Combination of Oncolytic Virotherapy with Different Antitumor Approaches against Glioblastoma
Abstract
1. Introduction
2. Virotherapy against Glioblastoma
2.1. Herpes Simplex Virus
2.2. Adenovirus
2.3. Poliovirus
2.4. Parvovirus
2.5. Other Oncolytic Viruses
3. Oncolytic Viruses Armed with Transgenes
3.1. Oncolytic Viruses with Immunotherapeutic Modulators
3.2. Oncolytic Viruses with Apoptotic Inducers
4. Combination of Oncolytic Virotherapy and Current Standard of Care for Glioblastoma
4.1. Virotherapy in Combination with Resection of the Tumor
4.2. Virotherapy in Combination with Chemotherapy
4.3. Virotherapy in Combination with Radiotherapy
5. Combination of Oncolytic Virotherapy and Immunotherapy for Glioblastoma
5.1. Immune Checkpoint Inhibitors
5.2. Adoptive Cell Therapy
5.3. Dendritic Cell Vaccines
5.4. Peptide Vaccines
6. Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Czarnywojtek, A.; Borowska, M.; Dyrka, K.; Van Gool, S.; Sawicka-Gutaj, N.; Moskal, J.; Kościński, J.; Graczyk, P.; Hałas, T.; Lewandowska, A.M.; et al. Glioblastoma Multiforme: The Latest Diagnostics and Treatment Techniques. Pharmacology 2023, 108, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Song, K.; Wu, S.; Hameed, N.U.F.; Kudulaiti, N.; Xu, H.; Qin, Z.-Y.; Wu, J.-S. The Prognosis of Glioblastoma: A Large, Multifactorial Study. Br. J. Neurosurg. 2021, 35, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wee, C.W. Treatment of Adult Gliomas: A Current Update. Brain Neurorehabil. 2022, 15, e24. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V.; Yang, Y.; Schubert, M.C.; Reyhan, E.; Tetzlaff, S.K.; Wißmann, N.; Botz, M.; Soyka, S.J.; Beretta, C.A.; Pramatarov, R.L.; et al. Glioblastoma Hijacks Neuronal Mechanisms for Brain Invasion. Cell 2022, 185, 2899–2917.e31. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma Multiforme (GBM): An Overview of Current Therapies and Mechanisms of Resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef]
- Daisy Precilla, S.; Kuduvalli, S.S.; Thirugnanasambandhar Sivasubramanian, A. Disentangling the Therapeutic Tactics in GBM: From Bench to Bedside and Beyond. Cell Biol. Int. 2021, 45, 18–53. [Google Scholar] [CrossRef]
- Shalhout, S.Z.; Miller, D.M.; Emerick, K.S.; Kaufman, H.L. Therapy with Oncolytic Viruses: Progress and Challenges. Nat. Rev. Clin. Oncol. 2023, 20, 160–177. [Google Scholar] [CrossRef]
- Lan, Q.; Xia, S.; Wang, Q.; Xu, W.; Huang, H.; Jiang, S.; Lu, L. Development of Oncolytic Virotherapy: From Genetic Modification to Combination Therapy. Front. Med. 2020, 14, 160–184. [Google Scholar] [CrossRef]
- Everts, B.; Van Der Poel, H.G. Replication-Selective Oncolytic Viruses in the Treatment of Cancer. Cancer Gene Ther. 2005, 12, 141–161. [Google Scholar] [CrossRef]
- Goradel, N.H.; Baker, A.T.; Arashkia, A.; Ebrahimi, N.; Ghorghanlu, S.; Negahdari, B. Oncolytic Virotherapy: Challenges and Solutions. Curr. Probl. Cancer 2021, 45, 100639. [Google Scholar] [CrossRef]
- Asija, S.; Chatterjee, A.; Goda, J.S.; Yadav, S.; Chekuri, G.; Purwar, R. Oncolytic Immunovirotherapy for High-Grade Gliomas: A Novel and an Evolving Therapeutic Option. Front. Immunol. 2023, 14, 1118246. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Pancer, K.W.; Wieczorek, M.; Staniszewska, M.; Salmaso, S.; Caliceti, P.; Kuryk, L. From Immunosuppression to Immunomodulation—Turning Cold Tumours into Hot. J. Cancer 2022, 13, 2884–2892. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, J.F.; De Vor, L.; Fouchier, R.A.M.; Van Den Hoogen, B.G. Armed Oncolytic Viruses: A Kick-Start for Anti-Tumor Immunity. Cytokine Growth Factor Rev. 2018, 41, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Chen, H.; Dai, S.-Z.; Huang, F.-Y.; Lin, Y.-Y.; Wang, C.-C.; Li, L.; Zheng, W.-P.; Tan, G.-H. Immunotherapy Combining Tumor and Endothelium Cell Lysis with Immune Enforcement by Recombinant MIP-3α Newcastle Disease Virus in a Vessel-Targeting Liposome Enhances Antitumor Immunity. J. Immunother. Cancer 2022, 10, e003950. [Google Scholar] [CrossRef] [PubMed]
- Todo, T.; Ino, Y.; Ohtsu, H.; Shibahara, J.; Tanaka, M. A Phase I/II Study of Triple-Mutated Oncolytic Herpes Virus G47∆ in Patients with Progressive Glioblastoma. Nat. Commun. 2022, 13, 4119. [Google Scholar] [CrossRef] [PubMed]
- Markert, J.M.; Liechty, P.G.; Wang, W.; Gaston, S.; Braz, E.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Lakeman, A.D.; Palmer, C.A.; et al. Phase Ib Trial of Mutant Herpes Simplex Virus G207 Inoculated Pre-and Post-Tumor Resection for Recurrent GBM. Mol. Ther. 2009, 17, 199–207. [Google Scholar] [CrossRef]
- Cheema, T.A.; Kanai, R.; Kim, G.W.; Wakimoto, H.; Passer, B.; Rabkin, S.D.; Martuza, R.L. Enhanced Antitumor Efficacy of Low-Dose Etoposide with Oncolytic Herpes Simplex Virus in Human Glioblastoma Stem Cell Xenografts. Clin. Cancer Res. 2011, 17, 7383–7393. [Google Scholar] [CrossRef]
- Kanai, R.; Rabkin, S.D.; Yip, S.; Sgubin, D.; Zaupa, C.M.; Hirose, Y.; Louis, D.N.; Wakimoto, H.; Martuza, R.L. Oncolytic Virus-Mediated Manipulation of DNA Damage Responses: Synergy with Chemotherapy in Killing Glioblastoma Stem Cells. JNCI J. Natl. Cancer Inst. 2012, 104, 42–55. [Google Scholar] [CrossRef]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell 2017, 32, 253–267.e5. [Google Scholar] [CrossRef]
- Alonso, M.M.; García-Moure, M.; Gonzalez-Huarriz, M.; Marigil, M.; Hernandez-Alcoceba, R.; Buñales, M.; Hervás, S.; Gallego, J.; Gomez-Manzano, C.; Fueyo, J.; et al. Abstract CT027: Oncolytic Virus DNX-2401 with a Short Course of Temozolomide for Glioblastoma at First Recurrence: Clinical Data and Prognostic Biomarkers. Cancer Res. 2017, 77, CT027. [Google Scholar] [CrossRef]
- Cassady, K.A.; Bauer, D.F.; Roth, J.; Chambers, M.R.; Shoeb, T.; Coleman, J.; Prichard, M.; Gillespie, G.Y.; Markert, J.M. Pre-Clinical Assessment of C134, a Chimeric Oncolytic Herpes Simplex Virus, in Mice and Non-Human Primates. Mol. Ther. Oncolytics 2017, 5, 1–10. [Google Scholar] [CrossRef]
- Holzmüller, R.; Mantwill, K.; Haczek, C.; Rognoni, E.; Anton, M.; Kasajima, A.; Weichert, W.; Treue, D.; Lage, H.; Schuster, T.; et al. YB-1 Dependent Virotherapy in Combination with Temozolomide as a Multimodal Therapy Approach to Eradicate Malignant Glioma. Int. J. Cancer 2011, 129, 1265–1276. [Google Scholar] [CrossRef]
- Alonso, M.M.; Gomez-Manzano, C.; Jiang, H.; Bekele, N.B.; Piao, Y.; Yung, W.K.A.; Alemany, R.; Fueyo, J. Combination of the Oncolytic Adenovirus ICOVIR-5 with Chemotherapy Provides Enhanced Anti-Glioma Effect in Vivo. Cancer Gene Ther. 2007, 14, 756–761. [Google Scholar] [CrossRef]
- Lamfers, M.L.M.; Grill, J.; Dirven, C.M.F.; Van Beusechem, V.W.; Geoerger, B.; Van Den Berg, J.; Alemany, R.; Fueyo, J.; Curiel, D.T.; Vassal, G.; et al. Potential of the Conditionally Replicative Adenovirus Ad5-Delta24RGD in the Treatment of Malignant Gliomas and Its Enhanced Effect with Radiotherapy. Cancer Res. 2002, 62, 5736–5742. [Google Scholar]
- Geoerger, B.; Grill, J.; Opolon, P.; Morizet, J.; Aubert, G.; Lecluse, Y.; Van Beusechem, V.W.; Gerritsen, W.R.; Kirn, D.H.; Vassal, G. Potentiation of Radiation Therapy by the Oncolytic Adenovirus Dl1520 (ONYX-015) in Human Malignant Glioma Xenografts. Br. J. Cancer 2003, 89, 577–584. [Google Scholar] [CrossRef][Green Version]
- Kieran, M.W.; Goumnerova, L.; Manley, P.; Chi, S.N.; Marcus, K.J.; Manzanera, A.G.; Polanco, M.L.S.; Guzik, B.W.; Aguilar-Cordova, E.; Diaz-Montero, C.M.; et al. Phase I Study of Gene-Mediated Cytotoxic Immunotherapy with AdV-Tk as Adjuvant to Surgery and Radiation for Pediatric Malignant Glioma and Recurrent Ependymoma. Neuro-Oncology 2019, 21, 537–546. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Z.; Zhong, K.; Wang, Z.; Yang, N.; Tang, X.; Li, H.; Lu, Q.; Wu, Z.; Yuan, B.; et al. CXCL11-Armed Oncolytic Adenoviruses Enhance CAR-T Cell Therapeutic Efficacy and Reprogram Tumor Microenvironment in Glioblastoma. Mol. Ther. 2023, 31, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zheng, M.; Zhang, Z.; Tang, X.; Chen, Y.; Peng, A.; Peng, X.; Tong, A.; Zhou, L. Interleukin-7-Loaded Oncolytic Adenovirus Improves CAR-T Cell Therapy for Glioblastoma. Cancer Immunol. Immunother. 2021, 70, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Brown, M.C.; Zhang, G.; Stevenson, K.; Mohme, M.; Kornahrens, R.; Bigner, D.D.; Ashley, D.M.; López, G.Y.; Gromeier, M. Polio Virotherapy Targets the Malignant Glioma Myeloid Infiltrate with Diffuse Microglia Activation Engulfing the CNS. Neuro-Oncology 2023, 25, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Foloppe, J.; Kempf, J.; Futin, N.; Kintz, J.; Cordier, P.; Pichon, C.; Findeli, A.; Vorburger, F.; Quemeneur, E.; Erbs, P. The Enhanced Tumor Specificity of TG6002, an Armed Oncolytic Vaccinia Virus Deleted in Two Genes Involved in Nucleotide Metabolism. Mol. Ther. Oncolytics 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Vasileva, N.; Ageenko, A.; Dmitrieva, M.; Nushtaeva, A.; Mishinov, S.; Kochneva, G.; Richter, V.; Kuligina, E. Double Recombinant Vaccinia Virus: A Candidate Drug against Human Glioblastoma. Life 2021, 11, 1084. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Zhang, C.; Zhang, N.; Wang, P.; Chu, Y.; Chard Dunmall, L.S.; Lemoine, N.R.; Wang, Y. An Effective Therapeutic Regime for Treatment of Glioma Using Oncolytic Vaccinia Virus Expressing IL-21 in Combination with Immune Checkpoint Inhibition. Mol. Ther. Oncolytics 2022, 26, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Guo, Z.S.; Bartlett, D.L.; Yan, D.Z.; Schane, C.P.; Thomas, D.L.; Liu, J.; McFadden, G.; Shisler, J.L.; Roy, E.J. Synergistic Combination of Oncolytic Virotherapy and Immunotherapy for Glioma. Clin. Cancer Res. 2020, 26, 2216–2230. [Google Scholar] [CrossRef]
- Lun, X.; Yang, W.; Alain, T.; Shi, Z.-Q.; Muzik, H.; Barrett, J.W.; McFadden, G.; Bell, J.; Hamilton, M.G.; Senger, D.L.; et al. Myxoma Virus Is a Novel Oncolytic Virus with Significant Antitumor Activity against Experimental Human Gliomas. Cancer Res. 2005, 65, 9982–9990. [Google Scholar] [CrossRef]
- Pisklakova, A.; McKenzie, B.; Zemp, F.; Lun, X.; Kenchappa, R.S.; Etame, A.B.; Rahman, M.M.; Reilly, K.; Pilon-Thomas, S.; McFadden, G.; et al. M011L-Deficient Oncolytic Myxoma Virus Induces Apoptosis in Brain Tumor-Initiating Cells and Enhances Survival in a Novel Immunocompetent Mouse Model of Glioblastoma. Neuro-Oncology 2016, 18, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Aldrak, N.; Alsaab, S.; Algethami, A.; Bhere, D.; Wakimoto, H.; Shah, K.; Alomary, M.N.; Zaidan, N. Oncolytic Herpes Simplex Virus-Based Therapies for Cancer. Cells 2021, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
- Jahan, N.; Ghouse, S.M.; Martuza, R.L.; Rabkin, S.D. In Situ Cancer Vaccination and Immunovirotherapy Using Oncolytic HSV. Viruses 2021, 13, 1740. [Google Scholar] [CrossRef] [PubMed]
- Kardani, K.; Sanchez Gil, J.; Rabkin, S.D. Oncolytic Herpes Simplex Viruses for the Treatment of Glioma and Targeting Glioblastoma Stem-like Cells. Front. Cell. Infect. Microbiol. 2023, 13, 1206111. [Google Scholar] [CrossRef]
- Friedman, G.K.; Johnston, J.M.; Bag, A.K.; Bernstock, J.D.; Li, R.; Aban, I.; Kachurak, K.; Nan, L.; Kang, K.-D.; Totsch, S.; et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N. Engl. J. Med. 2021, 384, 1613–1622. [Google Scholar] [CrossRef]
- Frampton, J.E. Teserpaturev/G47Δ: First Approval. BioDrugs 2022, 36, 667–672. [Google Scholar] [CrossRef]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral Oncolytic Herpes Virus G47∆ for Residual or Recurrent Glioblastoma: A Phase 2 Trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef]
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Lam, S.S.; Peng, W.; Aghbashlo, M.; Tabatabaei, M.; Guillemin, G.J. Oncolytic Viruses as a Promising Therapeutic Strategy against the Detrimental Health Impacts of Air Pollution: The Case of Glioblastoma Multiforme. Semin. Cancer Biol. 2022, 86, 1122–1142. [Google Scholar] [CrossRef]
- Kiyokawa, J.; Wakimoto, H. Preclinical and Clinical Development of Oncolytic Adenovirus for The Treatment of Malignant Glioma. Oncolytic Virother. 2019, 8, 27–37. [Google Scholar] [CrossRef]
- Lee, J.; Oh, G.-H.; Hong, J.A.; Choi, S.; Choi, H.J.; Song, J.J. Enhanced Oncolytic Adenoviral Production by Downregulation of Death-Domain Associated Protein and Overexpression of Precursor Terminal Protein. Sci. Rep. 2021, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Ene, C.I.; Fueyo, J.; Lang, F.F. Delta-24 Adenoviral Therapy for Glioblastoma: Evolution from the Bench to Bedside and Future Considerations. Neurosurg. Focus 2021, 50, E6. [Google Scholar] [CrossRef] [PubMed]
- Philbrick, B.; Adamson, D.C. DNX-2401: An Investigational Drug for the Treatment of Recurrent Glioblastoma. Expert Opin. Investig. Drugs 2019, 28, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- Fares, J.; Ahmed, A.U.; Ulasov, I.V.; Sonabend, A.M.; Miska, J.; Lee-Chang, C.; Balyasnikova, I.V.; Chandler, J.P.; Portnow, J.; Tate, M.C.; et al. Neural Stem Cell Delivery of an Oncolytic Adenovirus in Newly Diagnosed Malignant Glioma: A First-in-Human, Phase 1, Dose-Escalation Trial. Lancet Oncol. 2021, 22, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, C.; Song, Y.; Zhang, J.; Xu, J. Prognostic Role of Survivin in Patients with Glioma. Medicine 2018, 97, e0571. [Google Scholar] [CrossRef]
- Hammad, M.; Cornejo, Y.R.; Batalla-Covello, J.; Majid, A.A.; Burke, C.; Liu, Z.; Yuan, Y.-C.; Li, M.; Dellinger, T.H.; Lu, J.; et al. Neural Stem Cells Improve the Delivery of Oncolytic Chimeric Orthopoxvirus in a Metastatic Ovarian Cancer Model. Mol. Ther. Oncolytics 2020, 18, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Dighe, O.R.; Korde, P.; Bisen, Y.T.; Iratwar, S.; Kesharwani, A.; Vardhan, S.; Singh, A. Emerging Recombinant Oncolytic Poliovirus Therapies Against Malignant Glioma: A Review. Cureus 2023, 15, e34028. [Google Scholar] [CrossRef] [PubMed]
- Molfetta, R.; Zitti, B.; Lecce, M.; Milito, N.D.; Stabile, H.; Fionda, C.; Cippitelli, M.; Gismondi, A.; Santoni, A.; Paolini, R. CD155: A Multi-Functional Molecule in Tumor Progression. IJMS 2020, 21, 922. [Google Scholar] [CrossRef] [PubMed]
- Ghajar-Rahimi, G.; Kang, K.-D.; Totsch, S.K.; Gary, S.; Rocco, A.; Blitz, S.; Kachurak, K.; Chambers, M.R.; Li, R.; Beierle, E.A.; et al. Clinical Advances in Oncolytic Virotherapy for Pediatric Brain Tumors. Pharmacol. Ther. 2022, 239, 108193. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Z. IRES-Mediated Cap-Independent Translation, a Path Leading to Hidden Proteome. J. Mol. Cell Biol. 2019, 11, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Denniston, E.; Crewdson, H.; Rucinsky, N.; Stegman, A.; Remenar, D.; Moio, K.; Clark, B.; Higginbotham, A.; Keffer, R.; Brammer, S.; et al. The Practical Consideration of Poliovirus as an Oncolytic Virotherapy. Am. J. Virol. 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Walton, R.W.; Brown, M.C.; Sacco, M.T.; Gromeier, M. Engineered Oncolytic Poliovirus PVSRIPO Subverts MDA5-Dependent Innate Immune Responses in Cancer Cells. J. Virol. 2018, 92, e00879-e18. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Ferreira, T.; Kulkarni, A.; Bretscher, C.; Nazarov, P.; Hossain, J.; Ystaas, L.; Miletic, H.; Röth, R.; Niesler, B.; Marchini, A. Oncolytic H-1 Parvovirus Hijacks Galectin-1 to Enter Cancer Cells. Viruses 2022, 14, 1018. [Google Scholar] [CrossRef]
- Bretscher, C.; Marchini, A. H-1 Parvovirus as a Cancer-Killing Agent: Past, Present, and Future. Viruses 2019, 11, 562. [Google Scholar] [CrossRef]
- Geletneky, K.; Hajda, J.; Angelova, A.L.; Leuchs, B.; Capper, D.; Bartsch, A.J.; Neumann, J.-O.; Schöning, T.; Hüsing, J.; Beelte, B.; et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol. Ther. 2017, 25, 2620–2634. [Google Scholar] [CrossRef] [PubMed]
- Fields, B.N. Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Mercer, J.; Helenius, A. Vaccinia Virus Uses Macropinocytosis and Apoptotic Mimicry to Enter Host Cells. Science 2008, 320, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Béguin, J.; Foloppe, J.; Maurey, C.; Laloy, E.; Hortelano, J.; Nourtier, V.; Pichon, C.; Cochin, S.; Cordier, P.; Huet, H.; et al. Preclinical Evaluation of the Oncolytic Vaccinia Virus TG6002 by Translational Research on Canine Breast Cancer. Mol. Ther. Oncolytics 2020, 19, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Béguin, J.; Laloy, E.; Cochin, S.; Gantzer, M.; Farine, I.; Pichon, C.; Moreau, B.; Foloppe, J.; Balloul, J.-M.; Machon, C.; et al. Oncolytic Virotherapy with Intratumoral Injection of Vaccinia Virus TG6002 and 5-Fluorocytosine Administration in Dogs with Malignant Tumors. Mol. Ther. Oncolytics 2023, 30, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Koval, O.; Kochneva, G.; Tkachenko, A.; Troitskaya, O.; Sivolobova, G.; Grazhdantseva, A.; Nushtaeva, A.; Kuligina, E.; Richter, V. Recombinant Vaccinia Viruses Coding Transgenes of Apoptosis-Inducing Proteins Enhance Apoptosis but Not Immunogenicity of Infected Tumor Cells. BioMed Res. Int. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Semenov, D.V.; Fomin, A.S.; Kuligina, E.V.; Koval, O.A.; Matveeva, V.A.; Babkina, I.N.; Tikunova, N.V.; Richter, V.A. Recombinant Analogs of a Novel Milk Pro-Apoptotic Peptide, Lactaptin, and Their Effect on Cultured Human Cells. Protein J. 2010, 29, 174–180. [Google Scholar] [CrossRef]
- Koval, O.A.; Tkachenko, A.V.; Fomin, A.S.; Semenov, D.V.; Nushtaeva, A.A.; Kuligina, E.V.; Zavjalov, E.L.; Richter, V.A. Lactaptin Induces P53-Independent Cell Death Associated with Features of Apoptosis and Autophagy and Delays Growth of Breast Cancer Cells in Mouse Xenografts. PLoS ONE 2014, 9, e93921. [Google Scholar] [CrossRef]
- Rahman, M.M.; McFadden, G. Oncolytic Virotherapy with Myxoma Virus. JCM 2020, 9, 171. [Google Scholar] [CrossRef]
- Mercer, J.; Helenius, A. Virus Entry by Macropinocytosis. Nat. Cell Biol. 2009, 11, 510–520. [Google Scholar] [CrossRef]
- Aref, S.; Bailey, K.; Fielding, A. Measles to the Rescue: A Review of Oncolytic Measles Virus. Viruses 2016, 8, 294. [Google Scholar] [CrossRef]
- Hsu, E.C.; Sarangi, F.; Iorio, C.; Sidhu, M.S.; Udem, S.A.; Dillehay, D.L.; Xu, W.; Rota, P.A.; Bellini, W.J.; Richardson, C.D. A Single Amino Acid Change in the Hemagglutinin Protein of Measles Virus Determines Its Ability to Bind CD46 and Reveals Another Receptor on Marmoset B Cells. J. Virol. 1998, 72, 2905–2916. [Google Scholar] [CrossRef]
- Zeng, J.; Li, X.; Sander, M.; Zhang, H.; Yan, G.; Lin, Y. Oncolytic Viro-Immunotherapy: An Emerging Option in the Treatment of Gliomas. Front. Immunol. 2021, 12, 721830. [Google Scholar] [CrossRef]
- Dörig, R.E.; Marcil, A.; Chopra, A.; Richardson, C.D. The Human CD46 Molecule Is a Receptor for Measles Virus (Edmonston Strain). Cell 1993, 75, 295–305. [Google Scholar] [CrossRef]
- Letafati, A.; Ardekani, O.S.; Naderisemiromi, M.; Fazeli, M.M.; Jemezghani, N.A.; Yavarian, J. Oncolytic Viruses against Cancer, Promising or Delusion? Med. Oncol. 2023, 40, 246. [Google Scholar] [CrossRef]
- Mühlebach, M.D.; Mateo, M.; Sinn, P.L.; Prüfer, S.; Uhlig, K.M.; Leonard, V.H.J.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens Junction Protein Nectin-4 Is the Epithelial Receptor for Measles Virus. Nature 2011, 480, 530–533. [Google Scholar] [CrossRef]
- Melot, L.; Bankamp, B.; Rota, P.A.; Coughlin, M.M. Characterizing Infection of B Cells with Wild-Type and Vaccine Strains of Measles Virus. iScience 2023, 26, 107721. [Google Scholar] [CrossRef]
- DeRycke, M.S.; Pambuccian, S.E.; Gilks, C.B.; Kalloger, S.E.; Ghidouche, A.; Lopez, M.; Bliss, R.L.; Geller, M.A.; Argenta, P.A.; Harrington, K.M.; et al. Nectin 4 Overexpression in Ovarian Cancer Tissues and Serum: Potential Role as a Serum Biomarker. Am. J. Clin. Pathol. 2010, 134, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Fujiyuki, T.; Moritoh, K.; Akimoto, H.; Iizuka, K.; Sato, H.; Asano, K.; Yoneda, M.; Kai, C. Anti-Tumor Activity of a Recombinant Measles Virus against Canine Lung Cancer Cells. Sci. Rep. 2023, 13, 18168. [Google Scholar] [CrossRef]
- Allen, C.; Paraskevakou, G.; Liu, C.; Iankov, I.D.; Msaouel, P.; Zollman, P.; Myers, R.; Peng, K.W.; Russell, S.J.; Galanis, E. Oncolytic Measles Virus Strains in the Treatment of Gliomas. Expert Opin. Biol. Ther. 2008, 8, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Opyrchal, M.; Aderca, I.; Schroeder, M.A.; Sarkaria, J.N.; Domingo, E.; Federspiel, M.J.; Galanis, E. Oncolytic Measles Virus Strains Have Significant Antitumor Activity against Glioma Stem Cells. Gene Ther. 2013, 20, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Phuong, L.K.; Allen, C.; Peng, K.-W.; Giannini, C.; Greiner, S.; TenEyck, C.J.; Mishra, P.K.; Macura, S.I.; Russell, S.J.; Galanis, E.C. Use of a Vaccine Strain of Measles Virus Genetically Engineered to Produce Carcinoembryonic Antigen as a Novel Therapeutic Agent against Glioblastoma Multiforme. Cancer Res. 2003, 63, 2462–2469. [Google Scholar]
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral Vectored Vaccines: Design, Development, Preventive and Therapeutic Applications in Human Diseases. Signal Transduct. Target. Ther. 2023, 8, 149. [Google Scholar] [CrossRef]
- Wang, H.; Nan, F.; Zeng, Z.; Zhang, X.; Ke, D.; Zhang, S.; Zhou, X.; Niu, D.; Fan, T.; Jiang, S.; et al. Tumor Cell Vaccine Combined with Newcastle Disease Virus Promote Immunotherapy of Lung Cancer. J. Med. Virol. 2023, 95, e28554. [Google Scholar] [CrossRef]
- García-Romero, N.; Palacín-Aliana, I.; Esteban-Rubio, S.; Madurga, R.; Rius-Rocabert, S.; Carrión-Navarro, J.; Presa, J.; Cuadrado-Castano, S.; Sánchez-Gómez, P.; García-Sastre, A.; et al. Newcastle Disease Virus (NDV) Oncolytic Activity in Human Glioma Tumors Is Dependent on CDKN2A-Type I IFN Gene Cluster Codeletion. Cells 2020, 9, 1405. [Google Scholar] [CrossRef]
- Koks, C.A.; Garg, A.D.; Ehrhardt, M.; Riva, M.; Vandenberk, L.; Boon, L.; Vleeschouwer, S.D.; Agostinis, P.; Graf, N.; Van Gool, S.W. Newcastle Disease Virotherapy Induces Long-term Survival and Tumor-specific Immune Memory in Orthotopic Glioma through the Induction of Immunogenic Cell Death. Int. J. Cancer 2015, 136, E313–E325. [Google Scholar] [CrossRef]
- He, J.; An, Y.; Qi, J.; Cui, L.; Yang, K.; Liu, M.; Qu, B.; Yan, S.; Yin, J.; Jing, X.; et al. The Recombinant Newcastle Disease Virus Anhinga Strain Expressing Human TRAIL Exhibit Antitumor Effects on a Glioma Nude Mice Model. J. Med. Virol. 2021, 93, 3890–3898. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic Virus Therapy: A New Era of Cancer Treatment at Dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Li, Z.; Chiocca, E.A.; Caligiuri, M.A.; Yu, J. The Emerging Field of Oncolytic Virus-Based Cancer Immunotherapy. Trends Cancer 2023, 9, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Gujar, S.; Pol, J.G.; Kroemer, G. Heating It up: Oncolytic Viruses Make Tumors ‘Hot’ and Suitable for Checkpoint Blockade Immunotherapies. OncoImmunology 2018, 7, e1442169. [Google Scholar] [CrossRef] [PubMed]
- Woodell, A.S.; Landoni, E.; Valdivia, A.; Buckley, A.; Ogunnaike, E.A.; Dotti, G.; Hingtgen, S.D. Utilizing Induced Neural Stem Cell-based Delivery of a Cytokine Cocktail to Enhance Chimeric Antigen Receptor-modified T-cell Therapy for Brain Cancer. Bioeng. Transl. Med. 2023, 8, e10538. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-X.; Wang, S.-Q.; Lian, Z.-X.; Deng, S.-L.; Yu, K. Relationship between Tumor Infiltrating Immune Cells and Tumor Metastasis and Its Prognostic Value in Cancer. Cells 2022, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Voest, E.E.; Kenyon, B.M.; O’Reilly, M.S.; Truitt, G.; D’Amato, R.J.; Folkman, J. Inhibition of Angiogenesis In Vivo by Interleukin 12. JNCI J. Natl. Cancer Inst. 1995, 87, 581–586. [Google Scholar] [CrossRef]
- Asavarut, P.; Waramit, S.; Suwan, K.; Marais, G.J.K.; Chongchai, A.; Benjathummarak, S.; Al-Bahrani, M.; Vila-Gomez, P.; Williams, M.; Kongtawelert, P.; et al. Systemically Targeted Cancer Immunotherapy and Gene Delivery Using Transmorphic Particles. EMBO Mol. Med. 2022, 14, e15418. [Google Scholar] [CrossRef]
- Otani, T.; Nakamura, S.; Toki, M.; Motoda, R.; Kurimoto, M.; Orita, K. Identification of IFN-Gamma-Producing Cells in IL-12/IL-18-Treated Mice. Cell Immunol. 1999, 198, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Zwirner, N.W.; Ziblat, A. Regulation of NK Cell Activation and Effector Functions by the IL-12 Family of Cytokines: The Case of IL-27. Front. Immunol. 2017, 8, 25. [Google Scholar] [CrossRef]
- Omar, N.B.; Bentley, R.T.; Crossman, D.K.; Foote, J.B.; Koehler, J.W.; Markert, J.M.; Platt, S.R.; Rissi, D.R.; Shores, A.; Sorjonen, D.; et al. Safety and Interim Survival Data after Intracranial Administration of M032, a Genetically Engineered Oncolytic HSV-1 Expressing IL-12, in Pet Dogs with Sporadic Gliomas. Neurosurg. Focus. 2021, 50, E5. [Google Scholar] [CrossRef]
- Markert, J.M.; Cody, J.J.; Parker, J.N.; Coleman, J.M.; Price, K.H.; Kern, E.R.; Quenelle, D.C.; Lakeman, A.D.; Schoeb, T.R.; Palmer, C.A.; et al. Preclinical Evaluation of a Genetically Engineered Herpes Simplex Virus Expressing Interleukin-12. J. Virol. 2012, 86, 5304–5313. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.C.; Cassady, K.A.; Cody, J.J.; Parker, J.N.; Price, K.H.; Coleman, J.M.; Peggins, J.O.; Noker, P.E.; Powers, N.W.; Grimes, S.D.; et al. Evaluation of the Safety and Biodistribution of M032, an Attenuated Herpes Simplex Virus Type 1 Expressing hIL-12, After Intracerebral Administration to Aotus Nonhuman Primates. Human Gene Ther. Clin. Dev. 2014, 25, 16–27. [Google Scholar] [CrossRef]
- Cheema, T.A.; Wakimoto, H.; Fecci, P.E.; Ning, J.; Kuroda, T.; Jeyaretna, D.S.; Martuza, R.L.; Rabkin, S.D. Multifaceted Oncolytic Virus Therapy for Glioblastoma in an Immunocompetent Cancer Stem Cell Model. Proc. Natl. Acad. Sci. USA 2013, 110, 12006–12011. [Google Scholar] [CrossRef]
- Silvestre, R.N.; Eitler, J.; De Azevedo, J.T.C.; Tirapelle, M.C.; Fantacini, D.M.C.; De Souza, L.E.B.; Swiech, K.; Covas, D.T.; Calado, R.T.; Montero, P.O.; et al. Engineering NK-CAR.19 Cells with the IL-15/IL-15Rα Complex Improved Proliferation and Anti-Tumor Effect in Vivo. Front. Immunol. 2023, 14, 1226518. [Google Scholar] [CrossRef]
- Waldmann, T.A.; Tagaya, Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathoGENS. Annu. Rev. Immunol. 1999, 17, 19–49. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Lu, T.; Li, Z.; Teng, K.-Y.; Mansour, A.G.; Yu, M.; Tian, L.; Xu, B.; Ma, S.; Zhang, J.; et al. An Oncolytic Virus Expressing IL15/IL15Rα Combined with Off-the-Shelf EGFR-CAR NK Cells Targets Glioblastoma. Cancer Res. 2021, 81, 3635–3648. [Google Scholar] [CrossRef] [PubMed]
- Sarra, M.; Pallone, F.; Monteleone, G. Interleukin-21 in Chronic Inflammatory Diseases. BioFactors 2013, 39, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ding, X.; Liao, Q.; Gao, N.; Chen, Y.; Zhao, C.; Zhang, X.; Xu, J. IL-21 Arming Potentiates the Anti-Tumor Activity of an Oncolytic Vaccinia Virus in Monotherapy and Combination Therapy. J. Immunother. Cancer 2021, 9, e001647. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Anderson, G.P. Mini ReviewGM-CSF Biology. Growth Factors 2004, 22, 225–231. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Pala, L.; Conforti, F.; Cocorocchio, E. Talimogene Laherparepvec (T-VEC): An Intralesional Cancer Immunotherapy for Advanced Melanoma. Cancers 2021, 13, 1383. [Google Scholar] [CrossRef]
- Li, M.; Zhang, M.; Ye, Q.; Liu, Y.; Qian, W. Preclinical and Clinical Trials of Oncolytic Vaccinia Virus in Cancer Immunotherapy: A Comprehensive Review. Cancer Biol. Med. 2023, 20, 646–661. [Google Scholar] [CrossRef]
- Breitbach, C.J.; Burke, J.; Jonker, D.; Stephenson, J.; Haas, A.R.; Chow, L.Q.M.; Nieva, J.; Hwang, T.-H.; Moon, A.; Patt, R.; et al. Intravenous Delivery of a Multi-Mechanistic Cancer-Targeted Oncolytic Poxvirus in Humans. Nature 2011, 477, 99–102. [Google Scholar] [CrossRef]
- Heo, J.; Reid, T.; Ruo, L.; Breitbach, C.J.; Rose, S.; Bloomston, M.; Cho, M.; Lim, H.Y.; Chung, H.C.; Kim, C.W.; et al. Randomized Dose-Finding Clinical Trial of Oncolytic Immunotherapeutic Vaccinia JX-594 in Liver Cancer. Nat. Med. 2013, 19, 329–336. [Google Scholar] [CrossRef]
- Cripe, T.P.; Ngo, M.C.; Geller, J.I.; Louis, C.U.; Currier, M.A.; Racadio, J.M.; Towbin, A.J.; Rooney, C.M.; Pelusio, A.; Moon, A.; et al. Phase 1 Study of Intratumoral Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus, in Pediatric Cancer Patients. Mol. Ther. 2015, 23, 602–608. [Google Scholar] [CrossRef]
- Kochneva, G.; Sivolobova, G.; Tkacheva, A.; Grazhdantseva, A.; Troitskaya, O.; Nushtaeva, A.; Tkachenko, A.; Kuligina, E.; Richter, V.; Koval, O. Engineering of Double Recombinant Vaccinia Virus with Enhanced Oncolytic Potential for Solid Tumor Virotherapy. Oncotarget 2016, 7, 74171–74188. [Google Scholar] [CrossRef]
- Lisi, L. PI3K/AKT/mTOR Pathway in Tumor Progression of Oligodendrogliomas. Transl. Cancer Res. 2020, 9, 2161–2163. [Google Scholar] [CrossRef]
- Daniele, S.; Costa, B.; Zappelli, E.; Da Pozzo, E.; Sestito, S.; Nesi, G.; Campiglia, P.; Marinelli, L.; Novellino, E.; Rapposelli, S.; et al. Combined Inhibition of AKT/mTOR and MDM2 Enhances Glioblastoma Multiforme Cell Apoptosis and Differentiation of Cancer Stem Cells. Sci. Rep. 2015, 5, 9956. [Google Scholar] [CrossRef]
- Hernández Borrero, L.J.; El-Deiry, W.S. Tumor Suppressor P53: Biology, Signaling Pathways, and Therapeutic Targeting. Biochim. Biophys. Acta 2021, 1876, 188556. [Google Scholar] [CrossRef] [PubMed]
- Decraene, B.; Vanmechelen, M.; Clement, P.; Daisne, J.F.; Vanden Bempt, I.; Sciot, R.; Garg, A.D.; Agostinis, P.; De Smet, F.; De Vleeschouwer, S. Cellular and Molecular Features Related to Exceptional Therapy Response and Extreme Long-term Survival in Glioblastoma. Cancer Med. 2023, 12, 11107–11126. [Google Scholar] [CrossRef] [PubMed]
- Kyritsis, A.P.; Bondy, M.L.; Hess, K.R.; Cunningham, J.E.; Zhu, D.; Amos, C.J.; Yung, W.K.; Levin, V.A.; Bruner, J.M. Prognostic Significance of P53 Immunoreactivity in Patients with Glioma. Clin. Cancer Res. 1995, 1, 1617–1622. [Google Scholar]
- Fan, X.; Lu, H.; Cui, Y.; Hou, X.; Huang, C.; Liu, G. Overexpression of P53 Delivered Using Recombinant NDV Induces Apoptosis in Glioma Cells by Regulating the Apoptotic Signaling Pathway. Exp. Ther. Med. 2018, 15, 4522–4530. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Han, S.; Pan, D.; Liu, M.; Feng, X.; Dong, T.; Li, W.; Wei, X. Recombinant Adenoviral Vector Expressing Human Wild-Type P53, GM-CSF, and B7-1 Genes Suppresses the Growth of Glioma In Vivo. Tumor Biol. 2014, 35, 4411–4417. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Ravindran, J.; Prasad, S.; Pandey, M.K.; Aggarwal, B.B. Retraction: Gossypol Induces Death Receptor-5 through Activation of ROS-ERK-CHOP Pathway and Sensitizes Colon Cancer Cells to TRAIL. J. Biol. Chem. 2016, 291, 16923. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Venera, R.; Kapanova, G.; Tanbayeva, G.; Akhmetova, G.; Kudabayev, Y.; Turgambayeva, A. TRAIL-Mediated Signaling in Bladder Cancer: Realization of Clinical Efficacy of TRAIL-Based Therapeutics in Medical Oncology. Med. Oncol. 2023, 40, 236. [Google Scholar] [CrossRef]
- De Looff, M.; De Jong, S.; Kruyt, F.A.E. Multiple Interactions Between Cancer Cells and the Tumor Microenvironment Modulate TRAIL Signaling: Implications for TRAIL Receptor Targeted Therapy. Front. Immunol. 2019, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.K.; Rosewell-Shaw, A.; Suzuki, M. Modeling the Efficacy of Oncolytic Adenoviruses In Vitro and In Vivo: Current and Future Perspectives. Cancers 2020, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lang, F.; Xie, X.; Prabhu, S.; Xu, J.; Sampath, D.; Aldape, K.; Fuller, G.; Puduvalli, V.K. Efficacy of Adenovirally Expressed Soluble TRAIL in Human Glioma Organotypic Slice Culture and Glioma Xenografts. Cell Death Dis. 2011, 2, e121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shah, K. Encapsulated Stem Cells for Cancer Therapy. Biomatter 2013, 3, e24278. [Google Scholar] [CrossRef]
- Kim, K.-U.; Seo, S.-Y.; Heo, K.-Y.; Yoo, Y.-H.; Kim, H.-J.; Lee, H.-S.; Choi, S.-S.; Hwang, T.-H.; Lee, H.-J. Antitumor Activity of TRAIL Recombinant Adenovirus in Human Malignant Glioma Cells. J. Korean Med. Sci. 2005, 20, 1046. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsumae, M.; Nishiyama, J.; Kuroda, K. Intraoperative MR Imaging during Glioma Resection. Magn. Reson. Med. Sci. 2022, 21, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.P.; Watson, V.L.; Ryan, D.; Delfino, K.R.; Bekker, S.V.; Cozzens, J.W. Effects of 5-ALA Dose on Resection of Glioblastoma. J. Neuro-Oncol. 2019, 141, 523–531. [Google Scholar] [CrossRef]
- Hu, M.; Liao, X.; Tao, Y.; Chen, Y. Advances in Oncolytic Herpes Simplex Virus and Adenovirus Therapy for Recurrent Glioma. Front. Immunol. 2023, 14, 1285113. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Ou, A.; Yung, W.K.A.; Majd, N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. IJMS 2020, 22, 351. [Google Scholar] [CrossRef] [PubMed]
- Lipp, E.S.; Healy, P.; Austin, A.; Clark, A.; Dalton, T.; Perkinson, K.; Herndon, J.E.; Friedman, H.S.; Friedman, A.H.; Bigner, D.D.; et al. MGMT: Immunohistochemical Detection in High-Grade Astrocytomas. J. Neuropathol. Exp. Neurol. 2019, 78, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, L.E.; Von Wronski, M.A.; Bigner, S.H.; Rasheed, A.; Schold, S.C.; Brent, T.P.; Mitra, S.; Bigner, D.D. Expression of O. 6-Methylguanine-DNA Methyltransferase in Malignant Human Glioma Cell Lines. Carcinogenesis 1991, 12, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Ilkow, C.S.; Marguerie, M.; Batenchuk, C.; Mayer, J.; Ben Neriah, D.; Cousineau, S.; Falls, T.; Jennings, V.A.; Boileau, M.; Bellamy, D.; et al. Reciprocal Cellular Cross-Talk within the Tumor Microenvironment Promotes Oncolytic Virus Activity. Nat. Med. 2015, 21, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Kleijn, A.; Van Den Bossche, W.; Haefner, E.S.; Belcaid, Z.; Burghoorn-Maas, C.; Kloezeman, J.J.; Pas, S.D.; Leenstra, S.; Debets, R.; De Vrij, J.; et al. The Sequence of Delta24-RGD and TMZ Administration in Malignant Glioma Affects the Role of CD8+ T Cell Anti-Tumor Activity. Mol. Ther. Oncolytics 2017, 5, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.S.; Retz, M.; Gschwend, J.E.; Nawroth, R. YB-1-based virotherapy: A new therapeutic intervention for transitional cell carcinoma of the bladder? Urologe A 2016, 55, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.S.; Lage, H.; Bergmann, S.; Jürchott, K.; Glockzin, G.; Bernshausen, A.; Mantwill, K.; Ladhoff, A.; Wichert, A.; Mymryk, J.S.; et al. Multidrug-Resistant Cancer Cells Facilitate E1-Independent Adenoviral Replication. Cancer Res. 2004, 64, 322–328. [Google Scholar] [CrossRef][Green Version]
- Alkrekshi, A.; Wang, W.; Rana, P.S.; Markovic, V.; Sossey-Alaoui, K. A Comprehensive Review of the Functions of YB-1 in Cancer Stemness, Metastasis and Drug Resistance. Cell. Signal. 2021, 85, 110073. [Google Scholar] [CrossRef]
- Spiesschaert, B.; Angerer, K.; Park, J.; Wollmann, G. Combining Oncolytic Viruses and Small Molecule Therapeutics: Mutual Benefits. Cancers 2021, 13, 3386. [Google Scholar] [CrossRef]
- Burton, C.; Das, A.; McDonald, D.; Vandergrift, W.A.; Patel, S.J.; Cachia, D.; Bartee, E. Oncolytic Myxoma Virus Synergizes with Standard of Care for Treatment of Glioblastoma Multiforme. Oncolytic Virother. 2018, 7, 107–116. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, Y.; Hong, X.; Liu, X.; Su, X.; Li, S.; Dong, X.; Zhao, G.; Li, Y. Newcastle Disease Virus Enhances the Growth-Inhibiting and Proapoptotic Effects of Temozolomide on Glioblastoma Cells in Vitro and in Vivo. Sci. Rep. 2018, 8, 11470. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.; Leiser, D.; Weber, D.C. Oncological Outcomes, Long-Term Toxicities, Quality of Life and Sexual Health after Pencil-Beam Scanning Proton Therapy in Patients with Low-Grade Glioma. Cancers 2023, 15, 5287. [Google Scholar] [CrossRef]
- Karim, A.B.M.F.; Maat, B.; Hatlevoll, R.; Menten, J.; Rutten, E.H.J.M.; Thomas, D.G.T.; Mascarenhas, F.; Horiot, J.C.; Parvinen, L.M.; Van Reijn, M.; et al. A Randomized Trial on Dose-Response in Radiation Therapy of Low-Grade Cerebral Glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S.; Patil, C.G.; Chen, C.; Venteicher, A.S. Early versus Delayed Postoperative Radiotherapy for Treatment of Low-Grade Gliomas. Cochrane Database Syst. Rev. 2020, 1, CD009229. [Google Scholar] [CrossRef] [PubMed]
- Shoaf, M.L.; Desjardins, A. Oncolytic Viral Therapy for Malignant Glioma and Their Application in Clinical Practice. Neurotherapeutics 2022, 19, 1818–1831. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.D.; Kataoka, Y.; Advani, S.; Chung, S.M.; Arani, R.B.; Gillespie, G.Y.; Whitley, R.J.; Markert, J.M.; Roizman, B.; Weichselbaum, R.R. Ionizing Radiation Improves Survival in Mice Bearing Intracranial High-Grade Gliomas Injected with Genetically Modified Herpes Simplex Virus. Clin. Cancer Res. 1999, 5, 1517–1522. [Google Scholar] [PubMed]
- Gállego Pérez-Larraya, J.; Garcia-Moure, M.; Labiano, S.; Patiño-García, A.; Dobbs, J.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; Puigdelloses, M.; et al. Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma. N. Engl. J. Med. 2022, 386, 2471–2481. [Google Scholar] [CrossRef]
- Muthukutty, P.; Yoo, S.Y. Oncolytic Virus Engineering and Utilizations: Cancer Immunotherapy Perspective. Viruses 2023, 15, 1645. [Google Scholar] [CrossRef]
- Yun, C.-O.; Hong, J.; Yoon, A.-R. Current Clinical Landscape of Oncolytic Viruses as Novel Cancer Immunotherapeutic and Recent Preclinical Advancements. Front. Immunol. 2022, 13, 953410. [Google Scholar] [CrossRef]
- Kong, X.; Yu, D.; Wang, Z.; Li, S. Relationship between P53 Status and the Bioeffect of Ionizing Radiation. Oncol. Lett. 2021, 22, 661. [Google Scholar] [CrossRef]
- Alhaddad, L.; Osipov, A.N.; Leonov, S. The Molecular and Cellular Strategies of Glioblastoma and Non-Small-Cell Lung Cancer Cells Conferring Radioresistance. IJMS 2022, 23, 13577. [Google Scholar] [CrossRef]
- Liu, C.; Sarkaria, J.N.; Petell, C.A.; Paraskevakou, G.; Zollman, P.J.; Schroeder, M.; Carlson, B.; Decker, P.A.; Wu, W.; James, C.D.; et al. Combination of Measles Virus Virotherapy and Radiation Therapy Has Synergistic Activity in the Treatment of Glioblastoma Multiforme. Clin. Cancer Res. 2007, 13, 7155–7165. [Google Scholar] [CrossRef] [PubMed]
- Burchett, R.; Walsh, S.; Wan, Y.; Bramson, J.L. A Rational Relationship: Oncolytic Virus Vaccines as Functional Partners for Adoptive T Cell Therapy. Cytokine Growth Factor. Rev. 2020, 56, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Ghouzlani, A.; Kandoussi, S.; Tall, M.; Reddy, K.P.; Rafii, S.; Badou, A. Immune Checkpoint Inhibitors in Human Glioma Microenvironment. Front. Immunol. 2021, 12, 679425. [Google Scholar] [CrossRef]
- Caccese, M.; Indraccolo, S.; Zagonel, V.; Lombardi, G. PD-1/PD-L1 Immune-Checkpoint Inhibitors in Glioblastoma: A Concise Review. Crit. Rev. Oncol./Hematol. 2019, 135, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Harris-Bookman, S.; Mathios, D.; Martin, A.M.; Xia, Y.; Kim, E.; Xu, H.; Belcaid, Z.; Polanczyk, M.; Barberi, T.; Theodros, D.; et al. Expression of LAG-3 and Efficacy of Combination Treatment with anti-LAG-3 and anti-PD-1 Monoclonal Antibodies in Glioblastoma. Int. J. Cancer 2018, 143, 3201–3208. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Patel, M.A.; Mangraviti, A.; Kim, E.S.; Theodros, D.; Velarde, E.; Liu, A.; Sankey, E.W.; Tam, A.; Xu, H.; et al. Combination Therapy with Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clin. Cancer Res. 2017, 23, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.-H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; Vlahovic, G.; Lim, M.; Sahebjam, S.; Baehring, J.; Cloughesy, T.; Voloschin, A.; Ramkissoon, S.H.; Ligon, K.L.; Latek, R.; et al. Nivolumab with or without Ipilimumab in Patients with Recurrent Glioblastoma: Results from Exploratory Phase I Cohorts of CheckMate 143. Neuro-Oncology 2018, 20, 674–686. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients with Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003. [Google Scholar] [CrossRef]
- Samson, A.; Scott, K.J.; Taggart, D.; West, E.J.; Wilson, E.; Nuovo, G.J.; Thomson, S.; Corns, R.; Mathew, R.K.; Fuller, M.J.; et al. Intravenous Delivery of Oncolytic Reovirus to Brain Tumor Patients Immunologically Primes for Subsequent Checkpoint Blockade. Sci. Transl. Med. 2018, 10, eaam7577. [Google Scholar] [CrossRef]
- Hardcastle, J.; Mills, L.; Malo, C.S.; Jin, F.; Kurokawa, C.; Geekiyanage, H.; Schroeder, M.; Sarkaria, J.; Johnson, A.J.; Galanis, E. Immunovirotherapy with Measles Virus Strains in Combination with Anti–PD-1 Antibody Blockade Enhances Antitumor Activity in Glioblastoma Treatment. Neuro-Oncology 2016, 19, 493–502. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Mashouf, L.A.; Lim, M. CAR T Cell Therapy in Primary Brain Tumors: Current Investigations and the Future. Front. Immunol. 2022, 13, 817296. [Google Scholar] [CrossRef]
- Goff, S.L.; Morgan, R.A.; Yang, J.C.; Sherry, R.M.; Robbins, P.F.; Restifo, N.P.; Feldman, S.A.; Lu, Y.-C.; Lu, L.; Zheng, Z.; et al. Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor–Transduced T Cells Targeting EGFRvIII in Patients with Glioblastoma. J. Immunother. 2019, 42, 126–135. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor–Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094. [Google Scholar] [CrossRef]
- Fu, C.; Ma, T.; Zhou, L.; Mi, Q.-S.; Jiang, A. Dendritic Cell-Based Vaccines Against Cancer: Challenges, Advances and Future Opportunities. Immunol. Investig. 2022, 51, 2133–2158. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.J.; Zaupa, C.; Barnard, Z.; Maley, J.; Martuza, R.L.; Rabkin, S.D.; Curry, W.T. Combination Immunotherapy for Tumors via Sequential Intratumoral Injections of Oncolytic Herpes Simplex Virus 1 and Immature Dendritic Cells. Clin. Cancer Res. 2008, 14, 7711–7716. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Jamialahmadi, K.; Zamani, P.; Reza Jaafari, M. Improving the Efficacy of Peptide Vaccines in Cancer Immunotherapy. Int. Immunopharmacol. 2023, 123, 110721. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Zhang, Y.; Liu, Y.; Xie, J.; Wang, Y.; Hao, S.; Gao, Z. Heat Shock Protein Peptide Complex-96 Vaccination for Newly Diagnosed Glioblastoma: A Phase I, Single-Arm Trial. JCI Insight 2018, 3, e99145. [Google Scholar] [CrossRef] [PubMed]
- Bota, D.A.; Chung, J.; Dandekar, M.; Carrillo, J.A.; Kong, X.-T.; Fu, B.D.; Hsu, F.P.; Schönthal, A.H.; Hofman, F.M.; Chen, T.C.; et al. Phase II Study of ERC1671 plus Bevacizumab versus Bevacizumab plus Placebo in Recurrent Glioblastoma: Interim Results and Correlations with CD4+ T-Lymphocyte Counts. CNS Oncol. 2018, 7, CNS22. [Google Scholar] [CrossRef]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with Temozolomide for Patients with Newly Diagnosed, EGFRvIII-Expressing Glioblastoma (ACT IV): A Randomised, Double-Blind, International Phase 3 Trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- Boydell, E.; Marinari, E.; Migliorini, D.; Dietrich, P.-Y.; Patrikidou, A.; Dutoit, V. Exploratory Study of the Effect of IMA950/Poly-ICLC Vaccination on Response to Bevacizumab in Relapsing High-Grade Glioma Patients. Cancers 2019, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; Pongor, L.; Su, Y.-T.; Xi, L.; Raffeld, M.; Quezado, M.; Trepel, J.; Aldape, K.; Pommier, Y.; Wu, J. MGMT Status as a Clinical Biomarker in Glioblastoma. Trends Cancer 2020, 6, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Dhasmana, A.; Dhasmana, S.; Haque, S.; Cobos, E.; Yallapu, M.M.; Chauhan, S.C. Next-Generation Immune Checkpoint Inhibitors as Promising Functional Molecules in Cancer Therapeutics. Cancer Metastasis Rev. 2023, 42, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Jiao, M.; Xu, S.; Ismail, M.; Xie, X.; An, Y.; Guo, H.; Qian, R.; Shi, B.; Zheng, M. Brain-Targeted CRISPR/Cas9 Nanomedicine for Effective Glioblastoma Therapy. J. Control. Release 2022, 351, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Asmamaw Mengstie, M. Viral Vectors for the in Vivo Delivery of CRISPR Components: Advances and Challenges. Front. Bioeng. Biotechnol. 2022, 10, 895713. [Google Scholar] [CrossRef]
- Lindenboim, L.; Zohar, H.; Gundersen, G.G.; Worman, H.J.; Stein, R. LINC Complex Protein Nesprin-2 Has pro-Apoptotic Activity via Bcl-2 Family Proteins. Cell Death Discov. 2024, 10, 29. [Google Scholar] [CrossRef]
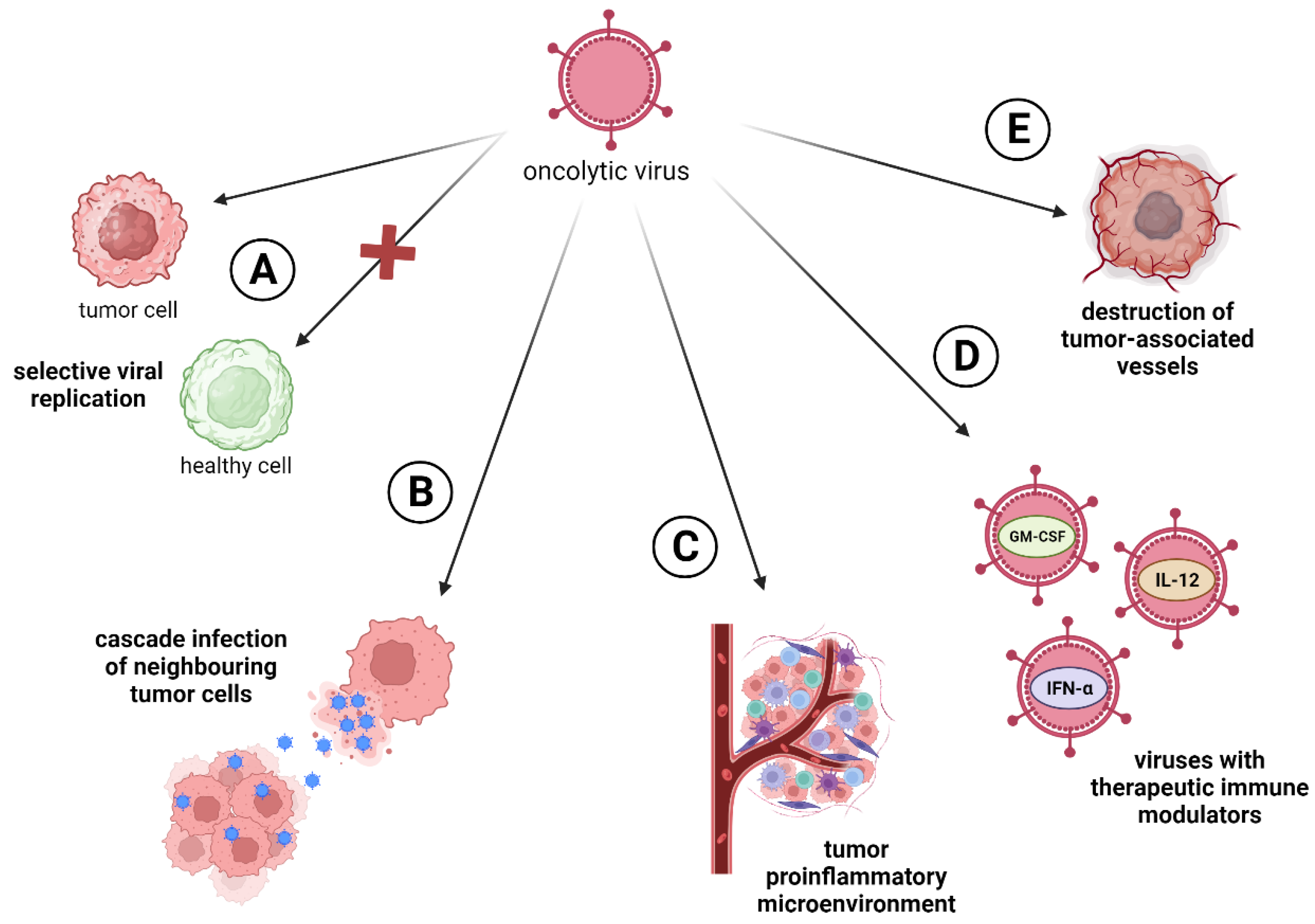
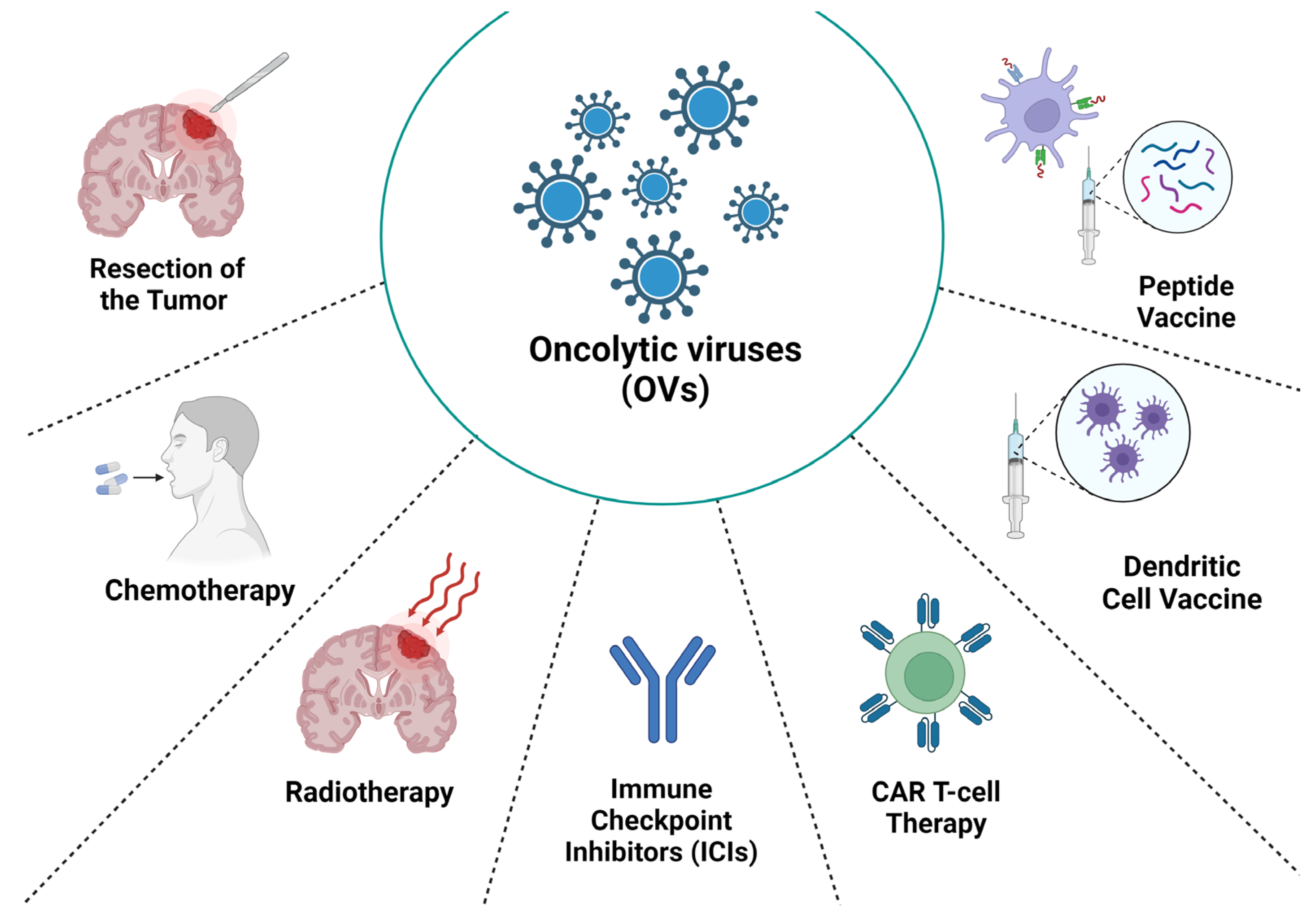

| Virus Type | Monotherapy | Resection of the Tumor | Chemotherapy | Radiotherapy | Immune Checkpoint Inhibitors (ICIs) | Adoptive Cell Therapy |
|---|---|---|---|---|---|---|
| Herpes Simplex Virus Type 1 (HSV-1) | G207 (NCT02457845) G47∆ (teserpaturev, DELYTACT) (UMIN-CTR Clinical Trial Registry UMIN000002661) [16] C134 (NCT03657576) | G207 [17] | G47∆ (etoposide), [18], (temozolomide) [19] | G207 (NCT02457845) (NCT04482933) | G47∆ [20] | |
| Adenovirus | DNX-2401 (Delta24-RGD, tasadenoturev) (NCT00805376) NSC-CRAd-S-pk7 (NCT05139056) | NSC-CRAd-S-pk7 (NCT03072134) | DNX-2401 [21,22] Ad-Delo3-RGD [23] ICOVIR-5 [24] NSC-CRAd-S-pk7 (NCT03072134) | DNX-2401 (NCT03178032) Ad5-Delta24RGD [25] ONYX-015 [26] AdV-tk [27] NSC-CRAd-S-pk7 (NCT03072134) | DNX-2401 (NCT02798406) | CXCL11-oAd [28] oAd-IL17 [29] |
| Poliovirus | PVSRIPO (NCT02986178) | PVSRIPO [30] | ||||
| Parvovirus | ParvOryx01 (NCT01301430) | |||||
| Vaccinia virus (VACV) | TG6002 [31] VV-GMCSF-Lact [32] | VVL∆TK-STC∆N1L-mIL21 [33] | vvDD-IL15Rα-YFP [34] | |||
| Myxoma virus (MYXM) | vMyxgfp [35] | vMyx-M011L-KO [36] | vMyx-IL15Rα-tdTr [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ageenko, A.; Vasileva, N.; Richter, V.; Kuligina, E. Combination of Oncolytic Virotherapy with Different Antitumor Approaches against Glioblastoma. Int. J. Mol. Sci. 2024, 25, 2042. https://doi.org/10.3390/ijms25042042
Ageenko A, Vasileva N, Richter V, Kuligina E. Combination of Oncolytic Virotherapy with Different Antitumor Approaches against Glioblastoma. International Journal of Molecular Sciences. 2024; 25(4):2042. https://doi.org/10.3390/ijms25042042
Chicago/Turabian StyleAgeenko, Alisa, Natalia Vasileva, Vladimir Richter, and Elena Kuligina. 2024. "Combination of Oncolytic Virotherapy with Different Antitumor Approaches against Glioblastoma" International Journal of Molecular Sciences 25, no. 4: 2042. https://doi.org/10.3390/ijms25042042
APA StyleAgeenko, A., Vasileva, N., Richter, V., & Kuligina, E. (2024). Combination of Oncolytic Virotherapy with Different Antitumor Approaches against Glioblastoma. International Journal of Molecular Sciences, 25(4), 2042. https://doi.org/10.3390/ijms25042042





