The Role of Rosavin in the Pathophysiology of Bone Metabolism
Abstract
1. Introduction
2. Biochemical Structure of Rosavin
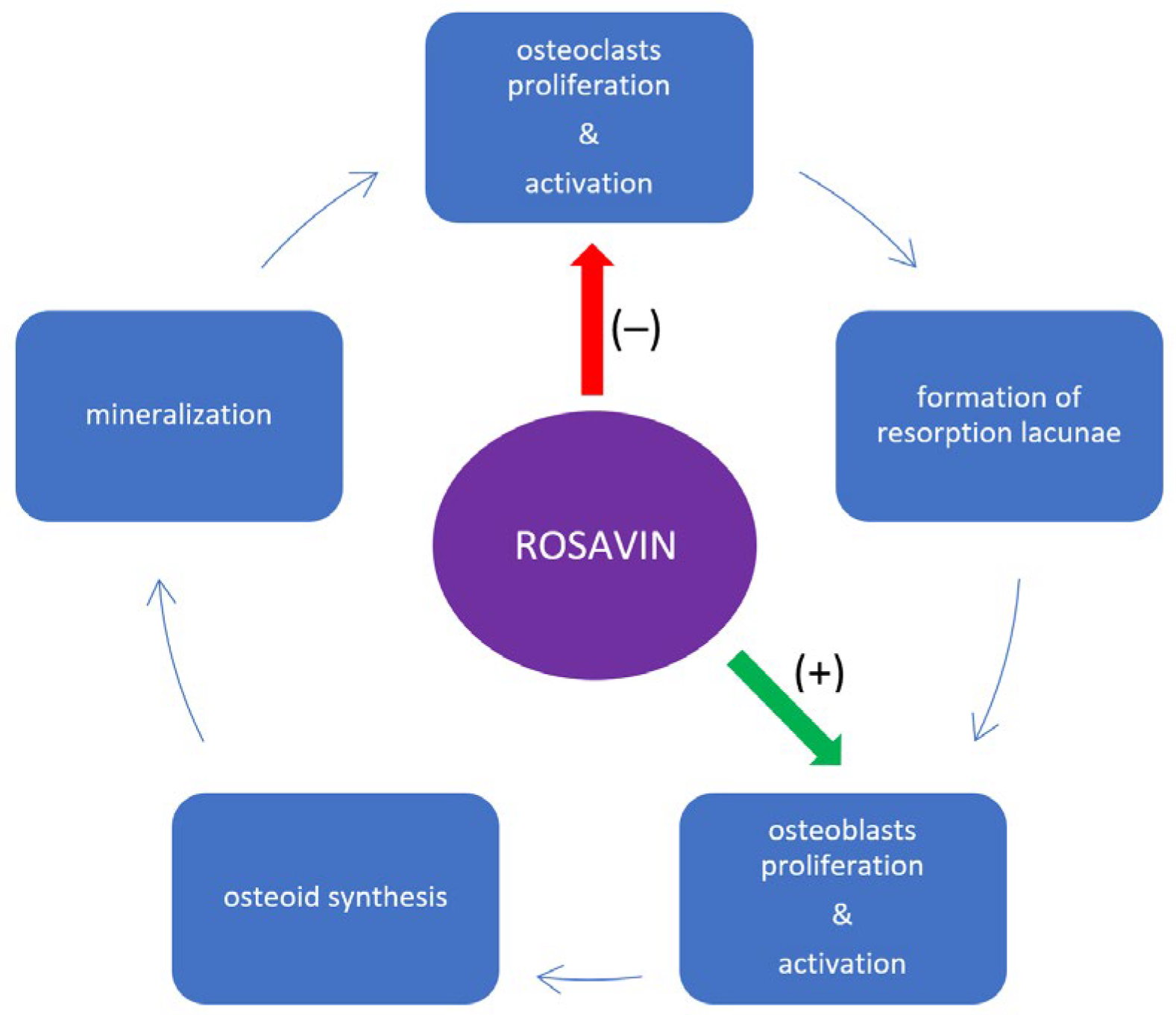
3. Impact of Rosavin on Bone Tissue Metabolism
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Feng, Y.; Zheng, L.; He, P.; Tan, J.; Cai, J.; Wu, M.; Ye, X. Rosavin: Research Advances in Extraction and Synthesis, Pharmacological Activities and Therapeutic Effects on Diseases of the Characteristic Active Ingredients of Rhodiola rosea L. Molecules 2023, 28, 7412. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Jagim, A.R.; Potter, G.D.M.; Garner, D.; Galpin, A.J. Rhodiola rosea as an adaptogen to enhance exercise performance: A review of the literature. Br. J. Nutr. 2023, 131, 461–473. [Google Scholar] [CrossRef]
- Ivanova Stojcheva, E.; Quintela, J.C. The Effectiveness of Rhodiola rosea L. Preparations in Alleviating Various Aspects of Life-Stress Symptoms and Stress-Induced Conditions—Encouraging Clinical Evidence. Molecules 2022, 27, 3902. [Google Scholar] [CrossRef]
- Kelly, G.S. Rhodiola rosea: A possible plant adaptogen. Altern. Med. Rev. 2001, 6, 293–302. [Google Scholar] [PubMed]
- European Medicines Agency; Committee on Herbal Medicinal Products (HMPC). Assessment Report on Rhodiola rosea L., Rhizoma et Radix; Document Reference EMA/HMPC/232100/2011; European Medicines Agency: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Khanna, K.; Mishra, K.P.; Ganju, L.; Singh, S.B. Golden root: A wholesome treat of immunity. Biomed. Pharmacother. 2017, 87, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Jakstas, V.; Kopustinskiene, D.M. Phenolic Compounds of Rhodiola rosea L. as the Potential Alternative Therapy in the Treatment of Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 12293. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pham, V.; Bui, M.; Song, L.; Wu, C.; Walia, A.; Uchio, E.; Smith-Liu, F.; Zi, X. Rhodiola rosea L.: An herb with anti-stress, anti-aging, and immunostimulating properties for cancer chemoprevention. Curr. Pharmacol. Rep. 2017, 3, 384–395. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, L.; Long, J.; Xie, Q.; Zheng, Y.; Liu, K.; Li, X. Salidroside: A review of its recent advances in synthetic pathways and pharmacological properties. Chem.-Biol. Interact. 2021, 339, 109268. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Zheng, C.; Meng, Y.; Yang, Y. Synthesis, biological activity of salidroside and its analogues. Chem. Pharm. Bull. 2010, 58, 1627–1629. [Google Scholar] [CrossRef]
- Huang, M.; Sui, R.; Zhang, L.; Zhu, Y.; Yuan, X.; Jiang, H.; Mao, X. Rosavin thwarts amyloid-β-induced macromolecular damages and neurotoxicity, exhibiting anti-Alzheimer’s disease activity in Wister rat model. Inflammopharmacology 2023. [Google Scholar] [CrossRef]
- Zou, H.; Li, L.; Yang, Z.; Tang, L.; Wang, C. Rosavin protects the blood-brain barrier against ischemia/reperfusion-induced cerebral injury by regulating MAPK-mediated MMPs pathway. Clin. Exp. Pharmacol. Physiol. 2023, 50, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Li, J.; Shi, L.; Hu, B. Rosavin inhibits neutrophil extracellular traps formation to ameliorate sepsis-induced lung injury by regulating the MAPK pathway. Allergol. Immunopathol. 2023, 51, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Jiang, C.; Zhao, Z.; Lv, Y.; Wang, G. Rosavin exerts an antitumor role and inactivates the MAPK/ERK pathway in small-cell lung carcinoma in vitro. Acta Pharm. 2023, 73, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Albadawy, R.; Hasanin, A.H.; Agwa, S.H.A.; Hamady, S.; Aboul-Ela, Y.M.; Raafat, M.H.; Kamar, S.S.; Othman, M.; Yahia, Y.A.; Matboli, M. Rosavin Ameliorates Hepatic Inflammation and Fibrosis in the NASH Rat Model via Targeting Hepatic Cell Death. Int. J. Mol. Sci. 2022, 23, 10148. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Choi, J.; Kwon, J.Y.; Jung, K.A.; Yang, C.W.; Park, S.H.; Cho, M.L. A probiotic complex, rosavin, zinc, and prebiotics ameliorate intestinal inflammation in an acute colitis mouse model. J. Transl. Med. 2018, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Buccino, F.; Colombo, C.; Duarte, D.H.L.; Rinaudo, L.; Ulivieri, F.M.; Vergani, L.M. 2D and 3D numerical models to evaluate trabecular bone damage. Med. Biol. Eng. Comput. 2021, 59, 2139–2152. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, I.; Albert, J.; Ritthaler, M.; Papastavrou, A.; Steinmann, P. Bone fracture healing within a continuum bone remodelling framework. Comput. Methods Biomech. Biomed. Eng. 2022, 25, 1040–1050. [Google Scholar] [CrossRef]
- Williams, S.A.; Daigle, S.G.; Weiss, R.; Wang, Y.; Arora, T.; Curtis, J.R. Economic Burden of Osteoporosis-Related Fractures in the US Medicare Population. Ann. Pharmacother. 2021, 55, 821–829. [Google Scholar] [CrossRef]
- Si, L.; Winzenberg, T.M.; Jiang, Q.; Chen, M.; Palmer, A.J. Projection of osteoporosis-related fractures and costs in China: 2010–2050. Osteoporos. Int. 2015, 26, 1929–1937. [Google Scholar] [CrossRef]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Lajas, C.; Abasolo, L.; Bellajdel, B.; Hernández-García, C.; Carmona, L.; Vargas, E.; Lázaro, P.; Jover, J.A. Costs and predictors of costs in rheumatoid arthritis: A prevalence-based study. Arthritis Rheum. 2003, 49, 64–70. [Google Scholar] [CrossRef]
- Rashki Kemmak, A.; Rezapour, A.; Jahangiri, R.; Nikjoo, S.; Farabi, H.; Soleimanpour, S. Economic burden of osteoporosis in the world: A systematic review. Med. J. Islam. Repub. Iran 2020, 34, 154. [Google Scholar] [CrossRef]
- Williamson, S.; Landeiro, F.; McConnell, T.; Fulford-Smith, L.; Javaid, M.K.; Judge, A.; Leal, J. Costs of fragility hip fractures globally: A systematic review and meta-regression analysis. Osteoporos. Int. 2017, 28, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Masi, F.; Chianese, G.; Hofstetter, R.K.; Cavallaro, A.L.; Riva, A.; Werz, O.; Taglialatela-Scafati, O. Phytochemical profile and anti-inflammatory activity of a commercially available Rhodiola rosea root extract. Fitoterapia 2023, 166, 105439. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 9823887, Rosavin. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Rosavin (accessed on 15 December 2023).
- Zhang, W.; Zhang, W.; Huo, L.; Chai, Y.; Liu, Z.; Ren, Z.; Yu, C. Rosavin suppresses osteoclastogenesis in vivo and in vitro by blocking the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways. Ann. Transl. Med. 2021, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of osteoblast differentiation by Runx2. Adv. Exp. Med. Biol. 2010, 658, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Sasso, G.R.; Florencio-Silva, R.; de Pizzol-Júnior, J.P.; Gil, C.D.; Simões, M.J.; Sasso-Cerri, E.; Cerri, P.S. Additional Insights into the Role of Osteocalcin in Osteoblast Differentiation and in the Early Steps of Developing Alveolar Process of Rat Molars. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2023, 71, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Garbe, A.I.; Roscher, A.; Schüler, C.; Lutter, A.H.; Glösmann, M.; Bernhardt, R.; Chopin, M.; Hempel, U.; Hofbauer, L.C.; Rammelt, S.; et al. Regulation of bone mass and osteoclast function depend on the F-actin modulator SWAP-70. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012, 27, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, X.; Liu, Y.; He, A.; Jia, R. NFATc1: Functions in osteoclasts. Int. J. Biochem. Cell Biol. 2010, 42, 576–579. [Google Scholar] [CrossRef]
- Takayanagi, H. The role of NFAT in osteoclast formation. Ann. N. Y. Acad. Sci. 2007, 1116, 227–237. [Google Scholar] [CrossRef]
- Omata, Y.; Tachibana, H.; Aizaki, Y.; Mimura, T.; Sato, K. Essentiality of Nfatc1 short isoform in osteoclast differentiation and its self-regulation. Sci. Rep. 2023, 13, 18797. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.; Youn, B.U.; Jin, H.M.; Kim, J.Y.; Moon, J.B.; Ko, A.; Seo, S.B.; Lee, K.Y.; Kim, N. RANKL induces NFATc1 acetylation and stability via histone acetyltransferases during osteoclast differentiation. Biochem. J. 2011, 436, 253–262. [Google Scholar] [CrossRef]
- McClung, M. Role of RANKL inhibition in osteoporosis. Arthritis Res. Ther. 2007, 9 (Suppl. S1), S3. [Google Scholar] [CrossRef]
- Ko, Y.; Lee, G.; Kim, B.; Park, M.; Jang, Y.; Lim, W. Modification of the RANKL-RANK-binding site for the immunotherapeutic treatment of osteoporosis. Osteoporos. Int. 2020, 31, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, Z.; Tang, E.; Fan, Z.; McCauley, L.; Franceschi, R.; Guan, K.; Krebsbach, P.H.; Wang, C.Y. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat. Med. 2009, 15, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Li, J.; Yao, Z.; Xing, L. Nuclear Factor-Kappa B Regulation of Osteoclastogenesis and Osteoblastogenesis. Endocrinol. Metab. 2023, 38, 504–521. [Google Scholar] [CrossRef] [PubMed]
- Cong, Q.; Jia, H.; Li, P.; Qiu, S.; Yeh, J.; Wang, Y.; Zhang, Z.L.; Ao, J.; Li, B.; Liu, H. p38α MAPK regulates proliferation and differentiation of osteoclast progenitors and bone remodeling in an aging-dependent manner. Sci. Rep. 2017, 7, 45964. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, N.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Ovariectomized rat model of osteoporosis: A practical guide. EXCLI J. 2020, 19, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Van Straalen, J.P.; Sanders, E.; Prummel, M.F.; Sanders, G.T. Bone-alkaline phosphatase as indicator of bone formation. Clin. Chim. Acta Int. J. Clin. Chem. 1991, 201, 27–33. [Google Scholar] [CrossRef]
- Nakamura, A.; Dohi, Y.; Akahane, M.; Ohgushi, H.; Nakajima, H.; Funaoka, H.; Takakura, Y. Osteocalcin secretion as an early marker of in vitro osteogenic differentiation of rat mesenchymal stem cells. Tissue Eng. Part C Methods 2009, 15, 169–180. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, L.; Wang, F.; Chen, M.; Li, H. Rosavin regulates bone homeostasis through HDAC1-induced epigenetic regulation of EEF2. Chem.-Biol. Interact. 2023, 384, 110696. [Google Scholar] [CrossRef]
- Shakespear, M.R.; Halili, M.A.; Irvine, K.M.; Fairlie, D.P.; Sweet, M.J. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011, 32, 335–343. [Google Scholar] [CrossRef]
- Cantley, M.D.; Zannettino, A.C.W.; Bartold, P.M.; Fairlie, D.P.; Haynes, D.R. Histone deacetylases (HDAC) in physiological and pathological bone remodelling. Bone 2017, 95, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Parveen, R.; Harihar, D.; Chatterji, B.P. Recent histone deacetylase inhibitors in cancer therapy. Cancer 2023, 129, 3372–3380. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Wu, X.; Simon, N.; Svensson, C.I.; Yuan, J.; Hart, D.A.; Ahmed, A.S.; Ackermann, P.W. eEF2 improves dense connective tissue repair and healing outcome by regulating cellular death, autophagy, apoptosis, proliferation and migration. Cell. Mol. Life Sci. CMLS 2023, 80, 128. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Lee, S.H.; Jhun, J.; Choi, J.; Jung, K.; Cho, K.H.; Kim, S.J.; Yang, C.W.; Park, S.H.; Cho, M.L. The Combination of Probiotic Complex, Rosavin, and Zinc Improves Pain and Cartilage Destruction in an Osteoarthritis Rat Model. J. Med. Food 2018, 21, 364–371. [Google Scholar] [CrossRef]
- Im, G.I.; Kim, M.K. The relationship between osteoarthritis and osteoporosis. J. Bone Miner. Metab. 2014, 32, 101–109. [Google Scholar] [CrossRef]
- Kaspiris, A.; Hadjimichael, A.C.; Lianou, I.; Iliopoulos, I.D.; Ntourantonis, D.; Melissaridou, D.; Savvidou, O.D.; Papadimitriou, E.; Chronopoulos, E. Subchondral Bone Cyst Development in Osteoarthritis: From Pathophysiology to Bone Microarchitecture Changes and Clinical Implementations. J. Clin. Med. 2023, 12, 815. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.R. Proinflammatory cytokines and osteoporosis. Curr. Osteoporos. Rep. 2009, 7, 134–139. [Google Scholar] [CrossRef]
- Jehan, F.; Zarka, M.; de la Houssaye, G.; Veziers, J.; Ostertag, A.; Cohen-Solal, M.; Geoffroy, V. New insights into the role of matrix metalloproteinase 3 (MMP3) in bone. FASEB Bioadv. 2022, 4, 524–538. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, B.; Yan, F.; Guo, J.; Zhu, X.; Ma, S.; Yang, W. Interleukin-10 inhibits bone resorption: A potential therapeutic strategy in periodontitis and other bone loss diseases. BioMed Res. Int. 2014, 2014, 284836. [Google Scholar] [CrossRef]
- Shen, Y.; Winkler, I.G.; Barbier, V.; Sims, N.A.; Hendy, J.; Lévesque, J.P. Tissue inhibitor of metalloproteinase-3 (TIMP-3) regulates hematopoiesis and bone formation in vivo. PLoS ONE 2010, 5, e13086. [Google Scholar] [CrossRef]
- Eek, D.; Krohe, M.; Mazar, I.; Horsfield, A.; Pompilus, F.; Friebe, R.; Shields, A.L. Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: A review of the literature. Patient Prefer. Adherence 2016, 10, 1609–1621. [Google Scholar] [CrossRef]
- Zhang, J.K.; Yang, L.; Meng, G.L.; Yuan, Z.; Fan, J.; Li, D.; Chen, J.Z.; Shi, T.Y.; Hu, H.M.; Wei, B.Y.; et al. Protection by salidroside against bone loss via inhibition of oxidative stress and bone-resorbing mediators. PLoS ONE 2013, 8, e57251. [Google Scholar] [CrossRef]
- Fu, S.; Yan, M.; Fan, Q.; Xu, J. Salidroside promotes osteoblast proliferation and differentiation via the activation of AMPK to inhibit bone resorption of knee osteoarthritis mice. Tissue Cell 2022, 79, 101917. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Qi, S.; Chen, C. Salidroside Improves Bone Histomorphology and Prevents Bone Loss in Ovariectomized Diabetic Rats by Upregulating the OPG/RANKL Ratio. Molecules 2018, 23, 2398. [Google Scholar] [CrossRef] [PubMed]
- Buccino, F.; Zagra, L.; Longo, E.; D’Amico, L.; Banfi, G.; Berto, F.; Tromba, G.; Vergani, L.M. Osteoporosis and COVID-19: Detected similarities in bone lacunar-level alterations via combined AI and advanced synchrotron testing. Mater. Des. 2023, 231, 112087. [Google Scholar] [CrossRef] [PubMed]
- Goff, E.; Cohen, A.; Shane, E.; Recker, R.R.; Kuhn, G.; Müller, R. Large-scale osteocyte lacunar morphological analysis of transiliac bone in normal and osteoporotic premenopausal women. Bone 2022, 160, 116424. [Google Scholar] [CrossRef] [PubMed]
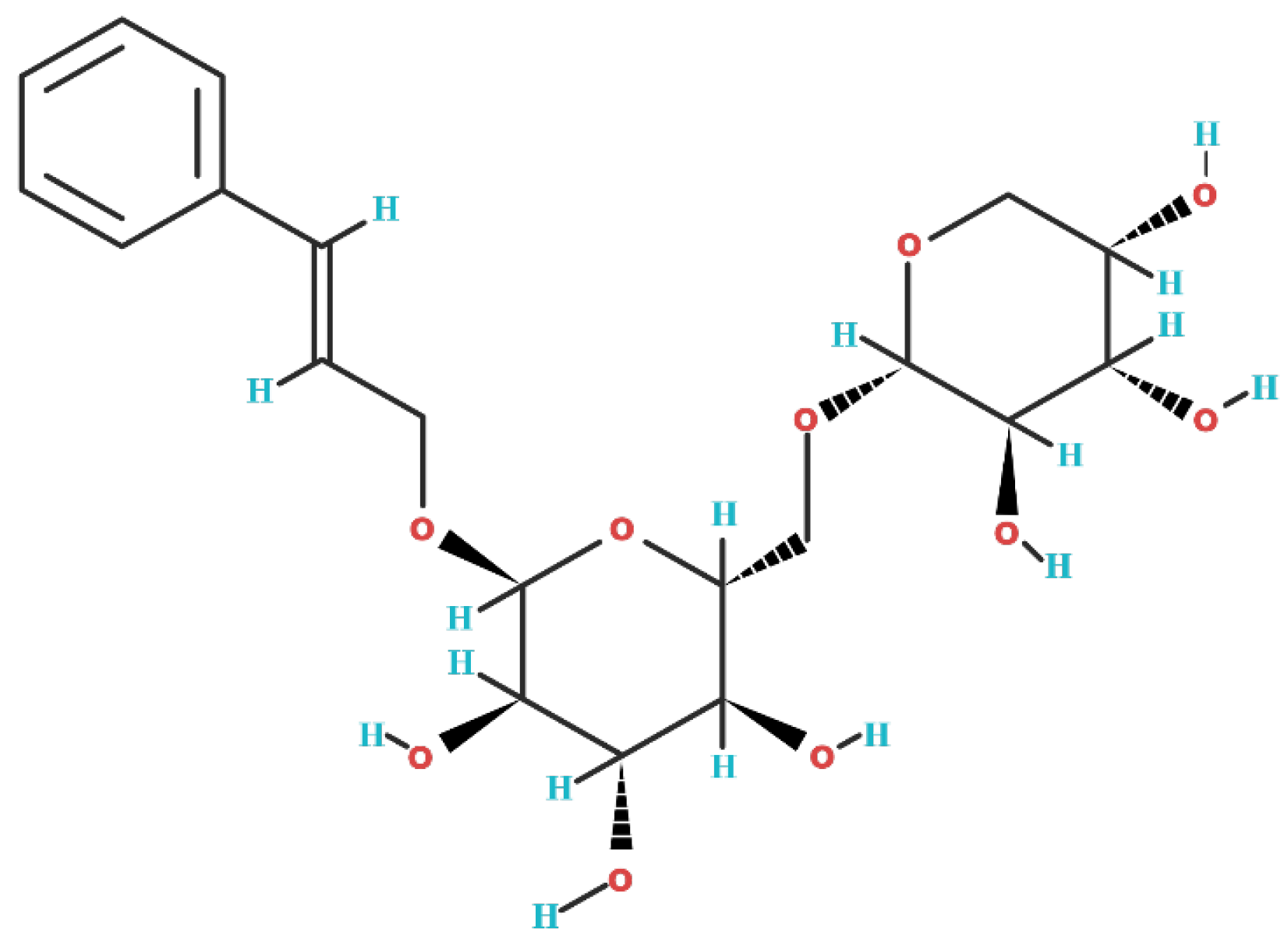

| BMMSCs (Isolated from the Femoral and Tibial Bone Marrow of C57BL/6 Mice) | RAW264.7 (Murine Macrophage Cells) | MC3T3-E (ATCC, Manassas, VA, USA) | |
|---|---|---|---|
| Osteoclastogenesis (number of TRAP-positive cells) | – | – | n/a |
| Osteoblast differentiation | + | n/a | + |
| Expressions of OCN and Runx2 | + | n/a | n/a |
| Expressions of c-fms and RANK | ↔ | n/a | n/a |
| Formation of F-actin rings | – | n/a | n/a |
| Expressions of cathepsin K, CTR, TRAF6, TRAP, and MMP-9 | – | – | n/a |
| Expression of NFATc1 | – | – | n/a |
| Activity of NF-κB and MAPK signaling pathways | n/a | – | n/a |
| Expression of c-Fos | n/a | – | n/a |
| Phosphorylation of ERK, p38, p56, and JNK | n/a | – | n/a |
| Phosphorylation of IκBα | n/a | – | n/a |
| mRNA expression of EEF2 | + | n/a | + |
| mRNA expression of HDAC1 | – | n/a | – |
| Blood Serum | Bone Tissue | Synovial Membrane | |
|---|---|---|---|
| C57BL/6 and OVX mice (intraperitoneal injections of rosavin (10 mg/kg)) | ↓ concentrations of CTX-1, TRAcp5b, RANKL, M-CSF, and TRAP ↑ concentrations of ALP and OCN | ↓ number of osteoclasts ↑ number of osteoblasts ↑ expression of EEF2 ↓ expression HDAC1 ↓ bone resorption ↑ number of calcium nodules | n/a |
| Male Wistar rats (Central Lab. Animal, Inc., Seoul, Korea) after intra-articular injection of monosodium iodoacetate (right knee) and oral administration of rosavin (100 mg/day) combined with zinc (20 mg/day) and a complex of probiotics (CNS, Pharm Korea Co., Ltd., Seoul, Korea) | n/a | ↓ bone resorption | ↓ expressions of MMP-3, IL-6, and TNF-α ↑ expressions IL-10 and TIMP3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojdasiewicz, P.; Turczyn, P.; Lach-Gruba, A.; Poniatowski, Ł.A.; Purrahman, D.; Mahmoudian-Sani, M.-R.; Szukiewicz, D. The Role of Rosavin in the Pathophysiology of Bone Metabolism. Int. J. Mol. Sci. 2024, 25, 2117. https://doi.org/10.3390/ijms25042117
Wojdasiewicz P, Turczyn P, Lach-Gruba A, Poniatowski ŁA, Purrahman D, Mahmoudian-Sani M-R, Szukiewicz D. The Role of Rosavin in the Pathophysiology of Bone Metabolism. International Journal of Molecular Sciences. 2024; 25(4):2117. https://doi.org/10.3390/ijms25042117
Chicago/Turabian StyleWojdasiewicz, Piotr, Paweł Turczyn, Anna Lach-Gruba, Łukasz A. Poniatowski, Daryush Purrahman, Mohammad-Reza Mahmoudian-Sani, and Dariusz Szukiewicz. 2024. "The Role of Rosavin in the Pathophysiology of Bone Metabolism" International Journal of Molecular Sciences 25, no. 4: 2117. https://doi.org/10.3390/ijms25042117
APA StyleWojdasiewicz, P., Turczyn, P., Lach-Gruba, A., Poniatowski, Ł. A., Purrahman, D., Mahmoudian-Sani, M.-R., & Szukiewicz, D. (2024). The Role of Rosavin in the Pathophysiology of Bone Metabolism. International Journal of Molecular Sciences, 25(4), 2117. https://doi.org/10.3390/ijms25042117






