Genome-Wide Identification of B-Box Gene Family and Candidate Light-Related Member Analysis of Tung Tree (Vernicia fordii)
Abstract
:1. Introduction
2. Results
2.1. Genome-Wide Identification B-Box (BBX) Family Members in Tung Tree
2.2. Conserved Domains and Gene Structure Analysis of VfBBX Genes in Tung Tree
2.3. Chromosomal Distribution and Interspecies Synteny Analyses for VfBBXs
2.4. Cis-Elements Analysis in the Promoter of VfBBXs
2.5. Expressions Analyzed of VfBBXs in Divergence Stages by RNA-seq Data
2.6. Different Light-Shading-Treatment of Tung Tree Seedlings
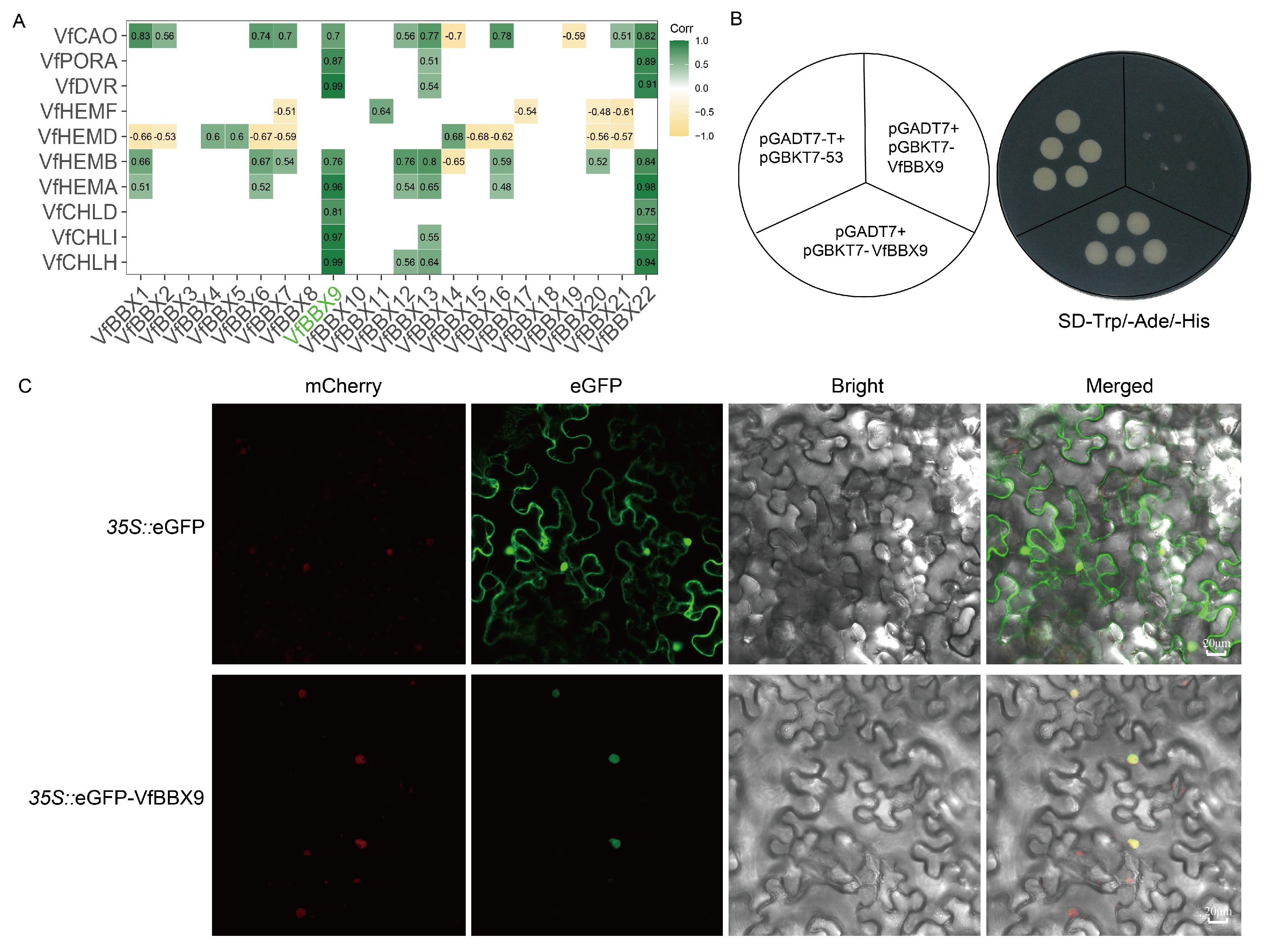
2.7. A Candidate VfBBX9 May Be Involved in Photomorphogenesis Process
3. Discussion
4. Materials and Methods
4.1. Identification of VfBBX Transcription Factors in Tung Tree
4.2. Conserved Motifs, Gene Structures, and Phylogenetic Analysis of VfBBX Members
4.3. Synteny Analysis and Cis-Elements Analysis of VfBBXs
4.4. Materials and RNA-seq Analysis
4.5. Different Light-Shading Treatment Analysis of Tung Tree Seedlings
4.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.7. Pearson Correlation Coefficient and Transcriptional Activity Analysis
4.8. Subcellular Localization Assay of VfBBX9
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knödler, M.; Rühl, C.; Emonts, J.; Buyel, J.F. Seasonal Weather Changes Affect the Yield and Quality of Recombinant Proteins Produced in Transgenic Tobacco Plants in a Greenhouse Setting. Front. Plant Sci. 2019, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Calderon, R.H.; Dalton, J.; Zhang, Y.; Quail, P.H. Shade triggers posttranscriptional PHYTOCHROME-INTERACTING FACTOR-dependent increases in H3K4 trimethylation. Plant Physiol. 2022, 190, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [PubMed]
- Cao, J.; Liang, Y.; Yan, T.; Wang, X.; Zhou, H.; Chen, C.; Zhang, Y.; Zhang, B.; Zhang, S.; Liao, J.; et al. The photomorphogenic repressors BBX28 and BBX29 integrate light and brassinosteroid signaling to inhibit seedling development in Arabidopsis. Plant Cell 2022, 34, 2266–2285. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.N.; Kathare, P.K.; Huq, E. Phytochromes and Phytochrome Interacting Factors. Plant Physiol. 2018, 176, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Li, Y.H.; Chen, L.T.; Chen, W.C.; Hsieh, W.P.; Shin, J.; Jane, W.N.; Chou, S.J.; Choi, G.; Hu, J.M.; et al. LZF1, a HY5-regulated transcriptional factor, functions in Arabidopsis de-etiolation. Plant J. 2008, 54, 205–219. [Google Scholar] [CrossRef]
- Crocco, C.D.; Holm, M.; Yanovsky, M.J.; Botto, J.F. AtBBX21 and COP1 genetically interact in the regulation of shade avoidance. Plant J. 2010, 64, 551–562. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Chen, Y.; Dai, Y.; Yuan, Q.; Shan, Q.; Pan, L.; Dai, L.; Zou, X.; Liu, F.; et al. Genome-Wide Characterization and Anthocyanin-Related Expression Analysis of the B-BOX Gene Family in Capsicum annuum L. Front. Genet. 2022, 13, 847328. [Google Scholar] [CrossRef]
- Xu, X.; Xu, J.; Yuan, C.; Chen, Q.; Liu, Q.; Wang, X.; Qin, C. BBX17 Interacts with CO and Negatively Regulates Flowering Time in Arabidopsis thaliana. Plant Cell Physiol. 2022, 63, 401–409. [Google Scholar] [CrossRef]
- Yadav, A.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. BBX31 promotes hypocotyl growth, primary root elongation and UV-B tolerance in Arabidopsis. Plant Signal. Behav. 2019, 14, e1588672. [Google Scholar] [CrossRef]
- Alvarez-Fernandez, R.; Penfold, C.A.; Galvez-Valdivieso, G.; Exposito-Rodriguez, M.; Stallard, E.J.; Bowden, L.; Moore, J.D.; Mead, A.; Davey, P.A.; Matthews, J.S.A.; et al. Time-series transcriptomics reveals a BBX32-directed control of acclimation to high light in mature Arabidopsis leaves. Plant J. 2021, 107, 1363–1386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Heng, Y.; Wang, X.; Deng, X.W.; Xu, D. A Positive Feedback Loop of BBX11-BBX21-HY5 Promotes Photomorphogenic Development in Arabidopsis. Plant Commun. 2020, 1, 100045. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.Y.; Sun, Y.; Cao, D.M.; Bai, M.Y.; Luo, X.M.; Yang, H.J.; Wei, C.Q.; Zhu, S.W.; Sun, Y.; Chong, K.; et al. BZS1, a B-box protein, promotes photomorphogenesis downstream of both brassinosteroid and light signaling pathways. Mol. Plant 2012, 5, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jiang, Y.; Li, J.; Lin, F.; Holm, M.; Deng, X.W. BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc. Natl. Acad. Sci. USA 2016, 113, 7655–7660. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, M.; Long, H.; Dong, W.; Pasha, A.; Esteban, E.; Li, W.; Yang, X.; Li, Z.; Song, A.; et al. Tung Tree (Vernicia fordii) Genome Provides A Resource for Understanding Genome Evolution and Improved Oil Production. Genom. Proteom. Bioinform. 2019, 17, 558–575. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Long, H.; Zhang, L.; Liu, Z.; Cao, H.; Shi, M.; Tan, X. The complete chloroplast genome sequence of tung tree (Vernicia fordii): Organization and phylogenetic relationships with other angiosperms. Sci. Rep. 2017, 7, 1869. [Google Scholar] [CrossRef]
- Gangappa, S.N.; Botto, J.F. The BBX family of plant transcription factors. Trends Plant Sci. 2014, 19, 460–470. [Google Scholar] [CrossRef]
- Khanna, R.; Kronmiller, B.; Maszle, D.R.; Coupland, G.; Holm, M.; Mizuno, T.; Wu, S.H. The Arabidopsis B-box zinc finger family. Plant Cell 2009, 21, 3416–3420. [Google Scholar] [CrossRef]
- Chu, Z.; Wang, X.; Li, Y.; Yu, H.; Li, J.; Lu, Y.; Li, H.; Ouyang, B. Genomic Organization, Phylogenetic and Expression Analysis of the B-BOX Gene Family in Tomato. Front. Plant Sci. 2016, 7, 1552. [Google Scholar] [CrossRef]
- Feng, Z.; Li, M.; Li, Y.; Yang, X.; Wei, H.; Fu, X.; Ma, L.; Lu, J.; Wang, H.; Yu, S. Comprehensive identification and expression analysis of B-Box genes in cotton. BMC Genom. 2021, 22, 439. [Google Scholar] [CrossRef]
- Haberer, G.; Hindemitt, T.; Meyers, B.C.; Mayer, K.F. Transcriptional similarities, dissimilarities, and conservation of cis-elements in duplicated genes of Arabidopsis. Plant Physiol. 2004, 136, 3009–3022. [Google Scholar] [CrossRef]
- Datta, S.; Hettiarachchi, C.; Johansson, H.; Holm, M. SALT TOLERANCE HOMOLOG2, a B-box protein in Arabidopsis that activates transcription and positively regulates light-mediated development. Plant Cell 2007, 19, 3242–3255. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Tang, B.; Dai, X.; Xie, L.; Liu, F.; Zou, X. Genome-Wide Identification and Capsaicinoid Biosynthesis-Related Expression Analysis of the R2R3-MYB Gene Family in Capsicum annuum L. Front. Genet. 2020, 11, 598183. [Google Scholar] [CrossRef]
- Xiong, C.; Luo, D.; Lin, A.; Zhang, C.; Shan, L.; He, P.; Li, B.; Zhang, Q.; Hua, B.; Yuan, Z.; et al. A tomato B-box protein SlBBX20 modulates carotenoid biosynthesis by directly activating PHYTOENE SYNTHASE 1, and is targeted for 26S proteasome-mediated degradation. New Phytol. 2019, 221, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Q.; Li, W.; Hu, T.; Wang, Q.; Yin, Y.; Liu, X.; He, S.; Zhang, M.; Liang, Y.; et al. Overexpression of SlBBX17 affects plant growth and enhances heat tolerance in tomato. Int. J. Biol. Macromol. 2022, 206, 799–811. [Google Scholar] [CrossRef]
- Chang, C.S.; Maloof, J.N.; Wu, S.H. COP1-mediated degradation of BBX22/LZF1 optimizes seedling development in Arabidopsis. Plant Physiol. 2011, 156, 228–239. [Google Scholar] [CrossRef]
- Lin, F.; Jiang, Y.; Li, J.; Yan, T.; Fan, L.; Liang, J.; Chen, Z.J.; Xu, D.; Deng, X.W. B-BOX DOMAIN PROTEIN28 Negatively Regulates Photomorphogenesis by Repressing the Activity of Transcription Factor HY5 and Undergoes COP1-Mediated Degradation. Plant Cell 2018, 30, 2006–2019. [Google Scholar] [CrossRef]
- Gangappa, S.N.; Holm, M.; Botto, J.F. Molecular interactions of BBX24 and BBX25 with HYH, HY5 HOMOLOG, to modulate Arabidopsis seedling development. Plant Signal. Behav. 2013, 8, e25208. [Google Scholar] [CrossRef]
- Buelbuel, S.; Sakuraba, Y.; Sedaghatmehr, M.; Watanabe, M.; Hoefgen, R.; Balazadeh, S.; Mueller-Roeber, B. Arabidopsis BBX14 negatively regulates nitrogen starvation- and dark-induced leaf senescence. Plant J. 2023, 116, 251–268. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tian, F.; Yang, D.C.; Meng, Y.Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef] [PubMed]

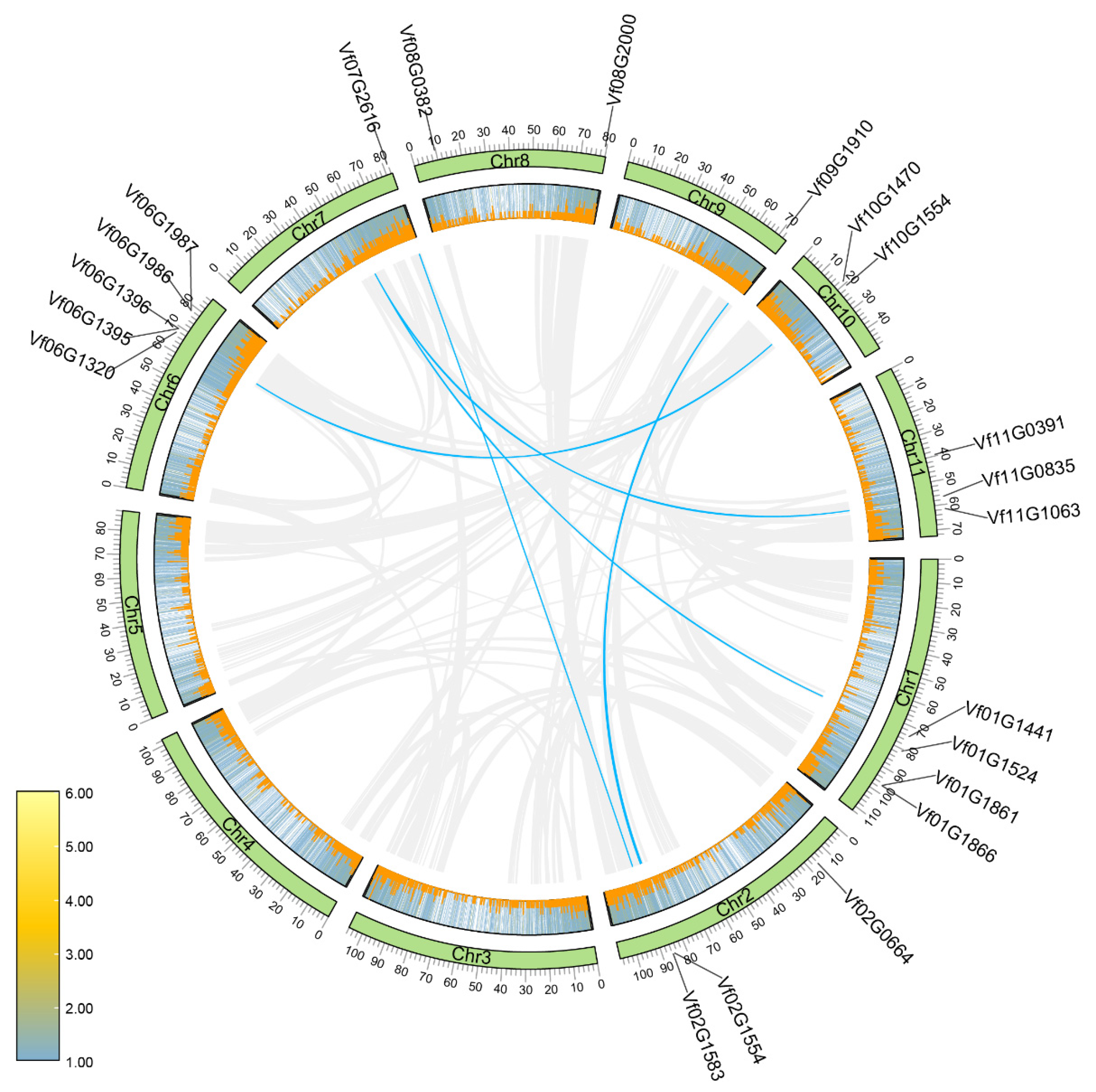



| ID | GeneID | Chr | Start | End | Strain | PI | Mw (Da) | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|
| VfBBX1 | Vf00G1104 | 0 | 89,662 | 91,738 | − | 6.01 | 45,087.65 | Nuclear |
| VfBBX2 | Vf01G1441 | 1 | 74,174,775 | 74,175,523 | − | 4.82 | 24,310.77 | Extracellular |
| VfBBX3 | Vf01G1524 | 1 | 80,724,519 | 80,726,304 | + | 5.14 | 48,109.19 | Nuclear |
| VfBBX4 | Vf01G1861 | 1 | 96,727,792 | 96,732,389 | − | 5.26 | 45,193.41 | Nuclear |
| VfBBX5 | Vf01G1866 | 1 | 96,872,982 | 96,873,350 | − | 7.52 | 13,502.70 | Nuclear |
| VfBBX6 | Vf02G0664 | 2 | 16,076,935 | 16,079,364 | + | 4.91 | 26,074.67 | Nuclear |
| VfBBX7 | Vf02G1554 | 2 | 84,978,268 | 84,980,266 | + | 6.30 | 23,345.27 | Nuclear |
| VfBBX8 | Vf02G1583 | 2 | 85,667,653 | 85,669,272 | − | 5.12 | 38,733.10 | Nuclear |
| VfBBX9 | Vf06G1320 | 6 | 67,314,277 | 67,315,841 | − | 5.64 | 51,051.01 | Nuclear |
| VfBBX10 | Vf06G1395 | 6 | 69,031,670 | 69,033,068 | + | 4.77 | 32,524.05 | Nuclear |
| VfBBX11 | Vf06G1396 | 6 | 69,040,070 | 69,041,484 | + | 5.61 | 39,894.44 | Nuclear |
| VfBBX12 | Vf06G1986 | 6 | 77,933,550 | 77,935,926 | − | 5.80 | 43,554.53 | Nuclear |
| VfBBX13 | Vf06G1987 | 6 | 77,938,312 | 77,940,688 | − | 5.80 | 43,538.49 | Nuclear |
| VfBBX14 | Vf07G2616 | 7 | 81,738,810 | 81,741,471 | − | 6.52 | 54,454.33 | Nuclear |
| VfBBX15 | Vf08G0382 | 8 | 9,584,101 | 9,585,313 | − | 6.35 | 41,569.61 | Nuclear |
| VfBBX16 | Vf08G2000 | 8 | 79,397,606 | 79,398,694 | − | 5.94 | 37,033.50 | Nuclear |
| VfBBX17 | Vf09G1910 | 9 | 70,869,957 | 70,871,084 | + | 6.35 | 34,220.51 | Nuclear |
| VfBBX18 | Vf10G1470 | 10 | 18,835,584 | 18,837,119 | + | 5.10 | 51,746.70 | Nuclear |
| VfBBX19 | Vf10G1554 | 10 | 20,915,531 | 20,922,830 | + | 5.91 | 57,450.27 | Extracellular |
| VfBBX20 | Vf11G0391 | 11 | 39,393,706 | 39,397,065 | + | 5.79 | 31,878.61 | Nuclear |
| VfBBX21 | Vf11G0835 | 11 | 56,711,530 | 56,712,533 | − | 4.40 | 29,473.33 | Extracellular |
| VfBBX22 | Vf11G1063 | 11 | 61,707,357 | 61,713,797 | − | 5.31 | 20,704.38 | Nuclear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Zhao, G.; Li, Z.; Zhou, J.; Wu, L.; Tan, X.; Yuan, J. Genome-Wide Identification of B-Box Gene Family and Candidate Light-Related Member Analysis of Tung Tree (Vernicia fordii). Int. J. Mol. Sci. 2024, 25, 1977. https://doi.org/10.3390/ijms25041977
Shi K, Zhao G, Li Z, Zhou J, Wu L, Tan X, Yuan J. Genome-Wide Identification of B-Box Gene Family and Candidate Light-Related Member Analysis of Tung Tree (Vernicia fordii). International Journal of Molecular Sciences. 2024; 25(4):1977. https://doi.org/10.3390/ijms25041977
Chicago/Turabian StyleShi, Kai, Guang Zhao, Ze Li, Junqin Zhou, Lingli Wu, Xiaofeng Tan, and Jun Yuan. 2024. "Genome-Wide Identification of B-Box Gene Family and Candidate Light-Related Member Analysis of Tung Tree (Vernicia fordii)" International Journal of Molecular Sciences 25, no. 4: 1977. https://doi.org/10.3390/ijms25041977





