A Blood Test for the Diagnosis of Multiple Sclerosis
Abstract
1. Introduction
2. Results
2.1. Peptide Designing
2.2. Set-up of ELISA for Detection of Auto-Antibodies in MS Patients
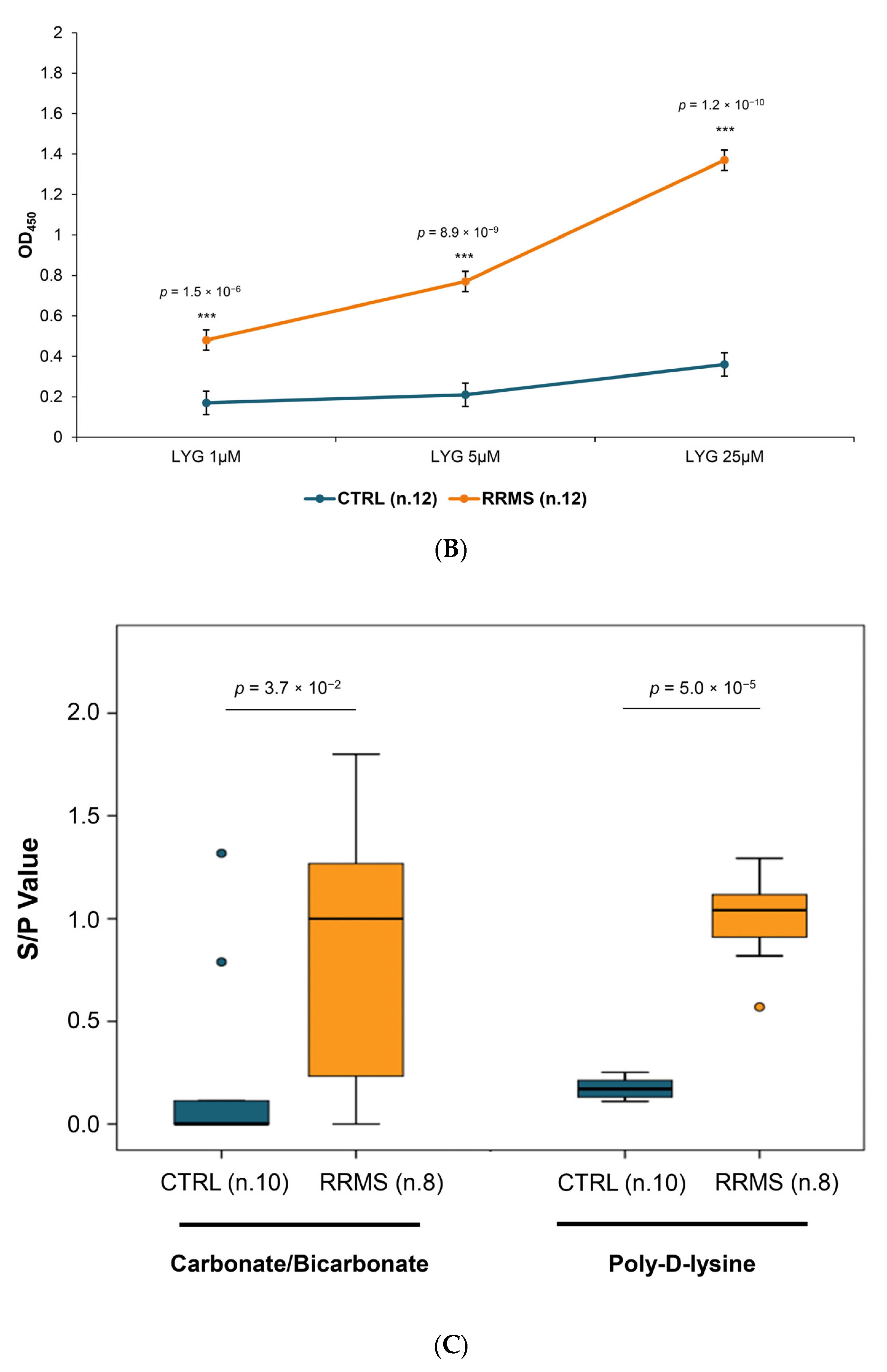
2.2.1. Establishment and Optimization of Peptide-ELISA
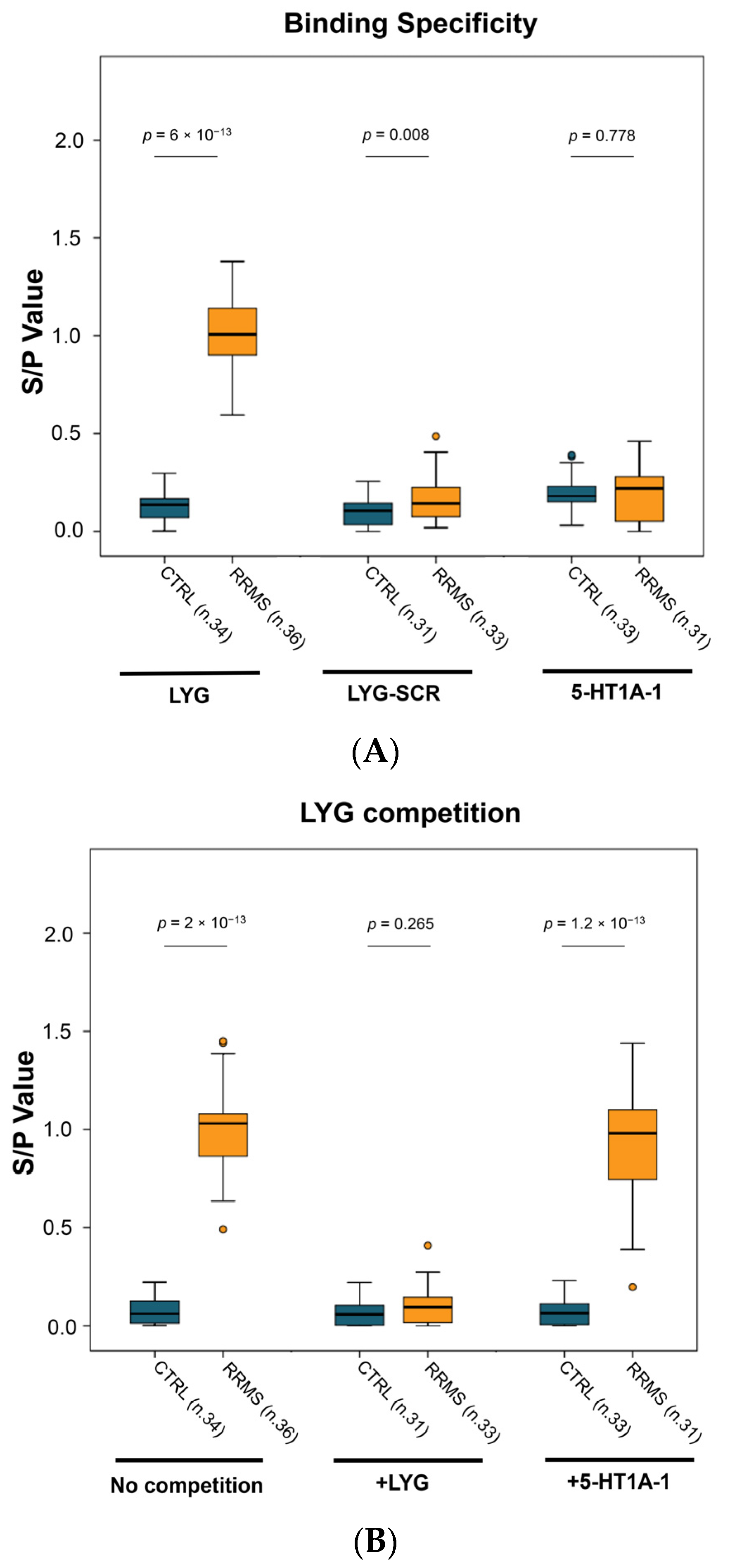
2.2.2. ELISA Specificity
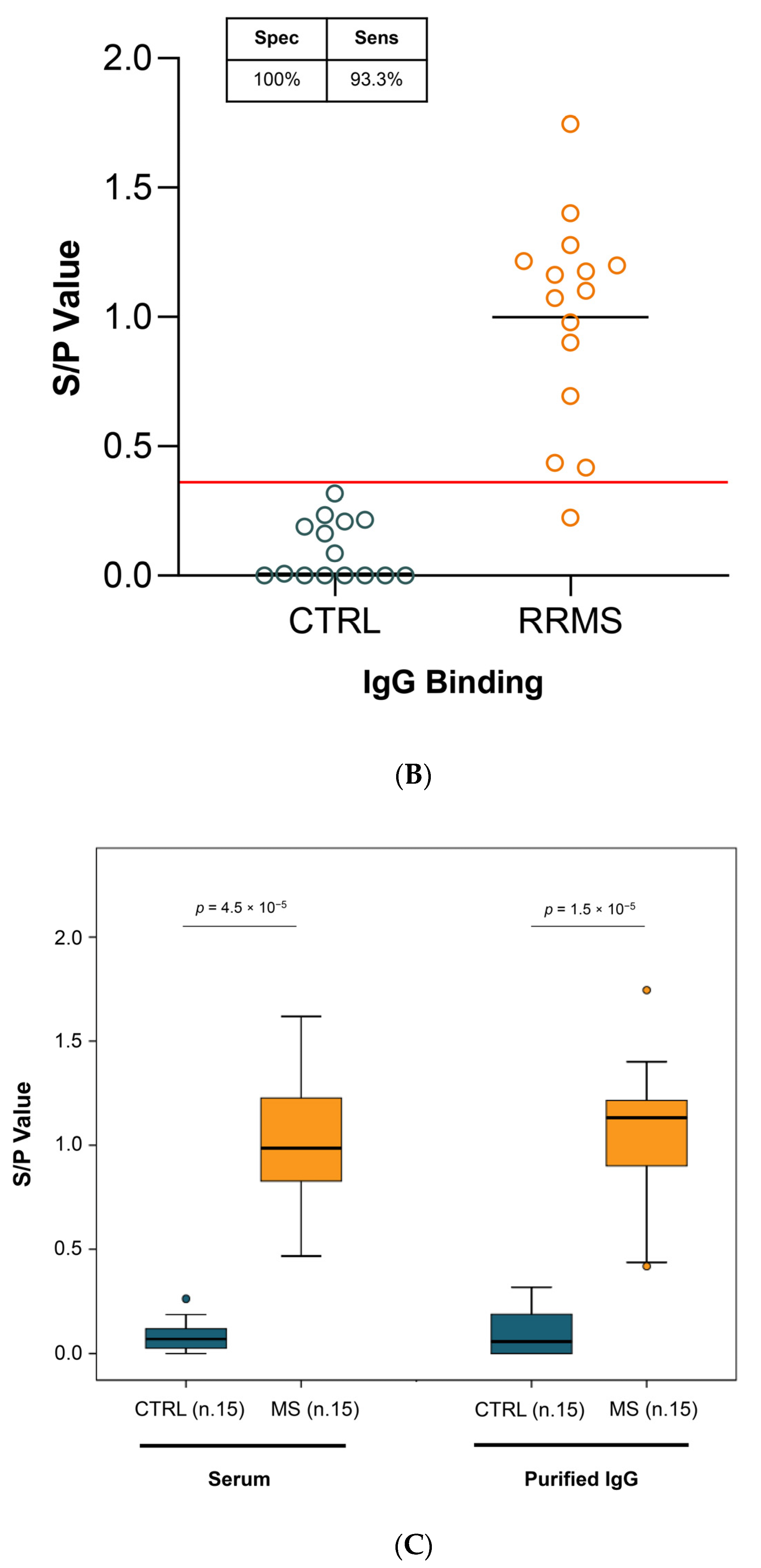
2.2.3. sIgG Binding
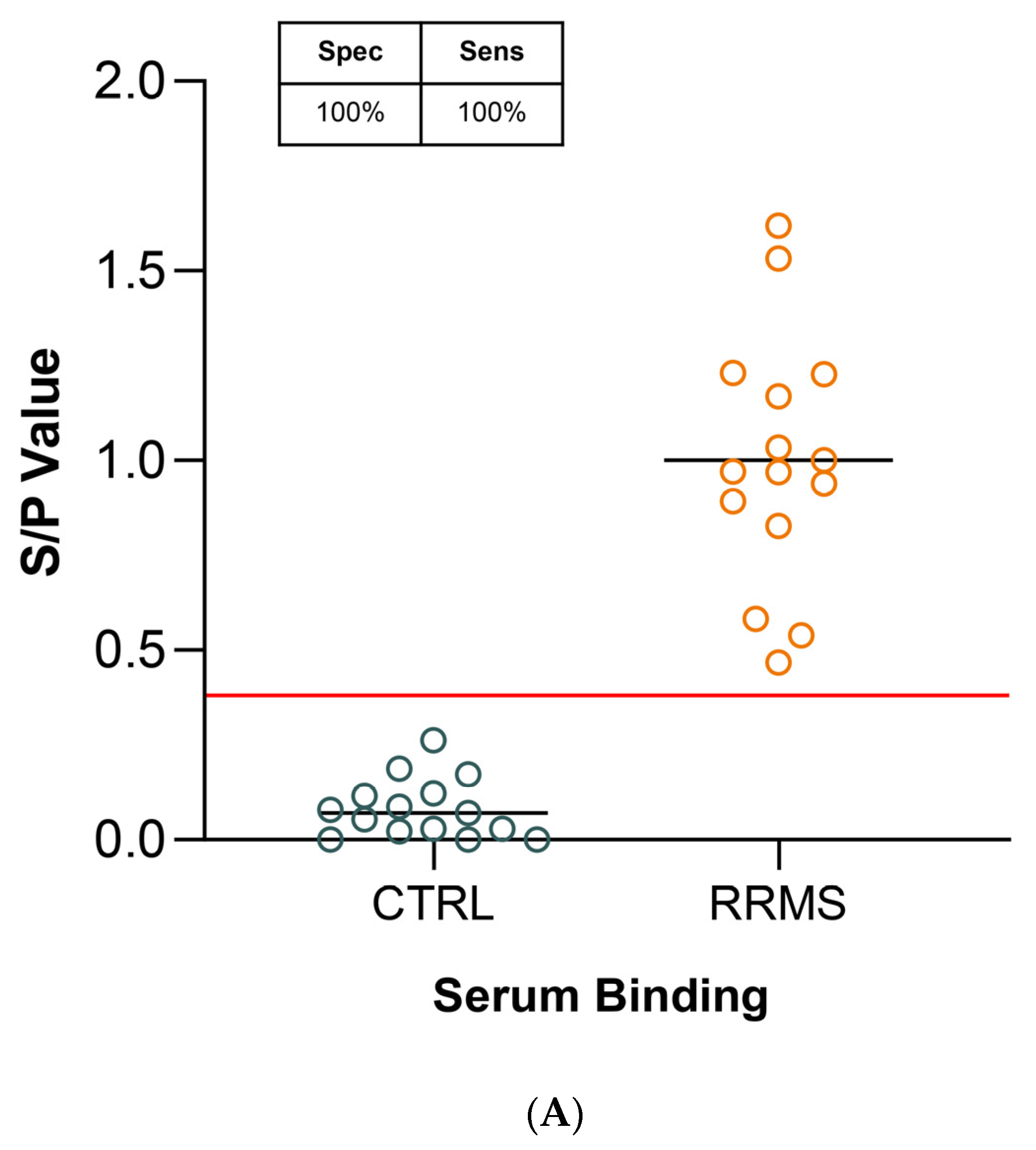
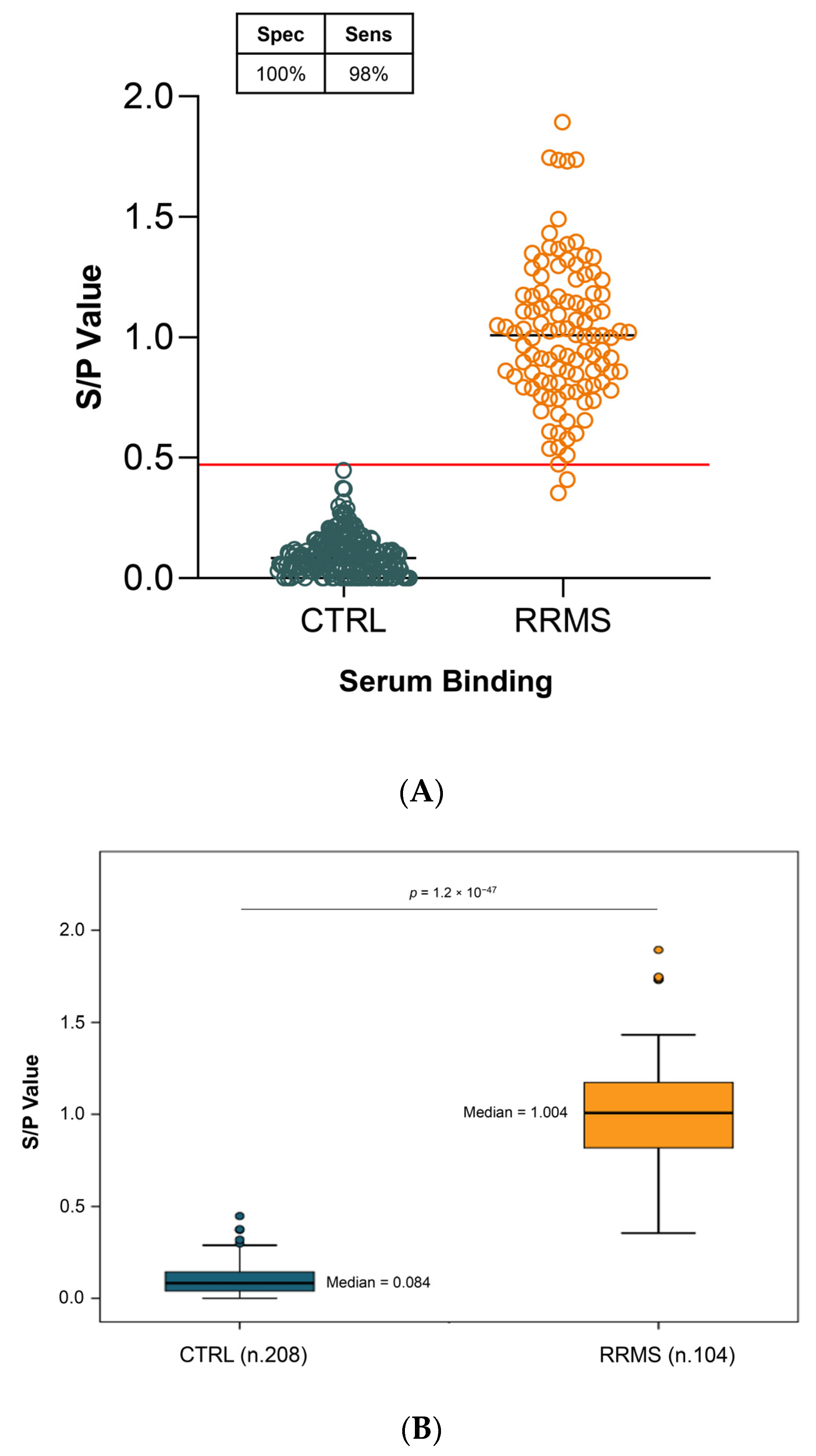
2.3. Diagnostic Sensitivity and Specificity
3. Discussion
4. Materials and Methods
4.1. Patients and Healthy Controls
4.2. Serum Sample Collection and Immunoglobulin Purification
4.3. PEPSCAN-Based ELISA
4.4. Synthetic Peptide Designing
4.5. Peptide ELISA Assay
4.6. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brownlee, W.J.; Hardy, T.A.; Fazekas, F.; Miller, D.H. Diagnosis of multiple sclerosis: Progress and challenges. Lancet 2017, 389, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.S.; Comi, G.; De Stefano, N.; Barkhof, F.; Polman, C.H.; Uitdehaag, B.M.; Lehr, L.; Stubinski, B.; Kappos, L. Moving toward earlier treatment of multiple sclerosis: Findings from a decade of clinical trials and implications for clinical practice. Mult. Scler. Relat. Disord. 2014, 3, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.J.; Corboy, J.R. The tension between early diagnosis and misdiagnosis of multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Kaisey, M.; Lashgari, G.; Fert-Bober, J.; Ontaneda, D.; Solomon, A.J.; Sicotte, N.L. An Update on Diagnostic Laboratory Biomarkers for Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2022, 22, 675–688. [Google Scholar] [CrossRef]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 25–36. [Google Scholar] [CrossRef]
- Levin, L.I.; Munger, K.L.; O’Reilly, E.J.; Falk, K.I.; Ascherio, A. Primary infection with the Epstein-Barr virus and risk of multiple sclerosis. Ann. Neurol. 2010, 67, 824–830. [Google Scholar] [CrossRef]
- Munger, K.L.; Levin, L.I.; O’Reilly, E.J.; Falk, K.I.; Ascherio, A. Anti-Epstein-Barr virus antibodies as serological markers of multiple sclerosis: A prospective study among United States military personnel. Mult. Scler. 2011, 17, 1185–1193. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L. EBV and Autoimmunity. Curr. Top. Microbiol. Immunol. 2015, 390, 365–385. [Google Scholar]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Haley, S.A.; Atwood, W.J. Progressive Multifocal Leukoencephalopathy: Endemic Viruses and Lethal Brain Disease. Annu. Rev. Virol. 2017, 4, 349–367. [Google Scholar] [CrossRef] [PubMed]
- Assetta, B.; Atwood, W.J. The biology of JC polyomavirus. Biol. Chem. 2017, 398, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Elphick, G.F.; Querbes, W.; Jordan, J.A.; Gee, G.V.; Eash, S.; Manley, K.; Dugan, A.; Stanifer, M.; Bhatnagar, A.; Kroeze, W.K.; et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science 2004, 306, 1380–1383. [Google Scholar] [CrossRef]
- Maginnis, M.S.; Haley, S.A.; Gee, G.V.; Atwood, W.J. Role of N-linked glycosylation of the 5-HT2A receptor in JC virus infection. J. Virol. 2010, 84, 9677–9684. [Google Scholar] [CrossRef] [PubMed]
- Assetta, B.; Maginnis, M.S.; Gracia Ahufinger, I.; Haley, S.A.; Gee, G.V.; Nelson, C.D.; O’Hara, B.A.; Allen Ramdial, S.A.; Atwood, W.J. 5-HT2 receptors facilitate JC polyomavirus entry. J. Virol. 2013, 87, 13490–13498. [Google Scholar] [CrossRef] [PubMed]
- Schaumburg, C.; O’Hara, B.A.; Lane, T.E.; Atwood, W.J. Human embryonic stem cell-derived oligodendrocyte progenitor cells express the serotonin receptor and are susceptible to JC virus infection. J. Virol. 2008, 82, 8896–8899. [Google Scholar] [CrossRef]
- Fan, L.W.; Bhatt, A.; Tien, L.T.; Zheng, B.; Simpson, K.L.; Lin, R.C.; Cai, Z.; Kumar, P.; Pang, Y. Exposure to serotonin adversely affects oligodendrocyte development and myelination in vitro. J. Neurochem. 2015, 133, 532–543. [Google Scholar] [CrossRef]
- Damiano, S.; La Rosa, G.; Sozio, C.; Cavaliere, G.; Trinchese, G.; Raia, M.; Paternò, R.; Mollica, M.P.; Avvedimento, V.E.; Santillo, M. 5-Hydroxytryptamine Modulates Maturation and Mitochondria Function of Human Oligodendrocyte Progenitor M03-13 Cells. Int. J. Mol. Sci. 2021, 22, 2621. [Google Scholar] [CrossRef]
- Markianos, M.; Koutsis, G.; Evangelopoulos, M.E.; Mandellos, D.; Karahalios, G.; Sfagos, C. Relationship of CSF neurotransmitter metabolite levels to disease severity and disability in multiple sclerosis. J. Neurochem. 2009, 108, 158–164. [Google Scholar] [CrossRef]
- Hofstetter, H.H.; Mössner, R.; Lesch, K.P.; Linker, R.A.; Toyka, K.V.; Gold, R. Absence of reuptake of serotonin influences susceptibility to clinical autoimmune disease and neuroantigen-specific interferon-gamma production in mouse EAE. Clin. Exp. Immunol. 2005, 142, 39–44. [Google Scholar] [CrossRef]
- O’Sullivan, D.; Green, L.; Stone, S.; Zareie, P.; Kharkrang, M.; Fong, D.; Connor, B.; La Flamme, A.C. Treatment with the antipsychotic agent, risperidone, reduces disease severity in experimental autoimmune encephalomyelitis. PLoS ONE 2014, 9, e104430. [Google Scholar] [CrossRef]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef] [PubMed]
- Geysen, H.M.; Rodda, S.J.; Mason, T.J.; Tribbick, G.; Schoofs, P.G. Strategies for epitope analysis using peptide synthesis. J. Immunol. Methods 1987, 102, 259–274. [Google Scholar] [CrossRef]
- Gegg, C.V.; Etzler, M.E. Directional coupling of synthetic peptides to poly-L-lysine and applications to the ELISA. Anal. Biochem. 1993, 210, 309–313. [Google Scholar] [CrossRef]
- Ball, J.M.; Henry, N.L.; Montelaro, R.C.; Newman, M.J. A versatile synthetic peptide-based ELISA for identifying antibody epitopes. J. Immunol. Methods 1994, 171, 37–44. [Google Scholar] [CrossRef]
- Stearns, N.A.; Zhou, S.; Petri, M.; Binder, S.R.; Pisetsky, D.S. The Use of Poly-L-Lysine as a Capture Agent to Enhance the Detection of Antinuclear Antibodies by ELISA. PLoS ONE 2016, 11, e0161818. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, R.S.; Bryant, S.C.; McKeon, A.; Weinshenker, B.; Murray, D.L.; Pittock, S.J.; Willrich, M.A.V. CSF Kappa Free Light Chains: Cutoff Validation for Diagnosing Multiple Sclerosis. Mayo Clin. Proc. 2022, 97, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Disanto, G.; Barro, C.; Benkert, P.; Naegelin, Y.; Schädelin, S.; Giardiello, A.; Zecca, C.; Blennow, K.; Zetterberg, H.; Leppert, D.; et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 2017, 81, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.R.; Garten, L.; Button, J.; O’Connor, J.; Kamath, V.; Frazier, C. Neurofilament light chain as an indicator of exacerbation prior to clinical symptoms in multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 31, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Dalla Costa, G.; Martinelli, V.; Sangalli, F.; Moiola, L.; Colombo, B.; Radaelli, M.; Leocani, L.; Furlan, R.; Comi, G. Prognostic value of serum neurofilaments in patients with clinically isolated syndromes. Neurology 2019, 92, e733–e741. [Google Scholar] [CrossRef] [PubMed]
- Ayrignac, X.; Le Bars, E.; Duflos, C.; Hirtz, C.; Maleska Maceski, A.; Carra-Dallière, C.; Charif, M.; Pinna, F.; Prin, P.; Menjot de Champfleur, N.; et al. Serum GFAP in multiple sclerosis: Correlation with disease type and MRI markers of disease severity. Sci. Rep. 2020, 10, 10923. [Google Scholar] [CrossRef]
- Niiranen, M.; Kontkanen, A.; Jääskeläinen, O.; Tertsunen, H.M.; Selander, T.; Hartikainen, P.; Huber, N.; Solje, E.; Haapasalo, A.; Kokkola, T.; et al. Serum GFAP and NfL levels in benign relapsing-remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 56, 103280. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Srivastava, R.; Aslam, M.; Kalluri, S.R.; Schirmer, L.; Buck, D.; Tackenberg, B.; Rothhammer, V.; Chan, A.; Gold, R.; Berthele, A.; et al. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N. Engl. J. Med. 2012, 367, 115–123. [Google Scholar] [CrossRef]
- Brickshawana, A.; Hinson, S.R.; Romero, M.F.; Lucchinetti, C.F.; Guo, Y.; Buttmann, M.; McKeon, A.; Pittock, S.J.; Chang, M.H.; Chen, A.P.; et al. Investigation of the KIR4.1 potassium channel as a putative antigen in patients with multiple sclerosis: A comparative study. Lancet Neurol. 2014, 13, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Brill, L.; Goldberg, L.; Karni, A.; Petrou, P.; Abramsky, O.; Ovadia, H.; Ben-Hur, T.; Karussis, D.; Vaknin-Dembinsky, A. Increased anti-KIR4.1 antibodies in multiple sclerosis: Could it be a marker of disease relapse? Mult. Scler. 2015, 21, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Chastre, A.; Hafler, D.A.; O’Connor, K.C. Evaluation of KIR4.1 as an Immune Target in Multiple Sclerosis. N. Engl. J. Med. 2016, 374, 1495–1496. [Google Scholar] [CrossRef] [PubMed]
- Ayoglu, B.; Häggmark, A.; Khademi, M.; Olsson, T.; Uhlén, M.; Schwenk, J.M.; Nilsson, P. Autoantibody profiling in multiple sclerosis using arrays of human protein fragments. Mol. Cell Proteom. 2013, 12, 2657–2672. [Google Scholar] [CrossRef] [PubMed]
- Ayoglu, B.; Mitsios, N.; Kockum, I.; Khademi, M.; Zandian, A.; Sjöberg, R.; Forsström, B.; Bredenberg, J.; Lima Bomfim, I.; Holmgren, E.; et al. Anoctamin 2 identified as an autoimmune target in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2016, 113, 2188–2193. [Google Scholar] [CrossRef] [PubMed]
- Tengvall, K.; Huang, J.; Hellström, C.; Kammer, P.; Biström, M.; Ayoglu, B.; Lima Bomfim, I.; Stridh, P.; Butt, J.; Brenner, N.; et al. Molecular mimicry between Anoctamin 2 and Epstein-Barr virus nuclear antigen 1 associates with multiple sclerosis risk. Proc. Natl. Acad. Sci. USA 2019, 116, 16955–16960. [Google Scholar] [CrossRef]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef]
- Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef]
- Geerligs, H.J.; Weijer, W.J.; Bloemhoff, W.; Welling, G.W.; Welling-Wester, S. The influence of pH and ionic strength on the coating of peptides of herpes simplex virus type 1 in an enzyme-linked immunosorbent assay. J. Immunol. Methods 1988, 106, 239–244. [Google Scholar] [CrossRef]
- Heyduk, E.; Hickey, R.; Pozzi, N.; Heyduk, T. Peptide ligand-based ELISA reagents for antibody detection. Anal. Biochem. 2018, 559, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Meng, X.; Zhang, J.; Xie, Q.; Zhang, W.; Li, T.; Shao, H.; Wan, Z.; Qin, A.; Ye, J. A novel S2-derived peptide-based ELISA for broad detection of antibody against infectious bronchitis virus. Poult. Sci. 2023, 102, 102661. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Zhang, X.; Zhang, W.; Lian, M.; Meng, X.; Li, T.; Xie, Q.; Shao, H.; Wan, Z.; Qin, A.; et al. A peptide-based ELISA for detection of antibodies against novel goose astrovirus type 1. J. Virol. Methods 2023, 312, 114646. [Google Scholar] [CrossRef] [PubMed]
- Slootstra, J.W.; Puijk, W.C.; Ligtvoet, G.J.; Langeveld, J.P.; Meloen, R.H. Structural aspects of antibody-antigen interaction revealed through small random peptide libraries. Mol. Divers. 1996, 1, 87–96. [Google Scholar] [CrossRef] [PubMed]


| Peptide Localization | Sequences PEPSCAN | Mean RRMS | Mean CTRL | p-Value |
|---|---|---|---|---|
| ECL-1 | LTILYGYRWPAASKL | 653 | 322 | 0.020 |
| ECL-1 | YRWPLPSKL | 566 | 286 | 0.027 |
| ECL-1 | LTILYGYRWPLPSKL | 647 | 340 | 0.03 |
| ECL-1 | GYRWPLPSK | 623 | 323 | 0.038 |
| N-term | TVDSENRTNLAAEGC | 587 | 348 | 0.043 |
| ECL-1 | TILYGYRWPLPSKLC | 652 | 378 | 0.046 |
| N-term | STTNSLMQLNAATRL | 612 | 385 | 0.047 |
| Sequence ECL-1 | Sequences PEPSCAN |
|---|---|
| LTILYGYRWP**SKLCAV | LTILYGYRWPAASKL |
| LTILYGYRWPLPSKLCAV | YRWPLPSKL |
| LTILYGYRWPLPSKLCAV | LTILYGYRWPLPSKL |
| LTILYGYRWPLPSKLCAV | GYRWPLPSK |
| LTILYGYRWPLPSKLCAV | TILYGYRWPLPSKLC |
| LTILYGYRWPLPSKLCAV | Sequence PRINDEX |
| Study Cohort | ||
|---|---|---|
| RRMS | CTRL | |
| Number (n°) | 104 | 208 |
| Females (n°; %) | 69 (66.6%) | 124 (59.7%) |
| Age (years ± SD) | 39.5 ± 8 | 42 ± 14 |
| Disease duration (years ± SD) | 9.9 ± 8.5 | |
| EDSS (mean ± SD) | 2.7 ± 1.9 | |
| Patients DMT treated | 93 (89.4%) | |
| DMT duration (years ± SD) | 8.01 ± 7.4 | |
| Patients relapse-free from DMT treatment | 85% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliano, P.; La Rosa, G.; Capozzi, S.; Cassano, E.; Damiano, S.; Habetswallner, F.; Iodice, R.; Marra, M.; Pavone, L.M.; Quarantelli, M.; et al. A Blood Test for the Diagnosis of Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 1696. https://doi.org/10.3390/ijms25031696
Giuliano P, La Rosa G, Capozzi S, Cassano E, Damiano S, Habetswallner F, Iodice R, Marra M, Pavone LM, Quarantelli M, et al. A Blood Test for the Diagnosis of Multiple Sclerosis. International Journal of Molecular Sciences. 2024; 25(3):1696. https://doi.org/10.3390/ijms25031696
Chicago/Turabian StyleGiuliano, Paola, Giuliana La Rosa, Serena Capozzi, Emanuele Cassano, Simona Damiano, Francesco Habetswallner, Rosa Iodice, Maurizio Marra, Luigi Michele Pavone, Mario Quarantelli, and et al. 2024. "A Blood Test for the Diagnosis of Multiple Sclerosis" International Journal of Molecular Sciences 25, no. 3: 1696. https://doi.org/10.3390/ijms25031696
APA StyleGiuliano, P., La Rosa, G., Capozzi, S., Cassano, E., Damiano, S., Habetswallner, F., Iodice, R., Marra, M., Pavone, L. M., Quarantelli, M., Vitelli, G., Santillo, M., & Paternò, R. (2024). A Blood Test for the Diagnosis of Multiple Sclerosis. International Journal of Molecular Sciences, 25(3), 1696. https://doi.org/10.3390/ijms25031696








