Machine Learning Methods for Gene Selection in Uveal Melanoma
Abstract
:1. Introduction
2. Results
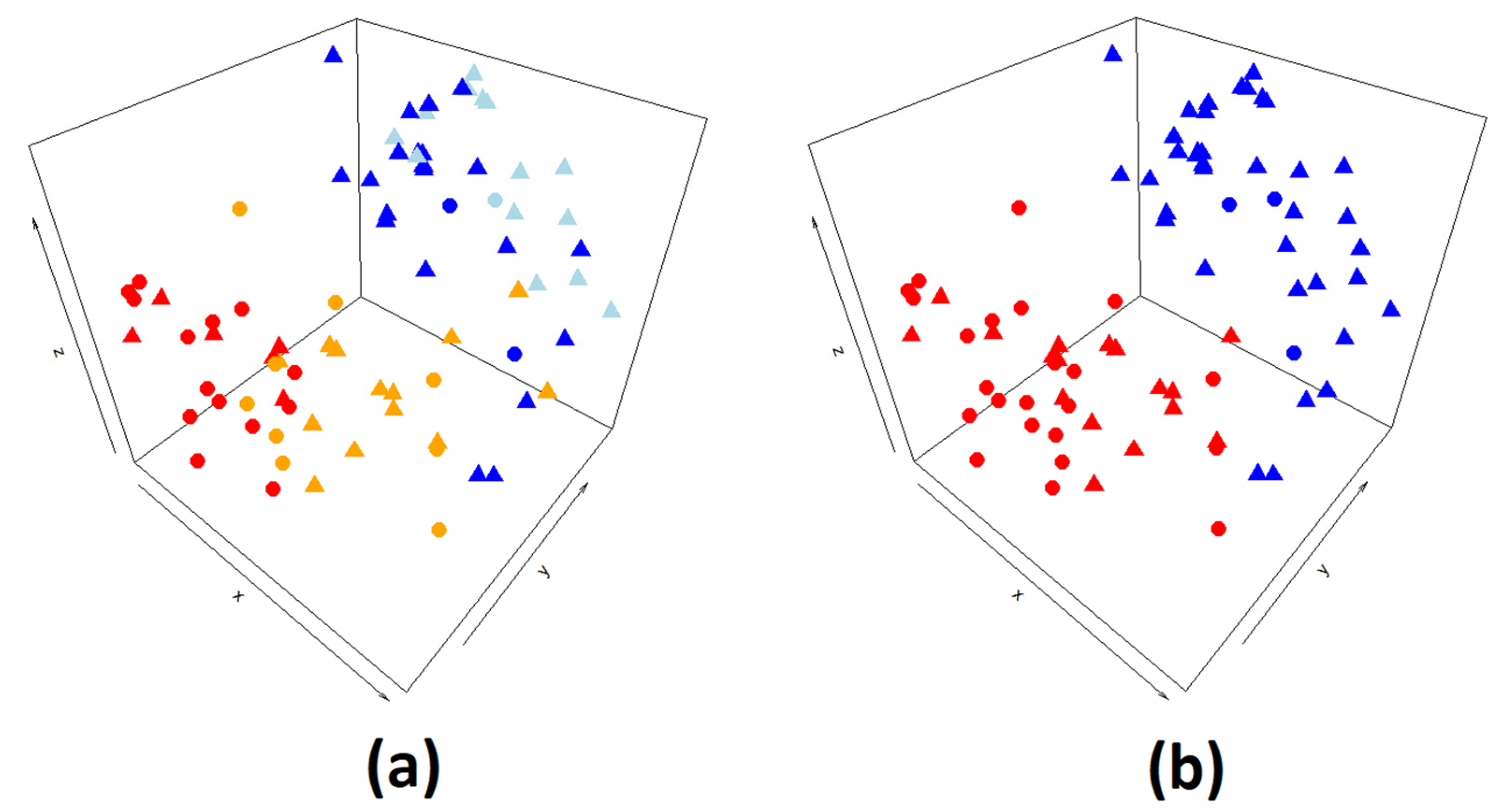
2.1. Gene Prioritization Methods
2.1.1. Data Fusion
2.1.2. CNA Analysis Methods
2.1.3. Methylation Analysis Methods
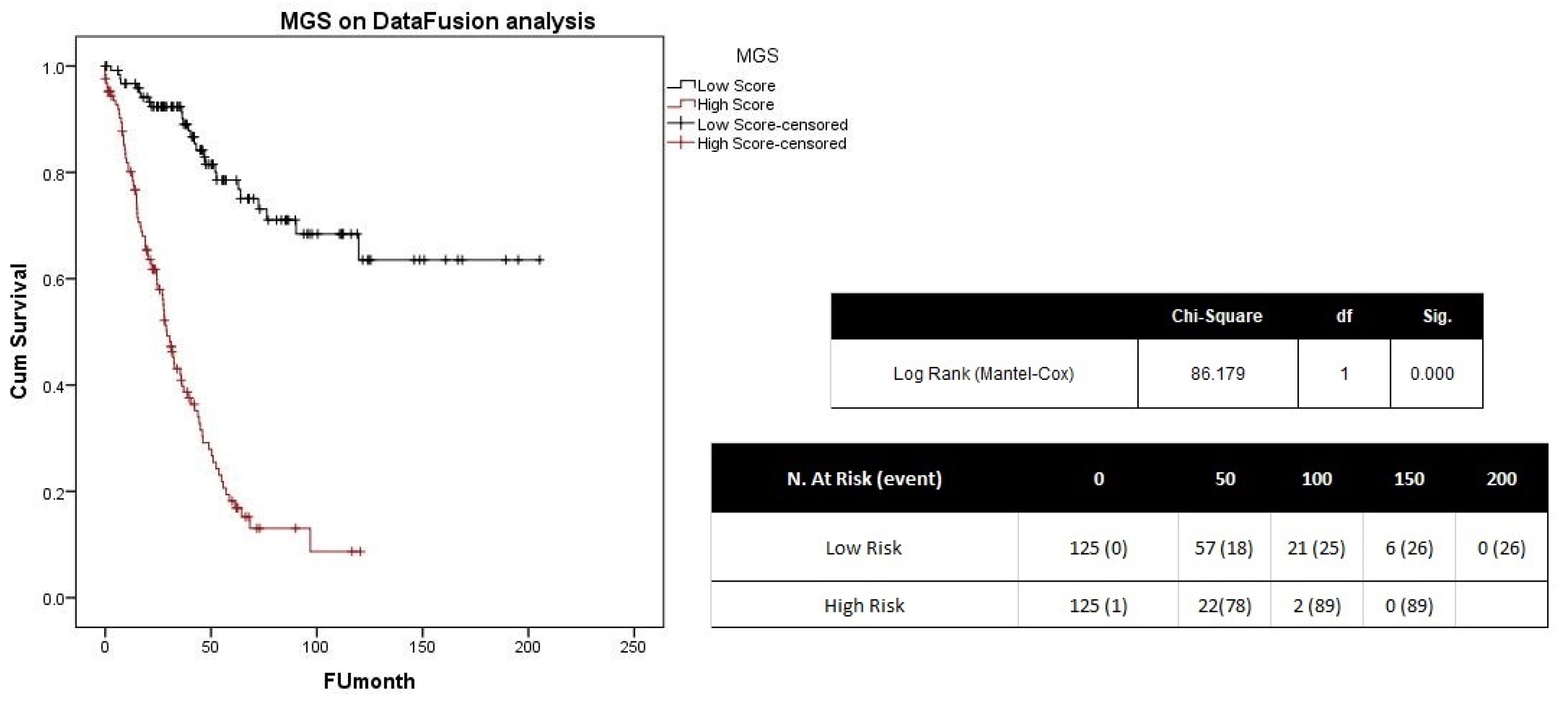
2.2. Integration of Results
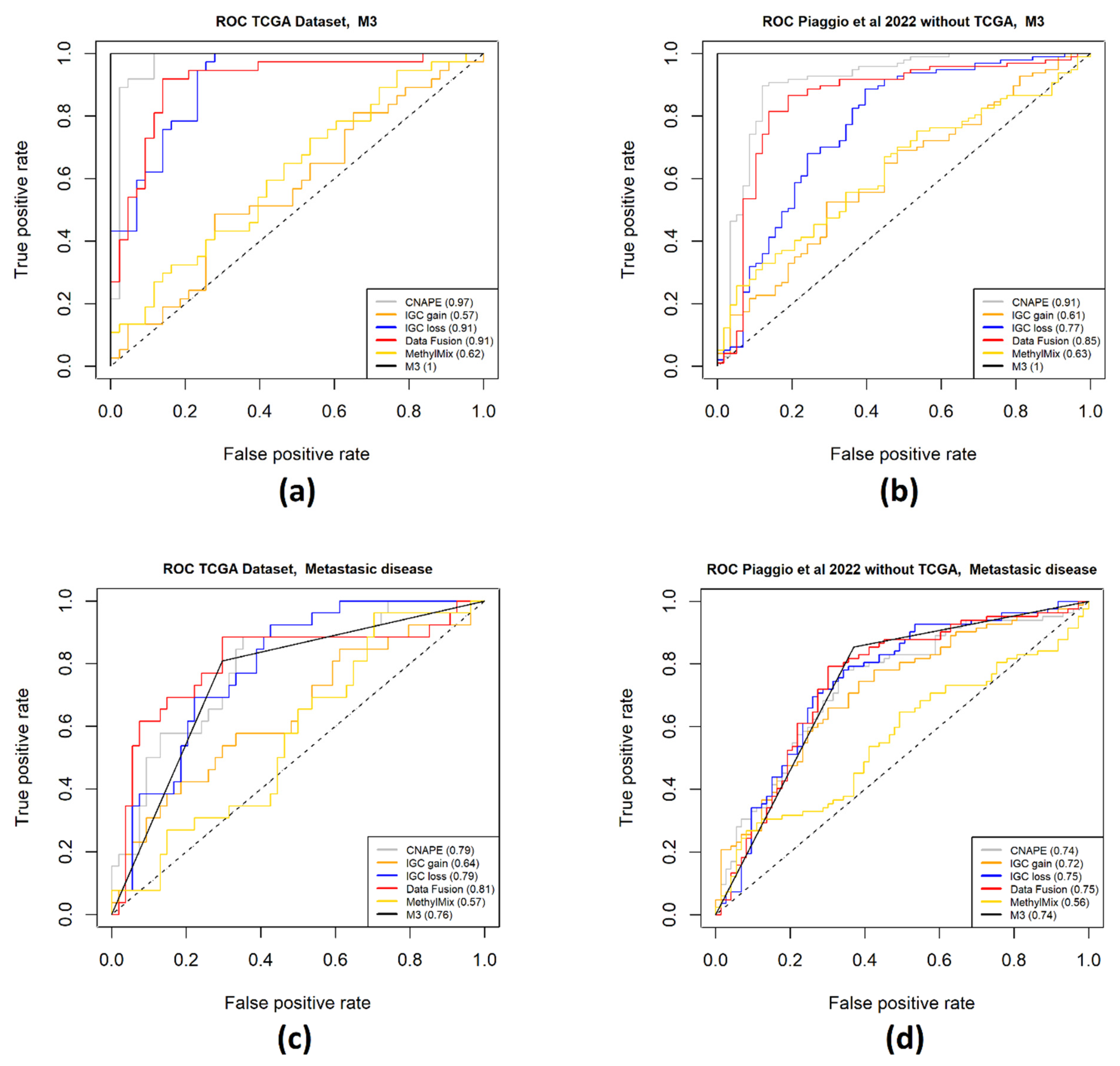
3. Discussion
4. Materials and Methods
4.1. jSVD Data Preparation and Analysis
4.2. CNAPE and IGC
4.3. MethylMix
4.4. Joint Singular Value Decomposition
4.5. Gene Signature Performance Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- The Cancer Genome Atlas Research Network; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer Analysis Project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Freeberg, M.A.; Fromont, L.A.; D’Altri, T.; Romero, A.F.; Ciges, J.I.; Jene, A.; Kerry, G.; Moldes, M.; Ariosa, R.; Bahena, S.; et al. The European Genome-Phenome Archive in 2021. Nucleic Acids Res. 2022, 50, D980–D987. [Google Scholar] [CrossRef] [PubMed]
- Church, G.M. The Personal Genome Project. Mol. Syst. Biol. 2005, 1, 2005.0030. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, P.; Chatzou, M.; Floden, E.W.; Barja, P.P.; Palumbo, E.; Notredame, C. Nextflow Enables Reproducible Computational Workflows. Nat. Biotechnol. 2017, 35, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Dotolo, S.; Esposito Abate, R.; Roma, C.; Guido, D.; Preziosi, A.; Tropea, B.; Palluzzi, F.; Giacò, L.; Normanno, N. Bioinformatics: From NGS Data to Biological Complexity in Variant Detection and Oncological Clinical Practice. Biomedicines 2022, 10, 2074. [Google Scholar] [CrossRef] [PubMed]
- Morabito, F.; Adornetto, C.; Monti, P.; Amaro, A.; Reggiani, F.; Colombo, M.; Rodriguez-Aldana, Y.; Tripepi, G.; D’Arrigo, G.; Vener, C.; et al. Genes Selection Using Deep Learning and Explainable Artificial Intelligence for Chronic Lymphocytic Leukemia Predicting the Need and Time to Therapy. Front. Oncol. 2023, 13, 1198992. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-P.; Wang, L.-B.; Wang, W.-A.; Lai, L.-C.; Tsai, M.-H.; Lu, T.-P.; Chuang, E.Y. iGC-an Integrated Analysis Package of Gene Expression and Copy Number Alteration. BMC Bioinform. 2017, 18, 35. [Google Scholar] [CrossRef]
- Pfeffer, M.; Uschmajew, A.; Amaro, A.; Pfeffer, U. Data Fusion Techniques for the Integration of Multi-Domain Genomic Data from Uveal Melanoma. Cancers 2019, 11, 1434. [Google Scholar] [CrossRef]
- Amaro, A.; Pfeffer, M.; Pfeffer, U.; Reggiani, F. Evaluation and Comparison of Multi-Omics Data Integration Methods for Subtyping of Cutaneous Melanoma. Biomedicines 2022, 10, 3240. [Google Scholar] [CrossRef]
- Duan, R.; Gao, L.; Gao, Y.; Hu, Y.; Xu, H.; Huang, M.; Song, K.; Wang, H.; Dong, Y.; Jiang, C.; et al. Evaluation and Comparison of Multi-Omics Data Integration Methods for Cancer Subtyping. PLoS Comput. Biol. 2021, 17, e1009224. [Google Scholar] [CrossRef]
- Leng, D.; Zheng, L.; Wen, Y.; Zhang, Y.; Wu, L.; Wang, J.; Wang, M.; Zhang, Z.; He, S.; Bo, X. A Benchmark Study of Deep Learning-Based Multi-Omics Data Fusion Methods for Cancer. Genome Biol. 2022, 23, 171. [Google Scholar] [CrossRef]
- Wang, B.; Mezlini, A.M.; Demir, F.; Fiume, M.; Tu, Z.; Brudno, M.; Haibe-Kains, B.; Goldenberg, A. Similarity Network Fusion for Aggregating Data Types on a Genomic Scale. Nat. Methods 2014, 11, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Croce, M.; Reggiani, F.; Schinzari, G.; Ambrosio, M.; Gangemi, R.; Tortora, G.; Pfeffer, U.; Amaro, A. Uveal Melanoma Metastasis. Cancers 2021, 13, 5684. [Google Scholar] [CrossRef] [PubMed]
- Amaro, A.; Gangemi, R.; Piaggio, F.; Angelini, G.; Barisione, G.; Ferrini, S.; Pfeffer, U. The Biology of Uveal Melanoma. Cancer Metastasis Rev. 2017, 36, 109–140. [Google Scholar] [CrossRef] [PubMed]
- Piaggio, F.; Tozzo, V.; Bernardi, C.; Croce, M.; Puzone, R.; Viaggi, S.; Patrone, S.; Barla, A.; Coviello, D.; Jager, M.J.; et al. Secondary Somatic Mutations in G-Protein-Related Pathways and Mutation Signatures in Uveal Melanoma. Cancers 2019, 11, 1688. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J.; Mungall, K.L.; Hess, J.M.; Uzunangelov, V.; Walter, V.; Danilova, L.; et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2017, 32, 204–220.e15. [Google Scholar] [CrossRef] [PubMed]
- Piaggio, F.; Croce, M.; Reggiani, F.; Monti, P.; Bernardi, C.; Ambrosio, M.; Banelli, B.; Dogrusöz, M.; Jockers, R.; Bordo, D.; et al. In Uveal Melanoma Gα-Protein GNA11 Mutations Convey a Shorter Disease-Specific Survival and Are More Strongly Associated with Loss of BAP1 and Chromosomal Alterations than Gα-Protein GNAQ Mutations. Eur. J. Cancer 2022, 170, 27–41. [Google Scholar] [CrossRef]
- Pfeffer, U.; Romeo, F.; Noonan, D.M.; Albini, A. Prediction of Breast Cancer Metastasis by Genomic Profiling: Where Do We Stand? Clin. Exp. Metastasis 2009, 26, 547–558. [Google Scholar] [CrossRef]
- Tibishirani, R.; Michael, J.; Seo, G.C.; Balasubramanian, N.; Jun, L. SAM: Significance Analysis of Microarrays R Package Version 2018, 3.0.
- Mu, Q.; Wang, J. CNAPE: A Machine Learning Method for Copy Number Alteration Prediction from Gene Expression. IEEE/ACM Trans. Comput. Biol. Bioinform. 2021, 18, 306–311. [Google Scholar] [CrossRef]
- Gevaert, O. MethylMix: An R Package for Identifying DNA Methylation-Driven Genes. Bioinformatics 2015, 31, 1839–1841. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Chen, L.; Zhang, J. Expression Analysis of Genes and Pathways Associated with Liver Metastases of the Uveal Melanoma. BMC Med. Genet. 2014, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Yu, Z.; Zhao, X.; Fu, X.; Liu, S.; Liang, S.; Deng, A. Immune Classification and Identification of Prognostic Genes for Uveal Melanoma Based on Six Immune Cell Signatures. Sci. Rep. 2021, 11, 22244. [Google Scholar] [CrossRef] [PubMed]
- Baqai, U.; Purwin, T.J.; Bechtel, N.; Chua, V.; Han, A.; Hartsough, E.J.; Kuznetsoff, J.N.; Harbour, J.W.; Aplin, A.E. Multi-Omics Profiling Shows BAP1 Loss Is Associated with Upregulated Cell Adhesion Molecules in Uveal Melanoma. Mol. Cancer Res. MCR 2022, 20, 1260–1271. [Google Scholar] [CrossRef]
- Geng, Y.; Geng, Y.; Liu, X.; Chai, Q.; Li, X.; Ren, T.; Shang, Q. PI3K/AKT/mTOR Pathway-Derived Risk Score Exhibits Correlation with Immune Infiltration in Uveal Melanoma Patients. Front. Oncol. 2023, 13, 1167930. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Ma, C. Identification of Prognostic Genes in Uveal Melanoma Microenvironment. PLoS ONE 2020, 15, e0242263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Deng, Y.; Wang, D.; Wang, S. Construction and Verification of the Molecular Subtype and a Novel Prognostic Signature Based on Inflammatory Response-Related Genes in Uveal Melanoma. J. Clin. Med. 2023, 12, 861. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, Z.; He, D.; Zhu, Y.; Gong, L.; Xiao, M.; Chen, X.; Cao, K. Analysis of Ferroptosis-Mediated Modification Patterns and Tumor Immune Microenvironment Characterization in Uveal Melanoma. Front. Cell Dev. Biol. 2021, 9, 685120. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, C.; Stella, M.; Broggi, G.; Russo, A.; Caltabiano, R.; Ragusa, M. Genetics and RNA Regulation of Uveal Melanoma. Cancers 2023, 15, 775. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S. The Prognostic Value of Immune-Related Genes AZGP1, SLCO5A1, and CTF1 in Uveal Melanoma. Front. Oncol. 2022, 12, 918230. [Google Scholar] [CrossRef]
- Shi, X.; Xia, S.; Chu, Y.; Yang, N.; Zheng, J.; Chen, Q.; Fen, Z.; Jiang, Y.; Fang, S.; Lin, J. CARD11 Is a Prognostic Biomarker and Correlated with Immune Infiltrates in Uveal Melanoma. PLoS ONE 2021, 16, e0255293. [Google Scholar] [CrossRef]
- Van Gils, W.; Lodder, E.M.; Mensink, H.W.; Kiliç, E.; Naus, N.C.; Brüggenwirth, H.T.; van Ijcken, W.; Paridaens, D.; Luyten, G.P.; de Klein, A. Gene Expression Profiling in Uveal Melanoma: Two Regions on 3p Related to Prognosis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4254–4262. [Google Scholar] [CrossRef]
- Yang, B.; Fan, Y.; Liang, R.; Wu, Y.; Gu, A. Identification of a Prognostic Six-Immune-Gene Signature and a Nomogram Model for Uveal Melanoma. BMC Ophthalmol. 2023, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Duan, J.; Luo, P.; Shao, L.; Chen, Q.; Tan, X.; Zhang, L.; Xu, X. SLC25A38 as a Novel Biomarker for Metastasis and Clinical Outcome in Uveal Melanoma. Cell Death Dis. 2022, 13, 330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Su, J.; Li, L.; Du, W. Identification of Precise Therapeutic Targets and Characteristic Prognostic Genes Based on Immune Gene Characteristics in Uveal Melanoma. Front. Cell Dev. Biol. 2021, 9, 666462. [Google Scholar] [CrossRef] [PubMed]
- Smit, K.N.; Boers, R.; Vaarwater, J.; Boers, J.; Brands, T.; Mensink, H.; Verdijk, R.M.; van IJcken, W.F.J.; Gribnau, J.; de Klein, A.; et al. Genome-Wide Aberrant Methylation in Primary Metastatic UM and Their Matched Metastases. Sci. Rep. 2022, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.-Y.; Xu, Y.-L.; Rong, H.; Yang, H. Low Level of PALMD Contributes to the Metastasis of Uveal Melanoma. Front. Oncol. 2022, 12, 802941. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zhang, Y. Integrative Analysis Identifies Key Genes Related to Metastasis and a Robust Gene-Based Prognostic Signature in Uveal Melanoma. BMC Med. Genom. 2022, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Steyaert, S.; Qiu, Y.L.; Zheng, Y.; Mukherjee, P.; Vogel, H.; Gevaert, O. Multimodal Deep Learning to Predict Prognosis in Adult and Pediatric Brain Tumors. Commun. Med. 2023, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, K.; Kong, W.; Huang, R.; Liu, L.; Wen, G.; Yu, Y. Multimodal Data Fusion Based on IGERNNC Algorithm for Detecting Pathogenic Brain Regions and Genes in Alzheimer’s Disease. Brief. Bioinform. 2023, 24, bbac515. [Google Scholar] [CrossRef]
- Yu, G.; Yang, Y.; Yan, Y.; Guo, M.; Zhang, X.; Wang, J. DeepIDA: Predicting Isoform-Disease Associations by Data Fusion and Deep Neural Networks. IEEE/ACM Trans. Comput. Biol. Bioinform. 2022, 19, 2166–2176. [Google Scholar] [CrossRef]
- Cai, Z.; Poulos, R.C.; Liu, J.; Zhong, Q. Machine Learning for Multi-Omics Data Integration in Cancer. iScience 2022, 25, 103798. [Google Scholar] [CrossRef] [PubMed]
- Turrisi, R.; Squillario, M.; Abate, G.; Uberti, D.; Barla, A. An Overview of Data Integration in Neuroscience with Focus on Alzheimer’s Disease. IEEE J. Biomed. Health Inform. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rappoport, N.; Shamir, R. NEMO: Cancer Subtyping by Integration of Partial Multi-Omic Data. Bioinformatics 2019, 35, 3348–3356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Parmigiani, G.; Johnson, W.E. ComBat-Seq: Batch Effect Adjustment for RNA-Seq Count Data. NAR Genom. Bioinform. 2020, 2, lqaa078. [Google Scholar] [CrossRef] [PubMed]
- De los Campos, G.; Gianola, D.; Rosa, G.J.M.; Weigel, K.A.; Crossa, J. Semi-Parametric Genomic-Enabled Prediction of Genetic Values Using Reproducing Kernel Hilbert Spaces Methods. Genet. Res. 2010, 92, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Berisha, V.; Krantsevich, C.; Hahn, P.R.; Hahn, S.; Dasarathy, G.; Turaga, P.; Liss, J. Digital Medicine and the Curse of Dimensionality. NPJ Digit. Med. 2021, 4, 153. [Google Scholar] [CrossRef] [PubMed]
- Ciaramella, A.; Nardone, D.; Staiano, A. Data Integration by Fuzzy Similarity-Based Hierarchical Clustering. BMC Bioinform. 2020, 21, 350. [Google Scholar] [CrossRef]
- Reggiani, F.; Ambrosio, M.; Croce, M.; Tanda, E.T.; Spagnolo, F.; Raposio, E.; Petito, M.; El Rashed, Z.; Forlani, A.; Pfeffer, U.; et al. Interdependence of Molecular Lesions That Drive Uveal Melanoma Metastasis. Int. J. Mol. Sci. 2023, 24, 15602. [Google Scholar] [CrossRef]
- Piovesan, D.; Hatos, A.; Minervini, G.; Quaglia, F.; Monzon, A.M.; Tosatto, S.C.E. Assessing Predictors for New Post Translational Modification Sites: A Case Study on Hydroxylation. PLoS Comput. Biol. 2020, 16, e1007967. [Google Scholar] [CrossRef]
- Ferrier, S.T.; Burnier, J.V. Novel Methylation Patterns Predict Outcome in Uveal Melanoma. Life 2020, 10, 248. [Google Scholar] [CrossRef]
- Koroknai, V.; Szász, I.; Hernandez-Vargas, H.; Fernandez-Jimenez, N.; Cuenin, C.; Herceg, Z.; Vízkeleti, L.; Ádány, R.; Ecsedi, S.; Balázs, M. DNA Hypermethylation Is Associated with Invasive Phenotype of Malignant Melanoma. Exp. Dermatol. 2020, 29, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Field, M.G.; Kuznetsov, J.N.; Bussies, P.L.; Cai, L.Z.; Alawa, K.A.; Decatur, C.L.; Kurtenbach, S.; Harbour, J.W. BAP1 Loss Is Associated with DNA Methylomic Repatterning in Highly Aggressive Class 2 Uveal Melanomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 5663–5673. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wilkerson, M.D.; Hayes, D.N. ConsensusClusterPlus: A Class Discovery Tool with Confidence Assessments and Item Tracking. Bioinformatics 2010, 26, 1572–1573. [Google Scholar] [CrossRef]
- Brock, G.; Pihur, V.; Datta, S.; Datta, S. clValid: An R Package for Cluster Validation. J. Stat. Softw. 2008, 25, 1–22. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Sato, H. Joint Singular Value Decomposition Algorithm Based on the Riemannian Trust-Region Method. JSIAM Lett. 2015, 7, 13–16. [Google Scholar] [CrossRef]
- Townsend, J.; Koep, N.; Weinchwald, S. Pymanopt: A Python Toolbox for Optimization on Manifolds Using Automatic Differentiation. J. Mach. Learn. Res. 2016, 17, 1–5. [Google Scholar]
- Mao, Y.; Gide, T.N.; Adegoke, N.A.; Quek, C.; Maher, N.; Potter, A.; Patrick, E.; Saw, R.P.M.; Thompson, J.F.; Spillane, A.J.; et al. Cross-Platform Comparison of Immune Signatures in Immunotherapy-Treated Patients with Advanced Melanoma Using a Rank-Based Scoring Approach. J. Transl. Med. 2023, 21, 257. [Google Scholar] [CrossRef]
- Vihinen, M. How to Evaluate Performance of Prediction Methods? Measures and Their Interpretation in Variation Effect Analysis. BMC Genom. 2012, 13 (Suppl. S4), S2. [Google Scholar] [CrossRef] [PubMed]
- Carraro, M.; Monzon, A.M.; Chiricosta, L.; Reggiani, F.; Aspromonte, M.C.; Bellini, M.; Pagel, K.; Jiang, Y.; Radivojac, P.; Kundu, K.; et al. Assessment of Patient Clinical Descriptions and Pathogenic Variants from Gene Panel Sequences in the CAGI-5 Intellectual Disability Challenge. Hum. Mutat. 2019, 40, 1330–1345. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, M.; Bhuva, D.D.; Lyu, R.; Horan, K.; Cursons, J.; Davis, M.J. Single Sample Scoring of Molecular Phenotypes. BMC Bioinform. 2018, 19, 404. [Google Scholar] [CrossRef] [PubMed]
- Bhuva, D.D.; Cursons, J.; Davis, M.J. Stable Gene Expression for Normalisation and Single-Sample Scoring. Nucleic Acids Res. 2020, 48, e113. [Google Scholar] [CrossRef] [PubMed]
- Sing, T.; Sander, O.; Beerenwinkel, N.; Lengauer, T. ROCR: Visualizing Classifier Performance in R. Bioinformatics 2005, 21, 3940–3941. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, E.; Ewens, K.; Richards-Yutz, J.; Ebrahimzedeh, J.; Terai, M.; Gonsalves, C.F.; Sato, T.; Shields, C.L.; Ganguly, A. Improved Uveal Melanoma Copy Number Subtypes Including an Ultra-High-Risk Group. Ophthalmol. Sci. 2022, 2, 100121. [Google Scholar] [CrossRef] [PubMed]
- Dogrusöz, M.; Ruschel Trasel, A.; Cao, J.; Çolak, S.; Van Pelt, S.I.; Kroes, W.G.M.; Teunisse, A.F.A.S.; Alsafadi, S.; Van Duinen, S.G.; Luyten, G.P.M.; et al. Differential Expression of DNA Repair Genes in Prognostically-Favorable versus Unfavorable Uveal Melanoma. Cancers 2019, 11, 1104. [Google Scholar] [CrossRef]
- Aryee, M.J.; Jaffe, A.E.; Corrada-Bravo, H.; Ladd-Acosta, C.; Feinberg, A.P.; Hansen, K.D.; Irizarry, R.A. Minfi: A Flexible and Comprehensive Bioconductor Package for the Analysis of Infinium DNA Methylation Microarrays. Bioinformatics 2014, 30, 1363–1369. [Google Scholar] [CrossRef]
- Dowle, M.; Srinivasan, A. Data.Table: Extension of “Data.Frame”. R Package Version 2021, 1.14.2.
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circlize Implements and Enhances Circular Visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Soetaert, K. plot3D: Plotting Multi-Dimensional Data. 2021. R Package Version 2022, 1.4.
- Hansen, K. IlluminaHumanMethylation450kanno.Ilmn12.Hg19: Annotation for Illumina’s 450k Methylation Arrays. R Package Version 2016, 0.6.0.
- Carlson, M. Org.Hs.Eg.Db: Genome Wide Annotation for Human. R Package Version 2019, 3.15.0.
- Wilke, O.C. Cowplot: Streamlined Plot Theme and Plot Annotations for “Ggplot2”. R package version 2020, 1.1.1.
- Yan, L. Ggvenn: Draw Venn Diagram by “Ggplot2”. R Package Version 2023, 0.1.10.
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]




| Number of k | 2 | 3 | 4 |
|---|---|---|---|
| Connectivity score | 2.25 | 6.27 | 15.66 |
| Silhouette score | 0.45 | 0.51 | 0.52 |
| GENE | CNAPE | IGC Loss | Data Fusion | n Overlap | Cytoband | MGS Score |
|---|---|---|---|---|---|---|
| ROBO1 | 1 | 1 | 1 | 3 | 3p12.3 | −0.241 |
| ROPN1 | 1 | 1 | 1 | 3 | 3q21.1 | 0.312 |
| CADM1 | 1 | 0 | 1 | 2 | 11q23.3 | 0.233 |
| ITPR2 | 1 | 0 | 1 | 2 | 12p12.1 | −0.323 |
| ISM1 | 1 | 0 | 1 | 2 | 20p12.1 | 0.213 |
| PDE4B | 1 | 0 | 1 | 2 | 1p31.3 | −0.291 |
| ACSF2 | 1 | 0 | 1 | 2 | 17q21.33 | 0.302 |
| BCHE | 0 | 1 | 1 | 2 | 3q26.1 | 0.274 |
| CHL1 | 0 | 1 | 1 | 2 | 3p26.3 | −0.152 |
| IL12RB2 | 0 | 0 | 1 | 1 | 1p31.3 | −0.225 |
| MTUS1 | 0 | 0 | 1 | 1 | 8p22 | −0.276 |
| CTF1 | 0 | 0 | 1 | 1 | 16p11.2 | −0.301 |
| CPS1 | 0 | 0 | 1 | 1 | 2q34 | 0.177 |
| HTR2B | 0 | 0 | 1 | 1 | 2q37.1 | 0.21 |
| CARD11 | 0 | 0 | 1 | 1 | 7p22.2 | −0.254 |
| TNFRSF19 | 0 | 0 | 1 | 1 | 13q12.12 | 0.125 |
| PTGER4 | 0 | 0 | 1 | 1 | 5p13.1 | 0.12 |
| Chr | CNAPE | IGC Gain | IGC Loss | Data Fusion | MethylMix |
|---|---|---|---|---|---|
| 1p | 9 | 0 | 606 | 5 | 6 |
| 1q | 8 | 0 | 0 | 1 | 4 |
| 3p | 71 | 0 | 301 | 7 | 4 |
| 3q | 53 | 0 | 285 | 5 | 0 |
| 6p | 11 | 265 | 0 | 2 | 5 |
| 6q | 3 | 0 | 244 | 1 | 2 |
| 8p | 5 | 1 | 0 | 2 | 1 |
| 8q | 11 | 236 | 0 | 3 | 3 |
| 16p | 3 | 0 | 0 | 2 | 2 |
| 16q | 2 | 0 | 98 | 1 | 0 |
| other | 123 | 0 | 0 | 48 | 63 |
| total | 299 | 502 | 1534 | 77 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reggiani, F.; El Rashed, Z.; Petito, M.; Pfeffer, M.; Morabito, A.; Tanda, E.T.; Spagnolo, F.; Croce, M.; Pfeffer, U.; Amaro, A. Machine Learning Methods for Gene Selection in Uveal Melanoma. Int. J. Mol. Sci. 2024, 25, 1796. https://doi.org/10.3390/ijms25031796
Reggiani F, El Rashed Z, Petito M, Pfeffer M, Morabito A, Tanda ET, Spagnolo F, Croce M, Pfeffer U, Amaro A. Machine Learning Methods for Gene Selection in Uveal Melanoma. International Journal of Molecular Sciences. 2024; 25(3):1796. https://doi.org/10.3390/ijms25031796
Chicago/Turabian StyleReggiani, Francesco, Zeinab El Rashed, Mariangela Petito, Max Pfeffer, Anna Morabito, Enrica Teresa Tanda, Francesco Spagnolo, Michela Croce, Ulrich Pfeffer, and Adriana Amaro. 2024. "Machine Learning Methods for Gene Selection in Uveal Melanoma" International Journal of Molecular Sciences 25, no. 3: 1796. https://doi.org/10.3390/ijms25031796






