Cellular Alterations Due to Direct and Indirect Interaction of Nanomaterials with Nucleic Acids
Abstract
1. Introduction
2. Computational Study of the Direct Interaction of NPs and DNA
3. Effects of NPs on DNA: In Vitro and In Vivo Studies
3.1. Silver Nanoparticles (Ag NPs)
3.2. Cerium Oxide Nanoparticles (CeO2 NPs/Nanoceria)
3.2.1. CeO2 NPs Cause DNA Damage
3.2.2. CeO2 NPs Protect from DNA Damage
3.3. Gold Nanoparticles (Au NPs)
3.3.1. Au NPs Induce DNA Damage
3.3.2. Nuclear Internalization of Au NPs
3.4. SiO2 NPs (Silica NPs)
3.5. Other Nanomaterials
3.5.1. Nanosized-Metal Organic Frameworks (NMOFs)
3.5.2. Magnetic Nanometer-Size Particles
3.5.3. Lipid-Based Nanoparticles (LPB NPs)
3.5.4. Nanoparticle Pollution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feynman, R.P. There’s Plenty of Room at the Bottom—An invitation to Enter a New Field of Physics. Eng. Sci. 1960, 23, 5. [Google Scholar]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Shafiq, M.; Anjum, S.; Hano, C.; Anjum, I.; Abbasi, B.H. An Overview of the Applications of Nanomaterials and Nanodevices in the Food Industry. Foods 2020, 9, 148. [Google Scholar] [CrossRef]
- Mazari, S.A.; Ali, E.; Abro, R.; Khan, F.S.A.; Ahmed, I.; Ahmed, M.; Nizamuddin, S.; Siddiqui, T.H.; Hossain, N.; Mubarak, N.M.; et al. Nanomaterials: Applications, waste-handling, environmental toxicities, and future challenges—A review. J. Environ. Chem. Eng. 2021, 9, 105028. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [PubMed]
- Martin-Pardillos, A.; Martin-Duque, P. Cellular Alterations in Carbohydrate and Lipid Metabolism Due to Interactions with Nanomaterials. J. Funct. Biomater. 2023, 14, 274. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.O.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Delgado, D.; del Pozo-Rodriguez, A.; Solinis, M.A.; Rodriguez-Gascon, A. Understanding the mechanism of protamine in solid lipid nanoparticle-based lipofection: The importance of the entry pathway. Eur. J. Pharm. Biopharm. 2011, 79, 495–502. [Google Scholar] [CrossRef]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Li, K.; Zhao, X.; Hammer, B.K.; Du, S.; Chen, Y. Nanoparticles Inhibit DNA Replication by Binding to DNA: Modeling and Experimental Validation. ACS Nano 2013, 7, 9664–9674. [Google Scholar] [CrossRef]
- Khan, S.T.; Malik, A.; Wahab, R.; Abd-Elkader, O.H.; Ahamed, M.; Ahmad, J.; Musarrat, J.; Siddiqui, M.A.; Al-Khedhairy, A.A. Synthesis and characterization of some abundant nanoparticles, their antimicrobial and enzyme inhibition activity. Acta Microbiol. Immunol. Hung. 2017, 64, 203–216. [Google Scholar] [CrossRef]
- Gao, C.H.; Mortimer, M.; Zhang, M.; Holden, P.A.; Cai, P.; Wu, S.; Xin, Y.; Wu, Y.; Huang, Q. Impact of metal oxide nanoparticles on in vitro DNA amplification. PeerJ 2019, 7, e7228. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yan, J.; Li, Y. Genotoxicity of titanium dioxide nanoparticles. J. Food Drug Anal. 2014, 22, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Carreiro, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Yang, W.; Wang, L.; Mettenbrink, E.M.; DeAngelis, P.L.; Wilhelm, S. Nanoparticle Toxicology. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 269–289. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Recordati, C.; De Maglie, M.; Bianchessi, S.; Argentiere, S.; Cella, C.; Mattiello, S.; Cubadda, F.; Aureli, F.; D’Amato, M.; Raggi, A.; et al. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: Nano-specific and size-dependent effects. Part. Fibre Toxicol. 2015, 13, 12. [Google Scholar] [CrossRef]
- Ibrahim, K.E.; Al-Mutary, M.G.; Bakhiet, A.O.; Khan, H.A. Histopathology of the Liver, Kidney, and Spleen of Mice Exposed to Gold Nanoparticles. Molecules 2018, 23, 1848. [Google Scholar] [CrossRef]
- Tripathy, N.; Hong, T.K.; Ha, K.T.; Jeong, H.S.; Hahn, Y.B. Effect of ZnO nanoparticles aggregation on the toxicity in RAW 264.7 murine macrophage. J. Hazard. Mater. 2014, 270, 110–117. [Google Scholar] [CrossRef]
- Albanese, A.; Chan, W.C.W. Effect of Gold Nanoparticle Aggregation on Cell Uptake and Toxicity. ACS Nano 2011, 5, 5478–5489. [Google Scholar] [CrossRef]
- Damasco, J.A.; Ravi, S.; Perez, J.D.; Hagaman, D.E.; Melancon, M.P. Understanding Nanoparticle Toxicity to Direct a Safe-by-Design Approach in Cancer Nanomedicine. Nanomaterials 2020, 10, 2186. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, S.J.; Yun, S.J.; Jang, J.Y.; Kang, H.; Kim, K.; Choi, I.H.; Park, S. Silver nanoparticles affect glucose metabolism in hepatoma cells through production of reactive oxygen species. Int. J. Nanomed. 2016, 11, 55–68. [Google Scholar] [CrossRef]
- Chang, X.; Wang, X.; Li, J.; Shang, M.; Niu, S.; Zhang, W.; Li, Y.; Sun, Z.; Gan, J.; Li, W.; et al. Silver nanoparticles induced cytotoxicity in HT22 cells through autophagy and apoptosis via PI3K/AKT/mTOR signaling pathway. Ecotoxicol. Environ. Saf. 2020, 208, 111696. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Kim, M.J.; Lee, S.Y.; Oh, S.M.; Chung, K.H. Genotoxic effects of silver nanoparticles stimulated by oxidative stress in human normal bronchial epithelial (BEAS-2B) cells. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 726, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Asare, N.; Instanes, C.; Sandberg, W.J.; Refsnes, M.; Schwarze, P.; Kruszewski, M.; Brunborg, G. Cytotoxic and genotoxic effects of silver nanoparticles in testicular cells. Toxicology 2012, 291, 65–72. [Google Scholar] [CrossRef]
- Butler, K.S.; Peeler, D.J.; Casey, B.J.; Dair, B.J.; Elespuru, R.K. Silver nanoparticles: Correlating nanoparticle size and cellular uptake with genotoxicity. Mutagenesis 2015, 30, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, T.; Ingle, T.; Yan, J.; He, W.; Yin, J.J.; Chen, T. Differential genotoxicity mechanisms of silver nanoparticles and silver ions. Arch. Toxicol. 2016, 91, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Nymark, P.; Catalan, J.; Suhonen, S.; Jarventaus, H.; Birkedal, R.; Clausen, P.A.; Jensen, K.A.; Vippola, M.; Savolainen, K.; Norppa, H. Genotoxicity of polyvinylpyrrolidone-coated silver nanoparticles in BEAS 2B cells. Toxicology 2013, 313, 38–48. [Google Scholar] [CrossRef]
- Li, Y.; Bhalli, J.A.; Ding, W.; Yan, J.; Pearce, M.G.; Sadiq, R.; Cunningham, C.K.; Jones, M.Y.; Monroe, W.A.; Howard, P.C.; et al. Cytotoxicity and genotoxicity assessment of silver nanoparticles in mouse. Nanotoxicology 2013, 8, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Dan, M.; Yang, Y.; Lyu, J.; Shao, A.; Cheng, X.; Chen, L.; Xu, L. Acute toxicity and genotoxicity of silver nanoparticle in rats. PLoS ONE 2017, 12, e0185554. [Google Scholar] [CrossRef]
- Loeschner, K.; Hadrup, N.; Qvortrup, K.; Larsen, A.; Gao, X.; Vogel, U.; Mortensen, A.; Lam, H.R.; Larsen, E.H. Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part. Fibre Toxicol. 2011, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lamana, J.; Laborda, F.; Bolea, E.; Abad-Alvaro, I.; Castillo, J.R.; Bianga, J.; He, M.; Bierla, K.; Mounicou, S.; Ouerdane, L.; et al. An insight into silver nanoparticles bioavailability in rats. Metallomics 2014, 6, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garraus, A.; Azqueta, A.; Vettorazzi, A.; Lopez de Cerain, A. Genotoxicity of Silver Nanoparticles. Nanomaterials 2020, 10, 251. [Google Scholar] [CrossRef]
- Liman, R.; Acikbas, Y.; Cigerci, I.H. Cytotoxicity and genotoxicity of cerium oxide micro and nanoparticles by Allium and Comet tests. Ecotoxicol. Environ. Saf. 2019, 168, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al-Salam, S.; Al Ansari, Z.; Alkharas, Z.A.; Al Ahbabi, R.M.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Ali, B.H. Impact of Pulmonary Exposure to Cerium Oxide Nanoparticles on Experimental Acute Kidney Injury. Cell. Physiol. Biochem. 2019, 52, 439–454. [Google Scholar] [CrossRef]
- Kuchma, M.H.; Komanski, C.B.; Colon, J.; Teblum, A.; Masunov, A.E.; Alvarado, B.; Babu, S.; Seal, S.; Summy, J.; Baker, C.H. Phosphate ester hydrolysis of biologically relevant molecules by cerium oxide nanoparticles. Nanomedicine 2010, 6, 738–744. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Comprehensive Screen of Metal Oxide Nanoparticles for DNA Adsorption, Fluorescence Quenching, and Anion Discrimination. ACS Appl. Mater. Interfaces 2015, 7, 24833–24838. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Xu, Z.; Liu, J. NiO Nanoparticles for Exceptionally Stable DNA Adsorption and Its Extraction from Biological Fluids. Langmuir 2018, 34, 9314–9321. [Google Scholar] [CrossRef] [PubMed]
- Link, N.; Brunner, T.J.; Dreesen, I.A.; Stark, W.J.; Fussenegger, M. Inorganic nanoparticles for transfection of mammalian cells and removal of viruses from aqueous solutions. Biotechnol. Bioeng. 2007, 98, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, D.; Anselmi-Tamburini, U.; Tredici, I.G.; Ricci, V.; Sommi, P. Overestimation of nanoparticles-induced DNA damage determined by the comet assay. Nanotoxicology 2016, 10, 861–870. [Google Scholar] [CrossRef]
- Kain, J.; Karlsson, H.L.; Moller, L. DNA damage induced by micro- and nanoparticles—Interaction with FPG influences the detection of DNA oxidation in the comet assay. Mutagenesis 2012, 27, 491–500. [Google Scholar] [CrossRef]
- Mittal, S.; Pandey, A.K. Cerium oxide nanoparticles induced toxicity in human lung cells: Role of ROS mediated DNA damage and apoptosis. BioMed Res. Int. 2014, 2014, 891934. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Alarifi, S.; Alkahtani, S.; AlKahtane, A.A.; Almalik, A. Cerium Oxide Nanoparticles Induce Oxidative Stress and Genotoxicity in Human Skin Melanoma Cells. Biochem. Biophys. 2014, 71, 1643–1651. [Google Scholar] [CrossRef]
- Preaubert, L.; Tassistro, V.; Auffan, M.; Sari-Minodier, I.; Rose, J.; Courbiere, B.; Perrin, J. Very low concentration of cerium dioxide nanoparticles induce DNA damage, but no loss of vitality, in human spermatozoa. Toxicol. In Vitro 2018, 50, 236–241. [Google Scholar] [CrossRef]
- Benameur, L.; Auffan, M.; Cassien, M.; Liu, W.; Culcasi, M.; Rahmouni, H.; Stocker, P.; Tassistro, V.; Bottero, J.Y.; Rose, J.; et al. DNA damage and oxidative stress induced by CeO2 nanoparticles in human dermal fibroblasts: Evidence of a clastogenic effect as a mechanism of genotoxicity. Nanotoxicology 2014, 9, 696–705. [Google Scholar] [CrossRef]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Fahim, M.A.; Ali, B.H. Cerium Oxide Nanoparticles in Lung Acutely Induce Oxidative Stress, Inflammation, and DNA Damage in Various Organs of Mice. Oxidative Med. Cell. Longev. 2017, 2017, 9639035. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Nuaman, S.A.; Kazim, M.; Mohamed, F.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Ali, B.H. Exacerbation of Coagulation and Cardiac Injury in Rats with Cisplatin-Induced Nephrotoxicity Following Intratracheal Instillation of Cerium Oxide Nanoparticles. Cell. Physiol. Biochem. 2021, 55, 1–16. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Beegam, S.; Yuvaraju, P.; Ali, B.H. Aortic Oxidative Stress, Inflammation and DNA Damage Following Pulmonary Exposure to Cerium Oxide Nanoparticles in a Rat Model of Vascular Injury. Biomolecules 2019, 9, 376. [Google Scholar] [CrossRef]
- Kumari, M.; Kumari, S.I.; Grover, P. Genotoxicity analysis of cerium oxide micro and nanoparticles in Wistar rats after 28 days of repeated oral administration. Mutagenesis 2014, 29, 467–479. [Google Scholar] [CrossRef]
- Rubio, L.; Annangi, B.; Vila, L.; Hernandez, A.; Marcos, R. Antioxidant and anti-genotoxic properties of cerium oxide nanoparticles in a pulmonary-like cell system. Arch. Toxicol. 2015, 90, 269–278. [Google Scholar] [CrossRef]
- Hashem, R.M.; Rashd, L.A.; Hashem, K.S.; Soliman, H.M. Cerium oxide nanoparticles alleviate oxidative stress and decreases Nrf-2/HO-1 in D-GALN/LPS induced hepatotoxicity. Biomed. Pharmacother. 2015, 73, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Vila, L.; Garcia-Rodriguez, A.; Cortes, C.; Velazquez, A.; Xamena, N.; Sampayo-Reyes, A.; Marcos, R.; Hernandez, A. Effects of cerium oxide nanoparticles on differentiated/undifferentiated human intestinal Caco-2 cells. Chem. Biol. Interact. 2018, 283, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ai, W.; Zhai, Y.; Li, H.; Zhou, K.; Chen, H. Effects of Nano-CeO2 with Different Nanocrystal Morphologies on Cytotoxicity in HepG2 Cells. Int. J. Environ. Res. Public Health 2015, 12, 10806–10819. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.R.H. Acute Oral Administration of Cerium Oxide Nanoparticles Suppresses Lead Acetate-Induced Genotoxicity, Inflammation, and ROS Generation in Mice Renal and Cardiac Tissues. Biol. Trace Element Res. 2021, 200, 3284–3293. [Google Scholar] [CrossRef] [PubMed]
- Goujon, G.; Baldim, V.; Roques, C.; Bia, N.; Seguin, J.; Palmier, B.; Graillot, A.; Loubat, C.; Mignet, N.; Margaill, I.; et al. Antioxidant Activity and Toxicity Study of Cerium Oxide Nanoparticles Stabilized with Innovative Functional Copolymers. Adv. Healthc. Mater. 2021, 10, 2100059. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; De Nicola, M.; Sienkiewicz, A.; Giovanetti, A.; Bejarano, I.; Licoccia, S.; Traversa, E.; Ghibelli, L. Cerium oxide nanoparticles, combining antioxidant and UV shielding properties, prevent UV-induced cell damage and mutagenesis. Nanoscale 2015, 7, 15643–15656. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Neal, C.J.; Sakthivel, T.S.; Seal, S.; Kean, T.; Razavi, M.; Coathup, M. Cerium oxide nanoparticles protect against irradiation-induced cellular damage while augmenting osteogenesis. Mater. Sci. Eng. C 2021, 126, 112145. [Google Scholar] [CrossRef]
- Wang, C.; Blough, E.; Dai, X.; Olajide, O.; Driscoll, H.; Leidy, J.W.; July, M.; Triest, W.E.; Wu, M. Protective Effects of Cerium Oxide Nanoparticles on MC3T3-E1 Osteoblastic Cells Exposed to X-Ray Irradiation. Cell. Physiol. Biochem. 2016, 38, 1510–1519. [Google Scholar] [CrossRef]
- Das, S.; Neal, C.J.; Ortiz, J.; Seal, S. Engineered nanoceria cytoprotection in vivo: Mitigation of reactive oxygen species and double-stranded DNA breakage due to radiation exposure. Nanoscale 2018, 10, 21069–21075. [Google Scholar] [CrossRef]
- Magogotya, M.; Vetten, M.; Roux-van der Merwe, M.P.; Badenhorst, J.; Gulumian, M. In vitro toxicity and internalization of gold nanoparticles (AuNPs) in human epithelial colorectal adenocarcinoma (Caco-2) cells and the human skin keratinocyte (HaCaT) cells. Mutat. Res. Toxicol. Environ. Mutagen 2022, 883–884, 503556. [Google Scholar] [CrossRef]
- Zhang, X.; Servos, M.R.; Liu, J. Surface science of DNA adsorption onto citrate-capped gold nanoparticles. Langmuir 2012, 28, 3896–3902. [Google Scholar] [CrossRef]
- Liu, Z.; Hettihewa, M.; Shu, Y.; Zhou, C.; Wan, Q.; Liu, L. The mechanism of the adsorption of dsDNA on citrate-stabilized gold nanoparticles and a colorimetric and visual method for detecting the V600E point mutation of the BRAF gene. Microchim. Acta 2018, 185, 240. [Google Scholar] [CrossRef]
- Nelson, E.M.; Rothberg, L.J. Kinetics and mechanism of single-stranded DNA adsorption onto citrate-stabilized gold nanoparticles in colloidal solution. Langmuir 2011, 27, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Paino, I.M.; Marangoni, V.S.; de Oliveira Rde, C.; Antunes, L.M.; Zucolotto, V. Cyto and genotoxicity of gold nanoparticles in human hepatocellular carcinoma and peripheral blood mononuclear cells. Toxicol. Lett. 2012, 215, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.; Faria, H.; Soares, M.E.; Duarte, J.A.; Soares, L.; Pereira, E.; Costa-Pereira, C.; Teixeira, J.P.; de Lourdes Bastos, M.; Carmo, H. Influence of the surface coating on the cytotoxicity, genotoxicity and uptake of gold nanoparticles in human HepG2 cells. J. Appl. Toxicol. 2013, 33, 1111–1119. [Google Scholar] [CrossRef]
- Mulder, D.; Taute, C.J.F.; van Wyk, M.; Pretorius, P.J. A Comparison of the Genotoxic Effects of Gold Nanoparticles Functionalized with Seven Different Ligands in Cultured Human Hepatocellular Carcinoma Cells. Nanomaterials 2022, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Lindeque, J.Z.; Matthyser, A.; Mason, S.; Louw, R.; Taute, C.J.F. Metabolomics reveals the depletion of intracellular metabolites in HepG2 cells after treatment with gold nanoparticles. Nanotoxicology 2018, 12, 251–262. [Google Scholar] [CrossRef] [PubMed]
- George, J.M.; Magogotya, M.; Vetten, M.A.; Buys, A.V.; Gulumian, M. From the Cover: An Investigation of the Genotoxicity and Interference of Gold Nanoparticles in Commonly Used In Vitro Mutagenicity and Genotoxicity Assays. Toxicol. Sci. 2017, 156, 149–166. [Google Scholar] [CrossRef]
- Geissler, D.; Wegmann, M.; Jochum, T.; Somma, V.; Sowa, M.; Scholz, J.; Frohlich, E.; Hoffmann, K.; Niehaus, J.; Roggenbuck, D.; et al. An automatable platform for genotoxicity testing of nanomaterials based on the fluorometric gamma-H2AX assay reveals no genotoxicity of properly surface-shielded cadmium-based quantum dots. Nanoscale 2019, 11, 13458–13468. [Google Scholar] [CrossRef]
- Cardoso, E.; Londero, E.; Ferreira, G.K.; Rezin, G.T.; Zanoni, E.T.; de Souza Notoya, F.; Leffa, D.D.; Damiani, A.P.; Daumann, F.; Rohr, P.; et al. Gold nanoparticles induce DNA damage in the blood and liver of rats. J. Nanopart. Res. 2014, 16, 2727. [Google Scholar] [CrossRef]
- Cardoso, E.; Rezin, G.T.; Zanoni, E.T.; de Souza Notoya, F.; Leffa, D.D.; Damiani, A.P.; Daumann, F.; Rodriguez, J.C.; Benavides, R.; da Silva, L.; et al. Acute and chronic administration of gold nanoparticles cause DNA damage in the cerebral cortex of adult rats. Mutat. Res. Mol. Mech. Mutagen. 2014, 766–767, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, X.; ZHang, H.; Lamn, J.; Huang, G.; Varin, E.; Lincet, H.; Poulain, L.; Icard, P. Citrate Induces Apoptotic Cell Death: A Promising Way to Treat Gastric Carcinoma? Anticancer Res. 2011, 31, 797–806. [Google Scholar] [PubMed]
- Uboldi, C.; Bonacchi, D.; Lorenzi, G.; Hermanns, M.I.; Pohl, C.; Baldi, G.; Unger, R.E.; Kirkpatrick, C.J. Gold nanoparticles induce cytotoxicity in the alveolar type-II cell lines A549 and NCIH441. Part. Fibre Toxicol. 2009, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Uertz, J.; Yohan, D.; Chithrani, B.D. Peptide modified gold nanoparticles for improved cellular uptake, nuclear transport, and intracellular retention. Nanoscale 2014, 6, 12026–12033. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.G.; Xie, H.; Coleman, D.; Glomm, W.; Ryan, J.; Anderson, M.F.; Franzen, S.; Feldheim, D.L. Multifunctional Gold Nanoparticle-Peptide Complexes for Nuclear Targeting. J. Am. Chem. Soc. 2003, 125, 4700–4701. [Google Scholar] [CrossRef] [PubMed]
- Boyoglu, C.; He, Q.; Willing, G.; Boyoglu-Barnum, S.; Dennis, V.A.; Pillai, S.; Singh, S.R. Microscopic Studies of Various Sizes of Gold Nanoparticles and Their Cellular Localizations. ISRN Nanotechnol. 2013, 2013, 123838. [Google Scholar] [CrossRef]
- Tsoli, M.; Kuhn, H.; Brandau, W.; Esche, H.; Schmid, G. Cellular uptake and toxicity of Au55 clusters. Small 2005, 1, 841–844. [Google Scholar] [CrossRef]
- Huo, S.; Shubin, J.; Ma, X.; Xue, X.; Yang, K.; Kumar, A.; Wang, P.C.; Zhang, J.; Hu, Z.; Liang, X.J. Ultrasmall Gold Nanoparticles as Carriers for Nucleus-Based Gene Therapy Due to Size-Dependent Nuclear Entry. ACS Nano 2014, 8, 5852–5862. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Mackey, M.A.; El-Sayed, M.A. Nuclear Targeting of Gold Nanoparticles in Cancer Cells Induces DNA Damage, Causing Cytokinesis Arrest and Apoptosis. J. Am. Chem. Soc. 2010, 132, 1517–1519. [Google Scholar] [CrossRef] [PubMed]
- Mackey, M.A.; Saira, F.; Mahmoud, M.A.; El-Sayed, M.A. Inducing cancer cell death by targeting its nucleus: Solid gold nanospheres versus hollow gold nanocages. Bioconjug. Chem. 2013, 24, 897–906. [Google Scholar] [CrossRef]
- Oyelere, A.K.; Chen, P.C.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Peptide-Conjugated Gold Nanorods for Nuclear Targeting. Bioconjug. Chem. 2007, 18, 1490–1497. [Google Scholar] [CrossRef]
- Huang, X.; Kang, B.; Qian, W.; Mackey, M.A.; Chen, P.C.; Oyelere, A.K.; El-Sayed, I.H.; El-Sayed, M.A. Comparative study of photothermolysis of cancer cells with nuclear-targeted or cytoplasm-targeted gold nanospheres: Continuous wave or pulsed lasers. J. Biomed. Opt. 2010, 15, 058002. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, A.; Bennie, L.; Coulter, J.A.; McCarthy, H.O.; Dromey, B.; Grimes, D.R.; Quinn, P.; Villagomez-Bernabe, B.; Currell, F. Nuclear Uptake of Gold Nanoparticles Deduced Using Dual-Angle X-Ray Fluorescence Mapping. Part. Part. Syst. Charact. 2019, 36, 1900140. [Google Scholar] [CrossRef]
- Bennie, L.A.; Feng, J.; Emmerson, C.; Hyland, W.B.; Matchett, K.B.; McCarthy, H.O.; Coulter, J.A. Formulating RALA/Au nanocomplexes to enhance nanoparticle internalisation efficiency, sensitising prostate tumour models to radiation treatment. J. Nanobiotechnol. 2021, 19, 279. [Google Scholar] [CrossRef]
- Gu, Y.J.; Cheng, J.; Lin, C.C.; Lam, Y.W.; Cheng, S.H.; Wong, W.T. Nuclear penetration of surface functionalized gold nanoparticles. Toxicol. Appl. Pharmacol. 2009, 237, 196–204. [Google Scholar] [CrossRef]
- Li, P.; Li, D.; Zhang, L.; Li, G.; Wang, E. Cationic lipid bilayer coated gold nanoparticles-mediated transfection of mammalian cells. Biomaterials 2008, 29, 3617–3624. [Google Scholar] [CrossRef]
- Castro-Smirnov, F.A.; Pietrement, O.; Aranda, P.; Bertrand, J.R.; Ayache, J.; Le Cam, E.; Ruiz-Hitzky, E.; Lopez, B.S. Physical interactions between DNA and sepiolite nanofibers, and potential application for DNA transfer into mammalian cells. Sci. Rep. 2016, 6, 36341. [Google Scholar] [CrossRef]
- Shi, B.; Shin, Y.K.; Hassanali, A.A.; Singer, S.J. DNA Binding to the Silica Surface. J. Phys. Chem. B 2015, 119, 11030–11040. [Google Scholar] [CrossRef]
- Reinhardt, N.; Adumeau, L.; Lambert, O.; Ravaine, S.; Mornet, S. Quaternary ammonium groups exposed at the surface of silica nanoparticles suitable for DNA complexation in the presence of cationic lipids. J. Phys. Chem. B 2015, 119, 6401–6411. [Google Scholar] [CrossRef]
- Nabeshi, H.; Yoshikawa, T.; Matsuyama, K.; Nakazato, Y.; Matsuo, K.; Arimori, A.; Isobe, M.; Tochigi, S.; Kondoh, S.; Hirai, T.; et al. Systemic distribution, nuclear entry and cytotoxicity of amorphous nanosilica following topical application. Biomaterials 2011, 32, 2713–2724. [Google Scholar] [CrossRef]
- Nabeshi, H.; Yoshikawa, T.; Arimori, A.; Yoshida, T.; Tochigi, S.; Hirai, T.; Akase, T.; Nagano, K.; Abe, Y.; Kamada, H.; et al. Effect of surface properties of silica nanoparticles on their cytotoxicity and cellular distribution in murine macrophages. Nanoscale Res. Lett. 2011, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Nabeshi, H.; Yoshikawa, T.; Matsuyama, K.; Nakazato, Y.; Arimori, A.; Isobe, M.; Tochigi, S.; Kondoh, S.; Hirai, T.; Akase, T.; et al. Size-dependent cytotoxic effects of amorphous silica nanoparticles on Langerhans cells. Pharmazie 2010, 65, 199–201. [Google Scholar] [CrossRef]
- Nabeshi, H.; Yoshikawa, T.; Matsuyama, K.; Nakazato, Y.; Tochigi, S.; Kondoh, S.; Hirai, T.; Akase, T.; Nagano, K.; Abe, Y.; et al. Amorphous nanosilica induce endocytosis-dependent ROS generation and DNA damage in human keratinocytes. Part. Fibre Toxicol. 2011, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Vonmikecz, A. Formation of nucleoplasmic protein aggregates impairs nuclear function in response to SiO nanoparticles. Exp. Cell Res. 2005, 305, 51–62. [Google Scholar] [CrossRef]
- Phonesouk, E.; Lechevallier, S.; Ferrand, A.; Rols, M.P.; Bezombes, C.; Verelst, M.; Golzio, M. Increasing Uptake of Silica Nanoparticles with Electroporation: From Cellular Characterization to Potential Applications. Materials 2019, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Bellard, E.; Tessié, J. Double Pulse Approach of Electropulsation: A Fluorescence Analysis of the Nucleus Perturbation at the Single Cell Level. Transactions on Dielectrics and Electrical Insulation. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1267–1272. [Google Scholar] [CrossRef]
- Duan, J.; Yu, Y.; Li, Y.; Yu, Y.; Li, Y.; Zhou, X.; Huang, P.; Sun, Z. Toxic effect of silica nanoparticles on endothelial cells through DNA damage response via Chk1-dependent G2/M checkpoint. PLoS ONE 2013, 8, e62087. [Google Scholar] [CrossRef]
- Sun, L.; Li, Y.; Liu, X.; Jin, M.; Zhang, L.; Du, Z.; Guo, C.; Huang, P.; Sun, Z. Cytotoxicity and mitochondrial damage caused by silica nanoparticles. Toxicol. In Vitro 2011, 25, 1619–1629. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Jin, M.; Du, Z.; Liu, X.; Guo, C.; Li, Y.; Huang, P.; Sun, Z. Size-dependent cytotoxicity of amorphous silica nanoparticles in human hepatoma HepG2 cells. Toxicol. In Vitro 2011, 25, 1343–1352. [Google Scholar] [CrossRef]
- Neuer, A.L.; Geck, D.; Gogos, A.; Kissling, V.M.; Balfourier, A.; Herrmann, I.K. Nanoanalytical Insights into the Stability, Intracellular Fate, and Biotransformation of Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2023, 15, 38367–38380. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sene, S.; Mielcarek, A.M.; Miraux, S.; Menguy, N.; Ihiawakrim, D.; Ersen, O.; Pechoux, C.; Guillou, N.; Scola, J.; et al. Hierarchical superparamagnetic metal-organic framework nanovectors as anti-inflammatory nanomedicines. J. Mater. Chem. B 2023, 11, 3195–3211. [Google Scholar] [CrossRef]
- Fytory, M.; Mansour, A.; El Rouby, W.M.A.; Farghali, A.A.; Zhang, X.; Bier, F.; Abdel-Hafiez, M.; El-Sherbiny, I.M. Core-Shell Nanostructured Drug Delivery Platform Based on Biocompatible Metal-Organic Framework-Ligated Polyethyleneimine for Targeted Hepatocellular Carcinoma Therapy. ACS Omega 2023, 8, 20779–20791. [Google Scholar] [CrossRef]
- Mosavi, S.H.; Zare-Dorabei, R. Synthesis of NMOF-5 Using Microwave and Coating with Chitosan: A Smart Biocompatible pH-Responsive Nanocarrier for 6-Mercaptopurine Release on MCF-7 Cell Lines. ACS Biomater. Sci. Eng. 2022, 8, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Xu, X.; Wang, M.F.; Xu, H.Z.; Peng, X.C.; Han, N.; Yu, T.T.; Li, L.G.; Li, Q.R.; Chen, X.; et al. A nanoreactor boosts chemodynamic therapy and ferroptosis for synergistic cancer therapy using molecular amplifier dihydroartemisinin. J. Nanobiotechnol. 2022, 20, 230. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Halder, S.; Mukherjee, S.; Debnath, U.; Misra, A.K.; Jana, K.; Das, D. Glutathione Depleting a Chemoselective Novel Pro-oxidant Nano Metal-Organic Framework Induced G2/M Arrest and ROS-Mediated Apoptotic Cell Death in a Human Triple-Negative Breast Cancer Cell Line. ACS Appl. Mater. Interfaces 2023, 15, 26442–26456. [Google Scholar] [CrossRef]
- Bao, J.; Zu, X.; Wang, X.; Li, J.; Fan, D.; Shi, Y.; Xia, Q.; Cheng, J. Multifunctional Hf/Mn-TCPP Metal-Organic Framework Nanoparticles for Triple-Modality Imaging-Guided PTT/RT Synergistic Cancer Therapy. Int. J. Nanomed. 2020, 15, 7687–7702. [Google Scholar] [CrossRef]
- Zhong, X.F.; Sun, X. Nanomedicines based on nanoscale metal-organic frameworks for cancer immunotherapy. Acta Pharmacol. Sin. 2020, 41, 928–935. [Google Scholar] [CrossRef]
- Shait Mohammed, M.R.; Ahmad, V.; Ahmad, A.; Tabrez, S.; Choudhry, H.; Zamzami, M.A.; Bakhrebah, M.A.; Ahmad, A.; Wasi, S.; Mukhtar, H.; et al. Prospective of nanoscale metal organic frameworks [NMOFs] for cancer therapy. Semin. Cancer Biol. 2019, 69, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Ruyra, A.; Yazdi, A.; Espin, J.; Carne-Sanchez, A.; Roher, N.; Lorenzo, J.; Imaz, I.; Maspoch, D. Synthesis, culture medium stability, and in vitro and in vivo zebrafish embryo toxicity of metal-organic framework nanoparticles. Chemistry 2014, 21, 2508–2518. [Google Scholar] [CrossRef]
- Cai, X.; Xie, Z.; Ding, B.; Shao, S.; Liang, S.; Pang, M.; Lin, J. Monodispersed Copper(I)-Based Nano Metal-Organic Framework as a Biodegradable Drug Carrier with Enhanced Photodynamic Therapy Efficacy. Adv. Sci. 2019, 6, 1900848. [Google Scholar] [CrossRef]
- Wen, T.; Quan, G.; Niu, B.; Zhou, Y.; Zhao, Y.; Lu, C.; Pan, X.; Wu, C. Versatile Nanoscale Metal-Organic Frameworks (nMOFs): An Emerging 3D Nanoplatform for Drug Delivery and Therapeutic Applications. Small 2021, 17, 2005064. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chawla, A.; Zhang, J.; Esa, A.; Jang, H.L.; Khademhosseini, A. Applications of Nanotechnology for Regenerative Medicine; Healing Tissues at the Nanoscale. Princ. Regen. Med. 2019, 485–504. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Pershina, A.G.; Sazonov, A.E.; Filimonov, V.D. Magnetic nanoparticles–DNA interactions: Design and applications of nanobiohybrid systems. Russ. Chem. Rev. 2014, 83, 299–322. [Google Scholar] [CrossRef]
- Tang, C.; He, Z.; Liu, H.; Xu, Y.; Huang, H.; Yang, G.; Xiao, Z.; Li, S.; Liu, H.; Deng, Y.; et al. Application of magnetic nanoparticles in nucleic acid detection. J. Nanobiotechnol. 2020, 18, 62. [Google Scholar] [CrossRef]
- Ishmukhametov, I.; Batasheva, S.; Rozhina, E.; Akhatova, F.; Mingaleeva, R.; Rozhin, A.; Fakhrullin, R. DNA/Magnetic Nanoparticles Composite to Attenuate Glass Surface Nanotopography for Enhanced Mesenchymal Stem Cell Differentiation. Polymers 2022, 14, 344. [Google Scholar] [CrossRef]
- Dass, C.R.; Walker, T.L.; DeCruz, E.E.; Burton, M.A. Cationic Liposomes and Gene Therapy for Solid Tumors. Drug Deliv. 1997, 4, 151–165. [Google Scholar] [CrossRef][Green Version]
- Ewert, K.K.; Zidovska, A.; Ahmad, A.; Bouxsein, N.F.; Evans, H.M.; McAllister, C.S.; Samuel, C.E.; Safinya, C.R. Cationic liposome-nucleic acid complexes for gene delivery and silencing: Pathways and mechanisms for plasmid DNA and siRNA. Top. Curr. Chem. 2010, 296, 191–226. [Google Scholar] [CrossRef]
- Moss, K.H.; Popova, P.; Hadrup, S.R.; Astakhova, K.; Taskova, M. Lipid Nanoparticles for Delivery of Therapeutic RNA Oligonucleotides. Mol. Pharm. 2019, 16, 2265–2277. [Google Scholar] [CrossRef]
- Sakurai, F.; Inoue, R.; Nishino, Y.; Okuda, A.; Matsumoto, O.; Taga, T.; Yamashita, F.; Takakura, Y.; Hashida, M. Effect of DNA/liposome mixing ratio on the physicochemical characteristics, cellular uptake and intracellular trafficking of plasmid DNA/cationic liposome complexes and subsequent gene expression. J. Control. Release 2000, 66, 255–269. [Google Scholar] [CrossRef]
- Dokka, S.; Toledo, D.; Shi, X.; Castranova, V.; Rojanasakul, Y. Oxygen Radical-Mediated Pulmonary Toxicity Induced by Some Cationic Liposomes. Pharm. Res. 2000, 17, 521–525. [Google Scholar] [CrossRef]
- Yan, W.; Chen, W.; Huang, L. Reactive oxygen species play a central role in the activity of cationic liposome based cancer vaccine. J. Control. Release 2008, 130, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.B.; Northeved, H.; Kumar, P.E.; Permin, A.; Gjetting, T.; Andresen, T.L.; Larsen, S.; Wegener, K.M.; Lykkesfeldt, J.; Jantzen, K.; et al. In vivo toxicity of cationic micelles and liposomes. Nanomedicine 2015, 11, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Zhanataev, A.K.; Anisina, E.A.; Kulakova, A.V.; Shilovskiy, I.P.; Lisitsyn, A.A.; Koloskova, O.O.; Khaitov, M.R.; Durnev, A.D. Genotoxicity of cationic lipopeptide nanoparticles. Toxicol. Lett. 2020, 328, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Leon-Mejia, G.; Quintana-Sosa, M.; de Moya Hernandez, Y.; Rodriguez, I.L.; Trindade, C.; Romero, M.A.; Luna-Carrascal, J.; Ortiz, L.O.; Acosta-Hoyos, A.; Ruiz-Benitez, M.; et al. DNA repair and metabolic gene polymorphisms affect genetic damage due to diesel engine exhaust exposure. Environ. Sci. Pollut. Res. 2020, 27, 20516–20526. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.H.; Wu, W.T.; Liao, H.Y.; Chen, C.Y.; Tsai, C.Y.; Jung, W.T.; Lee, H.L. Global DNA methylation and oxidative stress biomarkers in workers exposed to metal oxide nanoparticles. J. Hazard. Mater. 2017, 331, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Rossnerova, A.; Honkova, K.; Pelclova, D.; Zdimal, V.; Hubacek, J.A.; Chvojkova, I.; Vrbova, K.; Rossner, P., Jr.; Topinka, J.; Vlckova, S.; et al. DNA Methylation Profiles in a Group of Workers Occupationally Exposed to Nanoparticles. Int. J. Mol. Sci. 2020, 21, 2420. [Google Scholar] [CrossRef]
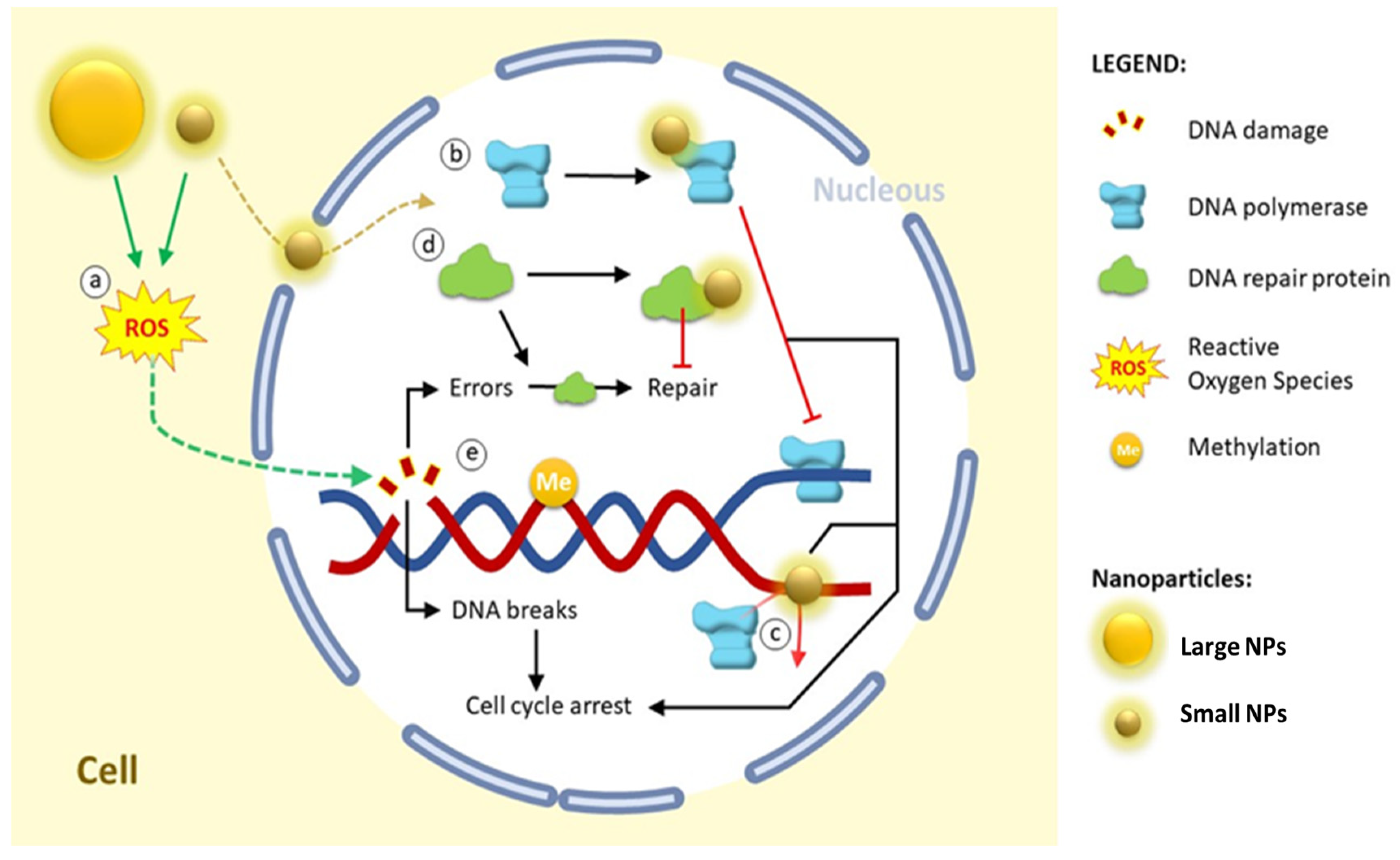
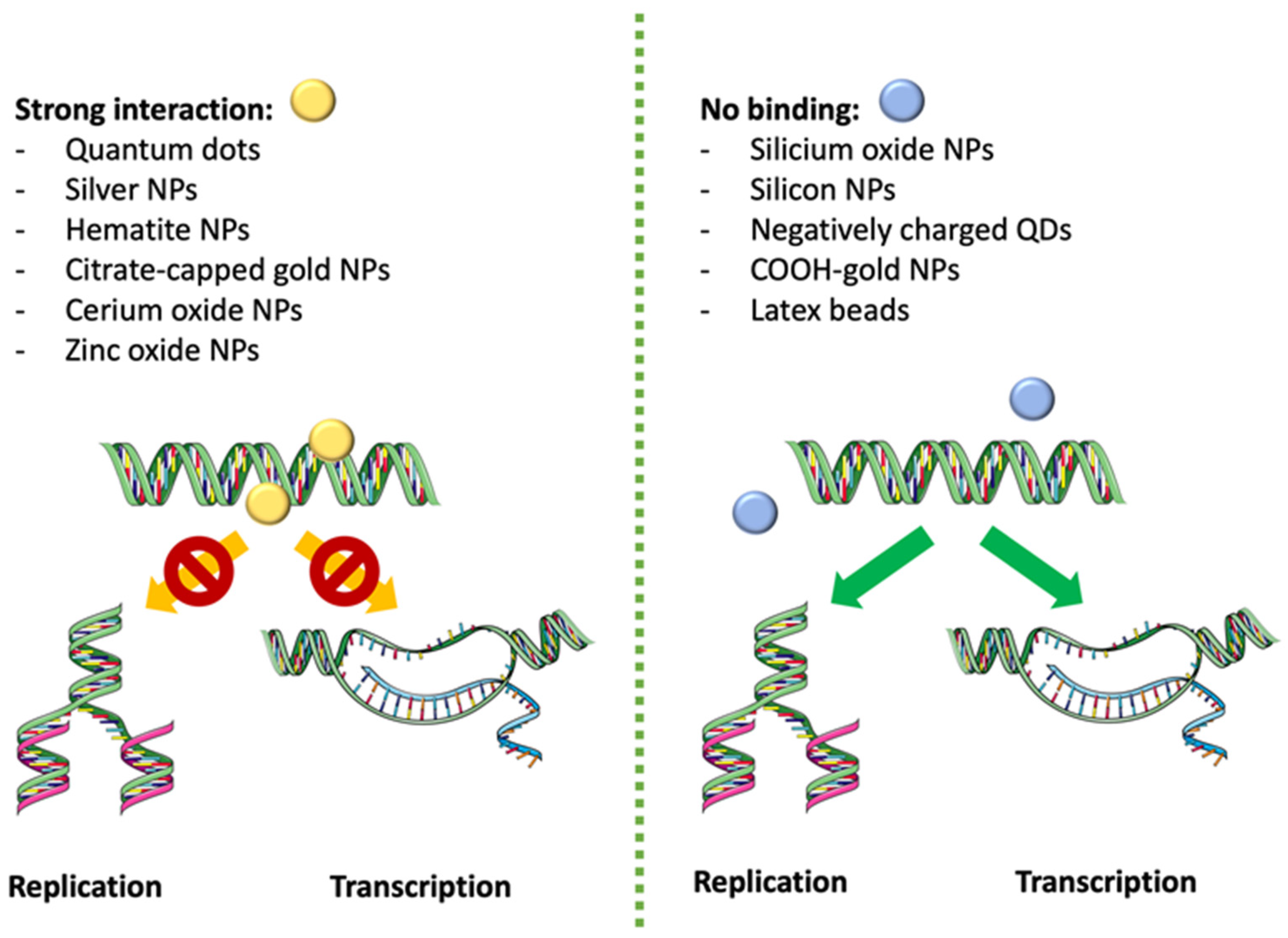
| Size | Stabilizer/Coating | Cell Line/Animal Model | Effect | Ref. |
|---|---|---|---|---|
| 6–20 nm | Sodium borohydride reduction Starch-capped | U251-MG, IMR-90 | Mitochondrial disfunction and increase in ROS production. DNA damage and chromosomal aberrations. Cell cycle arrest. | [24] |
| 5 nm | Commercial NPs (I&C Technology, Seoul, Republic of Korea) PVP-coated | HepG2, Huh7, THP-1 | Reduction in nuclear factor erythroid 2-like 2 expression after treatment with 5 nm Ag NPs in both hepatoma cell lines. Increase in ROS production after treatment with 5 nm Ag NPs in hepatoma cell lines Alteration of glucose metabolism after treatment with 5 nm Ag NPs in hepatoma cell lines and THP-1 cells. | [26] |
| 100 nm | ||||
| 20 nm | Commercial NPs (Shanghai YunfuNano Technology Co., Ltd., Shanghai, China) | HT22 | Cell viability reduction and membrane leakage induction in a dose-dependent manner. Increase in ROS production. Autophagy induction, upregulation of LC3 II/I, downregulation of p62. Upregulation of caspase-3 and Bax, downregulation of Bcl-2. Alteration of PI3K/AKT/mTOR signaling pathway. | [27] |
| 43–260 nm | Commercial NPs (Sigma Aldrich, St. Louis, MO, USA) | BEAS-2B | Stimulation of DNA breakage and micronuclei formation. Induction of increased oxidative DNA damage. Increase in reactive oxygen radicals. | [28] |
| 20 nm | Commercial NPs (Plasmachem GmbH, Berlin, Germany) | Ntera2 (NT2, human testicular embryonic carcinoma cell line) | Cytotoxic and cytostatic. Apoptosis and necrosis. Decreased proliferation. Concentration- and time-dependent manner. 200 nm Ag NP increase DNA-strand breaks. | [29] |
| 200 nm | ||||
| 10 nm | Commercial NPs (NanoComposix, San Diego, CA, USA) | Jurkat Clon E6-1, THP-1 | Micronuclei and DNA damage inversely correlated with Ag NP size. Suggestion that silver ions are the main cause of NP genotoxicity. | [30] |
| 20 nm | ||||
| 50 nm | ||||
| 100 nm | ||||
| 5 nm | Commercial NPs (NanoComposix, San Diego, CA, USA) PVP-coated | TK6 | Genotoxicity and cytotoxicity induction in a range of concentrations similar to silver nitrate (AgNO3). Induction of oxidative stress. Suggestion that silver ions are not the cause of NP genotoxicity. | [31] |
| 42.5 nm | Commercial NPs (NANOGAP, Milladoiro, Spain) PVP-coated | BEAS-2B | DNA damage induction in a dose-dependent manner. No induction of micronuclei or chromosomal aberrations observed. Lack of chromosomal damage may be due to PVP-coating protection from leaching or direct interaction with Ag NPs. | [32] |
| 5 nm | Commercial (NanoComposix, San Diego, CA, USA) PVP-coated | B6C3F1 mice—intravenous administration | Increase in oxidative damage with PVP- and silica-coated NPs of different sizes. Induction of toxicity in bone marrow caused by PVP-coated NPs but not by silica-coated NPs. No significant increase in mutant frequencies in the Pig-a gene or the percent of micronucleated reticulocytes. Induction of oxidative DNA damage in liver by PVP- and silicon-coated NPs. | [33] |
| 15–100 nm | ||||
| 10–80 nm | Commercial (NanoComposix, San Diego, CA, USA) Silicon-coated | |||
| 6.3–629 nm | Commercial NPs (Nanux, SL1105001, Gimhae, Republic of Korea) | Sprague-Dawley rats—intravenous administration | Highest Ag concentrations found in lung, spleen and liver. Marked increase in alanine aminotransferase (ALT), blood urea nitrogen (BUN), total bilirubin (TBil) and creatinine (Cre) after NP administration. Induction of extensive organ damages in liver, kidneys, thymus and spleen after NP administration. Increase in aberration and multiple aberrations cells, as well as polyploidy cells after NP administration. | [34] |
| 14 nm | Hydrazine reduction PVP-coated | Female Wistar Hannover Galas rats—oral administration | Largest silver concentrations found in intestinal system, liver and kidneys. Lower concentrations of absolute silver after administration of NPs than silver acetate (AgAc). Sulfur and selenium containing silver granules found in lysosomes of macrophages of the ileum. | [35] |
| 15 nm | Commercial (Collargol, Laboratorios Argenol S.L, Zaragoza, Spain.) | Weanling male Sprague–Dawley rats | Significant accumulation of silver found in liver and kidneys. Presence of Ag(I) complexed by high-molecular proteins in liver. | [36] |
| Size | Main Features | Cell Line/Animal Model | Main Effects and Conclusions | Ref. |
|---|---|---|---|---|
| 8–20 nm | Commercial NPs (Sigma Chemical Co., Ltd., St. Louis, MO, USA) | A549 | ROS generation, reduction in GSH concentration. Nuclear fragmentation and chromatin condensation induction. Apoptotic bodies generation. Caspase-3 and caspase-9 increased expression. PARP-1 cleavage and p53 phosphorylation. | [46] |
| 38 nm | Commercial NPs (Sigma Aldrich, St. Louis, MO, USA) | A375 | ROS generation, reduction in GSH concentration. Chromatin condensation induction. DNA double-strand break formation. Caspase-3 activity induction. | [47] |
| 7 nm | Commercial NPs (Rhodia Chemicals, Briton, UK) | Human spermatozoa fr0m healthy fertile donors | Significant induction of DNA damage. Genotoxicity inversely proportional to the concentration. Accumulation along the flagellum and no internalization in spermatozoa. | [48] |
| 7 nm | Commercial NPs (Rhodia Chemicals, Briton, UK) | Primary human foreskin fibroblasts | Micronuclei formation. Induction of lipid peroxidation. Intracellular GSH/GSSG ratio decrease. Clastogenic mechanism of chromosomal damage. | [49] |
| 20 nm | Commercial NPs (Sigma Aldrich, St. Louis, MO, USA) Intratracheal administration | BALB/C mice—intratracheal (IT) administration | ROS levels significantly increased in lung, heart, kidneys and brain. DNA damage in lung, heart, liver, kidneys, spleen and brain. Interleukin-6 (IL-6) increment in lung, heart, liver, kidneys, and spleen. Interleukin-1β (IL-1β) increment in lung, heart, kidneys, and spleen. | [50] |
| Male Wistar rats (acute kidney injury model)—IT administration | Significant exacerbation of DNA damage after treatment with NPs. Increment in Nrf2 expression in cardiac myocytes and endothelial cells. Elevation of coagulation function, troponin I, lactate dehydrogenase, IL-6 and TNFα in plasma. Increment in renal injury molecule-1, IL-6, tumoral necrosis factor α (TNFα) and glutathione concentrations in kidneys. Inhalation of nanomaterial has more serious effects in individuals with renal diseases. | [39,51] | ||
| Male Wistar rats (vascular damage model)—IT administration | Increment in DNA damage in aortic tissue and aggravation of vascular toxicity. Increment in Nrf2 expression in the nuclei of smooth muscles and endocardial cells | [52] | ||
| 24 nm | Commercial NPs (Sigma Chemical Co., Ltd., St. Louis, MO, USA) | Albino Wistar rats—oral administration | Increment in DNA damage in liver and peripheral blood leukocytes. Increment in micronuclei and chromosomal aberrations in bone marrow. Increment micronuclei in peripheral blood. | [53] |
| 9.5 nm | Commercial NPs (Sigma Aldrich, St. Louis, MO, USA) | BEAS-2B | Reduction in oxidative stress and DNA damage caused by KBrO3. Downregulation of the expression of Ho1 and Sod2 genes. | [54] |
| 25 nm | Commercial NPs (Sigma Aldrich, St. Louis, MO, USA) | Albino Westar rat—intraperitoneal administration | Alleviation of DNA fragmentation in liver caused by D-GALN/LPS-induced hepatotoxicity. Nrf-2 translocation and HO-1 gene expression decrease. Increment in GSH, GPX1, glutathione reductase, superoxide dismutase and catalase. | [55] |
| 70 nm | EU Joint Research Center (NM212) | Caco-2 | No induction of cytotoxic or genotoxic effects of NPs. | [56] |
| 10–30 nm | Octahedron-like hydrothermal synthesis | HepG2 | Cytotoxic effect inversely proportional to surface area: apoptosis induction; mitochondrial membrane potential (MMP), ROS and GSH increase, and reduction in cell ability to scavenge hydroxyl free radicals. Protection and inhibition of DNA damage by scavenging with octahedron-like and rod-like NPs. | [57] |
| 20–50 nm | Cube-like hydrothermal synthesis | |||
| 8 nm diameter 100–400 nm length | Rod-like Hydrothermal synthesis | |||
| 23 nm | Commercial NPs (Sigma Aldrich Chemical Company, St. Louis, MO, USA) | Swiss albino mice—oral administration | Amelioration of genotoxicity induced by lead acetate. Significant reduction in tail length, DNA% in tail, tail moment and percentage of fragmented DNA. Possible antioxidant capacity of NPs due to reversible switching between III and IV oxidation states. | [58] |
| 9 nm (DH) | Thermo-hydrolysis synthesis Polymer coating (MPEG2K-MPh, MPEG2K-MPEGa1K-MPh and MPEG2K-MPEGa2K-MPh) | bEnd.3 | Reduction in glutamate-induced intracellular production of ROS in endothelial cells by all NPs. Coated NPs are devoid of cytotoxicity. Lack of toxicity corroborated in vivo (male Swiss mice). | [59] |
| 27.2 nm (DH) | ||||
| 29.5 nm (DH) | ||||
| 31.5 nm (DH) | ||||
| 10 nm | Wet-chemical synthesis | Jurkat | Prevention of DNA damage and ROS production after UV-A irradiation. Prevention of micronuclei formation after UV-B irradiation. | [60] |
| 3–5 nm | Wet-chemical synthesis | hBMSCs | Reduction in ROS levels and DNA damage after irradiation. Increase in autophagy and bone matrix deposition after irradiation. | [61] |
| <25 nm | Commercial NPs (Sigma Aldrich, St. Louis, MO, USA) | MC3T3-E1 osteoblast-like cells | Attenuation of deteriorative effects of irradiation, alleviating cell viability, differentiation and mineralization. Alleviation of intracellular ROS production and extracellular hydrogen peroxide (H2O2) concentration. | [62] |
| 5–8 nm | Commercial NPs (Sigma Aldrich, St. Louis, MO, USA) | C57BL/6J male mice—intravenous administration | Significant reduction in tissue damage caused by irradiation. Substantial decrease in DNA damage and ROS. Sperm protection. | [63] |
| Size | Stabilizer/Coating | Cell Line/Animal Model | Effects | Ref. | |
|---|---|---|---|---|---|
| 10.9 nm | Poly(amidoamine) (PAMAM) | HepG2, PBMC | Interaction of both NPs with both cell lines and exhibition of geno- and cytotoxicity. Observation of higher sensitivity of HepG2 to Au NPs than PBMC. | [68] | |
| 18.2 nm | Citrate | ||||
| 18.4 nm | Citrate | HepG2 | No induction of significant cytotoxicity by both Au NPs. DNA damage production after treatment with citrate Au NPs but not after MUA-AuNP treatment. | [69] | |
| 11-mercaptoundecanoic acid (MUA) | |||||
| 18 nm | Citrate | Bovine Serum Albumin (BSA) | HepG2 | Differences in the biochemical effects produced by Au NPs depending on their coating. No overall toxicity of citrate Au NPs. Induction of DNA damage that could not be repaired by citrate Au NPs. Unbound citrate shows high toxicity. | [70] |
| Poly(sodium 4-styrene sulfonate) | |||||
| Citrate | |||||
| MUA | |||||
| GSH | |||||
| PVP | |||||
| Polyethylene Glycol (PEG) | |||||
| 14 nm | Citrate | Caco-2, HaCaT | Significant increase in micronuclei and nucleoplasmic bridge production at 5 nM concentration for both citrate NPs and both cell lines. Induction, by all tested AuNPs, of genotoxicity, indicating DNA damage. Induction of highest level of toxicity for 14 nm citrate Au NPs. | [64] | |
| 20 nm | |||||
| 14 nm | PEG | COOH ligand | |||
| NH2 ligand | |||||
| OH ligand | |||||
| 14 nm 20 nm | Citrate | CHO | No statistical significance in the genotoxic effect observed for citrate Au NPs at the tested concentrations. | [72] | |
| 10 nm 30 nm | Citrate | Wistar rats—acute and chronic intraperitoneal administration | Increase in the frequency of DNA damage and the damage index in blood and liver. DNA damage caused by of oxidative stress regardless of the type of administration and Au NP size. | [74] | |
| Au NPs induce DNA damage differently depending on the type of administration and the size of the Au NPs. There are higher levels of damage frequency and damage in the DNA by 30 nm Au NPs compared to 10 nm Au NPs. | [75] | ||||
| Size | Stabilizer/Coating | Cell Line | Effect | Ref. | |
|---|---|---|---|---|---|
| 15 nm | Citrate | RGD peptide | HeLa | Five-fold increase in NPs uptake and effective nuclear localization for peptide-capped Au NPs. Stabilization of conjugated Au NPs complex thanks to pentapeptide. Reduction in NPs exocytosis for peptide-capped NPs compared to citrate-capped ones. | [78] |
| NLS peptide | |||||
| Pentapeptide | |||||
| 35 nm | Citrate | mPEG-SH-5000 RGD peptide NLS peptide | HSC-3 | Co-localization of RGD/NLS-conjugated nanomaterials with the nucleus by confocal imaging. Induction of cell cycle changes and reduced ATP production when cells are treated with RGD/NLS-conjugated nanomaterials. Higher induction of apoptosis and necrosis by RGD/NLS-conjugated hollow gold nanocages than by peptide-conjugated solid gold nanospheres. | [84] |
| 45 nm | PVP | ||||
| 30 nm | Citrate | mPEG-SH-500 RGD peptide | HSC-3 | Dark-field light scattering confirmation that NLS-Au NPs were localized at the cell nucleus, while RGD-Au NPs were distributed throughout the cytoplasm. | [86] |
| mPEG-SH-500 NLS peptide | |||||
| 15 nm | Citrate | RALA peptide | PC-3 | Decoration with RALA peptide imparts nuclear targeting capabilities. Precise 3D location of Au NPs within the cell, showing evidence of the nuclear uptake of monodispersed Au NPs. 79% of RALA-Au NPs internalized by the nucleus are predicted to be monodispersed nanoparticles. | [87] |
| 15 nm | Citrate | RALA peptide | PC-3, DU145, PNT2-C2 | RALA stabilization of sub-110 nm complexes of several Au NPs. Validation of nuclear accumulation of RALA-Au NPs. Meaningful radiosensitization of cells using low microgram RALA-AuNP concentrations. | [88] |
| 4 nm | Citrate | PEG (MW 2000) MPA | HeLa | No induction of obvious cytotoxicity. Time-dependent cellular uptake of AuNP@MPA-PEG. Improvement of stability and biocompatibility due to surface modification. Potential nuclear-targeted drug delivery carrier. | [89] |
| Size | Stabilizer/Coating | Cell Line/Animal Model | Effect | Ref. |
|---|---|---|---|---|
| 70 nm | Commercial NPs (Micromod Partikeltechnologie GmbH, Rostock, Germany) | HaCat Raw246.7 Langerhans cell-like (XS52) BALB/c mice—Application in the inner side of both ears | Internalization of 70 nm SiO2 NPs in nucleus of HaCat cells, mice skin cells, cervical lymph node cells and parenchymal hepatocytes. Same observation for Langerhans cells and murine macrophages. Higher ROS generation after 70 nm SiO2 NPs in HaCat cells compared to the other sizes. DNA damage. | [94,95,96,97] |
| 300 nm | ||||
| 1000 nm | ||||
| 50 nm | Commercial NPs (Kisker (Steinfurt, Germany) and Postnova (Landsberg/Lech, Germany).) | HEp-2, A549, RLE-T6N, N2a | Nuclear localization observed in HaCat cells treated with 40 and 70 nm SIO2 NPs. Induction of aberrant nucleoplasmic protein aggregation in HEp-2 cells after treatment with 70 nm SiO2 NPs. | [98] |
| 70 nm | ||||
| 200 nm | ||||
| 500 nm | ||||
| 1000 nm | ||||
| 5000 nm | ||||
| 28 nm (LumiLys 650) | Commercial NPs (Chromalys, Toulouse, France) Functionalization with gadolinium—diethylene–triamine–pentaacetic acid) | HCT-116, RL | Nucleus internalization of LumyLys 650 NPs in both cell lines after electropermeabilization. Tumor cell tracking for 30 days, labeling cells with SiO2 NPs by electropermeabilization. | [99] |
| 30 nm (LumiLys 780) | ||||
| 62 nm | Stöber method (tetraethyl orthosilicate (TEOS) + ethanol/ammonia/water) | HUVECs | Induction of ROS generation and DNA damage response, causing endothelial cells toxic effect through Chk-1 dependent G2/M DNA damage checkpoint signaling pathway. | [101] |
| 43 nm | Stöber method (TEOS + ethanol/ammonia/water) | HepG2 | SiO2 NP induction of oxidative stress through ROS production, causing mitochondrial membrane potential decrease and apoptosis through mitochondrial pathway. | [102] |
| 19 nm | Provided by School of Chemistry, Jilin University, Changchun, China | HepG2 | Toxicity produced by SiO2 NPs was in a dose- and size-dependent manner. The smaller the size, the higher the ROS production. All four SiO2 NPs led to DNA damage, cell cycle arrest and apoptosis. | [103] |
| 43 nm | ||||
| 68 nm | ||||
| 498 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encinas-Gimenez, M.; Martin-Duque, P.; Martín-Pardillos, A. Cellular Alterations Due to Direct and Indirect Interaction of Nanomaterials with Nucleic Acids. Int. J. Mol. Sci. 2024, 25, 1983. https://doi.org/10.3390/ijms25041983
Encinas-Gimenez M, Martin-Duque P, Martín-Pardillos A. Cellular Alterations Due to Direct and Indirect Interaction of Nanomaterials with Nucleic Acids. International Journal of Molecular Sciences. 2024; 25(4):1983. https://doi.org/10.3390/ijms25041983
Chicago/Turabian StyleEncinas-Gimenez, Miguel, Pilar Martin-Duque, and Ana Martín-Pardillos. 2024. "Cellular Alterations Due to Direct and Indirect Interaction of Nanomaterials with Nucleic Acids" International Journal of Molecular Sciences 25, no. 4: 1983. https://doi.org/10.3390/ijms25041983
APA StyleEncinas-Gimenez, M., Martin-Duque, P., & Martín-Pardillos, A. (2024). Cellular Alterations Due to Direct and Indirect Interaction of Nanomaterials with Nucleic Acids. International Journal of Molecular Sciences, 25(4), 1983. https://doi.org/10.3390/ijms25041983






