Roles of the Caspase-11 Non-Canonical Inflammasome in Rheumatic Diseases
Abstract
:1. Introduction
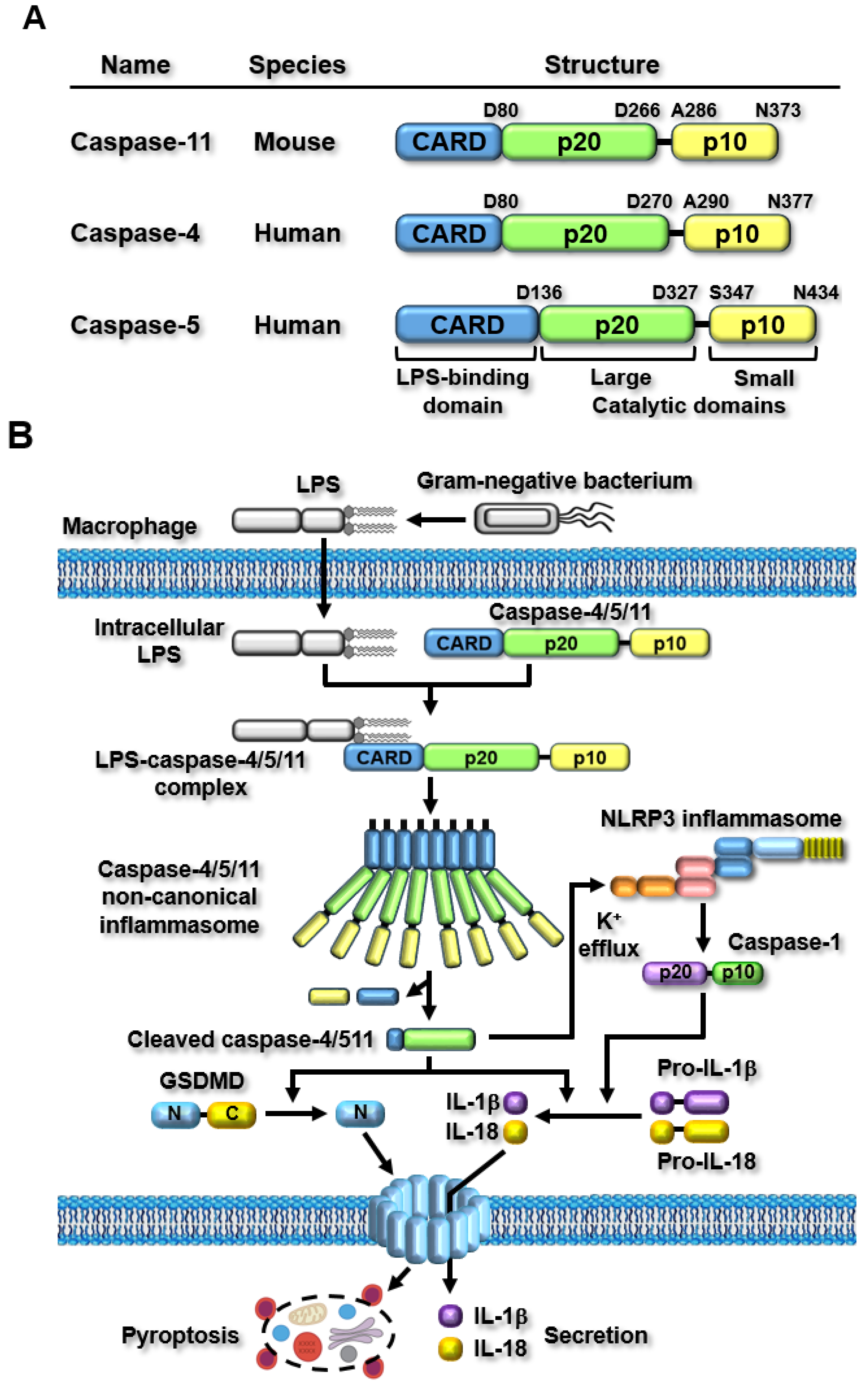
2. Caspase-11 Non-Canonical Inflammasome
2.1. Structure of Caspase-11 Non-Canonical Inflammasome
2.2. Caspase-11 Non-Canonical Inflammasome-Activated Inflammatory Signaling Pathways
3. The Roles of Caspase-11 Non-Canonical Inflammasome in Rheumatic Diseases
3.1. Inflammatory Arthritis—Rheumatoid Arthritis (RA) and Gouty Arthritis (GA)
3.2. Osteoarthritis (OA)
3.3. Systemic Lupus Erythematosus (SLE)
3.4. Sjögren’s Syndrome (SjS)
4. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RA | Rheumatoid arthritis |
| GA | Gouty arthritis |
| OA | Osteoarthritis |
| SLE | Systemic lupus erythematosus |
| SjS | Sjögren’s syndrome |
| PAMP | Pathogen-associated molecular pattern |
| DAMP | Damage-associated molecular pattern |
| PRR | Pattern-recognition receptor |
| NLR | Nucleotide oligomerization domain-like receptor |
| GSDMD | Gasdermin D |
| LPS | Lipopolysaccharide |
| RASF | Rheumatoid arthritis synovial fibroblast |
| ACLT | Anterior cruciate ligament transection |
References
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef]
- Yi, Y.S. Functional crosstalk between non-canonical caspase-11 and canonical NLRP3 inflammasomes during infection-mediated inflammation. Immunology 2020, 159, 142–155. [Google Scholar] [CrossRef]
- Barnett, K.C.; Li, S.; Liang, K.; Ting, J.P. A 360 degrees view of the inflammasome: Mechanisms of activation, cell death, and diseases. Cell 2023, 186, 2288–2312. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Enosi Tuipulotu, D.; Tan, W.H.; Kay, C.; Man, S.M. Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol. 2019, 40, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Kanneganti, T.D. Inflammasomes and the fine line between defense and disease. Curr. Opin. Immunol. 2020, 62, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Vande Walle, L.; Lamkanfi, M. Therapeutic modulation of inflammasome pathways. Immunol. Rev. 2020, 297, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Kayagaki, N.; Wong, M.T.; Stowe, I.B.; Ramani, S.R.; Gonzalez, L.C.; Akashi-Takamura, S.; Miyake, K.; Zhang, J.; Lee, W.P.; Muszynski, A.; et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 2013, 341, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Hagar, J.A.; Powell, D.A.; Aachoui, Y.; Ernst, R.K.; Miao, E.A. Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science 2013, 341, 1250–1253. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Yi, Y.S. Caspase-11 non-canonical inflammasome: A critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology 2017, 152, 207–217. [Google Scholar] [CrossRef]
- Shrestha, S.; Hong, C.W. Extracellular Mechanisms of Neutrophils in Immune Cell Crosstalk. Immune Netw. 2023, 23, e38. [Google Scholar] [CrossRef]
- Bulte, D.; Rigamonti, C.; Romano, A.; Mortellaro, A. Inflammasomes: Mechanisms of Action and Involvement in Human Diseases. Cells 2023, 12, 1766. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. MicroRNA-mediated epigenetic regulation of inflammasomes in inflammatory responses and immunopathologies. Semin. Cell Dev. Biol. 2024, 154, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Byun, D.J.; Lee, J.; Yu, J.W.; Hyun, Y.M. NLRP3 Exacerbate NETosis-Associated Neuroinflammation in an LPS-Induced Inflamed Brain. Immune Netw. 2023, 23, e27. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xie, F.; Zhou, X.; Wu, Y.; Yan, H.; Liu, T.; Huang, J.; Wang, F.; Zhou, F.; Zhang, L. Role of pyroptosis in inflammation and cancer. Cell Mol. Immunol. 2022, 19, 971–992. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Liu, B.; Zhang, Y.; Pan, X.; Yu, X.Y.; Shen, Z.; Song, Y.H. Inflammasomes as therapeutic targets in human diseases. Signal Transduct. Target. Ther. 2021, 6, 247. [Google Scholar] [CrossRef]
- Yi, Y.S. Potential benefits of ginseng against COVID-19 by targeting inflammasomes. J. Ginseng Res. 2022, 46, 722–730. [Google Scholar] [CrossRef]
- Yi, Y.S. Regulatory Roles of the Caspase-11 Non-Canonical Inflammasome in Inflammatory Diseases. Immune Netw. 2018, 18, e41. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, E.; Yi, Y.S. Korean Red Ginseng Saponins Play an Anti-Inflammatory Role by Targeting Caspase-11 Non-Canonical Inflammasome in Macrophages. Int. J. Mol. Sci. 2023, 24, 77. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, D.J.; Yi, Y.S. Anti-inflammatory activity of calmodulin-lysine N-methyltransferase through suppressing the caspase-11 non-canonical inflammasome. Immunobiology 2023, 228, 152758. [Google Scholar] [CrossRef]
- Kim, Y.B.; Cho, H.J.; Yi, Y.S. Anti-inflammatory role of Artemisia argyi methanol extract by targeting the caspase-11 non-canonical inflammasome in macrophages. J. Ethnopharmacol. 2023, 307, 116231. [Google Scholar] [CrossRef]
- Min, J.H.; Cho, H.J.; Yi, Y.S. A novel mechanism of Korean Red Ginseng-mediated anti-inflammatory action via targeting caspase-11 non-canonical inflammasome in macrophages. J. Ginseng Res. 2022, 46, 675–682. [Google Scholar] [CrossRef]
- Yi, Y.S. Regulatory Roles of Caspase-11 Non-Canonical Inflammasome in Inflammatory Liver Diseases. Int. J. Mol. Sci. 2022, 23, 4986. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. Dual roles of the caspase-11 non-canonical inflammasome in inflammatory bowel disease. Int. Immunopharmacol. 2022, 108, 108739. [Google Scholar] [CrossRef]
- Yi, Y.S. Regulatory Roles of Flavonoids in Caspase-11 Non-Canonical Inflammasome-Mediated Inflammatory Responses and Diseases. Int. J. Mol. Sci. 2023, 24, 402. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. Caspase-11 Noncanonical Inflammasome: A Novel Key Player in Murine Models of Neuroinflammation and Multiple Sclerosis. Neuroimmunomodulation 2021, 28, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Downs, K.P.; Nguyen, H.; Dorfleutner, A.; Stehlik, C. An overview of the non-canonical inflammasome. Mol. Asp. Med. 2020, 76, 100924. [Google Scholar] [CrossRef]
- Cyr, B.; Hadad, R.; Keane, R.W.; de Rivero Vaccari, J.P. The Role of Non-canonical and Canonical Inflammasomes in Inflammaging. Front. Mol. Neurosci. 2022, 15, 774014. [Google Scholar] [CrossRef]
- Matikainen, S.; Nyman, T.A.; Cypryk, W. Function and Regulation of Noncanonical Caspase-4/5/11 Inflammasome. J. Immunol. 2020, 204, 3063–3069. [Google Scholar] [CrossRef]
- Long, Z.; Zeng, L.; He, Q.; Yang, K.; Xiang, W.; Ren, X.; Deng, Y.; Chen, H. Research progress on the clinical application and mechanism of iguratimod in the treatment of autoimmune diseases and rheumatic diseases. Front. Immunol. 2023, 14, 1150661. [Google Scholar] [CrossRef]
- Badii, M.; Gaal, O.; Popp, R.A.; Crisan, T.O.; Joosten, L.A.B. Trained immunity and inflammation in rheumatic diseases. Joint Bone Spine 2022, 89, 105364. [Google Scholar] [CrossRef] [PubMed]
- Felten, R.; Mertz, P.; Sebbag, E.; Scherlinger, M.; Arnaud, L. Novel therapeutic strategies for autoimmune and inflammatory rheumatic diseases. Drug Discov. Today 2023, 28, 103612. [Google Scholar] [CrossRef]
- Moutsopoulos, H.M. Autoimmune rheumatic diseases: One or many diseases? J. Transl. Autoimmun. 2021, 4, 100129. [Google Scholar] [CrossRef] [PubMed]
- Vigano, E.; Diamond, C.E.; Spreafico, R.; Balachander, A.; Sobota, R.M.; Mortellaro, A. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat. Commun. 2015, 6, 8761. [Google Scholar] [CrossRef] [PubMed]
- Casson, C.N.; Yu, J.; Reyes, V.M.; Taschuk, F.O.; Yadav, A.; Copenhaver, A.M.; Nguyen, H.T.; Collman, R.G.; Shin, S. Human caspase-4 mediates noncanonical inflammasome activation against gram-negative bacterial pathogens. Proc. Natl. Acad. Sci. USA 2015, 112, 6688–6693. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.; Burgener, S.S.; Vezyrgiannis, K.; Wang, X.; Acklam, J.; Von Pein, J.B.; Pizzuto, M.; Labzin, L.I.; Boucher, D.; Schroder, K. Caspase-4 dimerisation and D289 auto-processing elicit an interleukin-1beta-converting enzyme. Life Sci. Alliance 2023, 6, e202301908. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Place, D.E.; Kanneganti, T.D. Toward targeting inflammasomes: Insights into their regulation and activation. Cell Res. 2020, 30, 315–327. [Google Scholar] [CrossRef]
- Lee, B.L.; Stowe, I.B.; Gupta, A.; Kornfeld, O.S.; Roose-Girma, M.; Anderson, K.; Warming, S.; Zhang, J.; Lee, W.P.; Kayagaki, N. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. J. Exp. Med. 2018, 215, 2279–2288. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Z.; Magupalli, V.G.; Pablo, J.L.; Dong, Y.; Vora, S.M.; Wang, L.; Fu, T.M.; Jacobson, M.P.; Greka, A.; et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 2021, 593, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, S.; Broz, P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur. J. Immunol. 2015, 45, 2927–2936. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Antonioli, L.; Lopez-Castejon, G.; Blandizzi, C.; Fornai, M. Canonical and Non-Canonical Activation of NLRP3 Inflammasome at the Crossroad between Immune Tolerance and Intestinal Inflammation. Front. Immunol. 2017, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, R.V.H.; Andrade, W.A.; Lima-Junior, D.S.; Dilucca, M.; de Oliveira, C.V.; Wang, K.; Nogueira, P.M.; Rugani, J.N.; Soares, R.P.; Beverley, S.M.; et al. Leishmania Lipophosphoglycan Triggers Caspase-11 and the Non-canonical Activation of the NLRP3 Inflammasome. Cell Rep. 2019, 26, 429–437.e425. [Google Scholar] [CrossRef] [PubMed]
- Moretti, J.; Jia, B.; Hutchins, Z.; Roy, S.; Yip, H.; Wu, J.; Shan, M.; Jaffrey, S.R.; Coers, J.; Blander, J.M. Caspase-11 interaction with NLRP3 potentiates the noncanonical activation of the NLRP3 inflammasome. Nat. Immunol. 2022, 23, 705–717. [Google Scholar] [CrossRef]
- Di Matteo, A.; Bathon, J.M.; Emery, P. Rheumatoid arthritis. Lancet 2023, 402, 2019–2033. [Google Scholar] [CrossRef]
- Almutairi, K.; Nossent, J.; Preen, D.; Keen, H.; Inderjeeth, C. The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatol. Int. 2021, 41, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Liu, N.; Sigdel, K.R.; Duan, L. Role of NLRP3 Inflammasome in Rheumatoid Arthritis. Front. Immunol. 2022, 13, 931690. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Inflammasomes and their roles in arthritic disease pathogenesis. Front. Mol. Biosci. 2022, 9, 1027917. [Google Scholar] [CrossRef]
- Yi, Y.S. Role of inflammasomes in inflammatory autoimmune rheumatic diseases. Korean J. Physiol. Pharmacol. 2018, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Spel, L.; Martinon, F. Inflammasomes contributing to inflammation in arthritis. Immunol. Rev. 2020, 294, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.H.; Starr, A.E.; Kappelhoff, R.; Yan, R.; Roberts, C.R.; Overall, C.M. Matrix metalloproteinase 8 deficiency in mice exacerbates inflammatory arthritis through delayed neutrophil apoptosis and reduced caspase 11 expression. Arthritis Rheum. 2010, 62, 3645–3655. [Google Scholar] [CrossRef] [PubMed]
- Muller-Ladner, U.; Ospelt, C.; Gay, S.; Distler, O.; Pap, T. Cells of the synovium in rheumatoid arthritis. Synovial fibroblasts. Arthritis Res. Ther. 2007, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, J.; Liu, C.; Grizzle, W.E.; Yu, S.; Zhang, S.; Barnes, S.; Koopman, W.J.; Mountz, J.D.; Kimberly, R.P.; et al. Cleavage of p53-vimentin complex enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis of rheumatoid arthritis synovial fibroblasts. Am. J. Pathol. 2005, 167, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Klotzsche, O.; Etzrodt, D.; Hohenberg, H.; Bohn, W.; Deppert, W. Cytoplasmic retention of mutant tsp53 is dependent on an intermediate filament protein (vimentin) scaffold. Oncogene 1998, 16, 3423–3434. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Chen, F.; Chang, R.; Trivedi, M.; Green, K.J.; Cryns, V.L. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ. 2001, 8, 443–450. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Chiniforush, N.; Partoazar, A.; Goudarzi, R. The role of bacterial infections in rheumatoid arthritis development and novel therapeutic interventions: Focus on oral infections. J. Clin. Lab. Anal. 2023, 37, e24897. [Google Scholar] [CrossRef]
- Li, S.; Yu, Y.; Yue, Y.; Zhang, Z.; Su, K. Microbial Infection and Rheumatoid Arthritis. J. Clin. Cell Immunol. 2013, 4, 174. [Google Scholar] [CrossRef]
- Lacey, C.A.; Mitchell, W.J.; Dadelahi, A.S.; Skyberg, J.A. Caspase-1 and Caspase-11 Mediate Pyroptosis, Inflammation, and Control of Brucella Joint Infection. Infect. Immun. 2018, 86, e00361-18. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Lee, H.Y.; Kim, N.Y.; Lee, D.S.; Yim, M. Extracellular Prdx1 mediates bacterial infection and inflammatory bone diseases. Life Sci. 2023, 333, 122140. [Google Scholar] [CrossRef]
- Dehlin, M.; Jacobsson, L.; Roddy, E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 2020, 16, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K. The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout. J. Rheum. Dis. 2022, 29, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.R.; Wang, J.Q.; Li, J. Role of NLRP3 in the pathogenesis and treatment of gout arthritis. Front. Immunol. 2023, 14, 1137822. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Szamosi, S.; Kovacs, G.E.; Kocsis, E.; Benko, S. The NLRP3 inflammasome—Interleukin 1 pathway as a therapeutic target in gout. Arch. Biochem. Biophys. 2019, 670, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yeon, S.H.; Lee, H.E.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Suppression of NLRP3 inflammasome by oral treatment with sulforaphane alleviates acute gouty inflammation. Rheumatology 2018, 57, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Caution, K.; Young, N.; Robledo-Avila, F.; Krause, K.; Abu Khweek, A.; Hamilton, K.; Badr, A.; Vaidya, A.; Daily, K.; Gosu, H.; et al. Caspase-11 Mediates Neutrophil Chemotaxis and Extracellular Trap Formation During Acute Gouty Arthritis through Alteration of Cofilin Phosphorylation. Front. Immunol. 2019, 10, 2519. [Google Scholar] [CrossRef]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef]
- Roskar, S.; Hafner-Bratkovic, I. The Role of Inflammasomes in Osteoarthritis and Secondary Joint Degeneration Diseases. Life 2022, 12, 731. [Google Scholar] [CrossRef]
- Ramirez-Perez, S.; Reyes-Perez, I.V.; Martinez-Fernandez, D.E.; Hernandez-Palma, L.A.; Bhattaram, P. Targeting inflammasome-dependent mechanisms as an emerging pharmacological approach for osteoarthritis therapy. iScience 2022, 25, 105548. [Google Scholar] [CrossRef]
- Han, X.; Lin, D.; Huang, W.; Li, D.; Li, N.; Xie, X. Mechanism of NLRP3 inflammasome intervention for synovitis in knee osteoarthritis: A review of TCM intervention. Front. Genet. 2023, 14, 1159167. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, W. The Role of AIM2 Inflammasome in Knee Osteoarthritis. J. Inflamm. Res. 2022, 15, 6453–6461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.R.; Xing, R.L.; Wang, P.M.; Zhang, N.S.; Yin, S.J.; Li, X.C.; Zhang, L. NLRP1 and NLRP3 inflammasomes mediate LPS/ATP-induced pyroptosis in knee osteoarthritis. Mol. Med. Rep. 2018, 17, 5463–5469. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Tang, H.; Ren, Y.; Yang, D.; Li, Y.; Huang, W.; Wu, Y.; Yin, Z. Melatonin promotes sirtuin 1 expression and inhibits IRE1alpha-XBP1S-CHOP to reduce endoplasmic reticulum stress-mediated apoptosis in chondrocytes. Front. Pharmacol. 2022, 13, 940629. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Ebata, T.; Terkawi, M.A.; Kitahara, K.; Yokota, S.; Shiota, J.; Nishida, Y.; Matsumae, G.; Alhasan, H.; Hamasaki, M.; Hontani, K.; et al. Noncanonical Pyroptosis Triggered by Macrophage-Derived Extracellular Vesicles in Chondrocytes Leading to Cartilage Catabolism in Osteoarthritis. Arthritis Rheumatol. 2023, 75, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, Y.; Ouyang, F.; Su, M.; Li, W.; Chen, J.; Xiao, H.; Zhou, X.; Liu, B. Extracellular vesicles derived from mesenchymal stem cells alleviate neuroinflammation and mechanical allodynia in interstitial cystitis rats by inhibiting NLRP3 inflammasome activation. J. Neuroinflamm. 2022, 19, 80. [Google Scholar] [CrossRef]
- Noonin, C.; Thongboonkerd, V. Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics 2021, 11, 4436–4451. [Google Scholar] [CrossRef]
- Cypryk, W.; Nyman, T.A.; Matikainen, S. From Inflammasome to Exosome-Does Extracellular Vesicle Secretion Constitute an Inflammasome-Dependent Immune Response? Front. Immunol. 2018, 9, 2188. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, D.; Yao, X.; Huang, Y.; Lu, Q. Global epidemiology of systemic lupus erythematosus: A comprehensive systematic analysis and modelling study. Ann. Rheum. Dis. 2023, 82, 351–356. [Google Scholar] [CrossRef]
- Chen, F.F.; Liu, X.T.; Tao, J.; Mao, Z.M.; Wang, H.; Tan, Y.; Qu, Z.; Yu, F. Renal NLRP3 Inflammasome activation is associated with disease activity in lupus nephritis. Clin. Immunol. 2023, 247, 109221. [Google Scholar] [CrossRef] [PubMed]
- Uresti-Rivera, E.E.; Garcia-Hernandez, M.H. AIM2-inflammasome role in systemic lupus erythematous and rheumatoid arthritis. Autoimmunity 2022, 55, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Mahonen, K.; Hau, A.; Bondet, V.; Duffy, D.; Eklund, K.K.; Panelius, J.; Ranki, A. Activation of NLRP3 Inflammasome in the Skin of Patients with Systemic and Cutaneous Lupus Erythematosus. Acta Derm. Venereol. 2022, 102, adv00708. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tao, X.; Tao, J. Strategies of Targeting Inflammasome in the Treatment of Systemic Lupus Erythematosus. Front. Immunol. 2022, 13, 894847. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.B.; Lima, C.A.D.; Vajgel, G.; Sandrin-Garcia, P. The Role of NLRP3 Inflammasome in Lupus Nephritis. Int. J. Mol. Sci. 2021, 22, 12476. [Google Scholar] [CrossRef] [PubMed]
- Kumpunya, S.; Thim-Uam, A.; Thumarat, C.; Leelahavanichkul, A.; Kalpongnukul, N.; Chantaravisoot, N.; Pisitkun, T.; Pisitkun, P. cGAS deficiency enhances inflammasome activation in macrophages and inflammatory pathology in pristane-induced lupus. Front. Immunol. 2022, 13, 1010764. [Google Scholar] [CrossRef]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef]
- Miao, N.; Wang, Z.; Wang, Q.; Xie, H.; Yang, N.; Wang, Y.; Wang, J.; Kang, H.; Bai, W.; Wang, Y.; et al. Oxidized mitochondrial DNA induces gasdermin D oligomerization in systemic lupus erythematosus. Nat. Commun. 2023, 14, 872. [Google Scholar] [CrossRef]
- Beydon, M.; McCoy, S.; Nguyen, Y.; Sumida, T.; Mariette, X.; Seror, R. Epidemiology of Sjogren syndrome. Nat. Rev. Rheumatol. 2023. [Google Scholar] [CrossRef]
- Carsons, S.E.; Patel, B.C. Sjogren Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Li, Z.; Guo, J.; Bi, L. Role of the NLRP3 inflammasome in autoimmune diseases. Biomed. Pharmacother. 2020, 130, 110542. [Google Scholar] [CrossRef]
- Vakrakou, A.G.; Boiu, S.; Ziakas, P.D.; Xingi, E.; Boleti, H.; Manoussakis, M.N. Systemic activation of NLRP3 inflammasome in patients with severe primary Sjogren’s syndrome fueled by inflammagenic DNA accumulations. J. Autoimmun. 2018, 91, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Vakrakou, A.G.; Svolaki, I.P.; Evangelou, K.; Gorgoulis, V.G.; Manoussakis, M.N. Cell-autonomous epithelial activation of AIM2 (absent in melanoma-2) inflammasome by cytoplasmic DNA accumulations in primary Sjogren’s syndrome. J. Autoimmun. 2020, 108, 102381. [Google Scholar] [CrossRef] [PubMed]
- Bulosan, M.; Pauley, K.M.; Yo, K.; Chan, E.K.; Katz, J.; Peck, A.B.; Cha, S. Inflammatory caspases are critical for enhanced cell death in the target tissue of Sjogren’s syndrome before disease onset. Immunol. Cell Biol. 2009, 87, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Crespo Yanguas, S.; Willebrords, J.; Johnstone, S.R.; Maes, M.; Decrock, E.; De Bock, M.; Leybaert, L.; Cogliati, B.; Vinken, M. Pannexin1 as mediator of inflammation and cell death. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 51–61. [Google Scholar] [CrossRef]
- Rusiecka, O.M.; Tournier, M.; Molica, F.; Kwak, B.R. Pannexin1 channels-a potential therapeutic target in inflammation. Front. Cell Dev. Biol. 2022, 10, 1020826. [Google Scholar] [CrossRef]
- Basova, L.V.; Tang, X.; Umasume, T.; Gromova, A.; Zyrianova, T.; Shmushkovich, T.; Wolfson, A.; Hawley, D.; Zoukhri, D.; Shestopalov, V.I.; et al. Manipulation of Panx1 Activity Increases the Engraftment of Transplanted Lacrimal Gland Epithelial Progenitor Cells. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5654–5665. [Google Scholar] [CrossRef]





| Diseases | Caspases | Roles | Models | Ref |
|---|---|---|---|---|
| RA | Caspase-4 |
| RASFs | [56] |
| Caspase-11 |
| FCA-induced arthritic mice Neutrophils | [54] | |
| Brucella-infected mice with joint arthritis BMDMs | [61] | ||
| Listeria- or Escherichia-infected mice with joint arthritis BMDMs | [62] | ||
| GA | Caspase-11 |
| MSU-induced mouse model of GA Neutrophils BMDMs | [68] |
| OA | Caspase-4 |
| OA patients Chondrocytes | [75] |
| Caspase-11 |
| Collagenase-induced and ACLT mouse models of OA Chondrocytes | [77] | |
| SLE | Caspase-11 |
| Pristane-induced mouse model of lupus BMDMs | [87] |
| SLE patients Pristane-induced mouse model of lupus MRL/lpr mouse model of lupus Neutrophils | [89] | ||
| SjS | Caspase-11 |
| SjS-prone C57BL/6.NOD-Aec1Aec2 mouse model | [95] |
| IL-1α-injured mouse lacrimal glands Lacrimal gland of TSP-1 KO mice | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Y.-S. Roles of the Caspase-11 Non-Canonical Inflammasome in Rheumatic Diseases. Int. J. Mol. Sci. 2024, 25, 2091. https://doi.org/10.3390/ijms25042091
Yi Y-S. Roles of the Caspase-11 Non-Canonical Inflammasome in Rheumatic Diseases. International Journal of Molecular Sciences. 2024; 25(4):2091. https://doi.org/10.3390/ijms25042091
Chicago/Turabian StyleYi, Young-Su. 2024. "Roles of the Caspase-11 Non-Canonical Inflammasome in Rheumatic Diseases" International Journal of Molecular Sciences 25, no. 4: 2091. https://doi.org/10.3390/ijms25042091





