Enemies or Allies? Hormetic and Apparent Non-Dose-Dependent Effects of Natural Bioactive Antioxidants in the Treatment of Inflammation
Abstract
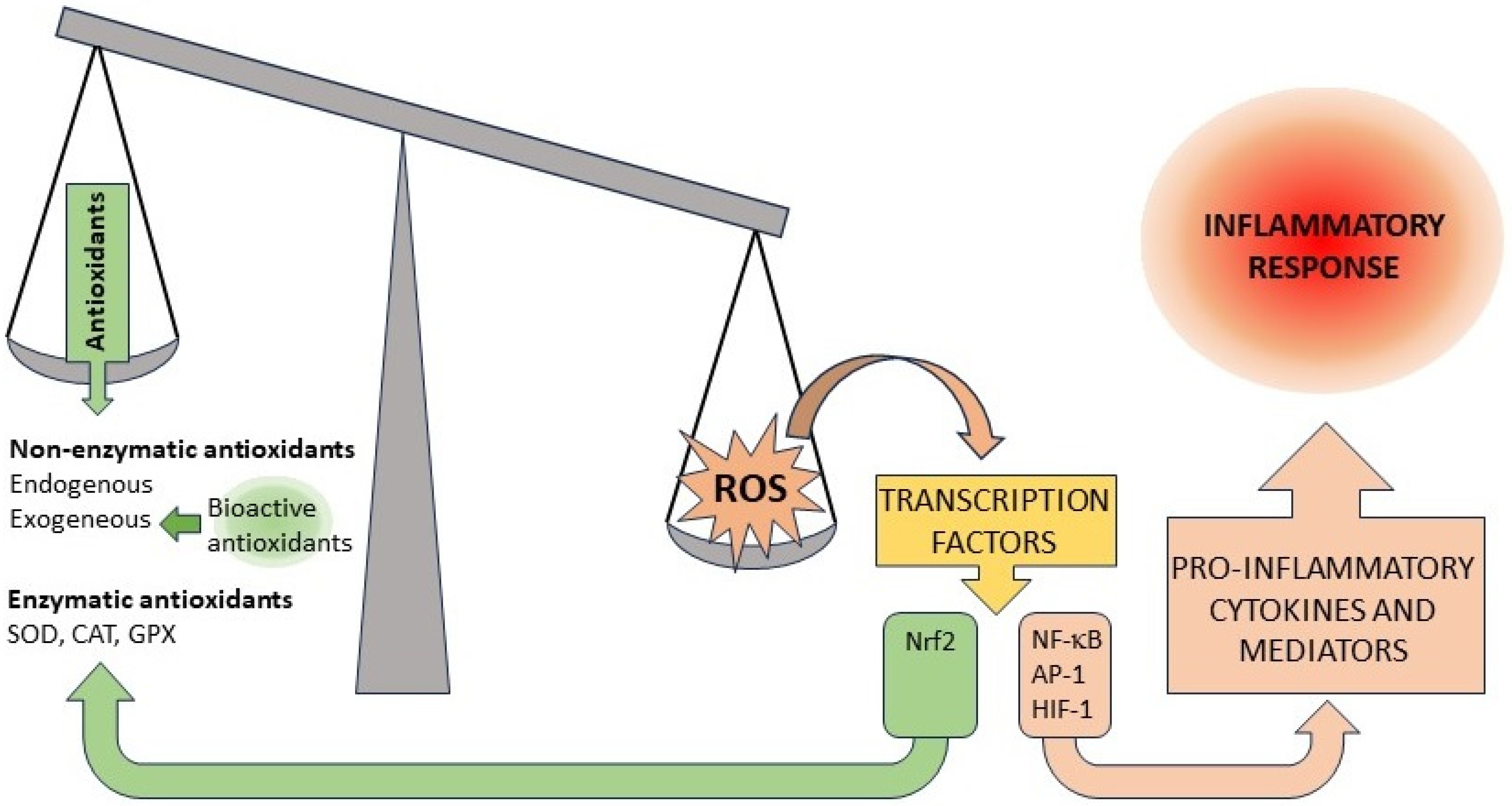
:1. Introduction
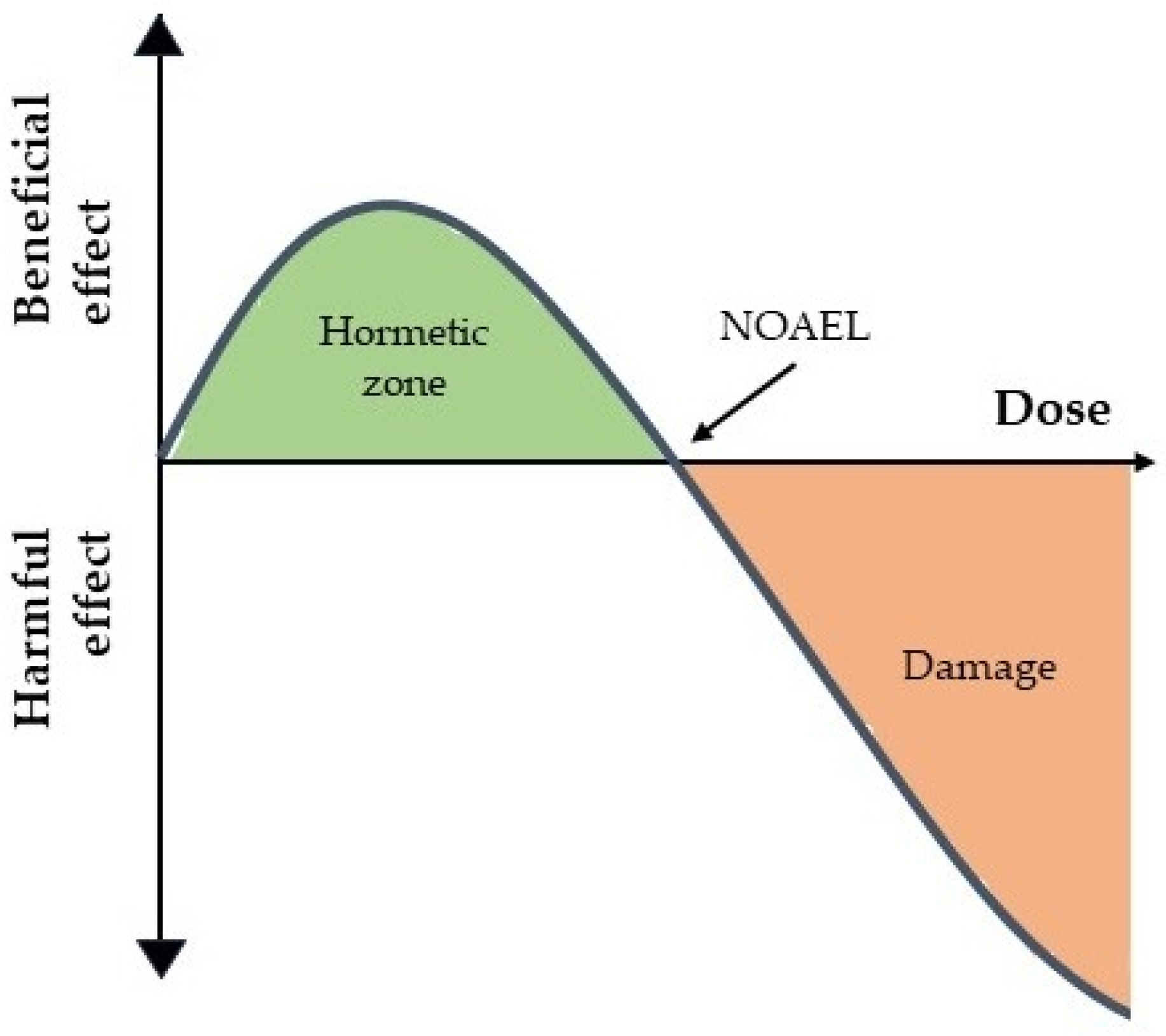
2. Dose–Response Curves, Hormesis and the Law of Mass Action

3. Unexpected Dose–Response Curves in the Antioxidant and Anti-Inflammatory Activity of Natural Bioactive Compounds
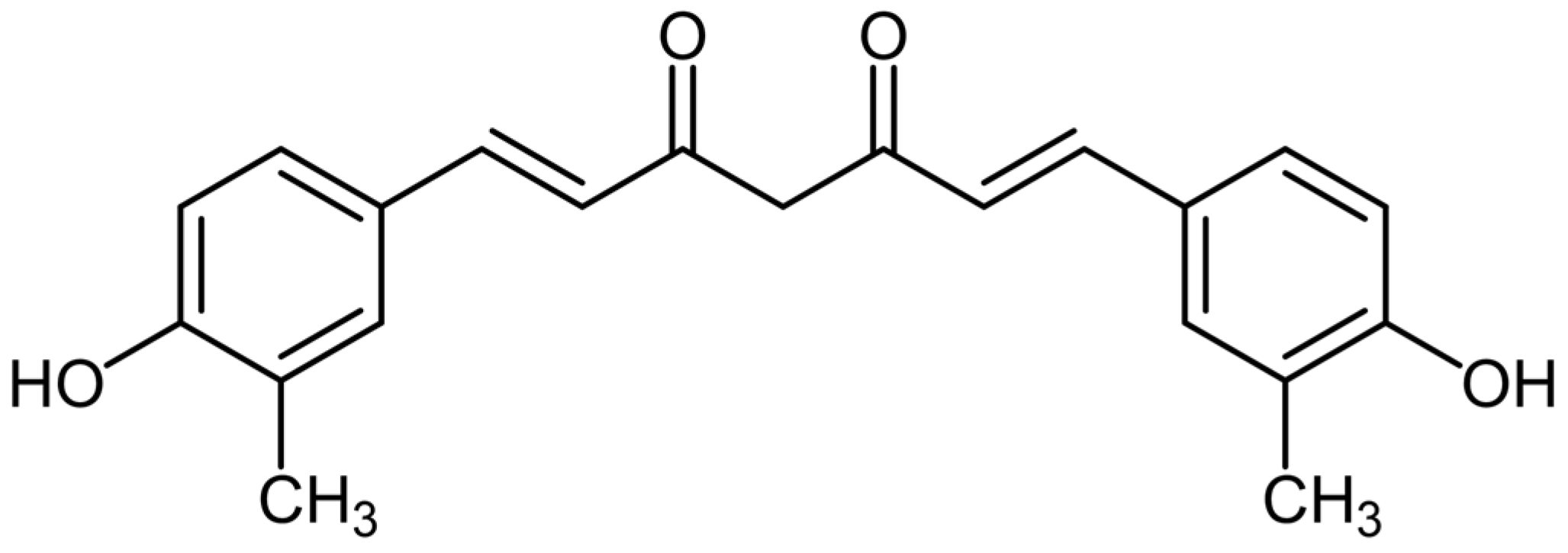
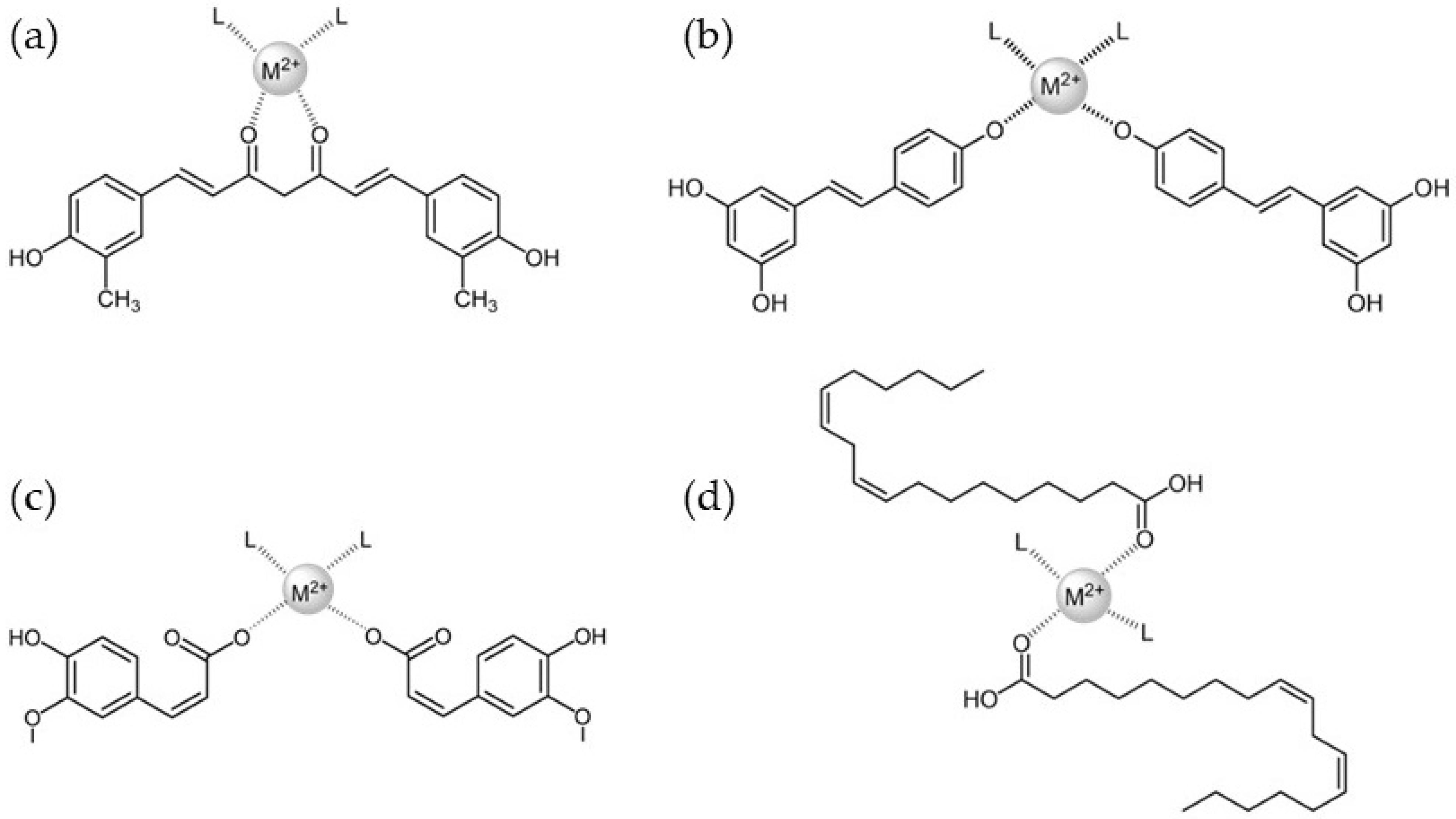
3.1. Curcumin
3.2. Resveratrol
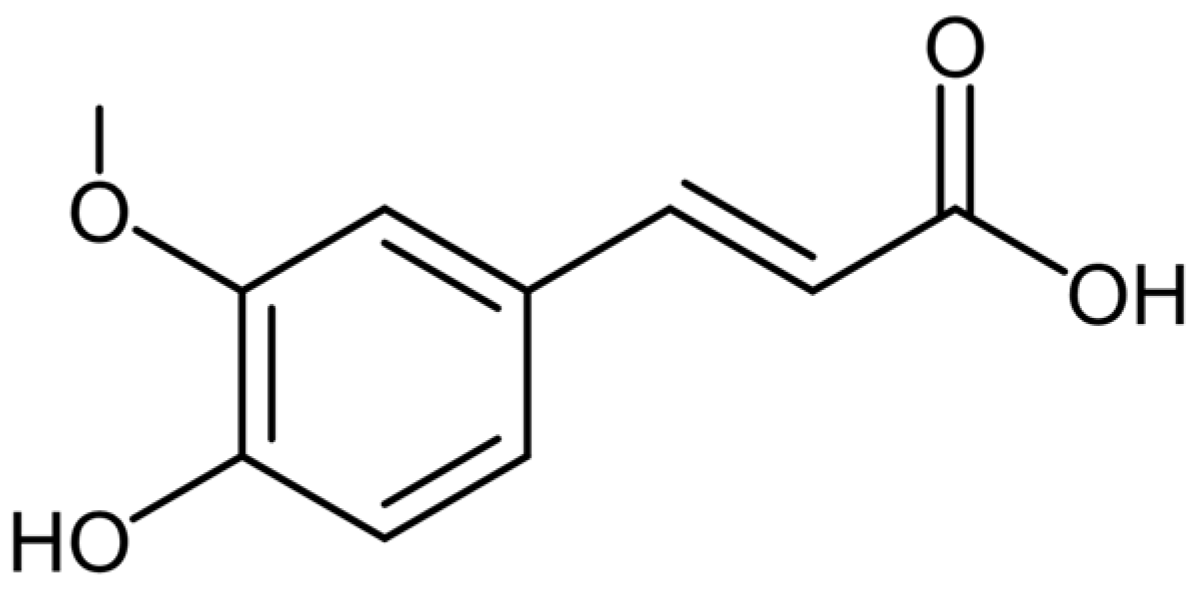
3.3. Ferulic Acid
3.4. Linoleic Acid
4. Discussing Unexpected Dose–Response Curves in Natural Bioactive Compounds
5. Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113, S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, K.; Akhtar, H. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Gupta, S.; Ahmed, M.; Dhar, M.K. Endophytic fungi from medicinal plants: A treasure hunt for bioactive metabolites. Phytochem. Rev. 2012, 11, 487–505. [Google Scholar] [CrossRef]
- Tiwari, M.; Kakkar, P. Plant derived antioxidants—Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. Vitr. 2009, 23, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, S.; Alu’datt, M.H.; Alhamad, M.N.; Tranchant, C.C.; Rababah, T.; Al-U’datt, D.; Hussein, N.; Alrosan, M.; Tan, T.-C.; Kubow, S.; et al. Functional and Bioactive Properties of Wheat Protein Fractions: Impact of Digestive Enzymes on Antioxidant, α-Amylase, and Angiotensin-Converting Enzyme Inhibition Potential. Molecules 2023, 28, 6012. [Google Scholar] [CrossRef] [PubMed]
- Chambon, M.; Ho, R.; Baghdikian, B.; Herbette, G.; Bun-Llopet, S.-S.; Garayev, E.; Raharivelomanana, P. Identification of Antioxidant Metabolites from Five Plants (Calophyllum inophyllum, Gardenia taitensis, Curcuma longa, Cordia subcordata, Ficus prolixa) of the Polynesian Pharmacopoeia and Cosmetopoeia for Skin Care. Antioxidants 2023, 12, 1870. [Google Scholar] [CrossRef]
- Cholet, J.; Decombat, C.; Delort, L.; Gainche, M.; Berry, A.; Ogeron, C.; Ripoche, I.; Vareille-Delarbre, M.; Vermerie, M.; Fraisse, D.; et al. Potential Anti-Inflammatory and Chondroprotective Effect of Luzula sylvatica. Int. J. Mol. Sci. 2023, 24, 127. [Google Scholar] [CrossRef]
- Villamena, F.A. Chemistry of Reactive Species. In Molecular Basis of Oxidative Stress: Chemistry, Mechanisms, and Disease Pathogenesis, 1st ed.; Villamena, F.A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 1–48. [Google Scholar]
- Cerutti, P.A. Oxidant stress and carcinogenesis. Eur. J. Clin. Investig. 1991, 21, 1–5. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants and human disease: A general introduction. Nutr. Rev. 1997, 55, S44–S49. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’arca, D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.B.; Liu, T.Y.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Rouco, L.; González-Noya, A.M.; Pedrido, R.; Maneiro, M. Pursuing the Elixir of Life: In vivo antioxidative effects of manganosalen complexes. Antioxidants 2020, 9, 727. [Google Scholar] [CrossRef]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.-F.; Teixeira, M.; Valentine, J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. The spectrum of inflammatory responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Saleh, J.; Peyssonanux, C.; Singh, K.K.; Edeas, M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion 2020, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef] [PubMed]
- Rafiyan, M.; Sadeghmousavi, S.; Akbarzadehmoallemkolaei, M.; Rezaei, N. Experimental animal models of chronic inflammation. Curr. Res. Immunol. 2023, 4, 100063. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Robinson, W.; Lepus, C.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Stebbing, A.R.D. Hormesis—The stimulation of growth by low levels of inhibitors. Sci. Total Environ. 1982, 22, 213–234. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Chemical hormesis: Its historical foundations as a biological hypothesis. Hum. Exp. Toxicol. 2000, 19, 2–31. [Google Scholar] [CrossRef]
- Hanberger, J.; Nilsson, L.E.; Maller, R.; Isaksson, B. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob. Agents Chemother. 1991, 35, 1710–1716. [Google Scholar] [CrossRef]
- Kojima, M. Data-dependent contrast test for dose-finding clinical trials. Contemp. Clin. Trials 2023, 131, 107265. [Google Scholar] [CrossRef] [PubMed]
- Tyuryaeva, I.; Lyublinskaya, O. Expected and unexpected effects of pharmacological antioxidants. Int. J. Mol. Sci. 2023, 24, 9303. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, Z.; Lin, A.; Li, X.; Li, K. Non-Dose-Dependent Relationship between Antipredator Behavior and Conspecific Alarm Substance in Zebrafish. Fishes 2023, 8, 76. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Agathokleous, E.; Calabrese, V. Hormesis: Wound healing and fibroblasts. Pharmacol. Res. 2022, 184, 106449. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, K.; Saul, N.; Chakrabarti, S.; Stürzenbaum, S.R.; Menzel, R.; Steinberg, C.E.W. Hormetins, antioxidants and prooxidants: Defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extensión in C. elegans. Biogerontology 2011, 12, 329–347. [Google Scholar] [CrossRef]
- Skaperda, Z.; Tekos, F.; Vardakas, P.; Nepka, C.; Kouretas, D. Reconceptualization of Hormetic Responses in the Frame of Redox Toxicology. Int. J. Mol. Sci. 2022, 23, 49. [Google Scholar] [CrossRef]
- Plauth, A.; Geikowski, A.; Cichon, S.; Wowro, S.J.; Liedgens, L.; Rousseau, M.; Weidner, C.; Fuhr, L.; Kliem, M.; Jenkins, G.; et al. Hormetic shifting of redox environment by pro-oxidative resveratrol protects cells against stress. Free Radic. Biol. Med. 2016, 99, 608–622. [Google Scholar] [CrossRef]
- Guldberg, C.M.; Waage, P. Études Sur Les Affinités Chimiques, 2nd ed.; Imprimerie de Brøgger & Christie: Oslo, Norway, 1867; ISBN 1166709043. [Google Scholar]
- McLean, F.C. Application of the law of chemical equilibrium (Law of Mass Action) to biological problems. Physiol. Rev. 1938, 18, 495–523. [Google Scholar] [CrossRef]
- Conolly, R.B.; Lutz, W.K. Nonmonotonic dose-response relationships: Mechanistic basis, kinetic modeling, and implications for risk assessment. Toxicol. Sci. 2004, 77, 151–157. [Google Scholar] [CrossRef]
- Andersen, M.E.; Lutz, R.W.; Liao, K.H.; Lutz, W.K. Dose-incidence modeling: Consequences of linking quantal measures of response to depletion of critical tissue targets. Toxicol. Sci. 2006, 89, 331–337. [Google Scholar] [CrossRef]
- Mayo, M.; Collier, Z.A.; Winton, C.; Chappell, M.A. Data-Driven Method to Estimate Nonlinear Chemical Equivalence. PLoS ONE 2015, 10, e0130494. [Google Scholar] [CrossRef]
- Di Veroli, G.Y.; Fornari, C.; Goldlust, I.; Mills, G.; Koh, S.B.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. An automated fitting procedure and software for dose-response curves with multiphasic features. Sci. Rep. 2015, 5, 14701. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K.; Ferner, R.E. The law of mass action and the pharmacological concentration–effect curve: Resolving the paradox of apparently non-dose-related adverse drug reactions. Br. J. Clin. Pharmacol. 2015, 81, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, L.; Hinrichs, M.J.; Skuba, E.V.; Iverson, W.O.; Ennulat, D. Interpreting and Integrating Clinical and Anatomic Pa-thology Results: Pulling It All Together. Toxicol. Pathol. 2017, 45, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, J.; Cheke, R.A.; Tang, S. A Universal Delayed Difference Model Fitting Dose-response Curves. Dose-Response 2021, 19, 15593258211062785. [Google Scholar] [CrossRef] [PubMed]
- Shubina, V.S.; Kozina, V.I.; Shatalin, Y.V. A Comparative Study of the Inhibitory Effect of Some Flavonoids and a Conjugate of Taxifolin with Glyoxylic Acid on the Oxidative Burst of Neutrophils. Int. J. Mol. Sci. 2023, 24, 15068. [Google Scholar] [CrossRef] [PubMed]
- Akeem, A.; Mohamed, K.B.; Asmawi, M.Z.; Sofiman, O.A. Mutagenic and Antimutagenic Potentials of Fruit Juices of Five Medicinal Plants in Allium Cepa L.: Possible Influence of DPPH Free Radical Scavengers. Afr. J. Biotechnol. 2011, 10, 10248–10257. [Google Scholar] [CrossRef]
- Varpe, S.S.; Juvekar, A.R.; Bidikar, M.P.; Juvekar, P.R. Evaluation of anti-inflammatory activity of Typha angustifolia pollen grains extracts in experimental animals. Indian J. Pharmacol. 2012, 44, 788–791. [Google Scholar] [CrossRef]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Zinellu, A.; Sotgia, S.; Vardeu, A.; Hoa, P.T.; Nguyen, L.H.V.; Carru, C.; Pintus, G. Resveratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem. Toxicol. 2015, 78, 10–16. [Google Scholar] [CrossRef]
- Pereira da Silva, C.; Soares-Freitas, R.A.M.; Rodrigues Sampaio, G.; Barros Santos, M.C.; Pimenta do Nascimento, T.; Cameron, L.C.; Larraz Ferreira, M.S.; Gomes Arêas, J.A. Identification and action of phenolic compounds of Jatobá-do-cerrado (Hymenaea stignocarpa Mart.) on α-amylase and α-glucosidase activities and flour effect on glycemic response and nutritional quality of breads. Food Res. Int. 2019, 116, 1076–1083. [Google Scholar] [CrossRef]
- Njinga, N.S.; Kola-Mustapha, A.T.; Quadri, A.L.; Atolani, O.; Ayanniyi, R.O.; Buhari, M.O.; Amusa, T.O.; Ajani, E.O.; Folaranmi, O.O.; Bakare-Odunola, M.T.; et al. Toxicity assessment of sub-acute and sub-chronic oral administration and diuretic potential of aqueous extract of Hibiscus sabdariffa calyces. Heliyon 2020, 6, e04853. [Google Scholar] [CrossRef]
- Bourebaba, L.; Gilbert-López, B.; Oukil, N.; Bedjou, F. Phytochemical composition of Ecballium elaterium extracts with antioxidant and anti-inflammatory activities: Comparison among leaves, flowers and fruits extracts. Arab. J. Chem. 2020, 13, 3286–3300. [Google Scholar] [CrossRef]
- Rouco, L.; Liberato, A.; Fernández-Trujillo, M.J.; Máñez, A.; Basallote, M.G.; Alvariño, R.; Alfonso, A.; Botana, L.M.; Maneiro, M. Salen-manganese complexes for controlling ROS damage: Neuroprotective effects, antioxidant activity and kinetic studies. J. Inorg. Biochem. 2020, 203, 110918. [Google Scholar] [CrossRef] [PubMed]
- Avoseh, O.N.; Mtunzi, F.M.; Ogunwande, I.A.; Ascrizzi, R.; Guido, F. Albizia lebbeck and Albizia zygia volatile oils exhibit anti-nociceptive and anti-inflammatory properties in pain models. J. Ethnopharmacol. 2021, 268, 113676. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, D.; Niculae, M.; Ielciu, I.; Olah, N.-K.; Munteanu, M.; Burtescu, R.; Ștefan, R.; Olar, L.; Pall, E.; Andrei, S.; et al. Chemical Profile, Cytotoxic Activity and Oxidative Stress Reduction of Different Syringa vulgaris L. Extracts. Molecules 2021, 26, 3104. [Google Scholar] [CrossRef] [PubMed]
- Rouco, L.; Alvariño, R.; Alfonso, A.; Romero, M.J.; Pedrido, R.; Maneiro, M. Neuroprotective effects of fluorophore-labelled manganese complexes: Determination of ROS production, mitochondrial membrane potential and confocal fluorescence microscopy studies in neuroblastoma cells. J. Inorg. Biochem. 2022, 227, 111670. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Okafor, I.A.; Okechukwu, G. The effect of the methanolic extract of Zingiber officinale on the duodenal histology, antioxidants and expression of inflammatory cytokines—A pilot study. J. Appl. Anim. Res. 2022, 50, 582–586. [Google Scholar] [CrossRef]
- Georgieva, M.; Vassileva, V. Stress Management in Plants: Examining Provisional and Unique Dose-Dependent Responses. Int. J. Mol. Sci. 2023, 24, 5105. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. The hormetic dose-response model is more common than the threshold model in toxicology. Toxicol. Sci. 2003, 71, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Stanek, E.J., III; Nascarella, M.A.; Hoffmann, G.R. Hormesis predicts low-dose responses better than threshold models. Int. J. Toxicol. 2008, 27, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis and medicine. Br. J. Clin. Pharmacol. 2008, 66, 594–617. [Google Scholar] [CrossRef] [PubMed]
- Maher, J.; Yamamoto, M. The rise of antioxidant signaling—The evolution and hormetic actions of Nrf2. Toxicol. Appl. Pharmacol. 2010, 1, 4–15. [Google Scholar] [CrossRef]
- Evgenios, A.; Calabrese, E.J. Hormesis: The dose response for the 21st century: The future has arrived. Toxicology 2019, 425, 152249. [Google Scholar] [CrossRef]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Hormetic and Mitochondria-Related Mechanisms of Antioxidant Action of Phytochemicals. Antioxidants 2019, 8, 373. [Google Scholar] [CrossRef]
- Iavicoli, I.; Fontana, L.; Santocono, C.; Guarino, D.; Laudiero, M.; Calabrese, E.J. The challenges of defining hormesis in epidemiological studies: The case of radiation hormesis. Sci. Total Environ. 2023, 902, 166030. [Google Scholar] [CrossRef]
- Bondy, S.C. The Hormesis Concept: Strengths and Shortcomings. Biomolecules 2023, 13, 1512. [Google Scholar] [CrossRef]
- Kyriazis, M.; Swas, L.; Orlova, T. The Impact of Hormesis, Neuronal Stress Response, and Reproduction, upon Clinical Aging: A Narrative Review. J. Clin. Med. 2023, 12, 5433. [Google Scholar] [CrossRef]
- Schulz, H. Ueber Hefegifte. Pflüg. Arch. Physiol. 1888, 42, 517–541. [Google Scholar] [CrossRef]
- Baldwin, J.; Grantham, V. Radiation Hormesis: Historical and Current Perspectives. J. Nucl. Med. Technol. 2015, 43, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis and homeopathy: A step forward. Homeopathy 2017, 106, 131–132. [Google Scholar] [CrossRef] [PubMed]
- De Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and Anthocyanins in Cardiovascular Health: A Review of Current Evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, N.; Gammone, M.A.; Gemello, E.; De Girolamo, M.; Cusenza, S.; Riccioni, G. Marine Bioactives: Pharmacological Properties and Potential Applications against Inflammatory Diseases. Mar. Drugs 2012, 10, 812–833. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.L.; Moreau, R. Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.T.; Kuo, C.Y. Bioactives and Inflammation. Curr. Issues Mol. Biol. 2023, 45, 5824–5829. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, Inflammation, and Chronic Diseases: How Are They Linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef]
- Moghaddam, N.S.A.; Oskouie, M.N.; Butler, A.E.; Petit, P.X.; Barreto, G.E.; Sahebkar, A. Hormetic effects of curcumin: What is the evidence? J. Cell. Physiol. 2019, 234, 10060–10071. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Mattson, M.P.; Rattan, S.I.S. Curcumin and hormesis with particular emphasis on neural cells. Food Chem. Toxicol. 2019, 129, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Scuto, M.C.; Mancuso, C.; Tomasello, B.; Ontario, M.L.; Cavallaro, A.; Frasca, F.; Maiolino, L.; Trovato Salinaro, A.; Calabrese, E.J.; Calabrese, V. Curcumin, Hormesis and the Nervous System. Nutrients 2019, 11, 2417. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis and Ginseng: Ginseng Mixtures and Individual Constituents Commonly Display Hormesis Dose Responses, Especially for Neuroprotective Effects. Molecules 2020, 25, 2719. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Petit, P.X. Curcumin, a Multifaceted Hormetic Agent, Mediates an Intricate Crosstalk between Mitochondrial Turnover, Autophagy, and Apoptosis. Oxidative Med. Cell. Longev. 2020, 2020, 3656419. [Google Scholar] [CrossRef]
- Kim, S.J.; Son, T.G.; Park, H.R.; Park, M.; Kim, M.-S.; Kim, H.S.; Chung, H.Y.; Mattson, M.P.; Lee, J. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J. Biol. Chem. 2008, 283, 14497–14505. [Google Scholar] [CrossRef]
- Santel, T.; Pflug, G.; Hemdan, N.Y.A.; Schäfer, A.; Hollenbach, M.; Buchold, M.; Birkenmeier, G.; Hintersdorf, A.; Lindner, I.; Otto, A.; et al. Curcumin inhibits glyoxalase 1—A possible link to its anti-inflammatory and anti-tumor activity. PLoS ONE 2008, 3, e3508. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Park, S.Y.; Kim, Y.M.; Park, O.J. Regulatory effect of the AMPK–COX-2 signaling pathway in curcumin-induced apoptosis in HT-29 colon cancer cells. Ann. N. Y. Acad. Sci. 2009, 1171, 489–494. [Google Scholar] [CrossRef]
- Son, S.; Kim, K.-T.; Cho, D.-C.; Kim, H.-J.; Sung, J.-K.; Bae, J.-S. Curcumin stimulates proliferation of spinal cord neural progenitor cells via a mitogen-activated protein kinase signaling pathway. J. Korean Neurosurg. Soc. 2014, 56, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.S.; Ali, R.E. Hormetic prevention of molecular damage during cellular aging of human skin fibroblasts and keratinocytes. Ann. N. Y. Acad. Sci. 2007, 1100, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.S.; Fernandes, R.A.; Demirovic, D.; Dymek, B.; Lima, C.F. Heat stress and hormetin-induced hormesis in human cells: Effects on aging, wound healing, angiogenesis, and differentiation. Dose-Response 2009, 7, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.S.; Demirovic, D. Curcumin induces stress response and hormetically modulates wound healing ability of human skin fibroblasts undergoing ageing in vitro. Biogerontology 2011, 12, 437–444. [Google Scholar] [CrossRef]
- Yu, T.; Dohl, J.; Wang, L.; Chen, Y.; Gasier, H.G.; Deuster, P.A. Curcumin Ameliorates Heat-Induced Injury through NADPH Oxidase–Dependent Redox Signaling and Mitochondrial Preservation in C2C12 Myoblasts and Mouse Skeletal Muscle. J. Nutr. 2020, 150, 2257–2267. [Google Scholar] [CrossRef] [PubMed]
- Stępień, K.; Wojdyła, D.; Nowak, K.; Mołoń, M. Impact of curcumin on replicative and chronological aging in the Saccharomyces cerevisiae yeast. Biogerontology 2020, 21, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Hang, T.T.T.; Molee, W.; Khempaka, S.; Paraksa, N. Supplementation with curcuminoids and tuna oil influenced skin yellowness, carcass composition, oxidation status, and meat fatty acids of slow-growing chickens. Poult. Sci. 2018, 97, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.L.N.; Zenha, R.S.S.; Antunes, A.H.; Faria, F.R.; Rezende, K.R.; de Souza, E.L.; Mota, J.F. Evaluation of the Impact of Different Doses of Curcuma longa L. on Antioxidant Capacity: A Randomized, Double-Blind, Crossover Pilot Trial. BioMed Res. Int. 2021, 2021, 3532864. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Tian, N.; Li, X.; Luo, Y.; Han, Z.; Li, Z.; Fan, C. Curcumin regulates the metabolism of low density lipoproteins by improving the C-to-U RNA editing efficiency of apolipoprotein B in primary rat hepatocytes. Mol. Med. Rep. 2014, 9, 132–136. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Chang, X.; Heene, E.; Qiao, F.; Nick, P. The phytoalexin resveratrol regulates the initiation of hypersensitive cell death in Vitis cell. PLoS ONE 2011, 6, e26405. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv. Nutr. 2016, 7, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Hum. Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, B.; Mukherjee, S.; Das, D.K. Hormetic response of resveratrol against cardioprotection. Exp. Clin. Cardiol. 2010, 15, e134–e138. [Google Scholar] [PubMed]
- Borriello, A.; Bencivenga, D.; Caldarelli, I.; Tramontano, A.; Borgia, A.; Pirozzi, A.V.A.; Oliva, A.; Ragione Della, F. Resveratrol and Cancer Treatment: Is Hormesis a Yet Unsolved Matter? Curr. Pharm. Des. 2013, 19, 5384–5393. [Google Scholar] [CrossRef] [PubMed]
- Arcanjo, N.M.O.; Luna, C.; Madruga, M.S.; Estévez, M. Antioxidant and pro-oxidant actions of resveratrol on human serum albumin in the presence of toxic diabetes metabolites: Glyoxal and methyl-glyoxal. Biochim. Biophys. Acta 2018, 1862, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Mukazhanova, Z.; Knut, E.; Turgumbayeva, A.; Kipchakbayeva, A.; Seitimova, G.; Mahomoodally, M.F.; Szopa, A.; Lobine, D.; et al. Resveratrol-Based Nanoformulations as an Emerging Therapeutic Strategy for Cancer. Front. Mol. Biosci. 2021, 8, 649395. [Google Scholar] [CrossRef]
- Sawan, A.; Davinelli, S.; Accardi, G.; Aiello, A.; Caruso, C.; Duro, G.; Ligotti, M.E.; Pojero, F.; Scapagnini, G.; Candore, G. Healthy ageing and Mediterranean diet: A focus on hormetic phytochemicals. Mech. Ageing Dev. 2021, 200, 111592. [Google Scholar] [CrossRef]
- Dai, Z.; Li, Y.; Quarles, L.D.; Song, T.; Pan, W.; Zhou, H.; Xiao, Z. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine 2007, 14, 806–814. [Google Scholar] [CrossRef]
- Caldarelli, I.; Speranza, M.C.; Bencivenga, D.; Tramontano, A.; Borgia, A.; Pirozzi, A.V.A.; Perrotta, S.; Oliva, A.; Della Ragione, F.; Borriello, A. Resveratrol mimics insulin activity in the adipogenic commitment of human bone marrow mesenchymal stromal cells. Int. J. Biochem. Cell Biol. 2015, 60, 60–72. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis and Endothelial Progenitor Cells. Dose-Response 2022, 20, 15593258211068625. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wang, C.Q.; Fan, H.H.; Ding, H.Y.; Xie, X.L.; Xu, Y.M.; Wang, B.Y.; Huang, D.J. Effects of Resveratrol on Endothelial Progenitor Cells and Their Contributions to Reendothelialization in Intima-injured Rats. J. Cardiovasc. Pharmacol. 2006, 47, 711–721. [Google Scholar] [CrossRef]
- Xia, L.; Wang, X.X.; Hu, X.S.; Guo, X.G.; Shang, Y.P.; Chen, H.J.; Zeng, C.L.; Zhang, F.R.; Chen, J.Z. Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br. J. Pharmacol. 2008, 155, 387–394. [Google Scholar] [CrossRef]
- Anton, S.D.; Embry, C.; Marsiske, M.; Lu, X.; Doss, H.; Leeuwenburgh, C.; Manini, T.M. Safety and metabolic outcomes of resveratrol supplementation in older adults: Results of a twelve-week, placebo-controlled pilot study. Exp. Gerontol. 2014, 57, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Morisco, F.; Lembo, V.; Ritieni, A. Effect of Red Wine Polyphenols on the Expression of Transthyretin in Murine Choroid Plexus. Curr. Pharm. Biotechnol. 2016, 17, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Loos, J.A.; Franco, M.; Chop, M.; Rodriguez Rodrigues, C.; Cumino, A.C. Resveratrol against Echinococcus sp.: Discrepancies between In Vitro and In Vivo Responses. Trop. Med. Infect. Dis. 2023, 8, 460. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 30, 1261–1269. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E.; Calabrese, V. Ferulic acid and hormesis: Biomedical and environmental implications. Mech. Ageing Dev. 2021, 198, 111544. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, F.H.; Mesbah-Ardakani, M.; Nasr-Esfahani, M.-H. Ferulic Acid exerts concentration-dependent anti-apoptotic and neuronal differentiation-inducing effects in PC12 and mouse neural stem cells. Eur. J. Pharmacol. 2018, 841, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Kenar, J.A.; Moser, B.R.; List, G.R. Naturally occurring fatty acids: Source, chemistry, and uses. In Fatty Acids; Ahmad, M.U., Ed.; AOCS Press, Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–82. [Google Scholar] [CrossRef]
- Sanders, T.A. Functional Dietary Lipids: Food Formulation, Consumer Issues and Innovation for Health, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 315–322. [Google Scholar]
- Choque, B.; Catheline, D.; Rioux, V.; Legrand, P. Linoleic acid: Between doubts and certainties. Biochimie 2014, 96, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Zhang, M.; Ren, F.Z.; Zhou, X.D. Lifelong diet including common unsaturated fatty acids extends the lifespan and affects oxidation in Caenorhabditis elegans consistently with hormesis model. Eur. J. Lipid Sci. Technol. 2016, 118, 1084–1092. [Google Scholar] [CrossRef]
- Di Cristofano, M.; Ferramosca, A.; Di Giacomo, M.; Fusco, C.; Boscaino, F.; Luongo, D.; Vera Rotondi, A.; Maurano, F.; Cocca, E.; Mazzarella, G.; et al. Mechanisms underlying the hormetic effect of conjugated linoleic acid: Focus on Nrf2, mitochondria and NADPH oxidases. Free. Radic. Biol. Med. 2021, 167, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Statistical Modeling: The Two Cultures (with comments and a rejoinder by the author). Stat. Sci. 2001, 16, 199–231. [Google Scholar] [CrossRef]
- Porkodi, J.; Raman, N. Synthesis, characterization and biological screening studies of mixed ligand complexes using flavonoids as precursors. Appl. Organomet. Chem. 2018, 32, e4030. [Google Scholar] [CrossRef]
- Kareem, A.; Khan, M.S.; Nami, S.A.A.; Bhat, S.A.; Mirza, A.U.; Nishat, N. Curcumin derived Schiff base ligand and their transition metal complexes: Synthesis, spectral characterization, catalytic potential and biological activity. J. Mol. Struct. 2018, 1167, 261–273. [Google Scholar] [CrossRef]
- Yan, F.-S.; Sun, J.-L.; Xie, W.-H.; Shen, L.; Ji, H.-F. Neuroprotective Effects and Mechanisms of Curcumin–Cu(II) and –Zn(II) Complexes Systems and Their Pharmacological Implications. Nutrients 2018, 10, 28. [Google Scholar] [CrossRef]
- Meza-Morales, W.; Estévez-Carmona, M.M.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Cassani, J.; Ramírez-Apan, M.T.; Escobedo-Martínez, C.; Soriano-García, M.; Reynolds, W.F.; Enríquez, R.G. Full Structural Characterization of Homoleptic Complexes of Diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-level Cytotoxicity in Vitro with Minimal Acute Toxicity in Vivo. Molecules 2019, 24, 1598. [Google Scholar] [CrossRef]
- Halevas, E.; Papadopoulos, T.A.; Swanson, C.H.; Smith, G.C.; Hatzidimitriou, A.; Katsipis, G.; Pantazaki, A.; Sanakis, I.; Mitrikas, G.; Ypsilantis, K.; et al. In-depth synthetic, physicochemical and in vitro biological investigation of a new ternary V(IV) antioxidant material based on curcumin. J. Inorg. Biochem. 2019, 191, 94–111. [Google Scholar] [CrossRef]
- Hieu, T.Q.; Thanh Thao, D.T. Enhancing the Solubility of Curcumin Metal Complexes and Investigating Some of Their Biological Activities. J. Chem. 2019, 2019, 8082195. [Google Scholar] [CrossRef]
- Călinescu, M.; Fiastru, M.; Bala, D.; Mihailciuc, C.; Negreanu-Pîrjol, T.; Jurcă, B. Synthesis, characterization, electrochemical behavior and antioxidant activity of new copper(II) coordination compounds with curcumin derivatives. J. Saudi Chem. Soc. 2019, 23, 817–827. [Google Scholar] [CrossRef]
- Tran, Q.H.; Doan, T.T. A novel study on curcumin metal complexes: Solubility improvement, bioactivity, and trial burn wound treatment in rats. New J. Chem. 2020, 44, 13036–13045. [Google Scholar] [CrossRef]
- Halevas, E.; Pekou, A.; Papi, R.; Mavroidi, B.; Hatzidimitriou, A.G.; Zahariou, G.; Litsardakis, G.; Sagnou, M.; Pelecanou, M.; Pantazaki, A.A. Synthesis, physicochemical characterization and biological properties of two novel Cu(II) complexes based on natural products curcumin and quercetin. J. Inorg. Biochem. 2020, 208, 111083. [Google Scholar] [CrossRef] [PubMed]
- Altundağ, E.M.; Özbilenler, C.; Ustürk, S.; Kerküklü, N.R.; Afshani, M.; Yilmaz, E. Metal-based curcumin and quercetin complexes: Cell viability, ROS production and antioxidant activity. J. Mol. Struct. 2021, 1245, 131107. [Google Scholar] [CrossRef]
- Li, S.; Mu, B.; Yan, P.; Kang, Y.; Wang, Q.; Wang, A. Incorporation of Different Metal Ion for Tuning Color and Enhancing Antioxidant Activity of Curcumin/Palygorskite Hybrid Materials. Front. Chem. 2021, 9, 760941. [Google Scholar] [CrossRef] [PubMed]
- Meza-Morales, W.; Alejo-Osorio, Y.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Machado-Rodriguez, J.C.; Arenaza-Corona, A.; Toscano, R.A.; Ramírez-Apan, M.T.; Enríquez, R.G. Homoleptic Complexes of Heterocyclic Curcuminoids with Mg(II) and Cu(II): First Conformationally Heteroleptic Case, Crystal Structures, and Biological Properties. Molecules 2023, 28, 1434. [Google Scholar] [CrossRef] [PubMed]
- Joice, M.V.; Metilda, P. Biologically important Schif base–metal complexes derived from arginine and curcumin derivatives. J. Iran. Chem. Soc. 2022, 19, 2495–2504. [Google Scholar] [CrossRef]
- Mari, M.; Carrozza, D.; Malavasi, G.; Venturi, E.; Avino, G.; Capponi, P.C.; Iori, M.; Rubagotti, S.; Belluti, S.; Asti, M.; et al. Curcumin-Based β-Diketo Ligands for Ga3+: Thermodynamic Investigation of Potential Metal-Based Drugs. Pharmaceuticals 2022, 15, 854. [Google Scholar] [CrossRef]
- Caligiuri, R.; Di Maio, G.; Godbert, N.; Scarpelli, F.; Candreva, A.; Rimoldi, I.; Faccheti, G.; Giovanna Lupo, M.; Sicilia, E.; Mazzone, G.; et al. Curcumin-based ionic Pt(II) complexes: Antioxidant and antimicrobial activity. Dalton Trans. 2022, 51, 16545–16556. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Lall, R. Zinc-curcumin based complexes in health and diseases: An approach in chemopreventive and therapeutic improvement. J. Trace Elements Med. Biol. 2022, 73, 127023. [Google Scholar] [CrossRef] [PubMed]
- Al-Thubaiti, E.H. Antibacterial and antioxidant activities of curcumin/Zn metal complex with its chemical characterization and spectroscopic studies. Heliyon 2023, 9, e17468. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Lehene, S.; Lasnapure, B.; Pawar, S.; Kandipati, D. Investigation of antioxidant, anti-ulcer, and analgesic potential of a metal-curcumin complex. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Arenaza-Corona, A.; Obregón-Mendoza, M.A.; Meza-Morales, W.; Ramírez-Apan, M.T.; Nieto-Camacho, A.; Toscano, R.A.; Pérez-González, L.L.; Sánchez-Obregón, R.; Enríquez, R.G. The Homoleptic Curcumin–Copper Single Crystal (ML2): A Long Awaited Breakthrough in the Field of Curcumin Metal Complexes. Molecules 2023, 28, 6033. [Google Scholar] [CrossRef] [PubMed]
- Dhatchinamoorthy, M.; Gobinath, E.; Parveen, S.; Dharani, S.; Kalaiarasi, G. New Ruthenium(II)-arene complexes appended curcumin based hydrazones: Synthesis, spectral characterization, anti-oxidant and anticancer studies. Chem. Pap. 2023, 77, 7725–7736. [Google Scholar] [CrossRef]
- Sumi, M.; Nevaditha, N.T.; Kumari, B.S. Synthesis, spectroscopic investigation and bioactivities of metal complexes from curcuma longa derivative. Inorg. Chim. Acta 2023, 549, 121397. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Hadadzadeh, H.; Mirahmadi-Zade, S.Z.; Farrokhpour, H.; Aboutalebi, F.; Morshedi, D. A curcumin-nicotinoyl derivative and its transition metal complexes: Synthesis, characterization, and in silico and in vitro biological behaviors. Dalton Trans. 2023, 52, 14477–14490. [Google Scholar] [CrossRef]
- Balasaheb, N.S.; Dilipkumar, P. Free radicals, Natural Antioxidants, and their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Purushothaman, A.; Teena Rose, K.S.; Jacob, J.M.; Varatharaj, R.; Shashikala, K.; Janardanan, D. Curcumin Analogues with Improved Antioxidant Properties: A Theoretical Exploration. Food Chem. 2022, 373, 131499. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-inflammatory Properties of Curcumin, a Major Constituent of Curcuma longa: A Review of Preclinical and Clinical Research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Dias, K.; Nikolau, S. Does the Combination of Resveratrol with Al (III) and Zn (II) Improve its Antioxidant Activity? Nat. Prod. Commun. 2011, 6, 1673–1676. [Google Scholar] [CrossRef]
- Qin, W.; Zou, B.; Zhang, Y.; Yi, X.; Pan, Z. UV-VIS, Fluorescence and Mass Spectrometry Investigation on the Transition Metal Ion Chelation of Two Bioisosteres of Resveratrol. Asian J. Chem. 2013, 25, 2185–2188. [Google Scholar] [CrossRef]
- Martínez, A.; Alcendor, R.; Rahman, T.; Podgorny, M.; Sanogo, I.; McCurdy, R. Ionophoric polyphenols selectively bind Cu2+, display potent antioxidant and anti-amyloidogenic properties, and are non-toxic toward Tetrahymena thermophila. Bioorganic Med. Chem. 2016, 25, 3657–3670. [Google Scholar] [CrossRef]
- Manfredi, C.; Trifuoggi, M.; Amoresano, A.; Vasca, E.; Pepe, C.; Volino, S.; Annetta, M. On Trans-Resveratrol in Aqueous Solutions. J. Solut. Chem. 2017, 6, 2214–2230. [Google Scholar] [CrossRef]
- Dytrtová, J.J.; Straka, M.; Bělonožníková, K.; Jakl, M.; Ryšlavá, H. Does resveratrol retain its antioxidative properties in wine? Redox behavior of resveratrol in the presence of Cu(II) and tebuconazole. Food Chem. 2018, 262, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Lima, J.L.F.C.; Pinto, I.; Reis, S.; Siquet, C. Application of a potentiometric system with data-analysis computer programs to the quantification of metal-chelating activity of two natural antioxidants: Caffeic acid and ferulic acid. Helvetica Chim. Acta 2003, 86, 3081–3087. [Google Scholar] [CrossRef]
- Ryan, P.; Hynes, M.J. The kinetics and mechanisms of the reactions of metal ions with naturally occurring antioxidants. J. Inorg. Biochem. 2008, 102, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, A.-D.M.; Durand, E.; Laguerre, M.; Bayrasy, C.; Lecomte, J.; Villeneuve, P.; Jacobsen, C. Antioxidant Properties and Efficacies of Synthesized Alkyl Caffeates, Ferulates, and Coumarates. J. Agric. Food Chem. 2014, 62, 12553–12562. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.P.; Kumar, S.; Kumar, J.; Venugopalan, P.; Aree, T. First structural evidence of biologically important dinegative ferulate ion: Synthesis, characterization, single crystal X-ray structure and DFT calculation of [Cu(en)2(H2O)2](fer). Polyhedron 2017, 126, 245–251. [Google Scholar] [CrossRef]
- Truong, D.H.; Nhung, N.T.A.; Dao, D.Q. Iron ions chelation-based antioxidant potential vs. pro-oxidant risk of ferulic acid: A DFT study in aqueous phase. Comput. Theor. Chem. 2020, 1185, 112905. [Google Scholar] [CrossRef]
- Mertens, T.; Kunz, T.; Methner, F.-J. Assessment of Chelators in Wort and Beer Model Solutions. BrewingScience 2020, 73, 58–67. [Google Scholar] [CrossRef]
- Shao, B.; Mao, L.; Tang, M.; Yan, Z.-Y.; Shao, J.; Huang, C.-H.; Sheng, Z.-G.; Zhu, B.-Z. Caffeic Acid Phenyl Ester (CAPE) Protects against Iron-Mediated Cellular DNA Damage through Its Strong Iron-Binding Ability and High Lipophilicity. Antioxidants 2021, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Arriaga, R.; Perez-Gonzalez, A.; Marino, T.; Russo, N.; Galano, A. Antioxidants into Nopal (Opuntia ficus-indica), Important Inhibitors of Free Radicals’ Formation. Antioxidants 2021, 10, 2006. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Gołębiewska, E.; Mazur, L.; Lewandowska, H.; Pruszyński, M.; Świderski, G.; Wyrwas, M.; Pawluczuk, N.; Lewandowski, W. Crystal Structure, Spectroscopic Characterization, Antioxidant and Cytotoxic Activity of New Mg(II) and Mn(II)/Na(I) Complexes of Isoferulic Acid. Materials 2021, 14, 3236. [Google Scholar] [CrossRef] [PubMed]
- Neopane, D.; Ansari, V.A.; Singh, A. Ferulic Acid: Signaling Pathways in Aging. Drug Res. 2023, 73, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Ha, Y.L.; Pariza, M.W. π-Complex formation of conjugated linoleic acid with iron. Food Chem. 2007, 100, 972–976. [Google Scholar] [CrossRef]
- Wang, Y.-E.; Zhai, J.; Zheng, Y.; Pan, J.; Liu, X.; Ma, Y.; Guan, S. Self-assembled iRGD-R7-LAHP-M nanoparticle induced sufficient singlet oxygen and enhanced tumor penetration immunological therapy. Nanoscale 2022, 14, 11388–11406. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Wu, H.; Tan, J.; Yi, W.; Wang, Z.; Yu, Z.; Wang, X. Self-assembly nanoplatform of platinum (Ⅳ) prodrug for enhanced ovarian cancer therapy. Mater. Today Bio 2023, 21, 100698. [Google Scholar] [CrossRef]




| Metal Ion/s | Biological Activity | Dose | Ref. |
|---|---|---|---|
| Co2+, Ni2+, Cu2+, Zn2+ | Enhance antioxidant and anti-inflammatory activity after complex formation. Albino rats were used for in vivo studies. | 10−4 mM for radical scavenger activity; 0.1 mL of a 1% w/v solution for in vivo studies. | [131] |
| Cu2+, Co2+, Ni2+, Zn2+ | Complexes better antioxidant activity than free curcumin. | 5–20 μM | [132] |
| Cu2+, Zn2+ | Complexes better antioxidant activity than free curcumin. Complexes exert neuroprotective effects. | 25 µM | [133] |
| Mn2+, Mg2+, Cu2+, Zn2+ | Antioxidant activity of the complexes inhibiting lipid peroxidation in the rat tissue model. | 0.1–10 μM | [134] |
| VO2+ | Complexes better antioxidant activity than free curcumin. | 1–25 μM | [135] |
| Zn2+, Cu2+, Fe3+ | Complexes better antioxidant activity than free curcumin. | 100 μg/mL | [136] |
| Cu2+ | Complex more potent antioxidant agents than curcumin. Activity influenced by the metal–ligand molar ratio. | 0.3–0.9 nM | [137] |
| Fe2+, Zn2+, Ca2+ | Complexes more potent antioxidant agents than free curcumin. Anti-inflammatory activity shown in Wistar albino rats. | 200 ppm, 3 times daily, 3–21 days. | [138] |
| Cu2+ | Complex more potent antioxidant agents than free curcumin. | 2–20 μM | [139] |
| Mg2+, Ca2+ | No difference in antioxidant activity between curcumin and metal complexes. | 19–21 μM | [140] |
| Cu2+, Zn2+, Mg2+, Al3+, Fe3+ | Complexes better antioxidant activity than free curcumin. | Complexes were incorporated to form hybrid composites. | [141] |
| Cu2+, Mg2+ | Complexes better antioxidant activity than free curcumin. | 10–11 μM | [142] |
| Cu2+, Co2+, Ni2+, Zn2+ | Complexes better antioxidant activity than free curcumin. | 250–1000 μg/mL | [143] |
| Ga3+ | Complexes antioxidant activity close to free curcumin. | 20–32 μM | [144] |
| Pt2+ | No difference in antioxidant activity between curcumin and metal complexes. | 10–50 μM | [145] |
| Zn2+ | Complexes better antioxidant activity than free curcumin. | 12–48 mg/kg Sprague–Dawley rats. | [146] |
| Zn2+ | Complexes better antioxidant activity than free curcumin. | 6.25–50 μM | [147] |
| Na+ | Complexes better antioxidant and analgesic activities than free curcumin. Sprague–Dawley rats used for anti-ulcer studies. | 0.1–15 µg/mL for antioxidant studies; 100 mg/kg body weight for in vivo studies. | [148] |
| Cu2+ | Complexes better antioxidant activity than free curcumin. | 1.24 μM | [149] |
| Ru2+ | Complexes better antioxidant activity than free curcumin. | 20–100 µg/mL | [150] |
| Mn2+, Co2+, Ni2+, Cu2+, Zn2+ | Free curcumin better antioxidant activity than metal complexes. | 6.25–100 μg/mL | [151] |
| Zn2+, Cu2+ | Complexes exhibit antioxidant activity. | 0.125–1.0 mg/mL | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreiro-Sisto, U.; Fernández-Fariña, S.; González-Noya, A.M.; Pedrido, R.; Maneiro, M. Enemies or Allies? Hormetic and Apparent Non-Dose-Dependent Effects of Natural Bioactive Antioxidants in the Treatment of Inflammation. Int. J. Mol. Sci. 2024, 25, 1892. https://doi.org/10.3390/ijms25031892
Barreiro-Sisto U, Fernández-Fariña S, González-Noya AM, Pedrido R, Maneiro M. Enemies or Allies? Hormetic and Apparent Non-Dose-Dependent Effects of Natural Bioactive Antioxidants in the Treatment of Inflammation. International Journal of Molecular Sciences. 2024; 25(3):1892. https://doi.org/10.3390/ijms25031892
Chicago/Turabian StyleBarreiro-Sisto, Uxía, Sandra Fernández-Fariña, Ana M. González-Noya, Rosa Pedrido, and Marcelino Maneiro. 2024. "Enemies or Allies? Hormetic and Apparent Non-Dose-Dependent Effects of Natural Bioactive Antioxidants in the Treatment of Inflammation" International Journal of Molecular Sciences 25, no. 3: 1892. https://doi.org/10.3390/ijms25031892
APA StyleBarreiro-Sisto, U., Fernández-Fariña, S., González-Noya, A. M., Pedrido, R., & Maneiro, M. (2024). Enemies or Allies? Hormetic and Apparent Non-Dose-Dependent Effects of Natural Bioactive Antioxidants in the Treatment of Inflammation. International Journal of Molecular Sciences, 25(3), 1892. https://doi.org/10.3390/ijms25031892







