A Generic Approach for Miniaturized Unbiased High-Throughput Screens of Bispecific Antibodies and Biparatopic Antibody–Drug Conjugates
Abstract
:1. Introduction
2. Results
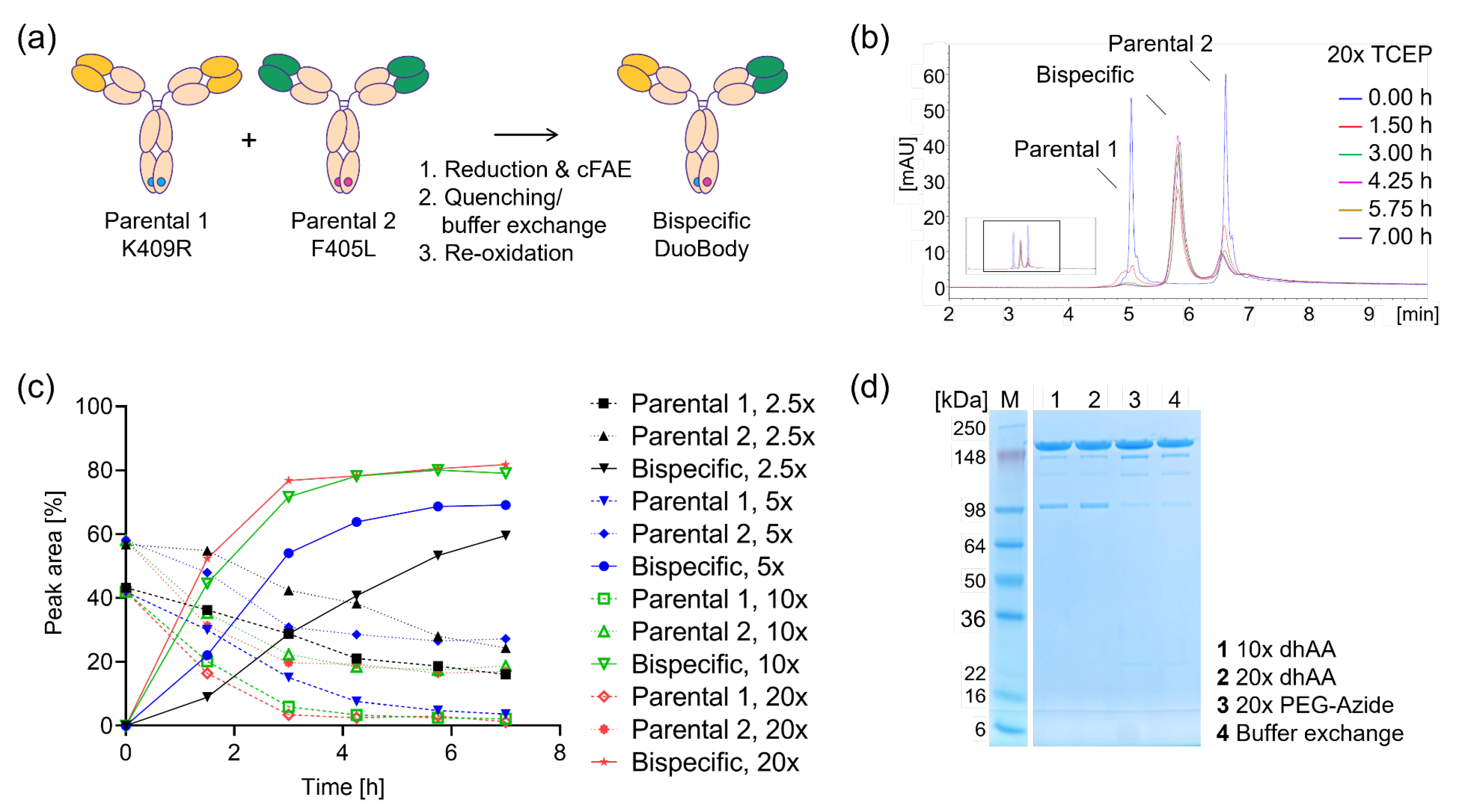
2.1. HTS-Compatible cFAE Setup
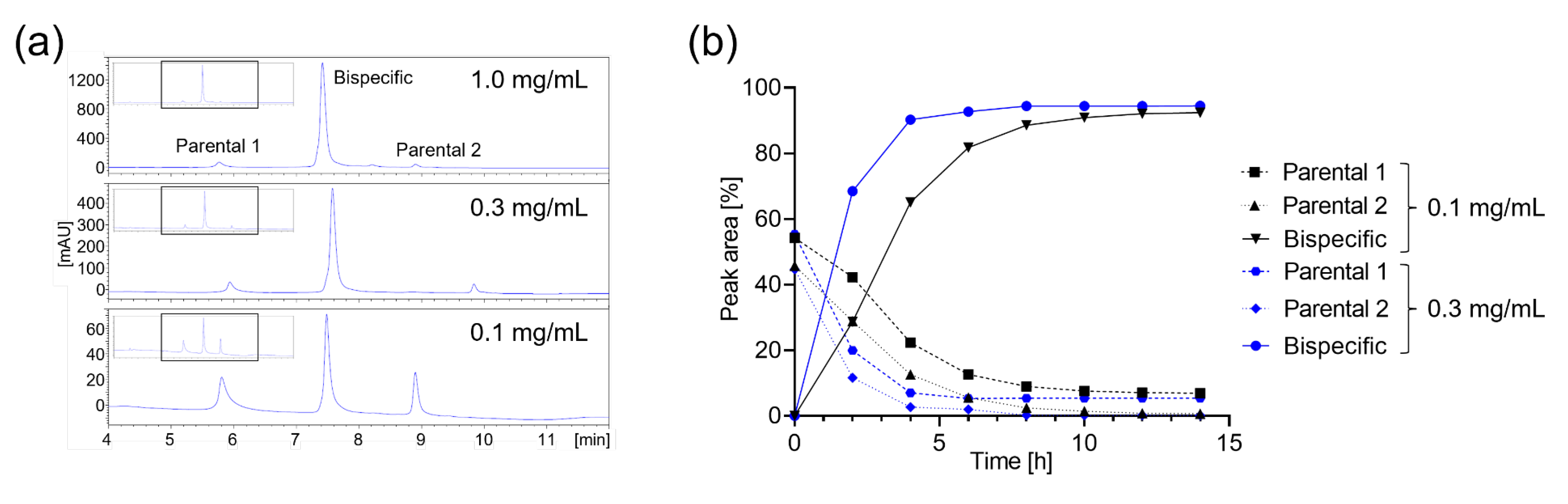
2.2. cFAE Optimization for Low Volumes or Concentrations
2.3. Automated Combinatorial Pipetting and cFAE
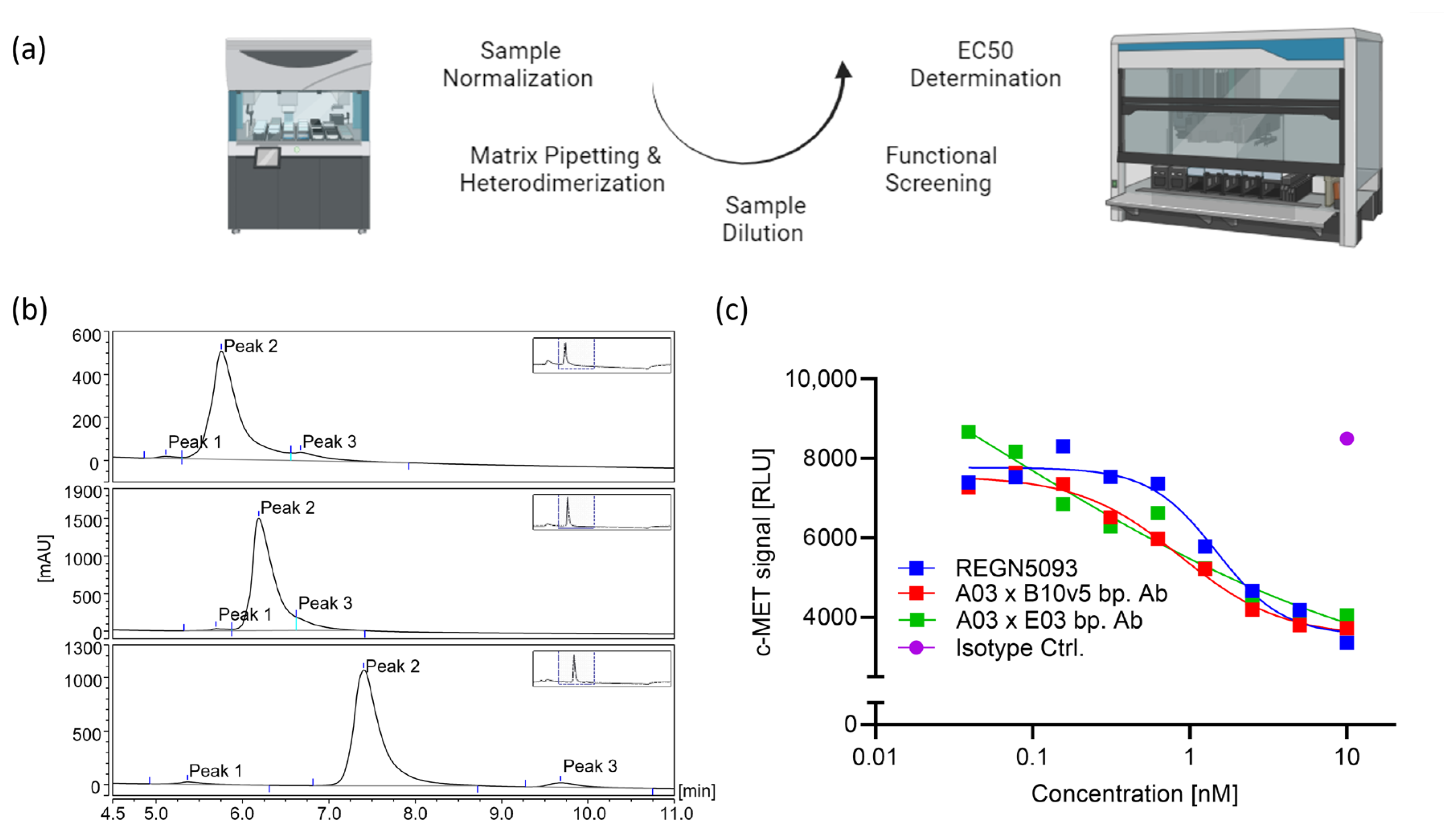
2.4. Applicability for Biparatopic ADC Screening
2.5. High-Throughput Functional Bispecific Screening
3. Discussion
4. Material and Methods
4.1. Manual cFAE
4.2. Miniaturized and Automated cFAE
4.3. Analytical SE-HPLC
4.4. Analytical HIC-HPLC
4.5. ADC Conjugation
4.6. Half-Conjugated ADC DuoBody Reaction
4.7. Analytical CIEX-HPLC
4.8. Cytotoxicity Evaluation
4.9. c-MET Degradation Screen
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-MEA | 2-mercaptoethylamine•HCl |
| ADC | antibody–drug conjugate |
| bpADCs | biparatopic antibody–drug conjugates |
| bsAb | bispecific antibody |
| cFAE | controlled Fab-arm exchange |
| CIEX | cation-exchange chromatography |
| dhAA | dehydroascorbic acid |
| Fc | fragment crystallizable |
| HIC | hydrophobic interaction chromatography |
| HTP | high throughput |
| HTS | high-throughput screening |
| IgG | immunoglobulin G |
| mAb | monoclonal antibody |
| MMAE | monomethyl auristatin E |
| MoA | mode of action |
| MW | mass weight |
| PEG-Azide | PEG-N3, polyethylene glycol–azide |
| PTM | post-translational modification |
| RP-HPLC | reverse-phase high-pressure liquid chromatography |
| TCEP | tris(2-carboxyethyl)phosphin |
References
- Kang, C. Teclistamab: First Approval. Drugs 2022, 82, 1613–1619. [Google Scholar] [CrossRef]
- Shirley, M. Glofitamab: First Approval. Drugs 2023, 83, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Kang, C. Mosunetuzumab: First Approval. Drugs 2022, 82, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Amivantamab: First Approval. Drugs 2021, 81, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Faricimab: First Approval. Drugs 2022, 82, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, U.; Kontermann, R.E. Bispecific Antibodies. Science 2021, 372, 916–917. [Google Scholar] [CrossRef]
- Brinkmann, U.; Kontermann, R.E. The Making of Bispecific Antibodies. MAbs 2017, 9, 182–212. [Google Scholar] [CrossRef]
- Merchant, A.M.; Zhu, Z.; Yuan, J.Q.; Goddard, A.; Adams, C.W.; Presta, L.G.; Carter, P. An Efficient Route to Human Bispecific IgG. Nat. Biotechnol. 1998, 16, 677–681. [Google Scholar] [CrossRef]
- De Nardis, C.; Hendriks, L.J.A.; Poirier, E.; Arvinte, T.; Gros, P.; Bakker, A.B.H.; de Kruif, J. A New Approach for Generating Bispecific Antibodies Based on a Common Light Chain Format and the Stable Architecture of Human Immunoglobulin G1. J. Biol. Chem. 2017, 292, 14706–14717. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Y.; Wang, G.; Liu, M. Current Landscape and Future Directions of Bispecific Antibodies in Cancer Immunotherapy. Front. Immunol. 2022, 13, 1035276. [Google Scholar] [CrossRef]
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12, 626616. [Google Scholar] [CrossRef]
- Scott, M.J.; Lee, J.A.; Wake, M.S.; Batt, K.V.; Wattam, T.A.; Hiles, I.D.; Batuwangala, T.D.; Ashman, C.I.; Steward, M. ‘In-Format’ Screening of a Novel Bispecific Antibody Format Reveals Significant Potency Improvements Relative to Unformatted Molecules. MAbs 2017, 9, 85–93. [Google Scholar] [CrossRef]
- Valldorf, B.; Hinz, S.C.; Russo, G.; Pekar, L.; Mohr, L.; Klemm, J.; Doerner, A.; Krah, S.; Hust, M.; Zielonka, S. Antibody Display Technologies: Selecting the Cream of the Crop. Biol. Chem. 2022, 403, 455–477. [Google Scholar] [CrossRef]
- Kitazawa, T.; Igawa, T.; Sampei, Z.; Muto, A.; Kojima, T.; Soeda, T.; Yoshihashi, K.; Okuyama-Nishida, Y.; Saito, H.; Tsunoda, H.; et al. A Bispecific Antibody to Factors IXa and X Restores Factor VIII Hemostatic Activity in a Hemophilia A Model. Nat. Med. 2012, 18, 1570–1574. [Google Scholar] [CrossRef]
- Furtmann, N.; Schneider, M.; Spindler, N.; Steinmann, B.; Li, Z.; Focken, I.; Meyer, J.; Dimova, D.; Kroll, K.; Leuschner, W.D.; et al. An End-to-End Automated Platform Process for High-Throughput Engineering of next-Generation Multi-Specific Antibody Therapeutics. MAbs 2021, 13, 1955433. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Dunshee, D.R.; Yee, A.; Tong, R.K.; Kim, I.; Farahi, F.; Hongo, J.A.; Ernst, J.A.; Sonoda, J.; Spiess, C. Tethered-Variable CL Bispecific IgG: An Antibody Platform for Rapid Bispecific Antibody Screening. Protein Eng. Des. Sel. 2017, 30, 627–637. [Google Scholar] [CrossRef]
- Invenra B-Body. Available online: https://invenra.com/our-science (accessed on 1 September 2023).
- Keeble, A.H.; Banerjee, A.; Ferla, M.P.; Reddington, S.C.; Anuar, I.N.A.K.; Howarth, M. Evolving Accelerated Amidation by SpyTag/SpyCatcher to Analyze Membrane Dynamics. Angew. Chem. Int. Ed. Engl. 2017, 56, 16521–16525. [Google Scholar] [CrossRef]
- Akiba, H.; Takayanagi, K.; Kusano-Arai, O.; Iwanari, H.; Hamakubo, T.; Tsumoto, K. Generation of Biparatopic Antibody through Two-Step Targeting of Fragment Antibodies on Antigen Using SpyTag and SpyCatcher. Biotechnol. Rep. 2020, 25, e00418. [Google Scholar] [CrossRef]
- Han, L.; Chen, J.; Ding, K.; Zong, H.; Xie, Y.; Jiang, H.; Zhang, B.; Lu, H.; Yin, W.; Gilly, J.; et al. Efficient Generation of Bispecific IgG Antibodies by Split Intein Mediated Protein Trans-Splicing System. Sci. Rep. 2017, 7, 8360. [Google Scholar] [CrossRef]
- Hofmann, T.; Schmidt, J.; Ciesielski, E.; Becker, S.; Rysiok, T.; Schütte, M.; Toleikis, L.; Kolmar, H.; Doerner, A. Intein Mediated High Throughput Screening for Bispecific Antibodies. MAbs 2020, 12, 1731938. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, P.; Whale, K.D.; Sawtell, A.K.; Thompson, C.L.; Rapecki, S.E.; Cook, D.A.; Twomey, B.M.; Mennecozzi, M.; Starkie, L.E.; Barry, E.M.C.; et al. Bispecific Antibody Target Pair Discovery by High-Throughput Phenotypic Screening Using in Vitro Combinatorial Fab Libraries. MAbs 2021, 13, 1859049. [Google Scholar] [CrossRef] [PubMed]
- Dengl, S.; Mayer, K.; Bormann, F.; Duerr, H.; Hoffmann, E.; Nussbaum, B.; Tischler, M.; Wagner, M.; Kuglstatter, A.; Leibrock, L.; et al. Format Chain Exchange (FORCE) for High-Throughput Generation of Bispecific Antibodies in Combinatorial Binder-Format Matrices. Nat. Commun. 2020, 11, 4974. [Google Scholar] [CrossRef] [PubMed]
- Gramer, M.J.; van den Bremer, E.T.; van Kampen, M.D.; Kundu, A.; Kopfmann, P.; Etter, E.; Stinehelfer, D.; Long, J.; Lannom, T.; Noordergraaf, E.H.; et al. Production of Stable Bispecific IgG1 by Controlled Fab-Arm Exchange. MAbs 2013, 5, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Labrijn, A.F.; Meesters, J.I.; De Goeij, B.E.C.G.; Van Den Bremer, E.T.J.; Neijssen, J.; Van Kampen, M.D.; Strumane, K.; Verploegen, S.; Kundu, A.; Gramer, M.J.; et al. Efficient Generation of Stable Bispecific IgG1 by Controlled Fab-Arm Exchange. Proc. Natl. Acad. Sci. USA 2013, 110, 5145–5150. [Google Scholar] [CrossRef] [PubMed]
- Labrijn, A.F.; Rispens, T.; Meesters, J.; Rose, R.J.; den Bleker, T.H.; Loverix, S.; van den Bremer, E.T.J.; Neijssen, J.; Vink, T.; Lasters, I.; et al. Species-Specific Determinants in the IgG CH3 Domain Enable Fab-Arm Exchange by Affecting the Noncovalent CH3–CH3 Interaction Strength. J. Immunol. 2011, 187, 3238–3246. [Google Scholar] [CrossRef]
- Neijssen, J.; Cardoso, R.M.F.; Chevalier, K.M.; Wiegman, L.; Valerius, T.; Anderson, G.M.; Moores, S.L.; Schuurman, J.; Parren, P.W.H.I.; Strohl, W.R.; et al. Discovery of Amivantamab (JNJ-61186372), a Bispecific Antibody Targeting EGFR and MET. J. Biol. Chem. 2021, 296, 100641. [Google Scholar] [CrossRef]
- Li, J.Y.; Perry, S.R.; Muniz-Medina, V.; Wang, X.; Wetzel, L.K.; Rebelatto, M.C.; Hinrichs, M.J.M.; Bezabeh, B.Z.; Fleming, R.L.; Dimasi, N.; et al. A Biparatopic HER2-Targeting Antibody-Drug Conjugate Induces Tumor Regression in Primary Models Refractory to or Ineligible for HER2-Targeted Therapy. Cancer Cell 2016, 29, 117–129. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, J.O.; Yang, K.; Perez Bay, A.E.; Andreev, J.; Ngoi, P.; Pyles, E.; Franklin, M.C.; Dudgeon, D.; Rafique, A.; Dore, A.; et al. A Biparatopic Antibody That Modulates MET Trafficking Exhibits Enhanced Efficacy Compared with Parental Antibodies in MET-Driven Tumor Models. Clin. Cancer Res. 2020, 26, 1408–1419. [Google Scholar] [CrossRef]
- Paul, D.; Stern, O.; Vallis, Y.; Dhillon, J.; Buchanan, A.; McMahon, H. Cell Surface Protein Aggregation Triggers Endocytosis to Maintain Plasma Membrane Proteostasis. Nat. Commun. 2023, 14, 947. [Google Scholar] [CrossRef]
- Kelton, C.; Wesolowski, J.S.; Soloviev, M.; Schweickhardt, R.; Fischer, D.; Kurosawa, E.; McKenna, S.D.; Gross, A.W. Anti-EGFR Biparatopic-SEED Antibody Has Enhanced Combination-Activity in a Single Molecule. Arch. Biochem. Biophys. 2012, 526, 219–225. [Google Scholar] [CrossRef]
- Oh, S.Y.; Lee, Y.W.; Lee, E.J.; Kim, J.H.; Park, Y.; Heo, S.G.; Yu, M.R.; Hong, M.H.; DaSilva, J.; Daly, C.; et al. Preclinical Study of a Biparatopic METxMET Antibody–Drug Conjugate, REGN5093-M114, Overcomes MET-Driven Acquired Resistance to EGFR TKIs in EGFR-Mutant NSCLC. Clin. Cancer Res. 2023, 29, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Evers, A.; Krah, S.; Demir, D.; Gaa, R.; Elter, D.; Schroeter, C.; Zielonka, S.; Rasche, N.; Dotterweich, J.; Knuehl, C.; et al. Engineering hydrophobicity and manufacturability for optimized biparatopic antibody-drug conjugates targeting c-MET. MAbs 2024, 16, 2302386. [Google Scholar] [CrossRef] [PubMed]
- Dickgiesser, S.; Rieker, M.; Mueller-Pompalla, D.; Schröter, C.; Tonillo, J.; Warszawski, S.; Raab-Westphal, S.; Kühn, S.; Knehans, T.; Könning, D.; et al. Site-Specific Conjugation of Native Antibodies Using Engineered Microbial Transglutaminases. Bioconjug. Chem. 2020, 31, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Mazor, Y.; Oganesyan, V.; Yang, C.; Hansen, A.; Wang, J.; Liu, H.; Sachsenmeier, K.; Carlson, M.; Gadre, D.V.; Borrok, M.J.; et al. Improving Target Cell Specificity Using a Novel Monovalent Bispecific IgG Design. MAbs 2015, 7, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Krah, S.; Sellmann, C.; Zielonka, S.; Doerner, A. Greatest Hits—Innovative Technologies for High Throughput Identification of Bispecific Antibodies. Int. J. Mol. Sci. 2020, 21, 6551. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.H.; Cable, J. Antibodies as Drugs-a Keystone Symposia Report. Ann. N. Y. Acad. Sci. 2023, 1519, 153–166. [Google Scholar] [CrossRef]
- Segaliny, A.I.; Jayaraman, J.; Chen, X.; Chong, J.; Luxon, R.; Fung, A.; Fu, Q.; Jiang, X.; Rivera, R.; Ma, X.; et al. A High Throughput Bispecific Antibody Discovery Pipeline. Commun. Biol. 2023, 6, 380. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, R.; Shen, B.; Li, N.; Zhou, H.; Wang, W.; Zhao, Y.; Huang, M.; Fang, P.; Wang, S.; et al. High-Throughput Functional Screening for next-Generation Cancer Immunotherapy Using Droplet-Based Microfluidics. Sci. Adv. 2021, 7, 3839–3850. [Google Scholar] [CrossRef]
- Gaa, R.; Mayer, H.M.; Noack, D.; Kumari, K.; Guenther, R.; Tsai, S.P.; Ji, Q.; Doerner, A. Mammalian display to secretion switchable libraries for antibody preselection and high throughput functional screening. MAbs. 2023, 15, 2251190. [Google Scholar] [CrossRef]
- Kolfschoten, M.V.D.N.; Schuurman, J.; Losen, M.; Bleeker, W.K.; Martínez-Martínez, P.; Vermeulen, E.; Den Bleker, T.H.; Wiegman, L.; Vink, T.; Aarden, L.A.; et al. Anti-Inflammatory Activity of Human IgG4 Antibodies by Dynamic Fab Arm Exchange. Science 2007, 317, 1554–1557. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Buijsse, A.O.; van den Bremer, E.T.J.; Verwilligen, A.Y.W.; Bleeker, W.K.; Thorpe, S.J.; Killestein, J.; Polman, C.H.; Aalberse, R.C.; Schuurman, J.; et al. Therapeutic IgG4 Antibodies Engage in Fab-Arm Exchange with Endogenous Human IgG4 in Vivo. Nat. Biotechnol. 2009, 27, 767–771. [Google Scholar] [CrossRef]
- Rose, R.J.; Labrijn, A.F.; Van Den Bremer, E.T.J.; Loverix, S.; Lasters, I.; Van Berkel, P.H.C.; Van De Winkel, J.G.J.; Schuurman, J.; Parren, P.W.H.I.; Heck, A.J.R. Quantitative Analysis of the Interaction Strength and Dynamics of Human IgG4 Half Molecules by Native Mass Spectrometry. Structure 2011, 19, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Goulet, D.R.; Orcutt, S.J.; Zwolak, A.; Rispens, T.; Labrijn, A.F.; De Jon, R.N.; Atkins, W.M.; Chiu, M.L. Kinetic Mechanism of Controlled Fab-Arm Exchange for the Formation of Bispecific Immunoglobulin G1 Antibodies. J. Biol. Chem. 2018, 293, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Junutula, J.R.; Raab, H.; Clark, S.; Bhakta, S.; Leipold, D.D.; Weir, S.; Chen, Y.; Simpson, M.; Tsai, S.P.; Dennis, M.S.; et al. Site-Specific Conjugation of a Cytotoxic Drug to an Antibody Improves the Therapeutic Index. Nat. Biotechnol. 2008, 26, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Kantner, T.; Alkhawaja, B.; Watts, A.G. In Situ Quenching of Trialkylphosphine Reducing Agents Using Water-Soluble PEG-Azides Improves Maleimide Conjugation to Proteins. ACS Omega 2017, 2, 5785–5791. [Google Scholar] [CrossRef]
- Wechtersbach, L.; Cigić, B. Reduction of Dehydroascorbic Acid at Low PH. J. Biochem. Biophys. Methods 2007, 70, 767–772. [Google Scholar] [CrossRef]
- Yanakieva, D.; Pekar, L.; Evers, A.; Fleischer, M.; Keller, S.; Mueller-Pompalla, D.; Toleikis, L.; Kolmar, H.; Zielonka, S.; Krah, S. Beyond Bispecificity: Controlled Fab Arm Exchange for the Generation of Antibodies with Multiple Specificities. MAbs 2022, 14, 2018960. [Google Scholar] [CrossRef] [PubMed]
- NCT05029882. Available online: https://www.clinicaltrials.gov/study/NCT05029882 (accessed on 1 September 2023).
- Lewis, S.M.; Wu, X.; Pustilnik, A.; Sereno, A.; Huang, F.; Rick, H.L.; Guntas, G.; Leaver-Fay, A.; Smith, E.M.; Ho, C.; et al. Generation of Bispecific IgG Antibodies by Structure-Based Design of an Orthogonal Fab Interface. Nat. Biotechnol. 2014, 32, 191–198. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barron, N.; Dickgiesser, S.; Fleischer, M.; Bachmann, A.-N.; Klewinghaus, D.; Hannewald, J.; Ciesielski, E.; Kusters, I.; Hammann, T.; Krause, V.; et al. A Generic Approach for Miniaturized Unbiased High-Throughput Screens of Bispecific Antibodies and Biparatopic Antibody–Drug Conjugates. Int. J. Mol. Sci. 2024, 25, 2097. https://doi.org/10.3390/ijms25042097
Barron N, Dickgiesser S, Fleischer M, Bachmann A-N, Klewinghaus D, Hannewald J, Ciesielski E, Kusters I, Hammann T, Krause V, et al. A Generic Approach for Miniaturized Unbiased High-Throughput Screens of Bispecific Antibodies and Biparatopic Antibody–Drug Conjugates. International Journal of Molecular Sciences. 2024; 25(4):2097. https://doi.org/10.3390/ijms25042097
Chicago/Turabian StyleBarron, Nadine, Stephan Dickgiesser, Markus Fleischer, Angelika-Nicole Bachmann, Daniel Klewinghaus, Jens Hannewald, Elke Ciesielski, Ilja Kusters, Til Hammann, Volker Krause, and et al. 2024. "A Generic Approach for Miniaturized Unbiased High-Throughput Screens of Bispecific Antibodies and Biparatopic Antibody–Drug Conjugates" International Journal of Molecular Sciences 25, no. 4: 2097. https://doi.org/10.3390/ijms25042097
APA StyleBarron, N., Dickgiesser, S., Fleischer, M., Bachmann, A.-N., Klewinghaus, D., Hannewald, J., Ciesielski, E., Kusters, I., Hammann, T., Krause, V., Fuchs, S. W., Siegmund, V., Gross, A. W., Mueller-Pompalla, D., Krah, S., Zielonka, S., & Doerner, A. (2024). A Generic Approach for Miniaturized Unbiased High-Throughput Screens of Bispecific Antibodies and Biparatopic Antibody–Drug Conjugates. International Journal of Molecular Sciences, 25(4), 2097. https://doi.org/10.3390/ijms25042097






