Plant-Origin Components: New Players to Combat Antibiotic Resistance in Klebsiella pneumoniae
Abstract
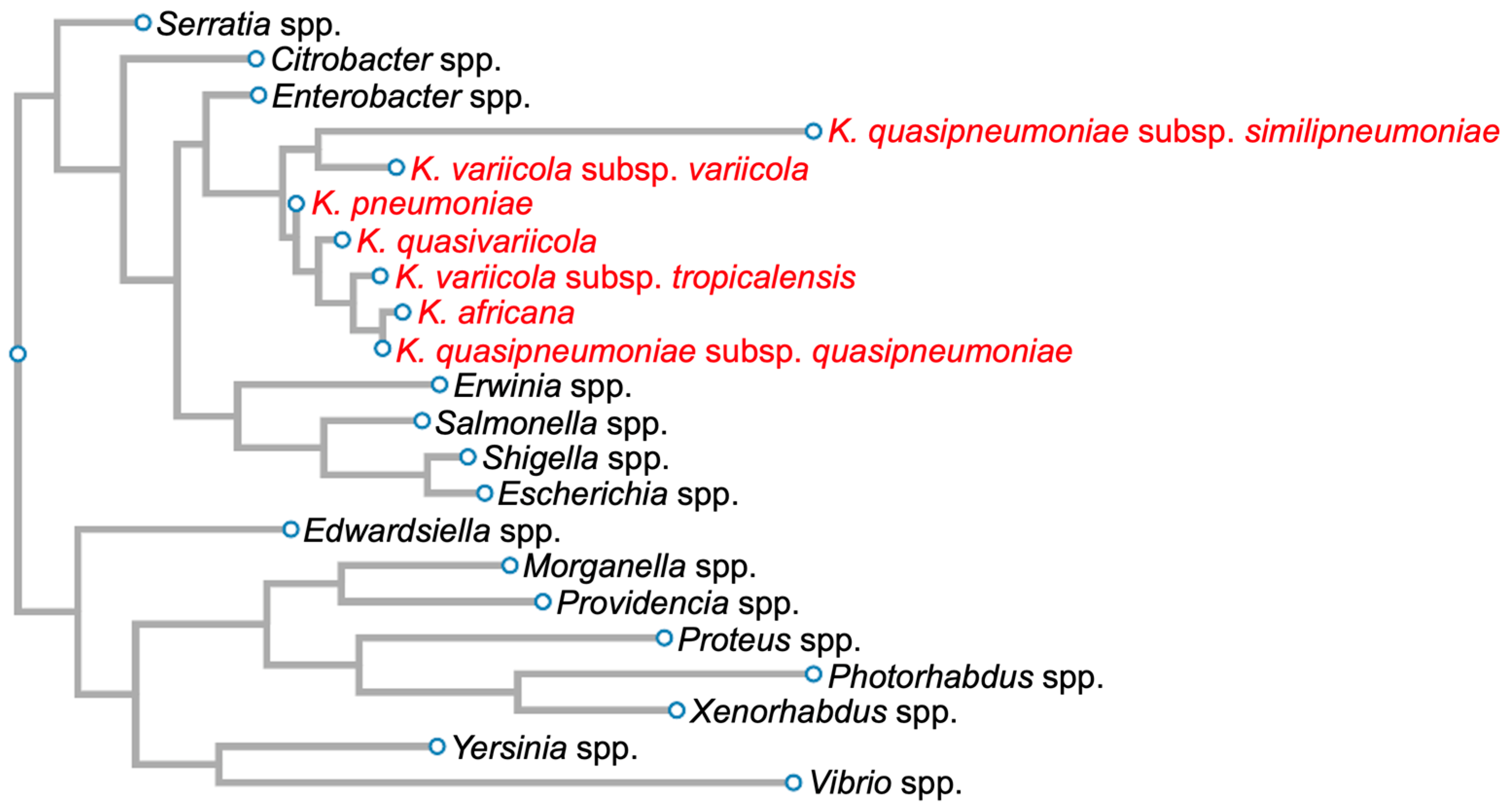
:1. Introduction: General Aspects of Klebsiella pneumoniae
2. Kpn Virulence Factors
3. Klebsiella pneumoniae and Public Health
4. Antibiotic Resistance
5. Treatment Development
6. The Potential of Plant-Origin Components as a New Treatment Source
7. Biotechnology Applying Plant-Origin Sources to Develop New Control Strategies against Kpn
8. Special Considerations in the Application of POCs in Medicine
9. Perspectives
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedlaender, C. Ueber Die Schizomyceten Bei Der Acuten Fibrösen Pneumonie. Arch. Pathol. Anat. Physiol. Klin. Med. 1882, 87, 319–324. [Google Scholar] [CrossRef]
- Brisse, S.; Grimont, F.; Grimont, P.A.D. The Genus Klebsiella. In The Prokaryotes; Springer: New York, NY, USA, 2006; pp. 159–196. [Google Scholar]
- Long, S.W.; Linson, S.E.; Ojeda Saavedra, M.; Cantu, C.; Davis, J.J.; Brettin, T.; Olsen, R.J. Whole-Genome Sequencing of Human Clinical Klebsiella pneumoniae Isolates Reveals Misidentification and Misunderstandings of Klebsiella pneumoniae, Klebsiella variicola, and Klebsiella quasipneumoniae. mSphere 2017, 2, 10–128. [Google Scholar] [CrossRef]
- Wyres, K.L.; Lam, M.M.C.; Holt, K.E. Population Genomics of Klebsiella pneumoniae. Nat. Rev. Microbiol. 2020, 18, 344–359. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.E.; Wertheim, H.; Zadoks, R.N.; Baker, S.; Whitehouse, C.A.; Dance, D.; Jenney, A.; Connor, T.R.; Hsu, L.Y.; Severin, J.; et al. Genomic Analysis of Diversity, Population Structure, Virulence, and Antimicrobial Resistance in Klebsiella pneumoniae, an Urgent Threat to Public Health. Proc. Natl. Acad. Sci. USA 2015, 112, E3574–E3581. [Google Scholar] [CrossRef]
- Catalán-Nájera, J.C.; Garza-Ramos, U.; Barrios-Camacho, H. Hypervirulence and Hypermucoviscosity: Two Different but Complementary Klebsiella spp. Phenotypes? Virulence 2017, 8, 1111–1123. [Google Scholar] [CrossRef]
- Chen, L.; Kreiswirth, B.N. Convergence of Carbapenem-Resistance and Hypervirulence in Klebsiella pneumoniae. Lancet Infect. Dis. 2018, 18, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Kong, X.; Hao, J.; Liu, J. Epidemiological Characteristics and Formation Mechanisms of Multidrug-Resistant Hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2020, 11, 581543. [Google Scholar] [CrossRef]
- Choby, J.E.; Howard-Anderson, J.; Weiss, D.S. Hypervirulent Klebsiella pneumoniae—Clinical and Molecular Perspectives. J. Intern. Med. 2020, 287, 283–300. [Google Scholar] [CrossRef]
- Hennequin, C.; Robin, F. Correlation between Antimicrobial Resistance and Virulence in Klebsiella pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 333–341. [Google Scholar] [CrossRef]
- Stojowska-Swędrzyńska, K.; Łupkowska, A.; Kuczyńska-Wiśnik, D.; Laskowska, E. Antibiotic Heteroresistance in Klebsiella pneumoniae. Int. J. Mol. Sci. 2021, 23, 449. [Google Scholar] [CrossRef]
- Savithramma, N.; Linga Rao, M.; Suhrulatha, D. Screening of Selected Medicinal Plants for Secondary Metabolites. Middle East J. Sci. Res. 2011, 8, 579–584. [Google Scholar]
- Struve, C.; Bojer, M.; Krogfelt, K.A. Characterization of Klebsiella pneumoniae Type 1 Fimbriae by Detection of Phase Variation during Colonization and Infection and Impact on Virulence. Infect. Immun. 2008, 76, 4055–4065. [Google Scholar] [CrossRef]
- Alcántar-Curiel, M.D.; Blackburn, D.; Saldaña, Z.; Gayosso-Vázquez, C.; Iovine, N.M.; De la Cruz, M.A.; Girón, J.A. Multi-Functional Analysis of Klebsiella pneumoniae Fimbrial Types in Adherence and Biofilm Formation. Virulence 2013, 4, 129–138. [Google Scholar] [CrossRef]
- Struve, C.; Bojer, M.; Krogfelt, K.A. Identification of a Conserved Chromosomal Region Encoding Klebsiella pneumoniae Type 1 and Type 3 Fimbriae and Assessment of the Role of Fimbriae in Pathogenicity. Infect. Immun. 2009, 77, 5016–5024. [Google Scholar] [CrossRef]
- Opoku-Temeng, C.; Kobayashi, S.D.; DeLeo, F.R. Klebsiella pneumoniae Capsule Polysaccharide as a Target for Therapeutics and Vaccines. Comput. Struct. Biotechnol. J. 2019, 17, 1360–1366. [Google Scholar] [CrossRef]
- Hsu, C.-R.; Pan, Y.-J.; Liu, J.-Y.; Chen, C.-T.; Lin, T.-L.; Wang, J.-T. Klebsiella pneumoniae Translocates across the Intestinal Epithelium via Rho GTPase- and Phosphatidylinositol 3-Kinase/Akt-Dependent Cell Invasion. Infect. Immun. 2015, 83, 769–779. [Google Scholar] [CrossRef]
- DeLeo, F.R.; Chen, L.; Porcella, S.F.; Martens, C.A.; Kobayashi, S.D.; Porter, A.R.; Chavda, K.D.; Jacobs, M.R.; Mathema, B.; Olsen, R.J.; et al. Molecular Dissection of the Evolution of Carbapenem-Resistant Multilocus Sequence Type 258 Klebsiella pneumoniae. Proc. Natl. Acad. Sci. USA 2014, 111, 4988–4993. [Google Scholar] [CrossRef]
- Regueiro, V.; Campos, M.A.; Pons, J.; Albertí, S.; Bengoechea, J.A. The Uptake of a Klebsiella pneumoniae Capsule Polysaccharide Mutant Triggers an Inflammatory Response by Human Airway Epithelial Cells. Microbiology 2006, 152, 555–566. [Google Scholar] [CrossRef]
- Pan, Y.-J.; Lin, T.-L.; Hsu, C.-R.; Wang, J.-T. Use of a Dictyostelium Model for Isolation of Genetic Loci Associated with Phagocytosis and Virulence in Klebsiella pneumoniae. Infect. Immun. 2011, 79, 997–1006. [Google Scholar] [CrossRef]
- You, H.S.; Lee, S.H.; Kang, S.S.; Hyun, S.H. OmpA of Klebsiella pneumoniae ATCC 13883 Induces Pyroptosis in HEp-2 Cells, Leading to Cell-Cycle Arrest and Apoptosis. Microbes Infect. 2020, 22, 432–440. [Google Scholar] [CrossRef]
- Rocker, A.; Lacey, J.A.; Belousoff, M.J.; Wilksch, J.J.; Strugnell, R.A.; Davies, M.R.; Lithgow, T. Global Trends in Proteome Remodeling of the Outer Membrane Modulate Antimicrobial Permeability in Klebsiella pneumoniae. mBio 2020, 11, 10–128. [Google Scholar] [CrossRef]
- Merino, S.; Camprubi, S.; Alberti, S.; Benedi, V.J.; Tomas, J.M. Mechanisms of Klebsiella pneumoniae Resistance to Complement-Mediated Killing. Infect. Immun. 1992, 60, 2529–2535. [Google Scholar] [CrossRef]
- Lawlor, M.S.; O’Connor, C.; Miller, V.L. Yersiniabactin Is a Virulence Factor for Klebsiella pneumoniae during Pulmonary Infection. Infect. Immun. 2007, 75, 1463–1472. [Google Scholar] [CrossRef]
- Bachman, M.A.; Oyler, J.E.; Burns, S.H.; Caza, M.; Lépine, F.; Dozois, C.M.; Weiser, J.N. Klebsiella pneumoniae Yersiniabactin Promotes Respiratory Tract Infection through Evasion of Lipocalin 2. Infect. Immun. 2011, 79, 3309–3316. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Marahiel, M.A. Siderophore-Based Iron Acquisition and Pathogen Control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Bachman, M.A.; Lenio, S.; Schmidt, L.; Oyler, J.E.; Weiser, J.N. Interaction of Lipocalin 2, Transferrin, and Siderophores Determines the Replicative Niche of Klebsiella pneumoniae during Pneumonia. mBio 2012, 3, 10–128. [Google Scholar] [CrossRef]
- Holden, V.I.; Wright, M.S.; Houle, S.; Collingwood, A.; Dozois, C.M.; Adams, M.D.; Bachman, M.A. Iron Acquisition and Siderophore Release by Carbapenem-Resistant Sequence Type 258 Klebsiella pneumoniae. mSphere 2018, 3, e00125-18. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Chang, H.-Y.; Lai, Y.-C.; Pan, C.-C.; Tsai, S.-F.; Peng, H.-L. Sequencing and Analysis of the Large Virulence Plasmid PLVPK of Klebsiella pneumoniae CG43. Gene 2004, 337, 189–198. [Google Scholar] [CrossRef]
- Russo, T.A.; Olson, R.; MacDonald, U.; Metzger, D.; Maltese, L.M.; Drake, E.J.; Gulick, A.M. Aerobactin Mediates Virulence and Accounts for Increased Siderophore Production under Iron-Limiting Conditions by Hypervirulent (Hypermucoviscous) Klebsiella pneumoniae. Infect. Immun. 2014, 82, 2356–2367. [Google Scholar] [CrossRef]
- Sarris, P.F.; Zoumadakis, C.; Panopoulos, N.J.; Scoulica, E.V. Distribution of the Putative Type VI Secretion System Core Genes in Klebsiella spp. Infect. Genet. Evol. 2011, 11, 157–166. [Google Scholar] [CrossRef]
- Liu, L.; Ye, M.; Li, X.; Li, J.; Deng, Z.; Yao, Y.-F.; Ou, H.Y. Identification and Characterization of an Antibacterial Type VI Secretion System in the Carbapenem-Resistant Strain Klebsiella pneumoniae HS11286. Front. Cell Infect. Microbiol. 2017, 7, 442. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Cao, S.; Ishaq, H.M.; Zhao, H.; Yang, F.; Liu, L. The Biological and Regulatory Role of Type VI Secretion System of Klebsiella pneumoniae. Infect. Drug Resist. 2023, 16, 6911–6922. [Google Scholar] [PubMed]
- Lu, M.-C.; Chen, Y.-T.; Chiang, M.-K.; Wang, Y.-C.; Hsiao, P.-Y.; Huang, Y.-J.; Lin, C.-T.; Cheng, C.-C.; Liang, C.-L.; Lai, Y.-C. Colibactin Contributes to the Hypervirulence of Pks(+) K1 CC23 Klebsiella pneumoniae in Mouse Meningitis Infections. Front. Cell. Infect. Microbiol. 2017, 7, 103. [Google Scholar] [CrossRef]
- Podschun, R.; Ullmann, U. Klebsiella spp. as Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef]
- Seguel, M.; Gottdenker, N.L.; Colegrove, K.; Johnson, S.; Struve, C.; Howerth, E.W. Hypervirulent Klebsiella pneumoniae in California Sea Lions (Zalophus californianus): Pathologic Findings in Natural Infections. Vet. Pathol. 2017, 54, 846–850. [Google Scholar] [CrossRef]
- Du, Q.; Pan, F.; Wang, C.; Yu, F.; Shi, Y.; Liu, W.; Li, Z.; He, P.; Han, D.; Zhang, H. Nosocomial Dissemination of Hypervirulent Klebsiella pneumoniae with High-Risk Clones among Children in Shanghai. Front. Cell. Infect. Microbiol. 2022, 12, 984180. [Google Scholar] [CrossRef] [PubMed]
- Siu, L.K.; Chang, F.-Y. Klebsiella pneumoniae Liver Abscesses--Authors’ Reply. Lancet Infect. Dis. 2013, 13, 393. [Google Scholar] [PubMed]
- Zhao, J.; Huo, T.; Luo, X.; Lu, F.; Hui, S.; Yang, B. Klebsiella pneumoniae-Related Brain Abscess and Meningitis in Adults: Case Report. Medicine 2022, 101, e28415. [Google Scholar] [CrossRef] [PubMed]
- Zandi, R.; Talebi, S.; Sheibani, S.; Ehsani, A. Klebsiella pneumoniae and Enterobacter cloacae Induced Septic Arthritis in a Healthy Adolescent: A Rare Case Report. Hip Pelvis 2022, 34, 185–190. [Google Scholar] [CrossRef]
- Kritikos, A.; Manuel, O. Bloodstream Infections after Solid-Organ Transplantation. Virulence 2016, 7, 329–340. [Google Scholar] [CrossRef]
- Bodro, M.; Sabé, N.; Tubau, F.; Lladó, L.; Baliellas, C.; Roca, J.; Cruzado, J.M.; Carratalà, J. Risk Factors and Outcomes of Bacteremia Caused by Drug-Resistant ESKAPE Pathogens in Solid-Organ Transplant Recipients. Transplantation 2013, 96, 843–849. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.-Y.; Song, W.-Q.; Wang, Y.; Dong, F.; Liu, G. Risk Factors for Carbapenem-Resistant K. pneumoniae Bloodstream Infection and Predictors of Mortality in Chinese Paediatric Patients. BMC Infect. Dis. 2018, 18, 248. [Google Scholar] [CrossRef]
- Caballero, S.; Carter, R.; Ke, X.; Sušac, B.; Leiner, I.M.; Kim, G.J.; Miller, L.; Ling, L.; Manova, K.; Pamer, E.G. Distinct but Spatially Overlapping Intestinal Niches for Vancomycin-Resistant Enterococcus faecium and Carbapenem-Resistant Klebsiella pneumoniae. PLoS Pathog. 2015, 11, e1005132. [Google Scholar] [CrossRef]
- Abril, D.; Vergara, E.; Palacios, D.; Leal, A.L.; Marquez-Ortiz, R.A.; Madroñero, J.; Corredor Rozo, Z.L.; De La Rosa, Z.; Nieto, C.A.; Vanegas, N.; et al. Within Patient Genetic Diversity of BlaKPC Harboring Klebsiella pneumoniae in a Colombian Hospital and Identification of a New NTEKPC Platform. Sci. Rep. 2021, 11, 21409. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, R.; Spinazzola, G.; Teofili, L.; Avolio, A.W.; Fiori, B.; Maresca, G.M.; Spanu, T.; Nicolotti, N.; de Pascale, G.; Antonelli, M. Protective Effect of SARS-CoV-2 Preventive Measures against ESKAPE and Escherichia coli Infections. Eur. J. Clin. Investig. 2021, 51, e13687. [Google Scholar] [CrossRef]
- Micozzi, A.; Assanto, G.M.; Cesini, L.; Minotti, C.; Cartoni, C.; Capria, S.; Ciotti, G.; Alunni Fegatelli, D.; Donzelli, L.; Martelli, M.; et al. Reduced Transmission of Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae (KPC-KP) in Patients with Haematological Malignancies Hospitalized in an Italian Hospital during the COVID-19 Pandemic. JAC Antimicrob. Resist. 2021, 3, dlab167. [Google Scholar] [CrossRef]
- Cerulli Irelli, E.; Morano, A.; Di Bonaventura, C. Reduction in Nosocomial Infections during the COVID-19 Era: A Lesson to Be Learned. Updates Surg. 2021, 73, 785–786. [Google Scholar] [CrossRef]
- Rawson, T.M.; Zhu, N.; Ranganathan, N.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Co-Infection in Individuals with Coronavirus: A Rapid Review to Support COVID-19 Antimicrobial Prescribing Timothy. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar]
- Salazar-Vizcaya, L.; Atkinson, A.; Kronenberg, A.; Plüss-Suard, C.; Kouyos, R.D.; Kachalov, V.; Troillet, N.; Marschall, J.; Sommerstein, R. The Impact of Public Health Interventions on the Future Prevalence of ESBL-Producing Klebsiella pneumoniae: A Population Based Mathematical Modelling Study. BMC Infect. Dis. 2022, 22, 487. [Google Scholar] [CrossRef]
- Cejas, D.; Magariños, F.; Elena, A.; Ferrara, M.; Ormazábal, C.; Yernazian, M.V.; Gutkind, G.; Radice, M. Emergence and Clonal Expansion of Klebsiella pneumoniae ST307, Simultaneously Producing KPC-3 and NDM-1. Rev. Argent Microbiol. 2022, 54, 288–292. [Google Scholar] [CrossRef]
- Peirano, G.; Chen, L.; Kreiswirth, B.N.; Pitout, J.D.D. Emerging Antimicrobial-Resistant High-Risk Klebsiella pneumoniae Clones ST307 and ST147. Antimicrob. Agents Chemother. 2020, 64, e01148-20. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, M.; Castillo-Polo, J.A.; Cordero, D.G.; Pérez-Viso, B.; García-Castillo, M.; Saez de la Fuente, J.; Morosini, M.I.; Cantón, R.; Ruiz-Garbajosa, P. Impact of Ceftazidime-Avibactam Treatment in the Emergence of Novel KPC Variants in the ST307-Klebsiella pneumoniae High-Risk Clone and Consequences for Their Routine Detection. J. Clin. Microbiol. 2022, 60, e0224521. [Google Scholar] [CrossRef]
- Mataseje, L.F.; Chen, L.; Peirano, G.; Fakharuddin, K.; Kreiswith, B.; Mulvey, M.; Pitout, J.D.D. Klebsiella pneumoniae ST147: And Then There Were Three Carbapenemases. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M. Antibiotic Resistance: The Last Resort. Nature 2013, 499, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and Resistance Mechanisms of Antibiotics: A Guide for Clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, W. The Mechanism of Bacterial Resistance and Potential Bacteriostatic Strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.B.; Venkataramaiah, M.; Mondal, A.; Vaidyanathan, V.; Govil, T.; Rajamohan, G. Functional Characterization of a Novel Outer Membrane Porin KpnO, Regulated by PhoBR Two-Component System in Klebsiella pneumoniae NTUH-K2044. PLoS ONE 2012, 7, e41505. [Google Scholar] [CrossRef]
- Srinivasan, V.B.; Rajamohan, G. KpnEF, a New Member of the Klebsiella pneumoniae Cell Envelope Stress Response Regulon, Is an SMR-Type Efflux Pump Involved in Broad-Spectrum Antimicrobial Resistance. Antimicrob. Agents Chemother. 2013, 57, 4449–4462. [Google Scholar] [CrossRef]
- Machulska, M.; Baraniak, A.; Żak, I.; Bojarska, K.; Żabicka, D.; Sowa-Sierant, I.; Hryniewicz, W.; Gniadkowski, M. KPC-2-Producing Klebsiella pneumoniae ST11 in a Children’s Hospital in Poland. Pol. J. Microbiol. 2017, 66, 401–404. [Google Scholar] [CrossRef]
- Kliebe, C.; Nies, B.A.; Meyer, J.F.; Tolxdorff-Neutzling, R.M.; Wiedemann, B. Evolution of Plasmid-Coded Resistance to Broad-Spectrum Cephalosporins. Antimicrob. Agents Chemother. 1985, 28, 302–307. [Google Scholar] [CrossRef]
- Sirot, D.; Sirot, J.; Labia, R.; Morand, A.; Courvalin, P.; Darfeuille-Michaud, A.; Perroux, R.; Cluzel, R. Transferable Resistance to Third-Generation Cephalosporins in Clinical Isolates of Klebsiella pneumoniae: Identification of CTX-1, a Novel β-Lactamase. J. Antimicrob. Chemother. 1987, 20, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Calbo, E.; Garau, J. The Changing Epidemiology of Hospital Outbreaks Due to ESBL-Producing Klebsiella pneumoniae: The CTX-M-15 Type Consolidation. Future Microbiol. 2015, 10, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.A.; Amyes, S.G.B. OXA β-Lactamases. Clin. Microbiol. Rev. 2014, 27, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.A. Extended-Spectrum β-Lactamases in the 21st Century: Characterization, Epidemiology, and Detection of This Important Resistance Threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef]
- Zamorano, L.; Miró, E.; Juan, C.; Gómez, L.; Bou, G.; González-López, J.J.; Martínez-Martínez, L.; Aracil, B.; Conejo, M.C.; Oliver, A.; et al. Mobile Genetic Elements Related to the Diffusion of Plasmid-Mediated AmpC β-Lactamases or Carbapenemases from Enterobacteriaceae: Findings from a Multicenter Study in Spain. Antimicrob. Agents Chemother. 2015, 59, 5260–5266. [Google Scholar] [CrossRef] [PubMed]
- Haruta, S.; Yamaguchi, H.; Yamamoto, E.T.; Eriguchi, Y.; Nukaga, M.; O’Hara, K.; Sawai, T. Functional Analysis of the Active Site of a Metallo-β-Lactamase Proliferating in Japan. Antimicrob. Agents Chemother. 2000, 44, 2304–2309. [Google Scholar] [CrossRef]
- Lee, C.-R.; Lee, J.H.; Park, K.S.; Kim, Y.B.; Jeong, B.C.; Lee, S.H. Global Dissemination of Carbapenemase-Producing Klebsiella pneumoniae: Epidemiology, Genetic Context, Treatment Options, and Detection Methods. Front. Microbiol. 2016, 7, 895. [Google Scholar] [CrossRef]
- Molton, J.S.; Tambyah, P.A.; Ang, B.S.P.; Ling, M.L.; Fisher, D.A. The Global Spread of Healthcare-Associated Multidrug-Resistant Bacteria: A Perspective From Asia. Clin. Infect. Dis. 2013, 56, 1310–1318. [Google Scholar] [CrossRef]
- Leavitt, A.; Chmelnitsky, I.; Colodner, R.; Ofek, I.; Carmeli, Y.; Navon-Venezia, S. Ertapenem Resistance among Extended-Spectrum-β-Lactamase-Producing Klebsiella pneumoniae Isolates. J. Clin. Microbiol. 2009, 47, 969–974. [Google Scholar] [CrossRef]
- Padilla, E.; Llobet, E.; Doménech-Sánchez, A.; Martínez-Martínez, L.; Bengoechea, J.A.; Albertí, S. Klebsiella pneumoniae AcrAB Efflux Pump Contributes to Antimicrobial Resistance and Virulence. Antimicrob. Agents Chemother. 2010, 54, 177–183. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Wachino, J.I.; Arakawa, Y. Aminoglycoside Resistance: The Emergence of Acquired 16S Ribosomal RNA Methyltransferases. Infect. Dis. Clin. 2016, 30, 523–537. [Google Scholar]
- Deguchi, T.; Fukuoka, A.; Yasuda, M.; Nakano, M.; Ozeki, S.; Kanematsu, E.; Nishino, Y.; Ishihara, S.; Ban, Y.; Kawada, Y. Alterations in the GyrA Subunit of DNA Gyrase and the ParC Subunit of Topoisomerase IV in Quinolone-Resistant Clinical Isolates of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 1997, 41, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Guillard, T.; de Jong, A.; Limelette, A.; Lebreil, A.L.; Madoux, J.; de Champs, C. Characterization of Quinolone Resistance Mechanisms in Enterobacteriaceae Recovered from Diseased Companion Animals in Europe. Vet. Microbiol. 2016, 194, 23–29. [Google Scholar] [CrossRef]
- Nam, Y.S.; Cho, S.Y.; Yang, H.Y.; Park, K.S.; Jang, J.-H.; Kim, Y.-T.; Jeong, J.; Suh, J.-T.; Lee, H.J. Investigation of Mutation Distribution in DNA Gyrase and Topoisomerase IV Genes in Ciprofloxacin-Non-Susceptible Enterobacteriaceae Isolated from Blood Cultures in a Tertiary Care University Hospital in South Korea, 2005–2010. Int. J. Antimicrob. Agents 2013, 41, 126–129. [Google Scholar] [CrossRef]
- Wong, M.H.Y.; Chan, E.W.C.; Chen, S. Evolution and Dissemination of OqxAB-Like Efflux Pumps, an Emerging Quinolone Resistance Determinant among Members of Enterobacteriaceae. Antimicrob. Agents Chemother. 2015, 59, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A.; Strahilevitz, J.; Hooper, D.C. Plasmid-Mediated Quinolone Resistance. Microbiol. Spectr. 2014, 2, 475–503. [Google Scholar] [CrossRef]
- Ruiz, E.; Sáenz, Y.; Zarazaga, M.; Rocha-Gracia, R.; Martínez-Martínez, L.; Arlet, G.; Torres, C. Qnr, Aac(6′)-Ib-Cr and QepA Genes in Escherichia Coli and Klebsiella spp.: Genetic Environments and Plasmid and Chromosomal Location. J. Antimicrob. Chemother. 2012, 67, 886–897. [Google Scholar] [CrossRef]
- De Majumdar, S.; Yu, J.; Fookes, M.; McAteer, S.P.; Llobet, E.; Finn, S.; Spence, S.; Monaghan, A.; Kissenpfennig, A.; Ingram, R.J.; et al. Elucidation of the RamA Regulon in Klebsiella pneumoniae Reveals a Role in LPS Regulation. PLoS Pathog. 2015, 11, e1004627. [Google Scholar] [CrossRef]
- Clements, A.; Tull, D.; Jenney, A.W.; Farn, J.L.; Kim, S.-H.; Bishop, R.E.; McPhee, J.B.; Hancock, R.E.W.; Hartland, E.L.; Pearse, M.J.; et al. Secondary Acylation of Klebsiella pneumoniae Lipopolysaccharide Contributes to Sensitivity to Antibacterial Peptides. J. Biol. Chem. 2007, 282, 15569–15577. [Google Scholar] [CrossRef]
- Mitrophanov, A.Y.; Jewett, M.W.; Hadley, T.J.; Groisman, E.A. Evolution and Dynamics of Regulatory Architectures Controlling Polymyxin B Resistance in Enteric Bacteria. PLoS Genet. 2008, 4, e1000233. [Google Scholar] [CrossRef]
- Llobet, E.; Campos, M.A.; Giménez, P.; Moranta, D.; Bengoechea, J.A. Analysis of the Networks Controlling the Antimicrobial-Peptide-Dependent Induction of Klebsiella pneumoniae Virulence Factors. Infect. Immun. 2011, 79, 3718–3732. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Jayol, A.; Poirel, L.; Brink, A.; Villegas, M.-V.; Yilmaz, M.; Nordmann, P. Resistance to Colistin Associated with a Single Amino Acid Change in Protein PmrB among Klebsiella pneumoniae Isolates of Worldwide Origin. Antimicrob. Agents Chemother. 2014, 58, 4762–4766. [Google Scholar] [CrossRef] [PubMed]
- De Majumdar, S.; Veleba, M.; Finn, S.; Fanning, S.; Schneiders, T. Elucidating the Regulon of Multidrug Resistance Regulator RarA in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2013, 57, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Osei Sekyere, J.; Govinden, U.; Bester, L.A.; Essack, S.Y. Colistin and Tigecycline Resistance in Carbapenemase-Producing Gram-Negative Bacteria: Emerging Resistance Mechanisms and Detection Methods. J. Appl. Microbiol. 2016, 121, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Villa, L.; Feudi, C.; Fortini, D.; García-Fernández, A.; Carattoli, A. Genomics of KPC-Producing Klebsiella pneumoniae Sequence Type 512 Clone Highlights the Role of RamR and Ribosomal S10 Protein Mutations in Conferring Tigecycline Resistance. Antimicrob. Agents Chemother. 2014, 58, 1707–1712. [Google Scholar] [CrossRef]
- Nielsen, L.E.; Snesrud, E.C.; Onmus-Leone, F.; Kwak, Y.I.; Avilés, R.; Steele, E.D.; Sutter, D.E.; Waterman, P.E.; Lesho, E.P. IS5 Element Integration, a Novel Mechanism for Rapid in Vivo Emergence of Tigecycline Nonsusceptibility in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2014, 58, 6151–6156. [Google Scholar] [CrossRef]
- Wilson, D.N.; Hauryliuk, V.; Atkinson, G.C.; O’Neill, A.J. Target Protection as a Key Antibiotic Resistance Mechanism. Nat. Rev. Microbiol. 2020, 18, 637–648. [Google Scholar] [CrossRef]
- Tran, J.H.; Jacoby, G.A.; Hooper, D.C. Interaction of the Plasmid-Encoded Quinolone Resistance Protein Qnr with Escherichia coli DNA Gyrase. Antimicrob. Agents Chemother. 2005, 49, 118–125. [Google Scholar] [CrossRef]
- Amereh, F.; Arabestani, M.R.; Hosseini, S.M.; Shokoohizadeh, L. Association of Qnr Genes and OqxAB Efflux Pump in Fluoroquinolone-Resistant Klebsiella pneumoniae Strains. Int. J. Microbiol. 2023, 2023, 9199108. [Google Scholar] [CrossRef]
- Gunn, J.S. Bacterial Modification of LPS and Resistance to Antimicrobial Peptides. J. Endotoxin Res. 2001, 7, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Mazzariol, A.; Zuliani, J.; Cornaglia, G.; Rossolini, G.M.; Fontana, R. AcrAB Efflux System: Expression and Contribution to Fluoroquinolone Resistance in Klebsiella spp. Antimicrob. Agents Chemother. 2002, 46, 3984–3986. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Ogawa, W.; Kuroda, T.; Tsuchiya, T. Gene Cloning and Characterization of KdeA, a Multidrug Efflux Pump from Klebsiella pneumoniae. Biol. Pharm. Bull. 2007, 30, 1962–1964. [Google Scholar] [CrossRef]
- Vuotto, C.; Longo, F.; Balice, M.P.; Donelli, G.; Varaldo, P.E. Antibiotic Resistance Related to Biofilm Formation in Klebsiella pneumoniae. Pathogens 2014, 3, 743–758. [Google Scholar] [CrossRef]
- Hadi, U.; Kuntaman, K.; Qiptiyah, M.; Paraton, H. Problem of Antibiotic Use and Antimicrobial Resistance in Indonesia: Are We Really Making Progress? Indones. J. Trop. Infect. Dis. 2013, 4, 5. [Google Scholar] [CrossRef]
- van der Meer, J.W.M.; Gyssens, I.C. Quality of Antimicrobial Drug Prescription in Hospital. Clin. Microbiol. Infect. 2001, 7, 12–15. [Google Scholar] [CrossRef]
- Diago-Navarro, E.; Calatayud-Baselg, I.; Sun, D.; Khairallah, C.; Mann, I.; Ulacia-Hernando, A.; Sheridan, B.; Shi, M.; Fries, B.C. Antibody-Based Immunotherapy to Treat and Prevent Infection with Hypervirulent Klebsiella pneumoniae. Clin. Vaccine Immunol. 2017, 24, e00456-16. [Google Scholar] [CrossRef]
- Herridge, W.P.; Shibu, P.; O’Shea, J.; Brook, T.C.; Hoyles, L. Bacteriophages of Klebsiella spp., Their Diversity and Potential Therapeutic Uses. J. Med. Microbiol. 2020, 69, 176–194. [Google Scholar] [CrossRef]
- Domingo-Calap, P.; Delgado-Martínez, J. Bacteriophages: Protagonists of a Post-Antibiotic Era. Antibiotics 2018, 7, 66. [Google Scholar] [CrossRef]
- Soleimani Sasani, M.; Eftekhar, F. Potential of a Bacteriophage Isolated from Wastewater in Treatment of Lobar Pneumonia Infection Induced by Klebsiella pneumoniae in Mice. Curr. Microbiol. 2020, 77, 2650–2655. [Google Scholar] [CrossRef] [PubMed]
- Arena, F.; Menchinelli, G.; di Pilato, V.; Torelli, R.; Antonelli, A.; Henrici De Angelis, L.; Coppi, M.; Sanguinetti, M.; Rossolini, G.M. Resistance and Virulence Features of Hypermucoviscous Klebsiella pneumoniae from Bloodstream Infections: Results of a Nationwide Italian Surveillance Study. Front. Microbiol. 2022, 13, 983294. [Google Scholar] [CrossRef]
- Verma, V.; Harjai, K.; Chhibber, S. Restricting Ciprofloxacin-Induced Resistant Variant Formation in Biofilm of Klebsiella pneumoniae B5055 by Complementary Bacteriophage Treatment. J. Antimicrob. Chemother. 2009, 64, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C. Natural Products Derived from Plants as a Source of Drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200. [Google Scholar] [CrossRef] [PubMed]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Muribeca, A.d.J.B.; Gomes, P.W.P.; Paes, S.S.; da Costa, A.P.A.; Gomes, P.W.P.; de S. Viana, J.; Reis, J.D.E.; Pamplona, S.d.G.S.R.; Silva, C.; Bauermeister, A.; et al. Antibacterial Activity from Momordica charantia L. Leaves and Flavones Enriched Phase. Pharmaceutics 2022, 14, 1796. [Google Scholar] [CrossRef]
- Nabi, M.; Tabassum, N.; Ganai, B.A. Phytochemical Screening and Antibacterial Activity of Skimmia anquetilia N.P. Taylor and Airy Shaw: A First Study from Kashmir Himalaya. Front. Plant Sci. 2022, 13, 937946. [Google Scholar] [CrossRef]
- Mehta, J.; Utkarsh, K.; Fuloria, S.; Singh, T.; Sekar, M.; Salaria, D.; Rolta, R.; Begum, M.Y.; Gan, S.H.; Rani, N.N.I.M.; et al. Antibacterial Potential of Bacopa monnieri (L.) Wettst. and Its Bioactive Molecules against Uropathogens-An In Silico Study to Identify Potential Lead Molecule(s) for the Development of New Drugs to Treat Urinary Tract Infections. Molecules 2022, 27, 4971. [Google Scholar] [CrossRef]
- Masota, N.E.; Ohlsen, K.; Schollmayer, C.; Meinel, L.; Holzgrabe, U. Isolation and Characterization of Galloylglucoses Effective against Multidrug-Resistant Strains of Escherichia coli and Klebsiella pneumoniae. Molecules 2022, 27, 5045. [Google Scholar] [CrossRef]
- Elamary, R.B.; Albarakaty, F.M.; Salem, W.M. Efficacy of Acacia Nilotica Aqueous Extract in Treating Biofilm-Forming and Multidrug Resistant Uropathogens Isolated from Patients with UTI Syndrome. Sci. Rep. 2020, 10, 11125. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.S.S.E.S.; Branco Santos, J.C.; Castro Junior, J.A.d.A.; Wakui, V.G.; Rodrigues, J.F.S.; Arruda, M.O.; Monteiro, A.d.S.; Monteiro-Neto, V.; Bomfim, M.R.Q.; Kato, L.; et al. Himatanthus drasticus Leaves: Chemical Characterization and Evaluation of Their Antimicrobial, Antibiofilm, Antiproliferative Activities. Molecules 2017, 22, 910. [Google Scholar] [CrossRef] [PubMed]
- Thinina, A.; Karim, H.; Alia, M.; Karim, A. Evaluation and Quantification of the Inhibition of Biofilm and Planktonic Forms of Klebsiella pneumoniae by the Polyphenolic Extract of Pulicaria crispa. J. Adv. Pharm. Technol. Res. 2020, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Sood, H.; Kumar, Y.; Gupta, V.K.; Arora, D.S. Bioprospecting the Antimicrobial, Antibiofilm and Antiproliferative Activity of Symplocos racemosa Roxb. Bark Phytoconstituents along with Their Biosafety Evaluation and Detection of Antimicrobial Components by GC-MS. BMC Pharmacol. Toxicol. 2020, 21, 78. [Google Scholar] [CrossRef]
- Gato, E.; Rosalowska, A.; Martínez-Guitián, M.; Lores, M.; Bou, G.; Pérez, A. Anti-Adhesive Activity of a Vaccinium corymbosum Polyphenolic Extract Targeting Intestinal Colonization by Klebsiella pneumoniae. Biomed. Pharmacother. 2020, 132, 110885. [Google Scholar] [CrossRef] [PubMed]
- Mozirandi, W.; Tagwireyi, D.; Mukanganyama, S. Evaluation of Antimicrobial Activity of Chondrillasterol Isolated from Vernonia adoensis (Asteraceae). BMC Complement. Altern. Med. 2019, 19, 249. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Silva, K.E.; Motta, M.L.L.; Maciel, W.G.; Limiere, L.C.; Simionatto, S. Origanum vulgare L. Essential Oil Inhibits the Growth of Carbapenem-Resistant Gram-Negative Bacteria. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180502. [Google Scholar] [CrossRef]
- Mujawah, A.A.H.; Abdallah, E.M.; Alshoumar, S.A.; Alfarraj, M.I.; Alajel, S.M.I.; Alharbi, A.L.; Alsalman, S.A.; Alhumaydhi, F.A. GC-MS and in Vitro Antibacterial Potential of Cinnamomum camphora Essential Oil against Some Clinical Antibiotic-Resistant Bacterial Isolates. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5372–5379. [Google Scholar] [CrossRef]
- Iseppi, R.; di Cerbo, A.; Aloisi, P.; Manelli, M.; Pellesi, V.; Provenzano, C.; Camellini, S.; Messi, P.; Sabia, C. In Vitro Activity of Essential Oils Against Planktonic and Biofilm Cells of Extended-Spectrum β-Lactamase (ESBL)/Carbapenamase-Producing Gram-Negative Bacteria Involved in Human Nosocomial Infections. Antibiotics 2020, 9, 272. [Google Scholar] [CrossRef]
- Romo-Castillo, M.; Flores-Bautista, V.A.; Guzmán-Gutiérrez, S.L.; Reyes-Chilpa, R.; León-Santiago, M.; Luna-Pineda, V.M. Synergy of Plant Essential Oils in Antibiotic Therapy to Combat Klebsiella pneumoniae Infections. Pharmaceuticals 2023, 16, 839. [Google Scholar] [CrossRef]
- Ginting, E.V.; Retnaningrum, E.; Widiasih, D.A. Antibacterial Activity of Clove (Syzygium aromaticum) and Cinnamon (Cinnamomum burmannii) Essential Oil against Extended-Spectrum β-Lactamase-Producing Bacteria. Vet. World 2021, 14, 2206–2211. [Google Scholar] [CrossRef]
- Wijesinghe, G.K.; Feiria, S.B.; Maia, F.C.; Oliveira, T.R.; Joia, F.; Barbosa, J.P.; Boni, G.C.; HÖfling, J.F. In-Vitro Antibacterial and Antibiofilm Activity of Cinnamomum verum Leaf Oil against Pseudomonas aeruginosa, Staphylococcus aureus and Klebsiella pneumoniae. Acad. Bras. Cienc. 2021, 93, e20201507. [Google Scholar] [CrossRef]
- Ramachandran, G.; Rajivgandhi, G.N.; Murugan, S.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Manoharan, N.; Li, W.-J. Anti-Carbapenamase Activity of Camellia japonica Essential Oil against Isolated Carbapenem Resistant Klebsiella pneumoniae (MN396685). Saudi J. Biol. Sci. 2020, 27, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.G.; Silva, K.E.; Croda, J.; Simionatto, S. Antibacterial Activity of Cinnamomum cassia L. Essential Oil in a Carbapenem- and Polymyxin-Resistant Klebsiella aerogenes Strain. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200032. [Google Scholar] [CrossRef]
- Meng, X.; Li, D.; Zhou, D.; Wang, D.; Liu, Q.; Fan, S. Chemical Composition, Antibacterial Activity and Related Mechanism of the Essential Oil from the Leaves of Juniperus rigida Sieb. et Zucc against Klebsiella pneumoniae. J. Ethnopharmacol. 2016, 194, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, T.B.; Braga, M.A.; de Oliveira, F.F.M.; Santiago, G.M.P.; Carvalho, C.B.M.; e Cabral, P.B.; Santiago, T.d.M.; Sousa, J.S.; Barros, E.B.; do Nascimento, R.F.; et al. Effect of Subinihibitory and Inhibitory Concentrations of Plectranthus amboinicus (Lour.) Spreng Essential Oil on Klebsiella pneumoniae. Phytomedicine 2012, 19, 962–968. [Google Scholar] [CrossRef]
- Yang, S.-K.; Yusoff, K.; Thomas, W.; Akseer, R.; Alhosani, M.S.; Abushelaibi, A.; Lim, S.-H.-E.; Lai, K.-S. Lavender Essential Oil Induces Oxidative Stress Which Modifies the Bacterial Membrane Permeability of Carbapenemase Producing Klebsiella pneumoniae. Sci. Rep. 2020, 10, 819. [Google Scholar] [CrossRef]
- Piperaki, E.-T.; Syrogiannopoulos, G.A.; Tzouvelekis, L.S.; Daikos, G.L. Klebsiella pneumoniae: Virulence, Biofilm and Antimicrobial Resistance. Pediatr. Infect. Dis. J. 2017, 36, 1002–1005. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Barrera-Ruiz, D.G.; Cuestas-Rosas, G.C.; Sánchez-Mariñez, R.I.; Álvarez-Ainza, M.L.; Moreno-Ibarra, G.M.; López-Meneses, A.K.; Plascencia-Jatomea, M.; Cortez-Rocha, M.O. Antibacterial Activity of Essential Oils Encapsulated in Chitosan Nanoparticles. Food Sci. Technol. 2020, 40, 568–573. [Google Scholar] [CrossRef]
- Oliva, A.; D’Abramo, A.; D’Agostino, C.; Iannetta, M.; Mascellino, M.T.; Gallinelli, C.; Mastroianni, C.M.; Vullo, V. Synergistic Activity and Effectiveness of a Double-Carbapenem Regimen in Pandrug-Resistant Klebsiella pneumoniae Bloodstream Infections. J. Antimicrob. Chemother. 2014, 69, 1718–1720. [Google Scholar] [CrossRef] [PubMed]
- de Souza, G.H.d.A.; dos Santos Radai, J.A.; Mattos Vaz, M.S.; Esther da Silva, K.; Fraga, T.L.; Barbosa, L.S.; Simionatto, S. In Vitro and in Vivo Antibacterial Activity Assays of Carvacrol: A Candidate for Development of Innovative Treatments against KPC-Producing Klebsiella pneumoniae. PLoS ONE 2021, 16, e0246003. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Sun, Z.; Wang, T.; Yang, M.; Liu, M.; Zhang, J.; Li, Y. Antimicrobial Activity of Eugenol against Carbapenem-Resistant Klebsiella pneumoniae and Its Effect on Biofilms. Microb. Pathog. 2020, 139, 103924. [Google Scholar] [CrossRef] [PubMed]
- Dhara, L.; Tripathi, A. Sub-Acute Toxicological and Behavioural Effects of Two Candidate Therapeutics, Cinnamaldehyde and Eugenol, for Treatment of ESBL Producing-Quinolone Resistant Pathogenic Enterobacteriaceae. Clin. Exp. Pharmacol. Physiol. 2020, 47, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Anwar, R.; Sukmasari, S.; Siti Aisyah, L.; Puspita Lestari, F.; Ilfani, D.; Febriani Yun, Y.; Diki Prestya, P. Antimicrobial Activity of β-Sitosterol Isolated from Kalanchoe tomentosa Leaves Against Staphylococcus aureus and Klebsiella pneumoniae. Pak. J. Biol. Sci. 2022, 25, 602–607. [Google Scholar] [CrossRef]
- Al-Qahtani, W.H.; Dinakarkumar, Y.; Arokiyaraj, S.; Saravanakumar, V.; Rajabathar, J.R.; Arjun, K.; Gayathri, P.K.; Nelson Appaturi, J. Phyto-Chemical and Biological Activity of Myristica fragrans, an Ayurvedic Medicinal Plant in Southern India and Its Ingredient Analysis. Saudi J. Biol. Sci. 2022, 29, 3815–3821. [Google Scholar] [CrossRef]
- Yang, S.-K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Akseer, R.; Lim, S.-H.E.; Lai, K.-S. Disruption of KPC-Producing Klebsiella pneumoniae Membrane via Induction of Oxidative Stress by Cinnamon Bark (Cinnamomum verum J. Presl) Essential Oil. PLoS ONE 2019, 14, e0214326. [Google Scholar] [CrossRef]
- Gopu, V.; Kothandapani, S.; Shetty, P.H. Quorum Quenching Activity of Syzygium cumini (L.) Skeels and Its Anthocyanin Malvidin against Klebsiella pneumoniae. Microb. Pathog. 2015, 79, 61–69. [Google Scholar] [CrossRef]
- Supardy, N.A. Inhibition of Klebsiella pneumoniae ATCC 13883 Cells by Hexane Extract of Halimeda discoidea (Decaisne) and the Identification of Its Potential Bioactive Compounds. J. Microbiol. Biotechnol. 2012, 22, 872–881. [Google Scholar] [CrossRef]
- Akbari, R.; Bafghi, M.F.; Fazeli, H. Nosocomial Infections Pathogens Isolated from Hospital Personnel, Hospital Environment and Devices. J. Med. Bacteriol. 2018, 7, 22–30. [Google Scholar]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Bharathala, S.; Sharma, P. Biomedical Applications of Nanoparticles. In Nanotechnology in Modern Animal Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–132. [Google Scholar]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Salomoni, R.; Léo, P.; Montemor, A.F.; Rinaldi, B.G.; Rodrigues, M.F.A. Antibacterial Effect of Silver Nanoparticles in Pseudomonas aeruginosa. Nanotechnol. Sci. Appl. 2017, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Chinnathambi, A.; Alharbi, S.A.; Joshi, D.; Saranya, V.; Jhanani, G.K.; On-Uma, R.; Jutamas, K.; Anupong, W. Synthesis of AgNPs from Leaf Extract of Naringi crenulata and Evaluation of Its Antibacterial Activity against Multidrug Resistant Bacteria. Environ. Res. 2022, 216, 114455. [Google Scholar] [CrossRef] [PubMed]
- Kabeerdass, N.; Murugesan, K.; Arumugam, N.; Almansour, A.I.; Kumar, R.S.; Djearamane, S.; Kumaravel, A.K.; Velmurugan, P.; Mohanavel, V.; Kumar, S.S.; et al. Biomedical and Textile Applications of Alternanthera sessilis Leaf Extract Mediated Synthesis of Colloidal Silver Nanoparticle. Nanomaterials 2022, 12, 2759. [Google Scholar] [CrossRef] [PubMed]
- Wali, S.; Zahra, M.; Okla, M.K.; Wahidah, H.A.; Tauseef, I.; Haleem, K.S.; Farid, A.; Maryam, A.; AbdElgawad, H.; Adetunji, C.O.; et al. Brassica oleracea L. (Acephala Group) Based Zinc Oxide Nanoparticles and Their Efficacy as Antibacterial Agent. Braz. J. Biol. 2022, 84, e259351. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.; Suganthy, N.; Shanmugam, S.; Brindhadevi, K.; Sabour, A.; Alshiekheid, M.; Lan Chi, N.T.; Pugazhendhi, A.; Shanmuganathan, R. Biosynthesis of Zirconium Nanoparticles (ZrO(2) NPs) by Phyllanthus niruri Extract: Characterization and Its Photocatalytic Dye Degradation Activity. Food Chem. Toxicol. 2022, 168, 113340. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, M.; Liu, Y.; Bai, Y.; Deng, Y.; Liu, J.; Chen, L. Green Synthesis of Se/Ru Alloy Nanoparticles Using Gallic Acid and Evaluation of Theiranti-Invasive Effects in HeLa Cells. Colloids Surf. B Biointerfaces 2016, 144, 118–124. [Google Scholar] [CrossRef]
- Hassan, H.U.; Raja, N.I.; Abasi, F.; Mehmood, A.; Qureshi, R.; Manzoor, Z.; Shahbaz, M.; Proćków, J. Comparative Study of Antimicrobial and Antioxidant Potential of Olea ferruginea Fruit Extract and Its Mediated Selenium Nanoparticles. Molecules 2022, 27, 5194. [Google Scholar] [CrossRef]
- Todorova, V.; Ivanov, K.; Georgieva, Y.; Karcheva-Bahchevanska, D.; Ivanova, S. Comparison between the Chemical Composition of Essential Oil from Commercial Products and Biocultivated Lavandula angustifolia Mill. Int. J. Anal. Chem. 2023, 2023, 1997157. [Google Scholar] [CrossRef]
- Chung, W.-Y.; Jadhav, S.; Hsu, P.-K.; Kuan, C.-M. Evaluation of Acute and Sub-Chronic Toxicity of Bitter Melon Seed Extract in Wistar Rats. Toxicol. Rep. 2022, 9, 1024–1034. [Google Scholar] [CrossRef]
- Sireeratawong, S.; Jaijoy, K.; Khonsung, P.; Lertprasertsuk, N.; Ingkaninan, K. Acute and Chronic Toxicities of Bacopa monnieri Extract in Sprague-Dawley Rats. BMC Complement Altern. Med. 2016, 16, 249. [Google Scholar] [CrossRef]
- Ahmad, F.; Tabassum, N. Preliminary Phytochemical, Acute Oral Toxicity and Antihepatotoxic Study of Roots of Paeonia officinalis Linn. Asian Pac. J. Trop. Biomed. 2013, 3, 64–68. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Gopalakrishnan, V.K. Radical Scavenging and In-Vitro Hemolytic Activity of Aqueous Extracts of Selected Acacia Species. J. Appl. Pharm. Sci. 2013, 3, 109–111. [Google Scholar]
- Liaqat, I.; Mahreen, A.; Arshad, M.; Arshad, N. Antimicrobial and Toxicological Evaluation of Origanum vulgare: An in Vivo Study. Braz. J. Biol. 2023, 83, e244551. [Google Scholar] [CrossRef]
- Rojas-Armas, J.; Arroyo-Acevedo, J.; Ortiz-Snchez, M.; Palomino-Pacheco, M.; Castro-Luna, A.; Ramos-Cevallos, N.; Justil-Guerrero, H.; Hilario-Vargas, J.; Herrera-Caldern, O. Acute and Repeated 28-Day Oral Dose Toxicity Studies of Thymus vulgaris L. Essential Oil in Rats. Toxicol. Res. 2019, 35, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of Clove (Syzygium aromaticum) Oil and Its Major Components to Human Skin Cells. Cell Prolif. 2006, 39, 241–248. [Google Scholar] [CrossRef]
- Wijesinghe, G.K.; Maia, F.C.; de Oliveira, T.R.; de Feiria, S.N.B.; Joia, F.; Barbosa, J.P.; Boni, G.C.; de Cássia Orlandi Sardi, J.; Rosalen, P.L.; Höfling, J.F. Effect of Cinnamomum verum Leaf Essential Oil on Virulence Factors of Candida Species and Determination of the In-Vivo Toxicity with Galleria Mellonella Model. Mem Inst Oswaldo Cruz 2020, 115. [Google Scholar] [CrossRef]
- Nair, B. Final Report on the Safety Assessment of Mentha piperita (Peppermint) Oil, Mentha piperita (Peppermint) Leaf Extract, Mentha piperita (Peppermint) Leaf, and Mentha piperita (Peppermint) Leaf Water. Int. J. Toxicol. 2001, 20 (Suppl. S3), 61–73. [Google Scholar] [PubMed]
- Fahim, F.; Esmat, A.; Fadel, H.; Hassan, K. Allied Studies on the Effect of Rosmarinus officinalis L. on Experimental Hepatotoxicity and Mutagenesis. Int. J. Food Sci. Nutr. 1999, 50, 413–427. [Google Scholar] [CrossRef]
- Aggarwal, M.L.; Chacko, K.M.; Kuruvilla, B.T. Systematic and Comprehensive Investigation of the Toxicity of Curcuminoid-Essential Oil Complex: A Bioavailable Turmeric Formulation. Mol. Med. Rep. 2016, 13, 592–604. [Google Scholar] [CrossRef]






| Antibiotic | Resistance Mechanisms | Related Genes | Reference |
|---|---|---|---|
| β-Lactams | Antibiotic inactivation | blaSHV-2 | [61] |
| blaTEM-3 | [62] | ||
| blaCTX-M | [63] | ||
| blaAMP | [64] | ||
| blaIMP-1 | [65] | ||
| blaKPC | [66] | ||
| blaGEN | [67] | ||
| blaOXA-48 | [68] | ||
| blaVIM-1 | [69] | ||
| Permeability alterations | blaACT-1 | [70] | |
| [71] | |||
| Aminoglycosides | Drug modification | aac | [72] |
| ant | |||
| aph | |||
| Target protection | armA | [73] | |
| rmt | [72] | ||
| npmA | |||
| Permeability alterations | kpnEF | [59] | |
| Quinolones | Target modification | gyrA 1 | [74] |
| gyrB 1 | [75] | ||
| parC 1 | [76] | ||
| parE 1 | |||
| Permeability alterations | Plasmid-mediated quinolone resistance genes (PMQR) | [77] | |
| Target protection | qnr | [78] | |
| Drug modification | aac(6′)-Ib-cr | [79] | |
| Polymyxin | LPS modification | LPS-MS 2 | [80] |
| lpxM | [81] | ||
| pbgP | [82] | ||
| pmrE | [83] | ||
| mcr-1 | [84] | ||
| pmrC | [85] | ||
| pagP | |||
| phoPQ | |||
| pmrA | |||
| pmrD | |||
| Physical barrier | CPSs 3 | [86] | |
| Tigecycline | Permeability alterations | rarA | [87] |
| ramA | [88] | ||
| ramR | [89] | ||
| acrR | |||
| rpsJ | |||
| kpgA | |||
| kpgB | |||
| kpgC |
| Plant | Type of Compound | Activity | Reference |
|---|---|---|---|
| Momordica charantia | Ethanolic leaf extract Ethyl acetate leaf extract | MIC = 625 µg/mL MIC = 156.2 µg/mL | [108] |
| Skimmia anquetilia | Ethyl acetate root extract | Zone diameter = 17.0 ± 1.0 mm MIC = 8 mg/mL | [109,110] |
| Bacopa monnieri | Ethanolic leaf extract Methanolic leaf extracts | Zone diameter =23.0 ± 0.4 mm Zone diameter = 25.0 ± 0.5 mm | [109,110] |
| Paeonia officinalis | Acetone leaf extract | MIC = 128 µg/mL | [111] |
| Acacia nilotica | Aqueous extract | MIC = 11.7 mg/mL MBC = 13.3 mg/mL Reduces biofilm by 59.03% | [112] |
| Himatanthus drasticus | Hydroalcoholic extract | Zone diameter = 16 ± 0.5 mm MIC and MBC = 6250 µg/mL Reduces biofilm by 50% | [113] |
| Pulicaria crispa | Polyphenolic extract | Zone diameter values vary between 12.55 ± 0.31 and 24.00 ± 0.02 mm. MIC values range from 0.1 to 0.425 mg/mL | [114] |
| Symplocos racemosa | Ethyl acetate extract | Zone diameter ranges from 14.33 to 25.66 mm MIC ranges from 0.5 to 10.0 mg/mL | [115] |
| Vaccinium corymbosum | Polyphenolic extract | Reduces the number of attached bacteria and biofilm production by 90% in vitro at 430 µg/mL | [116] |
| Vernonia adoensis | Chondrillasterol purified from acetone extract | Reduces bacterial growth by 38% at 100 µg/mL | [117] |
| Origanum vulgare | Essential oil | Zone diameter = 21 mm MIC = 0.059% (v/v) | [118] |
| Cinnamomum camphora | Essential oil | MIC = 6.25% (v/v) MBC = 12.5% (v/v) | [119] |
| Thymus vulgaris | Essential oil | Zone diameter of 21–30 mm MIC vary from 1 to 16 µg/mL | [120] |
| MIC = 0.15% (v/v) MBC = 0.45% (v/v) | [121] | ||
| Syzygium aromaticum | Essential oil | MIC = 0.078% (v/v) MBC = 0.156% (v/v) | [122,123,124,125] |
| Melaleuca alternifolia | Essential oil | Zone diameter of 31–40 mm MIC varies from 0.5 to 4.0 µg/mL | [120] |
| Cinnamomum burmanii | Essential oil | MIC = 0.078% (v/v) MBC = 0.156% (v/v) | [122,123,124,125] |
| Cinnamomum verum | Essential oil | MIC = 0.5 mg/mL MBC = 1.0 mg/mL | [122,123,124,125] |
| Mentha piperita | Essential oil | Zone diameter of 21–30 mm MIC varies from 8 to 128 µg/mL | [120] |
| MIC = 0.60% (v/v) MBC = 1.25% (v/v) | [121] | ||
| Camellia japonica | Essential oil | Zone diameter of 16 mm at 60 µg/mL MIC and MBC = 50 µg/mL | [122] |
| Rosmarinus officinalis | Essential oil | MIC = 0.45% (v/v) MBC = 3.75% (v/v) | [121] |
| Curcuma longa | Essential oil | MIC = 2.55% (v/v) MBC = 6.265% (v/v) | [121] |
| Juniperus rigida | Essential oil | Zone diameter = 16 ± 0.25 mm MIC and MBC = 3.125 mg/mL | [126] |
| Plectranthus amboinicus | Essential oil | MIC and MBC = 0.08% (700 µg/mL) | [127] |
| Lavandula angustifolia | Essential oil | MIC = 10% | [128] |
| Plant | Model | Activity | Reference |
|---|---|---|---|
| Momordica charantia | In vitro in lymphocytes | Lymphocyte viability was 98% at 12.5, 25, and 50 µg/mL), and micronucleus frequency was the same as in the negative control. M. charantia extracts did not affect IL-6 or IL-10 production. | [108] |
| In vivo in Wistar rats | The acute toxicity test revealed the manifestation of toxic signs in response to the hydroalcoholic extract of M. charantia, attributed to the presence of ethanol in the extract. A marginal reduction in body weight, although statistically nonsignificant, was observed. Conversely, administering the aqueous extract did not induce toxic signs or mortality. Both extracts were categorised as class 5, indicating their placement in the toxicity range with an LD50 greater than 2000 mg/kg. In the dermal and ocular irritation test, both extracts were deemed non-irritant. | [153] | |
| Bacopa monnieri | In vivo in Sprague–Dawley rats | B. monnieri extract (5000 mg/kg) did not cause a histopathological change in the internal organs, including the liver and the kidneys. Rats treated with B. monnieri extract at 30, 60, 300, and 1500 mg/kg dosages for 270 days did not present any toxic effect. | [154] |
| Paeonia officinalis | In vivo in Wistar rats | Aqueous extracts of the roots of P. officinalis in an acute oral toxicity test did not cause mortality in rats at a dose of 175 mg/kg, 550 mg/kg, or 2000 mg/kg and were considered safe. | [155] |
| Acacia nilotica | In vitro in freshly collected human red blood cells | A. nilotica at doses of 50 μg/mL, 100 μg/mL, 150 μg/mL, and 200 μg/mL were found to possess haemolytic activity. | [156] |
| Himatanthus drasticus | In vitro in human erythrocytes and peripheral blood mononuclear cells (PBMCs) | Himatanthus drasticus hydroalcoholic extract did not produce significant haemolysis at the concentrations tested, and no significant changes were detected in viability or nitric oxide (NO) production by PBMCs. | [113] |
| Symplocos racemosa | In vivo using Swiss albino mice | In vivo acute oral toxicity testing did not show any toxic effects | [115] |
| Origanum vulgare | In vivo using mice | Continuous use or high doses may deliver undesirable components causing liver and renal function impairment | [157] |
| Thymus vulgaris | In vivo using albino Holtzman rats | While the 28-day oral toxicity test indicated that the no-observed-adverse-effect level (NOAEL) was more than 250 mg/kg/day, Thymus vulgaris had moderate oral toxicity. | [158] |
| Syzygium aromaticum | In vitro against human normal dermal fibroblasts | Syzygium aromaticum oil cytotoxicity is dose-dependent at a concentration of 0.03%. | [159] |
| Cinnamomum verum | In vivo using G. mellonella larvae | C. verum leaf EO was non-toxic in the experimental model | [160] |
| Mentha piperita | In vivo and in vitro assays | Several (but not all) short-term and subchronic oral studies noted cystlike lesions in the cerebellum in rats that were given doses of Mentha piperita oil containing pulegone, pulegone alone, or large amounts (>200 mg/kg/day) of menthone. Thus, it is safe if the concentration of pulegone in these ingredients does not exceed 1%. | [161] |
| Rosmarinus officinalis | In vivo in Swiss albino mice | No significative changes were reported in relative liver, spleen, heart, or lung size and morphology, and there were changes in clinical chemistry parameters | [162] |
| Curcuma longa | In vivo in Wistar albino rats | No clinical signs of toxicity were observed in any of the treated or control mice at a dose of 5000 mg/kg body weight | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Pineda, V.M.; Rodríguez-Martínez, G.; Salazar-García, M.; Romo-Castillo, M. Plant-Origin Components: New Players to Combat Antibiotic Resistance in Klebsiella pneumoniae. Int. J. Mol. Sci. 2024, 25, 2134. https://doi.org/10.3390/ijms25042134
Luna-Pineda VM, Rodríguez-Martínez G, Salazar-García M, Romo-Castillo M. Plant-Origin Components: New Players to Combat Antibiotic Resistance in Klebsiella pneumoniae. International Journal of Molecular Sciences. 2024; 25(4):2134. https://doi.org/10.3390/ijms25042134
Chicago/Turabian StyleLuna-Pineda, Victor M., Griselda Rodríguez-Martínez, Marcela Salazar-García, and Mariana Romo-Castillo. 2024. "Plant-Origin Components: New Players to Combat Antibiotic Resistance in Klebsiella pneumoniae" International Journal of Molecular Sciences 25, no. 4: 2134. https://doi.org/10.3390/ijms25042134
APA StyleLuna-Pineda, V. M., Rodríguez-Martínez, G., Salazar-García, M., & Romo-Castillo, M. (2024). Plant-Origin Components: New Players to Combat Antibiotic Resistance in Klebsiella pneumoniae. International Journal of Molecular Sciences, 25(4), 2134. https://doi.org/10.3390/ijms25042134







