Inflammation in Development and Aging: Insights from the Zebrafish Model
Abstract
:1. Zebrafish as Inflammation Model
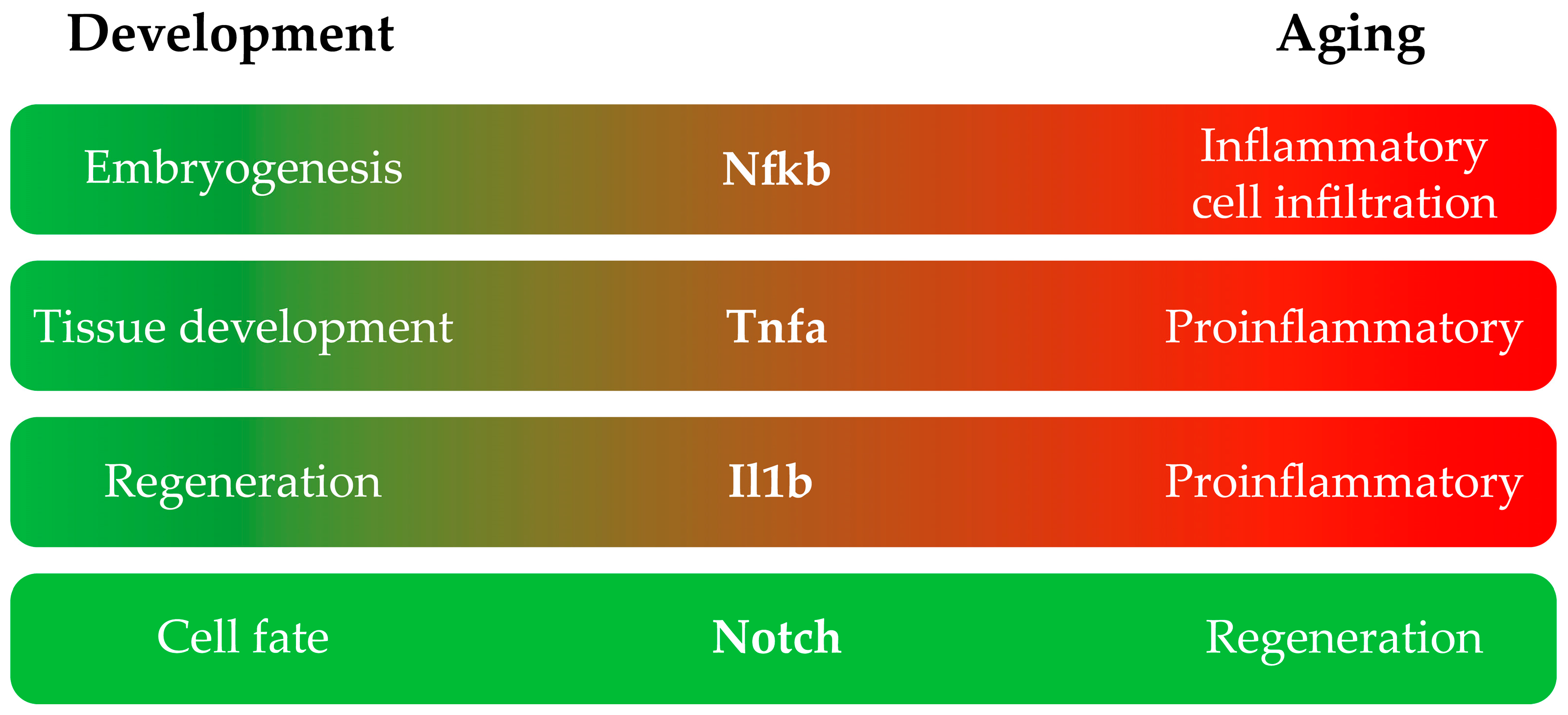
2. Inflammatory Pathways Are Involved in Zebrafish Development and Aging
2.1. NF-κB
2.2. TNF-α
2.3. IL-1β
2.4. Notch
3. Inflammation as a Developmental Mechanism for HSPC Emergence
3.1. Zebrafish Hematopoiesis
3.2. Sources of Developmental Inflammation in HSPC Emergence and Specification
3.3. Cytokines Role in HSPC Emergence
3.4. NF-κB Signaling in HSPC Emergence
3.5. Notch Signaling in HSPC Emergence
3.6. Demand-Driven Hematopoiesis Influences HSPC Lineage Commitment
4. Zebrafish as a Model to Study Inflammaging
4.1. Telomeres
4.2. TERRA
4.3. Senescence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Xie, Y.; Meijer, A.H.; Schaaf, M.J.M. Modeling Inflammation in Zebrafish for the Development of Anti-Inflammatory Drugs. Front. Cell Dev. Biol. 2020, 8, 620984. [Google Scholar] [CrossRef] [PubMed]
- Zanandrea, R.; Bonan, C.D.; Campos, M.M. Zebrafish as a model for inflammation and drug discovery. Drug Discov. Today 2020, 25, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Iribarne, M. Inflammation induces zebrafish regeneration. Neural Regen. Res. 2021, 16, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Kijima, Y.; Wantong, W.; Igarashi, Y.; Yoshitake, K.; Asakawa, S.; Suzuki, Y.; Watabe, S.; Kinoshita, S. Age-Associated Different Transcriptome Profiling in Zebrafish and Rats: An Insight into the Diversity of Vertebrate Aging. Mar. Biotechnol. 2022, 24, 895–910. [Google Scholar] [CrossRef]
- Janjuha, S.; Singh, S.P.; Tsakmaki, A.; Mousavy Gharavy, S.N.; Murawala, P.; Konantz, J.; Birke, S.; Hodson, D.J.; Rutter, G.A.; Bewick, G.A.; et al. Age-related islet inflammation marks the proliferative decline of pancreatic beta-cells in zebrafish. eLife 2018, 7, e32965. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.C.; de Castro, I.P.; Ferreira, M.G. Telomeres in aging and disease: Lessons from zebrafish. Dis. Models Mech. 2016, 9, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Herbomel, P.; Thisse, B.; Thisse, C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development 1999, 126, 3735–3745. [Google Scholar] [CrossRef]
- Gratacap, R.L.; Wheeler, R.T. Utilization of zebrafish for intravital study of eukaryotic pathogen-host interactions. Dev. Comp. Immunol. 2014, 46, 108–115. [Google Scholar] [CrossRef]
- Masud, S.; Torraca, V.; Meijer, A.H. Modeling Infectious Diseases in the Context of a Developing Immune System. Curr. Top. Dev. Biol. 2017, 124, 277–329. [Google Scholar] [CrossRef]
- Torraca, V.; Mostowy, S. Zebrafish Infection: From Pathogenesis to Cell Biology. Trends Cell Biol. 2018, 28, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Roca, F.J.; Ramakrishnan, L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 2013, 153, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Tyrkalska, S.D.; Candel, S.; Pedoto, A.; García-Moreno, D.; Alcaraz-Pérez, F.; Sánchez-Ferrer, Á.; Cayuela, M.L.; Mulero, V. Zebrafish models of COVID-19. FEMS Microbiol. Rev. 2023, 47, fuac042. [Google Scholar] [CrossRef] [PubMed]
- Kraus, A.; Huertas, M.; Ellis, L.; Boudinot, P.; Levraud, J.P.; Salinas, I. Intranasal delivery of SARS-CoV-2 spike protein is sufficient to cause olfactory damage, inflammation and olfactory dysfunction in zebrafish. Brain Behav. Immun. 2022, 102, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Sun, C.; Chen, Z.; Yang, D.; Zhou, Z.; Peng, X.; Tang, C. Alcohol Induces Zebrafish Skeletal Muscle Atrophy through HMGB1/TLR4/NF-κB Signaling. Life 2022, 12, 1211. [Google Scholar] [CrossRef]
- Onyenwoke, R.U.; Leung, T.; Huang, X.; Parker, D.; Shipman, J.G.; Alhadyan, S.K.; Sivaraman, V. An assessment of vaping-induced inflammation and toxicity: A feasibility study using a 2-stage zebrafish and mouse platform. Food Chem. Toxicol. 2022, 163, 112923. [Google Scholar] [CrossRef] [PubMed]
- Kasica-Jarosz, N.; Podlasz, P.; Kaleczyc, J. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) plays an inhibitory role against inflammation induced by chemical damage to zebrafish hair cells. PLoS ONE 2018, 13, e0198180. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Wang, B.; Lu, G.; Liu, J.; Wu, D.; Yan, Z. Toxicity comparison of perfluorooctanoic acid (PFOA), hexafluoropropylene oxide dimer acid (HFPO-DA), and hexafluoropropylene oxide trimer acid (HFPO-TA) in zebrafish gut. Aquat. Toxicol. 2023, 262, 106655. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Yazid, M.D.; Embong, H.; Othman, F. Zebrafish as a Model System to Study the Mechanism of Cutaneous Wound Healing and Drug Discovery: Advantages and Challenges. Pharmaceuticals 2021, 14, 1058. [Google Scholar] [CrossRef]
- Ellett, F.; Pase, L.; Hayman, J.W.; Andrianopoulos, A.; Lieschke, G.J. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 2011, 117, e49–e56. [Google Scholar] [CrossRef]
- Nguyen-Chi, M.; Laplace-Builhe, B.; Travnickova, J.; Luz-Crawford, P.; Tejedor, G.; Lutfalla, G.; Kissa, K.; Jorgensen, C.; Djouad, F. TNF signaling and macrophages govern fin regeneration in zebrafish larvae. Cell Death Dis. 2017, 8, e2979. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Chi, M.; Laplace-Builhe, B.; Travnickova, J.; Luz-Crawford, P.; Tejedor, G.; Phan, Q.T.; Duroux-Richard, I.; Levraud, J.P.; Kissa, K.; Lutfalla, G.; et al. Identification of polarized macrophage subsets in zebrafish. eLife 2015, 4, e07288. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, S.; Lopez-Munoz, A.; Martinez-Navarro, F.J.; Galindo-Villegas, J.; Mulero, V.; Calado, A. Cxcl8-l1 and Cxcl8-l2 are required in the zebrafish defense against Salmonella Typhimurium. Dev. Comp. Immunol. 2015, 49, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Anderson, R.; Mirmira, R.G. A zebrafish tailfin injury assay protocol for quantifying immune cell migration and infiltration. STAR Protoc. 2022, 3, 101196. [Google Scholar] [CrossRef] [PubMed]
- Michael, C.; de Oliveira, S. Exploring the dynamic behavior of leukocytes with zebrafish. Curr. Opin. Cell Biol. 2023, 85, 102276. [Google Scholar] [CrossRef] [PubMed]
- Boehm, T.; Iwanami, N.; Hess, I. Evolution of the immune system in the lower vertebrates. Annu. Rev. Genom. Hum. Genet. 2012, 13, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Boehm, T. Evolution of vertebrate immunity. Curr. Biol. 2012, 22, R722–R732. [Google Scholar] [CrossRef]
- Roca, F.J.; Mulero, I.; López-Muñoz, A.; Sepulcre, M.P.; Renshaw, S.A.; Meseguer, J.; Mulero, V. Evolution of the inflammatory response in vertebrates: Fish TNF-alpha is a powerful activator of endothelial cells but hardly activates phagocytes. J. Immunol. 2008, 181, 5071–5081. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. The Function of Fish Cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Langenau, D.M.; Ferrando, A.A.; Traver, D.; Kutok, J.L.; Hezel, J.P.; Kanki, J.P.; Zon, L.I.; Look, A.T.; Trede, N.S. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc. Natl. Acad. Sci. USA 2004, 101, 7369–7374. [Google Scholar] [CrossRef]
- Davidson, A.J.; Zon, L.I. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 2004, 23, 7233–7246. [Google Scholar] [CrossRef]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Lam, T.J.; Sin, Y.M. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef]
- Visekruna, A.; Volkov, A.; Steinhoff, U. A Key Role for NF-κB Transcription Factor c-Rel in T-Lymphocyte-Differentiation and Effector Functions. Clin. Dev. Immunol. 2012, 2012, 239368. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-κB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Espín-Palazón, R.; Traver, D. The NF-κB family: Key players during embryonic development and HSC emergence. Exp. Hematol. 2016, 44, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Beg, A.A.; Sha, W.C.; Bronson, R.T.; Ghosh, S.; Baltimore, D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 1995, 376, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Beg, A.A.; Sha, W.C.; Bronson, R.T.; Baltimore, D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 1995, 9, 2736–2746. [Google Scholar] [CrossRef]
- Takeda, K.; Takeuchi, O.; Tsujimura, T.; Itami, S.; Adachi, O.; Kawai, T.; Sanjo, H.; Yoshikawa, K.; Terada, N.; Akira, S. Limb and skin abnormalities in mice lacking IKKα. Science 1999, 284, 313–316. [Google Scholar] [CrossRef]
- Hu, Y.; Baud, V.; Delhase, M.; Zhang, P.; Deerinck, T.; Ellisman, M.; Johnson, R.; Karin, M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science 1999, 284, 316–320. [Google Scholar] [CrossRef]
- Correa, R.G.; Tergaonkar, V.; Ng, J.K.; Dubova, I.; Izpisua-Belmonte, J.C.; Verma, I.M. Characterization of NF-κΒ/IκΒ proteins in zebra fish and their involvement in notochord development. Mol. Cell. Biol. 2004, 24, 5257–5268. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Liao, Q.; Zhang, D.; Rong, F.; Cai, X.; Fan, S.; Zhu, J.; Wang, J.; Liu, X.; Liu, X.; et al. Zebrafish NF-κB/p65 Is Required for Antiviral Responses. J. Immunol. 2020, 204, 3019–3029. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.I.; Su, M.; Cantuti-Castelvetri, L.; Müller, S.A.; Schifferer, M.; Djannatian, M.; Alexopoulos, I.; van der Meer, F.; Winkler, A.; van Ham, T.J.; et al. Pro-inflammatory activation following demyelination is required for myelin clearance and oligodendrogenesis. J. Exp. Med. 2020, 217, e20191390. [Google Scholar] [CrossRef]
- Kim, D.H.; Jung, I.H.; Kim, D.H.; Park, S.W. Knockout of longevity gene Sirt1 in zebrafish leads to oxidative injury, chronic inflammation, and reduced life span. PLoS ONE 2019, 14, e0220581. [Google Scholar] [CrossRef]
- Wiltbank, A.T.; Steinson, E.R.; Criswell, S.J.; Piller, M.; Kucenas, S. Cd59 and inflammation regulate Schwann cell development. eLife 2022, 11, e76640. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chang, L.; Huang, A.; Liu, X.; Liu, X.; Zhou, H.; Liang, J.G.; Liang, P. Functional Detection of TNF Receptor Family Members by Affinity-Labeled Ligands. Sci. Rep. 2017, 7, 6944. [Google Scholar] [CrossRef]
- Su, Z.; Wu, Y. A computational model for understanding the oligomerization mechanisms of TNF receptor superfamily. Comput. Struct. Biotechnol. J. 2020, 18, 258–270. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef]
- MacEwan, D.J. TNF receptor subtype signalling: Differences and cellular consequences. Cell. Signal. 2002, 14, 477–492. [Google Scholar] [CrossRef]
- Tsarouchas, T.M.; Wehner, D.; Cavone, L.; Munir, T.; Keatinge, M.; Lambertus, M.; Underhill, A.; Barrett, T.; Kassapis, E.; Ogryzko, N.; et al. Dynamic control of proinflammatory cytokines Il-1β and Tnf-α by macrophages in zebrafish spinal cord regeneration. Nat. Commun. 2018, 9, 4670. [Google Scholar] [CrossRef] [PubMed]
- Bohaud, C.; Johansen, M.D.; Varga, B.; Contreras-Lopez, R.; Barthelaix, A.; Hamela, C.; Sapède, D.; Cloitre, T.; Gergely, C.; Jorgensen, C.; et al. Exploring Macrophage-Dependent Wound Regeneration during Mycobacterial Infection in Zebrafish. Front. Immunol. 2022, 13, 838425. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.D.; Sun, Y.; Cai, S.J.; Fang, Y.W.; Cui, J.L.; Li, Y.H. Role of tumor necrosis factor-alpha in zebrafish retinal neurogenesis and myelination. Int. J. Ophthalmol. 2016, 9, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Song, J.; Yang, H.; Gao, W.; Liu, N.A.; Zhang, B.; Lin, S. Mmp23b promotes liver development and hepatocyte proliferation through the tumor necrosis factor pathway in zebrafish. Hepatology 2010, 52, 2158–2166. [Google Scholar] [CrossRef] [PubMed]
- Espín, R.; Roca, F.J.; Candel, S.; Sepulcre, M.P.; González-Rosa, J.M.; Alcaraz-Pérez, F.; Meseguer, J.; Cayuela, M.L.; Mercader, N.; Mulero, V. TNF receptors regulate vascular homeostasis in zebrafish through a caspase-8, caspase-2 and P53 apoptotic program that bypasses caspase-3. Dis. Models Mech. 2013, 6, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.I.; Bryson-Richardson, R.J.; Daggett, D.F.; Gautier, P.; Keenan, D.G.; Currie, P.D. Dystrophin is required for the formation of stable muscle attachments in the zebrafish embryo. Development 2003, 130, 5851–5860. [Google Scholar] [CrossRef] [PubMed]
- Brogi, L.; Marchese, M.; Cellerino, A.; Licitra, R.; Naef, V.; Mero, S.; Bibbiani, C.; Fronte, B. β-Glucans as Dietary Supplement to Improve Locomotion and Mitochondrial Respiration in a Model of Duchenne Muscular Dystrophy. Nutrients 2021, 13, 1619. [Google Scholar] [CrossRef]
- Tobin, D.M.; Roca, F.J.; Oh, S.F.; McFarland, R.; Vickery, T.W.; Ray, J.P.; Ko, D.C.; Zou, Y.; Bang, N.D.; Chau, T.T.; et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 2012, 148, 434–446. [Google Scholar] [CrossRef]
- Roca, F.J.; Whitworth, L.J.; Prag, H.A.; Murphy, M.P.; Ramakrishnan, L. Tumor necrosis factor induces pathogenic mitochondrial ROS in tuberculosis through reverse electron transport. Science 2022, 376, eabh2841. [Google Scholar] [CrossRef]
- Yin, J.; Wang, A.P.; Li, W.F.; Shi, R.; Jin, H.T.; Wei, J.F. Time-response characteristic and potential biomarker identification of heavy metal induced toxicity in zebrafish. Fish. Shellfish. Immunol. 2018, 72, 309–317. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Wang, D.; Hu, G.; Liu, Z.; Yan, D.; Serikuly, N.; Alpyshov, E.T.; Demin, K.A.; Strekalova, T.; et al. Delayed behavioral and genomic responses to acute combined stress in zebrafish, potentially relevant to PTSD and other stress-related disorders: Focus on neuroglia, neuroinflammation, apoptosis and epigenetic modulation. Behav. Brain Res. 2020, 389, 112644. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.-K.; Kim, J.K.; Shin, D.-M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Masumoto, J.; Zhou, W.; Chen, F.F.; Su, F.; Kuwada, J.Y.; Hidaka, E.; Katsuyama, T.; Sagara, J.; Taniguchi, S.; Ngo-Hazelett, P.; et al. Caspy, a zebrafish caspase, activated by ASC oligomerization is required for pharyngeal arch development. J. Biol. Chem. 2003, 278, 4268–4276. [Google Scholar] [CrossRef]
- Kuri, P.; Schieber, N.L.; Thumberger, T.; Wittbrodt, J.; Schwab, Y.; Leptin, M. Dynamics of in vivo ASC speck formation. J. Cell Biol. 2017, 216, 2891–2909. [Google Scholar] [CrossRef]
- Li, J.Y.; Gao, K.; Shao, T.; Fan, D.D.; Hu, C.B.; Sun, C.C.; Dong, W.R.; Lin, A.F.; Xiang, L.X.; Shao, J.Z. Characterization of an NLRP1 Inflammasome from Zebrafish Reveals a Unique Sequential Activation Mechanism Underlying Inflammatory Caspases in Ancient Vertebrates. J. Immunol. 2018, 201, 1946–1966. [Google Scholar] [CrossRef]
- Vojtech, L.N.; Scharping, N.; Woodson, J.C.; Hansen, J.D. Roles of inflammatory caspases during processing of zebrafish interleukin-1beta in Francisella noatunensis infection. Infect. Immun. 2012, 80, 2878–2885. [Google Scholar] [CrossRef]
- Lanham, K.A.; Nedden, M.L.; Wise, V.E.; Taylor, M.R. Genetically inducible and reversible zebrafish model of systemic inflammation. Biol. Open 2022, 11, bio058559. [Google Scholar] [CrossRef] [PubMed]
- Sebo, D.J.; Fetsko, A.R.; Phipps, K.K.; Taylor, M.R. Functional identification of the zebrafish Interleukin-1 receptor in an embryonic model of Il-1β-induced systemic inflammation. Front. Immunol. 2022, 13, 1039161. [Google Scholar] [CrossRef]
- Yan, B.; Han, P.; Pan, L.; Lu, W.; Xiong, J.; Zhang, M.; Zhang, W.; Li, L.; Wen, Z. IL-1β and reactive oxygen species differentially regulate neutrophil directional migration and Basal random motility in a zebrafish injury-induced inflammation model. J. Immunol. 2014, 192, 5998–6008. [Google Scholar] [CrossRef]
- Delgadillo-Silva, L.F.; Tsakmaki, A.; Akhtar, N.; Franklin, Z.J.; Konantz, J.; Bewick, G.A.; Ninov, N. Modelling pancreatic β-cell inflammation in zebrafish identifies the natural product wedelolactone for human islet protection. Dis. Models Mech. 2019, 12, dmm036004. [Google Scholar] [CrossRef]
- Ibrahim, S.; Harris-Kawano, A.; Haider, I.; Mirmira, R.G.; Sims, E.K.; Anderson, R.M. A novel Cre-enabled tetracycline-inducible transgenic system for tissue-specific cytokine expression in the zebrafish: CETI-PIC3. Dis. Models Mech. 2020, 13, dmm042556. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Maddison, L.A.; Zaborska, K.E.; Dai, C.; Yin, L.; Tang, Z.; Zang, L.; Jacobson, D.A.; Powers, A.C.; Chen, W. RIPK3-mediated inflammation is a conserved β cell response to ER stress. Sci. Adv. 2020, 6, eabd7272. [Google Scholar] [CrossRef] [PubMed]
- Ogryzko, N.V.; Hoggett, E.E.; Solaymani-Kohal, S.; Tazzyman, S.; Chico, T.J.; Renshaw, S.A.; Wilson, H.L. Zebrafish tissue injury causes upregulation of interleukin-1 and caspase-dependent amplification of the inflammatory response. Dis. Models Mech. 2014, 7, 259–264. [Google Scholar] [CrossRef]
- Hasegawa, T.; Hall, C.J.; Crosier, P.S.; Abe, G.; Kawakami, K.; Kudo, A.; Kawakami, A. Transient inflammatory response mediated by interleukin-1β is required for proper regeneration in zebrafish fin fold. eLife 2017, 6, e22716. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Harper, S.L.; Yun, S.I. In Vivo toxicological assessment of biologically synthesized silver nanoparticles in adult Zebrafish (Danio rerio). J. Hazard. Mater. 2016, 301, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.C.; Gregorio, C.; Uribe-Cruz, C.; Guizzo, R.; Malysz, T.; Faccioni-Heuser, M.C.; Longo, L.; da Silveira, T.R. Chronic exposure to ethanol causes steatosis and inflammation in zebrafish liver. World J. Hepatol. 2017, 9, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Kemble, S.; Croft, A.P. Critical Role of Synovial Tissue-Resident Macrophage and Fibroblast Subsets in the Persistence of Joint Inflammation. Front. Immunol. 2021, 12, 715894. [Google Scholar] [CrossRef]
- Bruton, F.A.; Kaveh, A.; Ross-Stewart, K.M.; Matrone, G.; Oremek, M.E.M.; Solomonidis, E.G.; Tucker, C.S.; Mullins, J.J.; Lucas, C.D.; Brittan, M.; et al. Macrophages trigger cardiomyocyte proliferation by increasing epicardial vegfaa expression during larval zebrafish heart regeneration. Dev. Cell 2022, 57, 1512–1528.e1515. [Google Scholar] [CrossRef]
- Fogerty, J.; Song, P.; Boyd, P.; Grabinski, S.E.; Hoang, T.; Reich, A.; Cianciolo, L.T.; Blackshaw, S.; Mumm, J.S.; Hyde, D.R.; et al. Notch Inhibition Promotes Regeneration and Immunosuppression Supports Cone Survival in a Zebrafish Model of Inherited Retinal Dystrophy. J. Neurosci. 2022, 42, 5144–5158. [Google Scholar] [CrossRef]
- Kim, A.D.; Melick, C.H.; Clements, W.K.; Stachura, D.L.; Distel, M.; Panáková, D.; MacRae, C.; Mork, L.A.; Crump, J.G.; Traver, D. Discrete Notch signaling requirements in the specification of hematopoietic stem cells. EMBO J. 2014, 33, 2363–2373. [Google Scholar] [CrossRef]
- Kortschak, D.R.; Tamme, R.; Lardelli, M. Evolutionary analysis of vertebrate Notch Genes Dev. Genes Evol. 2001, 211, 350–354. [Google Scholar] [CrossRef]
- Lai, E.C. Notch signaling: Control of cell communication and cell fate. Development 2004, 131, 965–973. [Google Scholar] [CrossRef]
- Sharma, P.; Saraswathy, V.M.; Xiang, L.; Furthauer, M. Notch-mediated inhibition of neurogenesis is required for zebrafish spinal cord morphogenesis. Sci. Rep. 2019, 9, 9958. [Google Scholar] [CrossRef] [PubMed]
- Fazio, C.; Ricciardiello, L. Inflammation and Notch signaling: A crosstalk with opposite effects on tumorigenesis. Cell Death Dis. 2016, 7, e2515. [Google Scholar] [CrossRef] [PubMed]
- Quillien, A.; Moore, J.C.; Shin, M.; Siekmann, A.F.; Smith, T.; Pan, L.; Moens, C.B.; Parsons, M.J.; Lawson, N.D. Distinct Notch signaling outputs pattern the developing arterial system. Development 2014, 141, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Butko, E.; Pouget, C.; Traver, D. Complex regulation of HSC emergence by the Notch signaling pathway. Dev. Biol. 2016, 409, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Thambyrajah, R.; Bigas, A. Notch Signaling in HSC Emergence: When, Why and How. Cells 2022, 11, 358. [Google Scholar] [CrossRef]
- Lee, C.Y.; Vogeli, K.M.; Kim, S.H.; Chong, S.W.; Jiang, Y.J.; Stainier, D.Y.; Jin, S.W. Notch signaling functions as a cell-fate switch between the endothelial and hematopoietic lineages. Curr. Biol. 2009, 19, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Morales, R.A.; Rabahi, S.; Diaz, O.E.; Salloum, Y.; Kern, B.C.; Westling, M.; Luo, X.; Parigi, S.M.; Monasterio, G.; Das, S.; et al. Interleukin-10 regulates goblet cell numbers through Notch signaling in the developing zebrafish intestine. Mucosal Immunol. 2022, 15, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Münch, J.; Grivas, D.; González-Rajal, Á.; Torregrosa-Carrión, R.; de la Pompa, J.L. Notch signalling restricts inflammation and serpine1 expression in the dynamic endocardium of the regenerating zebrafish heart. Development 2017, 144, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Luodan, A.; Huang, X.; Chen, X.; Xu, H. Muller Glia-Mediated Retinal Regeneration. Mol. Neurobiol. 2021, 58, 2342–2361. [Google Scholar] [CrossRef] [PubMed]
- Chapple, R.H.; Tseng, Y.J.; Hu, T.; Kitano, A.; Takeichi, M.; Hoegenauer, K.A.; Nakada, D. Lineage tracing of murine adult hematopoietic stem cells reveals active contribution to steady-state hematopoiesis. Blood Adv. 2018, 2, 1220–1228. [Google Scholar] [CrossRef]
- Hofer, T.; Busch, K.; Klapproth, K.; Rodewald, H.R. Fate Mapping and Quantitation of Hematopoiesis In Vivo. Annu. Rev. Immunol. 2016, 34, 449–478. [Google Scholar] [CrossRef]
- Kondo, M. Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunol. Rev. 2010, 238, 37–46. [Google Scholar] [CrossRef]
- Lai, A.Y.; Kondo, M. Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. J. Exp. Med. 2006, 203, 1867–1873. [Google Scholar] [CrossRef]
- Cumano, A.; Godin, I. Ontogeny of the hematopoietic system. Annu. Rev. Immunol. 2007, 25, 745–785. [Google Scholar] [CrossRef]
- Orkin, S.H.; Zon, L.I. Hematopoiesis: An evolving paradigm for stem cell biology. Cell 2008, 132, 631–644. [Google Scholar] [CrossRef]
- Ketharnathan, S.; Rajan, V.; Prykhozhij, S.V.; Berman, J.N. Zebrafish models of inflammation in hematopoietic development and disease. Front. Cell Dev. Biol. 2022, 10, 955658. [Google Scholar] [CrossRef] [PubMed]
- Frame, J.M.; Kubaczka, C.; Long, T.L.; Esain, V.; Soto, R.A.; Hachimi, M.; Jing, R.; Shwartz, A.; Goessling, W.; Daley, G.Q.; et al. Metabolic Regulation of Inflammasome Activity Controls Embryonic Hematopoietic Stem and Progenitor Cell Production. Dev. Cell 2020, 55, 133–149.e136. [Google Scholar] [CrossRef] [PubMed]
- Espin-Palazon, R.; Weijts, B.; Mulero, V.; Traver, D. Proinflammatory Signals as Fuel for the Fire of Hematopoietic Stem Cell Emergence. Trends Cell Biol. 2018, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Crosier, P.; Crosier, K. Inflammatory cytokines provide both infection-responsive and developmental signals for blood development: Lessons from the zebrafish. Mol. Immunol. 2016, 69, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Nik, S.; Weinreb, J.T.; Bowman, T.V. Developmental HSC Microenvironments: Lessons from Zebrafish. Adv. Exp. Med. Biol. 2017, 1041, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Traver, D.; Herbomel, P.; Patton, E.E.; Murphey, R.D.; Yoder, J.A.; Litman, G.W.; Catic, A.; Amemiya, C.T.; Zon, L.I.; Trede, N.S. The zebrafish as a model organism to study development of the immune system. Adv. Immunol. 2003, 81, 253–330. [Google Scholar]
- Paik, E.J.; Zon, L.I. Hematopoietic development in the zebrafish. Int. J. Dev. Biol. 2010, 54, 1127–1137. [Google Scholar] [CrossRef]
- Jing, L.; Zon, L.I. Zebrafish as a model for normal and malignant hematopoiesis. Dis. Models Mech. 2011, 4, 433–438. [Google Scholar] [CrossRef]
- Jagannathan-Bogdan, M.; Zon, L.I. Hematopoiesis. Development 2013, 140, 2463–2467. [Google Scholar] [CrossRef]
- Gore, A.V.; Pillay, L.M.; Venero Galanternik, M.; Weinstein, B.M. The zebrafish: A fintastic model for hematopoietic development and disease. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e312. [Google Scholar] [CrossRef]
- Potts, K.S.; Bowman, T.V. Modeling Myeloid Malignancies Using Zebrafish. Front. Oncol. 2017, 7, 297. [Google Scholar] [CrossRef]
- Berman, J.; Payne, E.; Hall, C. The Zebrafish as a Tool to Study Hematopoiesis, Human Blood Diseases, and Immune Function. Adv. Hematol. 2012, 2012, 425345. [Google Scholar] [CrossRef]
- Ho, R.K.; Kimmel, C.B. Commitment of Cell Fate in the Early Zebrafish Embryo. Science 1993, 261, 109–111. [Google Scholar] [CrossRef]
- Kissa, K.; Herbomel, P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 2010, 464, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.Y.; Chi, N.C.; Santoso, B.; Teng, S.; Stainier, D.Y.; Traver, D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 2010, 464, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Murayama, E.; Kissa, K.; Zapata, A.; Mordelet, E.; Briolat, V.; Lin, H.F.; Handin, R.I.; Herbomel, P. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 2006, 25, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Kissa, K.; Murayama, E.; Zapata, A.; Cortés, A.; Perret, E.; Machu, C.; Herbomel, P. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood 2008, 111, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Espín-Palazón, R.; Stachura, D.L.; Campbell, C.A.; García-Moreno, D.; Del Cid, N.; Kim, A.D.; Candel, S.; Meseguer, J.; Mulero, V.; Traver, D. Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell 2014, 159, 1070–1085. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, C.; Wang, L.; Zhang, P.; Ma, D.; Lv, J.; Liu, F. Inflammatory signaling regulates hematopoietic stem and progenitor cell emergence in vertebrates. Blood 2015, 125, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Travnickova, J.; Tran Chau, V.; Julien, E.; Mateos-Langerak, J.; Gonzalez, C.; Lelièvre, E.; Lutfalla, G.; Tavian, M.; Kissa, K. Primitive macrophages control HSPC mobilization and definitive haematopoiesis. Nat. Commun. 2015, 6, 6227. [Google Scholar] [CrossRef]
- Yuan, H.; Gao, S.; Chen, H.; Liu, X.; Zhou, J.; de The, H.; Zhu, J. Primitive macrophages are dispensable for HSPC mobilization and definitive hematopoiesis. Blood 2019, 134, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Wattrus, S.J.; Smith, M.L.; Rodrigues, C.P.; Hagedorn, E.J.; Kim, J.W.; Budnik, B.; Zon, L.I. Quality assurance of hematopoietic stem cells by macrophages determines stem cell clonality. Science 2022, 377, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Esain, V.; Frechette, G.M.; Harris, L.J.; Cox, A.G.; Cortes, M.; Garnaas, M.K.; Carroll, K.J.; Cutting, C.C.; Khan, T.; et al. Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo. Blood 2013, 121, 2483–2493. [Google Scholar] [CrossRef]
- Lim, S.-E.; Esain, V.; Kwan, W.; Theodore, L.N.; Cortes, M.; Frost, I.M.; Liu, S.Y.; North, T.E. HIF1α-induced PDGFRβ signaling promotes developmental HSC production via IL-6 activation. Exp. Hematol. 2017, 46, 83–95.e86. [Google Scholar] [CrossRef]
- Yegutkin, G.; Bodin, P.; Burnstock, G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Br. J. Pharmacol. 2000, 129, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Lefkopoulos, S.; Polyzou, A.; Derecka, M.; Bergo, V.; Clapes, T.; Cauchy, P.; Jerez-Longres, C.; Onishi-Seebacher, M.; Yin, N.; Martagon-Calderón, N.A.; et al. Repetitive Elements Trigger RIG-I-like Receptor Signaling that Regulates the Emergence of Hematopoietic Stem and Progenitor Cells. Immunity 2020, 53, 934–951.e939. [Google Scholar] [CrossRef]
- Weinreb, J.T.; Ghazale, N.; Pradhan, K.; Gupta, V.; Potts, K.S.; Tricomi, B.; Daniels, N.J.; Padgett, R.A.; De Oliveira, S.; Verma, A.; et al. Excessive R-loops trigger an inflammatory cascade leading to increased HSPC production. Dev. Cell 2021, 56, 627–640.e625. [Google Scholar] [CrossRef]
- Sawamiphak, S.; Kontarakis, Z.; Stainier, D.Y. Interferon gamma signaling positively regulates hematopoietic stem cell emergence. Dev. Cell 2014, 31, 640–653. [Google Scholar] [CrossRef]
- Tie, R.; Li, H.; Cai, S.; Liang, Z.; Shan, W.; Wang, B.; Tan, Y.; Zheng, W.; Huang, H. Interleukin-6 signaling regulates hematopoietic stem cell emergence. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Monteiro, R.; Pinheiro, P.; Joseph, N.; Peterkin, T.; Koth, J.; Repapi, E.; Bonkhofer, F.; Kirmizitas, A.; Patient, R. Transforming Growth Factor β Drives Hemogenic Endothelium Programming and the Transition to Hematopoietic Stem Cells. Dev. Cell 2016, 38, 358–370. [Google Scholar] [CrossRef]
- Stachura, D.L.; Svoboda, O.; Campbell, C.A.; Espin-Palazon, R.; Lau, R.P.; Zon, L.I.; Bartunek, P.; Traver, D. The zebrafish granulocyte colony-stimulating factors (Gcsfs): 2 paralogous cytokines and their roles in hematopoietic development and maintenance. Blood 2013, 122, 3918–3928. [Google Scholar] [CrossRef]
- Robert-Moreno, A.; Guiu, J.; Ruiz-Herguido, C.; López, M.E.; Inglés-Esteve, J.; Riera, L.; Tipping, A.; Enver, T.; Dzierzak, E.; Gridley, T.; et al. Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J. 2008, 27, 1886–1895. [Google Scholar] [CrossRef]
- Cheng, X.; Barakat, R.; Pavani, G.; Usha, M.K.; Calderon, R.; Snella, E.; Gorden, A.; Zhang, Y.; Gadue, P.; French, D.L.; et al. Nod1-dependent NF-kB activation initiates hematopoietic stem cell specification in response to small Rho GTPases. Nat. Commun. 2023, 14, 7668. [Google Scholar] [CrossRef]
- Gering, M.; Patient, R. Notch signalling and haematopoietic stem cell formation during embryogenesis. J. Cell. Physiol. 2010, 222, 11–16. [Google Scholar] [CrossRef]
- Bigas, A.; Guiu, J.; Gama-Norton, L. Notch and Wnt signaling in the emergence of hematopoietic stem cells. Blood Cells Mol. Dis. 2013, 51, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Lomelí, H.; Castillo-Castellanos, F. Notch signaling and the emergence of hematopoietic stem cells. Dev. Dyn. 2020, 249, 1302–1317. [Google Scholar] [CrossRef]
- Sood, R.; English, M.A.; Belele, C.L.; Jin, H.; Bishop, K.; Haskins, R.; McKinney, M.C.; Chahal, J.; Weinstein, B.M.; Wen, Z.; et al. Development of multilineage adult hematopoiesis in the zebrafish with a runx1 truncation mutation. Blood 2010, 115, 2806–2809. [Google Scholar] [CrossRef]
- Parsons, M.J.; Pisharath, H.; Yusuff, S.; Moore, J.C.; Siekmann, A.F.; Lawson, N.; Leach, S.D. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech. Dev. 2009, 126, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Perlin, J.R.; Robertson, A.L.; Zon, L.I. Efforts to enhance blood stem cell engraftment: Recent insights from zebrafish hematopoiesis. J. Exp. Med. 2017, 214, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.E.; Traver, D.; Mayhall, E.; Shepard, J.L.; Zon, L.I. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005, 19, 2331–2342. [Google Scholar] [CrossRef]
- Bertrand, J.Y.; Cisson, J.L.; Stachura, D.L.; Traver, D. Notch signaling distinguishes 2 waves of definitive hematopoiesis in the zebrafish embryo. Blood 2010, 115, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.I.; Price, E.N.; Boatman, S.; Hagedorn, E.J.; Trompouki, E.; Satishchandran, S.; Carspecken, C.W.; Uong, A.; DiBiase, A.; Yang, S.; et al. Angiopoietin-like proteins stimulate HSPC development through interaction with notch receptor signaling. eLife 2015, 4, e05544. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, I.; Kobayashi-Sun, J.; Kim, A.D.; Pouget, C.; Fujita, N.; Suda, T.; Traver, D. Jam1a-Jam2a interactions regulate haematopoietic stem cell fate through Notch signalling. Nature 2014, 512, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, B.H.; Scott, M.L.; Cherry, S.R.; Bronson, R.T.; Baltimore, D. Failure of lymphopoiesis after adoptive transfer of NF-kappaB-deficient fetal liver cells. Immunity 1997, 6, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, C.; Zhang, Y.; Lin, C.; Zhang, Y.; Shu, L.; Luo, L.; Zhuo, J.; Li, L. Macrophage-Derived IL-1β Regulates Emergency Myelopoiesis via the NF-κB and C/ebpβ in Zebrafish. J. Immunol. 2020, 205, 2694–2706. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, M.L.; Claes, K.B.M.; Ferreira, M.G.; Henriques, C.M.; van Eeden, F.; Varga, M.; Vierstraete, J.; Mione, M.C. The Zebrafish as an Emerging Model to Study DNA Damage in Aging, Cancer and Other Diseases. Front. Cell Dev. Biol. 2018, 6, 178. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Fu, Y.; Ma, J.; Li, X.; Lu, C.; Zhang, R. Functional alterations and transcriptomic changes during zebrafish cardiac aging. Biogerontology 2020, 21, 637–652. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef]
- Van Houcke, J.; Bollaerts, I.; Geeraerts, E.; Davis, B.; Beckers, A.; Van Hove, I.; Lemmens, K.; De Groef, L.; Moons, L. Successful optic nerve regeneration in the senescent zebrafish despite age-related decline of cell intrinsic and extrinsic response processes. Neurobiol. Aging 2017, 60, 1–10. [Google Scholar] [CrossRef]
- Munzel, E.J.; Becker, C.G.; Becker, T.; Williams, A. Zebrafish regenerate full thickness optic nerve myelin after demyelination, but this fails with increasing age. Acta Neuropathol. Commun. 2014, 2, 77. [Google Scholar] [CrossRef]
- Blasco, M.A.; Lee, H.W.; Hande, M.P.; Samper, E.; Lansdorp, P.M.; DePinho, R.A.; Greider, C.W. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 1997, 91, 25–34. [Google Scholar] [CrossRef]
- Hayashi, M.T. Telomere biology in aging and cancer: Early history and perspectives. Genes Genet. Syst. 2018, 92, 107–118. [Google Scholar] [CrossRef]
- Anchelin, M.; Alcaraz-Perez, F.; Martinez, C.M.; Bernabe-Garcia, M.; Mulero, V.; Cayuela, M.L. Premature aging in telomerase-deficient zebrafish. Dis. Models Mech. 2013, 6, 1101–1112. [Google Scholar] [CrossRef]
- Blasco, M.A. Telomeres and human disease: Ageing, cancer and beyond. Nat. Rev. Genet. 2005, 6, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Calado, R.T.; Ly, H.; Kajigaya, S.; Baerlocher, G.M.; Chanock, S.J.; Lansdorp, P.M.; Young, N.S. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N. Engl. J. Med. 2005, 352, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Hallmarks of human “immunosenescence”: Adaptation or dysregulation? Immun. Ageing 2012, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Lex, K.; Maia Gil, M.; Lopes-Bastos, B.; Figueira, M.; Marzullo, M.; Giannetti, K.; Carvalho, T.; Ferreira, M.G. Telomere shortening produces an inflammatory environment that increases tumor incidence in zebrafish. Proc. Natl. Acad. Sci. USA 2020, 117, 15066–15074. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz-Perez, F.; Garcia-Castillo, J.; Garcia-Moreno, D.; Lopez-Munoz, A.; Anchelin, M.; Angosto, D.; Zon, L.I.; Mulero, V.; Cayuela, M.L. A non-canonical function of telomerase RNA in the regulation of developmental myelopoiesis in zebrafish. Nat. Commun. 2014, 5, 3228. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Balsalobre, E.; Garcia-Castillo, J.; Garcia-Moreno, D.; Naranjo-Sanchez, E.; Fernandez-Lajarin, M.; Blasco, M.A.; Alcaraz-Perez, F.; Mulero, V.; Cayuela, M.L. Telomerase RNA-based aptamers restore defective myelopoiesis in congenital neutropenic syndromes. Nat. Commun. 2023, 14, 5912. [Google Scholar] [CrossRef]
- Kirwan, M.; Dokal, I. Dyskeratosis congenita, stem cells and telomeres. Biochim. Biophys. Acta 2009, 1792, 371–379. [Google Scholar] [CrossRef]
- Trahan, C.; Dragon, F. Dyskeratosis congenita mutations in the H/ACA domain of human telomerase RNA affect its assembly into a pre-RNP. RNA 2009, 15, 235–243. [Google Scholar] [CrossRef]
- Schoeftner, S.; Blasco, M.A. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat. Cell Biol. 2008, 10, 228–236. [Google Scholar] [CrossRef]
- Azzalin, C.M.; Reichenbach, P.; Khoriauli, L.; Giulotto, E.; Lingner, J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 2007, 318, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, M.; Chartrand, P. TERRA, a Multifaceted Regulator of Telomerase Activity at Telomeres. J. Mol. Biol. 2020, 432, 4232–4243. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Deng, Z.; Dahmane, N.; Tsai, K.; Wang, P.; Williams, D.R.; Kossenkov, A.V.; Showe, L.C.; Zhang, R.; Huang, Q.; et al. Telomeric repeat-containing RNA (TERRA) constitutes a nucleoprotein component of extracellular inflammatory exosomes. Proc. Natl. Acad. Sci. USA 2015, 112, E6293–E6300. [Google Scholar] [CrossRef] [PubMed]
- Idilli, A.I.; Cusanelli, E.; Pagani, F.; Berardinelli, F.; Bernabe, M.; Cayuela, M.L.; Poliani, P.L.; Mione, M.C. Expression of tert Prevents ALT in Zebrafish Brain Tumors. Front. Cell Dev. Biol. 2020, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Bassing, C.H.; Swat, W.; Alt, F.W. The mechanism and regulation of chromosomal V(D)J recombination. Cell 2002, 109, S45–S55. [Google Scholar] [CrossRef] [PubMed]
- Wienholds, E.; Schulte-Merker, S.; Walderich, B.; Plasterk, R.H. Target-selected inactivation of the zebrafish rag1 gene. Science 2002, 297, 99–102. [Google Scholar] [CrossRef]
- Garcia-Valtanen, P.; Martinez-Lopez, A.; Lopez-Munoz, A.; Bello-Perez, M.; Medina-Gali, R.M.; Ortega-Villaizan, M.D.; Varela, M.; Figueras, A.; Mulero, V.; Novoa, B.; et al. Zebra Fish Lacking Adaptive Immunity Acquire an Antiviral Alert State Characterized by Upregulated Gene Expression of Apoptosis, Multigene Families, and Interferon-Related Genes. Front. Immunol. 2017, 8, 121. [Google Scholar] [CrossRef]
- Novoa, B.; Pereiro, P.; Lopez-Munoz, A.; Varela, M.; Forn-Cuni, G.; Anchelin, M.; Dios, S.; Romero, A.; Martinez-Lopez, A.; Medina-Gali, R.M.; et al. Rag1 immunodeficiency-induced early aging and senescence in zebrafish are dependent on chronic inflammation and oxidative stress. Aging Cell 2019, 18, e13020. [Google Scholar] [CrossRef]
- Michael, C.; Martinez-Navarro, F.J.; de Oliveira, S. Analysis of Liver Microenvironment during Early Progression of Non-Alcoholic Fatty Liver Disease-Associated Hepatocellular Carcinoma in Zebrafish. J. Vis. Exp. 2021. [Google Scholar] [CrossRef]
- Vergroesen, J.E.; Thee, E.F.; de Crom, T.O.E.; Kiefte-de Jong, J.C.; Meester-Smoor, M.A.; Voortman, T.; Klaver, C.C.W.; Ramdas, W.D. The inflammatory potential of diet is associated with the risk of age-related eye diseases. Clin. Nutr. 2023, 42, 2404–2413. [Google Scholar] [CrossRef]
- Rocha, I.; Torrinhas, R.; Fonseca, D.; Lyra, C.O.; de Sousa Alves Neri, J.L.; Balmant, B.D.; Callado, L.; Charlton, K.; Queiroz, N.; Waitzberg, D.L. Pro-Inflammatory Diet Is Correlated with High Veillonella rogosae, Gut Inflammation and Clinical Relapse of Inflammatory Bowel Disease. Nutrients 2023, 15, 4148. [Google Scholar] [CrossRef] [PubMed]
- Ganbaatar, G.; Okami, Y.; Kadota, A.; Ganbaatar, N.; Yano, Y.; Kondo, K.; Harada, A.; Okuda, N.; Yoshita, K.; Okamura, T.; et al. Association of Pro-Inflammatory Diet with Long-Term Risk of All-Cause and Cardiovascular Disease Mortality: NIPPON DATA80. J. Atheroscler. Thromb. 2023. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Silva, D.; Canton-Sandoval, J.; Martinez-Navarro, F.J.; Perez-Sanchez, H.; de Oliveira, S.; Mulero, V.; Alcaraz-Perez, F.; Cayuela, M.L. Senescence-Independent Anti-Inflammatory Activity of the Senolytic Drugs Dasatinib, Navitoclax, and Venetoclax in Zebrafish Models of Chronic Inflammation. Int. J. Mol. Sci. 2022, 23, 10468. [Google Scholar] [CrossRef] [PubMed]
- Mathias, J.R.; Dodd, M.E.; Walters, K.B.; Rhodes, J.; Kanki, J.P.; Look, A.T.; Huttenlocher, A. Live imaging of chronic inflammation caused by mutation of zebrafish Hai1. J. Cell Sci. 2007, 120, 3372–3383. [Google Scholar] [CrossRef] [PubMed]
- Carney, T.J.; von der Hardt, S.; Sonntag, C.; Amsterdam, A.; Topczewski, J.; Hopkins, N.; Hammerschmidt, M. Inactivation of serine protease Matriptase1a by its inhibitor Hai1 is required for epithelial integrity of the zebrafish epidermis. Development 2007, 134, 3461–3471. [Google Scholar] [CrossRef]
- Martinez-Navarro, F.J.; Martinez-Menchon, T.; Mulero, V.; Galindo-Villegas, J. Models of human psoriasis: Zebrafish the newly appointed player. Dev. Comp. Immunol. 2019, 97, 76–87. [Google Scholar] [CrossRef]
- Martinez-Navarro, F.J.; Martinez-Morcillo, F.J.; Lopez-Munoz, A.; Pardo-Sanchez, I.; Martinez-Menchon, T.; Corbalan-Velez, R.; Cayuela, M.L.; Perez-Oliva, A.B.; Garcia-Moreno, D.; Mulero, V. The vitamin B6-regulated enzymes PYGL and G6PD fuel NADPH oxidases to promote skin inflammation. Dev. Comp. Immunol. 2020, 108, 103666. [Google Scholar] [CrossRef]
- Martinez-Morcillo, F.J.; Canton-Sandoval, J.; Martinez-Navarro, F.J.; Cabas, I.; Martinez-Vicente, I.; Armistead, J.; Hatzold, J.; Lopez-Munoz, A.; Martinez-Menchon, T.; Corbalan-Velez, R.; et al. NAMPT-derived NAD+ fuels PARP1 to promote skin inflammation through parthanatos cell death. PLoS Biol. 2021, 19, e3001455. [Google Scholar] [CrossRef]
- Solman, M.; Blokzijl-Franke, S.; Piques, F.; Yan, C.; Yang, Q.; Strullu, M.; Kamel, S.M.; Ak, P.; Bakkers, J.; Langenau, D.M.; et al. Inflammatory response in hematopoietic stem and progenitor cells triggered by activating SHP2 mutations evokes blood defects. eLife 2022, 11, e73040. [Google Scholar] [CrossRef]
- Progatzky, F.; Sangha, N.J.; Yoshida, N.; McBrien, M.; Cheung, J.; Shia, A.; Scott, J.; Marchesi, J.R.; Lamb, J.R.; Bugeon, L.; et al. Dietary cholesterol directly induces acute inflammasome-dependent intestinal inflammation. Nat. Commun. 2014, 5, 5864. [Google Scholar] [CrossRef]
- Kulkarni, P.; Yellanki, S.; Medishetti, R.; Sriram, D.; Saxena, U.; Yogeeswari, P. Novel Zebrafish EAE model: A quick in vivo screen for multiple sclerosis. Mult. Scler. Relat. Disord. 2017, 11, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Imamura, S.; Uchiyama, J.; Koshimizu, E.; Hanai, J.; Raftopoulou, C.; Murphey, R.D.; Bayliss, P.E.; Imai, Y.; Burns, C.E.; Masutomi, K.; et al. A non-canonical function of zebrafish telomerase reverse transcriptase is required for developmental hematopoiesis. PLoS ONE 2008, 3, e3364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Morimoto, K.; Danilova, N.; Zhang, B.; Lin, S. Zebrafish models for dyskeratosis congenita reveal critical roles of p53 activation contributing to hematopoietic defects through RNA processing. PLoS ONE 2012, 7, e30188. [Google Scholar] [CrossRef] [PubMed]


| Inflammatory Signaling Pathway | Development | Adult |
|---|---|---|
| NF-κB | Antiviral responses [43] | Pancreatic beta-cells proliferation [6] |
| Oligodendrogenesis after myelin injury [44] | sirt1 knockout leads to oxidative injury, chronic inflammation, and a reduced life span [45] | |
| Mesoderm development and embryonic dorsalization [42] | ||
| TNF-α | Fin regeneration [21] | Heavy metal toxicity [60] |
| Retinal neurogenesis and optic myelination [53] | ||
| Liver development [54] | Delayed responses to acute stress [61] | |
| Blood vessel development [55] | ||
| Oligodendrogenesis after myelin injury [44] | ||
| IL-1β | Fin regeneration [77] | Inflammatory response in steatosis [79] |
| Inflammatory compartment via Notch | Cardiomyocyte proliferation and heart regeneration [81,93] | Regeneration of Inherited Retinal Dystrophy [82] |
| Arterial system development [88] | Endocardium regeneration [93] | |
| Endothelial cell and Hemogenic Endothelium formation [91] | Müller-Glia-mediated retinal regeneration [94] | |
| Intestinal goblet cell homeostasis [92] |
| Model | Generated by | References |
|---|---|---|
| Duchenne Muscular Dystrophy | sapje mutation | [56,57] |
| Myelinogenesis | cd59uva48 mutation | [46] |
| Psoriasis and atopic dermatitis | spint1a mutation | [180,181,182,183,184] |
| Noonan Syndrome-Myelomonocytic leukemia | Shp2D61G knock-in | [185] |
| Chronic inflammation and oxidative injury | sirt1 knock-out | [45] |
| IBD and MAFLD/MASH | High-cholesterol diet | [174,179,186] |
| EAE multiple sclerosis | Regimen of MOG | [187] |
| Skeletal muscle atrophy | Ethanol exposure | [15] |
| Model of Inherited Retinal Dystrophy | Eye lesion | [82] |
| Heavy metal induced toxicity | Immersion in heavy metal | [60] |
| PTSD and Stress related disorder | Stress stimuli | [61] |
| Alcohol-induced steatosis | 0.05 v/v ethanol | [79] |
| Systemic inflammation | Il1b induced secretion | [70] |
| Dyskeratosis congenita | tert/terc/dkc1 knock-out | [155,159,160,188,189] |
| Severe combined immunodeficiency | rag1 mutation | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastrogiovanni, M.; Martínez-Navarro, F.J.; Bowman, T.V.; Cayuela, M.L. Inflammation in Development and Aging: Insights from the Zebrafish Model. Int. J. Mol. Sci. 2024, 25, 2145. https://doi.org/10.3390/ijms25042145
Mastrogiovanni M, Martínez-Navarro FJ, Bowman TV, Cayuela ML. Inflammation in Development and Aging: Insights from the Zebrafish Model. International Journal of Molecular Sciences. 2024; 25(4):2145. https://doi.org/10.3390/ijms25042145
Chicago/Turabian StyleMastrogiovanni, Marta, Francisco Juan Martínez-Navarro, Teresa V. Bowman, and María L. Cayuela. 2024. "Inflammation in Development and Aging: Insights from the Zebrafish Model" International Journal of Molecular Sciences 25, no. 4: 2145. https://doi.org/10.3390/ijms25042145
APA StyleMastrogiovanni, M., Martínez-Navarro, F. J., Bowman, T. V., & Cayuela, M. L. (2024). Inflammation in Development and Aging: Insights from the Zebrafish Model. International Journal of Molecular Sciences, 25(4), 2145. https://doi.org/10.3390/ijms25042145








