Transmission-Blocking Vaccines against Schistosomiasis Japonica
Abstract
:1. Introduction
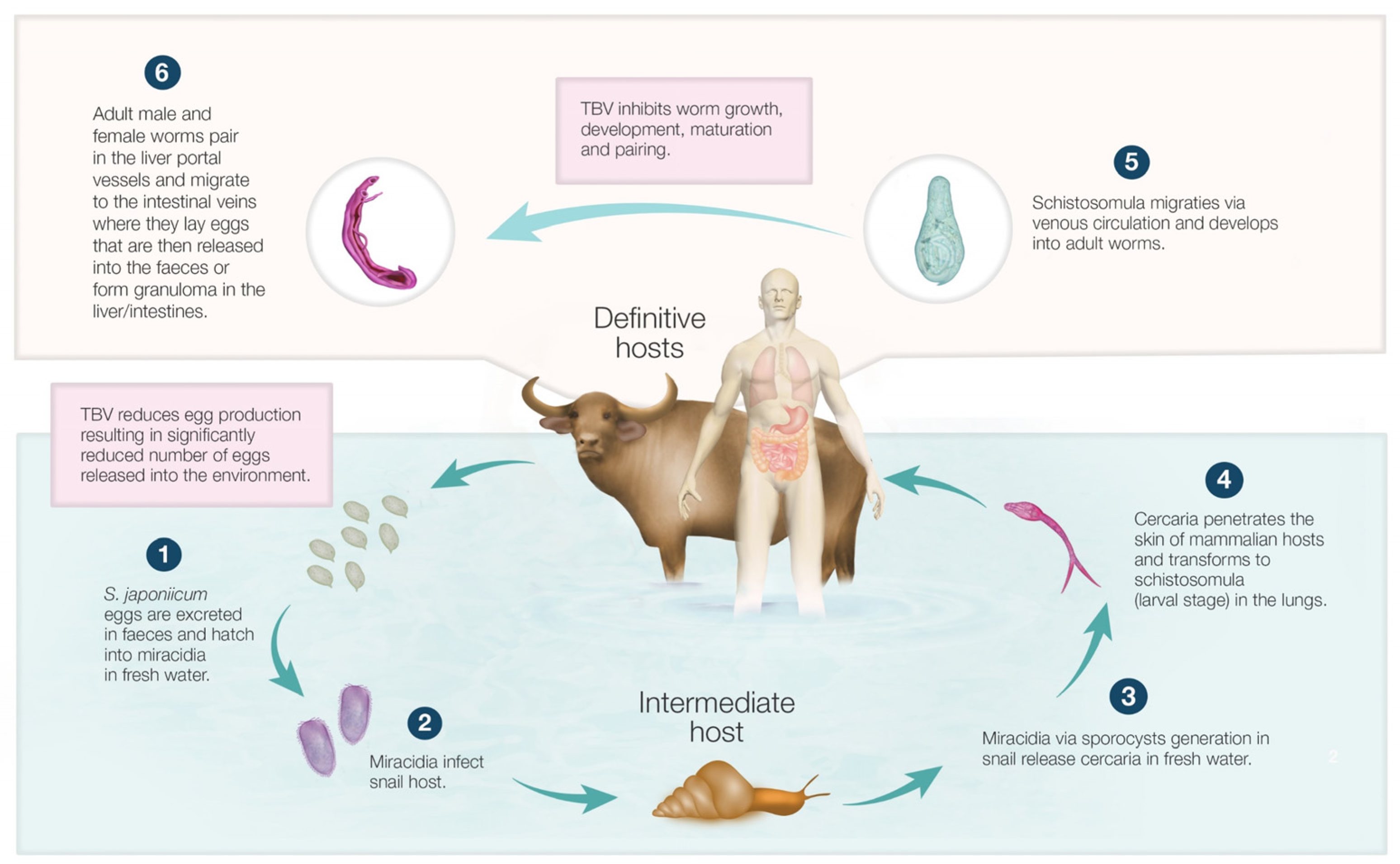
2. Life Cycle and Immunopathology
3. Rationale and Implications for the Development of a Veterinary-Based Transmission-Blocking Vaccine
4. The Role of Adjuvants in the Development of Schistosomiasis Veterinary Transmission-Blocking Vaccines
5. Current Status of Transmission-Blocking Vaccines against S. japonicum
5.1. Radiation-Attenuated Cercariae/Schistosomula Vaccines
5.2. S. japonicum Paramyosin (Sj97)
5.3. S. japonicum Triose Phosphate Isomerase (SjTPI)
5.4. S. japonicum 23-kDa Integral Membrane Protein (Sj23)
5.5. S. japonicum Glutathione-S-Transferase (SjGST)
6. Prospective Schistosoma japonicum Vaccine Candidates
6.1. S. japonicum Insulin Receptors
6.2. S. japonicum Calpains (Sj-p80)
| Candidate | Function | Immunization Strategy (Adjuvant) | Worm Burden Reduction (%) | Faecal Egg Reduction (%) | Liver Egg Reduction (%) | Intestinal Eggs Reduction (%) | Other Effects | Ref. |
|---|---|---|---|---|---|---|---|---|
| S. japonicum Calpain | Haemoglobin lysis, Inhibition of blood clotting, immune evasion. | rSm-p80 (GLA-se) | 46.75 | 4.74 | 39.9 | Balanced Th1 and Th2 responses. | [183] | |
| rSjp80 (Montanide ISA61) | 31.3–46.1 | 51.4 | 29.1–31.60% decrease in miracidial hatching. | [105] | ||||
| Ligand domain 1 and 2 of S. japonicum Insulin receptors | Glucose uptake. | rSjLD1 (Quil A) | 43.5–44 | 47–61 | ns, 44 | ns, 46 | 10–19% reduction in worm length, 56% reduction in intestinal granuloma | [104,107] |
| rSjLD1 (Montanide ISA 720) | 30 | 68 | 56 | 48 | [104] | |||
| rSjLD2 (Quil A) | - | 56–67 | 28 (ns) | 23 | 16–42% reduction in mean adult worm length | [175] | ||
| S. japonicum Triose Phosphate isomerase | Glucose metabolism. | rSjTPI (Montanide ISA 720) | ns | 51 (ns) | 21 (ns) | ns | [104] | |
| rAdV-SjTPI.opt | 36–50 | 41–52 | Th1 and Th2 cytokines induced, IgG levels elevated, Reduced liver granuloma | [144,145,185] | ||||
| rAdV-SjTPI.opt + rSjTPI boost | 72 | 72 | ||||||
| rAdV-SjTPI.opt + rAdV-SjTPI.opt boost | 44 | 57 | ||||||
| pcDNA-SjTPI.opt + rAdV-SjTPI.opt | 45.13 | 54.57 | ||||||
| S. japonicum membrane proteins | Regulation of cell-signalling, proliferation, adhesion, spreading, migration, and fusion. | pcDNA-Sj2-29 | 53.2 | 51.4 | Increased IgG, IL-4, and IFN-γ and reduction of the area of the granuloma | [186] | ||
| S. japonicum Glutathione-S-transferase | Detoxification of oxidative stress ligands. | pcDNA/Sj26GST | 62.02 | 17.08 (ns) | Increased titre of anti SjGST IgG. Induction of IL-2, IFN-γ, IL-4, and IL-10 | [110] | ||
| pcDNA/Sj26GST (IL-12) | 68.99 | 37.54 | Increased titre of anti SjGST IgG. Induction of IL-2, IFN-γ, IL-4, and IL-10 Increased IFN-γ, IL-2 | [110] | ||||
| pcDNA/Sj26GST + rSjGST | 72.33 | 34.46 | [110] | |||||
| rSj26GST (anti-CD25 mAb) | 47.09 | 50 | [65] | |||||
| S. japonicum Fatty acid binding protein | Binds and transports long-chain fatty acids, Larval migration, and immune evasion. | rSjFABP (Freund, anti-CD25 mAb) | 56.08 | 53.29 | Increased IFN-γ, IL-2, Il-4, and Il-5 | [63] | ||
| SjFABP (Freund’s, anti-CTLA-4 mAb) | 54.61 | 59.25 | [66] | |||||
| S. japonicum glyceraldehyde-3-phosphate dehydrogenase | Energy metabolism. | rSjGAPDH (anti-CTLA-4 mAb) | 59.36 | 62.45 | Increased IFN-γ, IL-2, Il-4, and Il-5 | [64] | ||
| S. japonicum Adenylate kinase 1 | Energy generation. | rSjAK1 (Freund) | 50 | 40 | 56% reduction in granuloma area. Increased IFN-γ and IL-2. | [187] | ||
| S. japonicum Lethal Giant Larvae | Apical-basal cell polarity, cell proliferation, differentiation, and tissue organization. | rSjLGL (ISA206) | 12.91–50.0 | 29.23–49.86 | 53–75% reduction in egg hatching rate. High IgG titre. | [188] | ||
| S. japonicum inhibitor apoptosis protein (SjIAP) | Regulates cell apoptosis. | Ad-rSjIAP | 34.4–41.5 | 23.8–39.6% | Increased IFN-γ, IL-2, IL-4, and IL-10 | [189] |
6.3. S. japonicum Glyceraldehyde Dehydrogenase (SjGAPDH)
6.4. S. japonicum Fatty Acid Binding Proteins (Sj14/SjFABP)
7. Challenges and Future Trends in the Development of Anti-Schistosome Vaccines
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020; p. 50.
- World Health Organization. Schistosomiasis. 1 February 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 1 October 2023).
- Ross, A.G.; Sleigh, A.C.; Li, Y.; Davis, G.M.; Williams, G.M.; Jiang, Z.; Feng, Z.; McManus, D.P. Schistosomiasis in the People’s Republic of China: Prospects and challenges for the 21st century. Clin. Microbiol. Rev. 2001, 14, 270–295. [Google Scholar] [CrossRef]
- McManus, D.P.; Bergquist, R.; Cai, P.; Ranasinghe, S.; Tebeje, B.M.; You, H. Schistosomiasis—From immunopathology to vaccines. Semin. Immunopathol. 2020, 42, 355–371. [Google Scholar] [CrossRef]
- Ogongo, P.; Nyakundi, R.K.; Chege, G.K.; Ochola, L. The Road to Elimination: Current State of Schistosomiasis Research and Progress Towards the End Game. Front. Immunol. 2022, 13, 846108. [Google Scholar] [CrossRef]
- Rajamanickam, A.; Munisankar, S.; Dolla, C.; Menon, P.A.; Nutman, T.B.; Babu, S. Helminth Coinfection Alters Monocyte Activation, Polarization, and Function in Latent Mycobacterium tuberculosis Infection. J. Immunol. 2020, 204, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Monin, L.; Griffiths, K.L.; Lam, W.Y.; Gopal, R.; Kang, D.D.; Ahmed, M.; Rajamanickam, A.; Cruz-Lagunas, A.; Zúñiga, J.; Babu, S.; et al. Helminth-induced arginase-1 exacerbates lung inflammation and disease severity in tuberculosis. J. Clin. Investig. 2015, 125, 4699–4713. [Google Scholar] [CrossRef] [PubMed]
- Diallo, T.O.; Remoue, F.; Schacht, A.-M.; Charrier, N.; Dompnier, J.P.; Pillet, S.; Garraud, O.; N’Diaye, A.A.; Capron, A.; Capron, M.; et al. Schistosomiasis co-infection in humans influences inflammatory markers in uncomplicated Plasmodium falciparum malaria. Parasite Immunol. 2004, 26, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.H. Impact of chronic schistosomiasis and HBV/HCV co-infection on the liver: Current perspectives. Hepat. Med. 2019, 11, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Bustinduy, A.; King, C.; Scott, J.; Appleton, S.; Sousa-Figueiredo, J.C.; Betson, M.; Stothard, J.R. HIV and schistosomiasis co-infection in African children. Lancet Infect. Dis. 2014, 14, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Kamau, E.; Yates, A.; Maisiba, R.; Singoei, V.; Opot, B.; Adeny, R.; Arima, C.O.; Otieno, V.; Sumbi, C.S.; Okoth, R.O.; et al. Epidemiological and clinical implications of asymptomatic malaria and schistosomiasis co-infections in a rural community in western Kenya. BMC Infect. Dis. 2021, 21, 937. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 34th meeting of the International Task Force for Disease Eradication, 19–20 September 2022. Weekly Epidemiol. Rec. 2023, 98, 41–50. [Google Scholar]
- Xu, J.; Dong, L.-L.; Sun, H.; Huang, P.; Zhang, R.-Z.; Wang, X.-Y.; Sun, D.-Q.; Xia, C.-M. Small change, big difference: A promising praziquantel derivative designated P96 with broad-spectrum antischistosomal activity for chemotherapy of schistosomiasis japonica. PLOS Neglected Trop. Dis. 2023, 17, e0011215. [Google Scholar] [CrossRef]
- Andressa Barban do, P. Schistosomiasis: Discovery of New Molecules for Disease Treatment and Vaccine Development. In New Horizons for Schistosomiasis Research; Tonay, I., Ed.; IntechOpen: Rijeka, Croatia, 2022; Chapter 6. [Google Scholar]
- Kura, K.; Truscott, J.E.; Toor, J.; Anderson, R.M. Modelling the impact of a Schistosoma mansoni vaccine and mass drug administration to achieve morbidity control and transmission elimination. PLOS Neglected Trop. Dis. 2019, 13, e0007349. [Google Scholar] [CrossRef]
- You, H.; Cai, P.; Tebeje, B.M.; Li, Y.; McManus, D.P. Schistosome Vaccines for Domestic Animals. Trop. Med. Infect. Dis. 2018, 3, 68. [Google Scholar] [CrossRef]
- Driciru, E.; Koopman, J.P.R.; Cose, S.; Siddiqui, A.A.; Yazdanbakhsh, M.; Elliott, A.M.; Roestenberg, M. Immunological Considerations for Schistosoma Vaccine Development: Transitioning to Endemic Settings. Front. Immunol. 2021, 12, 635985. [Google Scholar] [CrossRef]
- Hotez, P.J.; Bottazzi, M.E. Human Schistosomiasis Vaccines as Next Generation Control Tools. Trop. Med. Infect. Dis. 2023, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Santini-Oliveira, M.; Machado Pinto, P.; Santos, T.D.; Vilar, M.M.; Grinsztejn, B.; Veloso, V.; Paes-de-Almeida, E.C.; Amaral, M.A.Z.; Ramos, C.R.; Marroquin-Quelopana, M.; et al. Development of the Sm14/GLA-SE Schistosomiasis Vaccine Candidate: An Open, Non-Placebo-Controlled, Standardized-Dose Immunization Phase Ib Clinical Trial Targeting Healthy Young Women. Vaccines 2022, 10, 1724. [Google Scholar] [CrossRef] [PubMed]
- Keitel, W.A.; Potter, G.E.; Diemert, D.; Bethony, J.; El Sahly, H.M.; Kennedy, J.K.; Patel, S.M.; Plieskatt, J.L.; Jones, W.; Deye, G.; et al. A phase 1 study of the safety, reactogenicity, and immunogenicity of a Schistosoma mansoni vaccine with or without glucopyranosyl lipid A aqueous formulation (GLA-AF) in healthy adults from a non-endemic area. Vaccine 2019, 37, 6500–6509. [Google Scholar] [CrossRef] [PubMed]
- Riveau, G.; Schacht, A.M.; Dompnier, J.P.; Deplanque, D.; Seck, M.; Waucquier, N.; Senghor, S.; Delcroix-Genete, D.; Hermann, E.; Idris-Khodja, N.; et al. Safety and efficacy of the rSh28GST urinary schistosomiasis vaccine: A phase 3 randomized, controlled trial in Senegalese children. PLoS Neglected Trop. Dis. 2018, 12, e0006968. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Control of Schistosomiasis; WHO: Geneva, Switzerland, 1993. [Google Scholar]
- Van Dorssen, C.F.; Gordon, C.A.; Li, Y.; Williams, G.M.; Wang, Y.; Luo, Z.; Gobert, G.N.; You, H.; McManus, D.P.; Gray, D.J. Rodents, goats and dogs—Their potential roles in the transmission of schistosomiasis in China. Parasitology 2017, 144, 1633–1642. [Google Scholar] [CrossRef]
- Wang, T.-P.; Maria, V.J.; Zhang, S.-Q.; Wang, F.-F.; Wu, W.-D.; Zhang, G.-H.; Pan, X.-P.; Ju, Y.; Niels, Ø. Transmission of Schistosoma japonicum by humans and domestic animals in the Yangtze River valley, Anhui province, China. Acta Trop. 2005, 96, 198–204. [Google Scholar] [CrossRef]
- McManus, D.P.; Loukas, A. Current Status of Vaccines for Schistosomiasis. Clin. Microbiol. Rev. 2008, 21, 225–242. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, C.; Shi, Y.; Li, H.; Wang, L.; Qin, S.; Kang, S.; Huang, Y.; Jin, Y.; Lin, J. Surveillance of Schistosoma japonicum infection in domestic ruminants in the Dongting Lake region, Hunan province, China. PLoS ONE 2012, 7, e31876. [Google Scholar] [CrossRef]
- Gray, D.J.; Williams, G.M.; Li, Y.; Chen, H.; Li, R.S.; Forsyth, S.J.; Barnett, A.G.; Guo, J.; Feng, Z.; McManus, D.P. A cluster-randomized bovine intervention trial against Schistosoma japonicum in the People’s Republic of China: Design and baseline results. Am. J. Trop. Med. Hyg. 2007, 77, 866–874. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Gray, D.; Ning, A.; Hu, G.; Chen, H.; Davis, G.M.; Sleigh, A.C.; Feng, Z.; McManus, D.P.; et al. A drug-based intervention study on the importance of buffaloes for human Schistosoma japonicum infection around Poyang Lake, People’s Republic of China. Am. J. Trop. Med. Hyg. 2006, 74, 335–341. [Google Scholar] [CrossRef]
- Williams, G.M.; Li, Y.-S.; Gray, D.J.; Zhao, Z.-Y.; Harn, D.A.; Shollenberger, L.M.; Li, S.-M.; Yu, X.; Feng, Z.; Guo, J.-G.; et al. Field Testing Integrated Interventions for Schistosomiasis Elimination in the People’s Republic of China: Outcomes of a Multifactorial Cluster-Randomized Controlled Trial. Front. Immunol. 2019, 10, 645. [Google Scholar] [CrossRef]
- Gray, D.J.; Williams, G.M.; Li, Y.; McManus, D.P. Transmission Dynamics of Schistosoma japonicum in the Lakes and Marshlands of China. PLoS ONE 2009, 3, e4058. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.A.; Acosta, L.P.; Gray, D.J.; Olveda, R.M.; Jarilla, B.; Gobert, G.N.; Ross, A.G.; McManus, D.P. High prevalence of Schistosoma japonicum infection in Carabao from Samar Province, the Philippines: Implications for transmission and control. PLoS Neglected Trop. Dis. 2012, 6, e1778. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.J.; Williams, G.M.; Li, Y.; Chen, H.; Forsyth, S.J.; Li, R.S.; Barnett, A.G.; Guo, J.; Ross, A.G.; Feng, Z.; et al. A Cluster-Randomised Intervention Trial against Schistosoma japonicum in the Peoples’ Republic of China: Bovine and Human Transmission. PLoS ONE 2009, 4, e5900. [Google Scholar] [CrossRef] [PubMed]
- Budiono, N.G.; Satrija, F.; Ridwan, Y.; Handharyani, E.; Murtini, S. The contribution of domestic animals to the transmission of schistosomiasis japonica in the Lindu Subdistrict of the Central Sulawesi Province, Indonesia. Vet. World 2019, 12, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.K.; Candido, R.R.F.; Pierre, T.S.; Woodward, R.; Kusel, J.; Teixeira, C.G. Schistosome Egg. In Schistosoma: Biology, Pathology and Control, 1st ed.; Jamieson, B.G.M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 56–76. [Google Scholar]
- LoVerde, P.T. Schistosomiasis. In Digenetic Trematodes; Springer: Cham, Switzerland, 2019; Volume 1154, pp. 45–70. [Google Scholar] [CrossRef]
- Galanti, S.E.; Huang, S.C.-C.; Pearce, E.J. Cell Death and Reproductive Regression in Female Schistosoma mansoni. PLOS Neglected Trop. Dis. 2012, 6, e1509. [Google Scholar] [CrossRef]
- Kunz, W. Schistosome male–female interaction: Induction of germ-cell differentiation. Trends Parasitol. 2001, 17, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Luo, F.; You, Y.; Gu, M.; Yang, W.; Yi, C.; Zhang, W.; Feng, Z.; Wang, J.; Hu, W. MicroRNA-1 targets ribosomal protein genes to regulate the growth, development and reproduction of Schistosoma japonicum. Int. J. Parasitol. 2023, 53, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Qin, F.; Ren, Y.; Li, X.; Hou, L.; Gu, S.; Jin, Y. Functional characterization of differentially expressed proteins coming from unisexual and bisexual infected Schistosoma japonicum female worms. Exp. Parasitol. 2023, 248, 108504. [Google Scholar] [CrossRef]
- Cheever, A.W.; Macedonia, J.G.; Mosimann, J.E.; Cheever, E.A. Kinetics of egg production and egg excretion by Schistosoma mansoni and S. japonicum in mice infected with a single pair of worms. Am. J. Trop. Med. Hyg. 1994, 50, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.J.; MacDonald, A.S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002, 2, 499–511. [Google Scholar] [CrossRef]
- You, H.; McManus, D.P. Vaccines and diagnostics for zoonotic schistosomiasis japonica. Parasitology 2015, 142, 271–289. [Google Scholar] [CrossRef]
- Hedfi, M.; Debaibi, M.; Iahouel, S.B.; Chouchen, A. Gallbladder schistosomiasis: Rare but possible, a case report and review of the literature. Pan Afr. Med. J. 2019, 32, 91. [Google Scholar] [CrossRef]
- Olveda, D.U.; Li, Y.; Olveda, R.M.; Lam, A.K.; McManus, D.P.; Chau, T.N.P.; Harn, D.A.; Williams, G.M.; Gray, D.J.; Ross, A.G.P. Bilharzia in the Philippines: Past, present, and future. Int. J. Infect. Dis. 2014, 18, 52–56. [Google Scholar] [CrossRef]
- Molehin, A.J. Current Understanding of Immunity Against Schistosomiasis: Impact on Vaccine and Drug Development. Res. Rep. Trop. Med. 2020, 11, 119–128. [Google Scholar] [CrossRef]
- Colley, D.G.; Secor, W.E. Immunology of human schistosomiasis. Parasite Immunol. 2014, 36, 347–357. [Google Scholar] [CrossRef]
- Butrous, G. Schistosome infection and its effect on pulmonary circulation. Glob. Cardiol. Sci. Pract. 2019, 2019, 5. [Google Scholar] [CrossRef]
- Tebeje, B.M.; Harvie, M.; You, H.; Rivera, V.; McManus, D.P. T cell-mediated immunity in CBA mice during Schistosoma japonicum infection. Exp. Parasitol. 2019, 204, 107725. [Google Scholar] [CrossRef]
- Abdel Aziz, N.; Musaigwa, F.; Mosala, P.; Berkiks, I.; Brombacher, F. Type 2 immunity: A two-edged sword in schistosomiasis immunopathology. Trends Immunol. 2022, 43, 657–673. [Google Scholar] [CrossRef]
- Ye, Z.; Huang, S.; Zhang, Y.; Mei, X.; Zheng, H.; Li, M.; Chen, J.; Lu, F. Galectins, Eosinophiles, and Macrophages May Contribute to Schistosoma japonicum Egg-Induced Immunopathology in a Mouse Model. Front. Immunol. 2020, 11, 146. [Google Scholar] [CrossRef]
- Burke, M.L.; McManus, D.P.; Ramm, G.A.; Duke, M.; Li, Y.; Jones, M.K.; Gobert, G.N. Temporal expression of chemokines dictates the hepatic inflammatory infiltrate in a murine model of schistosomiasis. PLoS Neglected Trop. Dis. 2010, 4, e598. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.L.; Jones, M.K.; Gobert, G.N.; Li, Y.S.; Ellis, M.K.; McManus, D.P. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009, 31, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Llanwarne, F.; Helmby, H. Granuloma formation and tissue pathology in Schistosoma japonicum versus Schistosoma mansoni infections. Parasite Immunol. 2021, 43, e12778. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Guan, F.; Sun, L.; Zhang, Y.; Zhang, X.; Lu, S.; Liu, W. B cells induced by Schistosoma japonicum infection display diverse regulatory phenotypes and modulate CD4+ T cell response. Parasites Vectors 2020, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.J.; Qian, Q.F.; Zhou, J.R.; Sun, D.L.; Duan, Y.F.; Zhu, X.; Sartorius, K.; Lu, Y.J. Regulatory T cells (Tregs) in liver fibrosis. Cell Death Discov. 2023, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Y.; Liu, H.; Gong, W.; Hu, Y.; Shen, Y.; Cao, J. Granulocytic myeloid-derived suppressor cells inhibit T follicular helper cells during experimental Schistosoma japonicum infection. Parasites Vectors 2021, 14, 497. [Google Scholar] [CrossRef]
- Chen, X.; Yang, X.; Li, Y.; Zhu, J.; Zhou, S.; Xu, Z.; He, L.; Xue, X.; Zhang, W.; Dong, X.; et al. Follicular Helper T Cells Promote Liver Pathology in Mice during Schistosoma japonicum Infection. PLoS Pathog. 2014, 10, e1004097. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, J.; Chen, H.; Nie, H.; Miller, H.; Gong, Q.; Liu, C. T Lymphocyte-Mediated Liver Immunopathology of Schistosomiasis. Front. Immunol. 2020, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Tebeje, B.M. T Cell Mediated Immunity to Schistosoma japonicum Infection and Vaccination. Ph.D. Thesis, Faculty of Medicine, The University of Queensland, Brisbane, Australia, 2019. [Google Scholar]
- Jiz, M.; Friedman, J.F.; Leenstra, T.; Jarilla, B.; Pablo, A.; Langdon, G.; Pond-Tor, S.; Wu, H.W.; Manalo, D.; Olveda, R.; et al. Immunoglobulin E (IgE) responses to paramyosin predict resistance to reinfection with Schistosoma japonicum and are attenuated by IgG4. Infect. Immun. 2009, 77, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-L.; Lei, J.-H.; Wang, T.; Lu, S.-J.; Guan, F.; Liu, W.-Q.; Li, Y.-L. Effect of CD4+CD25+ regulatory T cells on the immune evasion of Schistosoma japonicum. Parasitol. Res. 2011, 108, 477–480. [Google Scholar] [CrossRef]
- Tang, C.-L.; Zhang, R.-H.; Liu, Z.-M.; Jin, H.; He, L. Effect of regulatory T cells on the efficacy of the fatty acid-binding protein vaccine against Schistosoma japonicum. Parasitol. Res. 2019, 118, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-L.; Yang, J.-F.; Pan, Q.; Zhang, R.-H.; Xie, Y.-P.; Xiong, Y.; Zhou, H.-H. Anti-CTLA-4 monoclonal antibody improves efficacy of the glyceraldehyde-3-phosphate dehydrogenase protein vaccine against Schistosoma japonicum in mice. Parasitol. Res. 2019, 118, 2287–2293. [Google Scholar] [CrossRef]
- Tang, C.-L.; Yang, J.; Cheng, L.-Y.; Cheng, L.-F.; Liu, Z.-M. Anti-CD25 monoclonal antibody enhances the protective efficacy of Schistosoma japonicum GST vaccine via inhibition of CD4+CD25+Foxp3+ regulatory T cells. Parasitol. Res. 2017, 116, 2727–2732. [Google Scholar] [CrossRef]
- Tang, C.-L.; Pan, Q.; Xie, Y.-P.; Xiong, Y.; Zhang, R.-H.; Huang, J. Effect of Cytotoxic T-Lymphocyte Antigen-4 on the Efficacy of the Fatty Acid-Binding Protein Vaccine against Schistosoma japonicum. Front. Immunol. 2019, 10, 1022. [Google Scholar] [CrossRef]
- McWilliam, H.E.G.; Piedrafita, D.; Li, Y.; Zheng, M.; He, Y.; Yu, X.; McManus, D.P.; Meeusen, E.N.T. Local immune responses of the Chinese water buffalo, Bubalus bubalis, against Schistosoma japonicum larvae: Crucial insights for vaccine design. PLoS Neglected Trop. Dis. 2013, 7, e2460. [Google Scholar] [CrossRef]
- He, C.; Mao, Y.; Zhang, X.; Li, H.; Lu, K.; Fu, Z.; Hong, Y.; Tang, Y.; Jin, Y.; Lin, J.; et al. High resistance of water buffalo against reinfection with Schistosoma japonicum. Vet. Parasitol. 2018, 261, 18–21. [Google Scholar] [CrossRef]
- Li, Y.-S.; McManus, D.P.; Lin, D.-D.; Williams, G.M.; Harn, D.A.; Ross, A.G.; Feng, Z.; Gray, D.J. The Schistosoma japonicum self-cure phenomenon in water buffaloes: Potential impact on the control and elimination of schistosomiasis in China. Int. J. Parasitol. 2014, 44, 167–171. [Google Scholar] [CrossRef]
- Da’dara, A.A.; Li, Y.S.; Xiong, T.; Zhou, J.; Williams, G.M.; McManus, D.P.; Feng, Z.; Yu, X.L.; Gray, D.J.; Harn, D.A. DNA-based vaccines protect against zoonotic schistosomiasis in water buffalo. Vaccine 2008, 26, 3617–3625. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.W.; Qin, Y.F.; Chu, K.; Meng, R.; Liu, Y.; McGarvey, S.T.; Olveda, R.; Acosta, L.; Ji, M.J.; Fernandez, T.; et al. High prevalence of Schistosoma japonicum infection in water buffaloes in the Philippines assessed by real-time polymerase chain reaction. Am. J. Trop. Med. Hyg. 2010, 82, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Jiz, M.; Mingala, C.; Fu, Z.-Q.; Adriatico, M.; Lu, K.; Jarilla, B.; Sagliba, M.; Moreno, A.; Park, S.; Lin, J.-J.; et al. High prevalence of Schistosoma japonicum by perfusion in naturally exposed water buffalo in a region of the Philippines endemic for human schistosomiasis. PLOS Neglected Trop. Dis. 2021, 15, e0009796. [Google Scholar] [CrossRef] [PubMed]
- Jumawan, J.C.; Estaño, L.A. Prevalence of Schistosoma japonicum in bovines and Oncomelania hupensis quadrasi from ricefields surrounding Lake Mainit, Philippines. J. Parasit. Dis. Off. Organ. Indian. Soc. Parasitol. 2021, 45, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Estaño, L.A.; Jumawan, J.C. The prevailing infection of Schistosoma japonicum and other zoonotic parasites in bubaline reservoir hosts in the ricefield of lake ecosystem: The case of Lake Mainit, Philippines. Parasitology 2023, 150, 786–791. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Gordon, C.A.; Williams, G.M.; Li, Y.; Wang, Y.; Hu, J.; Gray, D.J.; Ross, A.G.; Harn, D.; McManus, D.P. Real-time PCR diagnosis of Schistosoma japonicum in low transmission areas of China. Infect. Dis. Poverty 2018, 7, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bergquist, R.; King, C.H.; Yang, K. Elimination of schistosomiasis in China: Current status and future prospects. PLOS Neglected Trop. Dis. 2021, 15, e0009578. [Google Scholar] [CrossRef]
- Hong, Z.; Li, L.; Zhang, L.; Wang, Q.; Xu, J.; Li, S.; Zhou, X.-N. Elimination of Schistosomiasis Japonica in China: From the One Health Perspective. China CDC Wkly. 2022, 4, 130–134. [Google Scholar] [CrossRef]
- Li, F.-Y.; Hou, X.-Y.; Tan, H.-Z.; Williams, G.M.; Gray, D.J.; Gordon, C.A.; Kurscheid, J.; Clements, A.C.A.; Li, Y.-S.; McManus, D.P. Current Status of Schistosomiasis Control and Prospects for Elimination in the Dongting Lake Region of the People’s Republic of China. Front. Immunol. 2020, 11, 574136. [Google Scholar] [CrossRef]
- Zhou, X.N.; Guo, J.G.; Wu, X.H.; Jiang, Q.W.; Zheng, J.; Dang, H.; Wang, X.H.; Xu, J.; Zhu, H.Q.; Wu, G.L.; et al. Epidemiology of schistosomiasis in the People’s Republic of China, 2004. Emerg Infect Dis 2007, 13, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Dumag, P.U.; Gajudo, C.E.; Sena, C.Y.; Cardenas, E.C.; Fementira, E.B. Epidemiology of animal schistosomiasis in the Philippines. Philipp. J. Vet. Anim. Sci. 1980, 6, 410–425. [Google Scholar]
- Matsumoto, J.; Kirinoki, M.; Kawai, S.; Chigusa, Y.; Ilagan, E.J.; Ducusin, B.E.; Yasuraoka, K.; Matsuda, H. Prevalence of schistosomiasis japonica among schoolchildren and animal reservoirs in oriental Mindoro Philippines. Jpn. J. Trop. Med. Hyg. 1999, 27, 175–180. [Google Scholar] [CrossRef]
- Carabin, H.; Balolong, E.; Joseph, L.; McGarvey, S.; Johansen, M.; Fernandez, T.; Willingham, A.; Olveda, R.; Schistosomiasis Transmission and Ecology in the Philippines (STEP) Project. Estimating sensitivity and specificity of a faecal examination method for Schistosoma japonicum infection in cats, dogs, water buffaloes, pigs, and rats in Western Samar and Sorsogon Provinces, The Philippines. Int. J. Parasitol. 2005, 35, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.C.; de Cadiz, A.E.; Fronda, J.M.; Ong, L.A.D.; Belizario, V.Y., Jr. Prevalence of Schistosoma japonicum infection in water buffaloes in selected areas in Davao del Norte and Davao de Oro, the Philippines. Int. J. One Health 2021, 7, 12–18. [Google Scholar] [CrossRef]
- Tenorio, J.C.; Manalo, D.; Molina, E. Schistosomiasis japonica in animals in the Philippines and its veterinary public health importance. Philipp. J. Vet. Med. 2021, 58, 248–263. [Google Scholar]
- Wang, L.-D.; Chen, H.-G.; Guo, J.-G.; Zeng, X.-J.; Hong, X.-L.; Xiong, J.-J.; Wu, X.-H.; Wang, X.-H.; Wang, L.-Y.; Xia, G.; et al. A Strategy to Control Transmission of Schistosoma japonicum in China. N. Engl. J. Med. 2009, 360, 121–128. [Google Scholar] [CrossRef]
- McManus, D.P. The Search for a Schistosomiasis Vaccine: Australia’s Contribution. Vaccines 2021, 9, 872. [Google Scholar] [CrossRef]
- Ross, A.G.; Harn, D.A.; Chy, D.; Inobaya, M.; Guevarra, J.R.; Shollenberger, L.; Li, Y.; McManus, D.P.; Gray, D.J.; Williams, G.M. First bovine vaccine to prevent human schistosomiasis—A cluster randomised Phase 3 clinical trial. Int. J. Infect. Dis. 2023, 129, 110–117. [Google Scholar] [CrossRef]
- Williams, G.M.; Sleigh, A.C.; Li, Y.; Feng, Z.; Davis, G.M.; Chen, H.; Ross, A.G.; Bergquist, R.; McManus, D.P. Mathematical modelling of schistosomiasis japonica: Comparison of control strategies in the People’s Republic of China. Acta Trop. 2002, 82, 253–262. [Google Scholar] [CrossRef]
- Mo, A.X.; Agosti, J.M.; Walson, J.L.; Hall, B.F.; Gordon, L. Schistosomiasis elimination strategies and potential role of a vaccine in achieving global health goals. Am. J. Trop. Med. Hyg. 2014, 90, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Tebeje, B.M.; Harvie, M.; You, H.; Loukas, A.; McManus, D.P. Schistosomiasis vaccines: Where do we stand? Parasites Vectors 2016, 9, 528. [Google Scholar] [CrossRef]
- Olveda, D.U.; McManus, D.P.; Ross, A.G.P. Mass drug administration and the global control of schistosomiasis: Successes, limitations and clinical outcomes. Curr. Opin. Infect. Dis. 2016, 29, 595–608. [Google Scholar] [CrossRef]
- Bergquist, N.R.; Leonardo, L.R.; Mitchell, G.F. Vaccine-linked chemotherapy: Can schistosomiasis control benefit from an integrated approach? Trends Parasitol. 2005, 21, 112–117. [Google Scholar] [CrossRef]
- Guy, B. The perfect mix: Recent progress in adjuvant research. Nat. Rev. Microbiol. 2007, 5, 396–397. [Google Scholar] [CrossRef] [PubMed]
- Nooraei, S.; Sarkar Lotfabadi, A.; Akbarzadehmoallemkolaei, M.; Rezaei, N. Immunogenicity of Different Types of Adjuvants and Nano-Adjuvants in Veterinary Vaccines: A Comprehensive Review. Vaccines 2023, 11, 453. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Shi, S.; Zhu, H.; Xia, X.; Liang, Z.; Ma, X.; Sun, B. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178. [Google Scholar] [CrossRef]
- Panzner, U.; Excler, J.-L.; Kim, J.H.; Marks, F.; Carter, D.; Siddiqui, A.A. Recent Advances and Methodological Considerations on Vaccine Candidates for Human Schistosomiasis. Front. Trop. Dis. 2021, 2, 719369. [Google Scholar] [CrossRef]
- Riveau, G.; Deplanque, D.; Remoué, F.; Schacht, A.-M.; Vodougnon, H.; Capron, M.; Thiry, M.; Martial, J.; Libersa, C.; Capron, A. Safety and Immunogenicity of rSh28GST Antigen in Humans: Phase 1 Randomized Clinical Study of a Vaccine Candidate against Urinary Schistosomiasis. PLOS Neglected Trop. Dis. 2012, 6, e1704. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Zhang, W.; Ahmad, G.; Molehin, A.J.; Torben, W.; Le, L.; Kim, E.; Lazarus, S.; Siddiqui, A.J.; Carter, D.; Siddiqui, A.A. Schistosoma mansoni antigen Sm-p80: Prophylactic efficacy using TLR4 agonist vaccine adjuvant glucopyranosyl lipid A-Alum in murine and non-human primate models. J. Investig. Med. 2018, 66, 1124–1132. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Siddiqui, S.Z. Sm-p80-Based Schistosomiasis Vaccine: Preparation for Human Clinical Trials. Trends Parasitol. 2016, 33, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Gerdts, V. Adjuvants for veterinary vaccines–types and modes of action. Berl. Munch. Tierarztl. Wochenschr. 2015, 128, 456–463. [Google Scholar] [PubMed]
- Shi, F.; Zhang, Y.; Ye, P.; Lin, J.; Cai, Y.; Shen, W.; Bickle, Q.D.; Taylor, M.G. Laboratory and field evaluation of Schistosoma japonicum DNA vaccines in sheep and water buffalo in China. Vaccine 2001, 20, 462–467. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Harvie, M.; Du, X.; Rivera, V.; Zhang, P.; McManus, D.P. Protective immune responses generated in a murine model following immunization with recombinant Schistosoma japonicum insulin receptor. Int. J. Mol. Sci. 2018, 19, 3088. [Google Scholar] [CrossRef] [PubMed]
- Molehin, A.J.; Gray, S.A.; Turner, C.; Davis, J.; Zhang, W.; Khatoon, S.; Rattan, M.; Kernen, R.; Peterson, C.; Sennoune, S.R.; et al. Process Development of Sj-p80: A Low-Cost Transmission-Blocking Veterinary Vaccine for Asiatic Schistosomiasis. Front. Immunol. 2020, 11, 578715. [Google Scholar] [CrossRef] [PubMed]
- Jiz Ii, M.A.L.; Mingala, C.N.; Lopez, I.F.M.; Chua, M.; Gabonada, F.G., Jr.; Acosta, L.P.; Wu, H.; Kurtis, J.D. A field trial of recombinant Schistosoma japonicum paramyosin as a potential vaccine in naturally-infected water buffaloes. Ann. Parasitol. 2016, 62, 295–299. [Google Scholar] [CrossRef]
- You, H.; Gobert, G.N.; Cai, P.; Mou, R.; Nawaratna, S.; Fang, G.; Villinger, F.; McManus, D.P. Suppression of the Insulin Receptors in Adult Schistosoma japonicum Impacts on Parasite Growth and Development: Further Evidence of Vaccine Potential. PLoS Neglected Trop. Dis. 2015, 9, e0003730. [Google Scholar] [CrossRef]
- Ayele, G.; Getachew, B.; Bari, F.D.; Bayissa, B.; Muluneh, A.; Abayneh, T.; Gelaye, E.; Edao, B.M. Combined Adjuvant Formulations Enhanced an Immune Response of Trivalent Foot and Mouth Disease Vaccine in Cattle. Vet. Med. 2023, 14, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.M.; Xu, R.M.; Yuan, C.X.; Li, Y.Y.; Liu, Q.; Cheng, G.F.; Lin, J.-J.; Feng, X.G. SjHSP70, a recombinant Schistosoma japonicum heat shock protein 70, is immunostimulatory and induces protective immunity against cercarial challenge in mice. Parasitol. Res. 2015, 114, 3415–3429. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.-C.; Lin, C.-N.; Peng, S.-Y.; Kang, T.-F.; Lee, K.-M. Combined IL-12 Plasmid and Recombinant SjGST Enhance the Protective and Anti-pathology Effect of SjGST DNA Vaccine Against Schistosoma japonicum. PLoS Neglected Trop. Dis. 2016, 10, e0004459. [Google Scholar] [CrossRef] [PubMed]
- Da’Dara, A.A.; Li, C.; Yu, X.; Zheng, M.; Zhou, J.; Shollenberger, L.M.; Li, Y.-s.; Harn, D.A. Prime-Boost Vaccine Regimen for SjTPI and SjC23 Schistosome Vaccines, Increases Efficacy in Water Buffalo in a Field Trial in China. Front. Immunol. 2019, 10, 284. [Google Scholar] [CrossRef]
- Tang, C.L.; Xie, Y.P.; Yu, W.H.; Jin, L.; Xie, Z.L.; Li, X.R. Effects of regulatory T cells on glyceraldehyde-3-phosphate dehydrogenase vaccine efficacy against Schistosoma japonicum. Acta Trop. 2020, 202, 105239. [Google Scholar] [CrossRef]
- Al-Nazal, H.A.; Cooper, E.; Ho, M.F.; Eskandari, S.; Majam, V.; Giddam, A.K.; Hussein, W.M.; Islam, M.T.; Skwarczynski, M.; Toth, I.; et al. Pre-clinical evaluation of a whole-parasite vaccine to control human babesiosis. Cell Host Microbe 2021, 29, 894–903.e5. [Google Scholar] [CrossRef]
- Chatzikleanthous, D.; O’Hagan, D.T.; Adamo, R. Lipid-Based Nanoparticles for Delivery of Vaccine Adjuvants and Antigens: Toward Multicomponent Vaccines. Mol. Pharm. 2021, 18, 2867–2888. [Google Scholar] [CrossRef]
- Alameh, M.-G.; Tombácz, I.; Bettini, E.; Lederer, K.; Ndeupen, S.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Capron, A.; Riveau, G.J.; Bartley, P.B.; McManus, D.P. Prospects for a schistosome vaccine. Curr. Drug Targets Immune Endocr. Metab. Disord. 2002, 2, 281–290. [Google Scholar] [CrossRef]
- Mo, A.X.; Colley, D.G. Workshop report: Schistosomiasis vaccine clinical development and product characteristics. Vaccine 2016, 34, 995–1001. [Google Scholar] [CrossRef]
- Wu, H.W.; Fu, Z.-Q.; Lu, K.; Pond-tor, S.; Meng, R.; Hong, Y.; Chu, K.; Li, H.; Jiz, M.; Liu, J.-M.; et al. Vaccination with recombinant paramyosin in Montanide ISA206 protects against Schistosoma japonicum infection in water buffalo. Vaccine 2017, 35, 3409–3415. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; Song, G.; Luo, X.-S.; Xu, Y.; McManus, D.P. Anti-fecundity immunity to Schistosoma japonicum induced in Chinese water buffaloes (Bos buffelus) after vaccination with recombinant 26 kDa glutathione-S-transferase (reSjc26GST). Vet. Parasitol. 1997, 69, 39–47. [Google Scholar] [CrossRef]
- Xu, S.; Shi, F.; Shen, W.; Lin, J.; Wang, Y.; Lin, B.; Qian, C.; Ye, P.; Fu, L.; Shi, Y.; et al. Vaccination of bovines against Schistosomiasis japonica with cryopreserved-irradiated and freeze-thaw schistosomula. Vet. Parasitol. 1993, 47, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.E.; Jiang, C.F.; Han, J.J.; Li, Y.L.; Ruppel, A. Schistosoma japonicum: An ultraviolet-attenuated cercarial vaccine applicable in the field for water buffaloes. Exp. Parasitol. 1990, 71, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Eveland, L.K.; Morse, S.I. Schistosoma mansoni: Infectivity and immunizing effects of in vitro derived schistosomula attenuated by X irradiation. Exp. Parasitol. 1978, 45, 19–25. [Google Scholar] [CrossRef]
- Farias, L.P.; Vitoriano-Souza, J.; Cardozo, L.E.; Gama, L.D.R.; Singh, Y.; Miyasato, P.A.; Almeida, G.T.; Rodriguez, D.; Barbosa, M.M.F.; Fernandes, R.S.; et al. Systems Biology Analysis of the Radiation-Attenuated Schistosome Vaccine Reveals a Role for Growth Factors in Protection and Hemostasis Inhibition in Parasite Survival. Front. Immunol. 2021, 12, 624191. [Google Scholar] [CrossRef]
- Wilson, R.A. Models of Protective Immunity against Schistosomes: Implications for Vaccine Development. Pathogens 2023, 12, 1215. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.G.; James, E.R.; Nelson, G.S.; Bickle, Q.; Dunne, D.W.; Webbe, G. Immunisation of sheep against Schistosoma mattheei using either irradiated cercariae or irradiated schistosomula. J. Helminthol. 1976, 50, 1–9. [Google Scholar] [CrossRef]
- Taylor, M.G.; James, E.R.; Nelson, G.S.; Bickle, Q.; Andrews, B.J.; Dobinson, A.R.; Webbe, G. Immunisation of baboons against Schistosoma mansoni using irradiated S. mansoni cercariae and schistosomula and non-irradiated S. rodhaini cercariae. J. Helminthol. 1976, 50, 215–221. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H. Why the radiation-attenuated cercarial immunization studies failed to guide the road for an effective schistosomiasis vaccine: A review. J. Adv. Res. 2015, 6, 255–267. [Google Scholar] [CrossRef]
- Murrell, K.D.; Clark, S.S.; Dean, D.A.; Vannier, W.E. Schistosoma mansoni: Immunization of cynomolgus monkeys by injection of irradiated schistosomula. Exp. Parasitol. 1979, 48, 415–420. [Google Scholar] [CrossRef]
- Gobert, G.N.; Stenzel, D.J.; Jones, M.K.; Allen, D.E.; McManus, D.P. Schistosoma japonicum: Immunolocalization of paramyosin during development. Parasitology 1997, 114, 45–52. [Google Scholar] [CrossRef]
- Jiz, M.A.; Wu, H.; Olveda, R.; Jarilla, B.; Kurtis, J.D. Development of Paramyosin as a Vaccine Candidate for Schistosomiasis. Front. Immunol. 2015, 6, 347. [Google Scholar] [CrossRef]
- Hambrook, J.R.; Hanington, P.C. Immune Evasion Strategies of Schistosomes. Front. Immunol. 2021, 11, 624178. [Google Scholar] [CrossRef]
- Gobert, G.N.; McManus, D.P. Update on paramyosin in parasitic worms. Parasitol. Int. 2005, 54, 101–107. [Google Scholar] [CrossRef]
- Kang, J.M.; Lê, H.G.; Võ, T.C.; Yoo, W.G.; Sohn, W.M.; Na, B.K. Mapping of the Complement C9 Binding Region on Clonorchis sinensis Paramyosin. Korean J. Parasitol. 2022, 60, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Mulvenna, J.; Moertel, L.; Jones, M.K.; Nawaratna, S.; Lovas, E.M.; Gobert, G.N.; Colgrave, M.; Jones, A.; Loukas, A.; McManus, D.P. Exposed proteins of the Schistosoma japonicum tegument. Int. J. Parasitol. 2010, 40, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Janecharut, T.; Hata, H.; Niimura, M. Role of a mouse monoclonal IgE antibody in passive transfer of immunity to Schistosoma japonicum infection. Mem. Inst. Oswaldo Cruz 1987, 82 (Suppl. 4), 237–241. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.J.; James, S.L.; Hieny, S.; Lanar, D.E.; Sher, A. Induction of protective immunity against Schistosoma mansoni by vaccination with schistosome paramyosin (Sm97), a nonsurface parasite antigen. Proc. Natl. Acad. Sci. USA 1988, 85, 5678–5682. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, B.L.; Kurtis, J.D.; Wiest, P.M.; Arias, P.; Aligui, F.E.; Acosta, L.U.Z.; Peters, P.; Olds, G.R. Paramyosin: A candidate vaccine antigen against Schistosoma japonicum. Parasite Immunol. 1996, 18, 49–52. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Wong, J.Y.M.; Zhou, J.; Cai, C.; Zeng, Q.; Smyth, D.; Li, Y.; Kalinna, B.H.; Duke, M.J.; Yi, X. Recombinant paramyosin (rec-Sj-97) tested for immunogenicity and vaccine efficacy against Schistosoma japonicum in mice and water buffaloes. Vaccine 2001, 20, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Jiz, M.; Wu, H.-W.; Meng, R.; Pond-Tor, S.; Reynolds, M.; Friedman, J.F.; Olveda, R.; Acosta, L.; Kurtis, J.D. Pilot-Scale Production and Characterization of Paramyosin, a Vaccine Candidate for Schistosomiasis Japonica. Infect. Immun. 2008, 76, 3164–3169. [Google Scholar] [CrossRef]
- Bergquist, N.R.; Colley, D.G. Schistosomiasis Vaccine:Research to Development. Parasitol. Today 1998, 14, 99–104. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, X.; Zhang, F.; Zhu, Y.; Yang, B.; Hou, M.; Xu, Z.; Yu, C.; Chen, Y.; Chen, L.; et al. SjTat-TPI facilitates adaptive T-cell responses and reduces hepatic pathology during Schistosoma japonicum infection in BALB/c mice. Parasites Vectors 2015, 8, 664. [Google Scholar] [CrossRef]
- Zhu, Y.; Si, J.; Harn, D.A.; Xu, M.; Ren, J.; Yu, C.; Liang, Y.; Yin, X.; He, W.; Cao, G. Schistosoma japonicum triose-phosphate isomerase plasmid DNA vaccine protects pigs against challenge infection. Parasitology 2006, 132, 67–71. [Google Scholar] [CrossRef]
- You, H.; Zhang, W.; Jones, M.K.; Gobert, G.N.; Mulvenna, J.; Rees, G.; Spanevello, M.; Blair, D.; Duke, M.; Brehm, K.; et al. Cloning and characterisation of Schistosoma japonicum insulin receptors. PLoS ONE 2010, 5, e9868. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, X.; Tang, J.; Zhao, S.; Xing, Y.; Dai, J.; Jin, X.; Zhu, Y. Enhancement of protective efficacy through adenoviral vectored vaccine priming and protein boosting strategy encoding triosephosphate isomerase (SjTPI) against Schistosoma japonicum in mice. PLoS ONE 2015, 10, e0120792. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, S.; Tang, J.; Xing, Y.; Qu, G.; Dai, J.; Jin, X.; Wang, X. Evaluation of protective efficacy induced by different heterologous prime-boost strategies encoding triosephosphate isomerase against Schistosoma japonicum in mice. Parasites Vectors 2017, 10, 111. [Google Scholar] [CrossRef]
- Davern, K.M.; Wright, M.D.; Herrmann, V.R.; Mitchell, G.F. Further characterisation of the Schistosoma japonicum protein Sj23, a target antigen of an immunodiagnostic monoclonal antibody. Mol. Biochem. Parasitol. 1991, 48, 67–75. [Google Scholar] [CrossRef]
- Wang, J.; Yu, C.-X.; Yin, X.-R.; Zhang, W.; Qian, C.-Y.; Song, L.-J.; Ke, X.-D.; Xu, Y.-L.; He, W.; Cao, G.-Q. Monitoring specific antibody responses against the hydrophilic domain of the 23 kDa membrane protein of Schistosoma japonicum for early detection of infection in sentinel mice. Parasites Vectors 2011, 4, 172. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cai, P.; Chang, Q.; Hao, L.; Peng, S.; Sun, X.; Lu, H.; Yin, J.; Jiang, N.; Chen, Q. Mapping the Binding between the Tetraspanin Molecule (Sjc23) of Schistosoma japonicum and Human Non-Immune IgG. PLoS ONE 2011, 6, e19112. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.R.; Shoemaker, C.B.; Harn, D.A. T and B cell epitope mapping of SM23, an integral membrane protein of Schistosoma mansoni. J. Immunol. 1992, 149, 3995–4001. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Cai, P.; Yin, J.; Hao, L.; Lu, H.; Wang, X.; Wang, H.; Chen, Q. Characterization of antibody responses to the Sj23 antigen of Schistosoma japonicum after infection and immunization. Acta Trop. 2010, 116, 9–14. [Google Scholar] [CrossRef]
- Skelly, P.J. The Surface of Schistosomes within the Vertebrate Host. In Schistosomiasis; Secor, W.E., Colley, D.G., Eds.; Springer: Boston, MA, USA, 2005; pp. 81–100. [Google Scholar]
- Cruise, K.M.; Mitchell, G.F.; Garcia, E.G.; Tiu, W.U.; Hocking, R.E.; Ande, R.F. Sj23, the target antigen in Schistosoma japonicum adult worms of an immunodiagnostic hybridoma antibody. Parasite Immunol. 1983, 5, 37–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Taylor, M.G.; Johansen, M.V.; Bickle, Q.D. Vaccination of mice with a cocktail DNA vaccine induces a Th1-type immune response and partial protection against Schistosoma japonicum infection. Vaccine 2001, 20, 724–730. [Google Scholar] [CrossRef]
- Zhu, Y.; Ren, J.; Da’dara, A.; Harn, D.; Xu, M.; Si, J.; Yu, C.; Liang, Y.; Ye, P.; Yin, X.; et al. The protective effect of a Schistosoma japonicum Chinese strain 23 kDa plasmid DNA vaccine in pigs is enhanced with IL-12. Vaccine 2004, 23, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, L.; Liu, Z.; Zhu, L.; Hu, Y.; Zhu, M.; Zhu, X.; Shi, Y.; Meng, S. Schistosoma japonicum: The design and experimental evaluation of a multivalent DNA vaccine. Cell. Mol. Biol. Lett. 2006, 11, 449–460. [Google Scholar] [CrossRef]
- Scott, J.C.; McManus, D.P. Molecular cloning and enzymatic expression of the 28-kDa glutathione S-transferase of Schistosoma japonicum: Evidence for sequence variation but lack of consistent vaccine efficacy in the murine host. Parasitol. Int. 2000, 49, 289–300. [Google Scholar] [CrossRef]
- Platis, M.; Vlachakis, D.; Foudah, A.I.; Muharram, M.M.; Alqarni, M.H.; Papageorgiou, A.C.; Labrou, N.E. The Interaction of Schistosoma japonicum Glutathione Transferase with Cibacron Blue 3GA and its Fragments. Med. Chem. 2021, 17, 332–343. [Google Scholar] [CrossRef]
- Mazari, A.M.A.; Zhang, L.; Ye, Z.W.; Zhang, J.; Tew, K.D.; Townsend, D.M. The Multifaceted Role of Glutathione S-Transferases in Health and Disease. Biomolecules 2023, 13, 688. [Google Scholar] [CrossRef]
- Vaish, S.; Gupta, D.; Mehrotra, R.; Mehrotra, S.; Basantani, M.K. Glutathione S-transferase: A versatile protein family. 3 Biotech 2020, 10, 321. [Google Scholar] [CrossRef]
- Tang, C.L.; Zhou, H.H.; Zhu, Y.W.; Huang, J.; Wang, G.B. Glutathione S-transferase influences the fecundity of Schistosoma japonicum. Acta Trop. 2019, 191, 8–12. [Google Scholar] [CrossRef]
- Walker, J.; Crowley, P.; Moreman, A.D.; Barrett, J. Biochemical properties of cloned glutathione S-transferases from Schistosoma mansoni and Schistosoma japonicum. Mol. Biochem. Parasitol. 1993, 61, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Song, G.C.; Xu, Y.X.; Yang, W.; McManus, D.P. Anti-fecundity immunity induced in pigs vaccinated with recombinant Schistosoma japonicum 26kDa glutathione-S-transferase. Parasite Immunol. 1995, 17, 335–340. [Google Scholar] [CrossRef] [PubMed]
- He, Y.K.; Liu, S.X.; McManus, D.P.; Zhang, X.Y.; Song, G.C.; Luo, X.S.; Li, Y.S.; Xu, Y.X.; Yu, X.L.; Li, Y.; et al. Field assessment of recombinant Schistosoma japonicum 26 kDA glutathione S-transferase in Chinese water buffaloes. Southeast Asian J. Trop. Med. Public. Health 2003, 34, 473–479. [Google Scholar] [PubMed]
- Shi, F.; Zhang, Y.; Lin, J.; Zuo, X.; Shen, W.; Cai, Y.; Ye, P.; Bickle, Q.D.; Taylor, M.G. Field testing of Schistosoma japonicum DNA vaccines in cattle in China. Vaccine 2002, 20, 3629–3631. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, S.; Zhang, S.; Tong, H.; Gao, Z.; Liu, Y.; Lin, D.; Liu, Z.; Wu, G.; Yi, H.; et al. Persistence of the protective immunity to Schistosoma japonicum in Chinese yellow cattle induced by recombinant 26 kDa glutathione-S-transferase (reSjc26GST). Vet. Parasitol. 2004, 123, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Dai, Y.; Chen, J.; Wang, X.; Tang, B.; Zhu, Y.; Hua, Z. Oral delivery of the Sj23LHD-GST antigen by Salmonella typhimurium type III secretion system protects against Schistosoma japonicum infection in mice. PLoS Neglected Trop. Dis. 2011, 5, e1313. [Google Scholar] [CrossRef]
- Mbanefo, E.C.; Kumagai, T.; Kodama, Y.; Kurosaki, T.; Furushima-Shimogawara, R.; Cherif, M.S.; Mizukami, S.; Kikuchi, M.; Huy, N.T.; Ohta, N.; et al. Immunogenicity and anti-fecundity effect of nanoparticle coated glutathione S-transferase (Sj GST) DNA vaccine against murine Schistosoma japonicum infection. Parasitol. Int. 2015, 64, 24–31. [Google Scholar] [CrossRef]
- Abdul-Ghani, R.A.; Hassan, A.A. Murine schistosomiasis as a model for human schistosomiasis mansoni: Similarities and discrepancies. Parasitol. Res. 2010, 107, 1–8. [Google Scholar] [CrossRef]
- Wilson, R.A.; Li, X.H.; Castro-Borges, W. Do schistosome vaccine trials in mice have an intrinsic flaw that generates spurious protection data? Parasites Vectors 2016, 9, 89. [Google Scholar] [CrossRef]
- You, H.; Zhang, W.; Moertel, L.; McManus, D.P.; Gobert, G.N. Transcriptional profiles of adult male and female Schistosoma japonicum in response to insulin reveal increased expression of genes involved in growth and development. Int. J. Parasitol. 2009, 39, 1551–1559. [Google Scholar] [CrossRef]
- You, H.; Gobert, G.N.; Jones, M.K.; Zhang, W.; McManus, D.P. Signalling pathways and the host-parasite relationship: Putative targets for control interventions against schistosomiasis: Signalling pathways and future anti-schistosome therapies. Bioessays 2011, 33, 203–214. [Google Scholar] [CrossRef]
- Wang, S.; Luo, X.; Zhang, S.; Yin, C.; Dou, Y.; Cai, X. Identification of putative insulin-like peptides and components of insulin signaling pathways in parasitic platyhelminths by the use of genome-wide screening. FEBS J. 2014, 281, 877–893. [Google Scholar] [CrossRef]
- Stephenson, R.J.; Toth, I.; Liang, J.; Mangat, A.; McManus, D.P.; You, H. Identification of Host Insulin Binding Sites on Schistosoma japonicum Insulin Receptors. PLoS ONE 2016, 11, e0159704. [Google Scholar] [CrossRef]
- Du, X.; McManus, D.P.; Hu, W.; You, H. Identification and Functional Characterisation of Schistosome Insulin-like Peptide. Parasites Vectors 2017, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Gobert, G.N.; Duke, M.G.; Zhang, W.; Li, Y.; Jones, M.K.; McManus, D.P. The insulin receptor is a transmission blocking veterinary vaccine target for zoonotic Schistosoma japonicum. Int. J. Parasitol. 2012, 42, 801–807. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Zhou, Y.; Podesta, R.B.; Karcz, S.R.; Tognon, C.E.; Strejan, G.H.; Dekaban, G.A.; Clarke, M.W. Characterization of Ca2+-dependent neutral protease (calpain) from human blood flukes, Schistosoma mansoni. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1993, 1181, 37–44. [Google Scholar] [CrossRef]
- Kumagai, T.; Maruyama, H.; Hato, M.; Ohmae, H.; Osada, Y.; Kanazawa, T.; Ohta, N. Schistosoma japonicum: Localization of calpain in the penetration glands and secretions of cercariae. Exp. Parasitol. 2005, 109, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Shimogawara, R.; Ichimura, K.; Iwanaga, S. Calpain inhibitor suppresses both extracellular vesicle-mediated secretion of miRNAs and egg production from paired adults of Schistosoma japonicum. Parasitol. Int. 2022, 87, 102540. [Google Scholar] [CrossRef] [PubMed]
- Chaimon, S.; Limpanont, Y.; Reamtong, O.; Ampawong, S.; Phuphisut, O.; Chusongsang, P.; Ruangsittichai, J.; Boonyuen, U.; Watthanakulpanich, D.; O’Donoghue, A.J.; et al. Molecular characterization and functional analysis of the Schistosoma mekongi Ca2+-dependent cysteine protease (calpain). Parasites Vectors 2019, 12, 383. [Google Scholar] [CrossRef]
- Wang, Q.; Da’dara, A.A.; Skelly, P.J. The human blood parasite Schistosoma mansoni expresses extracellular tegumental calpains that cleave the blood clotting protein fibronectin. Sci. Rep. 2017, 7, 12912. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wu, K.; Zhang, C.; Batool, S.S.; Li, A.; Yu, Z.; Huang, J. Advances in new target molecules against schistosomiasis: A comprehensive discussion of physiological structure and nutrient intake. PLoS Pathog. 2023, 19, e1011498. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N.; Kumagai, T.; Maruyama, H.; Yoshida, A.; He, Y.; Zhang, R. Research on calpain of Schistosoma japonicum as a vaccine candidate. Parasitol. Int. 2004, 53, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Molehin, A.J.; Sennoune, S.R.; Zhang, W.; Rojo, J.U.; Siddiqui, A.J.; Herrera, K.A.; Johnson, L.; Sudduth, J.; May, J.; Siddiqui, A.A. Cross-species prophylactic efficacy of Sm-p80-based vaccine and intracellular localization of Sm-p80/Sm-p80 ortholog proteins during development in Schistosoma mansoni, Schistosoma japonicum, and Schistosoma haematobium. Parasitol. Res. 2017, 116, 3175–3188. [Google Scholar] [CrossRef]
- Molehin, A.J.; McManus, D.P.; You, H. Vaccines for Human Schistosomiasis: Recent Progress, New Developments and Future Prospects. Int. J. Mol. Sci. 2022, 23, 2255. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, X.; Zhao, S.; Tang, J.; Zhang, L.; Dai, J.; Zeng, M.; Lu, S.; Zhu, Y.; Su, C. Construction and evaluation of replication-defective recombinant optimized triosephosphate isomerase adenoviral vaccination in Schistosoma japonicum challenged mice. Vaccine 2013, 32, 771–778. [Google Scholar] [CrossRef]
- Lei, N.; Liu, F.-C.; Ren, C.-P.; Shen, J.-J.; Liu, M. An Efficient Schistosoma japonicum Bivalent Membrane Protein Antigen DNA Vaccine against Schistosomiasis in Mice. Med. Sci. Monit. 2019, 25, 9319–9326. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, X.; Wang, H.; Liu, R.; Ye, Q.; Zhao, Q.; Ming, Z.; Dong, H. Immunization with recombinant schistosome adenylate kinase 1 partially protects mice against Schistosoma japonicum infection. Parasitol. Res. 2017, 116, 1665–1674. [Google Scholar] [CrossRef]
- Cao, Y.; Qiao, H.; Shi, Y.; Han, Y.; Liu, J.; Li, H.; Lu, K.; Lin, J.; Jin, Y. Evaluation of Lethal Giant Larvae as a Schistosomiasis Vaccine Candidate. BioMed Res. Int. 2016, 2016, 4680812–4680817. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, L.; Luo, R.; Dao, J.; Zhao, J.; Shi, Y.; Li, H.; Lu, K.; Feng, X.; Lin, J.; et al. Evaluation of protective immune response in mice by vaccination the recombinant adenovirus for expressing Schistosoma japonicum inhibitor apoptosis protein. Parasitol. Res. 2014, 113, 4261–4269. [Google Scholar] [CrossRef] [PubMed]
- Perez-Casal, J.; Potter, A.A. Glyceradehyde-3-phosphate dehydrogenase as a suitable vaccine candidate for protection against bacterial and parasitic diseases. Vaccine 2016, 34, 1012–1017. [Google Scholar] [CrossRef]
- Jie, H.; Zhang, S.-M.; Ding, F.-R.; Tang, C.-L.; Li, X.-Y. Glyceraldehyde-3-phosphate dehydrogenase affects the growth of Schistosoma japonicum schistosomula. Acta Trop. 2022, 235, 106667. [Google Scholar] [CrossRef] [PubMed]
- Pirovich, D.B.; Da’dara, A.A.; Skelly, P.J. Schistosoma mansoni glyceraldehyde-3-phosphate dehydrogenase enhances formation of the blood-clot lysis protein plasmin. Biol. Open 2020, 9, bio050385. [Google Scholar] [CrossRef]
- Waine, G.J.; Becker, M.; Yang, W.; Kalinna, B.; McManus, D.P. Cloning, molecular characterization, and functional activity of Schistosoma japonicum glyceraldehyde-3-phosphate dehydrogenase, a putative vaccine candidate against schistosomiasis japonica. Infect. Immun. 1993, 61, 4716–4723. [Google Scholar] [CrossRef]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef]
- Tang, C.-l.; Zhao, C.-z.; Jie, H.; Zhang, S.-m.; Zhang, R.-h.; Lu, Y.; Pan, Q. Involvement of the fatty acid-binding protein in the growth of Schistosoma japonicum schistosomula. Parasitol. Res. 2021, 120, 3851–3856. [Google Scholar] [CrossRef]
- Castro-Borges, W.; Wilson, R.A. Schistosome proteomics: Updates and clinical implications. Expert Rev. Proteom. 2022, 19, 247–261. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for mRNA Vaccines. Curr. Top. Microbiol. Immunol. 2022, 440, 71–110. [Google Scholar] [CrossRef]
- Carson, J.P.; Robinson, M.W.; Hsieh, M.H.; Cody, J.; Le, L.; You, H.; McManus, D.P.; Gobert, G.N. A comparative proteomics analysis of the egg secretions of three major schistosome species. Mol. Biochem. Parasitol. 2020, 240, 111322. [Google Scholar] [CrossRef]
- Pearson, M.S.; Loukas, A.; Sotillo, J. Proteomic Analysis of Schistosoma mansoni Tegumental Proteins. Methods Mol. Biol. 2020, 2151, 85–92. [Google Scholar] [CrossRef]
- Cai, P.; Liu, S.; Piao, X.; Hou, N.; You, H.; McManus, D.P.; Chen, Q. A next-generation microarray further reveals stage-enriched gene expression pattern in the blood fluke Schistosoma japonicum. Parasites Vectors 2017, 10, 19. [Google Scholar] [CrossRef]
- You, H.; Mayer, J.U.; Johnston, R.L.; Sivakumaran, H.; Ranasinghe, S.; Rivera, V.; Kondrashova, O.; Koufariotis, L.T.; Du, X.; Driguez, P.; et al. CRISPR/Cas9-mediated genome editing of Schistosoma mansoni acetylcholinesterase. FASEB J. 2021, 35, e21205. [Google Scholar] [CrossRef] [PubMed]
- Ittiprasert, W.; Mann, V.H.; Karinshak, S.E.; Coghlan, A.; Rinaldi, G.; Sankaranarayanan, G.; Chaidee, A.; Tanno, T.; KumKhaek, C.; Prangtaworn, P.; et al. Programmed genome editing of the omega-1 ribonuclease of the blood fluke, Schistosoma mansoni. Elife 2019, 8, e41337. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, G.; Coghlan, A.; Driguez, P.; Lotkowska, M.E.; Sanders, M.; Holroyd, N.; Tracey, A.; Berriman, M.; Rinaldi, G. Large CRISPR-Cas-induced deletions in the oxamniquine resistance locus of the human parasite Schistosoma mansoni. Wellcome Open Res. 2020, 5, 178. [Google Scholar] [CrossRef]
- Du, X.; McManus, D.P.; French, J.D.; Jones, M.K.; You, H. CRISPR/Cas9: A new tool for the study and control of helminth parasites. Bioessays 2021, 43, e2000185. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Gordon, C.A.; MacGregor, S.R.; Cai, P.; McManus, D.P. Potential of the CRISPR-Cas system for improved parasite diagnosis: CRISPR-Cas mediated diagnosis in parasitic infections: CRISPR-Cas mediated diagnosis in parasitic infections. Bioessays 2022, 44, e2100286. [Google Scholar] [CrossRef]
- Li, X.-H.; Vance, G.M.; Cartwright, J.; Cao, J.-P.; Wilson, R.A.; Castro-Borges, W. Mapping the epitopes of Schistosoma japonicum esophageal gland proteins for incorporation into vaccine constructs. PLoS ONE 2020, 15, e0229542. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhong, Q.P.; Tang, H.B.; Dong, H.F. Comparative characterization of microRNAs of Schistosoma japonicum from SCID mice and BALB/c mice: Clues to the regulation of parasite growth and development. Acta Trop. 2022, 225, 106200. [Google Scholar] [CrossRef]
- Wu, H.W.; Park, S.; Pond-Tor, S.; Stuart, R.; Zhou, S.; Hong, Y.; Ruiz, A.E.; Acosta, L.; Jarilla, B.; Friedman, J.F.; et al. Whole-Proteome Differential Screening Identifies Novel Vaccine Candidates for Schistosomiasis japonica. J. Infect. Dis. 2021, 223, 1265–1274. [Google Scholar] [CrossRef]
- Hong, Y.; Sun, A.; Zhang, M.; Gao, F.; Han, Y.; Fu, Z.; Shi, Y.; Lin, J. Proteomics analysis of differentially expressed proteins in schistosomula and adult worms of Schistosoma japonicum. Acta Trop. 2013, 126, 1–10. [Google Scholar] [CrossRef]
- Bi, N.N.; Zhao, S.; Zhang, J.F.; Cheng, Y.; Zuo, C.Y.; Yang, G.L.; Yang, K. Proteomics Investigations of Potential Protein Biomarkers in Sera of Rabbits Infected With Schistosoma japonicum. Front. Cell. Infect. Microbiol. 2021, 11, 784279. [Google Scholar] [CrossRef]
- Al-Amrani, S.; Al-Jabri, Z.; Al-Zaabi, A.; Alshekaili, J.; Al-Khabori, M. Proteomics: Concepts and applications in human medicine. World J. Biol. Chem. 2021, 12, 57–69. [Google Scholar] [CrossRef]
- Cao, X.; Fu, Z.; Zhang, M.; Han, Y.; Han, H.; Han, Q.; Lu, K.; Hong, Y.; Lin, J. iTRAQ-based comparative proteomic analysis of excretory-secretory proteins of schistosomula and adult worms of Schistosoma japonicum. J. Proteom. 2016, 138, 30–39. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, M.; Yang, J.; Cao, X.; Han, Q.; Han, Y.; Qiu, C.; Zhu, C.; Lu, K.; Li, H.; et al. Immunoproteomic analysis of Schistosoma japonicum schistosomulum proteins recognized by immunoglobulin G in the sera of susceptible and non-susceptible hosts. J. Proteom. 2015, 124, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Asmamaw, M.; Zawdie, B. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biologics 2021, 15, 353–361. [Google Scholar] [CrossRef]
- Du, X.; McManus, D.P.; French, J.D.; Collinson, N.; Sivakumaran, H.; MacGregor, S.R.; Fogarty, C.E.; Jones, M.K.; You, H. CRISPR interference for sequence-specific regulation of fibroblast growth factor receptor A in Schistosoma mansoni. Front. Immunol. 2022, 13, 1105719. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Xiang, S.; Hu, Y.; Zhao, S.; Liao, Y.; Zhu, Z.; Wu, X. CRISPR/Cas9-mediated gene knockout of Sj16 in Schistosoma japonicum eggs upregulates the host-to-egg immune response. FASEB J. 2022, 36, e22615. [Google Scholar] [CrossRef] [PubMed]
- The Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature 2009, 460, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Berriman, M.; Haas, B.J.; LoVerde, P.T.; Wilson, R.A.; Dillon, G.P.; Cerqueira, G.C.; Mashiyama, S.T.; Al-Lazikani, B.; Andrade, L.F.; Ashton, P.D.; et al. The genome of the blood fluke Schistosoma mansoni. Nature 2009, 460, 352–358. [Google Scholar] [CrossRef]
- Young, N.D.; Jex, A.R.; Li, B.; Liu, S.; Yang, L.; Xiong, Z.; Li, Y.; Cantacessi, C.; Hall, R.S.; Xu, X.; et al. Whole-genome sequence of Schistosoma haematobium. Nat. Genet. 2012, 44, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Chivukula, S.; Plitnik, T.; Tibbitts, T.; Karve, S.; Dias, A.; Zhang, D.; Goldman, R.; Gopani, H.; Khanmohammed, A.; Sarode, A.; et al. Development of multivalent mRNA vaccine candidates for seasonal or pandemic influenza. NPJ Vaccines 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Jones, M.K.; Gordon, C.A.; Arganda, A.E.; Cai, P.; Al-Wassiti, H.; Pouton, C.W.; McManus, D.P. The mRNA Vaccine Technology Era and the Future Control of Parasitic Infections. Clin. Microbiol. Rev. 2023, 36, e0024121. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, M.A.; Oberli, A.M.; Jeklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Espeseth, A.S.; Cejas, P.J.; Citron, M.P.; Wang, D.; DiStefano, D.J.; Callahan, C.; Donnell, G.O.; Galli, J.D.; Swoyer, R.; Touch, S.; et al. Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. NPJ Vaccines 2020, 5, 16. [Google Scholar] [CrossRef]
- Liu, T.; Liang, Y.; Huang, L. Development and Delivery Systems of mRNA Vaccines. Front. Bioeng. Biotechnol. 2021, 9, 718753. [Google Scholar] [CrossRef]
- Mallory, K.L.; Taylor, J.A.; Zou, X.; Waghela, I.N.; Schneider, C.G.; Sibilo, M.Q.; Punde, N.M.; Perazzo, L.C.; Savransky, T.; Sedegah, M.; et al. Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice. NPJ Vaccines 2021, 6, 84. [Google Scholar] [CrossRef]
- Baeza Garcia, A.; Siu, E.; Sun, T.; Exler, V.; Brito, L.; Hekele, A.; Otten, G.; Augustijn, K.; Janse, C.J.; Ulmer, J.B.; et al. Neutralization of the Plasmodium-encoded MIF ortholog confers protective immunity against malaria infection. Nat. Commun. 2018, 9, 2714. [Google Scholar] [CrossRef] [PubMed]
- Duthie, M.S.; Van Hoeven, N.; MacMillen, Z.; Picone, A.; Mohamath, R.; Erasmus, J.; Hsu, F.-C.; Stinchcomb, D.T.; Reed, S.G. Heterologous Immunization With Defined RNA and Subunit Vaccines Enhances T Cell Responses That Protect Against Leishmania donovani. Front. Immunol. 2018, 9, 2420. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Zheng, L.; Hu, Y.; Liu, S.; Wang, Y.; Xiong, Z.; Hu, X.; Tan, F. Induction of Protective Immunity against Toxoplasma gondii in Mice by Nucleoside Triphosphate Hydrolase-II (NTPase-II) Self-amplifying RNA Vaccine Encapsulated in Lipid Nanoparticle (LNP). Front. Microbiol. 2017, 8, 605. [Google Scholar] [CrossRef] [PubMed]
- Sajid, A.; Matias, J.; Arora, G.; Kurokawa, C.; DePonte, K.; Tang, X.; Lynn, G.; Wu, M.J.; Pal, U.; Strank, N.O.; et al. mRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent. Sci. Transl. Med. 2021, 13, eabj9827. [Google Scholar] [CrossRef]
- Callaway, E.; Ledford, H. How to redesign COVID vaccines so they protect against variants. Nature 2021, 590, 15–16. [Google Scholar] [CrossRef]
- Muller, I.E.; Rubens, J.R.; Jun, T.; Graham, D.; Xavier, R.; Lu, T.K. Gene networks that compensate for crosstalk with crosstalk. Nat. Commun. 2019, 10, 4028. [Google Scholar] [CrossRef]
| Antigen | Immunization Strategy | Method of Immunization Regimen | Worm Burden Reduction % | Faecal Egg (FER) or Miracidia Hatching (MH) Reduction % | Ref. |
|---|---|---|---|---|---|
| Paramyosin | Recombinant | Montanide, ISA 206 | 52–61 | [118] | |
| SjTPI | DNA | Fused to bovine HSP70 | 41–51 | 33–52 (MH) | [70] |
| Sj23 | DNA | HSP70 and boosted with a plasmid DNA encoding IL-12 | 45–51 | 47–52 (MH) | [70] |
| Sj23 | DNA | 38 | 50 (MH), 60 (FE) | [103] | |
| Combination of SjTPI-Sj23 | DNA | Administered with pIL-12 and boosted with a combination of rSjC23 and rSjTPI | 55 | 57 (MH) | [111] |
| Combination of Sj23-Hsp70 | DNA | Administered with a plasmid DNA encoding IL-12 | 37–41 | 31–46 (MH) | [111] |
| Sj26GST | Recombinant protein | 22 | 50 (FE) | [119] | |
| Sj28GST | Recombinant protein | Injected with Freund’s adjuvants | 37 | 62 (MH) | [103] |
| Sj28 | DNA | 39 | 62(MH) | [103] | |
| Cryopreserved-irradiated/freeze-thaw schistosomula | Whole | Single/twice intradermal vaccination | 62–65 | [120] | |
| Cercariae UV irradiated | Whole | Six immunizations | 89 | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zumuk, C.P.; Jones, M.K.; Navarro, S.; Gray, D.J.; You, H. Transmission-Blocking Vaccines against Schistosomiasis Japonica. Int. J. Mol. Sci. 2024, 25, 1707. https://doi.org/10.3390/ijms25031707
Zumuk CP, Jones MK, Navarro S, Gray DJ, You H. Transmission-Blocking Vaccines against Schistosomiasis Japonica. International Journal of Molecular Sciences. 2024; 25(3):1707. https://doi.org/10.3390/ijms25031707
Chicago/Turabian StyleZumuk, Chika P., Malcolm K. Jones, Severine Navarro, Darren J. Gray, and Hong You. 2024. "Transmission-Blocking Vaccines against Schistosomiasis Japonica" International Journal of Molecular Sciences 25, no. 3: 1707. https://doi.org/10.3390/ijms25031707
APA StyleZumuk, C. P., Jones, M. K., Navarro, S., Gray, D. J., & You, H. (2024). Transmission-Blocking Vaccines against Schistosomiasis Japonica. International Journal of Molecular Sciences, 25(3), 1707. https://doi.org/10.3390/ijms25031707






