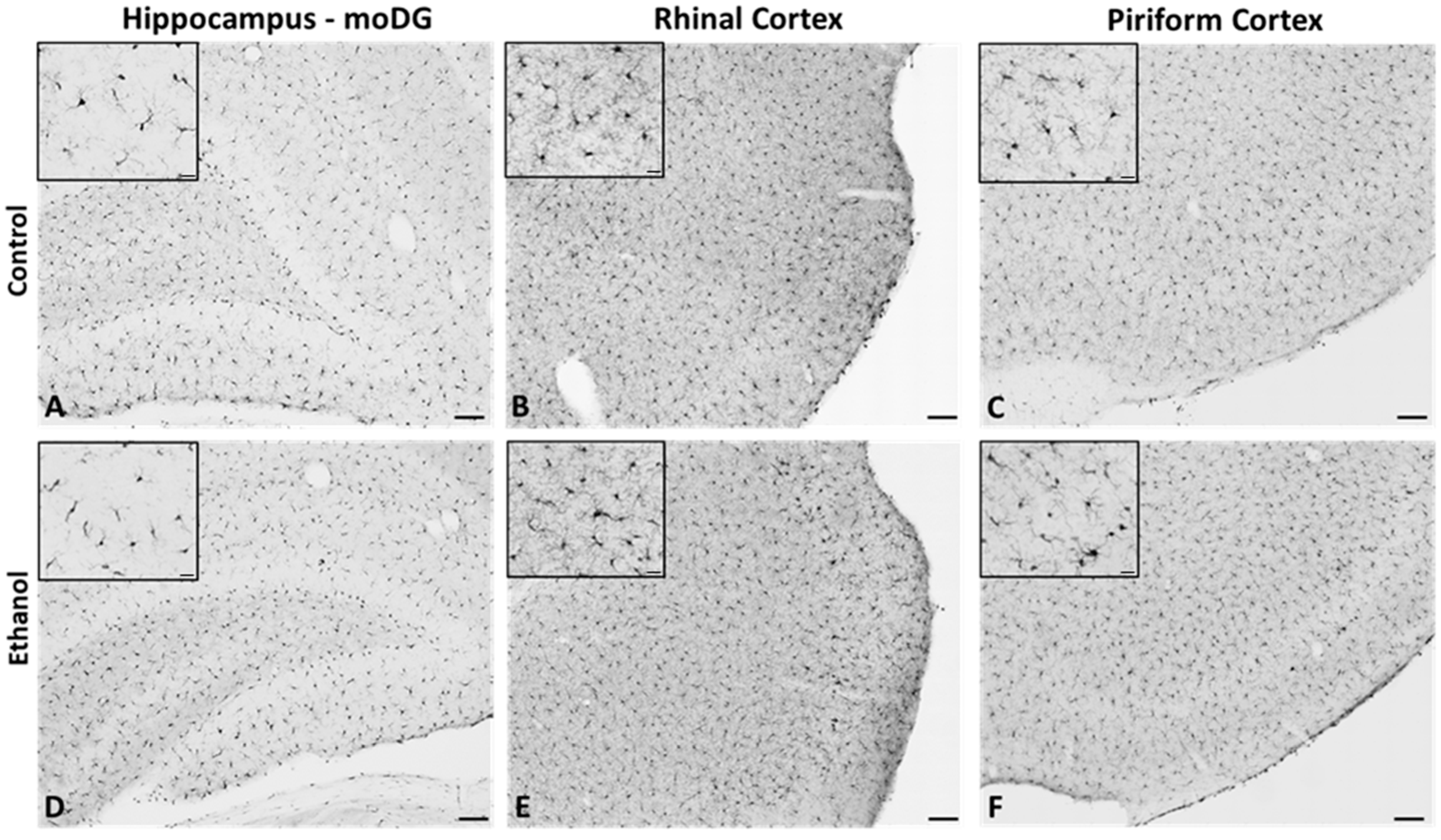
2.2. Microglia from Adult Female Rats Display a Reactive Morphology after 4-Day Binge Ethanol Exposure
2.3. Microglial Functional Phenotype Markers after a 4-Day Binge Ethanol Exposure in Adult Female Rats
2.4. Changes to Neuroimmune Markers after a 4-Day Binge Ethanol Exposure
3. Discussion
4. Materials and Methods
4.1. Binge Alcohol Model
4.2. Tissue Processing and Immunohistochemistry
4.3. Image Acquisition and Analysis
4.4. Enzyme-Linked Immunosorbent Assay
4.5. Statistical Approaches
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grant, B.F.; Goldstein, R.B.; Saha, T.D.; Chou, S.P.; Jung, J.; Zhang, H.; Pickering, R.P.; Ruan, W.J.; Smith, S.M.; Huang, B.; et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015, 72, 757–766. [Google Scholar] [CrossRef]
- White, A.M. Gender Differences in the Epidemiology of Alcohol Use and Related Harms in the United States. Alcohol Res. Curr. Rev. 2020, 40, 01. [Google Scholar] [CrossRef]
- Grant, B.F.; Chou, S.P.; Saha, T.D.; Pickering, R.P.; Kerridge, B.T.; Ruan, W.J.; Huang, B.; Jung, J.; Zhang, H.; Fan, A.; et al. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001-2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017, 74, 911–923. [Google Scholar] [CrossRef]
- Grucza, R.A.; Sher, K.J.; Kerr, W.C.; Krauss, M.J.; Lui, C.K.; McDowell, Y.E.; Hartz, S.; Virdi, G.; Bierut, L.J. Trends in Adult Alcohol Use and Binge Drinking in the Early 21st-Century United States: A Meta-Analysis of 6 National Survey Series. Alcohol Clin. Exp. Res. 2018, 42, 1939–1950. [Google Scholar] [CrossRef]
- Keyes, K.M.; Grant, B.F.; Hasin, D.S. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend. 2008, 93, 21–29. [Google Scholar] [CrossRef]
- Keyes, K.M.; Jager, J.; Mal-Sarkar, T.; Patrick, M.E.; Rutherford, C.; Hasin, D. Is There a Recent Epidemic of Women’s Drinking? A Critical Review of National Studies. Alcohol Clin. Exp. Res. 2019, 43, 1344–1359. [Google Scholar] [CrossRef] [PubMed]
- Slade, T.; Chapman, C.; Swift, W.; Keyes, K.; Tonks, Z.; Teesson, M. Birth cohort trends in the global epidemiology of alcohol use and alcohol-related harms in men and women: Systematic review and metaregression. BMJ Open 2016, 6, e011827. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.R.; Marks, C.; Becker, J.B.; Hurn, P.D.; Chen, W.J.; Woodruff, T.; McCarthy, M.M.; Sohrabji, F.; Schiebinger, L.; Wetherington, C.L.; et al. Considering sex as a biological variable in preclinical research. FASEB J. 2017, 31, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Rechlin, R.K.; Splinter, T.F.L.; Hodges, T.E.; Albert, A.Y.; Galea, L.A.M. An analysis of neuroscience and psychiatry papers published from 2009 and 2019 outlines opportunities for increasing discovery of sex differences. Nat. Comm. 2022, 13, 2137. [Google Scholar] [CrossRef]
- Cortez, I.; Rodgers, S.P.; Kosten, T.A.; Leasure, J.L. Sex and Age Effects on Neurobehavioral Toxicity Induced by Binge Alcohol. Brain Plast. 2020, 6, 5–25. [Google Scholar] [CrossRef]
- Rossetti, M.G.; Mackey, S.; Patalay, P.; Allen, N.B.; Batalla, A.; Bellani, M.; Chye, Y.; Conrod, P.; Cousijn, J.; Garavan, H.; et al. Sex and dependence related neuroanatomical differences in regular cannabis users: Findings from the ENIGMA Addiction Working Group. Transl. Psychiatry 2021, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Verplaetse, T.L.; Cosgrove, K.P.; Tanabe, J.; McKee, S.A. Sex/gender differences in brain function and structure in alcohol use: A narrative review of neuroimaging findings over the last 10 years. J. Neurosci. Res. 2021, 99, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Fama, R.; Le Berre, A.P.; Sullivan, E.V. Alcohol’s Unique Effects on Cognition in Women: A 2020 (Re)view to Envision Future Research and Treatment. Alcohol Res. Curr. Rev. 2020, 40, 03. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.; Borgonetti, V.; Bajo, M.; Roberto, M. Sex-dependent factors of alcohol and neuroimmune mechanisms. Neurobiol. Stress 2023, 26, 100562. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Nixon, K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009, 44, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, M.J.; Martin, P.R. Clinical assessment of cognition in alcoholism. Alcohol Clin. Exp. Res. 1986, 10, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.V.; Pfefferbaum, A. Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology 2005, 180, 583–594. [Google Scholar] [CrossRef]
- Zahr, N.M.; Kaufman, K.L.; Harper, C.G. Clinical and pathological features of alcohol-related brain damage. Nat. Rev. Neurol. 2011, 7, 284–294. [Google Scholar] [CrossRef]
- Parsons, O.A.; Nixon, S.J. Neurobehavioral sequelae of alcoholism. Neurol. Clin. 1993, 11, 205–218. [Google Scholar] [CrossRef]
- Sullivan, E.V.; Fama, R.; Rosenbloom, M.J.; Pfefferbaum, A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology 2002, 16, 74–83. [Google Scholar] [CrossRef]
- Wilson, S.; Bair, J.L.; Thomas, K.M.; Iacono, W.G. Problematic alcohol use and reduced hippocampal volume: A meta-analytic review. Psych. Med. 2017, 47, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Agartz, I.; Momenan, R.; Rawlings, R.R.; Kerich, M.J.; Hommer, D.W. Hippocampal volume in patients with alcohol dependence. Arch. Gen. Psychiatry 1999, 56, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Pfefferbaum, A.; Lim, K.O.; Zipursky, R.B.; Mathalon, D.H.; Rosenbloom, M.J.; Lane, B.; Ha, C.N.; Sullivan, E.V. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcohol Clin. Exp. Res. 1992, 16, 1078–1089. [Google Scholar] [CrossRef]
- Kril, J.J.; Halliday, G.M.; Svoboda, M.D.; Cartwright, H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience 1997, 79, 983–998. [Google Scholar] [CrossRef]
- Walker, D.W.; Barnes, D.E.; Zornetzer, S.F.; Hunter, B.E.; Kubanis, P. Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science 1980, 209, 711–713. [Google Scholar] [CrossRef]
- Harper, C.; Kril, J. If you drink your brain will shrink. Neuropathological considerations. Alcohol Alcohol. Suppl. 1991, 1, 375–380. [Google Scholar] [PubMed]
- Collins, M.A.; Corso, T.D.; Neafsey, E.J. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: Possible explanation for olfactory deficits in alcoholics. Alcohol Clin. Exp. Res. 1996, 20, 284–292. [Google Scholar] [CrossRef]
- Crews, F.T.; Braun, C.J.; Hoplight, B.; Switzer, R.C., 3rd; Knapp, D.J. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin. Exp. Res. 2000, 24, 1712–1723. [Google Scholar] [CrossRef]
- Kelso, M.L.; Liput, D.J.; Eaves, D.W.; Nixon, K. Upregulated vimentin suggests new areas of neurodegeneration in a model of an alcohol use disorder. Neuroscience 2011, 197, 381–393. [Google Scholar] [CrossRef]
- Guerin, S.P.; Melbourne, J.K.; Dang, H.Q.; Shaji, C.A.; Nixon, K. Astrocyte Reactivity and Neurodegeneration in the Female Rat Brain Following Alcohol Dependence. Neuroscience 2023, 529, 183–199. [Google Scholar] [CrossRef]
- Maynard, M.E.; Barton, E.A.; Robinson, C.R.; Wooden, J.I.; Leasure, J.L. Sex differences in hippocampal damage, cognitive impairment, and trophic factor expression in an animal model of an alcohol use disorder. Brain Struct. Funct. 2018, 223, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Silva-Gotay, A.; Davis, J.; Tavares, E.R.; Richardson, H.N. Alcohol drinking during early adolescence activates microglial cells and increases frontolimbic Interleukin-1 beta and Toll-like receptor 4 gene expression, with heightened sensitivity in male rats compared to females. Neuropharmacology 2021, 197, 108698. [Google Scholar] [CrossRef]
- Prendergast, M.A. Do women possess a unique susceptibility to the neurotoxic effects of alcohol? J. Am. Med. Womens Assoc. (1972) 2004, 59, 225–227. [Google Scholar] [PubMed]
- Alfonso-Loeches, S.; Pascual, M.; Guerri, C. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology 2013, 311, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Barton, E.A.; Baker, C.; Leasure, J.L. Investigation of Sex Differences in the Microglial Response to Binge Ethanol and Exercise. Brain Sci. 2017, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Vetreno, R.P. Neuroimmune basis of alcoholic brain damage. Int. Rev. Neurobiol. 2014, 118, 315–357. [Google Scholar] [CrossRef]
- Melbourne, J.K.; Thompson, K.R.; Peng, H.; Nixon, K. Its complicated: The relationship between alcohol and microglia in the search for novel pharmacotherapeutic targets for alcohol use disorders. Mol. Basis Neuropsychiatr. Disord. Bench Bedside 2019, 167, 179–221. [Google Scholar] [CrossRef]
- Kapoor, M.; Wang, J.C.; Farris, S.P.; Liu, Y.; McClintick, J.; Gupta, I.; Meyers, J.L.; Bertelsen, S.; Chao, M.; Nurnberger, J.; et al. Analysis of whole genome-transcriptomic organization in brain to identify genes associated with alcoholism. Transl. Psychiatry 2019, 9, 89. [Google Scholar] [CrossRef]
- Warden, A.S.; Wolfe, S.A.; Khom, S.; Varodayan, F.P.; Patel, R.R.; Steinman, M.Q.; Bajo, M.; Montgomery, S.E.; Vlkolinsky, R.; Nadav, T.; et al. Microglia Control Escalation of Drinking in Alcohol-Dependent Mice: Genomic and Synaptic Drivers. Biol. Psychiatry 2020, 88, 910–921. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Crews, F.T. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 2008, 210, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Araiz, A.; Porcu, F.; Perez-Hernandez, M.; Garcia-Gutierrez, M.S.; Aracil-Fernandez, M.A.; Gutierrez-Lopez, M.D.; Guerri, C.; Manzanares, J.; O’Shea, E.; Colado, M.I. Disruption of blood-brain barrier integrity in postmortem alcoholic brain: Preclinical evidence of TLR4 involvement from a binge-like drinking model. Addict. Biol. 2017, 22, 1103–1116. [Google Scholar] [CrossRef]
- Marshall, S.A.; McClain, J.A.; Kelso, M.L.; Hopkins, D.M.; Pauly, J.R.; Nixon, K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol. Dis. 2013, 54, 239–251. [Google Scholar] [CrossRef]
- Peng, H.; Geil Nickell, C.R.; Chen, K.Y.; McClain, J.A.; Nixon, K. Increased expression of M1 and M2 phenotypic markers in isolated microglia after four-day binge alcohol exposure in male rats. Alcohol 2017, 62, 29–40. [Google Scholar] [CrossRef]
- McCarthy, G.M.; Farris, S.P.; Blednov, Y.A.; Harris, R.A.; Mayfield, R.D. Microglial-specific transcriptome changes following chronic alcohol consumption. Neuropharmacology 2018, 128, 416–424. [Google Scholar] [CrossRef] [PubMed]
- West, R.K.; Rodgers, S.P.; Leasure, J.L. Neural Perturbations Associated With Recurrent Binge Alcohol in Male and Female Rats. Alcohol Clin. Exp. Res. 2021, 45, 365–374. [Google Scholar] [CrossRef]
- Walter, T.J.; Crews, F.T. Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. J. Neuroinflammation 2017, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Henriques, J.F.; Portugal, C.C.; Canedo, T.; Relvas, J.B.; Summavielle, T.; Socodato, R. Microglia and alcohol meet at the crossroads: Microglia as critical modulators of alcohol neurotoxicity. Toxicol. Lett. 2018, 283, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Achur, R.N.; Freeman, W.M.; Vrana, K.E. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J. Neuroimmune Pharmacol. 2010, 5, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Lin, A.; De Jong, R.; Vandoolaeghe, E.; Kenis, G.; Bosmans, E.; Whelan, A.; Scharpe, S.; Maes, M. Cytokines in detoxified patients with chronic alcoholism without liver disease: Increased monocytic cytokine production. Biol. Psychiatry 1999, 45, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Orio, L.; Alen, F.; Pavon, F.J.; Serrano, A.; Garcia-Bueno, B. Oleoylethanolamide, Neuroinflammation, and Alcohol Abuse. Front. Mol. Neurosci. 2018, 11, 490. [Google Scholar] [CrossRef]
- Marshall, S.A.; Geil, C.R.; Nixon, K. Prior Binge Ethanol Exposure Potentiates the Microglial Response in a Model of Alcohol-Induced Neurodegeneration. Brain Sci. 2016, 6, 16. [Google Scholar] [CrossRef]
- Ince, L.M.; Darling, J.S.; Sanchez, K.; Bell, K.S.; Melbourne, J.K.; Davis, L.K.; Nixon, K.; Gaudet, A.D.; Fonken, L.K. Sex differences in microglia function in aged rats underlie vulnerability to cognitive decline. Brain Behav. Immun. 2023, 114, 438–452. [Google Scholar] [CrossRef]
- Bollinger, J.L.; Collins, K.E.; Patel, R.; Wellman, C.L. Behavioral stress alters corticolimbic microglia in a sex- and brain region-specific manner. PLoS ONE 2017, 12, e0187631. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Perry, V.H. Microglial physiology: Unique stimuli, specialized responses. Annu. Rev. Immunol. 2009, 27, 119–145. [Google Scholar] [CrossRef]
- Hovens, I.; Nyakas, C.; Schoemaker, R. A novel method for evaluating microglial activation using ionized calcium-binding adaptor protein-1 staining: Cell body to cell size ratio. Neuroimmunol. Neuroinflammation 2014, 1, 82. [Google Scholar] [CrossRef]
- Raivich, G.; Jones, L.L.; Werner, A.; Bluthmann, H.; Doetschmann, T.; Kreutzberg, G.W. Molecular signals for glial activation: Pro- and anti-inflammatory cytokines in the injured brain. Acta Neurochir. Suppl. 1999, 73, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.; Guergues, J.; Pinho, J.P.C.; Zhang, P.; Nguyen, T.G.; MacFadyen, K.A.; Peris, J.; McLaughlin, J.P.; Stevens, S.M., Jr.; Liu, B. Chronic Voluntary Binge Ethanol Consumption Causes Sex-Specific Differences in Microglial Signaling Pathways and Withdrawal-associated Behaviors in Mice. Alcohol Clin. Exp. Res. 2020, 44, 1791–1806. [Google Scholar] [CrossRef] [PubMed]
- Bell-Temin, H.; Zhang, P.; Chaput, D.; King, M.A.; You, M.; Liu, B.; Stevens, S.M., Jr. Quantitative proteomic characterization of ethanol-responsive pathways in rat microglial cells. J. Proteome Res. 2013, 12, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Leasure, J.L.; Nixon, K. Exercise neuroprotection in a rat model of binge alcohol consumption. Alcohol Clin. Exp. Res. 2010, 34, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Itriago, A.; Radford, R.A.W.; Aramideh, J.A.; Maurel, C.; Scherer, N.M.; Don, E.K.; Lee, A.; Chung, R.S.; Graeber, M.B.; Morsch, M. Microglia morphophysiological diversity and its implications for the CNS. Front. Immunol. 2022, 13, 997786. [Google Scholar] [CrossRef]
- Marshall, S.A.; McClain, J.A.; Wooden, J.I.; Nixon, K. Microglia Dystrophy Following Binge-Like Alcohol Exposure in Adolescent and Adult Male Rats. Front. Neuroanat. 2020, 14, 52. [Google Scholar] [CrossRef]
- Hu, P.; Wang, D.; Zhang, Y.; Cai, Z.; Ye, T.; Tong, L.; Xu, X.; Lu, J.; Liu, F.; Lu, X.; et al. Apoptosis-triggered decline in hippocampal microglia mediates adolescent intermittent alcohol exposure-induced depression-like behaviors in mice. Neuropharmacology 2020, 170, 108054. [Google Scholar] [CrossRef]
- Nixon, K.; Kim, D.H.; Potts, E.N.; He, J.; Crews, F.T. Distinct cell proliferation events during abstinence after alcohol dependence: Microglia proliferation precedes neurogenesis. Neurobiol. Dis. 2008, 31, 218–229. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Liu, D.; Li, X.; He, L.; Pan, J.; Shen, Q.; Peng, Y. CB2R activation ameliorates late adolescent chronic alcohol exposure-induced anxiety-like behaviors during withdrawal by preventing morphological changes and suppressing NLRP3 inflammasome activation in prefrontal cortex microglia in mice. Brain Behav. Immun. 2023, 110, 60–79. [Google Scholar] [CrossRef]
- Avila, D.V.; Myers, S.A.; Zhang, J.; Kharebava, G.; McClain, C.J.; Kim, H.Y.; Whittemore, S.R.; Gobejishvili, L.; Barve, S. Phosphodiesterase 4b expression plays a major role in alcohol-induced neuro-inflammation. Neuropharmacology 2017, 125, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alavez, M.; Nguyen, W.; Mori, S.; Wills, D.N.; Otero, D.; Aguirre, C.A.; Singh, M.; Ehlers, C.L.; Conti, B. Time Course of Blood and Brain Cytokine/Chemokine Levels Following Adolescent Alcohol Exposure and Withdrawal in Rats. Alcohol Clin. Exp. Res. 2019, 43, 2547–2558. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alavez, M.; Nguyen, W.; Mori, S.; Wills, D.N.; Otero, D.; Ehlers, C.L.; Conti, B. Time course of microglia activation and brain and blood cytokine/chemokine levels following chronic ethanol exposure and protracted withdrawal in rats. Alcohol 2019, 76, 37–45. [Google Scholar] [CrossRef]
- Bajo, M.; Montgomery, S.E.; Cates, L.N.; Nadav, T.; Delucchi, A.M.; Cheng, K.; Yin, H.; Crawford, E.F.; Roberts, A.J.; Roberto, M. Evaluation of TLR4 Inhibitor, T5342126, in Modulation of Ethanol-Drinking Behavior in Alcohol-Dependent Mice. Alcohol Alcohol. 2016, 51, 541–548. [Google Scholar] [CrossRef]
- Crews, F.; Nixon, K.; Kim, D.; Joseph, J.; Shukitt-Hale, B.; Qin, L.; Zou, J. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin. Exp. Res. 2006, 30, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Yuan, L.; Lu, X.; Ding, H.; Luo, J.; Ke, Z.J. Binge Alcohol Exposure Causes Neurobehavioral Deficits and GSK3beta Activation in the Hippocampus of Adolescent Rats. Sci. Rep. 2018, 8, 3088. [Google Scholar] [CrossRef]
- Lowe, P.P.; Morel, C.; Ambade, A.; Iracheta-Vellve, A.; Kwiatkowski, E.; Satishchandran, A.; Furi, I.; Cho, Y.; Gyongyosi, B.; Catalano, D.; et al. Chronic alcohol-induced neuroinflammation involves CCR2/5-dependent peripheral macrophage infiltration and microglia alterations. J. Neuroinflamm. 2020, 17, 296. [Google Scholar] [CrossRef] [PubMed]
- Raivich, G.; Bohatschek, M.; Kloss, C.U.; Werner, A.; Jones, L.L.; Kreutzberg, G.W. Neuroglial activation repertoire in the injured brain: Graded response, molecular mechanisms and cues to physiological function. Brain Res. Rev. 1999, 30, 77–105. [Google Scholar] [CrossRef] [PubMed]
- Karavitis, J.; Kovacs, E.J. Macrophage phagocytosis: Effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J. Leukoc. Biol. 2011, 90, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, D.; Pirchl, M.; Humpel, C. Effects of long-term moderate ethanol and cholesterol on cognition, cholinergic neurons, inflammation, and vascular impairment in rats. Neuroscience 2012, 205, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.J.; Hashimoto, J.G.; Roberts, M.L.; Bloom, S.H.; Andrew, M.R.; Wiren, K.M. Astrocyte Dysfunction Induced by Alcohol in Females but Not Males. Brain Pathol. 2016, 26, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Montesinos, J.; Marcos, M.; Torres, J.L.; Costa-Alba, P.; Garcia-Garcia, F.; Laso, F.J.; Guerri, C. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict. Biol. 2017, 22, 1829–1841. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; Bilbo, S.D. Sex, glia, and development: Interactions in health and disease. Horm. Behav. 2012, 62, 243–253. [Google Scholar] [CrossRef]
- Bollinger, J.L.; Bergeon Burns, C.M.; Wellman, C.L. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav. Immun. 2016, 52, 88–97. [Google Scholar] [CrossRef]
- Alfonso-Loeches, S.; Pascual, M.; Gomez-Pinedo, U.; Pascual-Lucas, M.; Renau-Piqueras, J.; Guerri, C. Toll-like receptor 4 participates in the myelin disruptions associated with chronic alcohol abuse. Glia 2012, 60, 948–964. [Google Scholar] [CrossRef]
- Gildawie, K.R.; Orso, R.; Peterzell, S.; Thompson, V.; Brenhouse, H.C. Sex differences in prefrontal cortex microglia morphology: Impact of a two-hit model of adversity throughout development. Neurosci. Lett. 2020, 738, 135381. [Google Scholar] [CrossRef]
- Bobotis, B.C.; Braniff, O.; Gargus, M.; Akinluyi, E.T.; Awogbindin, I.O.; Tremblay, M.E. Sex differences of microglia in the healthy brain from embryonic development to adulthood and across lifestyle influences. Brain Res. Bull. 2023, 202, 110752. [Google Scholar] [CrossRef]
- Vegeto, E.; Bonincontro, C.; Pollio, G.; Sala, A.; Viappiani, S.; Nardi, F.; Brusadelli, A.; Viviani, B.; Ciana, P.; Maggi, A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J. Neurosci. 2001, 21, 1809–1818. [Google Scholar] [CrossRef]
- Becker, J.B.; Prendergast, B.J.; Liang, J.W. Female rats are not more variable than male rats: A meta-analysis of neuroscience studies. Biol. Sex Differ. 2016, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [PubMed]
- McKenna, I.M.; Waalkes, M.P.; Chen, L.C.; Gordon, T. Comparison of inflammatory lung responses in Wistar rats and C57 and DBA mice following acute exposure to cadmium oxide fumes. Toxicol. Appl. Pharmacol. 1997, 146, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, J.A.; Figueira, T.R.; Ravagnani, F.G.; Oliveira, H.C.; Vercesi, A.E.; Castilho, R.F. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radic. Biol. Med. 2013, 63, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Boschen, K.E.; Ptacek, T.S.; Berginski, M.E.; Simon, J.M.; Parnell, S.E. Transcriptomic analyses of gastrulation-stage mouse embryos with differential susceptibility to alcohol. Dis. Model. Mech. 2021, 14, dmm049012. [Google Scholar] [CrossRef] [PubMed]
- Melbourne, J.K.; Chandler, C.M.; Van Doorn, C.E.; Bardo, M.T.; Pauly, J.R.; Peng, H.; Nixon, K. Primed for addiction: A critical review of the role of microglia in the neurodevelopmental consequences of adolescent alcohol drinking. Alcohol Clin. Exp. Res. 2021, 45, 1908–1926. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Nixon, K. Microglia Phenotypes Following the Induction of Alcohol Dependence in Adolescent Rats. Alcohol Clin. Exp. Res. 2021, 45, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Gano, A.; Vore, A.S.; Sammakia, M.N.; Deak, T. Assessment of Extracellular Cytokines in the Hippocampus of the Awake Behaving Rat Using Large-Molecule Microdialysis Combined with Multiplex Arrays After Acute and Chronic Ethanol Exposure. Alcohol Clin. Exp. Res. 2019, 43, 640–654. [Google Scholar] [CrossRef]
- Gano, A.; Mondello, J.E.; Doremus-Fitzwater, T.L.; Deak, T. Rapid alterations in neuroimmune gene expression after acute ethanol: Timecourse, sex differences and sensitivity to cranial surgery. J. Neuroimmunol. 2019, 337, 577083. [Google Scholar] [CrossRef] [PubMed]
- Doremus-Fitzwater, T.L.; Gano, A.; Paniccia, J.E.; Deak, T. Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiol. Behav 2015, 148, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Doremus-Fitzwater, T.L.; Buck, H.M.; Bordner, K.; Richey, L.; Jones, M.E.; Deak, T. Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcohol Clin. Exp. Res. 2014, 38, 2186–2198. [Google Scholar] [CrossRef]
- Hammond, T.R.; Robinton, D.; Stevens, B. Microglia and the Brain: Complementary Partners in Development and Disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 523–544. [Google Scholar] [CrossRef] [PubMed]
- Socodato, R.; Henriques, J.F.; Portugal, C.C.; Almeida, T.O.; Tedim-Moreira, J.; Alves, R.L.; Canedo, T.; Silva, C.; Magalhaes, A.; Summavielle, T.; et al. Daily alcohol intake triggers aberrant synaptic pruning leading to synapse loss and anxiety-like behavior. Sci. Signal 2020, 13, aba5754. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Wang, H.; Zhang, X.; Shen, Q.; Li, X.; He, L.; Rong, X.; Peng, J.; Mo, J.; Peng, Y. Chronic exposure of alcohol triggers microglia-mediated synaptic elimination inducing cognitive impairment. Exp. Neurol. 2022, 353, 114061. [Google Scholar] [CrossRef] [PubMed]
- Blednov, Y.A.; Ponomarev, I.; Geil, C.; Bergeson, S.; Koob, G.F.; Harris, R.A. Neuroimmune regulation of alcohol consumption: Behavioral validation of genes obtained from genomic studies. Addict. Biol. 2012, 17, 108–120. [Google Scholar] [CrossRef]
- Hitzemann, R.; Bergeson, S.E.; Berman, A.E.; Bubier, J.A.; Chesler, E.J.; Finn, D.A.; Hein, M.; Hoffman, P.; Holmes, A.; Kisby, B.R.; et al. Sex Differences in the Brain Transcriptome Related to Alcohol Effects and Alcohol Use Disorder. Biol. Psychiatry 2022, 91, 43–52. [Google Scholar] [CrossRef]
- Cui, C.; Shurtleff, D.; Harris, R.A. Neuroimmune mechanisms of alcohol and drug addiction. Int. Rev. Neurobiol. 2014, 118, 1–12. [Google Scholar] [CrossRef]
- Agrawal, R.G.; Hewetson, A.; George, C.M.; Syapin, P.J.; Bergeson, S.E. Minocycline reduces ethanol drinking. Brain Behav. Immun. 2011, 25 (Suppl. S1), S165–S169. [Google Scholar] [CrossRef]
- Alfonso-Loeches, S.; Pascual-Lucas, M.; Blanco, A.M.; Sanchez-Vera, I.; Guerri, C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci. 2010, 30, 8285–8295. [Google Scholar] [CrossRef]
- Blednov, Y.A.; Benavidez, J.M.; Geil, C.; Perra, S.; Morikawa, H.; Harris, R.A. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav. Immun. 2011, 25 (Suppl. S1), S92–S105. [Google Scholar] [CrossRef]
- Warden, A.S.; Azzam, M.; DaCosta, A.; Mason, S.; Blednov, Y.A.; Messing, R.O.; Mayfield, R.D.; Harris, R.A. Toll-like receptor 3 activation increases voluntary alcohol intake in C57BL/6J male mice. Brain Behav. Immun. 2019, 77, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Warden, A.S.; Azzam, M.; DaCosta, A.; Mason, S.; Blednov, Y.A.; Messing, R.O.; Mayfield, R.D.; Harris, R.A. Toll-like receptor 3 dynamics in female C57BL/6J mice: Regulation of alcohol intake. Brain Behav. Immun. 2019, 77, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Majchrowicz, E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia 1975, 43, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Hunt, W.A. Are binge drinkers more at risk of developing brain damage? Alcohol 1993, 10, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.A.; Kelso, M.L.; Liput, D.J.; Marshall, S.A.; Nixon, K. Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol 2010, 44, 89–98. [Google Scholar] [CrossRef]







| Group | N (C, E) | Dose (g/kg/day) | BEC (mg/dL) | Peak WD (0–4 Scale) |
|---|---|---|---|---|
| T2–IHC | (8, 8) | 10.7 ± 0.7 | 402.4 ± 24.6 | 2.7 ± 0.4 |
| T2–ELISA (hippocampus) | (6, 6) | 11.5 ± 0.2 | 320.8 ± 16.8 | 2.1 ± 0.2 |
| T2–ELISA (rhinal cortex) | (7, 6) | 10.4 ± 0.6 | 403.7 ± 41.0 | 2.7 ± 0.5 |
| T7–IHC | (8, 8) | 10.2 ± 0.3 | 384.2 ± 6.9 | 3.3 ± 0.1 |
| T7–ELISA | (8, 8) | 10.6 ± 0.6 | 379.5 ± 28.4 | 2.2 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melbourne, J.K.; Wooden, J.I.; Carlson, E.R.; Anasooya Shaji, C.; Nixon, K. Neuroimmune Activation and Microglia Reactivity in Female Rats Following Alcohol Dependence. Int. J. Mol. Sci. 2024, 25, 1603. https://doi.org/10.3390/ijms25031603
Melbourne JK, Wooden JI, Carlson ER, Anasooya Shaji C, Nixon K. Neuroimmune Activation and Microglia Reactivity in Female Rats Following Alcohol Dependence. International Journal of Molecular Sciences. 2024; 25(3):1603. https://doi.org/10.3390/ijms25031603
Chicago/Turabian StyleMelbourne, Jennifer K., Jessica I. Wooden, Erika R. Carlson, Chinchusha Anasooya Shaji, and Kimberly Nixon. 2024. "Neuroimmune Activation and Microglia Reactivity in Female Rats Following Alcohol Dependence" International Journal of Molecular Sciences 25, no. 3: 1603. https://doi.org/10.3390/ijms25031603






