The Cyst Epithelium in Polycystic Kidney Disease Patients Displays Normal Apical-Basolateral Cell Polarity
Abstract
1. Introduction
2. Results
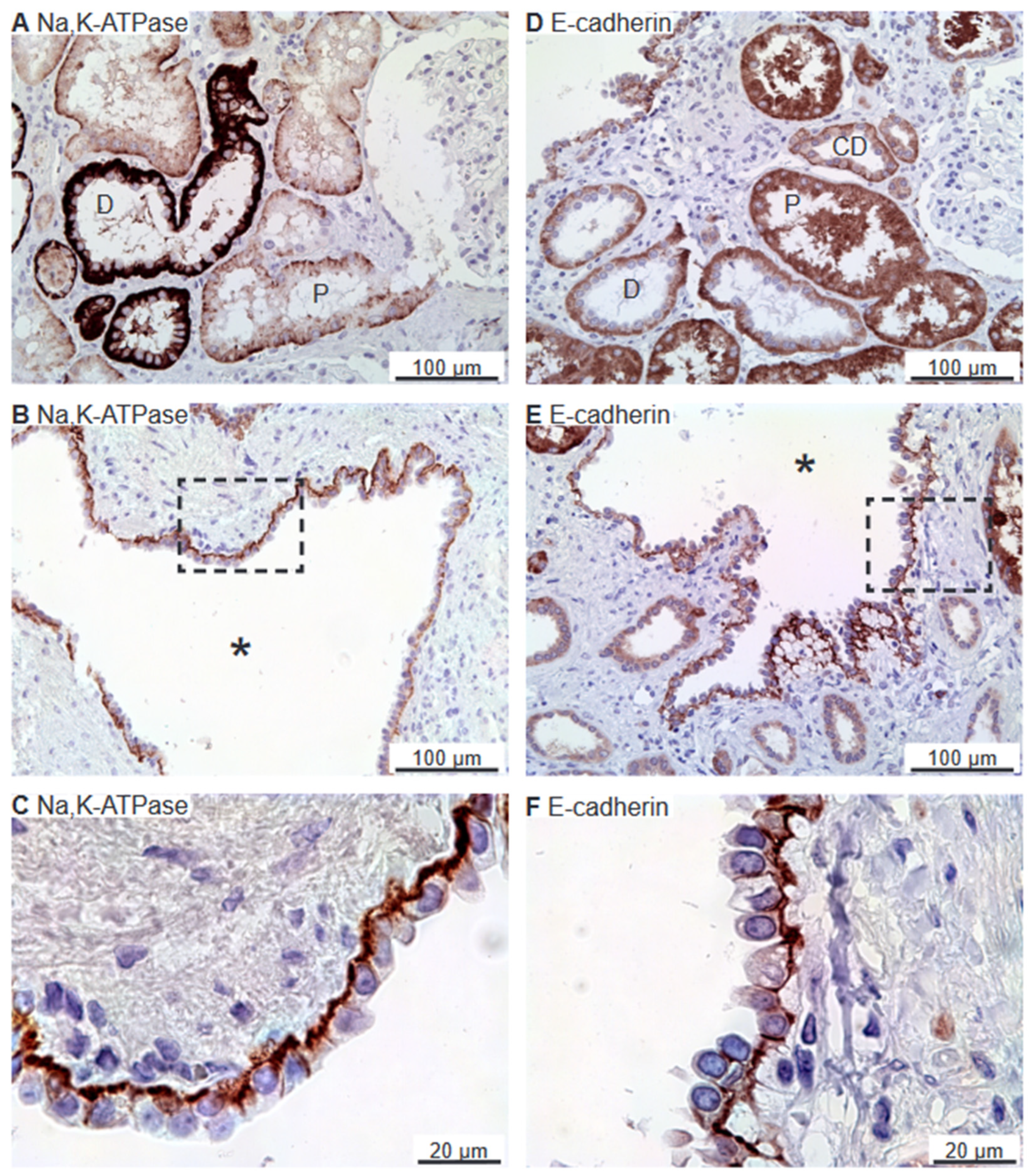
2.1. The Na,K-ATPase α1 Subunit and E-Cadherin Localize to the Basolateral Membranes of Renal Cyst Epithelia
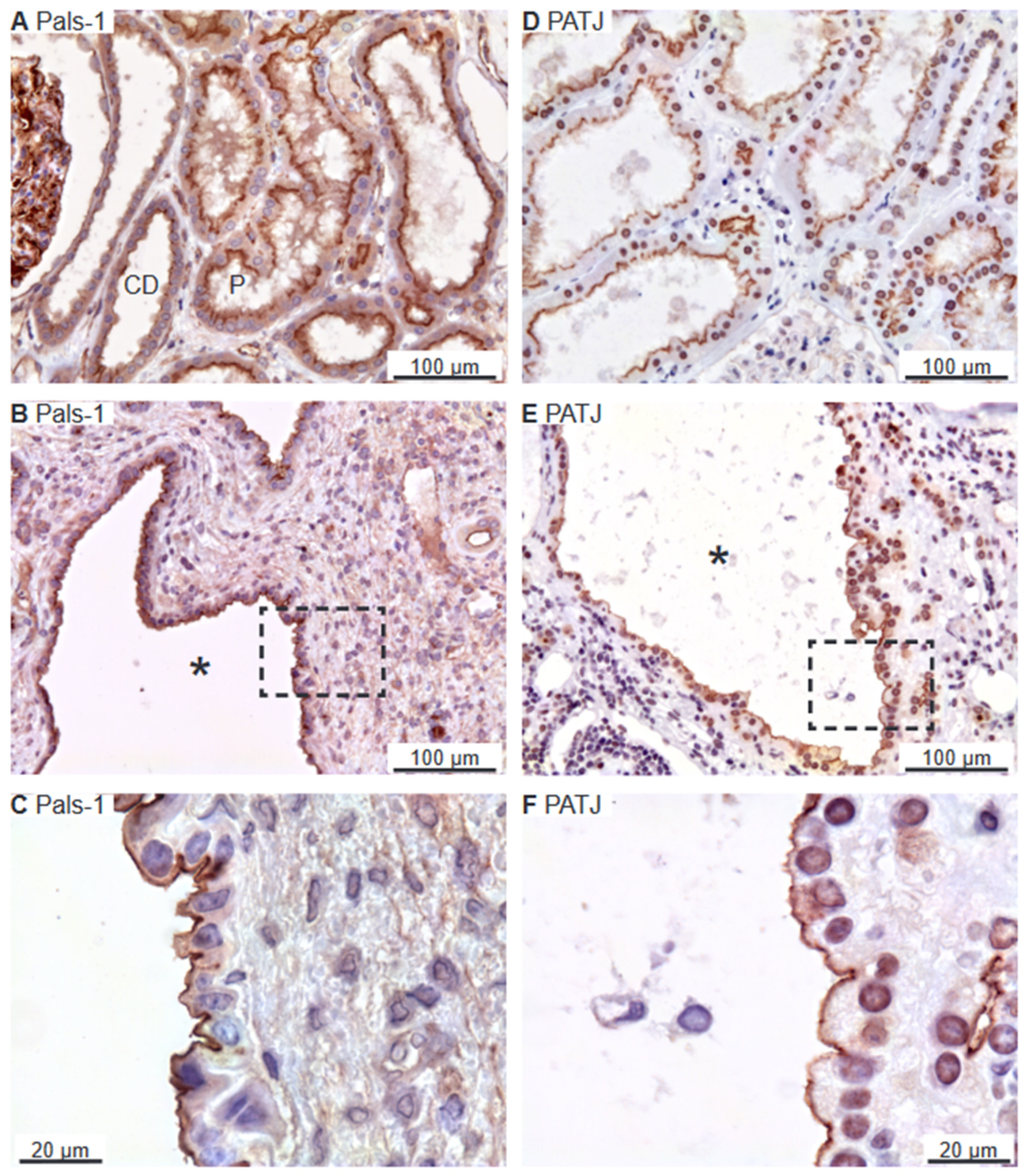
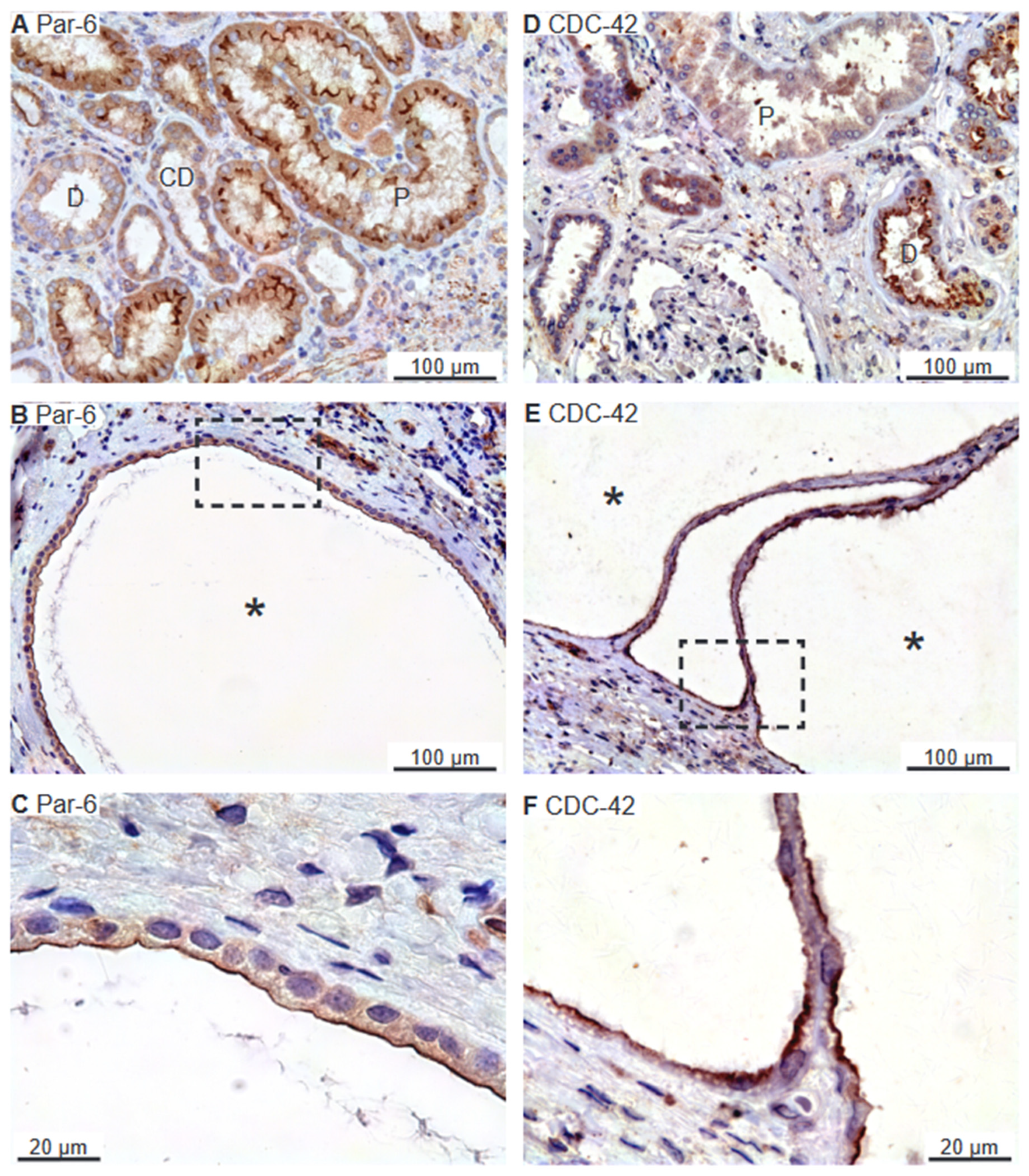
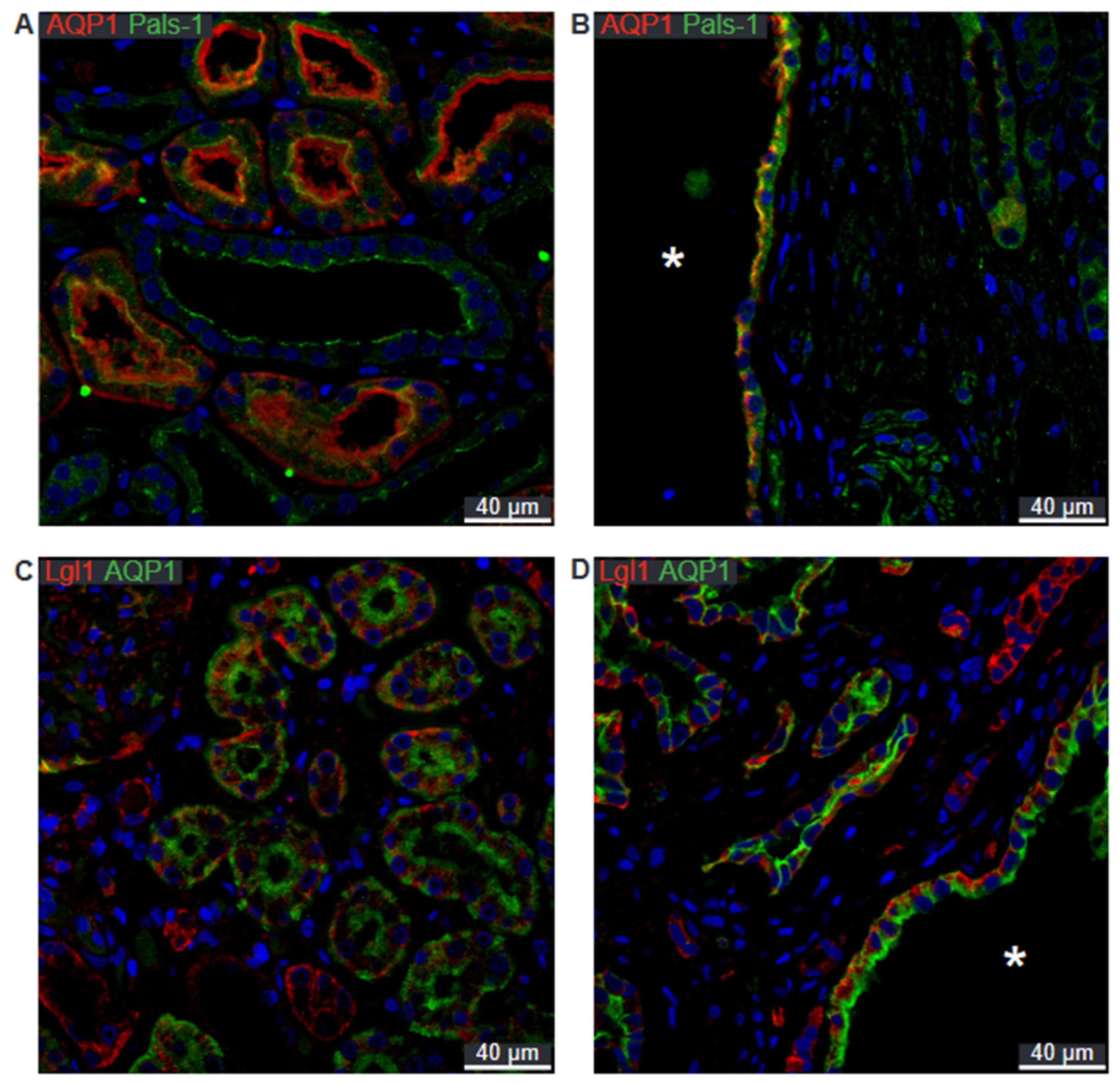
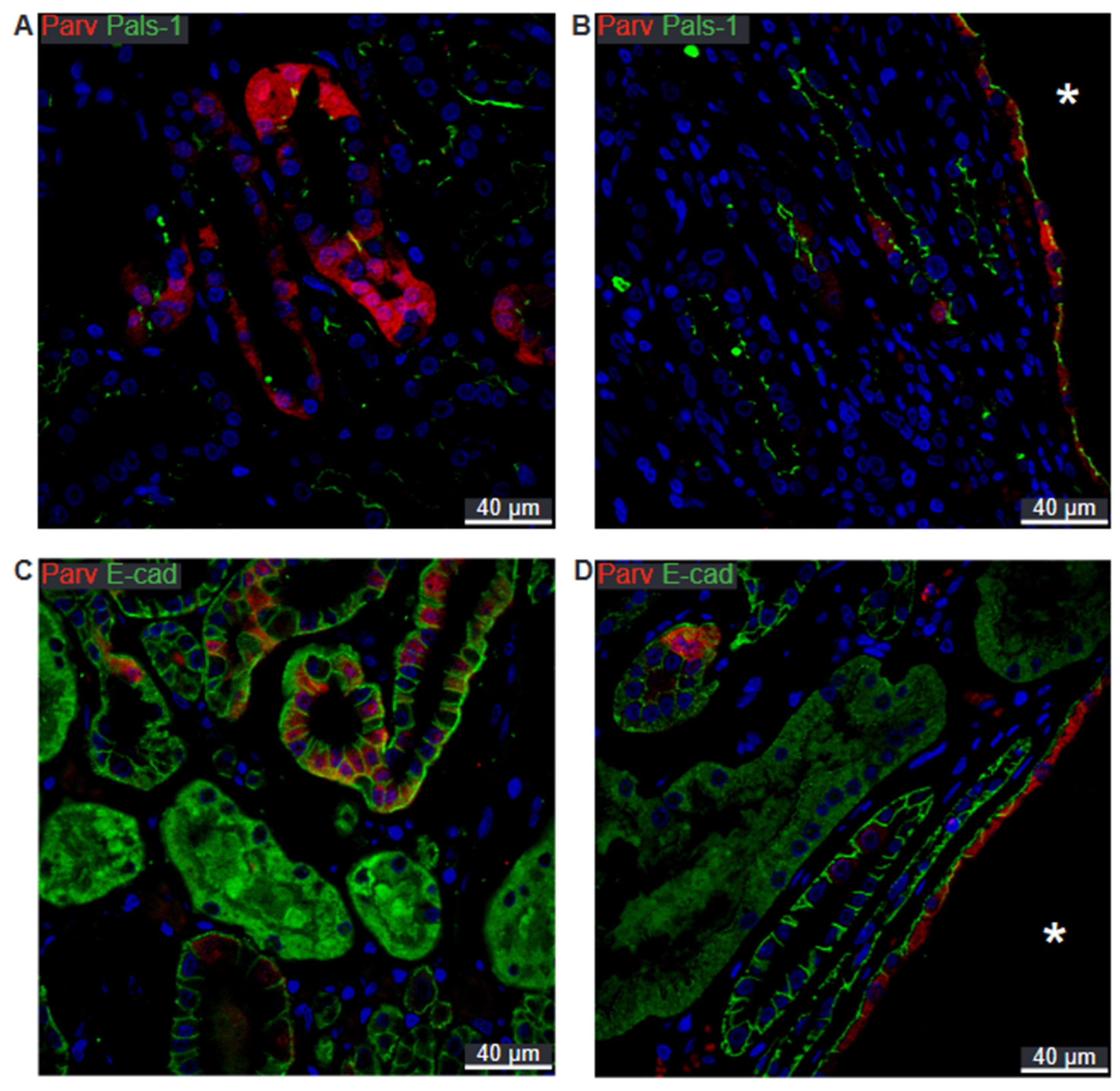
2.2. SNARE-Protein Syntaxin-3, Crumbs and Par Complexes Are Confined to the Apical Membranes of Renal Cyst Epithelia
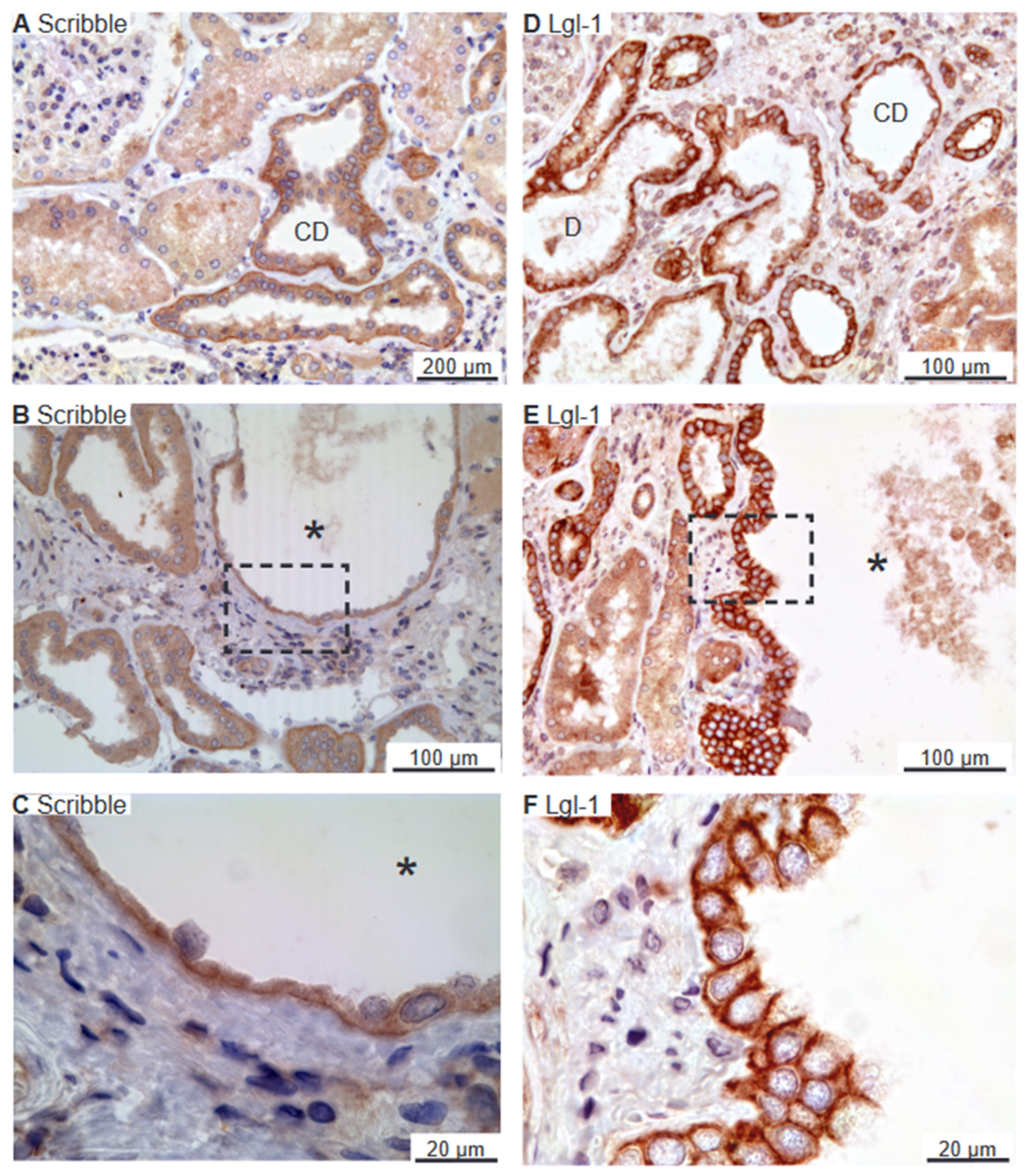
2.3. Scribble Complex Components Are Expressed in the Basolateral Domain of Renal Cysts
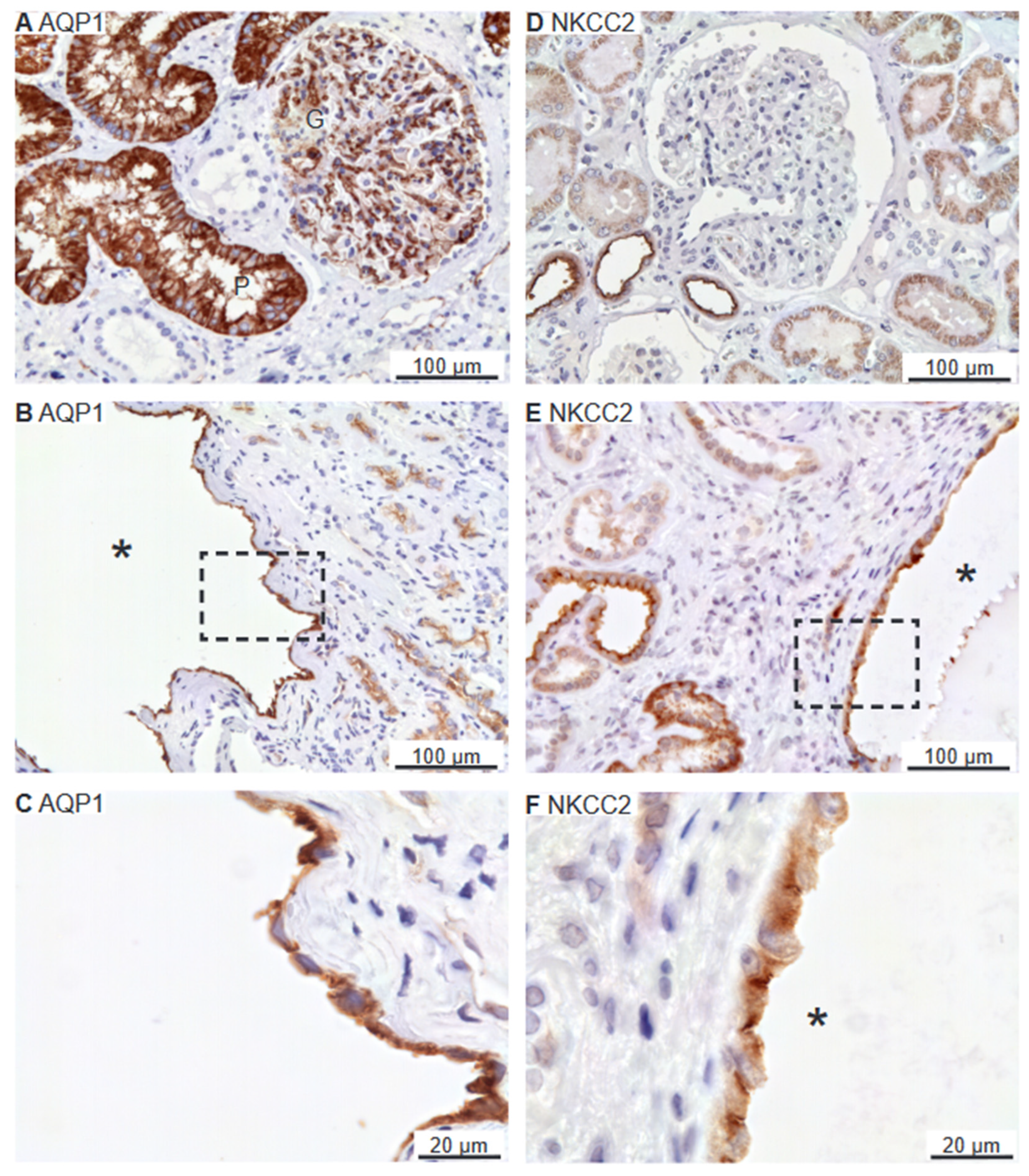
2.4. Renal Cysts Origin from More Tubular Segments
2.5. Preserved Cell Polarity in Cysts Derived from Different Segments
3. Discussion
4. Materials and Methods
4.1. Preparation of Patient Material
4.2. Immunohistochemistry
4.3. Cell Polarity Markers
4.4. Microscopy and Image Processing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres, V.E.; Harris, P.C.; Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 2007, 369, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers 2018, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.D.; Sherwood, A.C.; Palla, K.; Du, J.; Watson, R.; Norman, J.T. Reversed polarity of Na(+) -K(+) -ATPase: Mislocation to apical plasma membranes in polycystic kidney disease epithelia. Am. J. Physiol. 1991, 260, F420–F430. [Google Scholar] [CrossRef]
- Patel, V.; Li, L.; Cobo-Stark, P.; Shao, X.; Somlo, S.; Lin, F.; Igarashi, P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet. 2008, 17, 1578–1590. [Google Scholar] [CrossRef]
- Fischer, E.; Legue, E.; Doyen, A.; Nato, F.; Nicolas, J.-F.; Torres, V.; Yaniv, M.; Pontoglio, M. Defective planar cell polarity in polycystic kidney disease. Nat. Genet. 2006, 38, 21–23. [Google Scholar] [CrossRef]
- Mellman, I.; Nelson, W.J. Coordinated protein sorting, targeting and distribution in polarized cells. Nat. Rev. Mol. Cell Biol. 2008, 9, 833–845. [Google Scholar] [CrossRef]
- Stoops, E.H.; Caplan, M.J. Trafficking to the apical and basolateral membranes in polarized epithelial cells. J. Am. Soc. Nephrol. 2014, 25, 1375–1386. [Google Scholar] [CrossRef]
- Sharma, N.; Low, S.H.; Misra, S.; Pallavi, B.; Weimbs, T. Apical targeting of syntaxin 3 is essential for epithelial cell polarity. J. Cell Biol. 2006, 173, 937–948. [Google Scholar] [CrossRef] [PubMed]
- ter Beest, M.B.; Chapin, S.J.; Avrahami, D.; Mostov, K.E. The role of syntaxins in the specificity of vesicle targeting in polarized epithelial cells. Mol. Biol. Cell 2005, 16, 5784–5792. [Google Scholar] [CrossRef]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Roignot, J.; Peng, X.; Mostov, K. Polarity in mammalian epithelial morphogenesis. Cold Spring Harb. Perspect. Biol. 2013, 5, a013789. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.E.; St Johnston, D. Apical-basal polarity and the control of epithelial form and function. Nat. Rev. Mol. Cell Biol. 2022, 23, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Goehring, N.W. PAR polarity: From complexity to design principles. Exp. Cell Res. 2014, 328, 258–266. [Google Scholar] [CrossRef]
- Graybill, C.; Wee, B.; Atwood, S.X.; Prehoda, K.E. Partitioning-defective protein 6 (Par-6) activates atypical protein kinase C (aPKC) by pseudosubstrate displacement. J. Biol. Chem. 2012, 287, 21003–21011. [Google Scholar] [CrossRef]
- Whitney, D.S.; Peterson, F.C.; Kittell, A.W.; Egner, J.M.; Prehoda, K.E.; Volkman, B.F. Binding of Crumbs to the Par-6 CRIB-PDZ Module Is Regulated by Cdc42. Biochemistry 2016, 55, 1455–1461. [Google Scholar] [CrossRef]
- Plant, P.J.; Fawcett, J.P.; Lin, D.C.; Holdorf, A.D.; Binns, K.; Kulkarni, S.; Pawson, T. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat. Cell Biol. 2003, 5, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Hurov, J.B.; Watkins, J.L.; Piwnica-Worms, H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr. Biol. 2004, 14, 736–741. [Google Scholar] [CrossRef]
- Cereijido, M.; Valdés, J.; Shoshani, L.; Contreras, R.G. Role of tight junctions in establishing and maintaining cell polarity. Annu. Rev. Physiol. 1998, 60, 161–177. [Google Scholar] [CrossRef]
- Nejsum, L.N.; Nelson, W.J. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J. Cell Biol. 2007, 178, 323–335. [Google Scholar] [CrossRef]
- O’Brien, L.E.; Jou, T.S.; Pollack, A.L.; Zhang, Q.; Hansen, S.H.; Yurchenco, P.; Mostov, K.E. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 2001, 3, 831–838. [Google Scholar] [CrossRef]
- Grantham, J.J.; Geiser, J.L.; Evan, A.P. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 1987, 31, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.D.; Devuyst, O.; Li, X.; Gatti, L.; Falkenstein, D.; Robinson, S.; Fambrough, D.; Burrow, C.R. Apical Plasma Membrane Mispolarization of NaK-ATPase in Polycystic Kidney Disease Epithelia Is Associated with Aberrant Expression of the β2 Isoform. Am. J. Pathol. 2000, 156, 253–268. [Google Scholar] [CrossRef]
- van Roy, F.; Berx, G. The cell-cell adhesion molecule E-cadherin. Cell Mol. Life Sci. 2008, 65, 3756–3788. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Low, S.H.; Miura, M.; Weimbs, T. SNARE expression and localization in renal epithelial cells suggest mechanism for variability of trafficking phenotypes. Am. J. Physiol. Ren. Physiol. 2002, 283, F1111–F1122. [Google Scholar] [CrossRef]
- Horikoshi, Y.; Suzuki, A.; Yamanaka, T.; Sasaki, K.; Mizuno, K.; Sawada, H.; Yonemura, S.; Ohno, S. Interaction between PAR-3 and the aPKC-PAR-6 complex is indispensable for apical domain development of epithelial cells. J. Cell Sci. 2009, 122, 1595–1606. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Boulan, E.; Macara, I.G. Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 2014, 15, 225–242. [Google Scholar] [CrossRef]
- Leeuwen, I.S.L.-v.; Dauwerse, J.G.; Baelde, H.J.; Leonhard, W.N.; van de Wal, A.; Ward, C.J.; Verbeek, S.; DeRuiter, M.C.; Breuning, M.H.; de Heer, E.; et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Human Mol. Genet. 2004, 13, 3069–3077. [Google Scholar] [CrossRef]
- Jiang, S.T.; Chiou, Y.Y.; Wang, E.; Lin, H.K.; Lin, Y.T.; Chi, Y.C.; Wang, C.K.; Tang, M.J.; Li, H. Defining a link with autosomal-dominant polycystic kidney disease in mice with congenitally low expression of Pkd1. Am. J. Pathol. 2006, 168, 205–220. [Google Scholar] [CrossRef]
- Wilson, P.D.; Schrier, R.W.; Breckon, R.D.; Gabow, P.A. A new method for studying human polycystic kidney disease epithelia in culture. Kidney Int. 1986, 30, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Brill, S.R.; Ross, K.E.; Davidow, C.J.; Ye, M.; Grantham, J.J.; Caplan, M.J. Immunolocalization of ion transport proteins in human autosomal dominant polycystic kidney epithelial cells. Proc. Natl. Acad. Sci. USA 1996, 93, 10206–10211. [Google Scholar] [CrossRef]
- Carone, F.A.; Nakamura, S.; Caputo, M.; Bacallao, R.; Nelson, W.J.; Kanwar, Y.S. Cell polarity in human renal cystic disease. Lab. Investig. 1994, 70, 648–655. [Google Scholar]
- Kawa, G.; Nagao, S.; Yamamoto, A.; Omori, K.; Komatz, Y.; Takahashi, H.; Tashiro, Y. Sodium pump distribution is not reversed in the DBA/2FG-pcy, polycystic kidney disease model mouse. J. Am. Soc. Nephrol. 1994, 4, 2040–2049. [Google Scholar] [CrossRef]
- Takahashi, M.; Tsuchiya, K.; Komatsu, Y.; Nihei, H. A role for Na/K adenosine triphosphatase in the pathogenesis of cyst formation in experimental polycystic kidney disease. J. Lab. Clin. Med. 1997, 129, 517–526. [Google Scholar] [CrossRef]
- Wang, X.; Ward, C.J.; Harris, P.C.; Torres, V.E. Cyclic nucleotide signaling in polycystic kidney disease. Kidney Int. 2010, 77, 129–140. [Google Scholar] [CrossRef][Green Version]
- Gattone, V.H.; Wang, X.; Harris, P.C.; Torres, V.E. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat. Med. 2003, 9, 1323–1326. [Google Scholar] [CrossRef]
- Wallace, D.P.; Rome, L.A.; Sullivan, L.P.; Grantham, J.J. cAMP-dependent fluid secretion in rat inner medullary collecting ducts. Am. J. Physiol. Ren. Physiol. 2001, 280, F1019–F1029. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, K.; Devuyst, O.; Schwiebert, E.M.; Wilson, P.D.; Guggino, W.B. A role for CFTR in human autosomal dominant polycystic kidney disease. Am. J. Physiol. 1996, 270, C389–C399. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Lamar, P.C.; Appelt, D.M. Differential expression of E-cadherin, N-cadherin and beta-catenin in proximal and distal segments of the rat nephron. BMC Physiol. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Mandon, B.; Nielsen, S.; Kishore, B.K.; Knepper, M.A. Expression of syntaxins in rat kidney. Am. J. Physiol. Ren. Physiol. 1997, 273, F718–F730. [Google Scholar] [CrossRef] [PubMed]
- Breton, S.; Inoue, T.; Knepper, M.A.; Brown, D. Antigen retrieval reveals widespread basolateral expression of syntaxin 3 in renal epithelia. Am. J. Physiol. Ren. Physiol. 2002, 282, F523–F529. [Google Scholar] [CrossRef]
- Lin, F.; Hiesberger, T.; Cordes, K.; Sinclair, A.M.; Goldstein, L.S.B.; Somlo, S.; Igarashi, P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2003, 100, 5286–5291. [Google Scholar] [CrossRef]
- Lebeau, C.; Hanaoka, K.; Moore-Hoon, M.L.; Guggino, W.B.; Beauwens, R.; Devuyst, O. Basolateral chloride transporters in autosomal dominant polycystic kidney disease. Pflug. Arch. 2002, 444, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.M.; Nørregaard, R.; Topcu, S.O.; Frøkiær, J.; Pedersen, M. Oxygen tension correlates with regional blood flow in obstructed rat kidney. J. Exp. Biol. 2009, 212, 3156–3163. [Google Scholar] [CrossRef] [PubMed]
- Ecelbarger, C.A.; Terris, J.; Hoyer, J.R.; Nielsen, S.; Wade, J.B.; Knepper, M.A. Localization and regulation of the rat renal Na(+)-K(+)-2Cl- cotransporter, BSC-1. Am. J. Physiol. Ren. Physiol. 1996, 271, F619–F628. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Kwon, T.H.; Praetorius, J.; Frøkiaer, J.; Knepper, M.A.; Nielsen, S. Aldosterone increases urine production and decreases apical AQP2 expression in rats with diabetes insipidus. Am. J. Physiol. Ren. Physiol. 2006, 290, F438–F449. [Google Scholar] [CrossRef]




| Segment | Syntaxin-3 | E-Cadherin | Crumbs-3 | Pals-1 | PATJ | Par-6 | CDC-42 | Scribble | Lgl-1 |
|---|---|---|---|---|---|---|---|---|---|
| PT | x | x | x | x | x | ||||
| TAL | x | x | x | x | |||||
| DCT | x | x | x | x | x | x | x | x | |
| CCD | x | x | x | x | x | x | x |
| Target | Antibody No. | Dilution | Host | Source |
|---|---|---|---|---|
| Na/K-ATPase | 3B-0/56-0 | 1:5000 | Mouse | Forbush B III, New Haven, CT, USA |
| E-cadherin | LS-B12414-300 | 1:1000 | Goat | LS Bio, Seattle, WA, USA |
| Syntaxin-3 | sc-47437 | 1:25 | Goat | Santa Cruz Biotech, Dallas, TX, USA |
| Crumbs3a | crumbs 3a | 1:200 | Rat | Massey-Harroche & Le Bivic, Marseilles, France |
| Pals-1 | 17710-AP | 1:200 | Rabbit | Proteintech, Manchester, UK |
| PATJ | Anti-Patj | 1:125 | Rabbit | Massey-Harroche & Le Bivic, Marseilles, France |
| Par-6 | sc-166405 | 1:100 | Mouse | Santa Cruz Biotech, Dallas, TX, USA |
| CDC-42 | sc-34314 | 1:50 | Goat | Santa Cruz Biotech, Dallas, TX, USA |
| Scribble | sc-11048 | 1:10 | Goat | Santa Cruz Biotech, Dallas, TX, USA |
| Lgl-1 | UNC 17-35 | 1:25 | Mouse | Patrick Humbert, Melbourne, Victoria, Australia |
| AQP1 | NB6000-749 | 1:2000 | Rabbit | Novus Biologicals, Abingdon, UK |
| NKCC2 | 1495ap | 1:200 | Rabbit | [44,45] |
| NCC | SPC-402D | 1:10,000 | Rabbit | StressMarq Biosciences Victoria, BC, Canada |
| Parvalbumin | PV235 | 1:400 | Mouse | Swant AG, Burgdorf Switzerland |
| AQP2 | H7661 | 1:2000 | Rabbit | [46] |
| AQP2 | sc-9882 | 1:250 | Goat | Santa Cruz Biotech, Dallas, TX, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandegaard, S.L.; Riishede, A.; Birn, H.; Damkier, H.H.; Praetorius, J. The Cyst Epithelium in Polycystic Kidney Disease Patients Displays Normal Apical-Basolateral Cell Polarity. Int. J. Mol. Sci. 2024, 25, 1904. https://doi.org/10.3390/ijms25031904
Sandegaard SL, Riishede A, Birn H, Damkier HH, Praetorius J. The Cyst Epithelium in Polycystic Kidney Disease Patients Displays Normal Apical-Basolateral Cell Polarity. International Journal of Molecular Sciences. 2024; 25(3):1904. https://doi.org/10.3390/ijms25031904
Chicago/Turabian StyleSandegaard, Samuel Loft, Andreas Riishede, Henrik Birn, Helle Hasager Damkier, and Jeppe Praetorius. 2024. "The Cyst Epithelium in Polycystic Kidney Disease Patients Displays Normal Apical-Basolateral Cell Polarity" International Journal of Molecular Sciences 25, no. 3: 1904. https://doi.org/10.3390/ijms25031904
APA StyleSandegaard, S. L., Riishede, A., Birn, H., Damkier, H. H., & Praetorius, J. (2024). The Cyst Epithelium in Polycystic Kidney Disease Patients Displays Normal Apical-Basolateral Cell Polarity. International Journal of Molecular Sciences, 25(3), 1904. https://doi.org/10.3390/ijms25031904






