Classical and Innovative Evidence for Therapeutic Strategies in Retinal Dysfunctions
Abstract
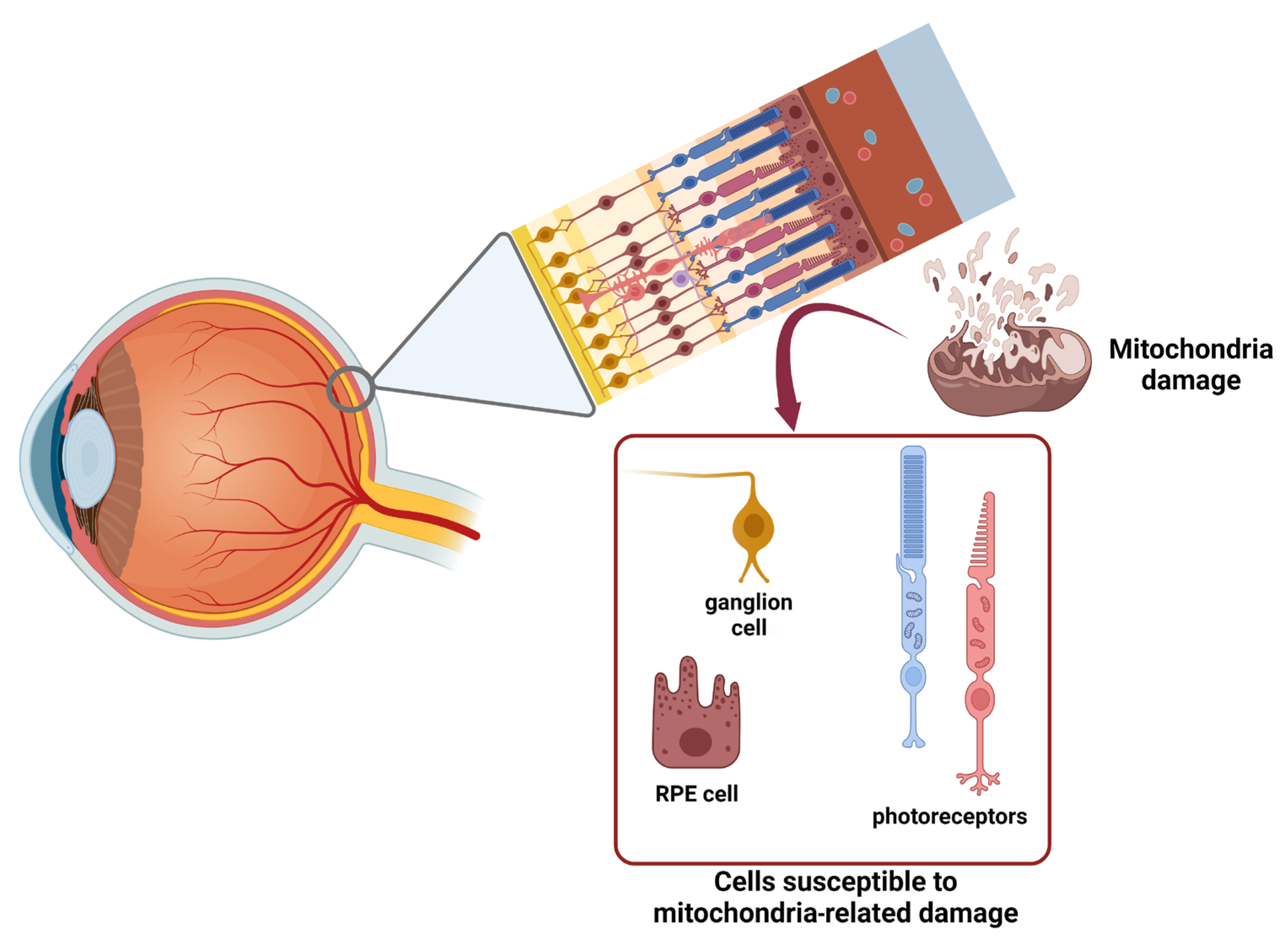
1. Functional Anatomy of Retina: Morphofunctional Characterization
2. Autophagy and Mitophagy: Programmed Cell ‘Amputations’ to Limit the Damage
3. Etiopathogenesis and Characterization of Retinopathies: Two Meaningful Examples
3.1. Glaucoma
3.2. Diabetic Retinopathy
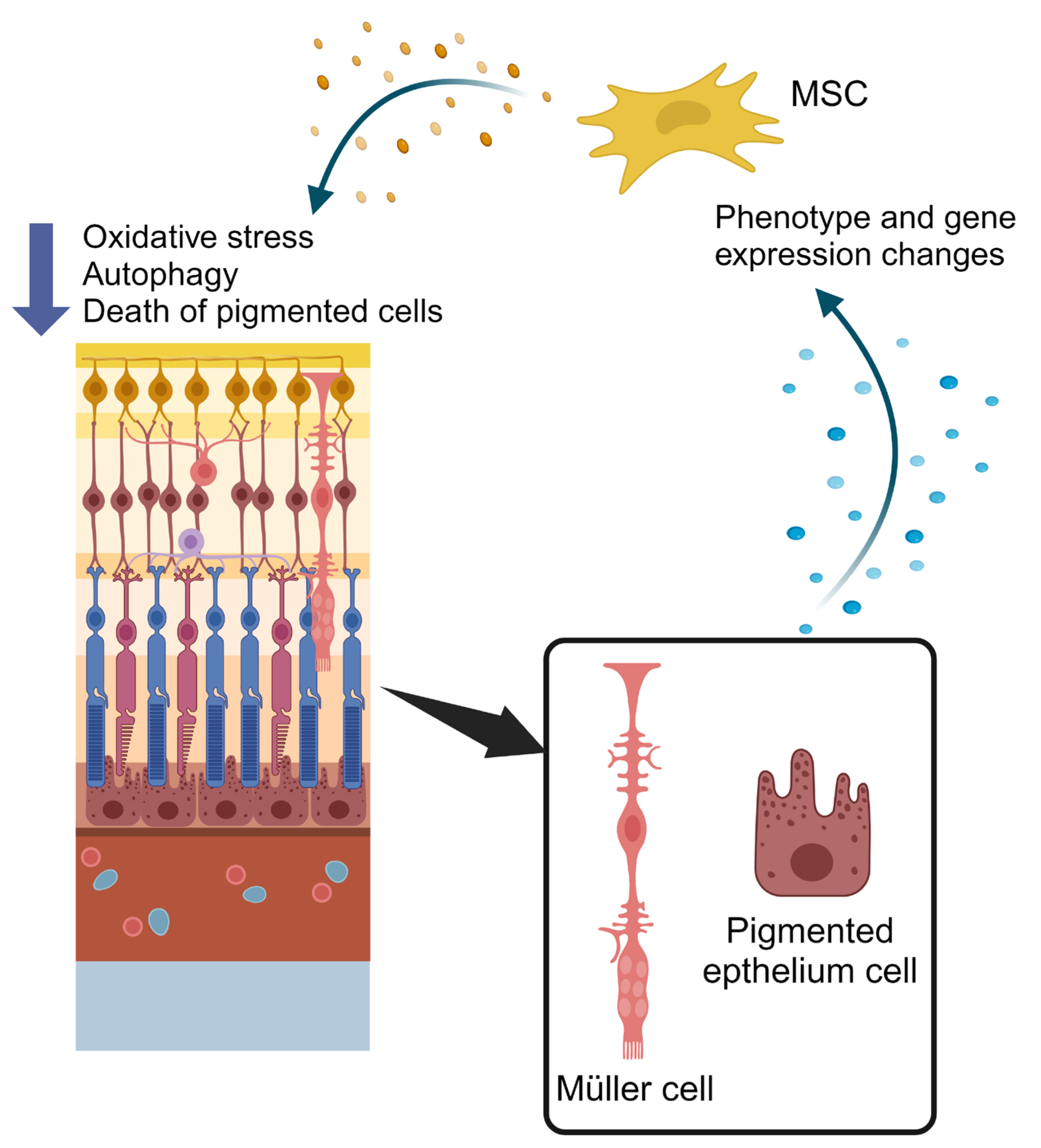
4. Therapeutic Applications of Extracellular Vesicles and Secretome Modulation from Mesenchymal Stem Cells in Retinopathies
5. Non-Coding RNAs as Innovative Diagnostic Tools and Therapeutic Molecules for Diabetic Retinopathy
5.1. Non-Coding RNAs
5.2. ncRNAs Involvement in Diabetic Retinopathy
5.3. ncRNAs as Predictive Biomarkers and Novel Therapeutics
6. The Gut–Retina Axis: A New Perspective in the Study of Retinal Dysfunction
6.1. Ocular Microbiota
6.2. Gut–Eye Axis
6.3. Potential Effectiveness of Probiotics and Prebiotics
7. Human Retinal Organoid: Is It a Useful New Tool for Preclinical Studies?
7.1. Retinal Organoids
7.2. Organoids as Models for Retinal Degenerative Diseases
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mahabadi, N.; Al Khalili, Y. Neuroanatomy, Retina. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- O’Leary, F.; Campbell, M. The blood-retina barrier in health and disease. FEBS J. 2023, 290, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H. The neural organization of the human retina. In Principles and Practice of Clinical Electrophysiology of Vision; Heckenlively, J.R., Arden, G.B., Eds.; Mosby Year Book: St. Louis, MO, USA, 1991; pp. 25–52. [Google Scholar]
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R.O. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res. 2014, 42, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Masland, R.H. The neuronal organization of the retina. Neuron 2012, 76, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Lamb, T.D. Why rods and cones? Eye 2016, 30, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.M. Phototransduction in ganglion-cell photoreceptors. Pflug. Arch. 2007, 454, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Euler, T.; Haverkamp, S.; Schubert, T.; Baden, T. Retinal bipolar cells: Elementary building blocks of vision. Nat. Rev. Neurosci. 2014, 15, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Masland, R.H. The tasks of amacrine cells. Vis. Neurosci. 2012, 29, 3–9. [Google Scholar] [CrossRef]
- Deniz, S.; Wersinger, E.; Schwab, Y.; Mura, C.; Erdelyi, F.; Szabó, G.; Rendon, A.; Sahel, J.-A.; Picaud, S.; Roux, M.J. Mammalian retinal horizontal cells are unconventional GABAergic neurons. J. Neurochem. 2011, 116, 350–362. [Google Scholar] [CrossRef]
- Kobat, S.G.; Turgut, B. Importance of Müller Cells. Beyoglu Eye J. 2020, 5, 59–63. [Google Scholar]
- Fanjul-Moles, M.L.; López-Riquelme, G.O. Relationship between Oxidative Stress, Circadian Rhythms, and AMD. Oxid. Med. Cell. Longev. 2016, 2016, 7420637. [Google Scholar] [CrossRef]
- Baba, K.; Goyal, V.; Tosini, G. Circadian Regulation of Retinal Pigment Epithelium Function. Int. J. Mol. Sci. 2022, 23, 2699. [Google Scholar] [CrossRef] [PubMed]
- DeVera, C.; Dixon, J.; Chrenek, M.A.; Baba, K.; Le, Y.Z.; Iuvone, P.M.; Tosini, G. The Circadian Clock in the Retinal Pigment Epithelium Controls the Diurnal Rhythm of Phagocytic Activity. Int. J. Mol. Sci. 2022, 23, 5302. [Google Scholar] [CrossRef] [PubMed]
- Country, M.W. Retinal metabolism: A comparative look at energetics in the retina. Brain Res. 2017, 1672, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Joyal, J.-S.; Sun, Y.; Gantner, M.L.; Shao, Z.; Evans, L.P.; Saba, N.; Fredrick, T.; Burnim, S.; Kim, J.-S.; Patel, G.; et al. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat. Med. 2016, 22, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.-K.; Perkins, G.A.; Kim, K.-Y.; Bastola, T.; Choi, W.-Y.; Choi, S.-H. Glaucomatous optic neuropathy: Mitochondrial dynamics, dysfunction and protection in retinal ganglion cells. Prog. Retin. Eye Res. 2023, 95, 101136. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Bouhamida, E.; Danese, A.; Previati, M.; Pinton, P.; Patergnani, S. Relevance of Autophagy and Mitophagy Dynamics and Markers in Neurodegenerative Diseases. Biomedicines 2021, 9, 149. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Heckmann, B.L.; Green, D.R. LC3-associated phagocytosis at a glance. J. Cell Sci. 2019, 132, jcs222984. [Google Scholar] [CrossRef]
- Frost, L.S.; Lopes, V.S.; Bragin, A.; Reyes-Reveles, J.; Brancato, J.; Cohen, A.; Mitchell, C.H.; Williams, D.S.; Boesze-Battaglia, K. The Contribution of Melanoregulin to Microtubule-Associated Protein 1 Light Chain 3 (LC3) Associated Phagocytosis in Retinal Pigment Epithelium. Mol. Neurobiol. 2015, 52, 1135–1151. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J. LAP it up, fuzz ball: A short history of LC3-associated phagocytosis. Curr. Opin. Immunol. 2018, 55, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Yefimova, M.G.; Ravel, C.; Rolland, A.D.; Bourmeyster, N.; Jégou, B. MERTK-Mediated LC3-Associated Phagocytosis (LAP) of Apoptotic Substrates in Blood-Separated Tissues: Retina, Testis, Ovarian Follicles. Cells 2021, 10, 1443. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Mitter, S.K.; Qi, X.; Beli, E.; Rao, H.V.; Ding, J.; Ip, C.S.; Gu, H.; Akin, D.; Dunn, W.A., Jr.; et al. Oxidative stress-mediated NFκB phosphorylation upregulates p62/SQSTM1 and promotes retinal pigmented epithelial cell survival through increased autophagy. PLoS ONE 2017, 12, e0171940. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, K.-K.; Tong, Y.; Zhou, Y.-L.; Wang, Y.-X.; Zhao, P.-Q.; Wang, Z.-Y. Exogenous NAD+ decreases oxidative stress and protects H2O2-treated RPE cells against necrotic death through the up-regulation of autophagy. Sci. Rep. 2016, 6, 26322. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, A.; Bell, B.A.; Peachey, N.S.; Daniele, L.L.; Reyes-Reveles, J.; Sharp, R.C.; Jun, B.; Bazan, N.G.; Sparrow, J.R.; Kim, H.J.; et al. Microtubule-Associated Protein 1 Light Chain 3B, (LC3B) Is Necessary to Maintain Lipid-Mediated Homeostasis in the Retinal Pigment Epithelium. Front. Cell. Neurosci. 2018, 12, 351. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2023, 19, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Popov, L.-D. Mitochondrial-derived vesicles: Recent insights. J. Cell. Mol. Med. 2022, 26, 3323–3328. [Google Scholar] [CrossRef]
- Panicker, N.; Ge, P.; Dawson, V.L.; Dawson, T.M. The cell biology of Parkinson’s disease. J. Cell Biol. 2021, 220, e202012095. [Google Scholar] [CrossRef]
- Jin, S.M.; Lazarou, M.; Wang, C.; Kane, L.A.; Narendra, D.P.; Youle, R.J. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 2010, 191, 933–942. [Google Scholar] [CrossRef]
- Deas, E.; Plun-Favreau, H.; Gandhi, S.; Desmond, H.; Kjaer, S.; Loh, S.H.; Renton, A.E.; Harvey, R.J.; Whitworth, A.J.; Martins, L.M.; et al. PINK1 cleavage at position A103 by the mitochondrial protease PARL. Hum. Mol. Genet. 2011, 20, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Yamano, K.; Youle, R.J. PINK1 is degraded through the N-end rule pathway. Autophagy 2013, 9, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Hasson, S.A.; Kane, L.A.; Yamano, K.; Huang, C.H.; Sliter, D.A.; Buehler, E.; Wang, C.; Heman-Ackah, S.M.; Hessa, T.; Guha, R.; et al. High-content genome-wide RNAi screens identify regulators of parkin upstream of mitophagy. Nature 2013, 504, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, M.; Jin, S.M.; Kane, L.A.; Youle, R.J. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev. Cell 2012, 22, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Okatsu, K.; Oka, T.; Iguchi, M.; Imamura, K.; Kosako, H.; Tani, N.; Kimura, M.; Go, E.; Koyano, F.; Funayama, M.; et al. PINK1 autophosphorylation upon membrane potential dissipation is essential for Parkin recruitment to damaged mitochondria. Nat. Commun. 2012, 3, 1016. [Google Scholar] [CrossRef] [PubMed]
- Kondapalli, C.; Kazlauskaite, A.; Zhang, N.; Woodroof, H.I.; Campbell, D.G.; Gourlay, R.; Burchell, L.; Walden, H.; Macartney, T.J.; Deak, M.; et al. PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol. 2012, 2, 120080. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Holmström, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef]
- Lazarou, M.; Sliter, D.A.; Kane, L.A.; Sarraf, S.A.; Wang, C.; Burman, J.L.; Sideris, D.P.; Fogel, A.I.; Youle, R.J. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 2015, 524, 309–314. [Google Scholar] [CrossRef]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal Diseases Associated with Oxidative Stress and the Effects of a Free Radical Scavenger (Edaravone). Oxid. Med. Cell. Longev. 2017, 2017, 9208489. [Google Scholar] [CrossRef]
- Yerramothu, P.; Vijay, A.K.; Willcox, M.D.P. Inflammasomes, the eye and anti-inflammasome therapy. Eye 2018, 32, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Marneros, A.G. Role of inflammasome activation in neovascular age-related macular degeneration. FEBS J. 2023, 290, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Wooff, Y.; Man, S.M.; Aggio-Bruce, R.; Natoli, R.; Fernando, N. IL-1 Family Members Mediate Cell Death, Inflammation and Angiogenesis in Retinal Degenerative Diseases. Front. Immunol. 2019, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Menini, S.; Iacobini, C.; Vitale, M.; Pugliese, G. The Inflammasome in Chronic Complications of Diabetes and Related Metabolic Disorders. Cells 2020, 9, 1812. [Google Scholar] [CrossRef] [PubMed]
- O’Koren, E.G.; Yu, C.; Klingeborn, M.; Wong, A.Y.W.; Prigge, C.L.; Mathew, R.; Kalnitsky, J.; Msallam, R.A.; Silvin, A.; Kay, J.N.; et al. Microglial Function Is Distinct in Different Anatomical Locations during Retinal Homeostasis and Degeneration. Immunity 2019, 50, 723–737.e7. [Google Scholar] [CrossRef] [PubMed]
- Eamegdool, S.S.; Sitiwin, E.I.; Cioanca, A.V.; Madigan, M.C. Extracellular matrix and oxidative stress regulate human retinal pigment epithelium growth. Free Radic. Biol. Med. 2020, 146, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Sene, A.; Apte, R.S. Inflammation-Induced Photoreceptor Cell Death. Adv. Exp. Med. Biol. 2018, 1074, 203–208. [Google Scholar] [PubMed]
- Yang, Y.; Jiang, G.; Huang, R.; Liu, Y.; Chang, X.; Fu, S. Targeting the NLRP3 inflammasome in diabetic retinopathy: From pathogenesis to therapeutic strategies. Biochem. Pharmacol. 2023, 212, 115569. [Google Scholar] [CrossRef]
- Chaurasia, S.S.; Lim, R.R.; Parikh, B.H.; Wey, Y.S.; Tun, B.B.; Wong, T.Y.; Luu, C.D.; Agrawal, R.; Ghosh, A.; Mortellaro, A.; et al. The NLRP3 Inflammasome May Contribute to Pathologic Neovascularization in the Advanced Stages of Diabetic Retinopathy. Sci. Rep. 2018, 8, 2847. [Google Scholar] [CrossRef]
- Liu, J.; Copland, D.A.; Theodoropoulou, S.; Chiu, H.A.A.; Barba, M.D.; Mak, K.W.; Mack, M.; Nicholson, L.B.; Dick, A.D. Impairing autophagy in retinal pigment epithelium leads to inflammasome activation and enhanced macrophage-mediated angiogenesis. Sci. Rep. 2016, 6, 20639. [Google Scholar] [CrossRef] [PubMed]
- Sui, A.; Zhong, Y.; Demetriades, A.M.; Lu, Q.; Cai, Y.; Gao, Y.; Zhu, Y.; Shen, X.; Xie, B. Inhibition of integrin α5β1 ameliorates VEGF-induced retinal neovascularization and leakage by suppressing NLRP3 inflammasome signaling in a mouse model. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Adornetto, A.; Rombolà, L.; Morrone, L.A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Russo, R. Natural Products: Evidence for Neuroprotection to Be Exploited in Glaucoma. Nutrients 2020, 12, 3158. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Lopez-Bernal, M.D.; Cobo-Martinez, A.; Zanon-Moreno, V.; Pinazo-Duran, M.D.; Del-Rio-Vellosillo, M. Glaucoma and Antioxidants: Review and Update. Antioxidants 2020, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- Hurley, D.J.; Normile, C.; Irnaten, M.; O’Brien, C. The Intertwined Roles of Oxidative Stress and Endoplasmic Reticulum Stress in Glaucoma. Antioxidants 2022, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amero, K.K.; Morales, J.; Bosley, T.M. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2533–2541. [Google Scholar] [CrossRef]
- Kim, K.-Y.; A Perkins, G.; Shim, M.S.; Bushong, E.; Alcasid, N.; Ju, S.; Ellisman, M.H.; Weinreb, R.N.; Ju, W.-K. DRP1 inhibition rescues retinal ganglion cells and their axons by preserving mitochondrial integrity in a mouse model of glaucoma. Cell Death Dis. 2015, 6, e1839. [Google Scholar] [CrossRef]
- Dai, Y.; Hu, X.; Sun, X. Overexpression of parkin protects retinal ganglion cells in experimental glaucoma. Cell Death Dis. 2018, 9, 88. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Liu, Y.; Huang, W.; Li, X.; Zhang, X. Inflammatory cytokine profiles in eyes with primary angle-closure glaucoma. Biosci. Rep. 2018, 38, BSR20181411. [Google Scholar] [CrossRef]
- Pronin, A.; Pham, D.; An, W.; Dvoriantchikova, G.; Reshetnikova, G.; Qiao, J.; Kozhekbaeva, Z.; Reiser, A.E.; Slepak, V.Z.; Shestopalov, V.I. Inflammasome Activation Induces Pyroptosis in the Retina Exposed to Ocular Hypertension Injury. Front. Mol. Neurosci. 2019, 12, 36. [Google Scholar] [CrossRef]
- Gong, Y.; Cao, X.; Gong, L.; Li, W. Sulforaphane alleviates retinal ganglion cell death and inflammation by suppressing NLRP3 inflammasome activation in a rat model of retinal ischemia/reperfusion injury. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419861777. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.A.; Kermany, D.S.; Waters, J.; Jansen, M.E.; Tyler, L. Diabetic macular edema: It is more than just VEGF. F1000Research 2016, 5, 1019. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, H.; Noma, H.; Mimura, T.; Eguchi, S.; Hori, S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology 2009, 116, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Boss, J.D.; Singh, P.K.; Pandya, H.K.; Tosi, J.; Kim, C.; Tewari, A.; Juzych, M.S.; Abrams, G.W.; Kumar, A. Assessment of Neurotrophins and Inflammatory Mediators in Vitreous of Patients with Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5594–5603. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Yamagishi, S.; Okamoto, T.; Inagaki, Y.; Amano, S.; Takeuchi, M.; Makita, Z. Serum levels of glucose-derived advanced glycation end products are associated with the severity of diabetic retinopathy in type 2 diabetic patients without renal dysfunction. Int. J. Clin. Pharmacol. Res. 2002, 22, 13–17. [Google Scholar]
- AnandBabu, K.; Sen, P.; Angayarkanni, N. Oxidized LDL, homocysteine, homocysteine thiolactone and advanced glycation end products act as pro-oxidant metabolites inducing cytokine release, macrophage infiltration and pro-angiogenic effect in ARPE-19 cells. PLoS ONE 2019, 14, e0216899. [Google Scholar] [CrossRef]
- Boya, P.; Esteban-Martínez, L.; Serrano-Puebla, A.; Gómez-Sintes, R.; Villarejo-Zori, B. Autophagy in the eye: Development, degeneration, and aging. Prog. Retin. Eye Res. 2016, 55, 206–245. [Google Scholar] [CrossRef]
- Russo, R.; Varano, G.P.; Adornetto, A.; Nazio, F.; Tettamanti, G.; Girardello, R.; Cianfanelli, V.; Cavaliere, F.; Morrone, L.A.; Corasaniti, M.T.; et al. Rapamycin and fasting sustain autophagy response activated by ischemia/reperfusion injury and promote retinal ganglion cell survival. Cell Death Dis. 2018, 9, 981. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Catalani, E.; Dal Monte, M.; Cammalleri, M.; Di Renzo, I.; Perrotta, C.; Cervia, D.; Casini, G. Autophagy-mediated neuroprotection induced by octreotide in an ex vivo model of early diabetic retinopathy. Pharmacol. Res. 2018, 128, 167–178. [Google Scholar] [CrossRef]
- Huang, C.; Lu, H.; Xu, J.; Yu, H.; Wang, X.; Zhang, X. Protective roles of autophagy in retinal pigment epithelium under high glucose condition via regulating PINK1/Parkin pathway and BNIP3L. Biol. Res. 2018, 51, 22. [Google Scholar] [CrossRef] [PubMed]
- Hombrebueno, J.R.; Cairns, L.; Dutton, L.R.; Lyons, T.J.; Brazil, D.P.; Moynagh, P.; Curtis, T.M.; Xu, H. Uncoupled turnover disrupts mitochondrial quality control in diabetic retinopathy. JCI Insight 2019, 4, e129760. [Google Scholar] [CrossRef]
- Patergnani, S.; Bonora, M.; Ingusci, S.; Previati, M.; Marchi, S.; Zucchini, S.; Perrone, M.; Wieckowski, M.R.; Castellazzi, M.; Pugliatti, M.; et al. Antipsychotic drugs counteract autophagy and mitophagy in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2020078118. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.L.; Walker, M.J.; Liu, N.-K.; Risberg, E.C.; Gao, X.; Chen, J.; Xu, X.-M. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS ONE 2012, 7, e30012. [Google Scholar] [CrossRef]
- Du, J.-H.; Li, X.; Li, R.; Cheng, B.-X.; Kuerbanjiang, M.; Ma, L. Role of Autophagy in Angiogenesis Induced by a High-Glucose Condition in RF/6A Cells. Ophthalmologica 2017, 237, 85–95. [Google Scholar] [CrossRef]
- Piano, I.; Novelli, E.; Della Santina, L.; Strettoi, E.; Cervetto, L.; Gargini, C. Involvement of Autophagic Pathway in the Progression of Retinal Degeneration in a Mouse Model of Diabetes. Front. Cell. Neurosci. 2016, 10, 42. [Google Scholar] [CrossRef]
- Madrakhimov, S.B.; Yang, J.Y.; Kim, J.H.; Han, J.W.; Park, T.K. mTOR-dependent dysregulation of autophagy contributes to the retinal ganglion cell loss in streptozotocin-induced diabetic retinopathy. Cell Commun. Signal. 2021, 19, 29. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Duan, Y.; Zhang, X.; Li, X. Mesenchymal-Stem-Cell-Based Strategies for Retinal Diseases. Genes 2022, 13, 1901. [Google Scholar] [CrossRef]
- Lechner, J.; Medina, R.J.; Lois, N.; Stitt, A.W. Advances in cell therapies using stem cells/progenitors as a novel approach for neurovascular repair of the diabetic retina. Stem Cell Res. Ther. 2022, 13, 388. [Google Scholar] [CrossRef]
- Saha, B.; Roy, A.; Beltramo, E.; Sahoo, O.S. Stem cells and diabetic retinopathy: From models to treatment. Mol. Biol. Rep. 2023, 50, 4517–4526. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Russo, C.; Longo, A.; Anfuso, C.D.; Lupo, G.; Lo Furno, D.; Giuffrida, R.; Giurdanella, G. Potential therapeutic applications of mesenchymal stem cells for the treatment of eye diseases. World J. Stem Cells 2021, 13, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jaganathan, B.G. Stem Cell Therapy for Retinal Degeneration: The Evidence to Date. Biol. Targets Ther. 2021, 15, 299–306. [Google Scholar] [CrossRef]
- Donato, L.; Scimone, C.; Alibrandi, S.; Scalinci, S.Z.; Mordà, D.; Rinaldi, C.; D’Angelo, R.; Sidoti, A. Human retinal secretome: A cross-link between mesenchymal and retinal cells. World J. Stem Cells 2023, 15, 665–686. [Google Scholar] [CrossRef] [PubMed]
- Moghadam Fard, A.; Mirshahi, R.; Naseripour, M.; Ghasemi Falavarjani, K. Stem Cell Therapy in Stargardt Disease: A Systematic Review. J. Ophthalmic Vis. Res. 2023, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Massoumi, H.; Amin, S.; Soleimani, M.; Momenaei, B.; Ashraf, M.J.; Guaiquil, V.H.; Hematti, P.; Rosenblatt, M.I.; Djalilian, A.R.; Jalilian, E. Extracellular-Vesicle-Based Therapeutics in Neuro-Ophthalmic Disorders. Int. J. Mol. Sci. 2023, 24, 9006. [Google Scholar] [CrossRef] [PubMed]
- Aguiar Koga, B.A.; Fernandes, L.A.; Fratini, P.; Sogayar, M.C.; Carreira, A.C.O. Role of MSC-derived small extracellular vesicles in tissue repair and regeneration. Front. Cell Dev. Biol. 2023, 10, 1047094. [Google Scholar] [CrossRef]
- Ozmert, E.; Arslan, U. Management of Retinitis Pigmentosa Via Wharton’s Jelly-Derived Mesenchymal Stem Cells or Combination with Magnovision: 3-Year Prospective Results. Stem Cells Transl. Med. 2023, 12, 631–650. [Google Scholar] [CrossRef]
- Nowroozzadeh, M.H.; Ghazanfari, S.; Sanie-Jahromi, F. Human Wharton’s Jelly Mesenchymal Stem Cell Secretome Modifies the Processes of Neuroprotection and Epithelial-Mesenchymal Transition in Retinal Pigment Epithelium at Transcriptional Level. Mol. Biol. Rep. 2023, 50, 5725–5732. [Google Scholar] [CrossRef]
- Abbasi, R.; Mesgin, R.M.; Nazari-Khanamiri, F.; Abdyazdani, N.; Imani, Z.; Talatapeh, S.P.; Nourmohammadi, A.; Nejati, V.; Rezaie, J. Mesenchymal stem cells-derived exosomes: Novel carriers for nanoparticle to combat cancer. Eur. J. Med. Res. 2023, 28, 579. [Google Scholar] [CrossRef]
- Sharma, S.; Bhonde, R. Applicability of mesenchymal stem cell-derived exosomes as a cell-free miRNA therapy and epigenetic modifiers for diabetes. Epigenomics 2023, 15, 1323–1336. [Google Scholar] [CrossRef]
- Ivosevic, Z.; Ljujic, B.; Pavlovic, D.; Matovic, V.; Jankovic, M.G. Mesenchymal Stem Cell-Derived Extracellular Vesicles: New Soldiers in the War on Immune-Mediated Diseases. Cell Transplant. 2023, 32, 9636897231207194. [Google Scholar] [CrossRef]
- Abdullaev, B.; Rasyid, S.A.; Ali, E.; Al-Dhalimy, A.M.B.; Mustafa, Y.F.; Fenjan, M.N.; Misra, N.; Al-Musawi, S.G.; Alawadi, A.; Alsalamy, A. Effective exosomes in breast cancer: Focusing on diagnosis and treatment of cancer progression. Pathol. Res. Pract. 2023, 253, 154995. [Google Scholar] [CrossRef]
- Hu, Z.; You, L.; Hu, S.; Yu, L.; Gao, Y.; Li, L.; Zhang, S. Hepatocellular carcinoma cell-derived exosomal miR-21-5p promotes the polarization of tumor-related macrophages (TAMs) through SP1/XBP1 and affects the progression of hepatocellular carcinoma. Int. Immunopharmacol. 2024, 126, 111149. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Du, Z.; Zhang, H.; Wang, B.; Xia, J. Exosomes derived from human umbilical cord mesenchymal stem cells loaded with RVG-Lamp2b and Netrin-1 promotes Schwann cell invasion and migration. Tissue Cell 2023, 85, 102219. [Google Scholar] [CrossRef] [PubMed]
- Panwar, D.; Shrivastava, D.; Kumar, A.; Gupta, L.K.; Kumar, N.S.S.; Chintagunta, A.D. Efficient strategy to isolate exosomes using anti-CD63 antibodies conjugated to gold nanoparticles. AMB Express 2023, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Usategui-Martin, R.; Fernandez-Bueno, I. Neuroprotective therapy for retinal neurodegenerative diseases by stem cell secretome. Neural Regen. Res. 2021, 16, 117–118. [Google Scholar] [PubMed]
- Bridoux, L.; Etique, N.; Lambert, E.; Thevenard, J.; Sowa, M.-L.; Belloy, N.; Dauchez, M.; Martiny, L.; Charpentier, E. A crucial role for Lyn in TIMP-1 erythroid cell survival signalling pathway. FEBS Lett. 2013, 587, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, L.; Angioni, R.; Calì, B.; Soldani, C.; Ploia, C.; Moalli, F.; Gargesha, M.; D’Amico, G.; Elliman, S.; Tedeschi, G.; et al. Mouse mesenchymal stem cells inhibit high endothelial cell activation and lymphocyte homing to lymph nodes by releasing TIMP-1. Leukemia 2016, 30, 1143–1154. [Google Scholar] [CrossRef]
- Li, L.; Hua, S.; You, L.; Zhong, T. Secretome Derived from Mesenchymal Stem/Stromal Cells: A Promising Strategy for Diabetes and its Complications. Curr. Stem Cell Res. Ther. 2023, 19, 1–23. [Google Scholar] [CrossRef]
- Szilágyi, M.; Pös, O.; Márton, É.; Buglyó, G.; Soltész, B.; Keserű, J.; Penyige, A.; Szemes, T.; Nagy, B. Circulating Cell-Free Nucleic Acids: Main Characteristics and Clinical Application. Int. J. Mol. Sci. 2020, 21, 6827. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-X.; Pu, S.-D.; Li, X.; Yu, Z.-W.; Zhang, Y.-T.; Tong, X.-W.; Shan, Y.-Y.; Gao, X.-Y. Exosomal ncRNAs: Novel therapeutic target and biomarker for diabetic complications. Pharmacol. Res. 2022, 178, 106135. [Google Scholar] [CrossRef]
- Chang, X.; Zhu, G.; Cai, Z.; Wang, Y.; Lian, R.; Tang, X.; Ma, C.; Fu, S. miRNA, lncRNA and circRNA: Targeted Molecules Full of Therapeutic Prospects in the Development of Diabetic Retinopathy. Front. Endocrinol. 2021, 12, 771552. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, Z.-W.; Wang, Y.; Fu, Y.-H.; Gao, X.-Y. MicroRNAs: Potential Targets in Diabetic Retinopathy. Horm. Metab. Res. 2020, 52, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Liu, Z. Effects of microRNA-217 on high glucose-induced inflammation and apoptosis of human retinal pigment epithelial cells (ARPE-19) and its underlying mechanism. Mol. Med. Rep. 2019, 20, 5125–5133. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Muraleedharan, C.K.; Xu, S. Intraocular Delivery of miR-146 Inhibits Diabetes-Induced Retinal Functional Defects in Diabetic Rat Model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.A.; Steinle, J.J. miR-146a Attenuates Inflammatory Pathways Mediated by TLR4/NF-κB and TNFα to Protect Primary Human Retinal Microvascular Endothelial Cells Grown in High Glucose. Mediat. Inflamm. 2016, 2016, 3958453. [Google Scholar] [CrossRef]
- Barutta, F.; Corbetta, B.; Bellini, S.; Guarrera, S.; Matullo, G.; Scandella, M.; Schalkwijk, C.; Stehouwer, C.D.; Chaturvedi, N.; Soedamah-Muthu, S.S.; et al. MicroRNA 146a is associated with diabetic complications in type 1 diabetic patients from the EURODIAB PCS. J. Transl. Med. 2021, 19, 475. [Google Scholar] [CrossRef]
- Xue, L.; Xiong, C.; Li, J.; Ren, Y.; Zhang, L.; Jiao, K.; Chen, C.; Ding, P. miR-200-3p suppresses cell proliferation and reduces apoptosis in diabetic retinopathy via blocking the TGF-β2/Smad pathway. Biosci. Rep. 2020, 40, BSR20201545. [Google Scholar] [CrossRef]
- Zeng, Q.; Luo, Y.; Fang, J.; Xu, S.; Hu, Y.H.; Yin, M. Circ_0000615 promotes high glucose-induced human retinal pigment epithelium cell apoptosis, inflammation and oxidative stress via miR-646/YAP1 axis in diabetic retinopathy. Eur. J. Ophthalmol. 2022, 32, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, F.F.; Wang, S.M. Circ-ITCH restrains the expression of MMP-2, MMP-9 and TNF-α in diabetic retinopathy by inhibiting miR-22. Exp. Mol. Pathol. 2021, 118, 104594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, L.; Xu, H.; Ling, L. Circ_0084043 Facilitates High Glucose-Induced Retinal Pigment Epithelial Cell Injury by Activating miR-128-3p/TXNIP-Mediated Wnt/β-Catenin Signaling Pathway. J. Cardiovasc. Pharmacol. 2021, 78, e112–e121. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhao, Y.; Xu, J.; Li, W.-J.; Chen, Y.; Sun, H.-J. NFE2/miR-423-5p/TFF1 axis regulates high glucose-induced apoptosis in retinal pigment epithelial cells. BMC Mol. Cell Biol. 2019, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, S.; Zhu, Y.; Shen, X. Significant role of microRNA-219-5p in diabetic retinopathy and its mechanism of action. Mol. Med. Rep. 2018, 18, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, T.; Cai, X.; Wang, X.; Li, S.; Xu, B.; Wu, Q. MicroRNA-203a-3p regulates CoCl2-induced apoptosis in human retinal pigment epithelial cells by targeting suppressor of cytokine signaling 3. J. Diabetes Complicat. 2020, 34, 107668. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, M. Circ_001209 aggravates diabetic retinal vascular dysfunction through regulating miR-15b-5p/COL12A1. J. Transl. Med. 2021, 19, 294. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Miao, Y.; Yan, P.; Wang, X.J.; Jiang, C.; Lei, Y. MiR-455-5p ameliorates HG-induced apoptosis, oxidative stress and inflammatory via targeting SOCS3 in retinal pigment epithelial cells. J. Cell. Physiol. 2019, 234, 21915–21924. [Google Scholar] [CrossRef]
- Zeng, Y.; Cui, Z.; Liu, J.; Chen, J.; Tang, S. MicroRNA-29b-3p Promotes Human Retinal Microvascular Endothelial Cell Apoptosis via Blocking SIRT1 in Diabetic Retinopathy. Front. Physiol. 2020, 10, 1621. [Google Scholar] [CrossRef]
- Chen, B.; Wu, L.; Cao, T.; Zheng, H.M.; He, T. MiR-221/SIRT1/Nrf2 signal axis regulates high glucose induced apoptosis in human retinal microvascular endothelial cells. BMC Ophthalmol. 2020, 20, 300. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Y.; Wang, K.; Li, J.; Chu, T.; Shen, H. MicroRNA-148a-3p alleviates high glucose-induced diabetic retinopathy by targeting TGFB2 and FGF2. Acta Diabetol. 2020, 57, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, C.; Li, C.-P.; Xu, S.-S.; Yao, M.-D.; Ge, H.-M.; Sun, Y.-N.; Li, X.-M.; Zhang, S.-J.; Shan, K.; et al. Circular RNA-ZNF532 regulates diabetes-induced retinal pericyte degeneration and vascular dysfunction. J. Clin. Investig. 2020, 130, 3833–3847. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, L.; Fu, J.-S.; Hu, Y.-X.; Luo, R. Regulation of the miR-19b-mediated SOCS6-JAK2/STAT3 pathway by lncRNA MEG3 is involved in high glucose-induced apoptosis in hRMECs. Biosci. Rep. 2020, 40, BSR20194370. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tan, C.; Wang, Y.; Zong, T.; Xie, T.; Yang, Q.; Wu, M.; Liu, Y.; Mu, T.; Wang, X.; et al. The circRNA MKLN1 regulates autophagy in the development of diabetic retinopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Qin, H.; Leng, Y.; Li, X.; Zhang, L.; Bai, D.; Meng, Y.; Wang, J. LncRNA MEG3 overexpression inhibits the development of diabetic retinopathy by regulating TGF-β1 and VEGF. Exp. Ther. Med. 2018, 16, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Kang, X. hsa_circ_0041795 contributes to human retinal pigment epithelial cells (ARPE 19) injury induced by high glucose via sponging miR-646 and activating VEGFC. Gene 2020, 747, 144654. [Google Scholar] [CrossRef]
- Tang, W.; Guo, J.; Gu, R.; Lei, B.; Ding, X.; Ma, J.; Xu, G. MicroRNA-29b-3p inhibits cell proliferation and angiogenesis by targeting VEGFA and PDGFB in retinal microvascular endothelial cells. Mol. Vis. 2020, 26, 64–75. [Google Scholar]
- Pan, Q.; Gao, Z.; Zhu, C.; Peng, Z.; Song, M.; Li, L. Overexpression of histone deacetylase SIRT1 exerts an antiangiogenic role in diabetic retinopathy via miR-20a elevation and YAP/HIF1α/VEGFA depletion. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E932–E943. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Qin, X.-H.; Zhang, J. MicroRNA-183 inhibition exerts suppressive effects on diabetic retinopathy by inactivating BTG1-mediated PI3K/Akt/VEGF signaling pathway. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E1050–E1060. [Google Scholar] [CrossRef]
- Biswas, S.; Feng, B.; Chen, S.; Liu, J.; Aref-Eshghi, E.; Gonder, J.; Ngo, V.; Sadikovic, B.; Chakrabarti, S. The Long Non-Coding RNA HOTAIR Is a Critical Epigenetic Mediator of Angiogenesis in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2021, 62, 20. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Guo, H.; Peng, Y.; Nie, D.; Mo, J.; Ye, L. Knockdown of MALAT1 attenuates high-glucose-induced angiogenesis and inflammation via endoplasmic reticulum stress in human retinal vascular endothelial cells. Biomed. Pharmacother. 2020, 124, 109699. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Tian, W.; Yu, N.; Yu, L. YAP1 is required for the angiogenesis in retinal microvascular endothelial cells via the inhibition of MALAT1-mediated miR-200b-3p in high glucose-induced diabetic retinopathy. J. Cell. Physiol. 2020, 235, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jia, S.B.; Shi, J.M.; Li, W.J.; Tang, L.S.; Zhu, X.H.; Tong, P. LncRNA-MALAT1 promotes neovascularization in diabetic retinopathy through regulating miR-125b/VE-cadherin axis. Biosci. Rep. 2019, 39, BSR20181469. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Wu, K.F.; Wang, D.D. A novel regulatory network of linc00174/miR-150-5p/VEGFA modulates pathological angiogenesis in diabetic retinopathy. Can. J. Physiol. Pharmacol. 2021, 99, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Liu, K.-C.; Wang, W.-P.; Xu, Y. Circular RNA COL1A2 promotes angiogenesis via regulating miR-29b/VEGF axis in diabetic retinopathy. Life Sci. 2020, 256, 117888. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lu, Y.; Lei, T. TPTEP1 suppresses high glucose-induced dysfunction in retinal vascular endothelial cells by interacting with STAT3 and targeting VEGFA. Acta Diabetol. 2021, 58, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, W.-X.; Huang, X.-G. MicroRNA-199a-3p inhibits angiogenesis by targeting the VEGF/PI3K/AKT signalling pathway in an in vitro model of diabetic retinopathy. Exp. Mol. Pathol. 2020, 116, 104488. [Google Scholar] [CrossRef]
- Han, N.; Xu, H.; Yu, N.; Wu, Y.; Yu, L. MiR-203a-3p inhibits retinal angiogenesis and alleviates proliferative diabetic retinopathy in oxygen-induced retinopathy (OIR) rat model via targeting VEGFA and HIF-1α. Clin. Exp. Pharmacol. Physiol. 2020, 47, 85–94. [Google Scholar] [CrossRef]
- Pan, T.; Wu, Y.; Zhang, X.; Wang, J.; Wang, X.; Gu, Q.; Xu, C.; Fan, Y.; Li, X.; Xie, P.; et al. Lens epithelial cell-derived exosome inhibits angiogenesis in ocular pathological neovascularization through its delivery of miR-146a-5p. FASEB J. 2023, 37, e23192. [Google Scholar] [CrossRef]
- Qiu, F.; Tong, H.; Wang, Y.; Tao, J.; Wang, H.; Chen, L. Inhibition of miR-21-5p suppresses high glucose-induced proliferation and angiogenesis of human retinal microvascular endothelial cells by the regulation of AKT and ERK pathways via maspin. Biosci. Biotechnol. Biochem. 2018, 82, 1366–1376. [Google Scholar] [CrossRef]
- Cai, F.; Jiang, H.; Li, Y.; Li, Q.; Yang, C. Upregulation of long non-coding RNA SNHG16 promotes diabetes-related RMEC dysfunction via activating NF-κB and PI3K/AKT pathways. Mol. Ther. Nucleic Acids 2021, 24, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Liao, X.; Liu, F.; Zhang, Q.; Qiu, J.-J.; Fu, S.-H. miR-132 mediates cell permeability and migration by targeting occludin in high-glucose-induced ARPE-19 cells. Endocr. J. 2021, 68, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Li, J.-L.; Yuan, X.-W. MicroRNA-411 plays a protective role in diabetic retinopathy through targeted regulating Robo4. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9171–9179. [Google Scholar] [PubMed]
- Gong, Q.; Xie, J.; Li, Y.; Liu, Y.; Su, G. Enhanced ROBO4 is mediated by up-regulation of HIF-1α/SP1 or reduction in miR-125b-5p/miR-146a-5p in diabetic retinopathy. J. Cell. Mol. Med. 2019, 23, 4723–4737. [Google Scholar] [CrossRef] [PubMed]
- Zampetaki, A.; Willeit, P.; Burr, S.; Yin, X.; Langley, S.R.; Kiechl, S.; Klein, R.; Rossing, P.; Chaturvedi, N.; Mayr, M. Angiogenic microRNAs Linked to Incidence and Progression of Diabetic Retinopathy in Type 1 Diabetes. Diabetes 2016, 65, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.-L.; An, M.-X.; Liu, Y.-L.; Xu, H.-C.; Lu, Z.-Q. MicroRNA-126: A promising novel biomarker in peripheral blood for diabetic retinopathy. Int. J. Ophthalmol. 2017, 10, 530–534. [Google Scholar] [PubMed]
- Fang, S.; Ma, X.; Guo, S.; Lu, J. MicroRNA-126 inhibits cell viability and invasion in a diabetic retinopathy model via targeting IRS-1. Oncol. Lett. 2017, 14, 4311–4318. [Google Scholar] [CrossRef]
- Liu, H.-N.; Cao, N.-J.; Li, X.; Qian, W.; Chen, X.-L. Serum microRNA-211 as a biomarker for diabetic retinopathy via modulating Sirtuin 1. Biochem. Biophys. Res. Commun. 2018, 505, 1236–1243. [Google Scholar] [CrossRef]
- Zou, H.-L.; Wang, Y.; Gang, Q.; Zhang, Y.; Sun, Y. Plasma level of miR-93 is associated with higher risk to develop type 2 diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1159–1166. [Google Scholar] [CrossRef]
- Long, J.; Wang, Y.; Wang, W.; Chang, B.H.J.; Danesh, F.R. Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J. Biol. Chem. 2010, 285, 23457–23465. [Google Scholar] [CrossRef] [PubMed]
- Pastukh, N.; Meerson, A.; Kalish, D.; Jabaly, H.; Blum, A. Serum miR-122 levels correlate with diabetic retinopathy. Clin. Exp. Med. 2019, 19, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-N.; Li, X.; Wu, N.; Tong, M.-M.; Chen, S.; Zhu, S.-S.; Qian, W.; Chen, X.-L. Serum microRNA-221 as a biomarker for diabetic retinopathy in patients associated with type 2 diabetes. Int. J. Ophthalmol. 2018, 11, 1889–1894. [Google Scholar] [PubMed]
- Niu, S.-R.; Hu, J.-M.; Lin, S.; Hong, Y. Research progress on exosomes/microRNAs in the treatment of diabetic retinopathy. Front. Endocrinol. 2022, 13, 935244. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, A.; Beltramo, E.; Lopatina, T.; Gai, C.; Trento, M.; Porta, M. Molecular and functional characterization of circulating extracellular vesicles from diabetic patients with and without retinopathy and healthy subjects. Exp. Eye Res. 2018, 176, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Q.; Jin, M.; Wang, Z.; Zhang, X.; Sun, X.; Luo, Y. Noncoding RNAs Are Promising Therapeutic Targets for Diabetic Retinopathy: An Updated Review (2017–2022). Biomolecules 2022, 12, 1774. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fang, Y.; Yu, J.; Chang, X. Hawthorn polyphenols reduce high glucose-induced inflammation and apoptosis in ARPE-19 cells by regulating miR-34a/SIRT1 to reduce acetylation. J. Food Biochem. 2021, 45, e13623. [Google Scholar] [CrossRef]
- Peng, Q.-H.; Tong, P.; Gu, L.M.; Li, W.-J. Astragalus polysaccharide attenuates metabolic memory-triggered ER stress and apoptosis via regulation of miR-204/SIRT1 axis in retinal pigment epithelial cells. Biosci. Rep. 2020, 40, BSR20192121. [Google Scholar] [CrossRef]
- Liu, P.; Peng, Q.-H.; Tong, P.; Li, W.-J. Astragalus polysaccharides suppresses high glucose-induced metabolic memory in retinal pigment epithelial cells through inhibiting mitochondrial dysfunction-induced apoptosis by regulating miR-195. Mol. Med. 2019, 25, 21. [Google Scholar] [CrossRef]
- Tu, Y.; Zhu, M.; Wang, Z.; Wang, K.; Chen, L.; Liu, W.; Shi, Q.; Zhao, Q.; Sun, Y.; Wang, X.; et al. Melatonin inhibits Müller cell activation and pro-inflammatory cytokine production via upregulating the MEG3/miR-204/Sirt1 axis in experimental diabetic retinopathy. J. Cell. Physiol. 2020, 235, 8724–8735. [Google Scholar] [CrossRef]
- Zeng, K.; Wang, Y.; Yang, N.; Wang, D.; Li, S.; Ming, J.; Wang, J.; Yu, X.; Song, Y.; Zhou, X.; et al. Resveratrol Inhibits Diabetic-Induced Müller Cells Apoptosis through MicroRNA-29b/Specificity Protein 1 Pathway. Mol. Neurobiol. 2017, 54, 4000–4014. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, C.; Gu, C.; Cui, X.; Wu, J. MiRNA-144-3p inhibits high glucose induced cell proliferation through suppressing FGF16. Biosci. Rep. 2019, 39, BSR20181788. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Sun, J.-J.; Jiang, Y.-Q.; Li, C.-F. MicroRNA-384-3p inhibits retinal neovascularization through targeting hexokinase 2 in mice with diabetic retinopathy. J. Cell. Physiol. 2018, 234, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Cazzolla, A.P.; Di Cosola, M.; Greco Lucchina, A.; Santacroce, L.; Charitos, I.A.; Topi, S.; Malcangi, G.; Hazballa, D.; Scarano, A.; et al. The integumentary system and its microbiota between health and disease. J. Biol. Regul. Homeost. Agents 2021, 35, 303–321. [Google Scholar] [PubMed]
- Gupta, A.; Singh, V.; Mani, I. Dysbiosis of human microbiome and infectious diseases. Prog. Mol. Biol. Transl. Sci. 2022, 192, 33–51. [Google Scholar] [PubMed]
- Ueta, M.; Kinoshita, S. Innate immunity of the ocular surface. Brain Res. Bull. 2010, 81, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D. Characterization of the normal microbiota of the ocular surface. Exp. Eye Res. 2013, 117, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Borroni, D.; Paytuví-Gallart, A.; Sanseverino, W.; Gómez-Huertas, C.; Bonci, P.; Romano, V.; Giannaccare, G.; Rechichi, M.; Meduri, A.; Oliverio, G.W.; et al. Exploring the Healthy Eye Microbiota Niche in a Multicenter Study. Int. J. Mol. Sci. 2022, 23, 10229. [Google Scholar] [CrossRef] [PubMed]
- Aragona, P.; Baudouin, C.; Benitez Del Castillo, J.M.; Messmer, E.; Barabino, S.; Merayo-Lloves, J.; Brignole-Baudouin, F.; Inferrera, L.; Rolando, M.; Mencucci, R.; et al. The ocular microbiome and microbiota and their effects on ocular surface pathophysiology and disorders. Surv. Ophthalmol. 2021, 66, 907–925. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, L.; Surh, L.; Baker, H.; Peterson, R.M.; Nyhan, W.L. Clinical and metabolic abnormalities in a boy with dietary deficiency of biotin. Pediatrics 1981, 68, 553–558. [Google Scholar] [CrossRef]
- Kugadas, A.; Gadjeva, M. Impact of Microbiome on Ocular Health. Ocul. Surf. 2016, 14, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Yi, S.; Wei, L. Ocular Microbiota and Intraocular Inflammation. Front. Immunol. 2020, 11, 609765. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.R.; Zhang, P.; Zhou, Y.; Yin, Y. Ocular surface microbiota in patients with varying degrees of dry eye severity. Int. J. Ophthalmol. 2023, 16, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jeyalatha M, V.; Qu, Y.; He, X.; Ou, S.; Bu, J.; Jia, C.; Wang, J.; Wu, H.; Liu, Z.; et al. Dry Eye Management: Targeting the Ocular Surface Microenvironment. Int. J. Mol. Sci. 2017, 18, 1398. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Vogt, J.K.; Dalgaard, M.D.; Pedersen, O.; Holmgaard, K.; Heegaard, S. Ocular surface microbiota in patients with aqueous tear-deficient dry eye. Ocul. Surf. 2021, 19, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.N.; Alvarenga, L.S.; Höfling-Lima, A.L.; Freitas, D.; Zorat-Yu, M.C.; Farah, M.E.; Mannis, M.J. Aerobic bacterial conjunctival flora in diabetic patients. Cornea 2004, 23, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Alfuzaie, R. The Link Between Gastrointestinal Microbiome and Ocular Disorders. Clin. Ophthalmol. 2023, 17, 2133–2140. [Google Scholar] [CrossRef]
- Zhang, H.; Mo, Y. The gut-retina axis: A new perspective in the prevention and treatment of diabetic retinopathy. Front. Endocrinol. 2023, 14, 1205846. [Google Scholar] [CrossRef]
- Nadeem, U.; Boachie-Mensah, M.; Zhang, J.; Skondra, D. Gut microbiome and retinal diseases: An updated review. Curr. Opin. Ophthalmol. 2022, 33, 195–201. [Google Scholar] [CrossRef]
- Scuderi, G.; Troiani, E.; Minnella, A.M. Gut Microbiome in Retina Health: The Crucial Role of the Gut-Retina Axis. Front. Microbiol. 2022, 12, 726792. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Koliarakis, I.; Messaritakis, I.; Nikolouzakis, T.K.; Hamilos, G.; Souglakos, J.; Tsiaoussis, J. Oral Bacteria and Intestinal Dysbiosis in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 4146. [Google Scholar] [CrossRef]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef]
- Xue, W.; Li, J.J.; Zou, Y.; Zou, B.; Wei, L. Microbiota and Ocular Diseases. Front. Cell. Infect. Microbiol. 2021, 11, 759333. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, K.; Ouwehand, A.C.; Rautonen, N. Human intestinal microbiota and healthy ageing. Ageing Res. Rev. 2010, 9, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.Z.; Plevy, S.E. The role of the macrophage in sentinel responses in intestinal immunity. Curr. Opin. Gastroenterol. 2010, 26, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.L.; Bernstein, C.N. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 2013, 144, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.; Reinisch, W. Intestinal microbiota: A source of novel biomarkers in inflammatory bowel diseases? Best Pract. Res. Clin. Gastroenterol. 2013, 27, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, Y.; Kanai, T.; Takahara, M.; Oshima, S.; Okamoto, R.; Tsuchiya, K.; Matsumoto, S.; Watanabe, M. Th1/Th17-mediated interstitial pneumonia in chronic colitis mice independent of intestinal microbiota. J. Immunol. 2013, 190, 6616–6625. [Google Scholar] [CrossRef] [PubMed]
- Chisari, G.; Chisari, E.M.; Francaviglia, A.; Chisari, C.G. The mixture of bifidobacterium associated with fructo-oligosaccharides reduces the damage of the ocular surface. Clin. Ter. 2017, 168, e181–e185. [Google Scholar]
- Iovieno, A.; Lambiase, A.; Sacchetti, M.; Stampachiacchiere, B.; Micera, A.; Bonini, S. Preliminary evidence of the efficacy of probiotic eye-drop treatment in patients with vernal keratoconjunctivitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Layús, B.I.; Gomez, M.A.; Cazorla, S.I.; Rodriguez, A.V. Drops of Lactiplantibacillus plantarum CRL 759 culture supernatant attenuates eyes inflammation induced by lipopolysaccharide. Benef. Microbes 2021, 12, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hu, H.; Kung, H.; Zou, R.; Dai, Y.; Hu, Y.; Wang, T.; Lv, T.; Yu, J.; Li, F. Organoids: The current status and biomedical applications. MedComm 2023, 4, e274. [Google Scholar] [CrossRef] [PubMed]
- Eiraku, M.; Sasai, Y. Self-formation of layered neural structures in three-dimensional culture of ES cells. Curr. Opin. Neurobiol. 2012, 22, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Ponnusamy, M.P.; Batra, S.K. Concise Review: Current Status of Three-Dimensional Organoids as Preclinical Models. Stem Cells 2018, 36, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Song, C.J.; Nguyen, T.; Cheng, S.Y.; McMahon, J.A.; Yang, R.; Guo, Q.; Der, B.; Lindström, N.O.; Lin, D.C.; et al. A scalable organoid model of human autosomal dominant polycystic kidney disease for disease mechanism and drug discovery. Cell Stem Cell 2022, 29, 1083–1101.e7. [Google Scholar] [CrossRef]
- Gupta, N.; Matsumoto, T.; Hiratsuka, K.; Saiz, E.G.; Galichon, P.; Miyoshi, T.; Susa, K.; Tatsumoto, N.; Yamashita, M.; Morizane, R. Modeling injury and repair in kidney organoids reveals that homologous recombination governs tubular intrinsic repair. Sci. Transl. Med. 2022, 14, eabj4772. [Google Scholar] [CrossRef]
- Hendriks, D.; Artegiani, B.; Hu, H.; Chuva de Sousa Lopes, S.; Clevers, H. Establishment of human fetal hepatocyte organoids and CRISPR-Cas9-based gene knockin and knockout in organoid cultures from human liver. Nat. Protoc. 2021, 16, 182–217. [Google Scholar] [CrossRef]
- Shinozawa, T.; Kimura, M.; Cai, Y.; Saiki, N.; Yoneyama, Y.; Ouchi, R.; Koike, H.; Maezawa, M.; Zhang, R.-R.; Dunn, A.; et al. High-Fidelity Drug-Induced Liver Injury Screen Using Human Pluripotent Stem Cell-Derived Organoids. Gastroenterology 2021, 160, 831–846.e10. [Google Scholar] [CrossRef] [PubMed]
- Altinisik, N.; Rathinam, D.; Tran, M.; Gopalakrishnan, J. Brain organoids restore cortical damage. Cell Stem Cell 2023, 30, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Ozaki, H.; Morikawa, A.; Shiraiwa, K.; Pin, A.P.; Salem, A.G.; Phommahasay, K.A.; Sugita, B.K.; Vu, C.H.; Mamoun Hammad, S.; et al. Brain organoid-on-a-chip to create multiple domains in forebrain organoids. bioRxiv 2023. [Google Scholar] [CrossRef]
- Bai, J.; Koos, D.S.; Stepanian, K.; Fouladian, Z.; Shayler, D.W.H.; Aparicio, J.G.; Fraser, S.E.; Moats, R.A.; Cobrinik, D. Episodic live imaging of cone photoreceptor maturation in GNAT2-EGFP retinal organoids. Dis. Models Mech. 2023, 16, dmm050193. [Google Scholar] [CrossRef] [PubMed]
- Dubaic, M.; Peskova, L.; Hampl, M.; Weissova, K.; Celiker, C.; Shylo, N.A.; Hruba, E.; Kavkova, M.; Zikmund, T.; Weatherbee, S.D.; et al. Role of ciliopathy protein TMEM107 in eye development: Insights from a mouse model and retinal organoid. Life Sci. Alliance 2023, 6, e202302073. [Google Scholar] [CrossRef] [PubMed]
- Marsee, A.; Roos, F.J.M.; Verstegen, M.M.A.; HPB Organoid Consortium; Gehart, H.; de Koning, E.; Lemaigre, F.; Forbes, S.J.; Peng, W.C.; Huch, M.; et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell 2021, 28, 816–832. [Google Scholar] [CrossRef] [PubMed]
- Gabbin, B.; Meraviglia, V.; Angenent, M.L.; Ward-van Oostwaard, D.; Sol, W.; Mummery, C.L.; Rabelink, T.J.; van Meer, B.J.; van den Berg, C.W.; Bellin, M. Heart and kidney organoids maintain organ-specific function in a microfluidic system. Mater. Today Bio 2023, 23, 100818. [Google Scholar] [CrossRef]
- Nguyen, V.V.T.; Ye, S.; Gkouzioti, V.; van Wolferen, M.E.; Yengej, F.Y.; Melkert, D.; Siti, S.; de Jong, B.; Besseling, P.J.; Spee, B.; et al. A human kidney and liver organoid-based multi-organ-on-a-chip model to study the therapeutic effects and biodistribution of mesenchymal stromal cell-derived extracellular vesicles. J. Extracell. Vesicles 2022, 11, e12280. [Google Scholar] [CrossRef]
- Koning, J.J.; Rodrigues Neves, C.T.; Schimek, K.; Thon, M.; Spiekstra, S.W.; Waaijman, T.; de Gruijl, T.D.; Gibbs, S. A Multi-Organ-on-Chip Approach to Investigate How Oral Exposure to Metals Can Cause Systemic Toxicity Leading to Langerhans Cell Activation in Skin. Front. Toxicol. 2022, 3, 824825. [Google Scholar] [CrossRef]
- Tao, T.; Deng, P.; Wang, Y.; Zhang, X.; Guo, Y.; Chen, W.; Qin, J. Microengineered Multi-Organoid System from hiPSCs to Recapitulate Human Liver-Islet Axis in Normal and Type 2 Diabetes. Adv. Sci. 2022, 9, 2103495. [Google Scholar] [CrossRef]
- Casey, M.A.; Lusk, S.; Kwan, K.M. Build me up optic cup: Intrinsic and extrinsic mechanisms of vertebrate eye morphogenesis. Dev. Biol. 2021, 476, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, A.; Zacchei, A.M.; Ceccherini, V. Retinal reconstitution in vitro after disaggregation of embryonic chicken eyes. Acta Embryol. Morphol. 1961, 4, 47–55. [Google Scholar]
- Layer, P.G.; Weikert, T.; Willbold, E. Chicken retinospheroids as developmental and pharmacological in vitro models: Acetylcholinesterase is regulated by its own and by butyrylcholinesterase activity. Cell Tissue Res. 1992, 268, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Takada, S.; Takada, R.; Takeichi, M. Identification of the laminar-inducing factor: Wnt-signal from the anterior rim induces correct laminar formation of the neural retina in vitro. Dev. Biol. 2003, 260, 414–425. [Google Scholar] [CrossRef]
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Ando, S.; Takata, N.; Kawada, M.; Muguruma, K.; Sekiguchi, K.; Saito, K.; Yonemura, S.; Eiraku, M.; Sasai, Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 2012, 10, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Gutierrez, C.; Xue, T.; Hampton, C.; Vergara, M.N.; Cao, L.-H.; Peters, A.; Park, T.S.; Zambidis, E.T.; Meyer, J.S.; et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun. 2014, 5, 4047. [Google Scholar] [CrossRef]
- Eldred, K.C.; Hadyniak, S.E.; Hussey, K.A.; Brenerman, B.; Zhang, P.W.; Chamling, X.; Sluch, V.M.; Welsbie, D.S.; Hattar, S.; Taylor, J.; et al. Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science 2018, 362, eaau6348. [Google Scholar] [CrossRef]
- Kim, S.; Lowe, A.; Dharmat, R.; Lee, S.; Owen, L.A.; Wang, J.; Shakoor, A.; Li, Y.; Morgan, D.J.; Hejazi, A.A.; et al. Generation, transcriptome profiling, and functional validation of cone-rich human retinal organoids. Proc. Natl. Acad. Sci. USA 2019, 116, 10824–10833. [Google Scholar] [CrossRef]
- Wolburg, H.; Willbold, E.; Layer, P.G. Müller glia endfeet, a basal lamina and the polarity of retinal layers form properly in vitro only in the presence of marginal pigmented epithelium. Cell Tissue Res. 1991, 264, 437–451. [Google Scholar] [CrossRef]
- Sheedlo, H.J.; Li, L.; Turner, J.E. Effects of RPE-cell factors secreted from permselective fibers on retinal cells in vitro. Brain Res. 1992, 587, 327–337. [Google Scholar] [CrossRef]
- Ghareeb, A.E.; Lako, M.; Steel, D.H. Coculture techniques for modeling retinal development and disease, and enabling regenerative medicine. Stem Cells Transl. Med. 2020, 9, 1531–1548. [Google Scholar] [CrossRef]
- Akhtar, T.; Xie, H.; Khan, M.I.; Zhao, H.; Bao, J.; Zhang, M.; Xue, T. Accelerated photoreceptor differentiation of hiPSC-derived retinal organoids by contact co-culture with retinal pigment epithelium. Stem Cell Res. 2019, 39, 101491. [Google Scholar] [CrossRef]
- Usui-Ouchi, A.; Giles, S.; Harkins-Perry, S.; Mills, E.A.; Bonelli, R.; Wei, G.; Ouchi, Y.; Ebihara, N.; Nakao, S.; Friedlander, M.; et al. Integrating human iPSC-derived macrophage progenitors into retinal organoids to generate a mature retinal microglial niche. Glia 2023, 10, 2372–2382. [Google Scholar] [CrossRef] [PubMed]
- Si, T.-E.; Li, Z.; Zhang, J.; Su, S.; Liu, Y.; Chen, S.; Peng, G.-H.; Cao, J.; Zang, W. Epigenetic mechanisms of Müller glial reprogramming mediating retinal regeneration. Front. Cell Dev. Biol. 2023, 11, 1157893. [Google Scholar] [CrossRef] [PubMed]
- Serjanov, D.; Hyde, D.R. Extracellular Matrix: The Unexplored Aspects of Retinal Pathologies and Regeneration. Adv. Exp. Med. Biol. 2023, 1415, 309–317. [Google Scholar] [PubMed]
- Volland, S.; Esteve-Rudd, J.; Hoo, J.; Yee, C.; Williams, D.S. A comparison of some organizational characteristics of the mouse central retina and the human macula. PLoS ONE 2015, 10, e0125631. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, K.; Nayak, D.; Debnath, J.; Das, D.; Shetty, R.; Ghosh, A. Retinal organoids in disease modeling and drug discovery: Opportunities and challenges. Surv. Ophthalmol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-M.; Ma, C.; Jin, K.; Jin, Z.-B. Retinal organoid and gene editing for basic and translational research. Vis. Res. 2023, 210, 108273. [Google Scholar] [CrossRef] [PubMed]
- Daiger, S.P.; Sullivan, L.S.; Bowne, S.J. Genes and mutations causing retinitis pigmentosa. Clin. Genet. 2013, 84, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, J.; Zeng, S.; Li, Z.; Liu, X.; Li, J.; Zhou, W.; Chai, Y.; Zhou, D. Retinal Organoid Models Show Heterozygous Rhodopsin Mutation Favors Endoplasmic Reticulum Stress-Induced Apoptosis in Rods. Stem Cells Dev. 2023, 32, 681–692. [Google Scholar] [CrossRef]
- Sanjurjo-Soriano, C.; Jimenez-Medina, C.; Erkilic, N.; Cappellino, L.; Lefevre, A.; Nagel-Wolfrum, K.; Wolfrum, U.; Van Wijk, E.; Roux, A.-F.; Meunier, I.; et al. USH2A variants causing retinitis pigmentosa or Usher syndrome provoke differential retinal phenotypes in disease-specific organoids. HGG Adv. 2023, 4, 100229. [Google Scholar] [CrossRef]
- Kandoi, S.; Martinez, C.; Chen, K.X.; Reddy, L.V.K.; Mehine, M.; Mansfield, B.C.; Duncan, J.L.; Lamba, D.A. Disease modeling and pharmacological rescue of autosomal dominant Retinitis Pigmentosa associated with RHO copy number variation. medRxiv 2023. [Google Scholar] [CrossRef]
- Deng, W.L.; Gao, M.L.; Lei, X.L.; Lv, J.N.; Zhao, H.; He, K.W.; Xia, X.X.; Li, L.Y.; Chen, Y.C.; Li, Y.P.; et al. Gene Correction Reverses Ciliopathy and Photoreceptor Loss in iPSC-Derived Retinal Organoids from Retinitis Pigmentosa Patients. Stem Cell Rep. 2018, 10, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-P.; Deng, W.-L.; Jin, Z.-B. Modeling retinitis pigmentosa through patient-derived retinal organoids. STAR Protoc. 2021, 2, 100438. [Google Scholar] [CrossRef]
- Georgiou, M.; Yang, C.; Atkinson, R.; Pan, K.T.; Buskin, A.; Molina, M.M.; Collin, J.; Al-Aama, J.; Goertler, F.; Ludwig, S.E.J.; et al. Activation of autophagy reverses progressive and deleterious protein aggregation in PRPF31 patient-induced pluripotent stem cell-derived retinal pigment epithelium cells. Clin. Transl. Med. 2022, 12, e759. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Slembrouck-Brec, A.; Nanteau, C.; Terray, A.; Tymoshenko, Y.; Zagar, Y.; Reichman, S.; Xi, Z.; Sahel, J.-A.; Fouquet, S.; et al. Modeling PRPF31 retinitis pigmentosa using retinal pigment epithelium and organoids combined with gene augmentation rescue. npj Regen. Med. 2022, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- da Costa, B.L.; Li, Y.; Levi, S.R.; Tsang, S.H.; Quinn, P.M.J. Generation of CRB1 RP Patient-Derived iPSCs and a CRISPR/Cas9-Mediated Homology-Directed Repair Strategy for the CRB1 c.2480G>T Mutation. Adv. Exp. Med. Biol. 2023, 1415, 571–576. [Google Scholar] [PubMed]
- Boon, N.; Lu, X.; Andriessen, C.A.; Orlovà, M.; Quinn, P.M.J.; Boon, C.J.F.; Wijnholds, J. Characterization and AAV-mediated CRB gene augmentation in human-derived CRB1KO and CRB1KOCRB2+/− retinal organoids. Mol. Ther. Methods Clin. Dev. 2023, 31, 101128. [Google Scholar] [CrossRef] [PubMed]
- Boon, N.; Lu, X.; Andriessen, C.A.; Moustakas, I.; Buck, T.M.; Freund, C.; Arendzen, C.H.; Böhringer, S.; Boon, C.J.; Mei, H.; et al. AAV-mediated gene augmentation therapy of CRB1 patient-derived retinal organoids restores the histological and transcriptional retinal phenotype. Stem Cell Rep. 2023, 18, 1123–1137. [Google Scholar] [CrossRef]
- Hirami, Y.; Mandai, M.; Sugita, S.; Maeda, A.; Maeda, T.; Yamamoto, M.; Uyama, H.; Yokota, S.; Fujihara, M.; Igeta, M.; et al. Safety and stable survival of stem-cell-derived retinal organoid for 2 years in patients with retinitis pigmentosa. Cell Stem Cell 2023, 30, 1585–1596. [Google Scholar] [CrossRef]
- Zerti, D.; Dorgau, B.; Felemban, M.; Ghareeb, A.E.; Yu, M.; Ding, Y.; Krasnogor, N.; Lako, M. Developing a simple method to enhance the generation of cone and rod photoreceptors in pluripotent stem cell-derived retinal organoids. Stem Cells 2020, 38, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Lu, Q.; Insinna-Kettenhofen, C.; Nagashima, K.; English, M.A.; Semler, E.M.; Mahgerefteh, J.; Cideciyan, A.V.; Li, T.; Brooks, B.P.; et al. In Vitro Modeling Using Ciliopathy-Patient-Derived Cells Reveals Distinct Cilia Dysfunctions Caused by CEP290 Mutations. Cell Rep. 2017, 20, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Rozanska, A.; Cerna-Chavez, R.; Queen, R.; Collin, J.; Zerti, D.; Dorgau, B.; Beh, C.S.; Davey, T.; Coxhead, J.; Hussain, R.; et al. pRB-Depleted Pluripotent Stem Cell Retinal Organoids Recapitulate Cell State Transitions of Retinoblastoma Development and Suggest an Important Role for pRB in Retinal Cell Differentiation. Stem Cells Transl. Med. 2022, 11, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Ajioka, I.; Martins, R.A.; Bayazitov, I.T.; Donovan, S.; Johnson, D.A.; Frase, S.; Cicero, S.A.; Boyd, K.; Zakharenko, S.S.; Dyer, M.A. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell 2007, 131, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Livne-bar, I.; Vanderluit, J.L.; Slack, R.S.; Agochiya, M.; Bremner, R. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell 2004, 5, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Singh, H.P.; Wang, L.; Qi, D.-L.; Poulos, B.K.; Abramson, D.H.; Jhanwar, S.C.; Cobrinik, D. Rb suppresses human cone-precursor-derived retinoblastoma tumours. Nature 2014, 514, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Zhang, Y.-Y.; Li, Y.-P.; Hua, Z.-Q.; Zhang, C.-J.; Wu, K.-C.; Yu, F.; Zhang, Y.; Su, J.; et al. Human embryonic stem cell-derived organoid retinoblastoma reveals a cancerous origin. Proc. Natl. Acad. Sci. USA 2020, 117, 33628–33638. [Google Scholar] [CrossRef]
- Kanber, D.; Woestefeld, J.; Döpper, H.; Bozet, M.; Brenzel, A.; Altmüller, J.; Kilpert, F.; Lohmann, D.; Pommerenke, C.; Steenpass, L. RB1-Negative Retinal Organoids Display Proliferation of Cone Photoreceptors and Loss of Retinal Differentiation. Cancers 2022, 14, 2166. [Google Scholar] [CrossRef]
- Saengwimol, D.; Rojanaporn, D.; Chaitankar, V.; Chittavanich, P.; Aroonroch, R.; Boontawon, T.; Thammachote, W.; Jinawath, N.; Hongeng, S.; Kaewkhaw, R. A three-dimensional organoid model recapitulates tumorigenic aspects and drug responses of advanced human retinoblastoma. Sci. Rep. 2018, 8, 15664. [Google Scholar] [CrossRef]
- Norrie, J.L.; Nityanandam, A.; Lai, K.; Chen, X.; Wilson, M.; Stewart, E.; Griffiths, L.; Jin, H.; Wu, G.; Orr, B.; et al. Retinoblastoma from human stem cell-derived retinal organoids. Nat. Commun. 2021, 12, 4535. [Google Scholar] [CrossRef]
- Gabriel, E.; Albanna, W.; Pasquini, G.; Ramani, A.; Josipovic, N.; Mariappan, A.; Schinzel, F.; Karch, C.M.; Bao, G.; Gottardo, M.; et al. Human brain organoids assemble functionally integrated bilateral optic vesicles. Cell Stem Cell 2021, 28, 1740–1757. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, L.; Fields, M.; Rimondi, E.; Zauli, G.; Longo, G.; Marcuzzi, A.; Previati, M.; Gonelli, A.; Zauli, E.; Milani, D. Classical and Innovative Evidence for Therapeutic Strategies in Retinal Dysfunctions. Int. J. Mol. Sci. 2024, 25, 2124. https://doi.org/10.3390/ijms25042124
Caruso L, Fields M, Rimondi E, Zauli G, Longo G, Marcuzzi A, Previati M, Gonelli A, Zauli E, Milani D. Classical and Innovative Evidence for Therapeutic Strategies in Retinal Dysfunctions. International Journal of Molecular Sciences. 2024; 25(4):2124. https://doi.org/10.3390/ijms25042124
Chicago/Turabian StyleCaruso, Lorenzo, Matteo Fields, Erika Rimondi, Giorgio Zauli, Giovanna Longo, Annalisa Marcuzzi, Maurizio Previati, Arianna Gonelli, Enrico Zauli, and Daniela Milani. 2024. "Classical and Innovative Evidence for Therapeutic Strategies in Retinal Dysfunctions" International Journal of Molecular Sciences 25, no. 4: 2124. https://doi.org/10.3390/ijms25042124
APA StyleCaruso, L., Fields, M., Rimondi, E., Zauli, G., Longo, G., Marcuzzi, A., Previati, M., Gonelli, A., Zauli, E., & Milani, D. (2024). Classical and Innovative Evidence for Therapeutic Strategies in Retinal Dysfunctions. International Journal of Molecular Sciences, 25(4), 2124. https://doi.org/10.3390/ijms25042124







