The Effect of Ethephon on Ethylene and Chlorophyll in Zoysia japonica Leaves
Abstract
1. Introduction
2. Results
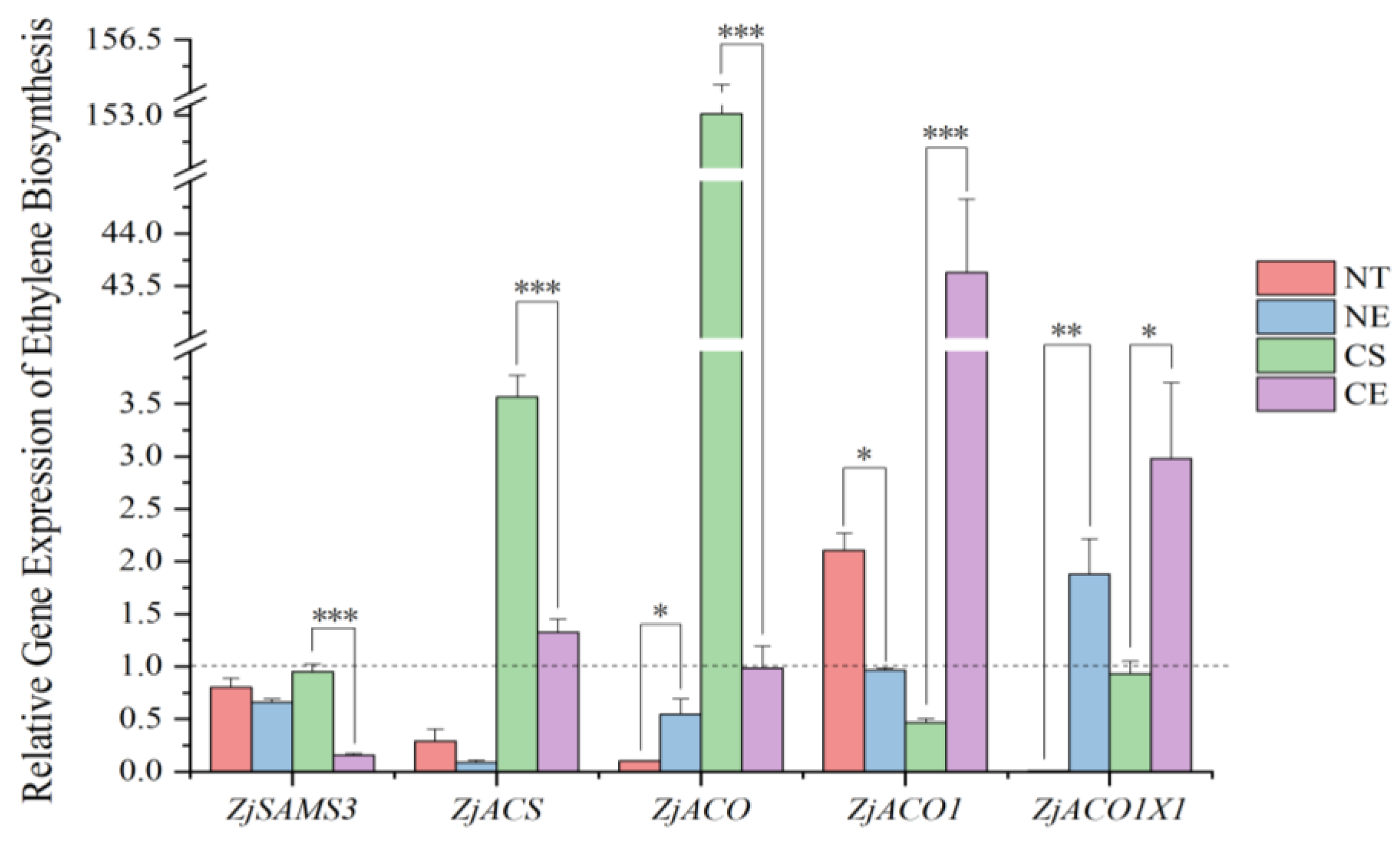
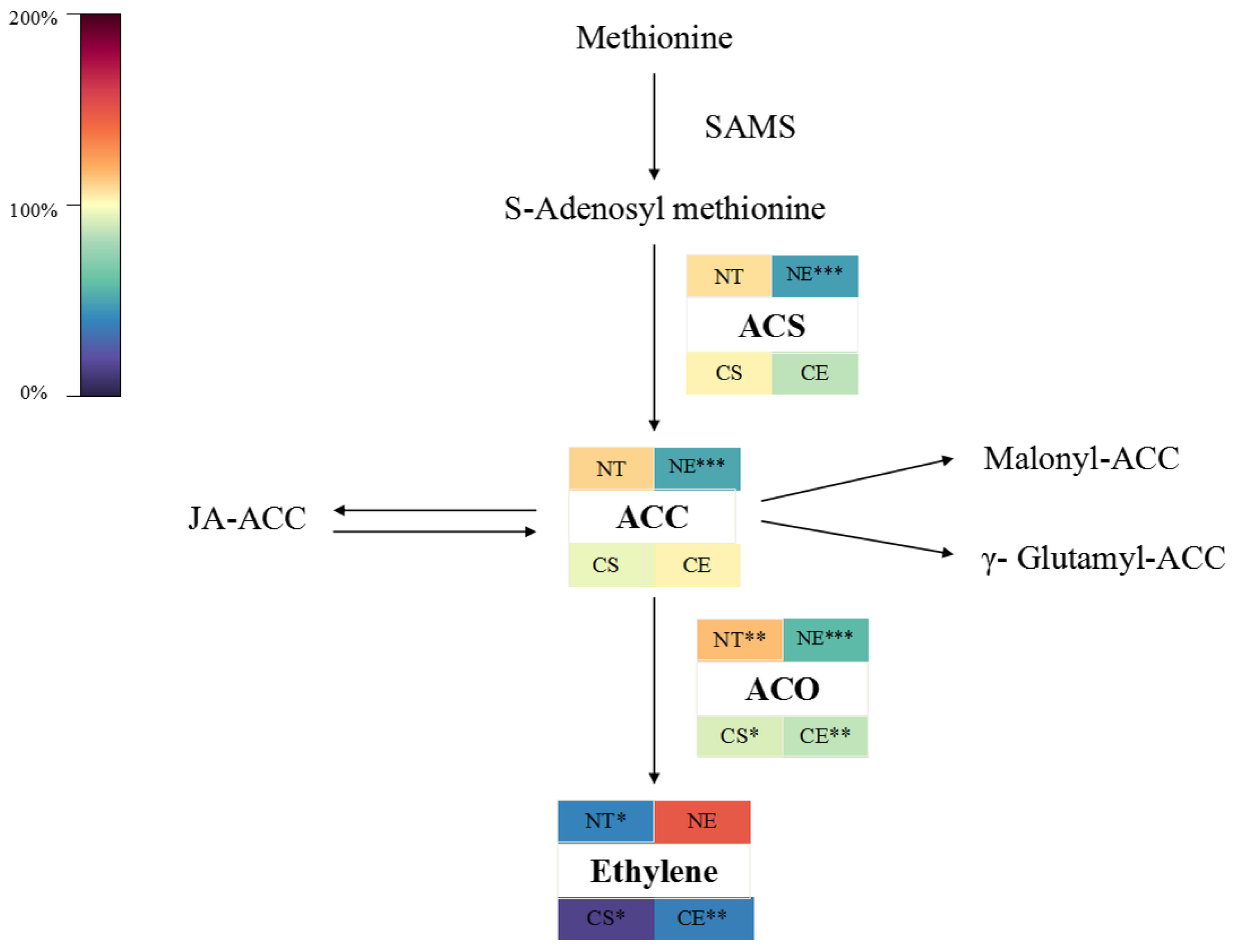
2.1. Effect of Ethephon Application on Ethylene Biosynthesis
2.1.1. Effect of Ethephon Application on Ethylene Biosynthesis under Non-Stressed Conditions
2.1.2. Effect of Ethephon Application on Ethylene Biosynthesis under Cold Stress
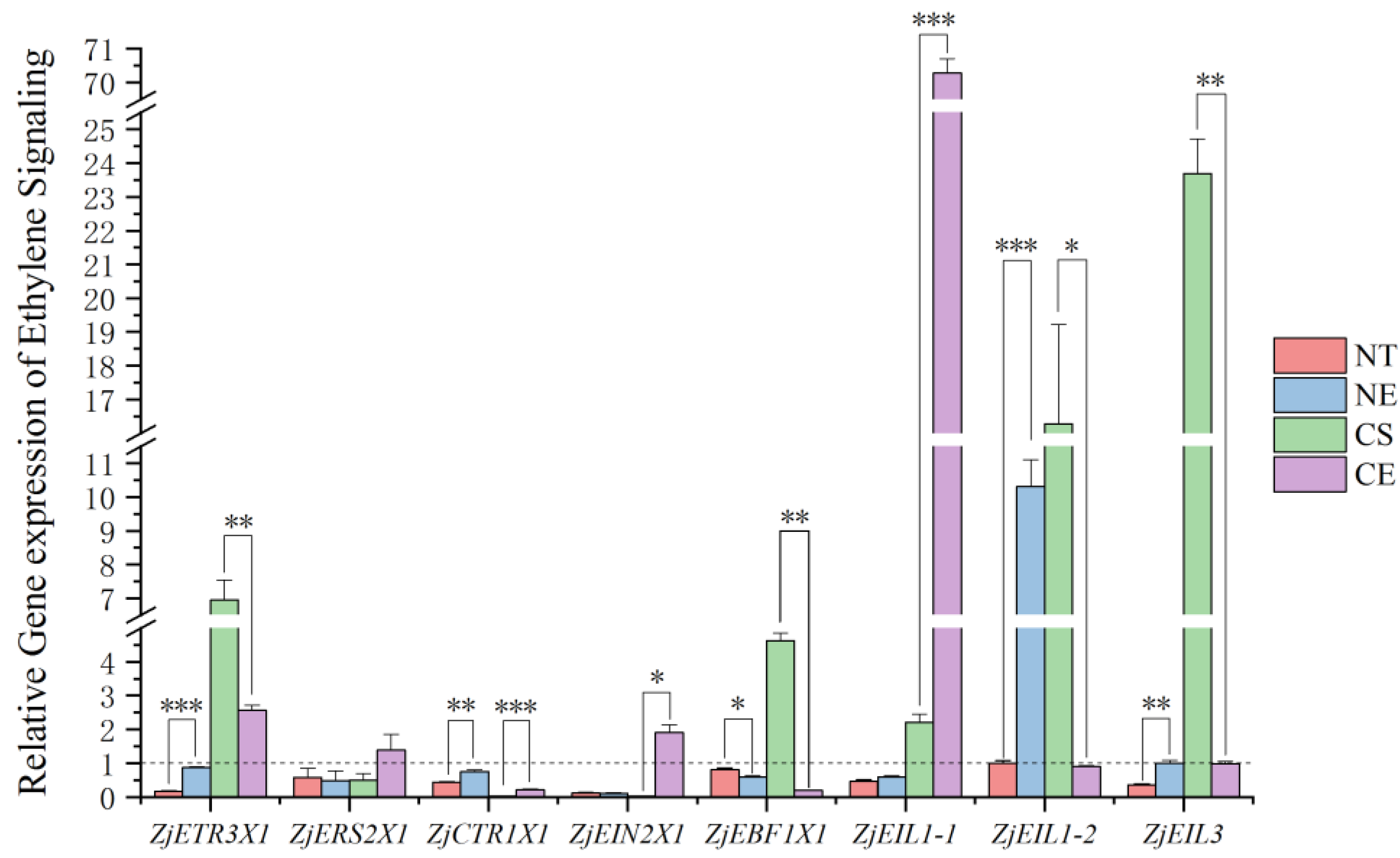
2.2. Effect of Ethephon Application on Ethylene Signaling under Non-Stressed Conditions and Cold Stress
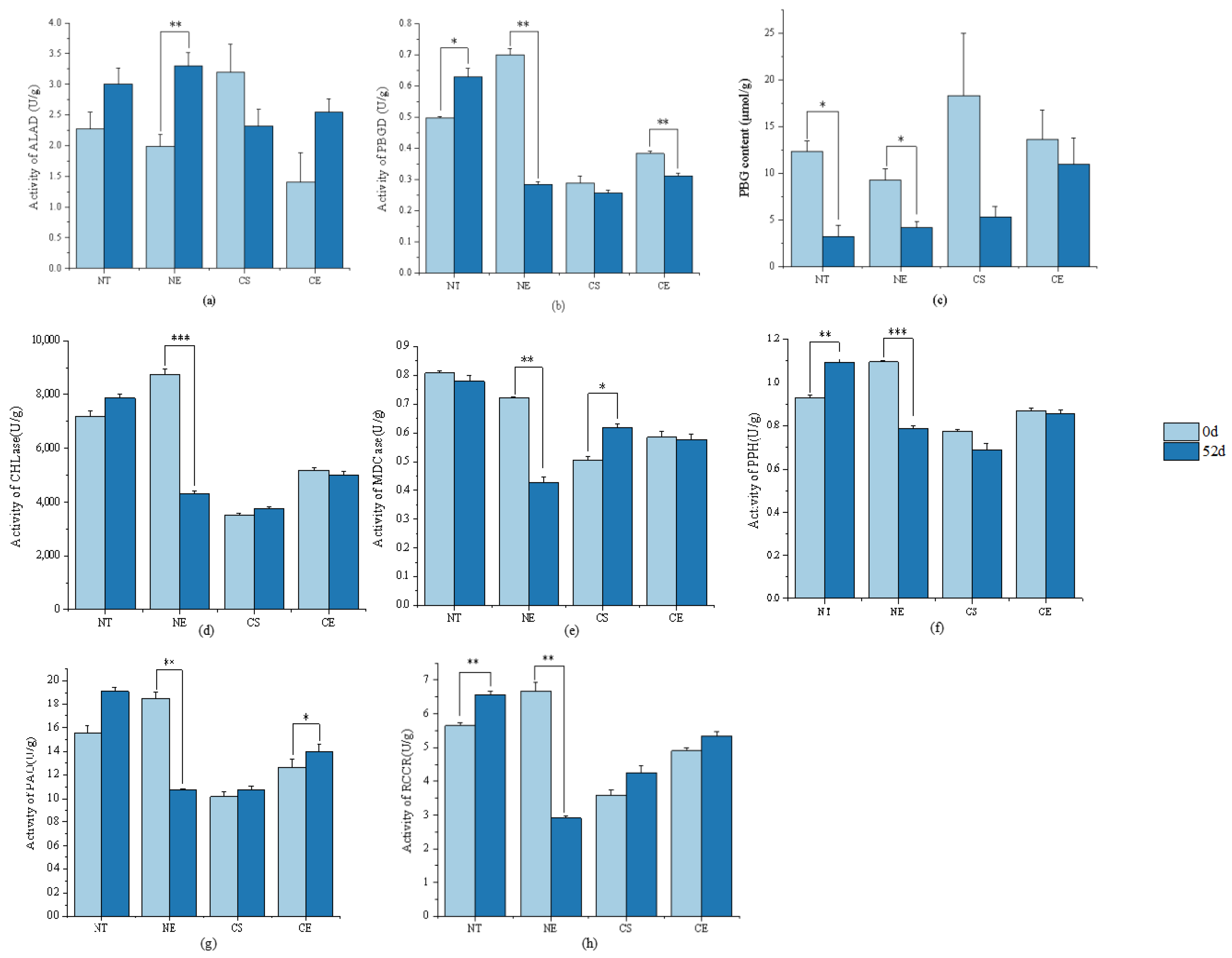
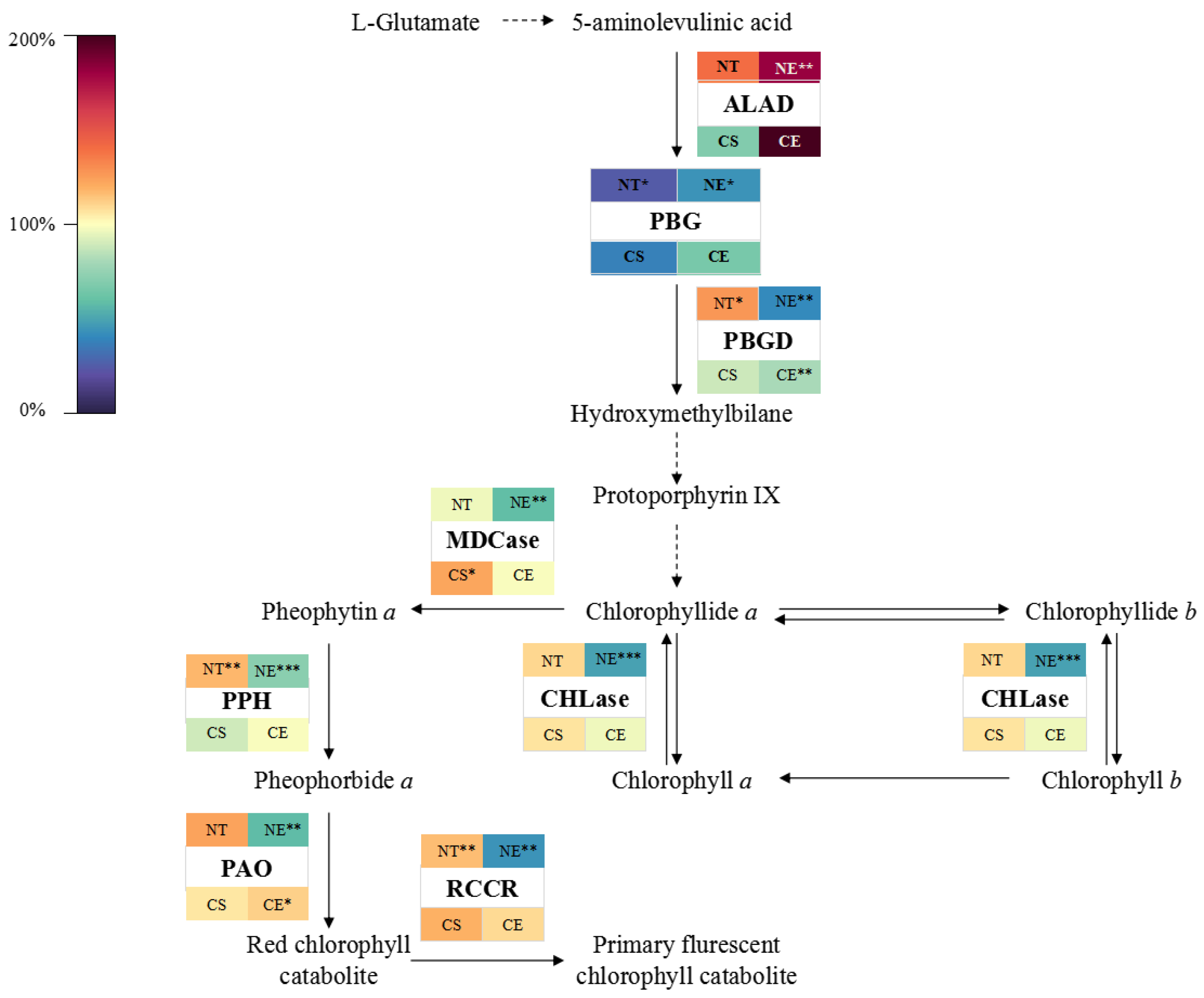
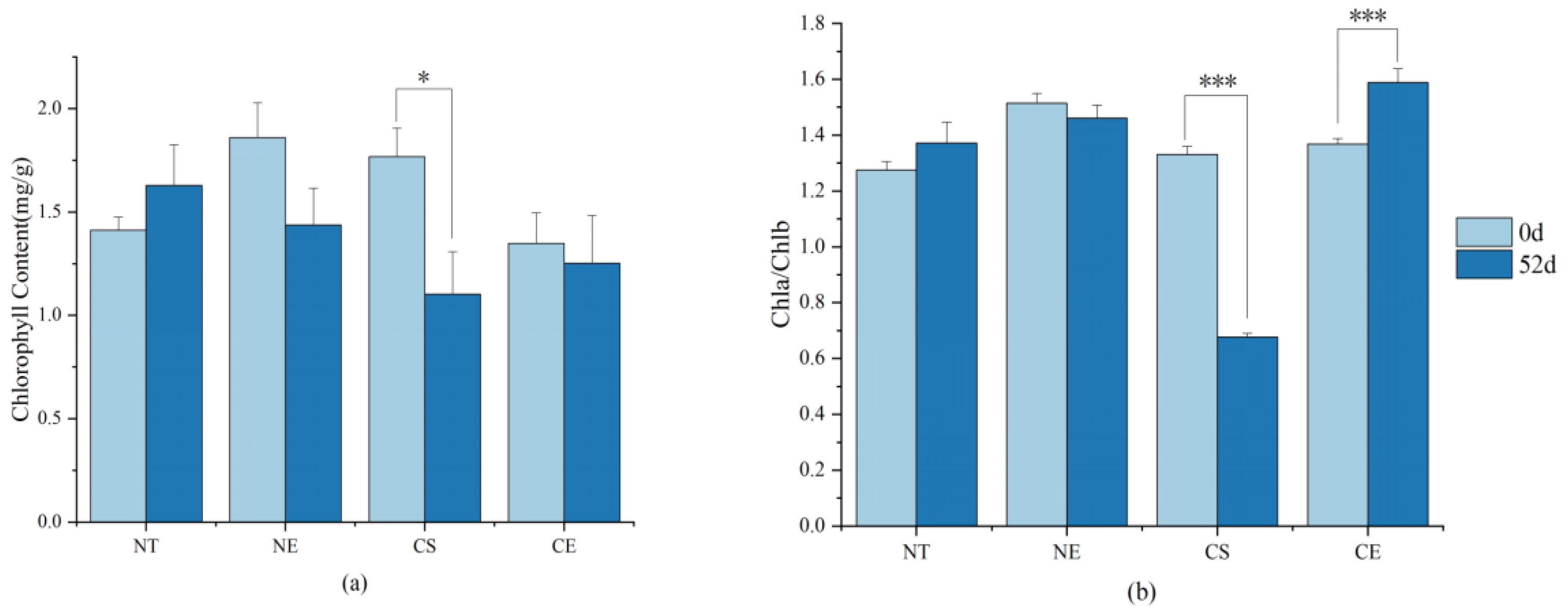
2.3. Effect of Ethephon Application on Chlorophyll Metabolism
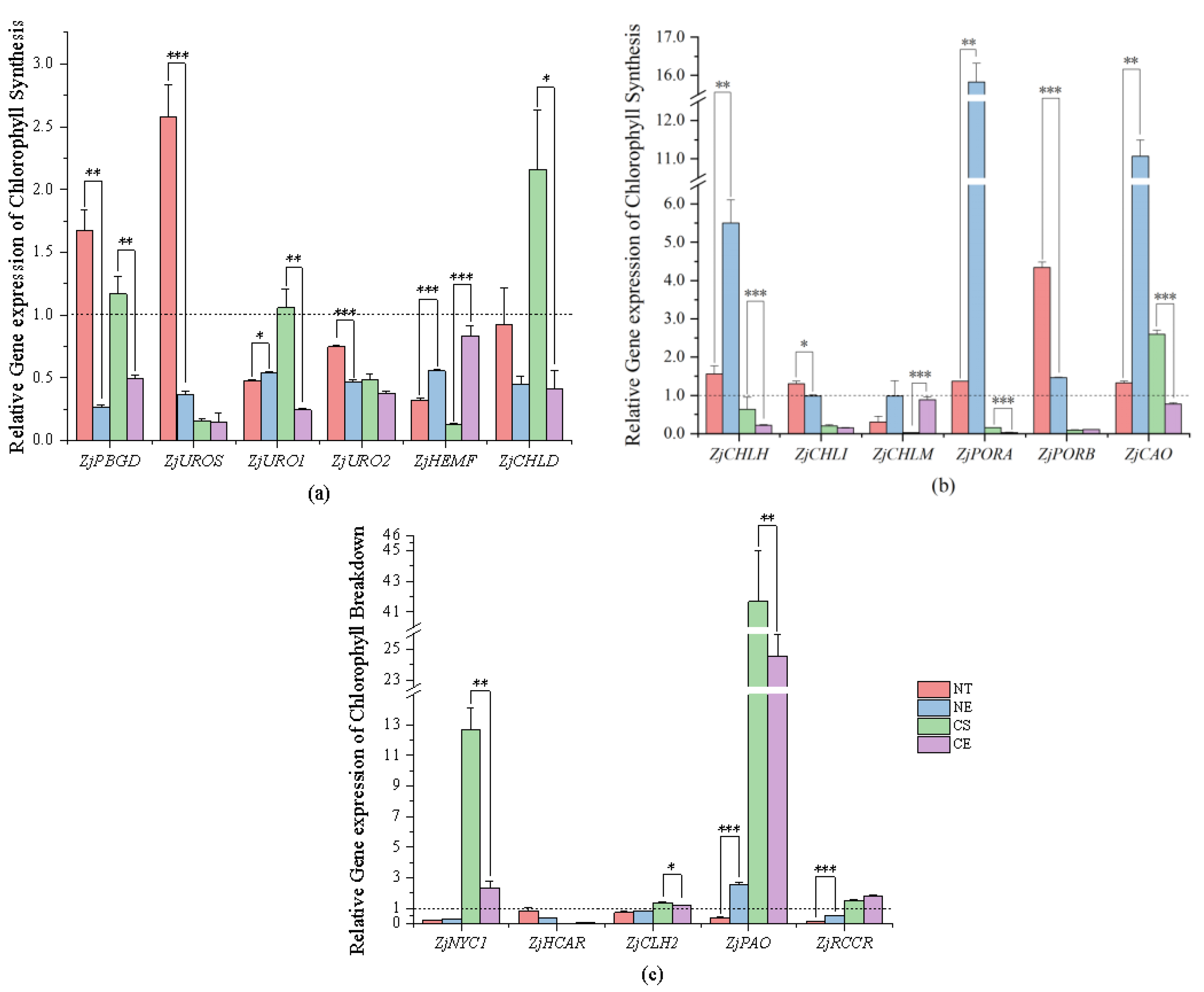
2.3.1. Effect of Ethephon Application on Chlorophyll Metabolism under Non-Stressed Conditions
2.3.2. Effect of Ethephon Application on Chlorophyll Metabolism under Cold Stress
3. Discussion
3.1. Ethephon Plays a Positive Role in Ethylene Releasing and Ethylene Signaling and It May Regulate Ethylene Biosynthesis in a Negative Feedback Loop in Z. japonica Leaves
3.2. Ethephon-Induced Ethylene Plays a Positive Role in Maintaining Chlorophyll Content in Z. japonica Leaves
4. Materials and Methods
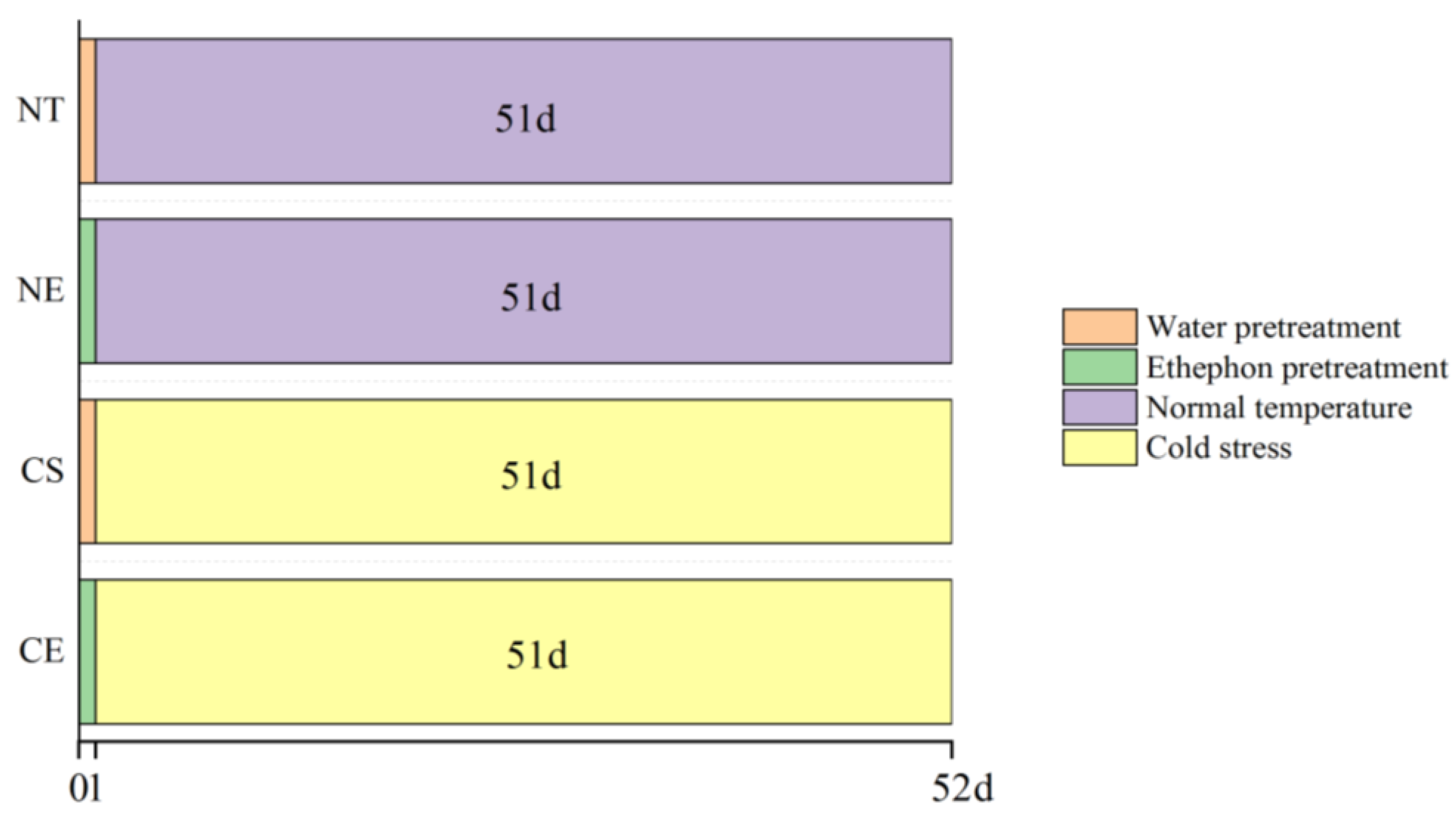
4.1. Plant Materials and Growth Conditions
4.2. Determination of Chlorophyll Content in Leaves
4.3. Method for Determination of Ethylene Production Rate
4.4. Determination of Enzyme Activities
4.5. Determination of the Ethylene Precursor and Chlorophyll Precursor
4.6. Methods for Determination of Relative Gene Expression
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manasa, S.L.; Panigrahy, M.; Panigrahi, K.C.S.; Rout, G.R. Overview of Cold Stress Regulation in Plants. Bot. Rev. 2022, 88, 359–387. [Google Scholar] [CrossRef]
- Bilska, A.; Sowiński, P. Closure of plasmodesmata in maize (Zea mays) at low temperature: A new mechanism for inhibition of photosynthesis. Ann. Bot. 2010, 106, 675–686. [Google Scholar] [CrossRef]
- Mandal, R.; Dutta, G. From photosynthesis to biosensing: Chlorophyll proves to be a versatile molecule. Sens. Int. 2020, 1, 100058. [Google Scholar] [CrossRef]
- Tanaka, R.; Tanaka, A. Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2011, 1807, 968–976. [Google Scholar] [CrossRef]
- Eckhardt, U.; Grimm, B.; Hörtensteiner, S. Recent advances in chlorophyll biosynthesis and breakdown in higher plants. Plant Mol. Biol. 2004, 56, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Han, Q.; Ding, C.; Huang, Y.; Liao, J.; Chen, T.; Feng, S.; Zhou, L.; Zhang, Z.; Chen, Y.; et al. Effect of Low Temperature on Chlorophyll Biosynthesis and Chloroplast Biogenesis of Rice Seedlings during Greening. Int. J. Mol. Sci. 2020, 21, 1390. [Google Scholar] [CrossRef] [PubMed]
- Brzezowski, P.; Ksas, B.; Havaux, M.; Grimm, B.; Chazaux, M.; Peltier, G.; Johnson, X.; Alric, J. The function of PROTOPORPHYRINOGEN IX OXIDASE in chlorophyll biosynthesis requires oxidised plastoquinone in Chlamydomonas reinhardtii. Commun. Biol. 2019, 2, 159. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, M.; Ito, H.; Morita, R.; Iida, S.; Sato, Y.; Fujimoto, M.; Kawasaki, S.; Tanaka, R.; Hirochika, H.; Nishimura, M.; et al. Rice NON-YELLOW COLORING1 Is Involved in Light-Harvesting Complex II and Grana Degradation during Leaf Senescence. Plant Cell 2007, 19, 1362–1375. [Google Scholar] [CrossRef]
- Meguro, M.; Ito, H.; Takabayashi, A.; Tanaka, R.; Tanaka, A. Identification of the 7-hydroxymethyl chlorophyll a reductase of the chlorophyll cycle in Arabidopsis. Plant Cell 2011, 23, 3442–3453. [Google Scholar] [CrossRef] [PubMed]
- Kuai, B.; Chen, J.; Hörtensteiner, S. The biochemistry and molecular biology of chlorophyll breakdown. J. Exp. Bot. 2018, 69, 751–767. [Google Scholar] [CrossRef]
- Shimoda, Y.; Ito, H.; Tanaka, A. Arabidopsis STAY-GREEN, Mendel’s Green Cotyledon Gene, Encodes Magnesium-Dechelatase. Plant Cell 2016, 28, 2147–2160. [Google Scholar] [CrossRef]
- Schelbert, S.; Aubry, S.; Burla, B.; Agne, B.; Kessler, F.; Krupinska, K.; Hörtensteiner, S. Pheophytin Pheophorbide Hydrolase (Pheophytinase) Is Involved in Chlorophyll Breakdown during Leaf Senescence in Arabidopsis. Plant Cell 2009, 21, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Pružinská, A.; Tanner, G.; Anders, I.; Roca, M.; Hörtensteiner, S. Chlorophyll breakdown: Pheophorbide a oxygenase is a Rieske-type iron–sulfur protein, encoded by the accelerated cell death 1 gene. Proc. Natl. Acad. Sci. USA 2003, 100, 15259–15264. [Google Scholar] [CrossRef]
- Pruzinská, A.; Anders, I.; Aubry, S.; Schenk, N.; Tapernoux-Lüthi, E.; Müller, T.; Kräutler, B.; Hörtensteiner, S. In Vivo Participation of Red Chlorophyll Catabolite Reductase in Chlorophyll Breakdown. Plant Cell 2007, 19, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Schelbert, S.; Aubry, S.; Süssenbacher, I.; Müller, T.; Kräutler, B.; Hörtensteiner, S. MES16, a Member of the Methylesterase Protein Family, Specifically Demethylates Fluorescent Chlorophyll Catabolites during Chlorophyll Breakdown in Arabidopsis. Plant Physiol. 2012, 158, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Hauenstein, M.; Christ, B.; Das, A.; Aubry, S.; Hörtensteiner, S. A Role for TIC55 as a Hydroxylase of Phyllobilins, the Products of Chlorophyll Breakdown during Plant Senescence. Plant Cell 2016, 28, 2510–2527. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Khan, M.I.R. Ethylene Potentiates Sulfur-Mediated Reversal of Cadmium Inhibited Photosynthetic Responses in Mustard. Front. Plant Sci. 2016, 7, 01628. [Google Scholar] [CrossRef] [PubMed]
- Zierer, W.; Hajirezaei, M.R.; Eggert, K.; Sauer, N.; von Wirén, N.; Pommerrenig, B. Phloem-Specific Methionine Recycling Fuels Polyamine Biosynthesis in a Sulfur-Dependent Manner and Promotes Flower and Seed Development. Plant Physiol. 2016, 170, 790–806. [Google Scholar] [CrossRef]
- Hua, J.; Meyerowitz, E.M. Ethylene Responses Are Negatively Regulated by a Receptor Gene Family in Arabidopsis thaliana. Cell 1998, 94, 261–271. [Google Scholar] [CrossRef]
- Tieman, D.M.; Taylor, M.G.; Ciardi, J.A.; Klee, H.J. The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc. Natl. Acad. Sci. USA 2000, 97, 5663–5668. [Google Scholar] [CrossRef]
- Cao, W.; Dong, Y.; Zhang, J.; Chen, S. Characterization of an ethylene receptor homolog gene from rice. Sci. China Ser. C Life Sci. 2003, 46, 370–378. [Google Scholar] [CrossRef]
- Watanabe, H.; Saigusa, M.; Hase, S.; Hayakawa, T.; Satoh, S. Cloning of a cDNA encoding an ETR2-like protein (Os-ERL1) from deep water rice (Oryza sativa L.) and increase in its mRNA level by submergence, ethylene, and gibberellin treatments. J. Exp. Bot. 2004, 55, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Yau, C.P.; Wang, L.; Yu, M.; Zee, S.Y.; Yip, W.K. Differential expression of three genes encoding an ethylene receptor in rice during development, and in response to indole-3-acetic acid and silver ions. J. Exp. Bot. 2004, 55, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Yoon, G.M.; Shemansky, J.M.; Lin, D.Y.; Ying, Z.I.; Chang, J.; Garrett, W.M.; Kessenbrock, M.; Groth, G.; Tucker, M.L.; et al. CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 19486–19491. [Google Scholar] [CrossRef]
- Qiao, H.; Shen, Z.; Huang, S.C.; Schmitz, R.J.; Urich, M.A.; Briggs, S.P.; Ecker, J.R. Processing and Subcellular Trafficking of ER-Tethered EIN2 Control Response to Ethylene Gas. Science 2012, 338, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, C.; Ji, Y.; Zhao, Q.; He, W.; An, F.; Jiang, L.; Guo, H. Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Res. 2012, 22, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Chang, C. Ethylene Signalling: The CTR1 Protein Kinase; McManus, M.T., Ed.; John Wiley & Sons, Inc.: Palmerston North, New Zealand, 2012; pp. 147–168. [Google Scholar]
- Guo, H.; Ecker, J.R. Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 2003, 115, 667–677. [Google Scholar] [CrossRef]
- An, F.; Zhao, Q.; Ji, Y.; Li, W.; Jiang, Z.; Yu, X.; Zhang, C.; Han, Y.; He, W.; Liu, Y.; et al. Ethylene-Induced Stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 Is Mediated by Proteasomal Degradation of EIN3 Binding F-Box 1 and 2 That Requires EIN2 in Arabidopsis. Plant Cell 2010, 22, 2384–2401. [Google Scholar] [CrossRef]
- Fujimoto, S.Y.; Ohta, M.; Usui, A.; Shinshi, H.; Ohme-Takagi, M. Arabidopsis Ethylene-Responsive Element Binding Factors Act as Transcriptional Activators or Repressors of GCC Box–Mediated Gene Expression. Plant Cell 2000, 12, 393–404. [Google Scholar] [CrossRef]
- Tieman, D.M.; Ciardi, J.A.; Taylor, M.G.; Klee, H.J. Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development. Plant J. 2001, 26, 47–58. [Google Scholar] [CrossRef]
- Tournier, B.; Sanchez-Ballesta, M.T.; Jones, B.; Pesquet, E.; Regad, F.; Latché, A.; Pech, J.; Bouzayen, M. New members of the tomato ERF family show specific expression pattern and diverse DNA-binding capacity to the GCC box element. FEBS Lett. 2003, 550, 149–154. [Google Scholar] [CrossRef]
- Liu, M.; Gomes, B.L.; Mila, I.; Purgatto, E.; Peres, L.E.P.; Frasse, P.; Maza, E.; Zouine, M.; Roustan, J.; Bouzayen, M.; et al. Comprehensive Profiling of Ethylene Response Factor Expression Identifies Ripening-Associated ERF Genes and Their Link to Key Regulators of Fruit Ripening in Tomato. Plant Physiol. 2016, 170, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Kim, S.T.; Gupta, R. Ethylene: A Master Regulator of Salinity Stress Tolerance in Plants. Biomolecules 2020, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol. Biol. 2010, 73, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, H.; Pan, X.; Chen, X.; Zhang, Z.; Lu, X.; Huang, R. Overexpression of ethylene response factor TERF2 confers cold tolerance in rice seedlings. Transgenic Res. 2011, 20, 857–866. [Google Scholar] [CrossRef]
- Teng, K.; Tan, P.; Guo, W.; Yue, Y.; Fan, X.; Wu, J. Heterologous Expression of a Novel Zoysia japonica C2H2 Zinc Finger Gene, ZjZFN1, Improved Salt Tolerance in Arabidopsis. Front. Plant Sci. 2018, 9, 01159. [Google Scholar] [CrossRef]
- Guan, J.; Teng, K.; Yue, Y.; Guo, Y.; Liu, L.; Yin, S.; Han, L. Zoysia japonica Chlorophyll b Reductase Gene NOL Participates in Chlorophyll Degradation and Photosynthesis. Front. Plant Sci. 2022, 13, 906018. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Zhu, J.; Huang, N.; Bian, X.; Li, H.; Wang, L.; Han, L. Polyamines and ethylene metabolism during cold acclimation in zoysiagrass (Zoysia Japonica Steud.). Acta Physiol. Plant. 2020, 42, 138. [Google Scholar] [CrossRef]
- Teng, K.; Tan, P.; Guan, J.; Dong, D.; Liu, L.; Guo, Y.; Guo, W.; Yuesen, Y.; Fan, X.; Wu, J. Functional characterization of the chlorophyll b reductase gene NYC1 associated with chlorophyll degradation and photosynthesis in Zoysia japonica. Environ. Exp. Bot. 2021, 191, 104607. [Google Scholar] [CrossRef]
- Liu, W.; Yu, K.; He, T.; Li, F.; Zhang, D.; Liu, J.; Wang, Z.; Huang, J. The Low Temperature Induced Physiological Responses of Avena nuda L., a Cold-Tolerant Plant Species. Sci. World J. 2013, 2013, 658793. [Google Scholar] [CrossRef]
- Cooke, A.R.; Randall, D.I. 2-Haloethanephosphonic acids as ethylene releasing agents for the induction of flowering in pineapples. Nature 1968, 218, 974–975. [Google Scholar] [CrossRef]
- Edgerton, L.J.; Blanpied, G.D. Regulation of Growth and Fruit Maturation with 2-Chloroethanephosphonic Acid. Nature 1968, 219, 1064–1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Islam, M.T.; Sapkota, S.; Ravindran, P.; Kumar, P.P.; Artlip, T.S.; Sherif, S.M. Ethylene-Mediated Modulation of Bud Phenology, Cold Hardiness, and Hormone Biosynthesis in Peach (Prunus persica). Plants 2021, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Z.; Liu, W.; Li, L.; Han, L.; Xu, L.; Zhao, Y. Transcriptome Analysis Revealed a Positive Role of Ethephon on Chlorophyll Metabolism of Zoysia japonica under Cold Stress. Plants 2022, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R. Sites of ethylene production in the pollinated and unpollinated senescing carnation (Dianthus caryophyllus) inflorescence. Planta 1977, 135, 155–159. [Google Scholar] [CrossRef]
- Shi, G.; Guo, X.; Li, C.; Fan, B.; Shi, J.; Bao, M. Changes on Ethylene Release and ACC Content of Peony Flowers at Different Development Stages. Acta Hortic. Sin. 2010, 37, 77–82. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Vaseva, I.; Vissenberg, K.; Van Der Straeten, D. Ethylene in vegetative development: A tale with a riddle. New Phytol. 2012, 194, 895–909. [Google Scholar] [CrossRef]
- Pattyn, J.; Vaughan-Hirsch, J.; Van de Poel, B. The regulation of ethylene biosynthesis: A complex multilevel control circuitry. New Phytol. 2021, 229, 770–782. [Google Scholar] [CrossRef]
- Wang, X.; Meng, H.; Tang, Y.; Zhang, Y.; He, Y.; Zhou, J.; Meng, X. Phosphorylation of an ethylene response factor by MPK3/MPK6 mediates negative feedback regulation of pathogen-induced ethylene biosynthesis in Arabidopsis. J. Genet. Genom. 2022, 49, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Malladi, A.; Doyle, J.W.; Scherm, H.; Nambeesan, S.U. The Effect of Ethephon, Abscisic Acid, and Methyl Jasmonate on Fruit Ripening in Rabbiteye Blueberry (Vaccinium virgatum). Horticulturae 2018, 4, 24. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, J.; Ren, H.; Li, Y.; Li, C.; Wang, H.; Wang, L.; Yang, Y.; Wang, X.; Hao, X. Exogenous Activation of the Ethylene Signaling Pathway Enhances the Freezing Tolerance of Young Tea Shoots by Regulating the Plant’s Antioxidant System. Horticulturae 2023, 9, 875. [Google Scholar] [CrossRef]
- Cui, Y.; Zhai, Y.; Flaishman, M.; Li, J.; Chen, S.; Zheng, C.; Ma, H. Ethephon induces coordinated ripening acceleration and divergent coloration responses in fig (Ficus carica L.) flowers and receptacles. Plant Mol. Biol. 2021, 105, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, H.; Mao, Z.; Liu, W.; Jiang, S.; Xu, H.; Su, M.; Zhang, J.; Wang, N.; Zhang, Z.; et al. Ethylene increases the cold tolerance of apple via the MdERF1B–MdCIbHLH1 regulatory module. Plant J. 2021, 106, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tang, M.; Huang, Z.; Song, J.; Xu, J.; Ahammed, G.J.; Yu, J.; Zhou, Y. The miR164a-NAM3 module confers cold tolerance by inducing ethylene production in tomato. Plant J. 2022, 111, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Griffith, M.; Wiseman, S.B. Ethylene Induces Antifreeze Activity in Winter Rye Leaves. Plant Physiol. 2001, 126, 1232–1240. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Sidhu, G.P.S.; Kumar, R.; Kohli, S.K.; Yadav, P.; Kapoor, D.; Bali, A.S.; Shahzad, B.; Khanna, K.; et al. Abiotic Stress Management in Plants: Role of Ethylene. Molecular In Plant Abiotic Stress: Biology and Biotechnology; Roychoudhury, A., Tripath, D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 185–208. [Google Scholar]
- Wei, C.; Ma, L.; Cheng, Y.; Guan, Y.; Guan, J. Exogenous ethylene alleviates chilling injury of ‘Huangguan’ pear by enhancing the proline content and antioxidant activity. Sci. Hortic. 2019, 257, 108671. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, S.; Hou, L.; Huang, X.; Zhang, X.; Guo, H.; Yang, S. Ethylene Signaling Negatively Regulates Freezing Tolerance by Repressing Expression of CBF and Type-A ARR Genes in Arabidopsis. Plant Cell 2012, 24, 2578–2595. [Google Scholar] [CrossRef]
- Ju, C.; Chang, C. Mechanistic Insights in Ethylene Perception and Signal Transduction. Plant Physiol. 2015, 169, 85–95. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Tanaka, A. Tetrapyrrole Biosynthesis in Higher Plants. Annu. Rev. Plant Biol. 2007, 58, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Hörtensteiner, S. Chlorophyll Degradation during Senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, C. Ethylene-mediated signaling confers thermotolerance and regulates transcript levels of heat shock factors in rice seedlings under heat stress. Bot. Stud. 2019, 60, 23. [Google Scholar] [CrossRef] [PubMed]
- Spano, A.J.; Timko, M.P. Isolation, characterization and partial amino acid sequence of a chloroplast-localized porphobilinogen deaminase from pea (Pisum sativum L.). Biochim. Et Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1991, 1076, 29–36. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Xie, J.; Li, J.; Zhang, J.; Yu, J.; Hu, L.; Zhang, G. The CaALAD Gene From Pepper (Capsicum annuum L.) Confers Chilling Stress Tolerance in Transgenic Arabidopsis Plants. Front. Plant Sci. 2022, 13, 884990. [Google Scholar] [CrossRef] [PubMed]
- Duggan, J.X.; Meller, E.; Gassman, M.L. Catabolism of Porphobilinogen by Etiolated Barley Leaves 1. Plant Physiol. 1982, 69, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Chen, L.; Meng, X.; Zhen, X.; Liang, Y.; Han, Y.; Li, H.; Zhang, B. Identification and function analysis of yellow-leaf mutant (YX-yl) of broomcorn millet. BMC Plant Biol. 2022, 22, 463. [Google Scholar] [CrossRef]
- Quesada, V.; Sarmiento-Mañús, R.; González-Bayón, R.; Hricová, A.; Ponce, M.R.; Micol, J.L. PORPHOBILINOGEN DEAMINASE Deficiency Alters Vegetative and Reproductive Development and Causes Lesions in Arabidopsis. PLoS ONE 2013, 8, e53378. [Google Scholar] [CrossRef]
- Al Ubeed, H.M.S.; Wills, R.B.H.; Bowyer, M.C.; Golding, J.B. Comparison of hydrogen sulphide with 1-methylcyclopropene (1-MCP) to inhibit senescence of the leafy vegetable, pak choy. Postharvest Biol. Technol. 2018, 137, 129–133. [Google Scholar] [CrossRef]
- Song, L.; Yi, R.; Luo, H.; Jiang, L.; Gu, S.; Yu, Z. Postharvest 1-methylcyclopropene application delays leaf yellowing of pak choi (Brassica rapa subsp. chinensis) by improving chloroplast antioxidant capacity and maintaining chloroplast structural integrity during storage at 20 °C. Sci. Hortic. 2020, 270, 109466. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, L.; Zhang, Y.; Guan, J. Effects of 1-MCP on softening, yellowing and H2O2 content in post-harvest ‘Jingbaili’ pear fruit during and after cold storage. Hortic. Environ. Biotechnol. 2014, 55, 404–409. [Google Scholar] [CrossRef]
- Tanaka, A.; Tanaka, R. Chapter Six—The biochemistry, physiology, and evolution of the chlorophyll cycle. Adv. Bot. Res. 2019, 90, 183–212. [Google Scholar] [CrossRef]
- Blankenship, R.E.; Chen, M. Spectral expansion and antenna reduction can enhance photosynthesis for energy production. Curr. Opin. Chem. Biol. 2013, 17, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Friedland, N.; Negi, S.; Vinogradova-Shah, T.; Wu, G.; Ma, L.; Flynn, S.; Kumssa, T.; Lee, C.H.; Sayre, R.T. Fine-tuning the photosynthetic light harvesting apparatus for improved photosynthetic efficiency and biomass yield. Sci. Rep. 2019, 9, 13028. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Li, M.; Duan, S.; Fu, M.; Dong, X.; Liu, B.; Feng, D.; Wang, J.; Wang, H. Optimization of Light-Harvesting Pigment Improves Photosynthetic Efficiency. Plant Physiol. 2016, 172, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, Y.; Schelbert, S.; Park, S.; Han, S.; Lee, B.; Andrès, C.B.; Kessler, F.; Hörtensteiner, S.; Paek, N. STAY-GREEN and Chlorophyll Catabolic Enzymes Interact at Light-Harvesting Complex II for Chlorophyll Detoxification during Leaf Senescence in Arabidopsis. Plant Cell 2012, 24, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhao, M.; Shi, T.; Shi, H.; An, F.; Zhao, Q.; Guo, H. EIN3/EIL1 cooperate with PIF1 to prevent photo-oxidation and to promote greening of Arabidopsis seedlings. Proc. Natl. Acad. Sci. USA 2009, 106, 21431–21436. [Google Scholar] [CrossRef]
- Zhong, S.; Shi, H.; Xi, Y.; Guo, H. Ethylene is crucial for cotyledon greening and seedling survival during de-etiolation. Plant Signal. Behav. 2010, 5, 739–742. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, M.S.; Bhowmick, T.K.; Gayen, K. Extraction of chlorophylls and carotenoids from dry and wet biomass of isolated Chlorella Thermophila: Optimization of process parameters and modelling by artificial neural network. Process Biochem. 2020, 96, 58–72. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Yan, J.; Wang, K.; Luo, H.; Zhang, W. MiR319 mediated salt tolerance by ethylene. Plant Biotechnol. J. 2019, 17, 2370–2383. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Li, Y.; Tan, H.; Yang, F.; Ma, N.; Gao, J. Expression of ethylene biosynthetic and receptor genes in rose floral tissues during ethylene-enhanced flower opening. J. Exp. Bot. 2008, 59, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Kohl, T.O.; Ascoli, C.A. Immunometric Double-Antibody Sandwich Enzyme-Linked Immunosorbent Assay. Cold Spring Harb. Protoc. 2017, 6, pdb.prot093724. [Google Scholar] [CrossRef] [PubMed]
- Mauzerall, D.; Granick, S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J. Biol. Chem. 1956, 219, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, L. [122] Porphyrin synthesis. Methods Enzymol. 1962, 5, 885–895. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, L.; Zhang, Z.; Han, L.; Xu, L. The Effect of Ethephon on Ethylene and Chlorophyll in Zoysia japonica Leaves. Int. J. Mol. Sci. 2024, 25, 1663. https://doi.org/10.3390/ijms25031663
Zhang J, Li L, Zhang Z, Han L, Xu L. The Effect of Ethephon on Ethylene and Chlorophyll in Zoysia japonica Leaves. International Journal of Molecular Sciences. 2024; 25(3):1663. https://doi.org/10.3390/ijms25031663
Chicago/Turabian StyleZhang, Jiahang, Lijing Li, Zhiwei Zhang, Liebao Han, and Lixin Xu. 2024. "The Effect of Ethephon on Ethylene and Chlorophyll in Zoysia japonica Leaves" International Journal of Molecular Sciences 25, no. 3: 1663. https://doi.org/10.3390/ijms25031663
APA StyleZhang, J., Li, L., Zhang, Z., Han, L., & Xu, L. (2024). The Effect of Ethephon on Ethylene and Chlorophyll in Zoysia japonica Leaves. International Journal of Molecular Sciences, 25(3), 1663. https://doi.org/10.3390/ijms25031663





