Abstract
Obstructive sleep apnea (OSA), a respiratory sleep disorder associated with cardiovascular diseases, is more prevalent in men. However, OSA occurrence in pregnant women rises to a level comparable to men during late gestation, creating persistent effects on both maternal and offspring health. The exact mechanisms behind OSA-induced cardiovascular diseases remain unclear, but inflammation and oxidative stress play a key role. Animal models using intermittent hypoxia (IH), a hallmark of OSA, reveal several pro-inflammatory signaling pathways at play in males, such as TLR4/MyD88/NF-κB/MAPK, miRNA/NLRP3, and COX signaling, along with shifts in immune cell populations and function. Limited evidence suggests similarities in pregnancies and offspring. In addition, suppressing these inflammatory molecules ameliorates IH-induced inflammation and tissue injury, providing new potential targets to treat OSA-associated cardiovascular diseases. This review will focus on the inflammatory mechanisms linking IH to cardiovascular dysfunction in males, pregnancies, and their offspring. The goal is to inspire further investigations into the understudied populations of pregnant females and their offspring, which ultimately uncover underlying mechanisms and therapeutic interventions for OSA-associated diseases.
1. Introduction
Obstructive sleep apnea (OSA) is a multifactorial respiratory disorder characterized by repeated episodes of airway collapse, resulting in intermittent hypoxemia, hypercapnia, and sleep fragmentation. OSA sex-specifically affects nearly 1 billion adults worldwide [1], with its occurrence ~2.5 times higher in men than in premenopausal women [2]. However, pregnancy increases the occurrence of OSA from 8.4% at baseline (first trimester) to 19.7% by the third trimester when adjusted for body mass index (BMI) [3], thus reaching a prevalence similar to men [4]. OSA has emerged as a public health burden because of its morbidities and associated complications [5], especially cardiovascular diseases such as systemic hypertension [6], stroke [7], and preeclampsia [8]. Notably, gestational OSA experienced by the mother has long-lasting deleterious consequences also for the offspring [9].
A typical pattern coupled with the majority of respiratory events during OSA involves repetitive, short cycles of desaturation followed by rapid reoxygenation [10]. Such a sequence of desaturation and reoxygenation exposes organs and tissues to episodic hypoxia and normoxia. Therefore, using intermittent hypoxia (IH) exposure to experimentally mimic the hypoxemic events experienced in OSA may provide valuable insights into OSA and its pathogenetic pathways. The exact mechanisms underlying OSA-induced cardiovascular disease remain elusive. However, inflammatory processes and oxidative stress are considered to play a key role [11,12].
This review will focus on the major effect of IH and its underlying inflammatory mechanisms linked to cardiovascular outcomes in males, pregnancies, and offspring. It aims to integrate the acquired knowledge on IH-induced inflammatory mechanisms in male subjects with the currently emerging knowledge on IH-triggered immune activation in pregnancies and consequent impacts on offspring. Major inflammatory pathways at play in males, including TLR4/MyD88/NF-κB/MAPK, miRNA/NLRP3, and COX signaling, will be discussed while concerning the therapeutic effects of suppressing specific inflammatory targets within these pathways. Additionally, the effects of IH on immune responses in pregnancies and offspring represent a relatively novel research area and are, consequently, less explored. This review will delve into the limited findings within these understudied populations and discuss the implications for potential new interventions. This may allow for the integration of massive amounts of existing data and further enhance our understanding of this expanding field of research. This, in turn, may springboard the exploration of potential protective mechanisms for treating cardiovascular diseases associated with OSA and the IH it causes, especially in bridging existing knowledge gaps.
2. Basic Aspects of IH Exposures and Associated Inflammation
IH, characterized by episodic exposure to hypoxia interspersed with short-term normoxia, is a major component of OSA [13]. Experimentally, IH profiles can be divided into three general exposure categories: (1) acute IH, which involves brief and mild IH exposures (typically above 10% O2 for less than 2 h/day) administered during wake cycles for therapeutic purposes [14,15]; (2) chronic IH, which consists of prolonged and severe exposures (usually 5–10% O2 and 8 h/day for a few weeks) administered during sleep to simulate OSA conditions; and (3) gestational IH, which models chronic IH exposure during pregnancy to mimic a gestational OSA scenario and to investigate the effects of in utero IH exposures on the offspring [16]. Nevertheless, direct comparisons across studies have been hindered because each laboratory uses a slightly different IH protocol, with diverse magnitudes of hypoxia exposure, number of cycles per hour, and number of hours per day.
IH is a well-recognized trigger of inflammation and oxidative stress, which are closely related during the pathogenesis of cardiovascular complications, especially myocardial infarction, hypertension, and preeclampsia [17,18,19,20]. The reciprocal relationship between inflammation and oxidative stress allows one to easily induce the other. For example, IH generates oxidative stress by decreasing the antioxidant mechanisms and increasing reactive oxygen species (ROS) production during hypoxia/reoxygenation periods [21,22]. The overproduced ROS may directly damage DNA, lipids, and proteins critical for membrane integrity and cellular activity. Imperfect repair of such damage can lead to cell malfunction and apoptosis [23]. Furthermore, ROS may stimulate the transcriptional activities of the pro-inflammatory nuclear factor-kappaB (NF-κB) and activator protein-1 (AP-1) factors, leading to excessive inflammatory cytokine production, adhesion molecule expression, and leukocyte activation [24]. Conversely, inflammation can, in turn, exacerbate oxidative stress [23]. Phagocyte activation undergoes respiratory bursts, during which large amounts of ROS are generated as part of their defense mechanisms against pathogens [25,26]. Further, inflammation can disrupt the normal functioning of mitochondria [27], leading to impaired bioenergetics and excessive ROS production [28,29].
3. Chronic IH and Immune Activation in Males
OSA is well-known to elicit systemic inflammation in males [30,31,32], the markers of which correlate with cardiovascular disease in both OSA and non-OSA cohorts [30,32]. In rat models, chronic IH induces aortic tunica media thickening and myocardial injuries, such as cardiac hypertrophy, fibrosis, and apoptosis [33,34,35]. Additionally, chronic IH-induced inflammation causes endothelial dysfunction and injury [36,37], contributing to hypertension and atherosclerosis associated with OSA [38].
3.1. TLR4/MyD88/NF-κB/MAPK Signaling
Chronic IH-associated inflammation is suggested to be mediated by Toll-like receptor 4 (TLR4) and NF-κB [33,34,35,39,40,41,42], which are significantly upregulated in apneic patients [43,44,45,46]. TLR4 is a prototypical pattern recognition receptor that senses both infectious stimuli and noninfectious molecules produced as a result of tissue injury and plays an essential role in promoting inflammation [47]. TLR4 requires the adaptor protein, myeloid differentiation factor 88 (MyD88), for effective signaling, leading to the rapid activation of NF-κB and subsequent inflammatory cytokine production [48,49].
In male rats and mice, chronic IH simulating the repeated apnea events of OSA increased TLR4 expression in the heart, liver, and hippocampus [33,39,40]. Chronic IH exposure also significantly increased NF-κB expression in the serum and various tissues such as the aorta, heart, and liver [34,35,39,41,42]. Increased TLR4 and NF-κB levels accompanied the increased expression of inflammatory mediators, including tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, IL-8, IL-1β, monocyte chemoattractant protein-1 (MCP-1), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), c-reactive protein (CRP), and regulated upon activation normal T cell expressed and secreted (RANTES) [33,34,35,39,40]. The downregulation of TLR4 using either atorvastatin or short hairpin RNA (shRNA) in rats and mice decreased NF-κB expression and attenuated IH-induced oxidative stress, inflammation, and tissue remodeling [33,39,40]. Similarly, NF-κB inhibition via either p50 knockout or IκBα mutant overexpression diminished IH-induced vascular inflammation and injury in mice [41,42].
Mitogen-activated protein kinase (MAPK) is another signaling pathway that can be triggered by TLR4/MyD88. In male rats, chronic IH led to the increased phosphorylation of p38, extracellular signal-regulated kinases (ERKs), and c-Jun N-terminal kinase (JNK) in pancreatic tissue, which were associated with pancreatic inflammation [50,51]. In male mice, chronic IH increased ERK phosphorylation in the liver, contributing to liver fibrosis [52], and increased p38 phosphorylation in brain tissues, contributing to brain injury [53]. A canine model of intermittent airway obstruction caused upregulation of p38 phosphorylation and apoptosis and fibrosis-related factors, which were associated with myocardial apoptosis and fibrosis [54]. Both in vivo [51,53,54] and in vitro [55,56,57,58] experiments showed that suppressing the MAPK pathway partially ameliorated IH-induced inflammation.
MAPK cascades can modulate downstream transcription factors, which ultimately increases the expression of the AP-1 complex [59]. AP-1 regulates critical processes such as proliferation, differentiation, inflammation, and apoptosis [60,61]. IH exposures in vitro induce oxidative stress and subsequently increase c-Fos AP-1 activity in rat PC12 cells [62]. Chronic IH exposures in vivo lead to the increased expression of AP-1 subunits (c-Fos and FosB/ΔFosB), associated with chronic elevations of sympathetic nerve activity and mean arterial pressure in male rats [63,64]. Although AP-1 suppression may be a useful therapeutic approach for treating inflammatory diseases such as asthma, arthritis, and Parkinson’s disease [65], no studies so far have investigated its effects in IH models.
3.2. miRNA/NLRP3 Signaling
Exosomes are extracellular vesicles that mediate cell-to-cell communication by delivering DNA, RNA, proteins, or lipids. MicroRNAs (miRNAs) are one of the cargos transported by exosomes and are endogenous non-coding RNAs that regulate gene expression [66]. The biogenesis and functioning of miRNAs can be modulated by oxidative stress [67].
OSA alters exosomal carriers in circulation, especially miRNAs, and promotes endothelial dysfunction [68]. Several in vivo studies have demonstrated the involvement of miRNAs and the nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome in chronic IH-induced inflammation and tissue injury [36,69,70,71,72,73,74,75]. During IH exposures, miRNAs such as miR-155 [69], miR-210 [70], and miR-144 [71] are upregulated in renal tissue, endothelium, and serum-derived extracellular vesicles, respectively; the silencing of these miRNAs ameliorate IH-induced inflammation, endothelial dysfunction, and tissue injury [69,70,71].
miRNAs exert post-transcriptional regulation over various genes, including the NLRP3 inflammasome [76]. miR-155, miR-210, and miR-144 all provide positive feedback to NLRP3 activation [77,78,79]. The NLRP3 inflammasome is an oligomeric molecular complex that responds to endogenous stress and triggers innate immune defenses through the maturation of the pro-inflammatory cytokines IL-1β and IL-18 [80].
Clinically, monocytes from individuals with severe OSA present with higher NLRP3 activity than those from control subjects, which directly correlates with the apnea–hypopnea index (AHI) and hypoxemic indices [81]. Monocytes cultured under IH with plasma from healthy humans increase intracellular NLRP3 expression, whereas culturing with plasma from OSA patients increases NLRP3 under both normoxic and IH conditions [81]. The NLRP3 upregulation in OSA patients can be attributed to several potential pathways. For example, NLRP3 overexpression is associated with high levels of oxidized low-density lipoprotein (oxLDL) in plasma from OSA patients with early subclinical atherosclerosis [82], indicating an interaction between dyslipidemia and inflammation in inducing tissue injury. Exposing monocytes from healthy volunteers concomitantly to oxLDL stimulation or plasma from OSA patients with early subclinical atherosclerosis, followed by 16 h of IH, significantly increases NLRP3 activation and IL-1β production, compared to IH exposure alone, suggesting a synergistic act of oxLDL and IH [82]. In addition, several mechanisms of ROS-mediated activation of NLRP3 inflammasome have recently been shown, where apoptosis and mitochondrial damage are potential stimuli leading to NLRP3 inflammasome activation [69,83,84,85,86].
In animal models, chronic IH exposure increases NLRP3 inflammasome expression, and NLRP3 deficiency or inhibition protects against IH-induced inflammation, oxidative stress, and apoptosis in the brain, heart, and vasculature [36,72,73,74,75]. Additionally, in vitro IH exposure fails to induce IL-1β overproduction in bone marrow-derived macrophages isolated from NLRP3 knockout male mice [87], reinforcing the involvement of NLRP3 in IH-induced inflammation.
Notably, NF-κB signaling upregulates miR-155 and miR-210 [88,89], promoting the inflammatory cascade via NLRP3 activation [49], which aggravates myocardial injury and vascular dysfunction [90,91,92]. This reinforces that crosstalk between TLR4/MyD88/NF-κB and miRNA/NLRP3 contributes to chronic IH-induced inflammation and cardiovascular dysfunctions.
Statins, also known as 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are a class of cholesterol-lowering medications that also possess cardiovascular protective effects [93]. Atorvastatin, which shows efficacy in attenuating chronic IH-induced myocardial and neural inflammation via suppressing TLR4/MyD88/NF-κB signaling pathways [33,40,94], also suppresses NLRP3 inflammasome activation [95], although this has not been tested in IH models. A recent clinical trial indicates promising protective effects of atorvastatin against cardiovascular risks in OSA patients [96]. However, some studies showed conflicting effects of atorvastatin, where it caused increased p38 phosphorylation, miR-155 upregulation, and NLRP3 activation in mice and rats, leading to inflammation in the adipose tissue and brain [97,98]. Administration of aspirin [98] or rapamycin [97] attenuated the deleterious effects of atorvastatin. This underscores the need for caution when considering atorvastatin usage in the OSA population.
3.3. COX-1/Thromboxane and COX-2/PGE2 Signaling
Cyclooxygenase 1 (COX-1) and 2 (COX-2) catalyze the rate-limiting step in the production of eicosanoids from arachidonic acid. While COX-1 is constitutively expressed in most tissues and contributes to tissue homeostasis, COX-2 is normally absent but is rapidly inducible at sites of inflammation [99]. COX-catalyzed eicosanoids play fundamental roles in inflammatory response and blood pressure regulation.
OSA subjects presenting with cardiovascular risk factors exhibit elevated urinary 11-dehydrothromboxane B2 (an inactive metabolite of thromboxane A2) compared to OSA subjects free of cardiovascular risk factors and controls [100]. In animal models, chronic IH exposure increased mRNA levels of COX-1 and thromboxane synthase, which correlated with IH-induced atherosclerotic lesion size; the administration of the COX-1 inhibitor SC-560 reduced lesion progression [100]. Additionally, both in vivo [51,101,102,103,104,105] and in vitro [106] studies show that COX-2 is upregulated in response to IH, and there is a concomitant increase in prostaglandin E2 (PGE2) production. PGE2 promotes inflammatory processes and modulates the function of multiple cells involved in the immune response [107].
While aspirin effectively inhibits COX activity and PGE2 production, and it is widely used clinically to prevent adverse cardiovascular events, surprisingly, no studies so far have investigated its effects in chronic IH-exposed animal models. This might be because aspirin resistance is prevalent among OSA patients [108]. Moreover, identifying an aspirin dose adequate to prevent inflammation without inducing additional side effects (such as increased bleeding risk) is crucial. A recent cohort study indicated that continuous aspirin use might elevate the incidence of adverse cardiovascular events in hypertensive patients with OSA [109], possibly due to the inhibitory effects of aspirin on platelet aggregation, though the dose of aspirin usage was not specified. Nevertheless, several studies have delved into the therapeutic anti-inflammatory potential of melatonin in mitigating IH-induced tissue injury. It is suggested that melatonin can suppress IH-induced COX-2 overexpression and protect against inflammation and tissue injury in the heart, vasculature, and adrenal medulla [103,104,105]. Melatonin may represent a safer modality than aspirin for suppressing COX-dependent prostaglandin production in OSA.
In addition, flavonoids with antioxidant and anti-inflammation properties may be promising in treating OSA-related cardiovascular diseases, as they target major signaling pathways involved in IH-associated inflammation. For instance, baicalin can reduce ROS production, inhibit p38 MAPK/NF-κB signaling and the NLRP3 inflammasome, and decrease pro-inflammatory cytokines in apolipoprotein E-deficient mice, therefore improving atherosclerosis [110,111]. Chrysoeriol inhibits COX-2 expression and PGE2 production without notable cytotoxicity in lipopolysaccharide (LPS)-treated RAW 264.7 cells by ameliorating TLR4/NF-κB, p38 MAPK, and AP-1 activation [112]. A recent study has discovered an inverse relationship between the dietary intake of flavonoids and the risk of sleep disorders [113]. It is worth investigating whether flavonoids show efficacy in IH-induced inflammation and cardiovascular injury.
3.4. Shifts in Immune Cell Population and Function
Leukocytes are one of the best-characterized sources of ROS formation and inflammation. Leukocyte accumulation, adhesion, and the initiation of leukocyte/endothelial cell interactions may critically impair endothelial cell function and propagate vascular pathogenesis.
OSA is associated with increased expression of the adhesion molecules MCP-1, CD15 and CD11c by monocytes, increased adherence of monocytes in culture to human endothelial cells and increased intracellular ROS production in some monocyte subpopulations [55,114]. Meanwhile, delayed apoptosis and increased CD15 expression are noted in granulocytes from OSA patients [115], contributing to the prolonged release of inflammatory cytokines and ROS [116,117]. In addition, OSA is associated with a shift in CD4 and CD8 T cells toward type 2 cytokine dominance and increased cytotoxicity [118]. OSA patients exhibit increased expression of natural killer (NK) receptors, CD40 ligand, perforin, and TNF-α in CD8 cytotoxic T lymphocytes [118,119]. Paradoxically, some studies suggest that monocytes from OSA patients exhibit an immunosuppressive phenotype, which includes increased expressions of programmed cell death (PD)-1 receptor and its ligand (PD-L1) [120], a co-inhibitory immune checkpoint to maintain the quiescence of autoreactive T cells [121], reduced CD4 CD8 T cell proliferation and CD8 cytotoxicity [120], and high levels of TGF-β which impairs NK cytotoxicity and maturation [122]. These findings link OSA with cancer incidence and tumor aggressiveness [123]. Alterations of the cellular immune system in OSA have recently been reviewed [124].
There are limited data regarding the effects of chronic IH on immune cell populations and function. In male mice, 4 weeks of IH augmented the macrophage population and ROS release in lung tissue [125]. However, 6 weeks of IH increased PD-L1 expression in splenocytes isolated from male mice, suggestive of an immunosuppressive phenotype, although circulating inflammatory markers were not evaluated [120]. In male rats, IH with a shorter duration (7 days) induced an increase in both circulating M1 (IL-6, TNF-α, IFN-γ, IL-5) and M2 (IL-4, IL-10, IL-13) inflammatory markers [126]. In a different rat model of OSA, 3 h of intermittent airway obstruction significantly increased systemic leukocyte activation and P-selectin expression [127].
Evidenced from in vitro studies, purified granulocytes from healthy humans exposed in vitro to 6 h of IH resulted in increased NF-κB nuclear translocation, p38 MAPK phosphorylation, and expression of IL-8 and its receptor CXCR2 [128]. Exposing purified monocytes from healthy humans to 4.5 h of IH alone had minimal effects on monocyte transcripts, but a combination of IH and IL-1β stimulation together significantly increased chemokine and cytokine gene expression, and led to increased release of MCP-1, IL-6 and TNF-α [129]. Exposing CD14 monocytes isolated from healthy volunteers to alternating preconditioned hypoxic and normoxic media, which mimics IH episodes, resulted in increased TGF-β1 and IL-10 expression, pointing to an NK-suppressing phenotype [122]. Moreover, THP-1 monocytes pre-treated with an inhibitor for either ERK1/2 MAPK or p38 MAPK suppressed the activation of MCP-1 and C-C chemokine receptor 5 (CCR5) expression by IH [55,57]. Using bone marrow-derived macrophages from male mice, IH over 8 h per day for two consecutive days led to a pro-inflammatory M1 phenotype characterized by increased inducible nitric oxide synthase (iNOS) and IL-6 mRNA expression, and a robust increase in NF-κB DNA-binding activity and IL-6 secretion [87]. However, whether these functional alterations are sustained during prolonged IH exposure in vivo remains to be tested.
4. Gestational IH and Immune Activation during Pregnancy
The occurrence of OSA increases as pregnancy progresses [3]. While OSA is associated with NF-κB activation, inflammation, oxidative stress, and endothelial dysfunction in a general population [46,130] and OSA is associated with inflammation in women between the ages of 20 and 70 [131], limited research has explored such associations in pregnant women. Recent studies indicate that OSA events positively correlate with systemic inflammation in both normal pregnancies and those with gestational diabetes mellitus (GDM), as indicated by higher circulating levels of TNF-α, IL-1β, IL-8, and IL-10 [132,133]. However, whether the OSA-associated inflammation correlates with compromised cardiovascular function in pregnancy has not yet been established.
OSA is a known risk factor for the development of hypertensive disorders of pregnancy [134,135], including gestational hypertension, preeclampsia, and eclampsia. The severity of OSA positively correlates with the development of gestational hypertension and the severity of preeclampsia [136,137]. Numerous human studies have shown an association between OSA in pregnancy and adverse fetal outcomes commonly seen in preeclampsia, such as intrauterine growth restriction, low Apgar scores, preterm births, and neonatal intensive care unit (NICU) admissions [138].
In murine models, gestational IH exposure serves as a causal factor for maternal development of preeclampsia-like symptoms, including hypertension, proteinuria, uterine artery dysfunction, placental enlargement and morphological change, and fetal growth restriction [139,140,141]. The hypertensive effect of gestational IH is associated with endothelial dysfunction and deficient endothelin relaxation signaling pathways in pregnant dams [142]. Furthermore, dams exposed to gestational IH exhibit increased systemic and placental oxidative stress, along with heightened TNF-α production [140]. However, whether similar inflammatory mechanisms in males and non-pregnant females are also triggered in pregnant females [143] and whether blocking maternal pro-inflammatory molecules can reverse the adverse effects of gestational IH on dam blood pressure, vasculature, and placenta remain to be investigated.
Interestingly, previous studies exposing placental tissue and human umbilical vein endothelial cells (HUVECs) to ischemia/reperfusion (I/R) mimicking placental insufficiency in preeclampsia showed increased oxidative stress and increased production of TNF-α [144,145]. The administration of a TNF-α blocking antibody significantly reduced HUVEC cell activation during I/R [145]. This suggests promising prospects for preventing IH-induced hypertensive disorders of pregnancy by targeting inflammatory signaling.
In addition, miR-155 and miR-210, which are upregulated in IH-exposed male subjects, are also increased in preeclampsia, and serve as predictive biomarkers for preeclampsia development [146,147]. miR-144, however, is downregulated in preeclamptic placentas and plays a protective role by inhibiting pro-apoptotic phosphatase and tensin homolog (PTEN) [148]. The downregulation of miR-144 in preeclampsia aligns with findings from prior in vitro studies using cardiac and intestinal I/R injury models [149,150] but contrasts with observations from the chronic IH model in vivo [71]. It is worth investigating whether gestational IH alters miRNA-mediated inflammation in pregnant females and whether this contributes to hypertensive disorders of pregnancy.
Melatonin has antioxidant, anti-inflammatory, and anti-apoptotic properties [151] and has been shown to exert pleiotropic effects in various endocrinology and cardiology studies [152]. Recent experiments have extensively explored melatonin’s role in pregnancy, and a growing understanding of its physiological functions and its potential therapeutic use to improve maternal and neonatal outcomes has become available [153,154,155]. Melatonin emerges as a new potential candidate in the prevention of pregnancy complications. For example, melatonin supplementation during pregnancy mitigates hypertension and enhances uterine artery endothelial function in hypertensive pregnant mice [156]. Similarly, in rat preeclampsia models, melatonin administration lowers blood pressure and reduces inflammation and oxidative stress in the plasma, placenta, and fetal brain [157,158]. Melatonin supplementation during pregnancy also increases offspring survival during LPS-induced inflammation [159] and reduces blood pressure in the adult offspring [160,161]. However, whether melatonin is protective during gestational IH-induced hypertension and preeclampsia-like symptoms is yet to be investigated. Since melatonin has demonstrated cardiovascular protective effects in chronic IH-exposed males [103,104,105] and hypertensive pregnant females [156,157,158], it is promising that melatonin may exert anti-inflammatory and antioxidant effects also in gestational IH-exposed females, thereby ameliorating adverse cardiovascular events.
5. Gestational IH and Offspring Immune Activation
Whether OSA during pregnancy is associated with fetoplacental hypoxia is unclear as there is evidence on both sides. In humans, maternal OSA is reportedly associated with fetal normoblastemia in the placenta, a marker of fetal hypoxia, and with increased placental immunoreactivity of the tissue hypoxia marker, carbonic anhydrase IX [162]. In pregnant mice, 14.5 days of IH exposure during early- to mid-gestation results in higher levels of oxidative stress and hypoxia markers in the placenta [140]. However, contrary evidence from animal models suggests that the fetus is at least partially protected from modest, transient maternal hypoxia [163,164], likely by the placenta. In anesthetized pregnant sheep, real-time partial pressure of oxygen (PO2) measurements indicate that a 25 s obstruction results in a ~21 mmHg decrease in maternal blood PO2 but only a ~3 mmHg drop in fetal blood [163]. Similarly, pregnant rats exposed to a 5% O2 gas mixture have significantly dampened decreases in placental oxygen saturation compared to maternal skin [164]. These data are consistent with findings that hypoxia-inducible factor 1 alpha (HIF-1α) mRNA and its target genes are not altered in placentas or fetal brain at E19 during gestational IH exposure, despite a decline in arterial oxygen saturation in the dam to ~80–85% with each hypoxic episode [165]. Typical oxygen saturation levels in pregnant women with sleep apnea range from 80 to 90% [166,167,168]. Further, the injection of dams with an oxygen-sensing probe (hydroxyprobe) that forms protein thiol adducts in hypoxic cells showed no change in gestational IH-exposed fetal brains relative to controls [165].
Despite the controversy over OSA-associated fetoplacental hypoxia, recent studies consistently highlight adverse fetal and offspring outcomes of gestational OSA. In humans, gestational OSA is associated with low birth weight, accelerated fetal growth, increased adiposity acquisition indicative of potential metabolic disorders, and impaired neurodevelopment in early childhood [169,170,171]. Correspondingly, animal models suggest that gestational IH leads to compromised social and cognitive function [165,172,173], as well as cardiovascular [174,175,176] and metabolic [177,178,179] dysfunctions.
In murine models, gestational IH induced hypertension and endothelial dysfunction, reduced perivascular adiponectin, and elevated TNF-α and oxidative stress marker, 4-hydroxynonenal (4-HNE) in adult male but not female offspring [174,175]. Additionally, gestational IH resulted in tunica intima thickening and the development of pre-atherosclerotic lesions in adult male offspring, which is associated with upregulated NF-κB translocation, p38 MAPK phosphorylation, and the production of TNF-α, IL-8, and CRP [176].
In adolescent male offspring, gestational IH exposure significantly lowered the expression of genes associated with glucose and lipid metabolism and capillary density in respiratory and limb muscles [177]. Impaired mitochondrial metabolism and alteration of oxidative myofibers of the geniohyoid muscle were observed, affecting tongue traction and respiration [178]. Moreover, gestational IH-induced metabolic dysfunction was associated with epigenomic alterations and increased macrophages in visceral white adipose tissue. The macrophage population shifted toward a pro-inflammatory phenotype, as indicated by an increased M1/M2 ratio [179].
The origin of this enduring, sex-specific effect of gestational IH in offspring is unknown, but evidence points to the influence of sex hormones and functional ovaries post-puberty [180]. Evidence suggests that female offspring are hypertensive in the prepubertal age, but after puberty, they become normotensive by 10 weeks of age, coinciding with increased levels of estradiol associated with puberty [180]; in contrast, male offspring are impacted more than their female counterparts in young adulthood, as evidenced by their hypertensive phenotypes [175] and subcortical brain maturation [181]. In addition, the disruption of the neuroendocrine stress pathways may also be a key mechanism by which gestational IH selectively increases disease risk in progeny since neuronal activity in the paraventricular nucleus (PVN) of the hypothalamus, an autonomic control center regulating stress response and blood pressure homeostasis, was specifically increased in male but not female offspring born to gestational IH-exposed dams [182].
The exact mechanisms underlying gestational IH-mediated cardiometabolic dysfunction in offspring, along with the associated changes in immune responses, remain less understood. Further investigations are necessary to elucidate how the effects of gestational IH are transmitted to the offspring and program long-lasting consequences. Interestingly, milk exosomes and their miRNA cargo can pass to the offspring through lactation and may impact the epigenetic programming of various organs and immune responses in the offspring [183], although this has not been tested in gestational IH models. It is worth investigating whether maternal-originated miRNAs contribute to cardiovascular and metabolic diseases in the offspring.
6. Conclusions
Inflammation induced by OSA-simulating IH is consistently evident in males, pregnant females, and their offspring and critically contributes to oxidative injury, apoptosis, and cardiovascular dysfunction. In males, the key contributors to chronic IH-induced cardiovascular dysfunction include TLR4/MyD88/NF-κB/MAPK, miRNA/NLRP3, and COX signaling (Figure 1). These pathways are associated with a shift in immune cell populations and function. Specific inhibition of these pathways using either pharmacological methods or transcriptive modifications successfully attenuated inflammation and oxidative stress in the cardiovascular system and improved cardiovascular function. On the other hand, there is a lack of research on the gestational effects of OSA-simulating IH in pregnant moms and their offspring despite the prevalence of OSA during gestation. This highlights the need for future investigations in these understudied populations. Based on the current studies, while it remains uncertain whether the same signaling pathways in males are activated in IH-exposed pregnant females and their offspring, noteworthy similarities have been identified, underscoring the necessity for more in-depth investigations into the underlying inflammatory pathways. More importantly, targeting these inflammatory signaling molecules holds promise for innovative interventions against IH-induced cardiovascular diseases in pregnancies and offspring. Future studies should consider evaluating translatable inflammatory targets in IH-exposed females and offspring.
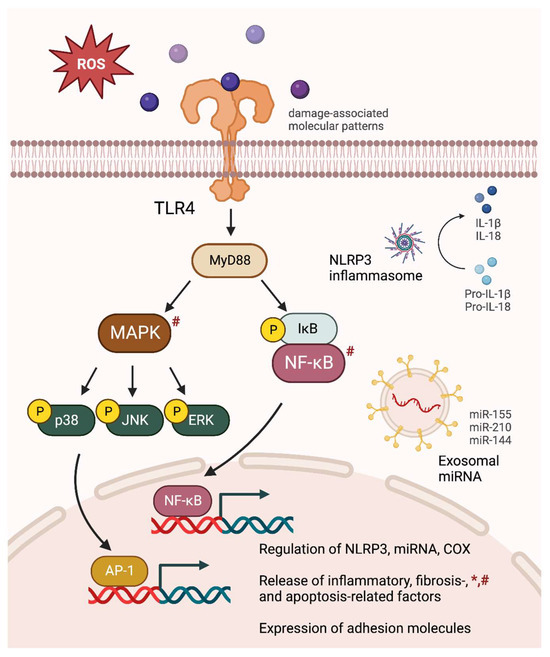
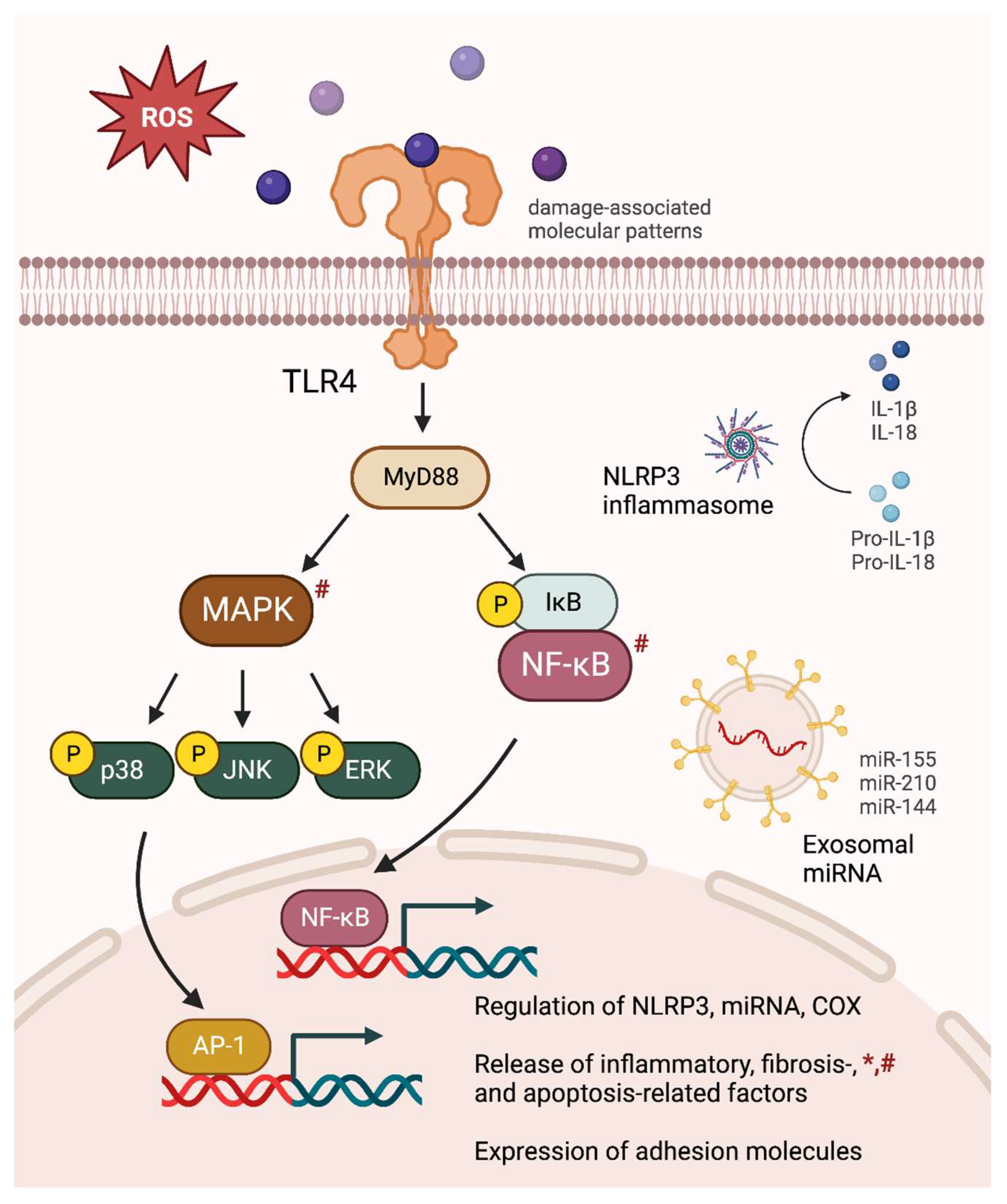
Figure 1.
Comprehensive inflammatory consequences of intermittent hypoxia (IH) exposures. IH-induced oxidative stress causes MAPK and NF-κB activation, leading to an upregulation of NLRP3 inflammasome, pro-inflammatory miRNA, and COX signaling. The releases of inflammatory cytokines, fibrosis- and apoptosis-related factors are increased, accompanied by an elevation in adhesion molecule expression. The figure specifically depicts major inflammatory signaling pathways observed in chronic IH-exposed male subjects. Similar alterations found in gestational IH-exposed pregnant females and their offspring are denoted by * and #, respectively.
Nevertheless, it is important to note that inflammation elicited by OSA may result from multiple factors beyond IH per se, such as obesity and nocturnal arousal. For example, 12 weeks of IH increased hepatic TNF-α gene expression only in mice fed a high-cholesterol diet [184]. In OSA patients, CRP levels are significantly correlated with BMI, hip/waist ratio, neck circumference, and frequent arousal [185,186]. This underscores the multifactorial nature of OSA-related inflammation and highlights the importance of considering these contributing factors in understanding and addressing cardiovascular consequences.
Author Contributions
Conceptualization, R.S. and S.K.; writing—original draft preparation, R.S.; writing—review and editing, T.L.B., J.J.W. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by R01ES033345, R01HL134779 and R01HL142752.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Pien, G.W.; Pack, A.I.; Jackson, N.; Maislin, G.; Macones, G.A.; Schwab, R.J. Risk factors for sleep-disordered breathing in pregnancy. Thorax 2014, 69, 371–377. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef]
- Redline, S.; Azarbarzin, A.; Peker, Y. Obstructive sleep apnoea heterogeneity and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 560–573. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef]
- Lungeanu-Juravle, L.; Patrascu, N.; Deleanu, O.C.; Cinteza, M. The Role of Obstructive Sleep Apnea in Developing Gestational Hypertension and Preeclampsia. Maedica 2016, 11, 330–333. [Google Scholar]
- Passarella, E.; Czuzoj-Shulman, N.; Abenhaim, H.A. Maternal and fetal outcomes in pregnancies with obstructive sleep apnea. J. Perinat Med. 2021, 49, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.; Pepin, J.L.; Arnaud, C.; Tamisier, R.; Borel, J.C.; Dematteis, M.; Godin-Ribuot, D.; Ribuot, C. Intermittent hypoxia and sleep-disordered breathing: Current concepts and perspectives. Eur. Respir. J. 2008, 32, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L. Intermittent hypoxia: The culprit of oxidative stress, vascular inflammation and dyslipidemia in obstructive sleep apnea. Expert Rev. Respir. Med. 2008, 2, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Nakano, H.; Maekawa, J.; Okamoto, Y.; Ohnishi, Y.; Suzuki, T.; Kimura, H. Oxidative stress in obstructive sleep apnea. Chest 2005, 127, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent hypoxemia and OSA: Implications for comorbidities. Chest 2015, 147, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, W.; Li, S.; Ding, Y.; Wang, Y.; Ji, X. Intermittent Hypoxia Conditioning: A Potential Multi-Organ Protective Therapeutic Strategy. Int. J. Med. Sci. 2023, 20, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic potential of intermittent hypoxia: A matter of dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1181–R1197. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Randhawa, K.S.; Epstein, J.J.; Gustafson, E.; Hocker, A.D.; Huxtable, A.G.; Baker, T.L.; Watters, J.J. Gestational intermittent hypoxia increases susceptibility to neuroinflammation and alters respiratory motor control in neonatal rats. Respir. Physiol. Neurobiol. 2018, 256, 128–142. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Phoswa, W.N.; Khaliq, O.P. The Role of Oxidative Stress in Hypertensive Disorders of Pregnancy (Preeclampsia, Gestational Hypertension) and Metabolic Disorder of Pregnancy (Gestational Diabetes Mellitus). Oxid. Med. Cell. Longev. 2021, 2021, 5581570. [Google Scholar] [CrossRef]
- Harmon, A.C.; Cornelius, D.C.; Amaral, L.M.; Faulkner, J.L.; Cunningham, M.W., Jr.; Wallace, K.; LaMarca, B. The role of inflammation in the pathology of preeclampsia. Clin. Sci. 2016, 130, 409–419. [Google Scholar] [CrossRef]
- Dinh, Q.N.; Drummond, G.R.; Sobey, C.G.; Chrissobolis, S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res. Int. 2014, 2014, 406960. [Google Scholar] [CrossRef]
- Yuan, G.; Nanduri, J.; Khan, S.; Semenza, G.L.; Prabhakar, N.R. Induction of HIF-1α expression by intermittent hypoxia: Involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J. Cell. Physiol. 2008, 217, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, J.; Wang, N.; Yuan, G.; Khan, S.A.; Souvannakitti, D.; Peng, Y.-J.; Kumar, G.K.; Garcia, J.A.; Prabhakar, N.R. Intermittent hypoxia degrades HIF-2α via calpains resulting in oxidative stress: Implications for recurrent apnea-induced morbidities. Proc. Natl. Acad. Sci. USA 2009, 106, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia—revisited—the bad ugly and good: Implications to the heart and brain. Sleep Med. Rev. 2015, 20, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.R.; Segal, A.W. The NADPH oxidase of professional phagocytes-prototype of the NOX electron transport chain systems. Biochim. Biophys. Acta 2004, 1657, 1–22. [Google Scholar] [CrossRef]
- Forman, H.J.; Torres, M. Reactive oxygen species and cell signaling: Respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 2002, 166, S4–S8. [Google Scholar] [CrossRef]
- van Horssen, J.; van Schaik, P.; Witte, M. Inflammation and mitochondrial dysfunction: A vicious circle in neurodegenerative disorders? Neurosci. Lett. 2019, 710, 132931. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Trombetti, F.; Pagliarani, A.; Ventrella, V.; Algieri, C.; Tioli, G.; Lenaz, G. Molecular and Supramolecular Structure of the Mitochondrial Oxidative Phosphorylation System: Implications for Pathology. Life 2021, 11, 242. [Google Scholar] [CrossRef]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef]
- Lavie, L. Obstructive sleep apnoea syndrome—An oxidative stress disorder. Sleep Med. Rev. 2003, 7, 35–51. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Kales, A.; Tyson, K.; Chrousos, G.P. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. J. Clin. Endocrinol. Metab. 1997, 82, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Minoguchi, K.; Yokoe, T.; Tazaki, T.; Minoguchi, H.; Tanaka, A.; Oda, N.; Okada, S.; Ohta, S.; Naito, H.; Adachi, M. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2005, 172, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Deng, Y.; Guo, X.; Shang, J.; Zhu, D.; Liu, H. Atorvastatin attenuates myocardial remodeling induced by chronic intermittent hypoxia in rats: Partly involvement of TLR-4/MYD88 pathway. Biochem. Biophys. Res. Commun. 2014, 446, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Yao, D.; Cai, X.D.; Ding, C.; Lin, Q.D.; Wang, L.X.; Huang, X.Y. Effect of chronic continual- and intermittent hypoxia-induced systemic inflammation on the cardiovascular system in rats. Sleep Breath. 2015, 19, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Bian, Y.; Yu, F.; Zhang, Q.; Zhang, G.; Li, Y.; Song, S.; Ren, X.; Tong, J. Chronic intermittent hypoxia induces cardiac inflammation and dysfunction in a rat obstructive sleep apnea model. J. Biomed. Res. 2016, 30, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.R.; Zhang, L.; Lin, Y.N.; Sun, X.W.; Ding, Y.J.; Li, N.; Li, H.P.; Li, S.Q.; Zhou, J.P.; Li, Q.Y. Chronic intermittent hypoxia-induced mitochondrial dysfunction mediates endothelial injury via the TXNIP/NLRP3/IL-1beta signaling pathway. Free. Radic. Biol. Med. 2021, 165, 401–410. [Google Scholar] [CrossRef]
- Badran, M.; Golbidi, S.; Devlin, A.; Ayas, N.; Laher, I. Chronic intermittent hypoxia causes endothelial dysfunction in a mouse model of diet-induced obesity. Sleep Med. 2014, 15, 596–602. [Google Scholar] [CrossRef]
- Tuleta, I.; Franca, C.N.; Wenzel, D.; Fleischmann, B.; Nickenig, G.; Werner, N.; Skowasch, D. Intermittent Hypoxia Impairs Endothelial Function in Early Preatherosclerosis. Adv. Exp. Med. Biol. 2015, 858, 1–7. [Google Scholar] [CrossRef]
- Lin, Z.P.; Lin, H.L.; Yu, X.P.; Zheng, Y.J.; Cheng, S.Y. TLR4 mediates inflammation and hepatic fibrosis induced by chronic intermittent hypoxia in rats. Mol. Med. Rep. 2020, 22, 651–660. [Google Scholar] [CrossRef]
- Deng, Y.; Yuan, X.; Guo, X.L.; Zhu, D.; Pan, Y.Y.; Liu, H.G. Efficacy of atorvastatin on hippocampal neuronal damage caused by chronic intermittent hypoxia: Involving TLR4 and its downstream signaling pathway. Respir. Physiol. Neurobiol. 2015, 218, 57–63. [Google Scholar] [CrossRef]
- Song, D.; Fang, G.; Mao, S.Z.; Ye, X.; Liu, G.; Gong, Y.; Liu, S.F. Chronic intermittent hypoxia induces atherosclerosis by NF-kappaB-dependent mechanisms. Biochim. Biophys. Acta 2012, 1822, 1650–1659. [Google Scholar] [CrossRef]
- Song, D.; Fang, G.; Mao, S.Z.; Ye, X.; Liu, G.; Miller, E.J.; Greenberg, H.; Liu, S.F. Selective inhibition of endothelial NF-kappaB signaling attenuates chronic intermittent hypoxia-induced atherosclerosis in mice. Atherosclerosis 2018, 270, 68–75. [Google Scholar] [CrossRef]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Predictors of elevated nuclear factor-κB–dependent genes in obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 824–830. [Google Scholar] [CrossRef]
- Viciani, E.; Montagnani, F.; Tavarini, S.; Tordini, G.; Maccari, S.; Morandi, M.; Faenzi, E.; Biagini, C.; Romano, A.; Salerni, L.; et al. Paediatric obstructive sleep apnoea syndrome (OSAS) is associated with tonsil colonisation by Streptococcus pyogenes. Sci. Rep. 2016, 6, 20609. [Google Scholar] [CrossRef]
- Akinnusi, M.; Jaoude, P.; Kufel, T.; El-Solh, A.A. Toll-like receptor activity in patients with obstructive sleep apnea. Sleep Breath. 2013, 17, 1009–1016. [Google Scholar] [CrossRef]
- Htoo, A.K.; Greenberg, H.; Tongia, S.; Chen, G.; Henderson, T.; Wilson, D.; Liu, S.F. Activation of nuclear factor κB in obstructive sleep apnea: A pathway leading to systemic inflammation. Sleep Breath. 2006, 10, 43–50. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Kuzmich, N.N.; Sivak, K.V.; Chubarev, V.N.; Porozov, Y.B.; Savateeva-Lyubimova, T.N.; Peri, F. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hai, B.; Niu, X.; Ai, L.; Cao, Y.; Li, R.; Li, Y. Chronic intermittent hypoxia disturbs insulin secretion and causes pancreatic injury via the MAPK signaling pathway. Biochem. Cell Biol. 2017, 95, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ai, L.; Hai, B.; Cao, Y.; Li, R.; Li, H.; Li, Y. Tempol alleviates chronic intermittent hypoxia-induced pancreatic injury through repressing inflammation and apoptosis. Physiol. Res. 2019, 68, 445–455. [Google Scholar] [CrossRef]
- Kang, H.H.; Kim, I.K.; Lee, H.I.; Joo, H.; Lim, J.U.; Lee, J.; Lee, S.H.; Moon, H.S. Chronic intermittent hypoxia induces liver fibrosis in mice with diet-induced obesity via TLR4/MyD88/MAPK/NF-kB signaling pathways. Biochem. Biophys. Res. Commun. 2017, 490, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, W.; Jin, H.; Nie, X.; Shen, H.; Li, E.; Wang, W. Curcumin attenuates chronic intermittent hypoxia-induced brain injuries by inhibiting AQP4 and p38 MAPK pathway. Respir. Physiol. Neurobiol. 2018, 255, 50–57. [Google Scholar] [CrossRef]
- Li, W.; Yan, S.; Zhao, J.; Ding, X.; Zhang, S.; Wang, D.; Liu, L.; Peng, W.; Li, H.; Wang, D.; et al. Metoprolol Inhibits Cardiac Apoptosis and Fibrosis in a Canine Model of Chronic Obstructive Sleep Apnea. Cell. Physiol. Biochem. 2015, 36, 1131–1141. [Google Scholar] [CrossRef]
- Chuang, L.P.; Chen, N.H.; Lin, Y.; Ko, W.S.; Pang, J.H. Increased MCP-1 gene expression in monocytes of severe OSA patients and under intermittent hypoxia. Sleep Breath. 2016, 20, 425–433. [Google Scholar] [CrossRef]
- Chen, J.K.; Guo, M.K.; Bai, X.H.; Chen, L.Q.; Su, S.M.; Li, L.; Li, J.Q. Astragaloside IV ameliorates intermittent hypoxia-induced inflammatory dysfunction by suppressing MAPK/NF-kappaB signalling pathways in Beas-2B cells. Sleep Breath. 2020, 24, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.P.; Chen, N.H.; Lin, S.W.; Hu, H.C.; Kao, K.C.; Li, L.F.; Yang, C.T.; Huang, C.C.; Pang, J.S. Monocytic C-C chemokine receptor 5 expression increases in in vitro intermittent hypoxia condition and in severe obstructive sleep apnea patients. Sleep Breath. 2019, 23, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, C.; Li, N.; Zhang, L. Propofol selectively inhibits nuclear factor-kappaB activity by suppressing p38 mitogen-activated protein kinase signaling in human EA.hy926 endothelial cells during intermittent hypoxia/reoxygenation. Mol. Med. Rep. 2014, 9, 1460–1466. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Kim, H.H.; Abusaliya, A.; Vetrivel, P.; Ha, S.E.; Park, M.Y.; Lee, H.J.; Kim, G.S. Structural and Functional Properties of Activator Protein-1 in Cancer and Inflammation. Evid. Based Complement. Altern. Med. 2022, 2022, 9797929. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Renoux, F.; Stellato, M.; Haftmann, C.; Vogetseder, A.; Huang, R.; Subramaniam, A.; Becker, M.O.; Blyszczuk, P.; Becher, B.; Distler, J.H.W.; et al. The AP1 Transcription Factor Fosl2 Promotes Systemic Autoimmunity and Inflammation by Repressing Treg Development. Cell Rep. 2020, 31, 107826. [Google Scholar] [CrossRef]
- Yuan, G.; Adhikary, G.; McCormick, A.A.; Holcroft, J.J.; Kumar, G.K.; Prabhakar, N.R. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J. Physiol. 2004, 557, 773–783. [Google Scholar] [CrossRef]
- Greenberg, H.E.; Sica, A.L.; Scharf, S.M.; Ruggiero, D.A. Expression of c-fos in the rat brainstem after chronic intermittent hypoxia. Brain Res. 1999, 816, 638–645. [Google Scholar] [CrossRef]
- Knight, W.D.; Little, J.T.; Carreno, F.R.; Toney, G.M.; Mifflin, S.W.; Cunningham, J.T. Chronic intermittent hypoxia increases blood pressure and expression of FosB/DeltaFosB in central autonomic regions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R131–R139. [Google Scholar] [CrossRef]
- Ye, N.; Ding, Y.; Wild, C.; Shen, Q.; Zhou, J. Small molecule inhibitors targeting activator protein 1 (AP-1). J. Med. Chem. 2014, 57, 6930–6948. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Olejniczak, M.; Kotowska-Zimmer, A.; Krzyzosiak, W. Stress-induced changes in miRNA biogenesis and functioning. Cell. Mol. Life Sci. 2018, 75, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.Y.; Zheng, Y.L.; Zhou, Y.F.; Wang, W.D.; Li, M.M.; Shi, Y.C.; Lin, H.L.; Lin, S. Research progress on the role of exosomes in obstructive sleep apnea-hypopnea syndrome-related atherosclerosis. Sleep Med. Rev. 2022, 66, 101696. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chang, S.C.; Jin, J.; Gu, W.; Li, S. NLRP3 in fl ammasome mediates chronic intermittent hypoxia-induced renal injury implication of the microRNA-155/FOXO3a signaling pathway. J. Cell. Physiol. 2018, 233, 9404–9415. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Wang, S.C.; Gongol, B.; Han, S.Y.; Cho, Y.; Schiavon, C.R.; Chen, L.; Xing, Y.; Zhao, Y.; Ning, M.; et al. Obstructive Sleep Apnea-induced Endothelial Dysfunction Is Mediated by miR-210. Am. J. Respir. Crit. Care Med. 2023, 207, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Peng, L.; Wang, Y.; Zhao, W.; Lau, W.B.; Wang, Y.; Li, Y.; Du, Y.; Li, L.; Huang, Y.; et al. Extracellular vesicle-derived miR-144 as a novel mechanism for chronic intermittent hypoxia-induced endothelial dysfunction. Theranostics 2022, 12, 4237–4249. [Google Scholar] [CrossRef]
- Wu, X.; Gong, L.; Xie, L.; Gu, W.; Wang, X.; Liu, Z.; Li, S. NLRP3 Deficiency Protects Against Intermittent Hypoxia-Induced Neuroinflammation and Mitochondrial ROS by Promoting the PINK1-Parkin Pathway of Mitophagy in a Murine Model of Sleep Apnea. Front. Immunol. 2021, 12, 628168. [Google Scholar] [CrossRef] [PubMed]
- She, N.; Shi, Y.; Feng, Y.; Ma, L.; Yuan, Y.; Zhang, Y.; Cao, Z.; Chen, X.; Zhao, B.; Liu, H.; et al. NLRP3 inflammasome regulates astrocyte transformation in brain injury induced by chronic intermittent hypoxia. BMC Neurosci. 2022, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Wang, J.; Han, Y.; Feng, J. Blocking the LncRNA MALAT1/miR-224-5p/NLRP3 Axis Inhibits the Hippocampal Inflammatory Response in T2DM with OSA. Front. Cell Neurosci. 2020, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xing, W.; Zhang, Y.; Wang, J.; Zuo, N.; Sun, F.; Liu, Q.; Liu, S. NLRP3/miR-223-3p axis attenuates neuroinflammation induced by chronic intermittent hypoxia. Funct. Integr. Genom. 2023, 23, 342. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, G.; Martynova, E.V.; Gilazieva, Z.E.; McIntyre, A.; Rizvanov, A.A.; Khaiboullina, S.F. MicroRNA Post-transcriptional Regulation of the NLRP3 Inflammasome in Immunopathologies. Front. Pharmacol. 2019, 10, 451. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zeng, J.; Li, W.; Lin, L.; Zhou, X.; Tian, X.; Liu, W.; Zhang, L.; Zhang, X. Silencing of miR-155 suppresses inflammatory responses in psoriasis through inflammasome NLRP3 regulation. Int. J. Mol. Med. 2018, 42, 1086–1095. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Ding, X.; Zhang, X.; Tang, J.; Lin, H. Plasma extracellular vesicle delivery of miR-210-3p by targeting ATG7 to promote sepsis-induced acute lung injury by regulating autophagy and activating inflammation. Exp. Mol. Med. 2021, 53, 1180–1191. [Google Scholar] [CrossRef]
- Jiang, J.M.; Mo, M.L.; Long, X.P.; Xie, L.H. MiR-144-3p induced by SP1 promotes IL-1beta-induced pyroptosis in chondrocytes via PTEN/PINK1/Parkin axis. Autoimmunity 2022, 55, 21–31. [Google Scholar] [CrossRef]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- Diaz-Garcia, E.; Garcia-Tovar, S.; Alfaro, E.; Jaureguizar, A.; Casitas, R.; Sanchez-Sanchez, B.; Zamarron, E.; Fernandez-Lahera, J.; Lopez-Collazo, E.; Cubillos-Zapata, C.; et al. Inflammasome Activation: A Keystone of Proinflammatory Response in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2022, 205, 1337–1348. [Google Scholar] [CrossRef]
- Diaz-Garcia, E.; Sanz-Rubio, D.; Garcia-Tovar, S.; Alfaro, E.; Cubero, P.; Gil, A.V.; Marin, J.M.; Cubillos-Zapata, C.; Garcia-Rio, F. Inflammasome activation mediated by oxidised low-density lipoprotein in patients with sleep apnoea and early subclinical atherosclerosis. Eur. Respir. J. 2023, 61, 2201401. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.M.; Guan, P.; Luo, L.F.; Qin, L.Y.; Wang, N.; Zhao, Y.S.; Ji, E.S. Resveratrol protects against CIH-induced myocardial injury by targeting Nrf2 and blocking NLRP3 inflammasome activation. Life Sci. 2020, 245, 117362. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.F.; King, A.D.; O’Donnell, C.; Roche, H.M.; Ryan, S. Mechanisms of intermittent hypoxia-mediated macrophage activation-potential therapeutic targets for obstructive sleep apnoea. J. Sleep Res. 2021, 30, e13202. [Google Scholar] [CrossRef] [PubMed]
- Markopoulos, G.S.; Roupakia, E.; Tokamani, M.; Alabasi, G.; Sandaltzopoulos, R.; Marcu, K.B.; Kolettas, E. Roles of NF-kappaB Signaling in the Regulation of miRNAs Impacting on Inflammation in Cancer. Biomedicines 2018, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Marwarha, G.; Slagsvold, K.H.; Hoydal, M.A. NF-kappaB Transcriptional Activity Indispensably Mediates Hypoxia-Reoxygenation Stress-Induced microRNA-210 Expression. Int. J. Mol. Sci. 2023, 24, 6618. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, P.; Li, P.; Feng, L.; Ren, Q.; Xie, X.; Xu, J. Ghrelin protects the heart against ischemia/reperfusion injury via inhibition of TLR4/NLRP3 inflammasome pathway. Life Sci. 2017, 186, 50–58. [Google Scholar] [CrossRef]
- Su, Q.; Li, L.; Sun, Y.; Yang, H.; Ye, Z.; Zhao, J. Effects of the TLR4/Myd88/NF-kappaB Signaling Pathway on NLRP3 Inflammasome in Coronary Microembolization-Induced Myocardial Injury. Cell. Physiol. Biochem. 2018, 47, 1497–1508. [Google Scholar] [CrossRef]
- Park, M.; Choi, S.; Kim, S.; Kim, J.; Lee, D.K.; Park, W.; Kim, T.; Jung, J.; Hwang, J.Y.; Won, M.H.; et al. NF-kappaB-responsive miR-155 induces functional impairment of vascular smooth muscle cells by downregulating soluble guanylyl cyclase. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Butler, A.E.; Eid, A.H.; Sahebkar, A. Pleiotropic properties of statins via angiogenesis modulation in cardiovascular disease. Drug Discov. Today 2022, 27, 103325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Cheng, H.J.; Yuan, Y.T.; Chen, Y.; Chen, Y.Y.; Chiu, K.Y.; Zeng, H.Q. Atorvastatin attenuates intermittent hypoxia-induced myocardial oxidative stress in a mouse obstructive sleep apnea model. Aging 2021, 13, 18870–18878. [Google Scholar] [CrossRef]
- Chen, D.; Sui, L.; Chen, C.; Liu, S.; Sun, X.; Guan, J. Atorvastatin suppresses NLRP3 inflammasome activation in intracerebral hemorrhage via TLR4- and MyD88-dependent pathways. Aging 2022, 14, 462–476. [Google Scholar] [CrossRef]
- Shah, R.; Patel, N.; Emin, M.; Celik, Y.; Jimenez, A.; Gao, S.; Garfinkel, J.; Wei, Y.; Jelic, S. Statins Restore Endothelial Protection against Complement Activity in Obstructive Sleep Apnea: A Randomized Clinical Trial. Ann. Am. Thorac. Soc. 2023, 20, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Henriksbo, B.D.; Tamrakar, A.K.; Phulka, J.S.; Barra, N.G.; Schertzer, J.D. Statins activate the NLRP3 inflammasome and impair insulin signaling via p38 and mTOR. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E110–E116. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.I.; Alaa El-Din Aly El-Waseef, D.; Nabih, E.S.; El-Kharashi, O.A.; Abd El-Kareem, H.F.; Abo Nahas, H.H.; Abdel-Wahab, B.A.; Helmy, Y.A.; Alshawwa, S.Z.; Saied, E.M. Acetylsalicylic Acid Suppresses Alcoholism-Induced Cognitive Impairment Associated with Atorvastatin Intake by Targeting Cerebral miRNA155 and NLRP3: In Vivo, and In Silico Study. Pharmaceutics 2022, 14, 529. [Google Scholar] [CrossRef] [PubMed]
- Picado, C.; Roca-Ferrer, J. Role of the Cyclooxygenase Pathway in the Association of Obstructive Sleep Apnea and Cancer. J. Clin. Med. 2020, 9, 3237. [Google Scholar] [CrossRef]
- Gautier-Veyret, E.; Arnaud, C.; Back, M.; Pepin, J.L.; Petri, M.H.; Baguet, J.P.; Tamisier, R.; Levy, P.; Stanke-Labesque, F. Intermittent hypoxia-activated cyclooxygenase pathway: Role in atherosclerosis. Eur. Respir. J. 2013, 42, 404–413. [Google Scholar] [CrossRef]
- Smith, S.M.; Friedle, S.A.; Watters, J.J. Chronic intermittent hypoxia exerts CNS region-specific effects on rat microglial inflammatory and TLR4 gene expression. PLoS ONE 2013, 8, e81584. [Google Scholar] [CrossRef]
- Li, R.C.; Row, B.W.; Gozal, E.; Kheirandish, L.; Fan, Q.; Brittian, K.R.; Guo, S.Z.; Sachleben, L.R., Jr.; Gozal, D. Cyclooxygenase 2 and intermittent hypoxia-induced spatial deficits in the rat. Am. J. Respir. Crit. Care Med. 2003, 168, 469–475. [Google Scholar] [CrossRef]
- Yeung, H.M.; Hung, M.W.; Lau, C.F.; Fung, M.L. Cardioprotective effects of melatonin against myocardial injuries induced by chronic intermittent hypoxia in rats. J. Pineal. Res. 2015, 58, 12–25. [Google Scholar] [CrossRef]
- Liu, Y.; Tipoe, G.L.; Fung, M.L. Melatonin attenuates intermittent hypoxia-induced lipid peroxidation and local inflammation in rat adrenal medulla. Int. J. Mol. Sci. 2014, 15, 18437–18452. [Google Scholar] [CrossRef]
- Hung, M.W.; Kravtsov, G.M.; Lau, C.F.; Poon, A.M.; Tipoe, G.L.; Fung, M.L. Melatonin ameliorates endothelial dysfunction, vascular inflammation, and systemic hypertension in rats with chronic intermittent hypoxia. J. Pineal. Res. 2013, 55, 247–256. [Google Scholar] [CrossRef]
- Daneau, G.; Boidot, R.; Martinive, P.; Feron, O. Identification of cyclooxygenase-2 as a major actor of the transcriptomic adaptation of endothelial and tumor cells to cyclic hypoxia: Effect on angiogenesis and metastases. Clin. Cancer Res. 2010, 16, 410–419. [Google Scholar] [CrossRef]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta 2015, 1851, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Scinico, M.; Sostin, O.V.; Agarwal, R.; Kapoor, A.D.; Petrini, J.R.; Mendez, J.L. A Pilot Study Of Aspirin Resistance In Obstructive Sleep Apnea Patients. Clin. Investig. Med. 2021, 44, E55–E63. [Google Scholar] [CrossRef]
- Li, N.; Wen, W.; Cai, X.; Zhu, Q.; Hu, J.; Heizhati, M.; Yuan, Y.; Gan, L.; Dang, Y.; Yang, W.; et al. The Use of Aspirin Increases the Risk of Major Adverse Cardiac and Cerebrovascular Events in Hypertensive Patients with Obstructive Sleep Apnea for the Primary Prevention of Cardiovascular Disease: A Real-World Cohort Study. J. Clin. Med. 2022, 11, 7066. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, F.; Fan, L.; Zhang, W.; Wang, T.; Du, Y.; Bai, X. Baicalin alleviates atherosclerosis by relieving oxidative stress and inflammatory responses via inactivating the NF-kappaB and p38 MAPK signaling pathways. Biomed. Pharmacother. 2018, 97, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Z.; Yuan, Z.; Lv, S.; Su, Q. Baicalin ameliorates atherosclerosis by inhibiting NLRP3 inflammasome in apolipoprotein E-deficient mice. Diab. Vasc. Dis. Res. 2020, 17, 1479164120977441. [Google Scholar] [CrossRef]
- Yoon, H.S.; Park, C.M. Chrysoeriol ameliorates COX-2 expression through NF-kappaB, AP-1 and MAPK regulation via the TLR4/MyD88 signaling pathway in LPS-stimulated murine macrophages. Exp. Ther. Med. 2021, 22, 718. [Google Scholar] [CrossRef]
- Wang, L.; Gui, J.; Ding, R.; Yang, X.; Yang, J.; Luo, H.; Huang, D.; Han, Z.; Jiang, L. Dietary Intake of Flavonoids Associated with Sleep Problems: An Analysis of Data from the National Health and Nutrition Examination Survey, 2007–2010. Brain Sci. 2023, 13, 873. [Google Scholar] [CrossRef]
- Dyugovskaya, L.; Lavie, P.; Lavie, L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am. J. Respir. Crit. Care Med. 2002, 165, 934–939. [Google Scholar] [CrossRef]
- Dyugovskaya, L.; Polyakov, A.; Lavie, P.; Lavie, L. Delayed neutrophil apoptosis in patients with sleep apnea. Am. J. Respir. Crit. Care Med. 2008, 177, 544–554. [Google Scholar] [CrossRef]
- Schulz, R.; Mahmoudi, S.; Hattar, K.; Sibelius, U.; Olschewski, H.; Mayer, K.; Seeger, W.; Grimminger, F. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am. J. Respir. Crit. Care Med. 2000, 162, 566–570. [Google Scholar] [CrossRef]
- Alzoghaibi, M.A.; Bahammam, A.S. Lipid peroxides, superoxide dismutase and circulating IL-8 and GCP-2 in patients with severe obstructive sleep apnea: A pilot study. Sleep Breath. 2005, 9, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Dyugovskaya, L.; Lavie, P.; Lavie, L. Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apnea. Ann. N. Y. Acad. Sci. 2005, 1051, 340–350. [Google Scholar] [CrossRef]
- Dyugovskaya, L.; Lavie, P.; Hirsh, M.; Lavie, L. Activated CD8+ T-lymphocytes in obstructive sleep apnoea. Eur. Respir. J. 2005, 25, 820–828. [Google Scholar] [CrossRef]
- Cubillos-Zapata, C.; Avendano-Ortiz, J.; Hernandez-Jimenez, E.; Toledano, V.; Casas-Martin, J.; Varela-Serrano, A.; Torres, M.; Almendros, I.; Casitas, R.; Fernandez-Navarro, I.; et al. Hypoxia-induced PD-L1/PD-1 crosstalk impairs T-cell function in sleep apnoea. Eur. Respir. J. 2017, 50, 1700833. [Google Scholar] [CrossRef]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Jimenez, E.; Cubillos-Zapata, C.; Toledano, V.; Perez de Diego, R.; Fernandez-Navarro, I.; Casitas, R.; Carpio, C.; Casas-Martin, J.; Valentin, J.; Varela-Serrano, A.; et al. Monocytes inhibit NK activity via TGF-beta in patients with obstructive sleep apnoea. Eur. Respir. J. 2017, 49, 1602456. [Google Scholar] [CrossRef] [PubMed]
- Marrone, O.; Bonsignore, M.R. Obstructive sleep apnea and cancer: A complex relationship. Curr. Opin. Pulm. Med. 2020, 26, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.; Huppertz, T.; Radsak, M.; Gouveris, H. Cellular Immune Dysfunction in Obstructive Sleep Apnea. Front. Surg. 2022, 9, 890377. [Google Scholar] [CrossRef] [PubMed]
- Tuleta, I.; Stockigt, F.; Juergens, U.R.; Pizarro, C.; Schrickel, J.W.; Kristiansen, G.; Nickenig, G.; Skowasch, D. Intermittent Hypoxia Contributes to the Lung Damage by Increased Oxidative Stress, Inflammation, and Disbalance in Protease/Antiprotease System. Lung 2016, 194, 1015–1020. [Google Scholar] [CrossRef]
- Snyder, B.; Shell, B.; Cunningham, J.T.; Cunningham, R.L. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol. Rep. 2017, 5, e13258. [Google Scholar] [CrossRef]
- Nacher, M.; Serrano-Mollar, A.; Farre, R.; Panes, J.; Segui, J.; Montserrat, J.M. Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea. Respir. Physiol. Neurobiol. 2007, 155, 93–96. [Google Scholar] [CrossRef]
- Dyugovskaya, L.; Polyakov, A.; Ginsberg, D.; Lavie, P.; Lavie, L. Molecular pathways of spontaneous and TNF-alpha-mediated neutrophil apoptosis under intermittent hypoxia. Am. J. Respir. Cell. Mol. Biol. 2011, 45, 154–162. [Google Scholar] [CrossRef]
- Wahlund, C.J.E.; Caglayan, S.; Czarnewski, P.; Hansen, J.B.; Snir, O. Sustained and intermittent hypoxia differentially modulate primary monocyte immunothrombotic responses to IL-1beta stimulation. Front. Immunol. 2023, 14, 1240597. [Google Scholar] [CrossRef]
- Jelic, S.; Padeletti, M.; Kawut, S.M.; Higgins, C.; Canfield, S.M.; Onat, D.; Colombo, P.C.; Basner, R.C.; Factor, P.; LeJemtel, T.H. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 2008, 117, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Venge, P.; Janson, C.; Lindberg, E. Relationship between sleep-disordered breathing and markers of systemic inflammation in women from the general population. J. Sleep Res. 2012, 21, 147–154. [Google Scholar] [CrossRef]
- Alonso-Fernandez, A.; Ribot Quetglas, C.; Herranz Mochales, A.; Alvarez Ruiz De Larrinaga, A.; Sanchez Baron, A.; Rodriguez Rodriguez, P.; Gil Gomez, A.V.; Pia Martinez, C.; Cubero Marin, J.P.; Barcelo Nicolau, M.; et al. Influence of Obstructive Sleep Apnea on Systemic Inflammation in Pregnancy. Front. Med. 2021, 8, 674997. [Google Scholar] [CrossRef] [PubMed]
- Serednytskyy, O.; Alonso-Fernandez, A.; Ribot, C.; Herranz, A.; Alvarez, A.; Sanchez, A.; Rodriguez, P.; Gil, A.V.; Pia, C.; Cubero, J.P.; et al. Systemic inflammation and sympathetic activation in gestational diabetes mellitus with obstructive sleep apnea. BMC Pulm. Med. 2022, 22, 94. [Google Scholar] [CrossRef] [PubMed]
- Facco, F.L.; Parker, C.B.; Reddy, U.M.; Silver, R.M.; Koch, M.A.; Louis, J.M.; Basner, R.C.; Chung, J.H.; Nhan-Chang, C.L.; Pien, G.W.; et al. Association between Sleep-Disordered Breathing and Hypertensive Disorders of Pregnancy and Gestational Diabetes Mellitus. Obs. Gynecol. 2017, 129, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Louis, J.M.; Mogos, M.F.; Salemi, J.L.; Redline, S.; Salihu, H.M. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998–2009. Sleep 2014, 37, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Suri, J.; Suri, J.C.; Arora, R.; Gupta, M.; Adhikari, T. The Impact of Sleep-Disordered Breathing on Severity of Pregnancy-Induced Hypertension and Feto-Maternal Outcomes. J. Obs. Gynaecol. India 2019, 69, 111–121. [Google Scholar] [CrossRef]
- Keshavarzi, F.; Mehdizadeh, S.; Khazaie, H.; Ghadami, M.R. Objective assessment of obstructive sleep apnea in normal pregnant and preeclamptic women. Hypertens. Pregnancy 2018, 37, 154–159. [Google Scholar] [CrossRef]
- Carnelio, S.; Morton, A.; McIntyre, H.D. Sleep disordered breathing in pregnancy: The maternal and fetal implications. J. Obs. Gynaecol. 2017, 37, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Huang, L.; Feng, H.; He, Q.; Lin, X.; Jiang, T.; Lin, J.; Wang, X.; Liu, Q. Gestational chronic intermittent hypoxia induces hypertension, proteinuria, and fetal growth restriction in mice. Sleep Breath. 2022, 26, 1661–1669. [Google Scholar] [CrossRef]
- Badran, M.; Abuyassin, B.; Ayas, N.; Laher, I. Intermittent hypoxia impairs uterine artery function in pregnant mice. J. Physiol. 2019, 597, 2639–2650. [Google Scholar] [CrossRef]
- Valverde-Perez, E.; Prieto-Lloret, J.; Gonzalez-Obeso, E.; Cabero, M.I.; Nieto, M.L.; Pablos, M.I.; Obeso, A.; Gomez-Nino, A.; Cardaba-Garcia, R.M.; Rocher, A.; et al. Effects of Gestational Intermittent Hypoxia on Placental Morphology and Fetal Development in a Murine Model of Sleep Apnea. Adv. Exp. Med. Biol. 2023, 1427, 73–81. [Google Scholar] [CrossRef]
- Song, R.; Yadav, P.; Dangudubiyyam, S.V.; Hofmann, A.; Mishra, J.S.; Kumar, S. Gestational intermittent hypoxia induces endothelial dysfunction and hypertension in pregnant rats: Role of endothelin type B receptordagger. Biol. Reprod. 2023. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yuan, Z.F.; Yang, C.H.; Shen, Y.J.; Lin, J.Y.; Lai, C.J. Estrogen Modulates the Sensitivity of Lung Vagal C Fibers in Female Rats Exposed to Intermittent Hypoxia. Front. Physiol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Hung, T.-H.; Skepper, J.N.; Burton, G.J. In Vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am. J. Pathol. 2001, 159, 1031–1043. [Google Scholar] [CrossRef]
- Hung, T.H.; Charnock-Jones, D.S.; Skepper, J.N.; Burton, G.J. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: A potential mediator of the inflammatory response in preeclampsia. Am. J. Pathol. 2004, 164, 1049–1061. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, H.; Wang, Y.; Liu, D.; Cao, C.; Dai, Y.; Zhao, G.; Gu, N.; Zhou, Y.; Zheng, M. Increased Circulating miR-155 identifies a subtype of preeclamptic patients. medRxiv 2022. medRxiv:2022.2003.2023.22272742. [Google Scholar]
- Jaszczuk, I.; Koczkodaj, D.; Kondracka, A.; Kwasniewska, A.; Winkler, I.; Filip, A. The role of miRNA-210 in pre-eclampsia development. Ann. Med. 2022, 54, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Tao, T.; Yin, Y.; Zhao, L.; Yang, L.; Hu, L. miR-144 may regulate the proliferation, migration and invasion of trophoblastic cells through targeting PTEN in preeclampsia. Biomed. Pharmacother. 2017, 94, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wan, Z.; Liu, Z.; Liu, D.; Zhao, Z.; Leng, Y. Exosomes Derived from BMSCs Ameliorate Intestinal Ischemia-Reperfusion Injury by Regulating miR-144-3p-Mediated Oxidative Stress. Dig. Dis. Sci. 2022, 67, 5090–5106. [Google Scholar] [CrossRef] [PubMed]
- E, L.; Jiang, H.; Lu, Z. MicroRNA-144 attenuates cardiac ischemia/reperfusion injury by targeting FOXO1. Exp. Ther. Med. 2019, 17, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.M. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef]
- Opie, L.H.; Lecour, S. Melatonin has multiorgan effects. Eur. Heart. J. Cardiovasc. Pharmacother. 2016, 2, 258–265. [Google Scholar] [CrossRef]
- Gomes, P.R.L.; Motta-Teixeira, L.C.; Gallo, C.C.; Carmo Buonfiglio, D.D.; Camargo, L.S.; Quintela, T.; Reiter, R.J.; Amaral, F.G.D.; Cipolla-Neto, J. Maternal pineal melatonin in gestation and lactation physiology, and in fetal development and programming. Gen. Comp. Endocrinol. 2021, 300, 113633. [Google Scholar] [CrossRef]
- Man, G.C.W.; Zhang, T.; Chen, X.; Wang, J.; Wu, F.; Liu, Y.; Wang, C.C.; Cheong, Y.; Li, T.C. The regulations and role of circadian clock and melatonin in uterine receptivity and pregnancy-An immunological perspective. Am. J. Reprod. Immunol. 2017, 78, e12715. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Rosales-Corral, S.A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update. 2014, 20, 293–307. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Zhang, N.; Liu, F. Melatonin ameliorates hypertension in hypertensive pregnant mice and suppresses the hypertension-induced decrease in Ca2+-activated K+ channels in uterine arteries. Hypertens Res. 2021, 44, 1079–1086. [Google Scholar] [CrossRef]
- Zuo, J.; Jiang, Z. Melatonin attenuates hypertension and oxidative stress in a rat model of L-NAME-induced gestational hypertension. Vasc. Med. 2020, 25, 295–301. [Google Scholar] [CrossRef]
- El-Malkey, N.F.; Aref, M.; Emam, H.; Khalil, S.S. Impact of Melatonin on Full-Term Fetal Brain Development and Transforming Growth Factor-beta Level in a Rat Model of Preeclampsia. Reprod Sci. 2021, 28, 2278–2291. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Xu, D.X.; Wang, J.P.; Wang, H.; Wei, L.Z.; Sun, M.F.; Wei, W. Melatonin protects against lipopolysaccharide-induced intra-uterine fetal death and growth retardation in mice. J. Pineal. Res. 2006, 40, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Chen, C.C.; Sheen, J.M.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Melatonin attenuates prenatal dexamethasone-induced blood pressure increase in a rat model. J. Am. Soc. Hypertens. 2014, 8, 216–226. [Google Scholar] [CrossRef]
- Lee, S.K.; Sirajudeen, K.N.; Sundaram, A.; Zakaria, R.; Singh, H.J. Effects of antenatal, postpartum and post-weaning melatonin supplementation on blood pressure and renal antioxidant enzyme activities in spontaneously hypertensive rats. J. Physiol. Biochem. 2011, 67, 249–257. [Google Scholar] [CrossRef]
- Ravishankar, S.; Bourjeily, G.; Lambert-Messerlian, G.; He, M.; De Paepe, M.E.; Gundogan, F. Evidence of Placental Hypoxia in Maternal Sleep Disordered Breathing. Pediatr. Dev. Pathol. 2015, 18, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Almendros, I.; Martinez-Ros, P.; Farre, N.; Rubio-Zaragoza, M.; Torres, M.; Gutierrez-Bautista, A.J.; Carrillo-Poveda, J.M.; Sopena-Juncosa, J.J.; Gozal, D.; Gonzalez-Bulnes, A.; et al. Placental oxygen transfer reduces hypoxia-reoxygenation swings in fetal blood in a sheep model of gestational sleep apnea. J. Appl. Physiol. 2019, 127, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Arthuis, C.J.; Novell, A.; Raes, F.; Escoffre, J.M.; Lerondel, S.; Le Pape, A.; Bouakaz, A.; Perrotin, F. Real-Time Monitoring of Placental Oxygenation during Maternal Hypoxia and Hyperoxygenation Using Photoacoustic Imaging. PLoS ONE 2017, 12, e0169850. [Google Scholar] [CrossRef] [PubMed]
- Vanderplow, A.M.; Kermath, B.A.; Bernhardt, C.R.; Gums, K.T.; Seablom, E.N.; Radcliff, A.B.; Ewald, A.C.; Jones, M.V.; Baker, T.L.; Watters, J.J.; et al. A feature of maternal sleep apnea during gestation causes autism-relevant neuronal and behavioral phenotypes in offspring. PLoS Biol. 2022, 20, e3001502. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Shinohara, H.; Kodama, H. Nocturnal oxygen desaturation in the late third trimester of uncomplicated pregnancy for prediction of late-onset gestational hypertension. J. Obs. Gynaecol. Res. 2020, 46, 1735–1743. [Google Scholar] [CrossRef]
- Pengo, M.F.; Banerjee, D.; Kaur, A.; Bourjeily, G. Sleep disordered breathing in pregnancy: Food for thought. Obs. Med. 2016, 9, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Zaidi, N.; Wroblewski, K.; Kay, H.H.; Ismail, M.; Ehrmann, D.A.; Van Cauter, E. Interactions between pregnancy, obstructive sleep apnea, and gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2013, 98, 4195–4202. [Google Scholar] [CrossRef] [PubMed]
- Telerant, A.; Dunietz, G.L.; Many, A.; Tauman, R. Mild Maternal Obstructive Sleep Apnea in Non-obese Pregnant Women and Accelerated Fetal Growth. Sci. Rep. 2018, 8, 10768. [Google Scholar] [CrossRef]
- Brener, A.; Lebenthal, Y.; Levy, S.; Dunietz, G.L.; Sever, O.; Tauman, R. Mild maternal sleep-disordered breathing during pregnancy and offspring growth and adiposity in the first 3 years of life. Sci. Rep. 2020, 10, 13979. [Google Scholar] [CrossRef]
- Tauman, R.; Zuk, L.; Uliel-Sibony, S.; Ascher-Landsberg, J.; Katsav, S.; Farber, M.; Sivan, Y.; Bassan, H. The effect of maternal sleep-disordered breathing on the infant’s neurodevelopment. Am. J. Obs. Gynecol. 2015, 212, e651–e657. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.M.; Wang, X.; Hao, K.; Yuan, Y.; Chen, X.Q.; Du, J.Z. Upregulation of PVN CRHR1 by gestational intermittent hypoxia selectively triggers a male-specific anxiogenic effect in rat offspring. Horm. Behav. 2013, 63, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Mabry, S.; Wilson, E.N.; Bradshaw, J.L.; Gardner, J.J.; Fadeyibi, O.; Vera, E., Jr.; Osikoya, O.; Cushen, S.C.; Karamichos, D.; Goulopoulou, S.; et al. Sex and age differences in social and cognitive function in offspring exposed to late gestational hypoxia. Biol. Sex. Differ. 2023, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.; Yassin, B.A.; Lin, D.T.S.; Kobor, M.S.; Ayas, N.; Laher, I. Gestational intermittent hypoxia induces endothelial dysfunction, reduces perivascular adiponectin and causes epigenetic changes in adult male offspring. J. Physiol. 2019, 597, 5349–5364. [Google Scholar] [CrossRef]
- Song, R.; Mishra, J.S.; Dangudubiyyam, S.V.; Antony, K.M.; Baker, T.L.; Watters, J.J.; Kumar, S. Gestational Intermittent Hypoxia Induces Sex-Specific Impairment in Endothelial Mechanisms and Sex Steroid Hormone Levels in Male Rat Offspring. Reprod. Sci. 2022, 29, 1531–1541. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, Y.; Lin, H. The effect of chronic intermittent hypoxia on atherosclerosis in rat offspring. Int. J. Cardiol. 2021, 335, 98–103. [Google Scholar] [CrossRef]
- Wongkitikamjorn, W.; Wada, E.; Hosomichi, J.; Maeda, H.; Satrawaha, S.; Hong, H.; Yoshida, K.I.; Ono, T.; Hayashi, Y.K. Metabolic dysregulation and decreased capillarization in skeletal muscles of male adolescent offspring rats exposed to gestational intermittent hypoxia. Front. Physiol. 2023, 14, 1067683. [Google Scholar] [CrossRef]
- Wongkitikamjorn, W.; Hosomichi, J.; Wada, E.; Maeda, H.; Satrawaha, S.; Hong, H.; Hayashi, Y.K.; Yoshida, K.I.; Ono, T. Gestational Intermittent Hypoxia Induces Mitochondrial Impairment in the Geniohyoid Muscle of Offspring Rats. Cureus 2022, 14, e25088. [Google Scholar] [CrossRef]
- Khalyfa, A.; Cortese, R.; Qiao, Z.; Ye, H.; Bao, R.; Andrade, J.; Gozal, D. Late gestational intermittent hypoxia induces metabolic and epigenetic changes in male adult offspring mice. J. Physiol. 2017, 595, 2551–2568. [Google Scholar] [CrossRef]
- Song, R.; Mishra, J.S.; Dangudubiyyam, S.V.; Baker, T.L.; Watters, J.J.; Kumar, S. Gestational Intermittent Hypoxia Programs Hypertensive Response in Female Rat Offspring: Impact of Ovaries. J. Womens Health Dev. 2022, 5, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.N.; Mabry, S.; Bradshaw, J.L.; Gardner, J.J.; Rybalchenko, N.; Engelland, R.; Fadeyibi, O.; Osikoya, O.; Cushen, S.C.; Goulopoulou, S.; et al. Gestational hypoxia in late pregnancy differentially programs subcortical brain maturation in male and female rat offspring. Biol. Sex. Differ. 2022, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Ambrozio-Marques, D.; Gagnon, M.; Radcliff, A.B.; Meza, A.L.; Baker, T.L.; Watters, J.J.; Kinkead, R. Gestational intermittent hypoxia increases FosB-immunoreactive perikaryas in the paraventricular nucleus of the hypothalamus of adult male (but not female) rats. Exp. Physiol. 2023, 108, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Stremmel, W.; Weiskirchen, R.; John, S.M.; Schmitz, G. Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development. Biomolecules 2021, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Savransky, V.; Nanayakkara, A.; Li, J.; Bevans, S.; Smith, P.L.; Rodriguez, A.; Polotsky, V.Y. Chronic intermittent hypoxia induces atherosclerosis. Am. J. Respir. Crit. Care Med. 2007, 175, 1290–1297. [Google Scholar] [CrossRef]
- Guilleminault, C.; Kirisoglu, C.; Ohayon, M.M. C-reactive protein and sleep-disordered breathing. Sleep 2004, 27, 1507–1517. [Google Scholar] [CrossRef]
- Meier-Ewert, H.K.; Ridker, P.M.; Rifai, N.; Regan, M.M.; Price, N.J.; Dinges, D.F.; Mullington, J.M. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J. Am. Coll. Cardiol. 2004, 43, 678–683. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).