Long-Term Effects of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease: Predictors of Treatment Response and Safety over 6 Years of Continuous Therapy
Abstract
1. Introduction
2. Results
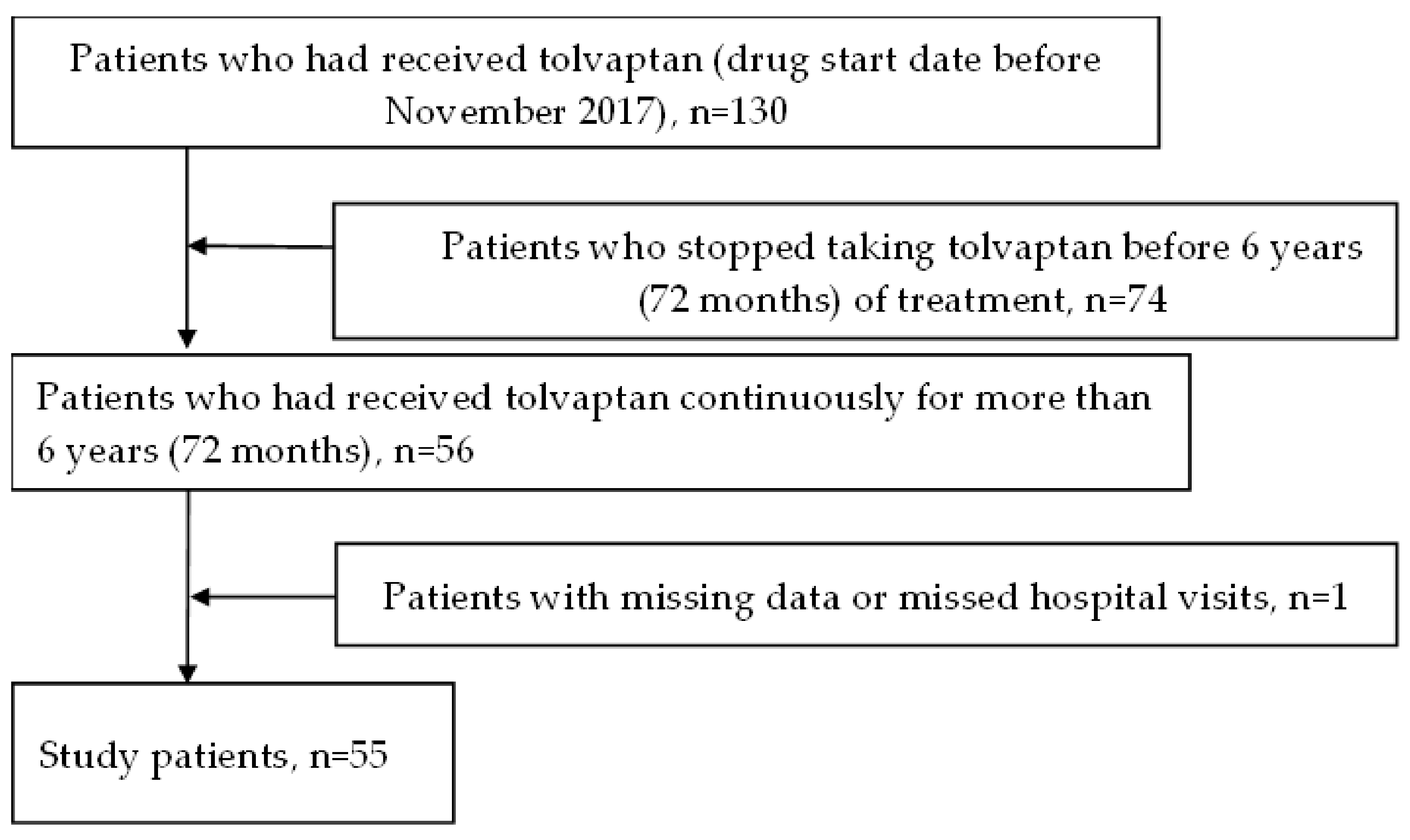
2.1. Patient Baseline Characteristics
2.2. Tolvaptan Dosing
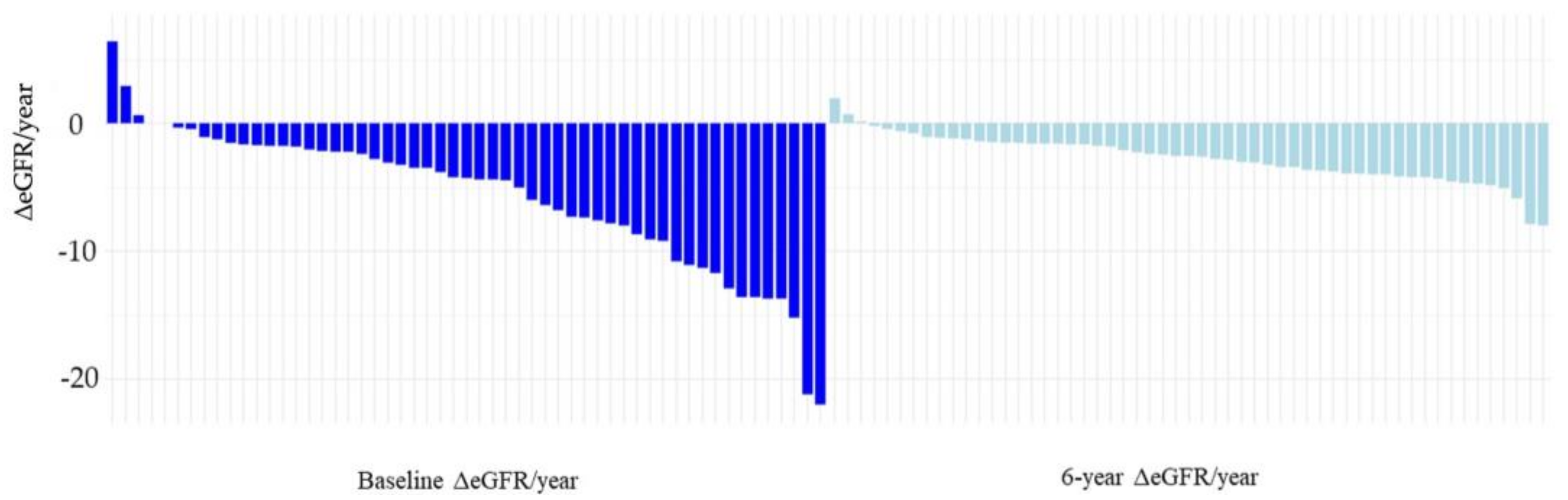
2.3. Effect of Tolvaptan on Renal Function
2.4. Predicted 6-Year eGFR and Actual 6-Year eGFR
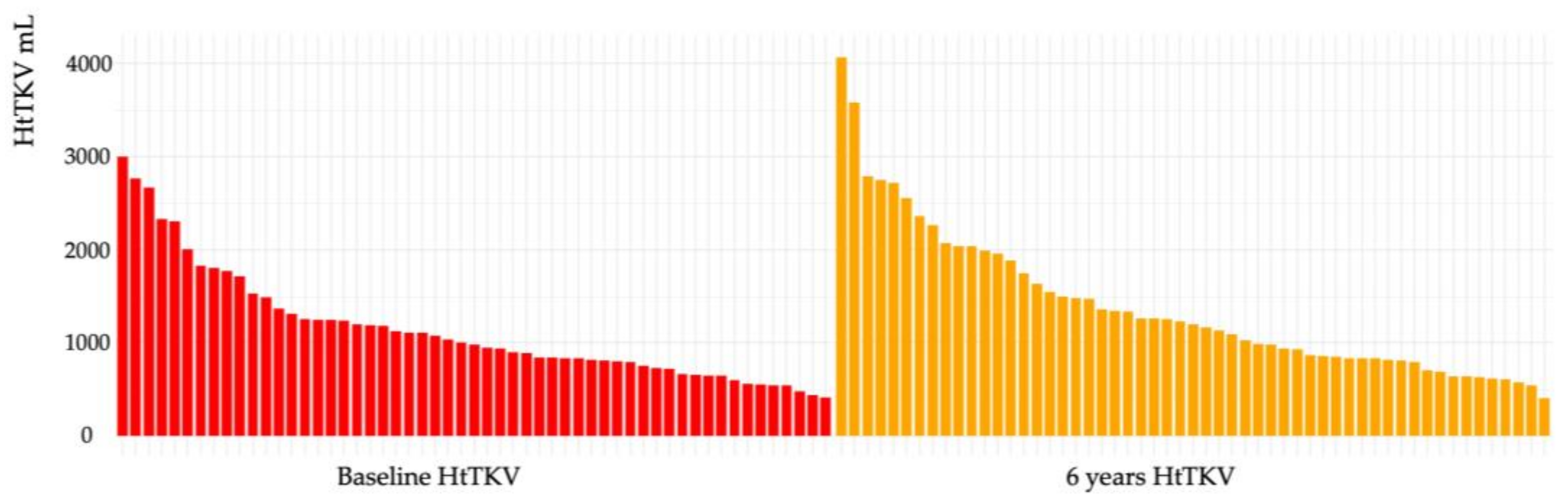
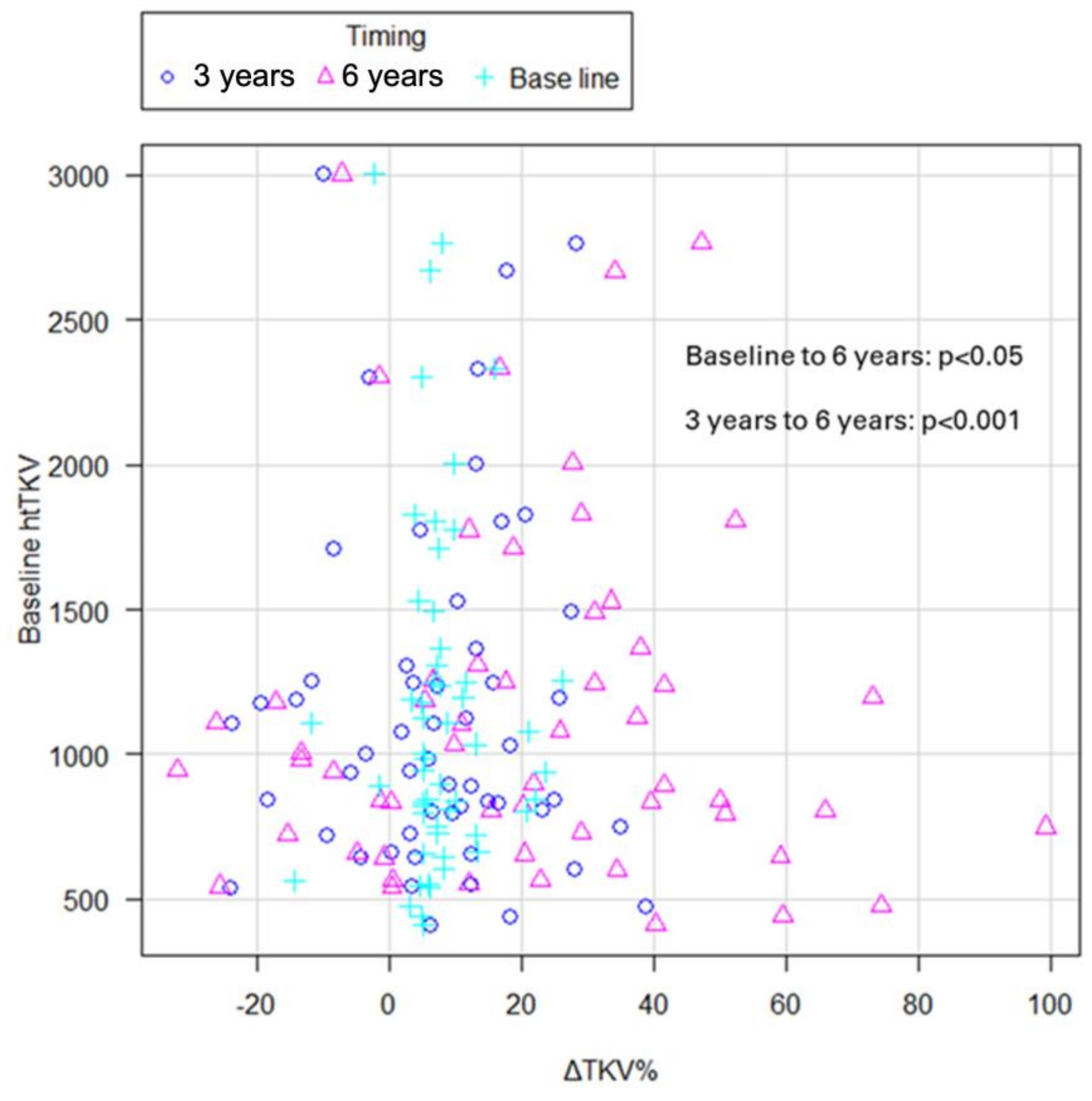
2.5. Effects of Tolvaptan on TKV
2.6. Analysis of Predictors of Treatment Efficacy of Tolvaptan
2.7. Safety and Long-Term Tolerability of Tolvaptan
3. Discussion
4. Materials and Methods
4.1. Study Population and Parameters
4.2. Data Collection and Calculations
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornec-Le Gall, E.; Alam, A.; Perrone, R.D. Autosomal dominant polycystic kidney disease. Lancet 2019, 393, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Gabow, P.A. Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 1993, 329, 332–342. [Google Scholar] [CrossRef]
- Grantham, J.J.; Torres, V.E.; Chapman, A.B.; Guay-Woodford, L.M.; Bae, K.T.; King, B.F.J.; Wetzel, L.H.; Baumgarten, D.A.; Kenney, P.J.; Harris, P.C.; et al. Volume Progression in Polycystic Kidney Disease. N. Engl. J. Med. 2006, 354, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J.J.; Chapman, A.B.; Torres, V.E. Volume progression in autosomal dominant polycystic kidney disease: The major factor determining clinical outcomes. Clin. J. Am. Soc. Nephrol. 2006, 1, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S.; et al. Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Koch, G.; Ouyang, J.; McQuade, R.D.; Blais, J.D.; Czerwiec, F.S.; et al. Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2017, 377, 1930–1942. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Davenport, E.; Ouyang, J.; Hoke, M.E.; Garbinsky, D.; Agarwal, I.; Krasa, H.B.; Oberdhan, D. Pooled Data Analysis of the Long-Term Treatment Effects of Tolvaptan in ADPKD. Kidney Int. Rep. 2022, 7, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Estilo, A.; Tracy, L.; Matthews, C.; Riggen, M.; Stemhagen, A.; Wilt, T.; Krakovich, A.; Jones-Burton, C.; George, V.; McQuade, R.; et al. Evaluating the impact of a Risk Evaluation and Mitigation Strategy with tolvaptan to monitor liver safety in patients with autosomal dominant polycystic kidney disease. Clin. Kidney J. 2022, 15, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.E.; Chebib, F.T.; Irazabal, M.V.; Ofstie, T.G.; Bungum, L.A.; Metzger, A.J.; Senum, S.R.; Hogan, M.C.; El-Zoghby, Z.M.; Kline, T.L.; et al. Long-Term Administration of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2018, 13, 1153–1161, Erratum in Clin. J. Am. Soc. Nephrol. 2019, 14, 910. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Lee, J.; Hoke, M.E.; Estilo, A.; Sergeyeva, O. Multicenter Study of Long-Term Safety of Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2020, 16, 48–58. [Google Scholar] [CrossRef]
- Torres, V.E.; Gansevoort, R.T.; Perrone, R.D.; Chapman, A.B.; Ouyang, J.; Lee, J.; Japes, H.; Nourbakhsh, A.; Wang, T. Tolvaptan in ADPKD Patients with Very Low Kidney Function. Kidney Int. Rep. 2021, 6, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Muto, S.; Kawano, H.; Higashihara, E.; Narita, I.; Ubara, Y.; Matsuzaki, T.; Ouyang, J.; Torres, V.E.; Horie, S. The effect of tolvaptan on autosomal dominant polycystic kidney disease patients: A subgroup analysis of the Japanese patient subset from TEMPO 3:4 trial. Clin. Exp. Nephrol. 2015, 19, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Devuyst, O.; Chapman, A.B.; Gansevoort, R.T.; Perrone, R.D.; Ouyang, J.; Blais, J.D.; Czerwiec, F.S.; Sergeyeva, O.; REPRISE Trial Investigators. Rationale and Design of a Clinical Trial Investigating Tolvaptan Safety and Efficacy in Autosomal Dominant Polycystic Kidney Disease. Am. J. Nephrol. 2017, 45, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Dandurand, A.; Ouyang, J.; Czerwiec, F.S.; Blais, J.D.; TEMPO 4:4 Trial Investigators. Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: The TEMPO 4:4 Trial. Nephrol. Dial. Transplant. 2017, 33, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Irazabal, M.V.; Rangel, L.J.; Bergstralh, E.J.; Osborn, S.L.; Harmon, A.J.; Sundsbak, J.L.; Bae, K.T.; Chapman, A.B.; Grantham, J.J.; Mrug, M.; et al. Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J. Am. Soc. Nephrol. 2015, 26, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.-U.; Messchendorp, A.L.; Birn, H.; Capasso, G.; Gall, E.C.-L.; Devuyst, O.; van Eerde, A.; Guirchoun, P.; Harris, T.; Hoorn, E.J.; et al. An update on the use of tolvaptan for autosomal dominant polycystic kidney disease: Consensus statement on behalf of the ERA Working Group on Inherited Kidney Disorders, the European Rare Kidney Disease Reference Network and Polycystic Kidney Disease International. Nephrol. Dial. Transplant. 2022, 37, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kawano, H.; Muto, S.; Muramoto, N.; Takano, T.; Lu, Y.; Eguchi, H.; Wada, H.; Okazaki, Y.; Ide, H.; et al. PKD1 Mutation Is a Biomarker for Autosomal Dominant Polycystic Kidney Disease. Biomolecules 2023, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
- Gall, E.C.-L.; Audrézet, M.-P.; Rousseau, A.; Hourmant, M.; Renaudineau, E.; Charasse, C.; Morin, M.-P.; Moal, M.-C.; Dantal, J.; Wehbe, B.; et al. The PROPKD Score: A New Algorithm to Predict Renal Survival in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 942–951. [Google Scholar] [CrossRef]
- Spithoven, E.M.; Kramer, A.; Meijer, E.; Orskov, B.; Wanner, C.; Caskey, F.; Collart, F.; Finne, P.; Fogarty, D.G.; Groothoff, J.W.; et al. Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int. 2014, 86, 1244–1252. [Google Scholar] [CrossRef]
- Chebib, F.T.; Torres, V.E. Assessing Risk of Rapid Progression in Autosomal Dominant Polycystic Kidney Disease and Special Considerations for Disease-Modifying Therapy. Am. J. Kidney Dis. 2021, 78, 282–292. [Google Scholar] [CrossRef]
- Choi, H.S.; Han, K.-D.; Oh, T.R.; Suh, S.H.; Kim, M.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Trends in the incidence and prevalence of end-stage renal disease with hemodialysis in entire Korean population: A nationwide population-based study. Medicine 2021, 100, e25293. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.R.; Dent, H.; McDonald, S.P.; Rangan, G.K. Incidence and survival of end-stage kidney disease due to polycystic kidney disease in Australia and New Zealand (1963–2014). Popul. Health Metr. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.K.; Ryu, H.; Ahn, C.; Park, H.C.; Ma, Y.; Xu, D.; Ecder, T.; Kao, T.-W.; Huang, J.-W.; Rangan, G.K.; et al. Clinical Characteristics of Rapid Progression in Asia-Pacific Patients With ADPKD. Kidney Int. Rep. 2023, 8, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, J.; Furlano, M.; Torres, F.; Hernandez, J.; Pybus, M.; Ejarque, L.; Cordoba, C.; Guirado, L.; Ars, E.; Torra, R. Comparative analysis of tools to predict rapid progression in autosomal dominant polycystic kidney disease. Clin. Kidney J. 2021, 15, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Tsuchiya, K.; Nakatani, S.; Muto, S.; Mochizuki, T.; Kawano, H.; Hanaoka, K.; Hidaka, S.; Ichikawa, D.; Ishikawa, E.; et al. A digest from evidence-based Clinical Practice Guideline for Polycystic Kidney Disease 2020. Clin. Exp. Nephrol. 2021, 25, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Solazzo, A.; Testa, F.; Giovanella, S.; Busutti, M.; Furci, L.; Carrera, P.; Ferrari, M.; Ligabue, G.; Mori, G.; Leonelli, M.; et al. The prevalence of autosomal dominant polycystic kidney disease (ADPKD): A meta-analysis of European literature and prevalence evaluation in the Italian province of Modena suggest that ADPKD is a rare and underdiagnosed condition. PLoS ONE 2018, 13, e0190430. [Google Scholar] [CrossRef] [PubMed]
- Gimpel, C.; Bergmann, C.; Bockenhauer, D.; Breysem, L.; Cadnapaphornchai, M.A.; Cetiner, M.; Dudley, J.; Emma, F.; Konrad, M.; Harris, T.; et al. International consensus statement on the diagnosis and management of autosomal dominant polycystic kidney disease in children and young people. Nat. Rev. Nephrol. 2019, 15, 713–726. [Google Scholar] [CrossRef]
- Iseki, K.; Ikemiya, Y.; Kinjo, K.; Inoue, T.; Iseki, C.; Takishita, S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004, 65, 1870–1876. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Song, Y.; Caballero, B.; Cheskin, L. Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int. 2008, 73, 19–33. [Google Scholar] [CrossRef]
- Rowe, I.; Chiaravalli, M.; Mannella, V.; Ulisse, V.; Quilici, G.; Pema, M.; Song, X.W.; Xu, H.; Mari, S.; Qian, F.; et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 2013, 19, 488–493. [Google Scholar] [CrossRef]
- Riwanto, M.; Kapoor, S.; Rodriguez, D.; Edenhofer, I.; Segerer, S.; Wüthrich, R.P. Inhibition of Aerobic Glycolysis Attenuates Disease Progression in Polycystic Kidney Disease. PLoS ONE 2016, 11, e0146654. [Google Scholar] [CrossRef] [PubMed]
- Warner, G.; Hein, K.Z.; Nin, V.; Edwards, M.; Chini, C.C.; Hopp, K.; Harris, P.C.; Torres, V.E.; Chini, E.N. Food Restriction Ameliorates the Development of Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2015, 27, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Kipp, K.R.; Rezaei, M.; Lin, L.; Dewey, E.C.; Weimbs, T.; Kruger, S.L.; Schimmel, M.F.; Parker, N.; Shillingford, J.M.; Leamon, C.P.; et al. A mild reduction of food intake slows disease progression in an orthologous mouse model of polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2016, 310, F726–F731. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Abebe, K.Z.; Perrone, R.D.; Torres, V.E.; Braun, W.E.; Steinman, T.I.; Winklhofer, F.T.; Brosnahan, G.; Czarnecki, P.G.; Hogan, M.C.; et al. Blood Pressure in Early Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2014, 371, 2255–2266. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.L.; You, Z.; Gitomer, B.; Brosnahan, G.; Torres, V.E.; Chapman, A.B.; Perrone, R.D.; Steinman, T.I.; Abebe, K.Z.; Rahbari-Oskoui, F.F.; et al. Overweight and Obesity Are Predictors of Progression in Early Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.L.; Steele, C.; Gitomer, B.; Wang, W.; Ouyang, J.; Chonchol, M.B. Overweight and Obesity and Progression of ADPKD. Clin. J. Am. Soc. Nephrol. 2021, 16, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.; Hein, K.Z.; Chini, C.C.; Lika, J.; Warner, G.M.; Bale, L.K.; Torres, V.E.; Harris, P.C.; Oxvig, C.; Conover, C.A.; et al. Metalloproteinase PAPP-A regulation of IGF-1 contributes to polycystic kidney disease pathogenesis. JCI Insight 2020, 5, e135700. [Google Scholar] [CrossRef]
- Watkins, P.B.; Lewis, J.H.; Kaplowitz, N.; Alpers, D.H.; Blais, J.D.; Smotzer, D.M.; Krasa, H.; Ouyang, J.; Torres, V.E.; Czerwiec, F.S.; et al. Clinical Pattern of Tolvaptan-Associated Liver Injury in Subjects with Autosomal Dominant Polycystic Kidney Disease: Analysis of Clinical Trials Database. Drug Saf. 2015, 38, 1103–1113. [Google Scholar] [CrossRef]
- Endo, M.; Katayama, K.; Matsuo, H.; Horiike, S.; Nomura, S.; Hayashi, A.; Ishikawa, E.; Harada, T.; Sugimoto, R.; Tanemura, A.; et al. Role of Liver Transplantation in Tolvaptan-Associated Acute Liver Failure. Kidney Int. Rep. 2019, 4, 1653–1657. [Google Scholar] [CrossRef]
- Combs, S.; Berl, T. Dysnatremias in Patients with Kidney Disease. Am. J. Kidney Dis. 2014, 63, 294–303. [Google Scholar] [CrossRef]
- Sterns, R.H.; Rondon-Berrios, H.; Adrogué, H.J.; Berl, T.; Burst, V.; Cohen, D.M.; Christ-Crain, M.; Cuesta, M.; Decaux, G.; Emmett, M.; et al. Treatment Guidelines for Hyponatremia: Stay the Course. Clin. J. Am. Soc. Nephrol. 2023, 19, 129–135. [Google Scholar] [CrossRef]
- Adrogué, H.J.; Tucker, B.M.; Madias, N.E. Diagnosis and Management of Hyponatremia: A Review. JAMA 2022, 328, 280–291. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
| Patient Characteristics | ||
|---|---|---|
| Patients | n (%) | 55 (100) |
| Age | Average (IQR) | 46.69 (24–72) |
| Sex | ||
| Female | n (%) | 15 (27.3) |
| Male | n (%) | 40 (72.7) |
| Height, m | average (IQR) | 1.69 (1.52–1.89) |
| BMI, kg/m2 | average (IQR) | 23.2 (17.7–32.5) |
| History of cerebral hemorrhage | n (%) | 4 (7.2) |
| Complications of liver cysts | n (%) | 51 (92.7) |
| History of heart valve disease | n (%) | 14 (25.4) |
| Family history of ADPKD | n (%) | 44 (80) |
| Genetic diagnosis | ||
| unknown or untested | n (%) | 15 (27.2) |
| PKD1 Truncated | n (%) | 22 (40) |
| PKD1 Non-Truncated | n (%) | 7 (12.7) |
| PKD2 Truncated | n (%) | 7 (12.7) |
| PKD2 Non-Truncated | n (%) | 4 (7.2) |
| Mayo subclass | ||
| Class 1A | n (%) | 0 (0) |
| Class 1B | n (%) | 11 (20) |
| Class 1C | n (%) | 17 (30.9) |
| Class 1D | n (%) | 19 (34.5) |
| Class 1E | n (%) | 8 (14.5) |
| Patient Parameters | Baseline | After 6 Years (72 Months) | |
|---|---|---|---|
| Comorbidities | |||
| Hypertension a | n (%) | 47 (85.4) | 49 (89.0) |
| Hyperuricemia b | n (%) | 17 (30.9) | 29 (59.7) |
| Hyperlipidemia c | n (%) | 18 (32.7) | 21 (38.1) |
| Cerebral aneurysm | n (%) | 12 (21.8) | 13 (23.6) |
| Malignant tumor | n (%) | 5 (9.0) | 8 (14.5) |
| Urologic d event | n (%) | 3 (5.4) | 3 (5.4) |
| eGFR, mL/min/1.73 m2 | average (IQR) | 55.5 (24.6–112.7) | 36.58 (6.2–81.9) |
| ΔeGFR/year, mL/min/1.73 m2 | average (IQR) | −4.29(−22–6.4) | −2.72 (−7.9–1.9) |
| TKV, mL | average (IQR) | 1935.49 (710.7–5255.5) | 2349.48 (682.2–6508.3) |
| HtTKV, mL/m | average (IQR) | 1146.61 (412–3003.1) | 1390.94 (406.0–4067.6) |
| ΔTKV%/year | average (IQR) | 7.64 (−14.2–26.1) | 20.74 (−32.0–74.3) |
| Odds Ratio | Lower 95% CI | Upper 95% CI | p-Value | Vif | |
|---|---|---|---|---|---|
| (Intercept) | 13.643 | 0.862 | 26.424 | 0.0370 | − |
| Mayo classification a | 1.939 | −1.529 | 5.407 | 0.2670 | 1.055886 |
| Tolvaptan b mg/day | 2.948 | −0.127 | 6.022 | 0.0600 | 1.069827 |
| Baseline BMI | −0.570 | −1.073 | −0.067 | 0.0271 * | 1.016598 |
| Family History | −3.607 | −7.118 | −0.095 | 0.0444 * | 1.082779 |
| Hypertension c | 2.196 | −1.745 | 6.138 | 0.2680 | 1.059599 |
| Adjusted R2:0.81 | * = p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, M.; Kawano, H.; Miyoshi, M.; Kimura, T.; Takahashi, K.; Muto, S.; Horie, S. Long-Term Effects of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease: Predictors of Treatment Response and Safety over 6 Years of Continuous Therapy. Int. J. Mol. Sci. 2024, 25, 2088. https://doi.org/10.3390/ijms25042088
Yamazaki M, Kawano H, Miyoshi M, Kimura T, Takahashi K, Muto S, Horie S. Long-Term Effects of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease: Predictors of Treatment Response and Safety over 6 Years of Continuous Therapy. International Journal of Molecular Sciences. 2024; 25(4):2088. https://doi.org/10.3390/ijms25042088
Chicago/Turabian StyleYamazaki, Mai, Haruna Kawano, Miho Miyoshi, Tomoki Kimura, Keiji Takahashi, Satoru Muto, and Shigeo Horie. 2024. "Long-Term Effects of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease: Predictors of Treatment Response and Safety over 6 Years of Continuous Therapy" International Journal of Molecular Sciences 25, no. 4: 2088. https://doi.org/10.3390/ijms25042088
APA StyleYamazaki, M., Kawano, H., Miyoshi, M., Kimura, T., Takahashi, K., Muto, S., & Horie, S. (2024). Long-Term Effects of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease: Predictors of Treatment Response and Safety over 6 Years of Continuous Therapy. International Journal of Molecular Sciences, 25(4), 2088. https://doi.org/10.3390/ijms25042088






