Extracellular Vesicles in the Central Nervous System: A Novel Mechanism of Neuronal Cell Communication
Abstract
:1. Introduction
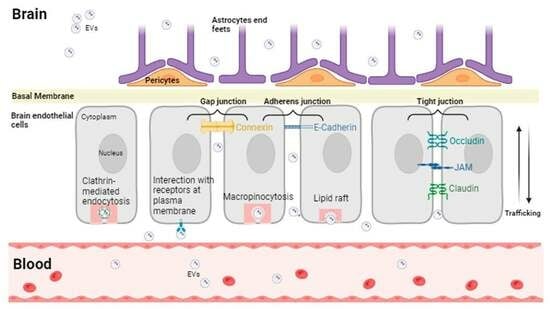
2. Extracellular Vesicles
3. EVs in the CNS
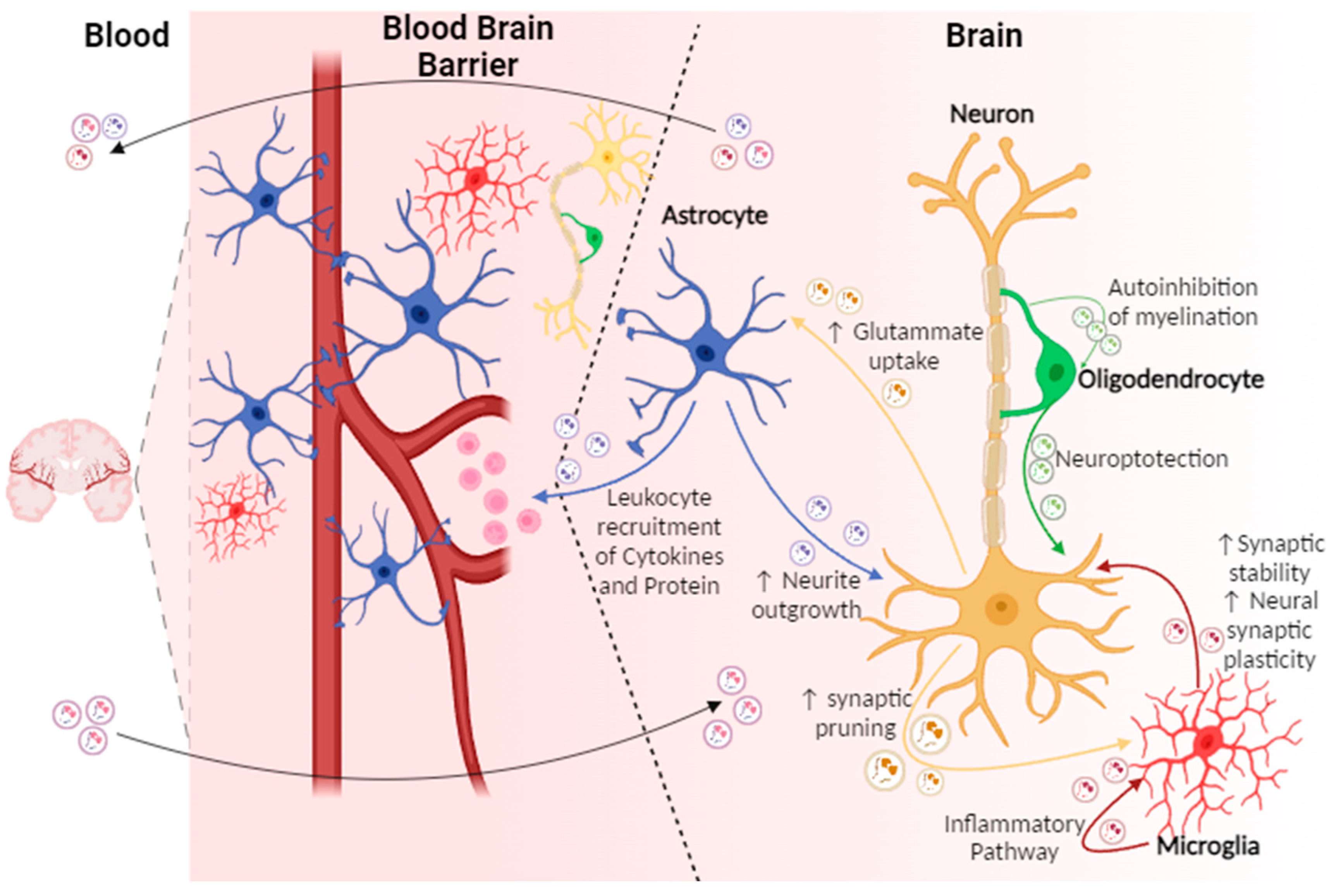
4. Neuron-Derived EVs
5. Astrocyte-Derived EVs
6. Microglia-Derived EVs
7. Oligodendrocytes and EVs
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, C.P.; Breakefield, X.O. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front. Physiol. 2012, 3, 228. [Google Scholar] [CrossRef] [PubMed]
- Krämer-Albers, E.M.; Hill, A.F. Extracellular vesicles: Interneural shuttles of complex messages. Curr. Opin. Neurobiol. 2016, 39, 101–107. [Google Scholar] [CrossRef]
- Budnik, V.; Ruiz-Cañada, C.; Wendler, F. Extracellular vesicles round off communication in the nervous system. Nat. Rev. Neurosci. 2016, 17, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J. Neuroinflammation 2017, 14, 47. [Google Scholar] [CrossRef]
- Chivet, M.; Javalet, C.; Laulagnier, K.; Blot, B.; Hemming, F.J.; Sadoul, R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J. Extracell. Vesicles 2014, 3, 24722. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Ghosh, S.; Bhuniya, T.; Koley, M.; Bera, A.; Guha, S.; Chakraborty, K.; Muthu, S.; Gorai, S.; Vorn, R.; et al. Clinical Theragnostic Signature of Extracellular Vesicles in Traumatic Brain Injury (TBI). ACS Chem. Neurosci. 2023, 14, 2981–2994. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guan, S.; Lu, P.; Li, Y.; Xu, H. Extracellular vesicles: Critical bilateral communicators in periphery-brain crosstalk in central nervous system disorders. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 160, 114354. [Google Scholar] [CrossRef]
- Ramos-Zaldívar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalán, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular vesicles through the blood-brain barrier: A review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef]
- Matsumoto, J.; Stewart, T.; Sheng, L.; Li, N.; Bullock, K.; Song, N. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: Another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol. Commun. 2017, 5, 71. [Google Scholar] [CrossRef]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef] [PubMed]
- Krämer-Albers, E.M. Extracellular Vesicles at CNS barriers: Mode of action. Curr. Opin. Neurobiol. 2022, 75, 102569. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood-brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Fitzner, D.; Schnaars, M.; van Rossum, D.; Krishnamoorthy, G.; Dibaj, P.; Bakhti, M.; Regen, T.; Hanisch, U.K.; Simons, M. Selective transfer of exosomes from oligodendrocytes to microglia by micropinocytosis. J. Cell Sci. 2011, 124, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Nikitidou, E.; Khoonsari, P.E.; Shevchenko, G.; Ingelsson, M.; Kultima, K.; Erlandsson, A. Increased Release of Apolipoprotein E in Extracellular Vesicles Following Amyloid-β Protofibril Exposure of Neuroglial Co-Cultures. J. Alzheimers Dis. 2017, 60, 305–321. [Google Scholar] [CrossRef]
- Simon, T.; Jackson, E.; Giamas, G. Breaking through the glioblastoma micro-environment via extracellular vesicles. Oncogene 2020, 39, 4477–4490. [Google Scholar] [CrossRef]
- Sardar Sinha, M.; Ansell-Schultz, A.; Civitelli, L.; Hildesjö, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2009, 12, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysisof ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [PubMed]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; DeGeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Mitsutake, S.; Igarashi, Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J. Biol. Chem. 2012, 287, 10977–10989. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-Regulated Shedding of Tumor Cell-Derived Plasma Membrane Microvesicles. Curr. Biol. 2009, 19, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- EV-TRACK Consortium; Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, S.; Jedrychowski, M.P.; Yanamandra, K.; Ikezu, S.; Gygi, S.P.; Ikezu, T. Proteomic Profiling of Extracellular Vesicles Derived from Cerebrospinal Fluid of Alzheimer’s Disease Patients: A Pilot Study. Cells 2020, 9, 1959. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Hao, H.; Zhang, Z.; Lv, Y.; Liang, X.; Liu, Q.; Liu, T.; Gong, P.; Zhang, L.; Cao, F.; et al. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics 2021, 11, 8570–8586. [Google Scholar] [CrossRef]
- Fraser, K.B.; Moehle, M.S.; Alcalay, R.N.; West, A.B.; LRRK2 Cohort Consortium. Urinary LRRK2 phosphorylation predicts parkinsonian phenotypes in G2019S LRRK2 carriers. Neurology 2016, 86, 994–999. [Google Scholar] [CrossRef]
- Pieragostino, D.; Lanuti, P.; Cicalini, I.; Cufaro, M.C.; Ciccocioppo, F.; Ronci, M.; Simeone, P.; Onofrj, M.; van der Pol, E.; Fontana, A.; et al. Proteomics characterization of extracellular vesicles sorted by flow cytometry reveals a disease-specific molecular cross-talk from cerebrospinal fluid and tears in multiple sclerosis. J. Proteom. 2019, 204, 103403. [Google Scholar] [CrossRef]
- Altıntaş, Ö.; Saylan, Y. Exploring the Versatility of Exosomes: A Review on Isolation, Characterization, Detection Methods, and Diverse Applications. Anal. Chem. 2023, 95, 16029–16048. [Google Scholar] [CrossRef]
- Lacroix, R.; Judicone, C.; Poncelet, P. Impact of pre-analytical parameters on the measurement of circulating microparticles: Towards standardization of protocol. J. Thromb. Haemost. 2012, 10, 437–446. [Google Scholar] [CrossRef]
- Morelli, A.E.; Larregina, A.T.; Shufesky, W.J. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 2004, 104, 3257–3266. [Google Scholar] [CrossRef]
- Noble, J.M.; Roberts, L.M.; Vidavsky, N. Direct comparison of optical and electron microscopy methods for structural characterization of extracellular vesicles. J. Struct. Biol. 2020, 210, 107474. [Google Scholar] [CrossRef]
- Nag, S.; Bhattacharya, B.; Dutta, S.; Mandal, D.; Mukherjee, S.; Anand, K.; Eswaramoorthy, R.; Thorat, N.; Jha, S.K.; Gorai, S. Clinical Theranostics Trademark of Exosome in Glioblastoma Metastasis. ACS Biomater. Sci. Eng. 2023, 9, 5205–5221. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhu, Y.L.; Zhou, Y.Y.; Liang, G.F.; Wang, Y.Y.; Hu, F.H.; Xiao, Z.D. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J. Biol. Chem. 2014, 289, 22258–22267. [Google Scholar] [CrossRef] [PubMed]
- Kalafatakis, I.; Karagogeos, D. Oligodendrocytes and Microglia: Key Players in Myelin Development, Damage and Repair. Biomolecules 2021, 11, 1058. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, W.L.; Ye, Y.Y.; Bai, X.C.; Liu, R.Q.; Chang, L.F.; Zhou, Q.; Sui, S.F. Cellular internalization of exosomes occurs through phagocytosis. Traffic 2010, 11, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Nanbo, A.; Kawanishi, E.; Yoshida, R.; Yoshiyama, H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J. Virol. 2013, 87, 10334–10347. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Useros, N.; Naranjo-Gómez, M.; Archer, J.; Hatch, S.C.; Erkizia, I.; Blanco, J.; Borràs, F.E.; Puertas, M.C.; Connor, J.H.; Fernández-Figueras, M.T.; et al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood 2009, 113, 2732–2741. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, F.; Turola, E.; Riganti, L.; Caleo, M.; Gabrielli, M.; Perrotta, C.; Novellino, L.; Clementi, E.; Giussani, P.; Viani, P.; et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012, 31, 1231–1240. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular vesicles: Composition, biological relevance, and methods of study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Panaro, M.A.; Benameur, T.; Porro, C. Extracellular vesicles mirna cargo for microglia polarization in traumatic brain injury. Biomolecules 2020, 10, 901. [Google Scholar] [CrossRef]
- Caby, M.P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef]
- Lässer, C.; Alikhani, V.S.; Ekström, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Street, J.M.; Barran, P.E.; Mackay, C.L.; Weidt, S.; Balmforth, C.; Walsh, T.S.; Chalmers, R.T.; Webb, D.J.; Dear, J.W. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J. Transl. Med. 2012, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Bátiz, L.F.; Castro, M.A.; Burgos, P.V.; Velásquez, Z.D.; Muñoz, R.I.; Lafourcade, C.A.; Troncoso-Escudero, P.; Wyneken, U. Exosomes as Novel Regulators of Adult Neurogenic Niches. Front. Cell Neurosci. 2016, 9, 501. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.S.; Singh, T.; Kayhanian, S.; Tjerkaski, J.; Garcia, N.M.; Carpenter, K.L.H.; Patani, R.; Lindblad, C.; Thelin, E.P.; Syed, Y.A.; et al. Modeling the Inflammatory Response of Traumatic Brain Injury Using Human Induced Pluripotent Stem Cell Derived Microglia. J. Neurotrauma 2023, 40, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zlokovic, B.V. Remote control of BBB: A tale of exosomes and microRNA. Cell Res. 2017, 27, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Meng, J.; Zhu, L.; Peng, Y. Exosomal noncoding RNAs in glioma: Biological functions and potential clinical applications. Mol. Cancer. 2020, 19, 66. [Google Scholar] [CrossRef]
- Rajendran, L.; Bali, J.; Barr, M.M.; Court, F.A.; Krämer-Albers, E.M.; Picou, F.; Raposo, G.; van der Vos, K.E.; van Niel, G.; Wang, J.; et al. Emerging roles of extracellular vesicles in the nervous system. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 15482–15489. [Google Scholar] [CrossRef]
- Ceman, S.; Saugstad, J. MicroRNAs: Meta-controllers of gene expression in synaptic activity emerge as genetic and diagnostic markers of human disease. Pharmacol. Ther. 2011, 130, 26–37. [Google Scholar] [CrossRef]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- Monteiro-Reis, S.; Carvalho-Maia, C.; Bart, G.; Vainio, S.J.; Pedro, J.; Silva, E.R.; Sales, G.; Henrique, R.; Jerónimo, C. Secreted Extracellular Vesicle Molecular Cargo as a Novel Liquid Biopsy Diagnostics of Central Nervous System Diseases. Int. J. Mol. Sci. 2021, 22, 3267. [Google Scholar] [CrossRef]
- Graykowski, D.R.; Wang, Y.Z.; Upadhyay, A.; Savas, J.N. The Dichotomous Role of Extracellular Vesicles in the Central Nervous System. iScience 2020, 23, 101456. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Sergeant, N.; Buee, L. Potential contribution of exosomes to the prion-like propagation of lesions in Alzheimer’s disease. Front. Physiol. 2012, 3, 229. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, A.; Rajendran, L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron 2009, 64, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Simons, M. Exosomes: Vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 2013, 352, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Sharples, R.A.; Vella, L.J.; Nisbet, R.M.; Naylor, R.; Perez, K.; Barnham, K.J.; Masters, C.L.; Hill, A.F. Inhibition of gamma-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 2008, 22, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.; Nam, H.; Moon, S.; Kwon, Y.J.; Lee, J.B. Engineered extracellular vesicles and their mimetics for clinical translation. Methods 2020, 177, 80–94. [Google Scholar] [CrossRef]
- . Vasconcelos, M.H.; Caires, H.R.; Ābols, A.; Xavier, C.P.R.; Linē, A. Extracellular vesicles as a novel source of biomarkers in liquid biopsies for monitoring cancer progression and drug resistance. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 2019, 47, 100647. [Google Scholar] [CrossRef]
- Trotta, T.; Panaro, M.A.; Cianciulli, A.; Mori, G.; Di Benedetto, A.; Porro, C. Microglia-derived extracellular vesicles in Alzheimer’s Disease: A double-edged sword. Biochem. Pharmacol. 2018, 148, 184–192. [Google Scholar] [CrossRef]
- Patnam, S.; Samal, R.; Koyyada, R.; Joshi, P.; Singh, A.D.; Nagalla, B.; Soma, M.R.; Sannareddy, R.R.; Ippili, K.; Raju, S.; et al. Exosomal PTEN as a predictive marker of aggressive gliomas. Neurol. India 2022, 70, 215–222. [Google Scholar] [PubMed]
- Driga, M.P.; Catalin, B.; Olaru, D.G.; Slowik, A.; Plesnila, N.; Hermann, D.M.; Popa-Wagner, A. The need for new biomarkers to assist with stroke prevention and prediction of post-stroke therapy based on plasma-derived extracellular vesicles. Biomedicines 2021, 9, 1226. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Rajadas, J.; Seifalian, A.M. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv. Drug Deliv. Rev. 2013, 65, 357–367. [Google Scholar] [CrossRef]
- Yasuda, T.; Ishimoto, T.; Baba, H. Conflicting metabolic alterations in cancer stem cells and regulation by the stromal niche. Regen. Ther. 2021, 17, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Nuzziello, N.; Liguori, M. The MicroRNA Centrism in the Orchestration of Neuroinflammation in Neurodegenerative Diseases. Cells 2019, 8, 1193. [Google Scholar] [CrossRef]
- Ma, F.; Sun, P.; Zhang, X.; Hamblin, M.H.; Yin, K.J. Endothelium-targeted deletion of the miR-15a/16-1 cluster ameliorates blood-brain barrier dysfunction in ischemic stroke. Sci. Signal. 2020, 13, eaay5686. [Google Scholar] [CrossRef]
- Ge, X.; Han, Z.; Chen, F.; Wang, H.; Zhang, B.; Jiang, R.; Lei, P.; Zhang, J. MiR-21 alleviates secondary blood-brain barrier damage after traumatic brain injury in rats. Brain Res. 2015, 1603, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Simeoli, R.; Montague, K.; Jones, H.R.; Castaldi, L.; Chambers, D.; Kelleher, J.H.; Vacca, V.; Pitcher, T.; Grist, J.; Al-Ahdal, H.; et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 2017, 8, 1778. [Google Scholar] [CrossRef]
- Raghav, A.; Singh, M.; Jeong, G.B.; Giri, R.; Agarwal, S.; Kala, S.; Gautam, K.A. Extracellular vesicles in neurodegenerative diseases: A systematic review. Front. Mol. Neurosci. 2022, 15, 1061076. [Google Scholar] [CrossRef]
- Veremeyko, T.; Kuznetsova, I.S.; Dukhinova, M.; Yung, A.W.Y.; Kopeikina, E.; Barteneva, N.S.; Ponomarev, E.D. Neuronal extra-cellular microRNAs miR-124 and miR-9 mediate cell-cell communication between neurons and microglia. J. Neurosci. Res. 2019, 97, 162–184. [Google Scholar] [CrossRef]
- Morel, L.; Regan, M.; Higashimori, H.; Ng, S.K.; Esau, C.; Vidensky, S.; Rothstein, J.; Yang, Y. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J. Biol. Chem. 2013, 288, 7105–7116. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Y.; Du, X.-F.; Li, J.; Zi, H.-X.; Bu, J.-W.; Yan, Y.; Han, H.; Du, J.-L. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. 2017, 27, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Hazelton, I.; Yates, A.; Dale, A.; Roodselaar, J.; Akbar, N.; Ruitenberg, M.J.; Anthony, D.C.; Couch, Y. Exacerbation of Acute Traumatic Brain Injury by Circulating Extracellular Vesicles. J. Neurotrauma 2018, 35, 639–651. [Google Scholar] [CrossRef]
- Varcianna, A.; Myszczynska, M.A.; Castelli, L.M. Micro-RNAs secreted through astrocyte-derived extracellular vesicles cause neuronal network degeneration in C9orf72 ALS. EBioMedicine 2019, 40, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhuang, Y.; Zhao, X.; Li, X. Long Noncoding RNA in Neuronal Development and Neurological Disorders. Front. Genet. 2019, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Canseco-Rodriguez, A.; Masola, V.; Aliperti, V.; Meseguer-Beltran, M.; Donizetti, A.; Sanchez-Perez, A.M. Long Non-Coding RNAs, Extracellular Vesicles and Inflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 13171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Han, C.L.; Wang, K.L.; Sui, Y.P.; Li, Z.B.; Chen, N.; Fan, S.Y.; Shimabukuro, M.; Wang, F.; Meng, F.G. Integrated analysis of exosomal LncRNA and MRNA expression profiles reveals the involvement of Lnc-MKRN2-42:1 in the pathogenesis of Parkinson’s disease. CNS Neurosci. Ther. 2020, 26, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Niu, F.; Dagur, R.S.; He, M.; Tian, C.; Hu, G. Intranasal Delivery of lincRNA-Cox2 siRNA Loaded Extracellular Vesicles Decreases Lipopolysaccharide-Induced Microglial Proliferation in Mice. J. Neuroimmune Pharmacol. Off. J. Soc. Neuro Immune Pharmacol. 2020, 15, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Zhang, Y.; Paudel, K.R.; Kachelmeier, A.; Hansbro, P.M.; Shi, X. The Emerging Role of Pericyte-Derived Extracellular Vesicles in Vascular and Neurological Health. Cells 2022, 11, 3108. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Di Liegro, I. Extracellular Vesicle-Associated RNA as a Carrier of Epigenetic Information. Genes. 2017, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular vesicles: A rising star for therapeutics and drug delivery. J. Nanobiotechnol. 2023, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Kar, R.; Dhar, R.; Mukherjee, S.; Nag, S.; Gorai, S.; Mukerjee, N.; Mukherjee, D.; Vatsa, R.; Chandrakanth Jadhav, M.; Ghosh, A.; et al. Exosome-Based Smart Drug Delivery Tool for Cancer Theranostics. ACS Biomater. Sci. Eng. 2023, 9, 577–594. [Google Scholar] [CrossRef]
- Harisa, G.I.; Faris, T.M.; Sherif, A.Y.; Alzhrani, R.F.; Alanazi, S.A.; Kohaf, N.A.; Alanazi, F.K. Coding Therapeutic Nucleic Acids from Recombinant Proteins to Next-Generation Vaccines: Current Uses, Limitations, and Future Horizons. Mol. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Wu, W.C.; Tian, J.; Xiao, D.; Guo, Y.X.; Xiao, Y.; Wu, X.Y.; Casella, G.; Rasouli, J.; Yan, Y.P.; Rostami, A.; et al. Engineered extracellular vesicles encapsulated Bryostatin-1 as therapy for neuroinflammation. Nanoscale 2022, 14, 2393–2410. [Google Scholar] [CrossRef]
- Zhang, C.; Li, D.; Hu, H.; Wang, Z.; An, J.; Gao, Z.; Zhang, K.; Mei, X.; Wu, C.; Tian, H. Engineered extracellular vesicles derived from primary M2 macrophages with anti-inflammatory and neuroprotective properties for the treatment of spinal cord injury. J. Nanobiotechnol. 2021, 19, 373. [Google Scholar] [CrossRef]
- Clinical Trials. Available online: https://clinicaltrials.gov (accessed on 20 January 2024).
- Xie, X.; Song, Q.; Dai, C.; Cui, S.; Tang, R.; Li, S.; Chang, J.; Li, P.; Wang, J.; Li, J.; et al. Clinical Safety and Efficacy of Allogenic Human Adipose Mesenchymal Stromal Cells-Derived Exosomes in Patients with Mild to Moderate Alzheimer’s Disease: A Phase I/II Clinical Trial. Gen. Psychiatr. 2023, 36, e101143. [Google Scholar] [CrossRef]
- Dehghani, L.; Khojasteh, A.; Soleimani, M.; Oraee-Yazdani, S.; Keshel, S.H.; Saadatnia, M.; Saboori, M.; Zali, A.; Hashemi, S.M.; Soleimani, R. Safety of Intraparenchymal Injection of Allogenic Placenta Mesenchymal Stem Cells Derived Exosome in Patients Undergoing Decompressive Craniectomy Following Malignan Middle Cerebral Artery Infarct, A Pilot Randomized Clinical Trial. Int. J. Prev. Med. 2022, 13, 7. [Google Scholar] [CrossRef]
- Picciolini, S.; Gualerzi, A.; Carlomagno, C.; Cabinio, M.; Sorrentino, S.; Baglio, F.; Bedoni, M. An SPRi-Based Biosensor Pilot Study: Analysis of Multiple Circulating Extracellular Vesicles and Hippocampal Volume in Alzheimer’s Disease. J. Pharm. Biomed. Anal. 2021, 192, 113649. [Google Scholar] [CrossRef]
- Gualerzi, A.; Picciolini, S.; Carlomagno, C.; Rodà, F.; Bedoni, M. Biophotonics for Diagnostic Detection of Extracellular Vesicles. Adv. Drug Deliv. Rev. 2021, 174, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Dong, J.-F.; Zhang, J. Roles and Therapeutic Potential of Different Extracellular Vesicle Subtypes on Traumatic Brain Injury. Cell Commun. Signal. 2023, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Beard, K.; Meaney, D.F.; Issadore, D. Clinical Applications of Extracellular Vesicles in the Diagnosis and Treatment of Traumatic Brain Injury. J. Neurotrauma 2020, 37, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, F.; Bai, X.; Jia, H.; Wang, C.; Li, P.; Zhang, Q.; Guan, S.; Peng, R.; Zhang, S.; et al. Circulating Extracellular Vesicles from Patients with Traumatic Brain Injury Induce Cerebrovascular Endothelial Dysfunction. Pharmacol. Res. 2023, 192, 106791. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, C.R.; Rodal, A.A. Mechanisms for biogenesis and release of neuronal extracellular vesicles. Curr. Opin. Neurobiol. 2020, 63, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Keshava, S.; Mukherjee, T.; Wang, J.; Magisetty, J.; Kolesnick, R.; Pendurthi, U.R.; Rao, L.V.M. Factor VIIa releases phosphatidylserine-enriched extracellular vesicles from endothelial cells by activating acid sphingomyelinase. J. Thromb. Haemost. 2023, 21, 3414–3431. [Google Scholar] [CrossRef] [PubMed]
- Sohda, M.; Misumi, Y.; Oda, K. TNFα triggers release of extracellular vesicles containing TNFR1 and TRADD, which can modulate TNFα responses of the parental cells. Arch. Biochem. Biophys. 2015, 587, 31–37. [Google Scholar] [CrossRef]
- Crivelli, S.M.; Quadri, Z.; Vekaria, H.J.; Zhu, Z.; Tripathi, P.; Elsherbini, A.; Zhang, L.; Sullivan, P.G.; Bieberich, E. Inhibition of acid sphingomyelinase reduces reactive astrocyte secretion of mitotoxic extracellular vesicles and improves Alzheimer’s disease pathology in the 5xFAD mouse. Acta Neuropathol. Commun. 2023, 11, 135. [Google Scholar] [CrossRef]
- Polanco, J.C.; Li, C.; Durisic, N.; Sullivan, R.; Götz, J. Exosomes taken up by neurons hijack the endosomal pathway to spread to interconnected neurons. Acta Neuropathol. Commun. 2018, 6, 10. [Google Scholar] [CrossRef]
- Lachenal, G.; Pernet-Gallay, K.; Chivet, M.; Hemming, F.J.; Belly, A.; Bodon, G.; Blot, B.; Haase, G.; Goldberg, Y.; Sadoul, R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell Neurosci. 2011, 46, 409–418. [Google Scholar] [CrossRef]
- Wilkerson, J.R.; Albanesi, J.P.; Huber, K.M. Roles for Arc in metabotropic glutamate receptor-dependent LTD and synapse elimination: Implications in health and disease. Semin. Cell Dev. Biol. 2018, 77, 51–62. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, S.M.; Zhong, P.; Kim, H.T.; Kim, D.I.; Kim, J.M.; Heo, W.D.; Kim, D.W.; Yeo, C.Y.; Kim, C.H.; et al. Reciprocal control of excitatory synapse numbers by Wnt and Wnt inhibitor PRR7 secreted on exosomes. Nat. Commun. 2018, 9, 3434. [Google Scholar] [CrossRef] [PubMed]
- Nieves Torres, D.; Lee, S.H. Inter-neuronal signaling mediated by small extracellular vesicles: Wireless communication? Front. Mol. Neurosci. 2023, 16, 1187300. [Google Scholar] [CrossRef] [PubMed]
- Korkut, C.; Ataman, B.; Ramachandran, P.; Ashley, J.; Barria, R.; Gherbesi, N.; Budnik, V. Trans-synaptic transmission of vesicular Wnt signalling by Evi/Wntless. Cells 2009, 139, 393–404. [Google Scholar] [CrossRef] [PubMed]
- McLeod, F.; Bossio, A.; Marzo, A.; Ciani, L.; Sibilla, S.; Hannan, S.; Wilson, G.A.; Palomer, E.; Smart, T.G.; Gibb, A.; et al. Wnt Signaling Mediates LTP-Dependent Spine Plasticity and AMPAR Localization through Frizzled-7 Receptors. Cell Rep. 2018, 23, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Orefice, L.L.; Chiu, S.L.; LeGates, T.A.; Hattar, S.; Huganir, R.L.; Zhao, H.; Xu, B.; Kuruvilla, R. Wnt5a is essential for hippocampal dendritic maintenance and spatial learning and memory in adult mice. Proc. Natl. Acad. Sci. USA 2017, 114, E619–E628. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.C. Extracellular WNTs: Trafficking, Exosomes, and Ligand-Receptor Interaction. Handb. Exp. Pharmacol. 2021, 269, 29–43. [Google Scholar] [CrossRef]
- Bassani, S.; Folci, A.; Zapata, J.; Passafaro, M. AMPAR trafficking in synapse maturation and plasticity. Cell. Mol. Life Sci. 2013, 70, 4411–4430. [Google Scholar] [CrossRef]
- Castillo, P.E.; Younts, T.J.; Chavez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef]
- Gong, J.; Korner, R.; Gaitanos, L.; Klein, R. Exosomes mediate cell contact-independent ephrin-Eph signaling during axon guidance. J. Cell Biol. 2016, 214, 35–44. [Google Scholar]
- Escudero, C.A.; Lazo, O.M.; Galleguillos, C.; Parraguez, J.I.; Lopez-Verrilli, M.A.; Cabeza, C.; Leon, L.; Saeed, U.; Retamal, C.; Gonzalez, A.; et al. The p75 neurotrophin receptor evades the endolysosomal route in neuronal cells favouring multivesicular bodies specialised for exosomal release. J. Cell Sci. 2014, 127, 1966–1979. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Liu, D.Z.; Jickling, G.C.; Sharp, F.R.; Yin, K.J. MicroRNA-based therapeutics in central nervous system injuries. J. Cereb. Blood Flow. Metab. 2018, 38, 1125–1148. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Z.; He, T.; Qu, M.; Jiang, L.; Li, W.; Shi, X.; Pan, J.; Zhang, L.; Wang, Y.; et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 2019, 9, 2910–2923. [Google Scholar] [CrossRef]
- Qing, L.; Chen, H.; Tang, J.; Jia, X. Exosomes and Their MicroRNA Cargo: New Players in Peripheral Nerve Regeneration. Neurorehabil. Neural Repair. 2018, 32, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.; Auderset, L.; Kaurani, L.; Sebastian, E.; Zeng, Y.; Allahham, M.; Cases-Cunillera, S.; Schoch, S.; Gründemann, J.; Fischer, A.; et al. Neuronal extracellular vesicles and associated microRNAs induce circuit connectivity downstream BDNF. Cell Rep. 2023, 42, 112063. [Google Scholar] [CrossRef]
- Hornung, S.; Dutta, S.; Bitan, G. CNS-Derived Blood Exosomes as a Promising Source of Biomarkers: Opportunities and Challenges. Front. Mol. Neurosci. 2020, 13, 38. [Google Scholar] [CrossRef]
- Goldie, B.J.; Dun, M.D.; Lin, M.; Smith, N.D.; Verrills, N.M.; Dayas, C.V.; Cairns, M.J. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014, 42, 9195–9208. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R.; Sheridan, G.K. Extracellular vesicles and intercellular communication in the central nervous system. FEBS Lett. 2021, 595, 1391–1410. [Google Scholar] [CrossRef]
- Xu, M.; Feng, T.; Liu, B.; Qiu, F.; Xu, Y.; Zhao, Y.; Zheng, Y. Engineered exosomes: Desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics 2021, 11, 8926–8944. [Google Scholar] [CrossRef]
- Korkut, C.; Li, Y.; Koles, K.; Brewer, C.; Ashley, J.; Yoshihara, M.; Budnik, V. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron 2013, 77, 1039–1046. [Google Scholar] [CrossRef]
- Gassama, Y.; Favereaux, A. Emerging Roles of Extracellular Vesicles in the Central Nervous System: Physiology, Pathology, and Therapeutic Perspectives. Front. Cell Neurosci. 2021, 15, 626043. [Google Scholar] [CrossRef]
- Li, X.H.; Zhang, J.; Li, D.F.; Wu, W.; Xie, Z.W.; Liu, Q. Physiological and pathological insights into exosomes in the brain. Zool. Res. 2020, 41, 365–372. [Google Scholar] [CrossRef]
- Zumkehr, J.; Rodriguez-Ortiz, C.J.; Medeiros, R.; Kitazawa, M. Inflammatory Cytokine, IL-1β, Regulates Glial Glutamate Transporter via microRNA-181a in vitro. J. Alzheimers Dis. 2018, 63, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gozen, O.; Vidensky, S.; Robinson, M.B.; Rothstein, J.D. Epigenetic regulation of neuron-dependent induction of astroglial synaptic protein GLT1. Glia 2010, 58, 277–286. [Google Scholar] [CrossRef]
- Men, Y.; Yelick, J.; Jin, S.; Tian, Y.; Chiang, M.S.R.; Higashimori, H.; Brown, E.; Jarvis, R.; Yang, Y. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat. Commun. 2019, 10, 4136. [Google Scholar] [CrossRef]
- Bahrini, I.; Song, J.H.; Diez, D.; Hanayama, R. Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci. Rep. 2015, 5, 7989. [Google Scholar] [CrossRef]
- Becker-Krail, D.D.; Ketchesin, K.D.; Burns, J.N.; Zong, W.; Hildebrand, M.A.; DePoy, L.M.; Vadnie, C.A.; Tseng, G.C.; Logan, R.W.; Huang, Y.H.; et al. Astrocyte Molecular Clock Function in the Nucleus Accumbens Is Important for Reward-Related Behavior. Biol. Psychiatry 2022, 92, 68–80. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Borgmann, K.; Edara, V.V.; Stacy, S.; Ghorpade, A.; Ikezu, T. Activated human astrocyte-derived extracellular vesicles modulate neuronal uptake, differentiation and firing. J. Extracell. Vesicles 2019, 9, 1706801. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10, 1114. [Google Scholar] [CrossRef]
- . Upadhya, R.; Zingg, W.; Shetty, S.; Shetty, A.K. Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders. J. Control. Release Off. J. Control. Release Soc. 2020, 323, 225–239. [Google Scholar] [CrossRef]
- Szpakowski, P.; Ksiazek-Winiarek, D.; Czpakowska, J.; Kaluza, M.; Milewska-Jedrzejczak, M.; Glabinski, A. Astrocyte-Derived Exosomes Differentially Shape T Cells’ Immune Response in MS Patients. Int. J. Mol. Sci. 2023, 24, 7470. [Google Scholar] [CrossRef] [PubMed]
- Ponath, G.; Park, C.; Pitt, D. The Role of Astrocytes in Multiple Sclerosis. Front. Immunol. 2018, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Song, P.; Wu, Z.; Wang, C.; Liu, Y.; Ying, W.; Li, K.; Shen, C. Inflammatory stimulation of astrocytes affects the expression of miRNA-22-3p within NSCs-EVs regulating remyelination by targeting KDM3A. Stem Cell Res. Ther. 2023, 14, 52. [Google Scholar] [CrossRef]
- Peng, D.; Wang, Y.; Xiao, Y.; Peng, M.; Mai, W.; Hu, B.; Jia, Y.; Chen, H.; Yang, Y.; Xiang, Q.; et al. Extracellular vesicles derived from astrocyte-treated with haFGF14-154 attenuate Alzheimer phenotype in AD mice. Theranostics 2022, 12, 3862–3881. [Google Scholar] [CrossRef]
- Liao, K.; Niu, F.; Hu, G.; Yang, L.; Dallon, B.; Villarreal, D.; Buch, S. Morphine-mediated release of miR-138 in astrocyte-derived extracellular vesicles promotes microglial activation. J. Extracell. Vesicles 2020, 10, e12027. [Google Scholar] [CrossRef] [PubMed]
- Ruhela, D.; Bhopale, V.M.; Kalakonda, S.; Thom, S.R. Astrocyte-derived microparticles initiate a neuroinflammatory cycle due to carbon monoxide poisoning. Brain Behav. Immun. Health 2021, 18, 100398. [Google Scholar] [CrossRef] [PubMed]
- Sidoryk-Wegrzynowicz, M.; Wegrzynowicz, M.; Lee, E.; Bowman, A.B.; Aschner, M. Role of astrocytes in brain function and disease. Toxicol. Pathol. 2011, 39, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Phatnani, H.; Maniatis, T. Astrocytes in neurodegenerative disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a020628. [Google Scholar] [CrossRef]
- Brandebura, A.N.; Paumier, A.; Onur, T.S.; Allen, N.J. Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat. Rev. Neurosci. 2023, 24, 23–39. [Google Scholar] [CrossRef]
- Pistono, C.; Bister, N.; Stanová, I.; Malm, T. Glia-Derived Extracellular Vesicles: Role in Central Nervous System Communication in Health and Disease. Front. Cell Dev. Biol. 2021, 8, 623771. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, M.E.; Sutter, P.A.; Durham, O.R.; Willis, C.M.; Crocker, S.J. Astrocyte-Derived Extracellular Vesicles (ADEVs): Deciphering their Influences in Aging. Aging Dis. 2021, 12, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wang, Y.; Zheng, J.C. Extracellular vesicles, from the pathogenesis to the therapy of neurodegenerative diseases. Transl. Neurodegener. 2022, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Sheng, S.; Wang, Y.; Ding, L.; Xu, X.; Xia, X.; Zheng, J.C. Astrocyte-derived extracellular vesicles: A double-edged sword in central nervous system disorders. Neurosci. Biobehav. Rev. 2021, 125, 148–159. [Google Scholar] [CrossRef] [PubMed]
- García-Bueno, B.; Caso, J.R.; Leza, J.C. Stress as a neuroinflammatory condition in brain: Damaging and protective mechanisms. Neurosci. Biobehav. Rev. 2008, 32, 1136–1151. [Google Scholar] [CrossRef] [PubMed]
- Wallensten, J.; Nager, A.; Åsberg, M.; Borg, K.; Beser, A.; Wilczek, A.; Mobarrez, F. Leakage of astrocyte-derived extracellular vesicles in stress-induced exhaustion disorder: A cross-sectional study. Sci. Rep. 2021, 11, 2009, Erratum in Sci. Rep. 2023, 13, 10211. [Google Scholar] [CrossRef]
- Nekludov, M.; Bellander, B.M.; Gryth, D.; Wallen, H.; Mobarrez, F. Brain-Derived Microparticles in Patients with Severe Isolated TBI. Brain Inj. 2017, 31, 1856–1862. [Google Scholar] [CrossRef]
- Hering, C.; Shetty, A.K. Extracellular Vesicles Derived from Neural Stem Cells, Astrocytes, and Microglia as Therapeutics for Easing TBI-Induced Brain Dysfunction. Stem Cells Transl. Med. 2023, 12, 140–153. [Google Scholar] [CrossRef]
- Zhang, W.; Hong, J.; Zhang, H.; Zheng, W.; Yang, Y. Astrocyte-derived exosomes protect hippocampal neurons after traumatic brain injury by suppressing mitochondrial oxidative stress and apoptosis. Aging 2021, 13, 21642–21658. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Keyhanian, K.; Douthwright, C. Glial Cell Dysfunction in C9orf72-Related Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Cells 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Priller, J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014, 15, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Porro, C.; Calvello, R.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Microglia Mediated Neuroinflammation: Focus on PI3K Modulation. Biomolecules 2020, 10, 137. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, M.H.; Shim, W.H.; Kim, J.K.; Jeon, E.Y.; Kim, D.H.; Yoon, S.Y. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol. Psychiatry 2016, 22, 1576–1584. [Google Scholar] [CrossRef]
- Reichert, F.; Rotshenker, S. Galectin-3 (MAC-2) Controls Microglia Phenotype Whether Amoeboid and Phagocytic or Branched and Non-phagocytic by Regulating the Cytoskeleton. Front. Cell Neurosci. 2019, 13, 90. [Google Scholar] [CrossRef]
- Ransohoff, R.M. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 2016, 19, 987–991. [Google Scholar] [CrossRef]
- Porro, C.; Cianciulli, A.; Calvello, R.; Panaro, M.A. Reviewing the Role of Resveratrol as a Natural Modulator of Microglial Activities. Curr. Pharm. Des. 2015, 21, 5277–5291. [Google Scholar] [CrossRef]
- Calvello, R.; Porro, C.; Lofrumento, D.D.; Ruggiero, M.; Panaro, M.A.; Cianciulli, A. Decoy Receptors Regulation by Resveratrol in Lipopolysaccharide-Activated Microglia. Cells 2023, 12, 681. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Battista, N.; Riganti, L.; Prada, I.; Antonucci, F.; Cantone, L.; Matteoli, M.; Maccarrone, M.; Verderio, C. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep. 2015, 16, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Pravettoni, E.; Colombo, A.; Schenk, U.; Möller, T.; Matteoli, M.; Verderio, C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J. Immunol. 2005, 174, 7268–7277. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, T.; Tsukimoto, M.; Iwamaru, Y.; Sugama, S.; Sekiyama, K.; Sato, M.; Kojima, S.; Hashimoto, M.; Kitani, H. Extracellular ATP induces unconventional release of glyceraldehyde-3-phosphate dehydrogenase from microglial cells. Immunol. Lett. 2015, 167, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Colonna, M. Two-faced behavior of microglia in Alzheimer’s disease. Nat. Neurosci. 2022, 25, 3–4. [Google Scholar] [CrossRef]
- Glebov, K.; Löchner, M.; Jabs, R.; Lau, T.; Merkel, O.; Schloss, P.; Steinhäuser, C.; Walter, J. Serotonin stimulates secretion of exosomes from microglia cells. Glia 2015, 63, 626–634. [Google Scholar] [CrossRef]
- Hooper, C.; Sainz-Fuertes, R.; Lynham, S.; Hye, A.; Killick, R.; Warley, A.; Bolondi, C.; Pocock, J.; Lovestone, S. Correction to: Wnt3a induces exosome secretion from primary cultured rat microglia. BMC Neurosci. 2020, 21, 10, Erratum in BMC Neurosci. 2012, 13, 144.. [Google Scholar] [CrossRef]
- Chang, C.; Lang, H.; Geng, N.; Wang, J.; Li, N.; Wang, X. Exosomes of BV-2 cells induced by alpha-synuclein: Important mediator of neurodegeneration in PD. Neurosci. Lett. 2013, 548, 190–195. [Google Scholar] [CrossRef]
- Cianciulli, A.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A. Folic Acid Is Able to Polarize the Inflammatory Response in LPS Activated Microglia by Regulating Multiple Signaling Pathways. Mediat. Inflamm. 2016, 2016, 5240127. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Cao, Y.; Jia, H.; Li, X.; Li, F.; Zhang, S.; Zhang, J. Neuroinflammation of traumatic brain injury: Roles of extracellular vesicles. Front. Immunol. 2023, 13, 1088827. [Google Scholar] [CrossRef] [PubMed]
- La Torre, M.E.; Panaro, M.A.; Ruggiero, M.; Polito, R.; Cianciulli, A.; Filannino, F.M.; Lofrumento, D.D.; Antonucci, L.; Benameur, T.; Monda, V.; et al. Extracellular Vesicles Cargo in Modulating Microglia Functional Responses. Biology 2022, 11, 1426. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Bastoni, M.; Nigro, A.; Podini, P.; Finardi, A.; Casella, G.; Ramesh, M.; Farina, C.; Verderio, C.; Furlan, R. Cytokines Stimulate the Release of Microvesicles from Myeloid Cells Independently from the P2X7 Receptor/Acid Sphingomyelinase Pathway. Front. Immunol. 2018, 9, 204. [Google Scholar] [CrossRef]
- Prada, I.; Gabrielli, M.; Turola, E.; Iorio, A.; D’Arrigo, G.; Parolisi, R.; De Luca, M.; Pacifici, M.; Bastoni, M.; Lombardi, M.; et al. Glia-to-neuron transfer of miRNAs via extracellular vesicles: A new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol. 2018, 135, 529–550. [Google Scholar] [CrossRef]
- Lombardi, M.; Parolisi, R.; Scaroni, F.; Bonfanti, E.; Gualerzi, A.; Gabrielli, M.; Kerlero de Rosbo, N.; Uccelli, A.; Giussani, P.; Viani, P.; et al. Detrimental and protective action of microglial extracellular vesicles on myelin lesions: Astrocyte involvement in remyelination failure. Acta Neuropathol. 2019, 138, 987–1012. [Google Scholar] [CrossRef]
- Raffaele, S.; Lombardi, M.; Verderio, C.; Fumagalli, M. TNF Production and Release from Microglia via Extracellular Vesicles: Impact on Brain Functions. Cells 2020, 9, 2145. [Google Scholar] [CrossRef] [PubMed]
- Marrone, M.C.; Morabito, A.; Giustizieri, M.; Chiurchiù, V.; Leuti, A.; Mattioli, M.; Marinelli, S.; Riganti, L.; Lombardi, M.; Murana, E.; et al. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat. Commun. 2017, 8, 15292. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, J.; Zhao, Y.; Feng, Y.; Han, S.; Dong, Q.; Cui, M.; Tieu, K. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain 2020, 143, 1476–1497. [Google Scholar] [CrossRef]
- Mukherjee, S.; Cabrera, M.A.; Boyadjieva, N.I.; Berger, G.; Rousseau, B.; Sarkar, D.K. Alcohol Increases Exosome Release from Microglia to Promote Complement C1q-Induced Cellular Death of Proopiomelanocortin Neurons in the Hypothalamus in a Rat Model of Fetal Alcohol Spectrum Disorders. J. Neurosci. 2020, 40, 7965–7979. [Google Scholar] [CrossRef]
- Sarkar, S.; Rokad, D.; Malovic, E.; Luo, J.; Harischandra, D.S.; Jin, H.; Anantharam, V.; Huang, X.; Lewis, M.; Kanthasamy, A.; et al. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci. Signal. 2019, 12, eaat9900. [Google Scholar] [CrossRef]
- Stadelmann, C.; Timmler, S.; Barrantes-Freer, A.; Simons, M. Myelin in the central nervous system: Structure, function, and 853 pathology. Physiol. Rev. 2019, 99, 1381–1431. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, A.D.; David, S.; Bennett, F.C. Immune cell regulation of glia during CNS injury and disease. Nat. Rev. Neurosci. 2020, 21, 139–152. [Google Scholar] [CrossRef]
- Kang, M.; Yao, Y. Oligodendrocytes in intracerebral hemorrhage. CNS Neurosci. Ther. 2019, 25, 1075–1084. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Hughes, A.N.; Appel, B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 2020, 23, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Bakhti, M.; Winter, C.; Simons, M. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J. Biol. Chem. 2011, 286, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, D.; Kuo, W.P.; Frühbeis, C.; Sun, J.J.; Zehendner, C.M.; Luhmann, H.J.; Pinto, S.; Toedling, J.; Trotter, J.; Krämer-Albers, E.M. Multifaceted effects of oligodendroglial exosomes on neurons: Impact on neuronal firing rate, signal transduction and gene regulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130510. [Google Scholar] [CrossRef] [PubMed]
- Frühbeis, C.; Fröhlich, D.; Kuo, W.P.; Amphornrat, J.; Thilemann, S.; Saab, A.S.; Kirchhoff, F.; Möbius, W.; Goebbels, S.; Nave, K.A.; et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013, 11, e1001604. [Google Scholar] [CrossRef]
- Zeke, A.; Misheva, M.; Reményi, A.; Bogoyevitch, M.A. JNK Signaling: Regulation and Functions Based on Complex Protein-Protein Partnerships. Microbiol. Mol. Biol. Rev. 2016, 80, 793–835. [Google Scholar] [CrossRef]

| EVs miRNA Name | EVs Origin | Target Pathways/CNS Component | Major Function | Ref. |
|---|---|---|---|---|
| miR-15a miR-15b | Endothelial Cells | Microglia |
| [10,68] |
| miR-21 | Endothelial Cells | SNC, Microglia |
| [10,69] |
| miR-21-5p | Neuron | Microglia, Astrocytes, Endothelial Cells |
| [70] |
| miR-92b-3p | Neuron | Neuron |
| [71] |
| miR-124 | Neuron | Microglia, Astrocytes, Endothelial Cells |
| [72] |
| miR-124a | Neuron | Astrocytes |
| [73] |
| miR-132 | Neuron | Ctbp2 on glial progenitor cells |
| [74] |
| miR-155 | Microglia | Microglia |
| [75] |
| miR-494-3p | Astrocytes | Neuron |
| [76] |
| EVs Type/Origin | Potential Application | Cargo/Delivered Drug | Study Identifier of Clinical Trials | Ref. |
|---|---|---|---|---|
| MSCs | Alzheimer disease | No | NCT04388982 | [91] |
| MSCs | Stroke/Cerebrovascular disease | miR-124 | NCT03384433 | [92] |
| Multiple origins | Stroke | Prognostic biomarkers, profiling biomarkers | NCT05370105 | Ongoing |
| Neurons, astrocytes, microglia and oligodendrocytes | Neurodegenerative diseases (Alzheimer’s disease) | Circulating biomarkers | NA | [93] |
| Multiple origins (blood) | Neurological disorders and other disorders | Circulating biomarkers | NA | [94] |
| Multiple origins (saliva) | Parkinson disease | Saliva-based biomarker | NCT05320250 | Ongoing |
| Multiple origins (blood) | brain Huntingtin (HTT) | Blood-based biomarker of disease progression or conversion | NCT06082713 | Ongoing |
| Multiple origins (blood) | meningioma | Biomarkers of (early) tumour progression. DNA methylation profiling of plasma EVs | NCT06104930 | Ongoing |
| Multiple origins (blood) | Traumatic brain injury | Early disease assessment and biomarker of prognosis following traumatic brain injury;HMGB1 | NCT05279599 | [95,96,97] |
| Multiple origins (blood, CSF) | Alzheimer’s disease, neurofibrillary degeneration (NFD) | Detection of early markers of (NFD) such as Tau, therapeutic targets | NCT03381482 | Ongoing |
| Origin | Effects | Ref. |
|---|---|---|
| Neuron-derived EVs | Synapse maintenance in central neurons. | [110] |
| Synaptogenesis, synapse, and dendrite maintenance, spatial learning and memory, formation of hippocampal long-term potentiation. | [167,168] | |
| Axon guidance and synapse formation. | [113] | |
| Neurite outgrowth, neuronal survival, and death. | [114] | |
| Modulation of microglial activity | [116,117,119,120,121,126] | |
| Synaptic plasticity, helping astrocytes to maintain the homeostasis of neurotransmission. | [122,128] | |
| Formation of excitatory synapse in recipient neurons | [118] | |
| Synaptic plasticity, memory formation, facilitating synaptic tuning. | [111,123,124] | |
| Prevention of neuronal excitotoxicity | [127] | |
| Astrocyte-derived EVs | Helping to strengthen tight junctions between endothelial cells in brain capillaries. | [123,131] |
| Neurogenesis, synaptogenesis, and synaptic plasticity. | [135] | |
| Mediation of pathological processes and neuroinflammatory disorders. | [145,146] | |
| Neuroprotection. | [133,152] | |
| Microglia-derived EVs | Regulation and propagation of neuroinflammatory response | [156,157,158,162,163] |
| Modulation of inflammatory mediators | [156,157,158,162,163] | |
| Support neuroprotection | [41] | |
| Oligodendrocytes | Helping in myelin clereance | [185] |
| Transfer stress-protective enzymes | [186,187,188] | |
| Neuron protection | [186] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filannino, F.M.; Panaro, M.A.; Benameur, T.; Pizzolorusso, I.; Porro, C. Extracellular Vesicles in the Central Nervous System: A Novel Mechanism of Neuronal Cell Communication. Int. J. Mol. Sci. 2024, 25, 1629. https://doi.org/10.3390/ijms25031629
Filannino FM, Panaro MA, Benameur T, Pizzolorusso I, Porro C. Extracellular Vesicles in the Central Nervous System: A Novel Mechanism of Neuronal Cell Communication. International Journal of Molecular Sciences. 2024; 25(3):1629. https://doi.org/10.3390/ijms25031629
Chicago/Turabian StyleFilannino, Francesca Martina, Maria Antonietta Panaro, Tarek Benameur, Ilaria Pizzolorusso, and Chiara Porro. 2024. "Extracellular Vesicles in the Central Nervous System: A Novel Mechanism of Neuronal Cell Communication" International Journal of Molecular Sciences 25, no. 3: 1629. https://doi.org/10.3390/ijms25031629
APA StyleFilannino, F. M., Panaro, M. A., Benameur, T., Pizzolorusso, I., & Porro, C. (2024). Extracellular Vesicles in the Central Nervous System: A Novel Mechanism of Neuronal Cell Communication. International Journal of Molecular Sciences, 25(3), 1629. https://doi.org/10.3390/ijms25031629