The Metabolic Syndrome, a Human Disease
Abstract
:1. Introduction
1.1. The Human Nature of MS
1.2. The Complexity of MS
- (a)
- The human-made environment: the direct relationship of MS incidence with the highly evolved/refined social control conditions (safety, nutrition, reproduction, environmental dominance) that define contemporary humans. The MS can be easily recognized in human-modulated animals and experimental models; for them, at least, MS is a human-transmitted or caused disorder [31,32].
- (b)
- (c)
- (d)
- (e)
- Senescence, the biological transition from maturity to old age: this process is somehow accelerated by MS, essentially along the lines of inflammation, global metabolic control of substrate partition, and overall physiological activity [49,50,51]. Both paths and effects have been found to be shared by MS and senescence [52,53].
- (f)
2. Pathologic Traits of the MS Cluster
2.1. Components of the MS
2.2. MS Main Paths and Mechanisms
| food → nutrients → partition: | → to oxidation (yielding NADH, ATP, heat) |
| → or energy storage (as fat, glycogen) |
| brain (food intake, endocrine control) → hormones (insulin, T, E2, GC) → nutrient partition |
| food intake → digestion/assimilation of food → splanchnic triage and partition of nutrients → oxidation for energy of substrates and/or storage, of the energy balance. |
- (a)
- Hepatic steatosis, with loss of hepatic function, disorders of redox status, and energy partition.Interrelated with disorders of lipid synthesis disposal, transport, and deposition, such as obesity and hyperlipidemia [156,168], as well as alterations in supporting metabolism, such as that of purines/urate [169,170].The liver is the main site for digested substrate-handling; liver damage results in the rapid extension of a wave of altered functionality to peripheral tissues, eventually affecting the regulation of whole systems. Liver steatosis (i.e., hepatic parenchyma accumulation of unused triacylglycerols –TAG– and altered two-carbon catabolites –2C– handling), also known as NAFLD (non-alcoholic fatty liver disease), has been proposed as the mainstay (and/or core initial metabolic disorder) of MS [104,171] because it affects the critical node of energy triage and partition between intestine/diet and the systemic circulation [133]. But also because, in the core, NAFLD is a consequence of altered insulin system function, resulting in the inability to dispose of excess 2C energy at the main point of intersection of human systems with the main body (gut) microbiota. This results in an excessive export of TAG-laden lipoproteins that the liver secretes because it cannot handle them [133,152,168]. Chronic inflammation of the liver can induce a domino effect on many other homeostatic systems, giving way to the appearance of known MS component complications/pathologies [172].
- (b)
- Insulin resistance, intolerance of glucose, and type 2 diabetes.Related to hepatic function, steroid hormone disorders, and energy partition, especially the handling of carbohydrates and its regulation [173,174,175], including the deposition of TAGs as energy reserves (or ectopic deposition of TAGs anywhere as a way to dispose of excess of unneeded 2C) [152,176,177].
- (c)
- Hypoandrogenism, plus low estrogen levels and disordered gonadal and adrenal estrogen and androgen synthesis.Related to brain function, genetic inheritance of metabolic alterations, and inherited patterns and functional blueprints for metabolic responses to alterations in normalcy [184,185,186], as well as the adjustment to biological/metabolic rhythms [187] and bone protection [188].Related to the regulation of insulin handling of glucose [189,190,191], control of 3C (three-carbon gluconeogenesis-related substrates) to 2C conversion and disposal of 2C for energy in the mitochondria [192,193,194], as well as keeping redox status and energy partition [133,195,196].
- (d)
- Disorders of corticosteroid synthesis and function and adrenal redox state.Affecting rhythms, behavior, and additional regulation of hormones, but also the differentiation and growth of cells and tissues [203,204]. Corticosteroids play a critical role in the defense system [205] by modulating and adjusting the immune response [206,207], both downplaying the immune response [208,209] but enhancing its effectivity [210,211,212]. Their function is necessary to prevent/correct the damages caused by inflammation [213,214,215].Interrelated with all the previous points presented here (a–d) and the next one (e). An important aspect of corticosteroids is their regulation of blood vessel reactivity, affecting blood flow and pressure [203,216], largely in contraposition to DHEA (dehydroepiandrosterone) [217,218]; interacting with T (and other androgens), E2 [110,188,219], and insulin [220,221]; and even acting in coordination with catecholamines [222,223].Corticosteroids also show a critical effect on behavior, in conjunction with other steroid hormones [224,225], including appetite (via a myriad of additional cytokines, non-coding RNAs, and other protein factors). The relationship with neural transmission and the modulation of the complex nature of depression has been also acknowledged, but the mechanisms have not yet been fully established [226,227,228].
- (e)
- Disorders of cell and tissue matrix, interrelationships, and interstitial space/tissue.
- (f)
- Indirect relationship with the microbiota, as well as defense reactions to infection and the effect of alien (not necessarily active or toxic) agents.Consequences of immune overreaction such as asthma, allergy [235,236], and dermatitis, including the classical “four Ds” of deficit (pellagra) or acute insult affecting, essentially, cells derived from the ectodermic layer: dermatitis (skin), diarrhea (digestive canal), dementia (brain, nervous system), and death (global irrecoverable disorders or disproportionate reactions to them). This is a relatively localized response (often autolytic).
- (g)
- The probability of additional ties with rheumatic diseases (via tissue redox and immune homeostasis).This part, including metabolic stress, is currently being studied at speed, probably prompting its incorporation into this group of rheumatic diseases such as osteoarthritis [237,238,239], but also psoriasis [125,126,240], as two representative syndromic effects on bone and (primarily) the skin, both of developmental epithelial origin.
- (h)
- Probable incorporation of the ‘metabolism’ of some essential minerals, such as Mg, Zn, Cu, and, especially, Fe.
- (i)
- Implication of most of the disorders exposed here with cancer and other altered mechanisms of cell tissue proliferation, growth, and altered control [249,250].These mechanisms are many and diverse, including the loss of tight metabolic–hormonal control and/or severe nutrient dysfunctions, mainly due to excess/disarray of dietary nutrients (and/or their handling in the gut and liver). Interactions with tumor tissue may result in the opportunistic hijacking of pathways [251,252,253], organelles [254,255], or signals to promote neoplastic growth [256] and, especially, cells: fibroblasts [257,258,259], adipocytes [260,261,262], stem cells [263,264,265], macrophages [266], and other defense-related cells [267]. This is quite a different type of disorder compared with the previous ones but shares its global activation by the syndromic pathologies that constitute MS in its wider definition.In sum, these overlapping mechanisms and actions are directly related to the fracas of the specific cell and tissue mechanisms of defense, and to the evolved facility of some types of cancer (including that provoked by viral or comparable means of infective transmission of the attacking blueprint) to grow as if its nature were simply parasitic [268]. The key point here is not the diverse metabolic approaches taken by the modified cells, but the biological meaning of this complex group of syndromic disorders. The possibility of them forming part of a coordinated culling mechanism as that postulated for the entirety of MS cannot be, right now, ruled out.
2.3. Other Aspects of MS
- (a)
- Human behavioral patterns affected by MS.These encompass the whole spectrum of eating disorders, often accompanied by well-defined behavioral or psychiatric pathologies [269,270]. In most patients, social rejection aggravates their problems, but this reaction is often compounded by other pathologies. Probably, altered behavior is (at least in a significant part) a consequence of the deep changes that MS (enhanced by age) induces in the lifestyle of those affected [271,272]. The current appreciation of this group of alterations is largely derived from these parameters.
- (b)
- Cardiovascular diseases.They are almost omnipresent in populations with MS, and constitute, perhaps, its most common consequences [273,274,275] and the subject of a few apparently beneficial ‘paradoxes’ [276,277]. They can be summarized by an increased risk of AHT [278,279,280,281], atherosclerosis [282,283], stroke [284,285], arrhythmias [286,287], and heart failure: coronary insufficiency or claudication [288,289], loss of heart function because of damaged tissue or contractile rhythm, signal transmission, or bringing up altered function because of degenerative processes [290,291]. Cardiovascular diseases are a main cause of death and incapacity attributable to MS [107,292], but the total risk of MS remains lower than those of these individual disorders taken independently [293]. The origin of these cardiovascular pathologies is a consequence of distorted metabolic function and regulation, which may result in sarcopenia [294,295], damaged tissue [296,297], altered vessel function [298,299], loss of heartbeat rhythm or dysfunctional signal transmission [300,301], altered blood coagulation [302,303], etc. These disorders constitute a legion of related, coordinated, or secondary actions which have already been presented as part/consequence of the main MS pathological traits. They are indeed a sum of different pathological paths (in the purest syndromic style), but their causes (and treatments) are to be found in the core of the main groups of mechanisms described in Section 2.2.
- (c)
- Mass-dependent problems ‘package’.Related to consequent, parallel, or causing damages that affect the operation of the circulatory system and, consequently, alter the organ functions: kidney [304,305], retina [306,307], adipose tissue [308,309], and lung insufficiency [310,311]. Pulmonary function is critically affected by MS [312,313,314], in line with generalized cardio-vascular alteration [292,300]. Inflammation in the lung [315] probably enhances the chronicity and severity of infective, toxic, autoimmune, or occlusive diseases of quite different origins, causing fibrosis and irreversible loss of function, such as asthma and pulmonary chronic obstructive disease [316,317]. MS also induces a higher lung susceptibility to infection [318,319] and cancer [320].
- (d)
- Common (or not) disorders being ascribed to the MS spectrum, but which mechanisms are not clear fully clarified.This group includes the classic acanthosis nigricans [321], directly related to obesity and insulin resistance [322,323]. Obstructive sleep apnea is a widely extended disorder, related to obesity [324] or MS [325,326], which has been linked to insulin resistance [327]. Sleep apnea has been postulated as a cause of insulin resistance [328], but the nature of this relationship has not been fully clarified [329]. Sleep apnea and its consequences on adrenergic signaling have also been related to AHT [330] and other cardiovascular effects [331,332]. The induction of atrial fibrillation is a known path for these interactions [301,333,334].
- (e)
- Immediate physiological consequences of disturbed blood circulation, including the loss of heart pumping capacity and the related appearance of edema (or, even, lipedema).These are direct physiological dysfunctions/responses to the insult of dysfunctional circulation [335,336]. We can also include here the renal [337,338], respiratory (pulmonary hypertension [339]), eye (glaucoma [337]), or adipose [340] problems caused by altered organ circulation and fluid handling. Most of these effects, despite their importance (and often crippling loss of function), are the consequences of fairly known mechanisms. Unfortunately, this ‘knowledge’ does not mean that the effects can, consequently, be successfully treated, since this depends on the particular nature of the core problem causing the disorder.Obesity often carries with it severe additional consequences, including the loss of mobility [341,342] and misadjusted water/electrolyte handling [343] or thermoregulation [344,345]. Some of these effects can be attributable simply to the effect of the extra body mass to be held, physically supported/carried, and provided with adequate nutrients and oxygen supply through an (also altered) blood flow; a burden additional to the metabolic and regulatory distortions that cause the obesity hypertrophic and/or hyperplastic disordered growth. There is a biological (albeit not fully mechanistic) parallelism with tumor growth, in which the migration of adipocytes and fibroblasts helps nurture and consolidate the neoplastic growth (Section 2.2 (i)).
- (f)
- The expanding field of diet- or regulatory-induced microbiota alterations.
- (g)
- In an indirect way, the MS-induced changes in body function have a direct translation into behavior, including society-generalized changes in habits.This question is in line with the possible interpretation of MS along a ‘planned obsolescence’ hypothesis, requiring—and provoked by—social interrelationships and the requirements of basic social structures [201], which antecedents are even previous to our present-day species. In addition, the often superfluous (when not utterly negative) intents to treat deep metabolic processes using prematurely launched, simplistic, and even non-knowledge-supported procedures to treat the MS spectrum of diseases have deep and long-lasting consequences to our health. The spot (and often aleatory or ‘ideological’) changes in the diet (e.g., ‘zero’, carbohydrate-free, dissociated, lactic, nutrient-restricted diets) may result in further damages to the health, hope, patience, economy, and trust of the patients, despite the huge amounts of resources invested in their apparently proven ‘healthy’ results (and almost nil effectiveness on MS) [352,353]. In this complicated path, there have been considerable setbacks (mostly because too often hope has substituted knowledge and proof). In this list we can include the fracas of sustained hypo-energetic diets, the β3-adrenergic agonists’ boom, the hopes put on leptin, ghrelin, and other hopeful miracle treatments… and thousands of compounds, procedures, and guides that, at most, have had only marginal effects on the ‘cure’ of obesity. Most of these failed intents are the fruit of the ‘need’ to treat millions of patients asking for a rapid and effective solution to their crippling health problems. This desire is compounded by the frustration of health professionals and the persistent lack of effective knowledge-based canonic treatments. It is often ‘forgotten’ that the bottom line for this lack of responses lies in the inadequacy of the research conducted and the real interference of corporate interest-focused financing [354,355], but also in of the piecemeal cumbersome system of scientific diffusion and the final control of omnipresent spurious interests [356,357]. We can only hope that this situation will eventually subside when our scientific knowledge (and the unconditioned access to it) reach the critical mass needed for solving a problem practically created by humans and which essentially affects humans: the MS.
3. Inflammation
3.1. Inflammation, Concept, and Types
- Classical or clinical inflammation [CI] (basically that of Celsus). The term ‘Clinical Inflammation’ may be preferable, since its descriptors fit, at the level of organization and context, with common clinical symptoms, with its descriptors (mechanism and function) being interchangeable.
- ‘Biochemical Inflammation’ [BI] is adequate to describe the ‘inflammatory’ phenomena occurring at the molecular and structural cell levels, i.e., the common realm of biochemistry.
3.2. Tissue-Related Inflammation Mechanisms: CI, Clinical Inflammation
- (a)
- Increased capillary permeability.This results in an increase in capillary fluid leaking into the interstitial space, swelling the tissue area (i.e., mostly enlarging the interstitial, intercellular, or tissue extracellular spaces) and also allowing for a number of proteins, largely related to defensive recognition, binding, and inactivation of potentially harmful elements, to pass from plasma to tissue. The increase in volume cannot be rapidly corrected because the main way-out of fluid, osmotic uptake in the capillary venous side, is slow and has been altered; furthermore, lymph efflux is also diminished, especially in lymphedema [400,401]. Brain tissue inflammation is peculiar, since the swelling cannot be readily corrected by the slow CSF dynamics but is instead by the long cycles of glymphatic wave-washing [402,403]. Extravasation also allows for the incorporation of coagulation factors to the interstitial extracellular space, which, when and if necessary, clot to prevent hemorrhages and to help maintain the isolated inflamed space [404,405] in which limited, controlled, and largely unconnected efflux helps to prevent the dissemination of the ‘perceived danger’ agents (and the responses to them). The swelling and turgid stasis results in the partial loss of function and homeostatic equilibrium, at least until (if) full working order in the tissue is recovered [375].
- (b)
- Local vasodilation.It occurs mainly at the arteriole level [406,407], and results in a locally increased capillary blood flow [406,408]. This helps activate the extravasation described in point (a), and the maintenance of turgidity, which also limits the clearance of excess fluid and, thus, helps the maintenance of the inflammatory theatre limits. In addition to raised blood pressure, the increased blood flow results in a higher supply of oxygen and nutrients (including specially required amino acids such as histidine and arginine) to the tissue [409], as well as eliciting a higher temperature than that of the surrounding tissue and even higher than that of the body’s core [410]. The higher temperature, oxygen, and energy supply of substrates increase the metabolic activity within the circumscribed space and also enhance the activity of defense system agents, such as phagocytic and antigen/antibody-related cells [411,412]. Inflammation is often related to fever, an increase in temperature (and BMR) which is centrally controlled and helps to globally activate the defense system, i.e., enhance phagocytosis [413].
- (c)
- Recruiting of phagocytic cells in the inflammation theatre.This includes the recruitment and selective polarization of macrophages [414,415,416,417] and their chemotactic (or electrophilic) migration from neighboring spaces or tissues [418,419,420], including different types of leukocytes, and, especially, those arriving with the raised inflow of blood [421,422,423]. The cells bind or cross the capillary walls (via diapedesis) in high numbers [424], a process facilitated by their increased abundance in the blood influx to the affected zone. The inflamed tissue draws blood monocytes and converts them into macrophages, but also receives, especially, neutrophilic leukocytes, which fight infections close to cell membranes in massive waves of intense phagocytic activity [425,426] The increased concentration of phagocytes and higher temperature (oxygen plus energy substrates) multiply their eventual activity, killing microorganisms or just embedding any suspicious particulate matter [427,428]. These actions result in the massive accumulation of tissue debris, dead cells full of partly digested microorganisms or other materials, altered matrix components, and inactivated alien structures (i.e., pus), which marks the end of the defensive part of the battle [375].
3.3. Cell-Related Mechanisms: BI, Biochemical Inflammation
- (a)
- Modulation of gene expression because of genetic and epigenetic signals.Resulting in cascade effects induced by differential expression of agents favoring inflammation or not. There is considerable bibliography on these mechanisms [443,444,445,446], but despite the biological reasoning that stresses their importance, the difficulties inherent to their exposure, tracing, and understanding have prevented most of them being a part of already mainstream knowledge.
- (b)
- Dysoxia-related processes.Showing alterations in the availability or handling of oxygen. The effects of hypoxia considerably limit the oxidative generation of energy and, thus, the effects extend to all corners of intermediate metabolism [447,448]. Changes in the redox state of cells and cellular systems also condition metabolic pathway shifting and/or modulation of (or disordered) substrate utilization and regulative systems shifts [449,450,451,452,453].
- (c)
- Accumulation of free radicals.
- (d)
- Alteration in metal ion roles.In relation to membrane barrier/transport/gate control functions, which modulate the cell compartment distribution of, mainly, calcium [460,461], magnesium [462,463], zinc [464,465,466], and iron [393,467] as part of their signaling/cofactor functions. Evidently, the Na+/K+ equilibrium is another critical factor, often altered over the development of inflammation [468,469] because of its signaling and transport functions.
- (e)
- Cell cycle modifications.Elicited by external factors spurning inflammation or internal factors, such as disorders of regulation, substrate energy handling, or genetic/epigenetic modulation [452,470,471]. These mechanisms often include the modulation of cell differentiation (i.e., fibroblast transformation [472,473]) or response to stimuli (i.e., macrophage polarization [416,417]), but also include changes in cell-type distribution in tissues (i.e., in adipose or bone marrow [474,475,476]) or the elimination and deep reconstruction of cell patterns (i.e., such as apoptosis [477], ferroptosis [245], remodeling of adipose tissue [478,479]), changes in mitochondria numbers and functions [480,481], and, especially, the transfer [482,483] and biogenesis of mitochondria [484,485]. This is one of the main cutting-edge areas in cell biology at present, and is probably the main focus of attention on inflammation because of the coincidence of three main vectors: (a) tissue reshaping/remodeling as an adaptation to shifting needs [483,486,487]; (b) adjustments in energy and protein/energy accrual or wasting [488,489,490,491]; and (c) the production of specialized cells, mainly in the orbit of defense systems (immunity, phagocytosis) [492,493].
- (f)
- Control of possible incorporations to the working genome (eventually including DNA from alien sources).This refers to the Lamarckian adaptations of live human beings—and, especially, of their descendants—to the conditions of the medium and their genetic [494,495] (but mainly epigenetic [496,497]) modulation. The implication of the diverse niches of the human microbiota has extended our horizons to the modulation not only of our cells and tissues, but also to the commensal, symbiotic, and even parasitic cells that do not share our DNA but live within us and are a necessary part of us (as a biocenosis) [498,499]. The transfer and handling of cell organelles (mainly mitochondria, so far) may be contemplated within this context of tissue structure, function, and cell composition shifting [482,500,501,502].
- (g)
- Handling of body (i.e., cells and tissue matrix) protein.In the complex context of protection/sparing of amino N sparing [503,504], including the retention and reuse of amino nitrogen and, especially, essential amino acid hydrocarbon structures [505,506]. These complex processes occur within the context of omnivorous feeding, which includes the use of protein as a common/usual staple for energy generation. Our bodies need to maintain their total protein mass/pool as fully functional at any time, despite senescence [507,508] and even under the influence of MS [509,510]. This issue has, so far, been poorly studied for obvious methodological constrictions and even within the context of dietary needs, since many “recommendations” are no longer sustained by current knowledge. Nevertheless, there is information available on structural problems in the cell handling of proteins, such as protein folding errors [511,512] and their elimination [513]. There is, also, a growing interest in the metabolic remodeling of intercellular matrix proteins, including the action of some cathepsins [514,515]. Recently, as an example of an expansive analysis of our biocenosis, it has been found that some viruses may affect/interfere with the fine-tuned system of selection of mRNAs to be translated into newly formed proteins [516].
- (h)
- Excess of energy-providing nutrients.Mammals have evolved to develop successful strategies to endure the harsh (albeit common) conditions of insufficient availability of food [517,518]. Adaptation to food deprivation elicits a rapid, compensatory reduction in energy expenditure [519]; when needed, the use of body reserves of energy substrates is activated [518,520], eventually carrying on the deconstructive oxidation of body structures. All of these actions are centered on the priority of maintaining our essential functions in order to keep surviving by using the metabolic energy remaining in the body as life-sustaining fuel [517,521]. The reverse condition, i.e., “how to cope with an ‘excess’ of energy available” is so rare in any ecosystems’ context that no evolutionary mechanisms have been developed yet to face such a biologically improbable situation [5]. Unfortunately, the biological success of humans has been (so far) overwhelming, and has developed within an extremely short (historical, not biological or evolutionary) period; the maintained availability of food has ceased to be a main problem for a large part of humankind. In fact, a consequence of affluence, the inordinate accumulation of body energy reserves—in practice never to be used, stored in counterproductively large amounts—has become a serious health problem by itself [522,523]. Now, obesity is a main component of MS, a disease of ‘excess’ availability of energy, protein, diet micro-components, etc., but lacking a possible counter-regulatory escalation of the basic and well-regulated energy expenditure system of mammals [524]. This is probably a critical factor for the development (and maintenance) of MS. The question of energy balance, diet, and MS is further developed in Section 4.4.
4. Metabolic Regulation-Derived Inflammatory States
4.1. Energy Balance
| E-intake crude: food − E-excreted heat and waste − [E-transfer] = E-net-balance loss/accrual |

4.2. Dietary Energy Assimilation, Triage, and Partition
- Excretion/waste through the bile to the gut. The bile is a partly toxic mixture but also contains bile salts that act as emulsifying detergent [541].
- Metabolic transformation, in which the input molecules are modified by the liver cells and finally driven to one of two main functional fates [133]:
- 4.1
- Oxidation: to obtain energy, e.g., conversion of substrates to 2C and oxidation to CO2 through the Krebs cycle.
- 4.2
- Storage: to save energy in the form of depot substrates (TAGs, glycogen) stored in the liver or exported (point 1).
4.3. The Importance of Protein as Energy-Providing Nutrient in Humans

4.4. Influence of the Timing of the Digestive/Absorptive Process on Nutrient Energy Partition

- (a)
- The distribution of the daily food allotment should be spaced over time to allow for smaller portion sizes of food in each meal as a way to facilitate streamlined digestive processes (and thus their timing) and to prevent substrate overloads. Large portions and a higher frequency of meals both increase the net energy balance [636], and large servings also increase insulin resistance [637], but the effect on total energy intake in a higher number of meals is more limited on these parameters [638].
- (b)
- All meals should contain a varied mix of nutrients for optimal assimilation, but (in humans) the ingestion of carbohydrates (in special digestible starches and sugars taken in large amounts) should be combined with the presence of protein. This protein component may provide the KCAI and other small molecules that prevent the almost exclusive and massive availability of 6C (eventually building an excess of 2C). Meals with a large carbohydrate and protein presence actually facilitate adipose tissue lipolysis [639]. Diets laden with carbohydrates and lipids, on the other hand, distort the maintenance of glycaemia [640]. The differential increases in protein, globally, do not alter energy intake [641].
- (c)
- The proteins referred to in point (b) should contain a reasonable diversity of molecular species (translatable to quite different velocities of hydrolysis) to facilitate the distribution, over time, of their digestive breakup products. Easily digestible proteins such as whey (as compared to the slower digestion of casein) [632] may help fast-start the protein effect on carbohydrate digestion. The strict N protein needs are easily covered with equilibrated omnivorous diets, and the body total protein mass is maintained despite important variations in the amount of dietary protein ingested [642]. However, dietary deficiency in protein may affect growth and body protein when not corrected [579], which hints at the primary importance of protein as a key factor in facilitating the energy use of dietary carbohydrates, since the preservation of dietary protein amino acids (as newly synthesized body proteins) to maintain body N homeostasis may be also affected by persistent dietary amino acid deficits.
- (d)
- Meals should contain enough protein (and/or lipid) to pace the stomach voiding in order to (at least) prevent fast and steep rises in circulating 6C, as explained above. The presence of fiber (instead of highly hydrolysable purified starches or oligosaccharides) may also help slow the overall process of digestion and, thus, allow for a smaller gap between the blood peak of sugars and that of amino acids.
4.5. Main Constrictions and Rules Governing the Partition, Use, and Disposal of Nutrients
- (a)
- Glucose is a highly reactive molecule that spontaneously glycosylates proteins and other compounds [644]; in addition, its two main isomeric forms (in biological fluids) are maintained in equilibrium, but only the β-pyranose form is actively used by most metabolic pathways, with the α-isomer being slowly reconverted, thus slowing (and diluting) glucose utilization [645]. Glucose is, by far, not an ideal substrate, but it is the best one (and the only one) available. Eons of evolution of photosynthesis have produced this particular compound, which is now the main energy staple of plant metabolism. Simply, there is no alternative for heterotrophic organisms, such as humans. Thus, we have had to rely on plant-synthetized glucose as our main energy staple despite its many inconveniences. Consequently all animals’ energy homeostasis is centered on glucose [150].
- (b)
- Glycogen is the main glucose (6C) form of storage in animals and is related to plant starches. It is found in limited amounts in mammal tissues; its maximal levels (mainly in liver and skeletal muscle) amount to a tiny proportion of all body energy reserves. Glucose availability from glycogen is relatively fast (but not sustainable over time) [646] and, thus, amino acids (and other 3C sources) may be used for gluconeogenesis to cover on-spot needs or starvation-related deficits in glucose [647,648].
- (c)
- The main energy reserves of mammals, including humans, are TAGs and body protein. In fact, protein is not a canonical reserve as such, but its utilization allows for a margin of consumption linked to normal turnover. This arrangement includes a large part of body protein as a variable unit of energy (and amino-N) and dynamic reserve. Lipids are the best option for energy storage in animals because of their high energy-packing capability in an unbeatably smaller added weight, several-fold lower than that of glycogen and protein (i.e., hydrated vs. fully lipophilic, and energy-dense TAG structures); thus, lipids are the energy reserve substrate of choice [649].
- (d)
- The availability for use of the energy of lipid reserves is slower than that of glycogen, but both are coordinated [650]. To use the 2C pool of our fat reserves requires a progressive activation system compared with the direct glycolytic consumption of dietary 6C for energy. Despite the complex timing, application of catabolic protocols, and partial sparing of nutrients, at the end of triage, all nutrient sources marked for oxidation to obtain the energy we need to function are converted into 3C or 2C, but, eventually, to 2C, which is oxidized to CO2 in the mitochondria to provide energy [133].
- (e)
- Under conditions of energy deficit, the body’s reserves, especially protein [651], tend to be spared by decreasing energy consumption, leaving their use for energy metabolism mainly to cope with “non-consuetudinary situations” but common situations, such as starvation [608,652] or increased energy needs; energy transfer (reproduction) [653,654]; travel and migration (i.e., of birds) [655,656]; or illness [657,658]. The level of reserves should maintain a balance between possible time-extended needs and the mass of fat weight, i.e., the cost of storing and moving around the fat reserves as a hindrance to fight or flee, both as predator or prey, as well as migration and survival during the sparse-food-availability periods [656,658]. Protein “reserves”, such as those of plant seeds, are not present in humans, as the term ‘reserve’ refers to the whole-body protein content, maintained through a constant turnover from amino acids and energy. Deficits in dietary N sources ease the use of amino acids for energy instead of protein turnover, diminishing whole-body protein in a multi-fractioned way. Excesses of energy and amino acids facilitate the accrual/growth of protein, depending on a strict genetic control.
- (f)
- All systems are conditioned to save/spare and defend some strategic nutrients from wasting, especially those more difficult to obtain and retain, to maintain working pools and to prevent deficits. The list includes minerals, such as P, Ca, Mg, Zn, Fe, I [659,660,661], and ‘essential’ structures, such as some amino acids, most vitamins [662,663], and polyunsaturated fatty acids [664].
- (g)
- The preferred hierarchy of use of external (diet) substrates for energy depends on their availability, the need for energy, the metabolic context, ability and predisposition to use them [665], and the immediacy of their presence already in the gut. The first staple to prioritize for energy production should be the dietary fatty acids (PUFAs and others are spared, and in fact picked up, to a point), oxidized to obtain energy (via 2C). The second, depending on the overall availability, are the carbohydrates (6C and their derived 3C); meanwhile, protein is, assumedly, the last to be massively consumed. In fact, a whole-body energy status is reached in which the flows of substrates are mixed and adjusted to an optimal efficiency and preservation of both energy and selected components [666,667]. Nevertheless, these considerations are essentially theoretical and, in practice, a sizeable part of dietary fatty acids may be saved, favoring TAG deposition [668,669], or protein may be oxidized to facilitate carbohydrate disposal, as indicated in Section 4.4.
- (h)
- Under the conditions of starvation and low dietary energy availability, the body applies a protocol of energy saving, lowering exercise, reproduction, thermogenesis, and maintaining activity and function barely over the survival level until (if) external conditions improve [520,665]. If no 6C are available, then TAGs (from food and/or reserves) are the substrate of choice [670]. In the last stages of starvation, body protein is more actively used [670,671], albeit in higher proportions when there is no concomitant presence of body fat [672]. The diet and functional orientation of different species deeply affect their management and homeostatic resilience to starvation [673].
- (i)
- Under conditions of high food availability (i.e., 6C, TAG, and protein available in excess of assumed N needs in every meal), the order of use in humans shifts to using excess 6C first, either to oxidize it or to eventually store part of its energy as TAG. Nevertheless, it has been known for long time that gross excess of protein in a meal (i.e., in the absence of large loads of carbohydrates) results in the selective rapid oxidation of the proteinaceous overload (i.e., a shift to ‘carnivore mode’ over the basic ‘omnivore’). The general indications of metabolic priorities must be taken only as general indications, since it is the complete metabolic panorama that in fact drives the mobilization of supplements [674]. Surplus dietary protein is used for energy, essentially, to prevent the accumulation of amino acids [557] (i.e., disposing of unwanted excess N), which results in the need to also oxidize their hydrocarbon structures. In part, this process accelerates the oxidation of 2C, but also helps create an unwanted overload of 3C (and, thus, potentially of 2C again), which may compete/interfere with the process of disposing of dietary 6C [675].
- (j)
- Dietary TAG, the main canonical source of 2C, may be spared in part because their availability for oxidation may interfere with the disposal of excess 6C, with both processes fueling the mitochondrial oxidative machine with 2C. The sidetracking of β-fatty acid oxidation is the lesser of two evils since its ‘preventive or temporal’ incorporation into the whole body TAG pool may give time for the maintenance of the postprandial energy equilibrium, [669,676] despite being a harbinger of delayed severe metabolic consequences [677]. But, a rise in circulating 6C and insulin resistance [678,679] are more urgent problems to solve than the eventual increase in fat stores [680].
- A timing coordination between the arrival of portal blood substrates to the liver and their release (or that of their metabolites) to the systemic bloodstream. The rate of handling of these substrates by the liver should be sufficient to adapt the flows (in/out of the liver) to those of the transforming processes: 6C glycolysis to 3C; oxidation of 3C to 2C; oxidation of fatty acids to 2C; (eventually) lipogenesis from 2C; esterification of fatty acids to TAG; incorporation of TAG to VLDL; (eventually) synthesis of ketone bodies from 2C; oxidative catabolism of amino acids, starting by the disposal of their amino-N; and, then, oxidation to 3C, 2C, and KCAI. These transformations are coupled to the release of (mainly) glucose, VLDL-TAG, 3C (mainly lactate), amino acids, (eventually) ketone bodies, and waste catabolites. The liver retains (optimally) little in the form of net reserves, other than glycogen (if needed). The release is regulated to maintain the systemic blood levels of metabolites within homeostatic parameters.
- The liver fuels cover its needs for energy via oxidation of 3C to 2C and the oxidation of 2C through the Krebs cycle. The insulin-E2 regulative mechanisms must be sufficiently operative to allow for the sustained operation activity of the pyruvate dehydrogenase complex (3C → 2C) [184].E2 also favors the use of protein amino acids as energy substrates through the enhanced elimination of their N moiety. Consequently, dietary protein, irrespective of its constitutive N, must be catabolized, along the process of triage, to prevent the bottleneck of 2C accumulation (Section 4.4). Necessarily, this rapid use of amino acids requires an equally efficient system of amino N disposal, coordinated with the estrogen-insulin process of 3C oxidation, and circumventing the key rule of N preservation [152,196,692]. If all these premises are accomplished, then there should be a sufficient parallel supply of anaplerotic substrates (KCAI) to facilitate the rapid oxidation of 2C to CO2 and, obviously, more than sufficient biochemically available (NADH and ATP) to the liver, coupled, especially, with an efficient distribution of substrates to all organs and tissues.
5. The Connective Tissue: Role in Energy Handling and Inflammation
5.1. Structure and Functions of the Connective Tissue
- A main cell type, adapted to the main function of the specialized form of CT (such as fibroblasts, adipocytes, mast cells, macrophages, and leukocytes).
- A number of other cell types, formed elsewhere and later migrated into the CT, but which definitive functions are largely carried out as part of the CT (such as different types of leukocytes: neutrophils, basophils, eosinophils, monocytes or lymphocytes) [727].
- 3.
- 4.
| Type/Subtype | Main Junction(s) | Main Cell Type | Other Cells * | Matrix (Largely Amorphous) | Fiber Types | Location |
|---|---|---|---|---|---|---|
| loose/areolar | cover, bind, hold organs | fibroblasts | macrophage, stem cells, adipocytes, leukocytes | proteo-glycans and glycosamino-glycans | collagen loose, elastin | skin, binding/holding most organs |
| loose/reticular | holding cells, filtering cells and lymph | reticular cells (and phagocytes) | macrophage, immune cells, leukocytes | proteo-glycans and glycosamino-glycans | collagen (III) | lymph nodes, thymus, liver, spleen |
| dense/white fibrous [NBV] | hard–delicate organ protection, heavy-duty mechanical work | fibroblasts | macrophages, adipocytes, mesenchymal cells | proteo-glycans and glycosamino-glycans | collagen (I) packed, (elastin) | fascia, duramater, spinal sheath, pericardium, cornea, tendons, periostium |
| dense/yellow fibrous | Hard–fine repetitive mechanical work | fibroblasts | macrophages, adipocytes, mesenchymal cells | proteo-glycans and glycosamino-glycans, chondroitins | elastin, collagen | ligaments, vessel walls, vocal cords, lung, respiratory tract |
| cartilage/hyaline [NBV] | structural, shock-absorbing cartilage | chondrocytes | chondroblasts | proteo-glycans and glycosamino-glycans, collagen | collagen (II), (elastin) | costal cartilage, nose, trachea, larynx |
| cartilage/elastic | very flexible structural cartilage | chondrocytes | chondroblasts | proteo-glycans and glycosamino-glycans, collagen | elastin | epiglotis, larynx, ear pavilion |
| fibrocartilage [NBV] | friction-resistant joint surfaces | chondrocytes | chondroblasts | proteo-glycans and glycosamino-glycans | collagen, (elastin) | meniscus, intervertebral discs |
| bone (and teeth) | maintenance of bone structures, support, armor protection | osteocytes, osteoblasts, osteoclasts | stem cells, macrophages | hydroxyapatite, dentin in teeth, proteo-glycans and glycosaminoglycans, collagens | collagen, elastin (mineralized) | bones, teeth |
| bone marrow hematopoietic | hematopoiesis, regeneration | reticular cells, hematopoietic, and blood series | stem cells, macrophages | interstitial lymph | collagen, elastin | bone marrow |
| adipose white | fat storage, insulation, endocrine, immune control, regeneration | adipocytes | macrophage, stem cells, leukocytes, immune cells | interstitial lymph | collagen, elastin | adipose tissue masses, organ interstitial fat |
| adipose brown | thermogenesis, heat diffusion | brown/beige adipocytes | macrophage, stem cells, leukocytes, immune cells | proteoglycans, glycosaminoglycans, glycoproteins | collagen, elastin | brown adipose tissue sites; often mixed with white |
| blood (and lymph) | oxygen, nutrient, and cell transport and delivery, coagulation | erythrocytes, leukocytes, platelets | other immune system cells | absence of solid matrix, just blood plasma (and its proteins) | no fibers | within blood vessels, heart |
5.2. The Adipose Organ, an Energy-Handling Specialization of the CT
6. The MS Disease
6.1. Pattern of Development of the MS
- (a)
- Age; senescence.Typically, the first markers of MS appear early in men. Lore informally establishes the fourth decade of life as when (essentially men) become aware of ‘becoming old’ through a number of functional, psychological, social, and health shortcomings, often eliciting drastic worsening changes in their psychology and activity [867,868]. This phenomenon also occurs in women, albeit being more diluted over their mature years because of higher social environmental pressure and also higher resilience [42,47]. In women, the ‘discovery of aging’ is often acknowledged later, close to menopause. In both genders, after these crises comes an interlude for acceptation and adaptation to the altered concept of their life/personality (or for its negative psychological blockage). Advancement of the MS condition, especially that of obesity (which can be easily detected/commented on by others) [869,870] and liver steatosis [871], are also accompanied by stress [872], and then by other MS components, that elicit additional consequences of (or caused by) changes in social behavior [43,873,874]. These changes may bring in a new phase, the conscience of bearing a progressively severe crippling (and eventually terminal) disease, can be socially devastating for many patients. Often, this awakening does not take place this way, because one of the main MS spectrum diseases is the (practically unique) focus on health-menace attention; it is ‘the disease’, and thus the other manifestations are taken as subordinate, not as syndromic partners of generalized inflammation consequences [875]. This point of view is in part also shared by a high number of health professionals, and may result in the establishment of conflicting therapeutic priorities that should be carefully pondered when treating a polymorphic pathologic state, largely relying on damaging, ineffectual, and expensive polypharmacy [876]. At this phase, most disorders are already treated in a ‘sustaining’ or ’palliative’ way because of a lack of effective alternatives, i.e., treating the conditions as best as possible to gain time and improve the living conditions of the patients. Unfortunately, this humane and ethical way forfeits real healing due to the intertwined nature of the illnesses implicated [28], and their extension and transcendence (e.g., sleep apnea [325,877], type 2 diabetes [878,879,880], and most MS-related heart and circulatory conditions [275,288]). There are, too, complicated avenues for treatment that are not always well defined, applicable, or even available to most patients, such as depression [881,882] (not even agreeing on its relationships with MS [883,884]), osteoarthritis [238,239], psoriasis [126], or even severe obesity [885,886], which is the leader of medical, social, and economic studies (and failures). This situation continues until tumors, dementias, aggravation of a principal disorder, or, commonly, vascular or heart failure cuts a life, between late maturity and old age, after a course/curse of (often) many years.
- (b)
- Sex; planned obsolescence.Women constitute the bulk of patients with diagnosed MS [887,888], but men often present more severe (and lethal) manifestations of MS [866,889]. The superposition of menopause, senescence and MS in women, compared with the earlier onset of severity in men [48], somehow obscures the choral effects of MS [866,890]. The higher capability of women to limit the ravages of disordered lifestyle involves a careful, often restrictive, choice of food [891], together with higher psychological fortitude [892], an ‘intimate’ relationship with E2 [893], higher X chromosome reliability [894,895], metabolic resilience, and lower MS-related cardiovascular consequences [48], and mortality rates than men [866,896]. In men, MS and most of its morbidities are related to low blood T [897,898,899]; a marked lack of enough T also results in lower dihydrotestosterone effectiveness [900] and a parallel decrease in E2, which [95,124] results in a further lack of control of the insulin–glucose system [898,901] and a decrease in the flow of 2C through the Krebs cycle (Section 4.2 and Section 4.3). This consequence is a marked incapacity for processing the excess 2C induced by diets rich in 6C, but even affecting diets that are not hyperenergetic at all [902]. The deficit in T is also crippling because hormone production is not sufficient to sustain an adequate mass and turnover of body protein [903,904,905], which may eventually result in overall protein loss and sarcopenia [50,295,906], a characteristic of advanced senescence [907,908]. A deficit in T (controlled by the brain, via the hypothalamus–hypophysis–gonadal axis) at mature age results in a marked decrease in sexual interest, arousal, frequency, and effectiveness of sex [909,910,911], but also a progressive loss of muscle and whole-body protein [912,913] (including that of the heart [914]), higher insulin resistance [915] plus lower capability to oxidize 2C (thus favoring fat deposition) [916,917,918], depression [919,920,921], and deep hormonal disturbances [50,922,923]. The consequence is, usually, an earlier than expectable demise. These extended effects, at least in men, may be compared to those of a programmed obsolescence [201], which shortens the life of individuals of the species when they are no longer “needed” by the group, thus saving resources for the rest. In fact, senescence is exactly that, a genetically planned mechanism to cull the individuals which no longer reproduce, take care/protect the pregnant, younger/elder, or obtain food, defend the tribe, etc., but consume otherwise (collectively) needed resources. Women’s lives endure a stop to ovulation (and related hormonal milieu) when their remaining lifespan (and physique) cannot safely guarantee their contribution to sire new individuals (neither, the continuous provision of care and protection to them). However, (older) women’s resilience is (apparently) relied upon by our innate biology rules, by allowing them to continue help caring... for other women’s children; they already have almost a lifetime of training, retain the capacity to carry on these tasks, and commonly live up to fairly more advanced ages that men [924,925,926]. Anyway, some sort of gender-related planned obsolescence is probably set in our genes, and may be detrimental for men, at least in the question of survival/longevity (via resilience?). The question of whether MS is an instrumental agent for the implementation of a gender-related planned obsolescence, remains, thus, a plausibly probable (but so far unprovable) hypothesis.
- (c)
- Diet and food security.The direct relationship of MS with the problems caused by excess dietary energy supply, as well as the effects of inadequate structuration of nutrient availability, have been described and explained in Section 4. The relationship between the development of MS (liver steatosis, type 2 diabetes, and obesity, at least) may be traced to the repeated use of diets which cannot be properly handled under the metabolic (and, especially, regulatory) conditions affecting individuals with (or prone to) MS [927,928,929]. The nexus of the insulin–glycaemia system with the estrogenic regulation of 2C disposal (and, by defect, of enhanced synthesis of fatty acids) supports a clear endocrine basis for MS [133,152,192], more so when we include the compounding deleterious effect of insufficient availability of T cited in point b). Glucocorticoids tend to antagonize testosterone in the preservation of protein [99,110] and facilitate hyperglycemia against the reverse effect of T [930,931,932]. The equilibrium of these classes of steroid hormones is essentially controlled by the brain, thus adding another level of regulation (nervous) to the core mechanisms of energy partition. The brain also has an important role in the control of appetite [160,161], including complex mechanisms based on energy function markers, but also on hedonic signals and memory [933,934,935]. Another factor related with food use is the control of stress caused by the imprecise availability of food, food security [8,936,937], which has not been yet made universal for humans, but which, from the ‘human’ perspective of MS (described in Section 1.1), the anxiety for obtaining the next meal is a condition relegated to the rest of animals, since human ingenuity has worked over thousands of years to close on this ultimate level of security. Unfortunately, the fight and strife between human groups has prevented, so far, a complete universalization of this primary objective. Food insecurity is a main cause of stress [938,939], which represents a corticosteroid-dominated regulatory milieu [940,941] in the context of the modulation of feeding by the brain [942,943,944].
- (d)
- Social enviroment and its effects on psychology and behavior.Social constrictions affect the consumption of certain types of foods, a signal of identity, tradition, or secular availability (including religious and social habits and mandates) [945,946,947]. These are aspects deeply affected by the availability of food [948,949], its variety [950], and the regular supply affecting the food security aspects presented above [951]. The epidemiology of MS shows that, in a few generations, a society may often pass from a survival stage, under the permanent danger of famine, (nutrition transition) to high food availability [952] and gluttony-overeating [953], helping to induce a massive development of obesity [954,955]. Probably, the sociobiological roots of these (social evolution) sudden shifts may lie in ‘epigenetic hunger’: memories and functional/behavioral patterns acquired from prenatal experiences or transmitted from previous generations [956,957]. The relative coincidence of increases in MS expansion in countries that have recently endured harsh stress and starvation conditions hints at learned overeating as a precaution [953], but also at overfeeding of children, as is the common situation of proudly showing-off chubby babies (linked to a positive view of children obesity) [958]. These attitudes have been in use as ancient recipes for survival, and remain clearly marked in our genetic background. Nowadays, in most communities and close-knit groups, any meeting justifies excess eating [959,960]. Food sharing promotes feasting, overeating [961], access to more varied foods [962], and is an element of social unity, hospitality, friendship, and positive relationships. As such, this universal—and quite diverse—behavior is a critical part of social groups’ cohesion via tradition and heritage. In many social events, the excesses (aka “justified” exceptions) in energy intake (and loose behavior) are part of the expected (i.e., “normal”) behavior. Nevertheless, socialization should not become a disrupting factor in regular eating patterns, especially when the risk of famine is low and that of MS is skyrocketing.
6.2. MS as a Branching Monophyletic Disease

6.3. Conclusions and Perspectives
- (a)
- Type 2 diabetes and obesity.So far, this is the (apparently) most medically internalized mechanism used to explain the development of MS. In its favor, we can include the pivotal role of insulin on carbohydrate metabolism, energy partition and supply, the use of glucose, and the 6C–3C relationships to sustain a large part of the body’s energy needs. It is directly related to the insulin-favored deposition of excess energy as TAG, and the correlation of obesity and insulin resistance (and inflammation) is strong. In most cases of MS, insulin resistance is present, often accompanied by type 2 diabetes and obesity [135,870]; in fact, most people with MS are directly treated for diabetes and obesity. Type 2 diabetes and obesity are mainstays of MS, and are related to most of its other components and consequences, including their incidence in cardiovascular disorders. Nevertheless, type 2 diabetes also exists as a more-or-less standalone disease [1019,1020], and obesity may be (albeit in a small proportion) a consequence of other disorders [1021]. There are common cases of obesity (metabolic) without excess fat deposition (anatomic), as is the case of “normal-weight obesity” [1022], an oxymoron indeed, of which existence is not yet fully acknowledged [1023,1024] despite the finding of a number of complications affecting the ‘normal-weight obese’ [1024,1025,1026]. The timing may be important, since clinically defined MS is often declared later than obesity and/or diabetes. The main factor against a simple sequential hypothesis is the diverse (and complex) pathological signature of MS compared with the more lineal development of diabetes and obesity-related complications. Their causes (genetic, endocrine, dietary, etc.) are also often traceable. In any case, the question of them inducing a trigger effect remains, since the type 2 diabetes/obesity tandem appears, more often than not, as a harbinger of full MS [1027].
- (b)
- Hypotestosteronemia.In general, MS can be traced (or found to be parallel) to low T availability in mature (or old) age [94,899]. T has been intensively (and almost exclusively) related with sex (as also occurs with E2) [1028], but, nowadays, its metabolic maintenance function is fully recognized [201]. The need for E2 to facilitate the initial phases of energy partition is known but not yet fully integrated in clinical treatments [133,195,1029], in part because of prevalent bias against the use of ‘sex hormones’ in the treatment of metabolic (not explicitly sex-function-related) disorders [152]. The development of this line is just one step ahead of the preceding case in relation to medium- and long-term regulation: low T results in low E2, which then cannot maintain a tight control of the insulin–glycaemia system regulating dietary energy partition. The main real argument against this line of thought lies precisely in the wide extension (and roots) of ‘sex hormones’ as a ‘group’ of hormones identified solely with the gender-related regulation of sexual/reproductive functions. Consequently, both androgens’ and estrogens’ functions are contemplated mostly as gender-differentiating agents, minimizing the specifically metabolic regulatory roles shared by females and males outside of the additional limitations of age, sex, and maintenance of energy metabolism. In sum, sex is sufficiently transcendental (the future of the species depends on it) by itself to also include the control of energy metabolism. For a large portion of clinicians and basic scientists, energy metabolism is already controlled by appetite, insulin, and a large (and growing) number of additional known and largely independent mechanisms. However, the need for E2 for male reproduction and T for that of females, androgen and GH for body protein maintenance, and estrogens (and androgens) interacting between them and with glucocorticoids (and many other regulatory molecules), constitutes a whole intertwined set of medium-term hormonal control processes that affect both male and female individuals from cradle to grave. Additionally, both T and E2 are needed in both sexes for effective reproduction, with androgens eliciting a ‘male’ phenotype and ‘estrogens’ favoring a ‘female’ phenotype. Even in the absence of truly sexual development (or outside of the ‘reproductive window’, in practice including about half of a lifetime or less), androgens and estrogens keep maintaining their metabolic homeostatic functions (albeit impaired by eventual insufficient secretion).
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1C | one-carbon fragment |
| 2C | two-(hydro)carbon molecules (e.g., acetyl-CoA) |
| 3C | three-(hydro)carbon molecules related to glycolysis and gluconeogenesis |
| 6C | six-(hydro)carbon carbohydrate units (oses) and other oses yielding them (such as pentoses) via the pentose-P pathway |
| AHT | arterial hypertension |
| BAT | brown adipose tissue |
| BI | biochemical inflammation |
| BMR | basal metabolic rate |
| CI | clinical/classic/Celsus inflammation |
| CSF | cerebrospinal fluid |
| CT | connective tissue |
| DHEA | dehydroepiandrosterone |
| E2 | 17β-estradiol |
| GC | glucocorticoids |
| KCAI | Krebs cycle anaplerotic intermediates |
| MS | metabolic syndrome |
| NAFLD | non-alcoholic fatty liver disease |
| OSA | obstructive sleep apnea |
| TAG | triacylglycerols |
| UCP-1 | uncoupling protein 1 |
| WAT | white adipose tissue |
References
- Miranda, P.J.; DeFronzo, R.A.; Califf, R.M.; Guyton, J.R. Metabolic syndrome: Definition, pathophysiology, and mechanisms. Am. Heart J. 2005, 149, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, M.; Leonhardt, W. Das metabolische syndrom. Dtsch. Gesundheitsw. Z. Klin. Med. 1981, 36, 545–551. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.K. Metabolic syndrome or insulin resistance: Evolution, controversies and association with cardiovascular disease risk. Indian J. Clin. Cardiol. 2020, 1, 77–85. [Google Scholar] [CrossRef]
- Alemany, M. Metabolic syndrome: A multifaceted disease of affluence. J. Endocrinol. Metab. 2012, 2, 155–165. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.P. A brief review of the archaeological evidence for Palaeolithic and Neolithic subsistence. Eur. J. Clin. Nutr. 2002, 56, 1270–1278. [Google Scholar] [CrossRef]
- Johnston, J.L.; Fanzo, J.C.; Cogill, B. Understanding sustainable diets: A descriptive analysis of the determinants and processes that influence diets and their impact on health, food security, and environmental sustainability. Adv. Nutr. 2014, 5, 418–429. [Google Scholar] [CrossRef]
- Loftus, J.P.; Wakshlag, J.J. Canine and feline obesity: A review of pathophysiology, epidemiology, and clinical management. Vet. Med. Res. Rep. 2014, 6, 49–60. [Google Scholar] [CrossRef]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Marañón, G. Über Hypertonie und Zuckerkrankheit. Zentralblatt Für Inn. Med. 1922, 43, 169–176. [Google Scholar]
- Kahn, R.; Buse, J.; Ferrannini, E.; Stern, M. The metabolic syndrome: Time for a critical appraisal. Diabetologia 2005, 48, 1684–1699. [Google Scholar] [CrossRef] [PubMed]
- Penno, G.; Miccoli, R.; Pucci, L.; del Prato, S.T. The metabolic syndrome—Beyond the insulin resistance syndrome. Pharmacol. Res. 2006, 53, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.M. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia and hypertension. Arch. Intern. Med. 1989, 149, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Groop, L.; Orho-Melander, M. The dysmetabolic syndrome. J. Intern. Med. 2001, 250, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Kylin, E. Studien über das hypertonie-hyperglykämie-hyperurikämiesyndrom. Zentralblatt Für Inn. Med. 1923, 44, 105–127. [Google Scholar]
- Dobrowolski, P.; Prejbisz, A.; Kurylowicz, A.; Baska, A.; Burchardt, P.; Chlebus, K.; Dzida, G.; Jankowski, P.; Jaroszewicz, J.; Jaworski, P.; et al. Metabolic syndrome—A new definition and management guidelines. Arter. Hypertens. 2022, 26, 99–122. [Google Scholar] [CrossRef]
- Montecucco, F.; Mach, F.; Pende, A. Inflammation is a key pathophysiological feature of metabolic syndrome. Mediat. Inflamm. 2013, 2013, 135984. [Google Scholar] [CrossRef] [PubMed]
- Desroches, S.; Lamarche, B. The evolving definitions and increasing prevalence of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 23–32. [Google Scholar] [CrossRef]
- Bremer, A.A.; Mietus-Snyder, M.; Lustig, R.H. Toward a unifying hypothesis of metabolic syndrome. Pediatrics 2012, 129, 557–570. [Google Scholar] [CrossRef]
- Monnerie, S.; Comte, B.; Ziegler, D.; Morais, J.A.; Pujos-Guillot, E.; Gaudreau, P. Metabolomic and lipidomic signatures of metabolic syndrome and its physiological components in adults: A systematic review. Sci. Rep. 2020, 10, 669. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Chien, K.-L.; Chiu, Y.-H.; Chuang, P.-C.; Yen, M.-F.; Chen, H.-H. Dynamics of detailed components of metabolic syndrome associated with the risk of cardiovascular disease and death. Sci. Rep. 2021, 11, 3677. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.R.; Hu, W.J.; Yang, S.; Dai, H.; Xu, S.C.; Yang, G.Y.; Li, L.; Tang, S.G.; Wang, Y. Association of metabolic syndrome components with circulating levels of cytokine clusters in young women. Endocr. Connect. 2021, 10, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Meng, F.; Xu, D.; Xu, L.; Qiu, J.; Shu, X. Synergism between the metabolic syndrome components and cancer incidence: Results from a prospective nested case-control study based on the China Health and Retirement Longitudinal Study (CHARLS). BMJ Open 2022, 12, e061362. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ortí, J.V.; Balanzá-Martínez, V.; Correa-Ghisays, P.; Selva-Vera, G.; Vila-Francés, J.; Magdalena-Benedito, R.; San-Martin, C.; Victor, V.M.; Escribano-López, I.; Hernández-Mijares, A.; et al. Specific metabolic syndrome components predict cognition and social functioning in people with type 2 diabetes mellitus and severe mental disorders. Acta Psychiatr. Scand. 2022, 146, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Zhao, Y.; Calton, E.; Pathak, K.; Ping-Delfos, W.; She, C.; Cummings, N.; Nsatimba, P. The impact of the metabolic syndrome and its components on resting energy expenditure. Metabolites 2022, 12, 722. [Google Scholar] [CrossRef]
- Carmen Zaha, D.; Vesa, C.; Uivarosan, D.; Bratu, O.; Fratila, O.; Mirela Tit, D.; Pantis, C.; Diaconu, C.C.; Bungau, S. Influence of inflammation and adipocyte biochemical markers on the components of metabolic syndrome. Exp. Ther. Med. 2020, 20, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.-M.; Sibianu, M.; Hutanu, A.; Beresescu, F.G.; Sala, D.T.; Flavius, M.; Rosca, A.; Constantin, C.; Scurtu, A.; Moriczi, R.; et al. A comprehensive summary of the current understanding of the relationship between severe obesity, metabolic syndrome, and inflammatory status. J. Clin. Med. 2023, 12, 3818. [Google Scholar] [CrossRef]
- Dulloo, A.G.; Montani, J.P. Body composition, inflammation and thermogenesis in pathways to obesity and the metabolic syndrome: An overview. Obes. Rev. 2012, 13 (Suppl. S2), 1–5. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A chronic low-grad inflammation and its markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Zoran, D.L. Obesity in dogs and cats: A metabolic and endocrine disorder. Vet. Clin. N. Am.-Small Anim. Pract. 2010, 40, 221–239. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Bautista-Castaño, I.; Peña, C.; Suárez, L.; Juste, M.C.; Tvarijonaviciute, A. Prevalence of canine obesity, obesity-related metabolic dysfunction, and relationship with owner obesity in an obesogenic region of Spain. Front. Vet. Sci. 2017, 4, e59. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Metabolic syndrome: From the genetics to the pathophysiology. Curr. Hypertens. Rep. 2011, 13, 149–157. [Google Scholar] [CrossRef]
- Stancáková, A.; Laakso, M. Genetics of metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 243–252. [Google Scholar] [CrossRef]
- Rana, S.; Ali, S.; Ahmad Wani, H.; Danish Mushtaq, Q.; Sharma, S.; Rehman, M.U. Metabolic syndrome and underlying genetic determinants—A systematic review. J. Diabetes Metab. Disord. 2022, 21, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Bruce, K.D.; Cagampang, F.R. Epigenetic priming of the metabolic syndrome. Toxicol. Mech. Methods 2011, 21, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Nuotio, M.L.; Pervjakova, N.; Joensuu, A.; Karhunen, V.; Hiekkalinna, T.; Milani, L.; Kettunen, J.; Järvelin, M.R.; Jousilahti, P.; Metspalu, A.; et al. An epigenome-wide association study of metabolic syndrome and its components. Sci. Rep. 2020, 10, 20567. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Rich, S.S. Genetics of glucose homeostasis. Implications for insulin resistance and metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C. Genetics of obesity: What we have learned over decades of research. Obesity 2021, 29, 802–820. [Google Scholar] [CrossRef]
- Sivasubramanian, R.; Malhotra, S. Genetic contributors to obesity. Gastroenterol. Clin. N. Am. 2023, 52, 323–332. [Google Scholar] [CrossRef]
- Yin, R.; Wu, J.; Lin, W.; Chen, Y.; Yang, D.; Pan, S. The environmental and genetic evidence for the association of hyperlipidemia and hypertension. J. Hypertens. 2009, 27, 251–258. [Google Scholar] [CrossRef]
- Lidfeldt, J.; Nyberg, P.; Nerbrand, C.; Samsioe, G.; Scherstén, B.; Agardh, C.D. Socio-demographic and psychosocial factors are associated with features of the metabolic syndrome. The Women’s Health in the Lund Area (WHILA) study. Diabetes Obes. Metab. 2003, 5, 106–112. [Google Scholar] [CrossRef]
- Sanghez, V.; Razzoli, M.; Carobbio, S.; Campbell, M.; McCallum, J.; Cero, C.; Ceresini, G.; Cabassi, A.; Govoni, P.; Franceschini, P.; et al. Psychosocial stress induces hyperphagia and exacerbates diet-induced insulin resistance and the manifestations of the Metabolic Syndrome. Psychoneuroendocrinology 2013, 38, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Strauss, S.M. Food insecurity as a predictor of metabolic syndrome in U. S. female adults. Public Health Nurs. 2020, 37, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Onat, A.; Hergenç, G.; Keleş, I.; Doǧan, Y.; Türkmen, S.; Sansoy, V. Sex difference in development of diabetes and cardiovascular disease on the way from obesity and metabolic syndrome—Prospective study of a cohort with normal glucose metabolism. Metab. Clin. Exp. 2005, 54, 800–808. [Google Scholar] [CrossRef]
- Antonio, L.; Wu, F.C.W.; O’Neill, T.W.; Pye, S.R.; Carter, E.L.; Finn, J.D.; Rutter, M.K.; Laurent, M.R.; Huhtaniemi, I.T.; Han, T.S.; et al. Associations between sex steroids and the development of metabolic syndrome: A longitudinal study in European men. J. Clin. Endocrinol. Metab. 2015, 100, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Rochlani, Y.; Pothineni, N.; Mehta, J. Metabolic syndrome: Does it differ between women and men? Cardiovasc. Drugs Ther. 2015, 29, 329–338. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Lehmkuhl, E.; Weickert, M.O. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin. Res. Cardiol. 2006, 95, 136–147. [Google Scholar] [CrossRef]
- McCarthy, K.; Laird, E.; O’Halloran, A.M.; Fallon, P.; Romero Ortuño, R.; Kenny, R.A. Association between metabolic syndrome and risk of both prevalent and incident frailty in older adults: Findings from The Irish Longitudinal Study on Ageing (TILDA). Exp. Gerontol. 2023, 172, 112056. [Google Scholar] [CrossRef]
- Scott, D.; Cumming, R.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Handelsman, D.J.; Seibel, M.; Waite, L.M.; Hirani, V. Associations of sarcopenic obesity with the metabolic syndrome and insulin resistance over five years in older men: The Concord Health and Ageing in Men Project. Exp. Gerontol. 2018, 108, 99–105. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Scafato, E.; Capurso, C.; d’Introno, A.; Colacicco, A.M.; Frisardi, V.; Vendemiale, G.; Baldereschi, M.; Crepaldi, G.; Di Carlo, A.; et al. Metabolic syndrome and the risk of vascular dementia: The Italian Longitudinal Study on Ageing. J. Neurol. Neurosurg. Psychiatry 2010, 81, 433–440. [Google Scholar] [CrossRef]
- Schroen, B.; Heymans, S. Small but smart-microRNAs in the centre of inflammatory processes during cardiovascular diseases, the metabolic syndrome, and ageing. Cardiovasc. Res. 2012, 93, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Su, Q.Z. MicroRNA regulation of mitochondrial and ER stress signaling pathways: Implications for lipoprotein metabolism in metabolic syndrome. Am. J. Physiol. 2014, 307, E729–E737. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; St Onge, M.-P.; Heshka, S.; Heymsfield, S.B. Lifestyle behaviors associated with lower risk of having the metabolic syndrome. Metabolism 2004, 53, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Sénéchal, M.; Bouchard, D.R.; Dionne, I.J.; Brochu, M. Lifestyle habits and physical capacity in patients with moderate or severe metabolic syndrome. Metab. Syndr. Relat. Disord. 2012, 10, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Sprini, D.; Grosso, G.; Galvano, F.; Nicolucci, A.; Lucisano, G.; Massenti, F.M.; Amodio, E.; Rini, G.B. Impact of lifestyle on metabolic syndrome in apparently healthy people. Eat. Weight Disord. 2014, 19, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Suliga, E.; Ciesla, E.; Lelonek, M.; Piechowska, A.; Gluszek, S. Lifestyle elements and risk of metabolic syndrome in adults. PLoS ONE 2022, 17, e0275510. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Ceolotto, G.; Pagnin, E.; de Kreutzenberg, S.; Avogaro, A. At the crossroads of longevity and metabolism: The metabolic syndrome and lifespan determinant pathways. Aging Cell 2011, 10, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Maralani, H.G.; Tai, B.C.; Wong, T.Y.; Tai, E.S.; Li, J.L.; Wang, J.J.; Mitchell, P. Metabolic syndrome and mortality in the elderly: A time-dependent association. Diabetes Res. Clin. Pract. 2013, 99, 209–216. [Google Scholar] [CrossRef]
- Wen, C.-P.; Chan, H.-T.; Tsai, M.-K.; Cheng, T.-Y.D.; Chung, W.-S.I.; Chang, Y.-C.; Hsu, H.-L.; Tsai, S.-P.; Tsao, C.-K.; Man Wai, J.P.; et al. Attributable mortality burden of metabolic syndrome: Comparison with its individual components. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 561–573. [Google Scholar] [CrossRef]
- Pannier, B.; Thomas, F.; Bean, K.; Jégo, B.; Benetos, A.; Guize, L. The metabolic syndrome: Similar deleterious impact on all-cause mortality in hypertensive and normotensive subjects. J. Hypertens. 2008, 26, 1223–1228. [Google Scholar] [CrossRef]
- Loucks, E.B.; Rehkopf, D.H.; Thurston, R.C.; Kawachi, I. Socioeconomic disparities in metabolic syndrome differ by gender: Evidence from NHANES III. Ann. Epidemiol. 2007, 17, 19–26. [Google Scholar] [CrossRef]
- Schultz, A.B.; Edington, D.W. Metabolic syndrome in a workplace: Prevalence, co-morbidities, and economic impact. Metab. Syndr. Relat. Disord. 2009, 7, 459–468. [Google Scholar] [CrossRef]
- Krijnen, H.K.; Hoveling, L.A.; Liefbroer, A.C.; Bültmann, U.; Smidt, N. Socioeconomic differences in metabolic syndrome development among males and females, and the mediating role of health literacy and self-management skills. Prev. Med. 2022, 161, 107140. [Google Scholar] [CrossRef]
- Scholze, J.; Alegria, E.; Ferri, C.; Langham, S.; Stevens, W.; Jeffries, D.; Uhl-Hochgraeber, K. Epidemiological and economic burden of metabolic syndrome and its consequences in patients with hypertension in Germany, Spain and Italy; a prevalence-based model. BMC Public Health 2010, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, D.M.; Malone, D.C.; Raebel, M.A.; Fishman, P.A.; Nicholls, G.A.; Feldstein, A.C.; Boscoe, A.N.; Ben-Joseph, R.H.; Magid, D.J.; Okamoto, L.J. Health care utilization and costs by metabolic syndrome risk factors. Metab. Syndr. Relat. Disord. 2009, 7, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Tamashiro, K.L.; Sakai, R.R.; Shively, C.A.; Karatsoreos, I.N.; Reagan, L.P. Chronic stress, metabolism, and metabolic syndrome. Stress 2011, 14, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, N.; Gyntelberg, F.; Faber, J. The appraisal of chronic stress and the development of the metabolic syndrome: A systematic review of prospective cohort studies. Endocr. Connect. 2014, 3, R55–R80. [Google Scholar] [CrossRef] [PubMed]
- Bruce, K.D.; Byrne, C.D. The metabolic syndrome: Common origins of a multifactorial disorder. Postgrad. Med. J. 2009, 85, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Cagampang, F.R.; Poore, K.R.; Hanson, M.A. Developmental origins of the metabolic syndrome: Body clocks and stress responses. Brain Behav. Immun. 2011, 25, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, G.F.; Leunissen, R.W.J.; Hokken-Koelega, A.C.S. Early origins of the metabolic syndrome: Role of small size at birth, early postnatal weight gain, and adult IGF-I. J. Clin. Endocrinol. Metab. 2012, 97, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Seda, O. Parental overnutrition by carbohydrates in developmental origins of metabolic syndrome. Physiol. Res. 2021, 70 (Suppl. S4), S585–S596. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Valdez, R.A.; Hazuda, H.P.; Mitchell, B.D.; Morales, P.A.; Stern, M.P. Prospective analysis of the insulin-resistance syndrome (Syndrome X). Diabetes 1992, 41, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Finer, N. Redefining type 2 diabetes: “diabesity” or “obesity dependent diabetes Mellitus”? Obes. Rev. 2000, 1, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.C.T.; Delgado, V.; Borlaug, B.A.; Bax, J.J. Diabesity: The combined burden of obesity and diabetes on heart disease and the role of imaging. Nat. Rev. Cardiol. 2021, 18, 291–304. [Google Scholar] [CrossRef]
- Farag, Y.M.K.; Gaballa, M.R. Diabesity: An overview of a rising epidemic. Nephrol. Dial. Transplant. 2010, 26, 28–35. [Google Scholar] [CrossRef]
- Spiegelman, B.M.; Flier, J.S. Obesity and the regulation of energy balance. Cell 2001, 104, 531–543. [Google Scholar] [CrossRef]
- Yun, J.E.; Won, S.; Sung, J.; Jee, S.H. Impact of metabolic syndrome independent of insulin resistance on the development of cardiovascular disease. Circ. J. 2012, 76, 2443–2448. [Google Scholar] [CrossRef]
- Brietzke, S.A. Controversy in diagnosis and management of the metabolic syndrome. Med. Clin. N. Am. 2007, 91, 1041–1061. [Google Scholar] [CrossRef]
- Chateau-Degat, M.L.; Dewailly, E.; Poirier, P.; Gingras, S.; Egeland, G.M. Comparison of diagnostic criteria of the metabolic syndrome in 3 ethnic groups of Canada. Metabolism 2008, 57, 1526–1532. [Google Scholar] [CrossRef]
- Strazzullo, P.; Barbato, A.; Siani, A.; Cappuccio, F.P.; Versiero, M.; Schiattarella, P.; Russo, O.; Avallone, S.; della Valle, E.; Farinaro, E. Diagnostic criteria for metabolic syndrome: A comparative analysis in an unselected sample of adult male population. Metabolism 2008, 57, 355–361. [Google Scholar] [CrossRef]
- Engin, A. The definition and prevalence of obesity and metabolic syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef]
- Stunkard, A.J. Some perspectives on human obesity: Its causes. Bull. N. Y. Acad. Med. 1988, 64, 902–923. [Google Scholar]
- Blüher, M. Adipose tissue inflammation: A cause or consequence of obesity-related insulin resistance? Clin. Sci. 2016, 130, 1603–1614. [Google Scholar] [CrossRef]
- Pedersen, D.J.; Guilherme, A.; Danai, L.V.; Heyda, L.; Matevossian, A.; Cohen, J.; Nicoloro, S.M.; Straubhaar, J.; Noh, H.L.; Jung, D.; et al. A major role of insulin in promoting obesity-associated adipose tissue inflammation. Mol. Metab. 2015, 4, 507–518. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Aeberli, I. Dietary determinants of subclinical inflammation, dyslipidemia and components of the metabolic syndrome in overweight children: A review. Int. J. Obes. 2008, 32 (Suppl. S6), S11–S18. [Google Scholar] [CrossRef]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Nishiguchi, S.; Higuchi, K. Metabolic syndrome and sarcopenia. Nutrients 2021, 13, 3519. [Google Scholar] [CrossRef]
- Kim, S.P.; Ellmerer, M.; Van Citters, G.W.; Bergman, R.N. Primacy of hepatic insulin resistance in the development of the metabolic syndrome induced by an isocaloric moderate-fat diet in the dog. Diabetes 2003, 52, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Hutchison, S.; Zoungas, S.; Meyer, C. Insulin resistance, the metabolic syndrome, diabetes, and cardiovascular disease risk in women with PCOS. Endocrine 2006, 30, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, U. Hyperinsulinaemia, a key factor of the metabolic syndrome in postmenopausal women. Maturitas 2009, 62, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Muzurovic, E.; Mikhailidis, D.P.; Mantzoros, C. Non-alcoholic fatty liver disease, insulin resistance, metabolic syndrome and their association with vascular risk. Metab. Clin. Exp. 2021, 119, 154770. [Google Scholar] [CrossRef]
- Gould, D.C.; Amoroso, P.; Kirby, R.S. Hypoandrogen-metabolic syndrome: A significant issue for men’s health. BJU Int. 2006, 98, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-W.; Lee, J.-K.; Wu, C.-K.; Caffrey, J.L.; Chang, M.-H.; Hwang, J.-J.; Dowling, N.; Lin, Y.-S. Metabolic syndrome, testosterone, and cardiovascular mortality in men. J. Sex. Med. 2011, 8, 2350–2360. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.-R.; Yang, H.-H.; Liao, C.-H.; Yang, D.-H.; Tu, S.-K.; Hung, C.-L.; Liao, C.-C. Association between low serum testosterone and the development of metabolic syndrome in elderly Taiwanese men. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; Lauretani, F.; Ceda, G.P.; Bandinelli, S.; Basaria, S.; Paolisso, G.; Giumelli, C.; Luci, M.; Najjar, S.S.; Metter, E.J.; et al. Estradiol and metabolic syndrome in older italian men: The InCHIANTI study. J. Androl. 2010, 31, 155–162. [Google Scholar] [CrossRef]
- Matic, M.; Bryzgalova, G.; Gao, H.; Antonson, P.; Humire, P.; Omoto, Y.; Portwood, N.; Pramfalk, C.; Efendic, S.; Berggren, P.-O.; et al. Estrogen signalling and the metabolic syndrome: Targeting the hepatic estrogen receptor alpha action. PLoS ONE 2013, 8, e57458. [Google Scholar] [CrossRef]
- Duclos, M.; Márquez Pereira, P.; Barat, P.; Gatta, B.; Roger, P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes. Res. 2005, 13, 1157–1166. [Google Scholar] [CrossRef]
- Esteghamati, A.; Morteza, A.; Khalilzadeh, O.; Noshad, S.; Novin, L.; Nakhjavani, M. Association of serum cortisol levels with parameters of metabolic syndrome in men and women. Clin. Investig. Med. 2011, 34, E131–E137. [Google Scholar] [CrossRef]
- Brindley, D.N. Role of glucocorticoids and fatty acids in the impairment of lipid metabolism observed in the metabolic syndrome. Int. J. Obes. 1995, 19 (Suppl. S1), S69–S75. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, M.A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef]
- Mantha, O.L.; Huneau, J.F.; Mathe, V.; Hermier, D.; Khodorova, N.; Mariotti, F.; Fouillet, H. Differential changes to splanchnic and peripheral protein metabolism during the diet-induced development of metabolic syndrome in rats. Am. J. Physiol. 2020, 319, E175–E186. [Google Scholar] [CrossRef]
- Hanley, A.J.G.; Williams, K.; Festa, A.; Wagenknecht, L.E.; d’Agostino, R.B., Jr.; Haffner, S.M. Liver markers and development of the metabolic syndrome. The insulin resistance atherosclerosis study. Diabetes 2005, 54, 3140–3147. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig. Liver Dis. 2015, 47, 181–190. [Google Scholar] [CrossRef]
- Yoo, J.-J.; Cho, E.J.; Chung, G.E.; Chang, Y.; Cho, Y.; Park, S.-H.; Jeong, S.-M.; Kim, B.-Y.; Shin, D.W.; Kim, Y.J.; et al. Nonalcoholic fatty liver disease is a precursor of new-onset metabolic syndrome in metabolically healthy young adults. J. Clin. Med. 2022, 11, 935. [Google Scholar] [CrossRef]
- Bonora, E. The metabolic syndrome and cardiovascular disease. Ann. Med. 2006, 38, 64–80. [Google Scholar] [CrossRef]
- Vazzana, N.; Santilli, F.; Sestili, S.; Cuccurullo, C.; Davi, G. Determinants of increased cardiovascular disease in obesity and metabolic syndrome. Curr. Med. Chem. 2011, 18, 5267–5280. [Google Scholar] [CrossRef]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef]
- Nichols, G.A.; Amitay, E.L.; Chatterjee, S.; Steubl, D. The bidirectional association of chronic kidney disease, type 2 diabetes, atherosclerotic cardiovascular disease, and heart failure: The cardio-renal-metabolic syndrome. Metab. Syndr. Relat. Disord. 2023, 21, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Larqué, C.; Velasco, M.; Navarro-Tableros, V.; Duhne, M.; Aguirre, J.; Gutiérrez-Reyes, G.; Moreno, J.; Robles-Díaz, G.; Hong, E.; Hiriart, M. Early endocrine and molecular changes in metabolic syndrome models. IUBMB Life 2011, 63, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Alemany, M. Do the interactions between glucocorticoids and sex hormones regulate the development of the metabolic syndrome? Front. Endocrinol. 2012, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, R.; Tanaka, M.; Itoh, M.; Suganami, T. Molecular mechanism of crosstalk between immune and metabolic systems in metabolic syndrome. Inflamm. Regen. 2022, 42, 13. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J.; Murphy, K.E.; Fernández, M.L. Impact of obesity and metabolic syndrome on immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Sala, M.; de Roos, A.; van den Berg, A.; Altmann-Schneider, I.; Slagboom, P.E.; Westendorp, R.G.; van Buchem, M.A.; de Craen, A.J.M.; van der Grond, J. Microstructural brain tissue damage in metabolic syndrome. Diabetes Care 2014, 37, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Kazamel, M.; Stino, A.M.; Smith, A.G. Metabolic syndrome and peripheral neuropathy. Muscle Nerve 2021, 63, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.P.; Patel, T.V.; Farag, Y.M.K.; Florez, A.; Rennke, H.G.; Singh, A.K. Kidney pathological changes in metabolic syndrome: A cross-sectional study. Am. J. Kidney Dis. 2009, 53, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Marette, A.; Liu, Y.; Sweeney, G. Skeletal muscle glucose metabolism and inflammation in the development of the metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 299–305. [Google Scholar] [CrossRef]
- Kruszewska, J.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Remodeling and fibrosis of the cardiac muscle in the course of obesity-pathogenesis and involvement of the extracellular matrix. Int. J. Mol. Sci. 2022, 23, 4195. [Google Scholar] [CrossRef]
- García Nores, G.D.; Cuzzone, D.A.; Albano, N.J.; Hespe, G.E.; Kataru, R.P.; Torrisi, J.S.; Gardenier, J.C.; Savetsky, I.L.; Aschen, S.Z.; Nitti, M.D.; et al. Obesity but not high-fat diet impairs lymphatic function. Int. J. Obes. 2016, 40, 1582–1590. [Google Scholar] [CrossRef]
- Oda, E. The metabolic syndrome as a concept of adipose tissue disease. Hypertens. Res. 2008, 31, 1283–1291. [Google Scholar] [CrossRef]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef]
- Leone, N.; Courbon, D.; Thomas, F.; Bean, K.; Jego, B.; Leynaert, B.; Guize, L.; Zureik, M. Lung function impairment and metabolic syndrome- The critical role of abdominal obesity. Am. J. Respir. Crit. Care Med. 2009, 179, 509–516. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, B.; Han, K.; Kim, S.W. The relationship between metabolic syndrome and obstructive sleep apnea syndrome: A nationwide population-based study. Sci. Rep. 2021, 11, 8751. [Google Scholar] [CrossRef]
- Puenpatom, R.A.; Victor, T.W. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: An analysis of NHANES III data. Postgrad. Med. 2009, 121, 9–20. [Google Scholar] [CrossRef]
- Ornstrup, M.J.; Kjaer, T.N.; Harslof, T.; Stodkilde-Jorgensen, H.; Hougaard, D.M.; Cohen, A.; Pedersen, S.B.; Langdahl, B.L. Adipose tissue, estradiol levels, and bone health in obese men with metabolic syndrome. Eur. J. Endocrinol. 2015, 172, 205–216. [Google Scholar] [CrossRef]
- Hao, Y.; Zhu, Y.-J.; Zou, S.; Zhou, P.; Hu, Y.-W.; Zhao, Q.-X.; Gu, L.-N.; Zhang, H.-Z.; Wang, Z.; Li, J. Metabolic syndrome and psoriasis: Mechanisms and future directions. Front. Immunol. 2021, 12, 711060. [Google Scholar] [CrossRef]
- Qiao, J.; Jia, Q.-N.; Jin, H.-Z. Association between metabolic syndrome and psoriasis: A meta-analysis of observational studies with non-psoriasis control groups. Arch. Med. Sci. 2021, 17, 1558–1565. [Google Scholar] [CrossRef]
- Brandsma, E.; Houben, T.; Fu, J.; Shiri-Sverdlov, R.; Hofker, M.H. The immunity-diet-microbiota axis in the development of metabolic syndrome. Curr. Opin. Lipidol. 2015, 26, 73–81. [Google Scholar] [CrossRef]
- Araujo, R.; Borges-Canha, M.; Pimentel-Nunes, P. Microbiota modulation in patients with metabolic syndrome. Nutrients 2022, 14, 4490. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarlata, G.G.M.; Scarpellini, E.; Boccuto, L.; Spagnuolo, R.; Tilocca, B.; Roncada, P.; Luzza, F. Metabolic-dysfunction-associated fatty liver disease and gut microbiota: From fatty liver to dysmetabolic syndrome. Med. Lith. 2023, 59, 594. [Google Scholar] [CrossRef]
- Bryan, S.; Baregzay, B.; Spicer, D.; Singal, P.K.; Khaper, N. Redox-inflammatory synergy in the metabolic syndrome. Can. J. Physiol. Pharmacol. 2013, 91, 22–30. [Google Scholar] [CrossRef]
- Korac, B.; Kalezic, A.; Pekovic-Vaughan, V.; Korac, A.; Jankovic, A. Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biol. 2021, 42, e101887. [Google Scholar] [CrossRef]
- Denis, R.G.P.; Joy-Amado, A.; Cansell, C.; Castel, J.; Martinez, S.; Delbes, A.S.; Luquet, S. Central orchestration of peripheral nutrient partitioning and substrate utilization: Implications for the metabolic syndrome. Diabetes Metab. 2014, 40, 191–197. [Google Scholar] [CrossRef]
- Remesar, X.; Alemany, M. Dietary energy partition: The central role of glucose. Int. J. Mol. Sci. 2020, 21, 7729. [Google Scholar] [CrossRef]
- Zimmet, P.; Boyko, E.J.; Collier, G.R.; de Courten, M. Etiology of the metabolic syndrome: Potential role of insulin resistance, leptin resistance, and other players. Ann. N. Y. Acad. Sci. 1999, 892, 25–44. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D.; Karnieli, E. Insulin resistance in obesity as the underlying cause for the metabolic syndrome. Mt. Sinai J. Med. 2010, 77, 511–523. [Google Scholar] [CrossRef]
- Romeo, G.R.; Lee, J.; Shoelson, S.E. Metabolic syndrome, insulin resistance, and roles of inflammation—Mechanisms and therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1771–1776. [Google Scholar] [CrossRef]
- Nolan, C.J.; Prentki, M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diabetes Vasc. Dis. Res. 2019, 16, 118–127. [Google Scholar] [CrossRef]
- Anderson, P.J.; Critchley, J.A.J.H.; Chan, J.C.N.; Cockram, C.S.; Lee, Z.S.K.; Thomas, G.N.; Tomlinson, B. Factor analysis of the metabolic syndrome: Obesity vs insulin resistance as the central abnormality. Int. J. Obes. 2001, 25, 1782–1788. [Google Scholar] [CrossRef]
- Shirai, K. Obesity as the core of the metabolic syndrome and the management of coronary heart disease. Curr. Med. Res. Opin. 2004, 20, 295–304. [Google Scholar] [CrossRef]
- Després, J.-P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Bloomgarden, Z.T. Dyslipidemia and the metabolic syndrome. Diabetes Care 2004, 27, 3009–3016. [Google Scholar] [CrossRef]
- De Souza, J.A.; Vindis, C.; Hansel, B.; Negre-Salvayre, A.; Therond, P.; Serrano, C.V., Jr.; Chantepie, S.; Salvayre, R.; Bruckert, E.; Chapman, M.J.; et al. Metabolic syndrome features small, apolipoprotein A-I-poor, triglyceride-rich HDL3 particles with defective anti-apoptotic activity. Atherosclerosis 2008, 197, 84–94. [Google Scholar] [CrossRef]
- Arsenault, B.J.; Despres, J.-P.; Stroes, E.S.G.; Wareham, N.J.; Kastelein, J.J.P.; Khaw, K.-T.; Boekholdt, S.M. Lipid assessment, metabolic syndrome and coronary heart disease risk. Eur. J. Clin. Investig. 2010, 40, 1081–1093. [Google Scholar] [CrossRef]
- Tarantino, G.; Saldalamacchia, G.; Conca, P.; Arena, A. Non-alcoholic fatty liver disease: Further expression of the metabolic syndrome. J. Gastroenterol. Hepatol. 2007, 22, 293–303. [Google Scholar] [CrossRef]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef]
- Yip, T.C.-F.; Wong, G.L.-H.; Wong, V.W.-S.; Goh, G.B.-B.; Chan, W.-K. Nonalcoholic fatty liver disease a unique entity or part of the metabolic syndrome or both. Med. Clin. N. Am. 2023, 107, 449–463. [Google Scholar] [CrossRef]
- Aizawa, Y.; Kamimura, N.; Watanabe, H.; Aizawa, Y.; Makiyama, Y.; Usuda, Y.; Watanabe, T.O.; Kurashina, Y. Cardiovascular risk factors are really linked in the metabolic syndrome: This phenomenon suggests clustering rather than coincidence. Int. J. Cardiol. 2006, 109, 213–218. [Google Scholar] [CrossRef]
- Obunai, K.; Jani, S.; Dangas, G.D. Cardiovascular morbidity and mortality of the metabolic syndrome. Med. Clin. N. Am. 2007, 91, 1169–1184. [Google Scholar] [CrossRef]
- Tamariz, L.; Hassan, B.; Palacio, A.; Arcement, L.; Horswell, R.; Hebert, K. Metabolic syndrome increases mortality in heart failure. Clin. Cardiol. 2009, 32, 327–331. [Google Scholar] [CrossRef]
- Alemany, M. Utilization of dietary glucose in the metabolic syndrome. Nutr. Metab. 2011, 8, 74. [Google Scholar] [CrossRef]
- Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165838. [Google Scholar] [CrossRef]
- Alemany, M. Estrogens and the regulation of glucose metabolism. World J. Diabetes 2021, 12, 1622–1654. [Google Scholar] [CrossRef]
- Grossmann, M.; Hoermann, R.; Wittert, G.; Yeap, B.B. Effects of testosterone treatment on glucose metabolism and symptoms in men with type 2 diabetes and the metabolic syndrome: A systematic review and meta-analysis of randomized controlled clinical trials. Clin. Endocrinol. 2015, 83, 344–351. [Google Scholar] [CrossRef]
- Seeley, R.J.; York, D.A. Fuel sensing and the central nervous system (CNS): Implications for the regulation of energy balance and the treatment for obesity. Obes. Rev. 2005, 6, 259–265. [Google Scholar] [CrossRef]
- Stefater, M.A.; Seeley, R.J. Central nervous system nutrient signaling: The regulation of energy balance and the future of dietary therapies. Annu. Rev. Nutr. 2010, 30, 219–235. [Google Scholar] [CrossRef]
- Rasouli, N.; Molavi, B.; Elbein, S.C.; Kern, P.A. Ectopic fat accumulation and metabolic syndrome. Diabetes Obes. Metab. 2007, 9, 1–10. [Google Scholar] [CrossRef]
- Migrenne, S.; le Foll, C.; Levin, B.E.; Magnan, C. Brain lipid sensing and nervous control of energy balance. Diabetes Metab. 2011, 37, 83–88. [Google Scholar] [CrossRef]
- Schneeberger, M.; Gomis, R.; Claret, M. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J. Endocrinol. 2014, 220, T25–T46. [Google Scholar] [CrossRef]
- Anthony, K.; Reed, L.J.; Dunn, J.T.; Bingham, E.; Hopkins, D.; Marsden, P.K.; Amiel, S.A. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance. The cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes 2006, 55, 2986–2992. [Google Scholar] [CrossRef]
- Ahima, R.S.; Antwi, D.A. Brain regulation of appetite and satiety. Endocrinol. Metab. Clin. N. Am. 2008, 37, 811–823. [Google Scholar] [CrossRef]
- Van de Wouw, M.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Microbiota-gut-brain axis: Modulator of host metabolism and appetite. J. Nutr. 2017, 147, 727–745. [Google Scholar] [CrossRef]
- Ahn, B.H.; Kim, M.; Kim, S.-Y. Brain circuits for promoting homeostatic and non-homeostatic appetites. Exp. Mol. Med. 2022, 54, 349–357. [Google Scholar] [CrossRef]
- Pasquali, R.; Vicennati, V.; Cacciari, M.; Pagotto, U. The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann. N. Y. Acad. Sci. 2006, 1083, 111–128. [Google Scholar] [CrossRef]
- Michalakis, K.; Mintziori, G.; Kaprara, A.; Tarlatzis, B.C.; Goulis, D.G. The complex interaction between obesity, metabolic syndrome and reproductive axis: A narrative review. Metab. Clin. Exp. 2013, 62, 457–478. [Google Scholar] [CrossRef]
- Rettberg, J.R.; Yao, J.; Brinton, R.D. Estrogen: A master regulator of bioenergetic. systems in the brain and body. Front. Neuroendocrinol. 2014, 35, 8–30. [Google Scholar] [CrossRef]
- Nogueiras, R. Mechanisms in Endocrinology. The gut–brain axis: Regulating energy balance independent of food intake. Eur. J. Endocrinol. 2021, 185, R75–R91. [Google Scholar] [CrossRef]
- Aguilera, C.M.; Gil-Campos, M.; Cañete, R.; Gil, A. Alterations in plasma and tissue lipids associated with obesity and metabolic syndrome. Clin. Sci. 2008, 114, 183–193. [Google Scholar] [CrossRef]
- Adiels, M.; Olofsson, S.O.; Taskinen, M.R.; Borén, J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1225–1236. [Google Scholar] [CrossRef]
- Johnson, R.J.; Lanaspa, M.A.; Gaucher, E.A. Uric acid: A danger signal from the RNA world that may have a role in the epidemic of obesity, metabolic syndrome, and cardiorenal disease: Evolutionary considerations. Semin. Nephrol. 2011, 31, 394–399. [Google Scholar] [CrossRef]
- Raya-Cano, E.; Vaquero-Abellán, M.; Molina-Luque, R.; de Pedro-Jiménez, D.; Molina-Recio, G.; Romero-Saldaña, M. Association between metabolic syndrome and uric acid: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 18412. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Silva, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; Acierno, C.; Nevola, R.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Salvatore, T.; Adinolfi, L.E.; Sasso, F.C. Non-alcoholic fatty liver disease: From pathogenesis to clinical impact. Processes 2021, 9, 135. [Google Scholar] [CrossRef]
- Inoue, H. Regulation of glucose metabolism by central insulin action. Biomed. Rev. 2011, 22, 31–39. [Google Scholar] [CrossRef]
- Lundsgaard, A.-M.; Sjoberg, K.A.; Hoeg, L.D.; Jeppesen, J.; Jordy, A.B.; Serup, A.K.; Fritzen, A.M.; Pilegaard, H.; Myrmel, L.S.; Madsen, L.; et al. Opposite regulation of insulin sensitivity by dietary lipid versus carbohydrate excess. Diabetes 2017, 66, 2583–2595. [Google Scholar] [CrossRef] [PubMed]
- London, A.; Lundsgaard, A.-M.; Kiens, B.; Bojsen-Møller, K.N. The role of hepatic fat accumulation in glucose and insulin homeostasis-dysregulation by the liver. J. Clin. Med. 2021, 10, 390. [Google Scholar] [CrossRef]
- Abildgaard, J.; Rubaek Danielsen, E.; Dorph, E.; Thomsen, C.; Juul, A.; Ewertsen, C.; Klarlund Pedersen, B.; Tonnes Pedersen, A.; Ploug, T.; Lindegaard, B. Ectopic lipid deposition is associated with insulin resistance in postmenopausal women. J. Clin. Endocrinol. Andm. 2018, 103, 3394–3404. [Google Scholar] [CrossRef]
- Moran, C.; McEniery, C.M.; Schoenmakers, N.; Mitchell, C.; Sleigh, A.; Watson, L.; Lyons, G.; Burling, K.; Barker, P.; Chatterjee, K. Dyslipidemia, insulin resistance, ectopic lipid accumulation, and vascular function in resistance to thyroid hormone β. J. Clin. Endocrinol. Metab. 2021, 106, E2005–E2014. [Google Scholar] [CrossRef]
- Yki-Järvinen, H. Ectopic fat accumulation: An important cause of insulin resistance in humans. J. R. Soc. Med. 2002, 95 (Suppl. S42), 39–45. [Google Scholar]
- Angelico, F.; del Ben, M.; Conti, R.; Francioso, S.; Feole, K.; Fiorello, S.; Cavallo, M.G.; Zalunardo, B.; Lirussi, F.; Alessandri, C.; et al. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2005, 90, 1578–1582. [Google Scholar] [CrossRef]
- Whaley-Connell, A.; Sowers, J.R. Insulin resistance in kidney disease: Is there a distinct role separate from that of diabetes or obesity. Cardiorenal Med. 2017, 8, 41–49. [Google Scholar] [CrossRef]
- Hoscheidt, S.M.; Kellawan, J.M.; Berman, S.E.; Rivera-Rivera, L.A.; Krause, R.A.; Oh, J.M.; Beeri, M.S.; Rowley, H.A.; Wieben, O.; Carlsson, C.M.; et al. Insulin resistance is associated with lower arterial blood flow and reduced cortical perfusion in cognitively asymptomatic middle-aged adults. J. Cereb. Blood Flow Metab. 2017, 37, 2249–2261. [Google Scholar] [CrossRef]
- Wiernsperger, N.F.; Bouskela, E. Microcirculation in insulin resistance and diabetes: More than just a complication. Diabetes Metab. 2003, 29 Pt 2, 6S77–6S87. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.F.; Imig, J.D. Obesity, insulin resistance, and renal function. Microcirculation 2007, 14, 349–362. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, K.; Sadana, P.; Chowdhury, F.; Gaillard, S.; Wang, F.; McDonnell, D.P.; Unterman, T.G.; Elam, M.B.; Park, E.A. Estrogen-related receptors stimulate pyruvate dehydrogenase kinase isoform 4 gene expression. J. Biol. Chem. 2006, 281, 39897–39906. [Google Scholar] [CrossRef] [PubMed]
- Maher, A.C.; Akhtar, M.; Tarnopolsky, M.A. Men supplemented with 17b-estradiol have increased beta-oxidation capacity in skeletal muscle. Physiol. Genom. 2010, 42, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, A.; Sampath, S.; Jayaraman, S.; Karundevi, B. Estradiol favors glucose oxidation in gastrocnemius muscle through modulation of insulin signaling molecules in adult female rats. Endocr. Res. 2013, 38, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Omotola, O.; Legan, S.; Slade, E.; Adekunle, A.; Pendergast, J.S. Estradiol regulates daily rhythms underlying diet-induced obesity in female mice. Am. J. Physiol. 2019, 317, E1172–E1181. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Hentunen, T.A.; Nars, M.; Härkönen, P.L.; Väänänen, H.K. Estrogen protects primary osteocytes against glucocorticoid-induced apoptosis. Apoptosis 2005, 10, 583–595. [Google Scholar] [CrossRef]
- Kapoor, D.; Goodwin, E.; Channer, K.S.; Jones, T.H. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur. J. Endocrinol. 2006, 154, 899–906. [Google Scholar] [CrossRef]
- Kumar, R.; Balhuizen, A.; Amisten, S.; Lundquist, I.; Salehi, A. Insulinotropic and antidiabetic effects of 17b-estradiol and the GPR30 agonist G-1 on human pancreatic islets. Endocrinology 2011, 152, 2568–2579. [Google Scholar] [CrossRef]
- Kim, C.; Halter, J.B. Endogenous sex hormones, metabolic syndrome, and diabetes in men and women. Curr. Cardiol. Rep. 2014, 16, 467. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Sowers, J.R. Estrogen and mitochondria function in cardiorenal metabolic syndrome. Prog. Mol. Biol. Transl. Sci. 2014, 127, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Galmés-Pascual, B.M.; Nadal-Casellas, A.; Bauzá-Thorbrugge, M.; Sbert-Roig, M.; García-Palmer, F.J.; Proenza, A.M.; Gianotti, M.; Lladó, I. 17b-estradiol improves hepatic mitochondrial biogenesis and function through PGC1B. J. Endocrinol. 2017, 232, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogenic control of mitochondrial function. Redox Biol. 2020, 31, 101435. [Google Scholar] [CrossRef]
- d’Eon, T.M.; Souza, S.C.; Aronovitz, M.; Obin, M.S.; Fried, S.K.; Greenberg, A.S. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J. Biol. Chem. 2005, 280, 35983–35991. [Google Scholar] [CrossRef]
- Oliva, L.; Alemany, M.; Fernández-López, J.-A.; Remesar, X. Circulating oestradiol determines liver lipid deposition in rats fed standard diets partially unbalanced with higher lipid or protein proportions. Br. J. Nutr. 2022, 128, 1499–1508. [Google Scholar] [CrossRef]
- Howard, E.E.; Shankaran, M.; Evans, W.J.; Berryman, C.E.; Margolis, L.M.; Lieberman, H.R.; Karl, J.P.; Young, A.J.; Montano, M.A.; Matthews, M.D.; et al. Effects of testosterone on mixed-muscle protein synthesis and proteome dynamics during energy deficit. J. Clin. Endocrinol. Metab. 2022, 107, E3254–E3263. [Google Scholar] [CrossRef]
- Birzniece, V.; Meinhardt, U.J.; Umpleby, M.A.; Handelsman, D.J.; Ho, K.K.Y. Interaction between testosterone and growth hormone on whole-body protein anabolism occurs in the liver. J. Clin. Endocrinol. Metab. 2011, 96, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Giannoulis, M.G.; Jackson, N.; Shojaee-Moradie, F.; Nair, K.S.; Sonksen, P.H.; Martin, F.C.; Umpleby, A.M. The effects of growth hormone and/or testosterone on whole body protein kinetics and skeletal muscle gene expression in healthy elderly men: A randomized controlled trial. J. Clin. Endocrinol. Metab. 2008, 93, 3066–3074. [Google Scholar] [CrossRef]
- Zhou, L.; Han, L.; Liu, M.; Lu, J.; Pan, S. Impact of metabolic syndrome on sex hormones and reproductive function: A meta-analysis of 2923 cases and 14062 controls. Aging 2021, 13, 1962–1971. [Google Scholar] [CrossRef]
- Alemany, M. The roles of androgens in humans: Biology, metabolic regulation and health. Int. J. Mol. Sci. 2022, 23, 11952. [Google Scholar] [CrossRef]
- Yassin, D.-J.; Doros, G.; Hammerer, P.G.; Yassin, A.A. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J. Sex. Med. 2014, 11, 1567–1576. [Google Scholar] [CrossRef]
- Hauner, H.; Entenmann, G.; Wabitsch, M.; Gaillard, D.; Ailhaud, G.; Negrel, R.; Pfeiffer, E.F. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J. Clin. Investig. 1989, 84, 1663–1670. [Google Scholar] [CrossRef]
- Casteilla, L.; Nougues, J.; Reyne, Y.; Ricquier, D. Differentiation of ovine brown adipocyte precursor cells in a chemically defined serum-free medium-importance of glucocorticoids and age of animals. Eur. J. Biochem. 1991, 198, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Tait, A.S.; Butts, C.L.; Sternberg, E.M. The role of glucocorticoids and progestins in inflammatory, autoimmune, and infectious disease. J. Leukoc. Biol. 2008, 84, 924–931. [Google Scholar] [CrossRef]
- Shimba, A.; Ikuta, K. Control of immunity by glucocorticoids in health and disease. Semin. Immunopathol. 2020, 42, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Zen, M.; Canova, M.; Campana, C.; Bettio, S.; Nalotto, L.; Rampudda, M.; Ramonda, R.; Iaccarino, L.; Doria, A. The kaleidoscope of glucorticoid effects on immune system. Autoimmun. Rev. 2011, 10, 305–310. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Franchimont, D. Overview of the actions of glucocorticoids on the immune response. A good model to characterize new pathways of immunosuppression for new treatment strategies. Ann. N. Y. Acad. Sci. 2004, 1024, 124–137. [Google Scholar] [CrossRef]
- Busillo, J.M.; Azzam, K.M.; Cidlowski, J.A. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J. Biol. Chem. 2011, 286, 38703–38713. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Sapolsky, R.M. Glucocorticoids can arm macrophages for innate immune battle. Brain Behav. Immun. 2010, 24, 17–18. [Google Scholar] [CrossRef]
- Taves, M.D.; Ashwell, J.D. Glucocorticoids in T cell development, differentiation and function. Nat. Rev. Immunol. 2021, 21, 233–243. [Google Scholar] [CrossRef]
- Ehrchen, J.; Steinmuller, L.; Barczyk, K.; Tenbrock, K.; Nacken, W.; Eisenacher, M.; Nordhues, U.; Sorg, C.; Sunderkotter, C.; Roth, J. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood 2007, 109, 1265–1274. [Google Scholar] [CrossRef]
- Ingawale, D.K.; Mandlik, S.K. New insights into the novel anti-inflammatory mode of action of glucocorticoids. Immunopharmacol. Immunotoxicol. 2020, 42, 59–73. [Google Scholar] [CrossRef]
- Syed, A.P.; Greulich, F.; Ansari, S.A.; Uhlenhaut, N.H. Anti-inflammatory glucocorticoid action: Genomic insights and emerging concepts. Curr. Opin. Pharmacol. 2020, 53, 35–44. [Google Scholar] [CrossRef]
- Borst, O.; Schaub, M.; Walker, B.; Schmid, E.; Münzer, P.; Voelkl, J.; Alesutan, I.; Rodríguez, J.M.; Vogel, S.; Schoenberger, T.; et al. Pivotal role of serum- and glucocorticoid-inducible kinase 1 in vascular inflammation and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 547–557. [Google Scholar] [CrossRef]
- Kalimi, M.; Shafagoj, Y.; Loria, R.; Padgett, D.; Regelson, W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Mol. Cell. Biochem. 1994, 131, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Araneo, B.; Daynes, R. Dehydropepiandrosterone functions as more than an antiglucocorticoid in preserving immunocompetence after thermal injury. Endocrinology 1995, 136, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Forbes, C.; Miller, N.L.; Strand, D.; Case, T.; Cates, J.M.; Kim, H.-Y.H.; Wages, P.; Porter, N.A.; Mantione, K.M.; et al. Glucocorticoids are induced while dihydrotestosterone levels are suppressed in 5α reductase inhibitor treated human benign prostate hyperplasia patients. Prostate 2022, 82, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Abulizi, A.; Camporez, J.-P.; Jurczak, M.J.; Høyer, K.F.; Zhang, D.; Cline, G.W.; Samuel, V.T.; Shulman, G.I.; Vatner, D.F. Adipose glucocorticoid action influences whole-body metabolism via modulation of hepatic insulin action. FASEB J. 2019, 33, 8174–8185. [Google Scholar] [CrossRef] [PubMed]
- Beaupère, C.; Liboz, A.; Fève, B.; Blondeau, B.; Guillemain, G. Molecular mechanisms of glucocorticoid-induced insulin resistance. Int. J. Mol. Sci. 2021, 22, 623. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Tamura, E.K.; d’Argenio-Garcia, L.; Muxel, S.M.; da Silveira Cruz-Machado, S.; Marçola, M.; Carvalho-Sousa, C.E.; Cecon, E.; Ferreira, Z.S.; Markus, R.P. Dual effect of catecholamines and corticosterone crosstalk on pineal gland melatonin synthesis. Neuroendocrinology 2017, 104, 126–134. [Google Scholar] [CrossRef]
- Grishin, S.N.; Gabdrakhmanov, A.I.; Khairullin, A.E.; Ziganshin, A.U. The influence of glucocorticoids and catecholamines on the neuromuscular transmission. Biochem. Mosc. Suppl. Ser. Membr. Cell Biol. 2017, 11, 253–260. [Google Scholar] [CrossRef]
- Ahnert, L.; Deichmann, F.; Bauer, M.; Supper, B.; Piskernik, B. Fathering behavior, attachment, and engagement in childcare predict testosterone and cortisol. Dev. Psychobiol. 2021, 63, e22149. [Google Scholar] [CrossRef]
- Ullah Khan, S.; Jannat, S.; Shaukat, H.; Tanzeela, S.; Unab, S.; Akram, M.; Khan Khattak, M.N.; Vizcara Soto, M.; Fiaz Khan, M.; Ali, A.; et al. Stress induced cortisol release depresses the secretion of testosterone in patients with type 2 diabetes mellitus. Clin. Med. Insights-Endocrinol. Diabetes 2023, 16, 11795514221145841. [Google Scholar] [CrossRef]
- Ma, L.; Shen, Q.; Yang, S.; Xie, X.; Xiao, Q.; Yu, C.; Cao, L.; Fu, Z. Effect of chronic corticosterone-induced depression on circadian rhythms and age-related phenotypes in mice. Acta Biochim. Biophys. Sin. 2018, 50, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Skorzewska, A. Glucocorticoid-induced depression. The role of the dopaminergic system and microRNAs. Postępy Psychiatr. I Neurol. 2021, 30, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Zajkowska, Z.; Gullett, N.; Walsh, A.; Zonca, V.; Pedersen, G.A.; Souza, L.; Kieling, C.; Fisher, H.L.; Kohrt, B.A.; Mondelli, V. Cortisol and development of depression in adolescence and young adulthood-a systematic review and meta-analysis. Psychoneuroendocrinology 2022, 136, 105625. [Google Scholar] [CrossRef] [PubMed]
- Purslow, P.P. The structure and role of intramuscular connective tissue in muscle function. Front. Physiol. 2020, 11, 495. [Google Scholar] [CrossRef]
- Halper, J. Basic components of connective tissues and extracellular matrix: Fibronectin, fibrinogen, laminin, elastin, fibrillins, fibulins, matrilins, tenascins and thrombospondins. Adv. Exp. Med. Biol. 2021, 1348, 105–126. [Google Scholar] [CrossRef]
- Muntz, I.; Fenu, M.; van Osch, G.J.V.M.; Koenderink, G.H. The role of cell-matrix interactions in connective tissue mechanics. Phys. Biol. 2022, 19, 021001. [Google Scholar] [CrossRef] [PubMed]
- Smith, U. Abdominal obesity: A marker of ectopic fat accumulation. J. Clin. Investig. 2015, 125, 1790–1792. [Google Scholar] [CrossRef] [PubMed]
- Asser Karsdal, M.; Byers Kraus, V.; Shevell, D.; Bay-Jensen, A.C.; Schattenberg, J.; Rambabu Surabattula, R.; Schuppan, D. Profiling and targeting connective tissue remodeling in autoimmunity—A novel paradigm for diagnosing and treating chronic diseases. Autoimmun. Rev. 2021, 20, 102706. [Google Scholar] [CrossRef] [PubMed]
- Fede, C.; Fan, C.; Pirri, C.; Petrelli, L.; Biz, C.; Porzionato, A.; Macchi, V.; de Caro, R.; Stecco, C. The effects of aging on the intramuscular connective tissue. Int. J. Mol. Sci. 2022, 23, 11061. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Caldez, M.J.; Akira, S. Innate immunity in allergy. Allergy 2019, 74, 1660–1674. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, D.T.; McIntire, J.J.; Akbari, O.; Macaubas, C.; DeKruyff, R.H. Asthma: An epidemic of dysregulated immunity. Nat. Immunol. 2002, 3, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Jiang, A.; Li, W.; Song, C.; Leng, H. Metabolic syndrome and osteoarthritis: Possible mechanisms and management strategies. Med. Nov. Technol. Devices 2021, 9, 100052. [Google Scholar] [CrossRef]
- Zhuo, Q.; Yang, W.; Chen, J.; Wang, Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 729–737. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Katz, J.D. Osteoarthritis: Another component of metabolic syndrome? Metab. Syndr. Relat. Disord. 2010, 8, 295–305. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Wu, C.-Y.; Shen, J.-L.; Chu, S.-Y.; Chen, C.-K.; Chang, Y.-T.; Chen, C.-M. Psoriasis independently associated with hyperleptinemia contributing to metabolic syndrome. Arch. Dermatol. 2008, 144, 1571–1575. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Fracanzani, A.L.; Fargion, S.; Valenti, L. Iron in fatty liver and in the metabolic syndrome: A promising therapeutic target. J. Hepatol. 2011, 55, 920–932. [Google Scholar] [CrossRef]
- Britton, L.J.; Subramaniam, V.N.; Crawford, D.H. Iron and non-alcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 8112–8122. [Google Scholar] [CrossRef]
- Deltreggia Benites, B.; Olenscki Gilli, S.C.; Olalla Saad, S.T. Obesity and inflammation and the effect on the hematopoietic system. Rev. Bras. Hematol. Hemoter. 2014, 36, 147–151. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; Stroes, E.S.G.; Meijers, J.C.M.; Büller, H. Hypercoagulability in the metabolic syndrome. Curr. Opin. Pharmacol. 2005, 5, 155–159. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.; Arab, N.T.T.; Greenwood, M.T. Iron mediated toxicity and programmed cell death: A review and a re-examination of existing paradigms. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 399–430. [Google Scholar] [CrossRef]
- Miao, X.; Sun, W.; Fu, Y.; Miao, L.; Cai, L. Zinc homeostasis in the metabolic syndrome and diabetes. Front. Med. 2013, 7, 31–52. [Google Scholar] [CrossRef]
- Hashemipour, M.; Kelishadi, R.; Shapouri, J.; Sarrafzadegan, N.; Amini, M.; Tavakoli, N.; Movahedian-Attar, A.; Mirmoghtadaee, P.; Poursafa, P. Effect of zinc supplementation on insulin resistance and components of the metabolic syndrome in prepubertal obese children. Hormones 2009, 8, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef] [PubMed]
- Schiliro, C.; Firestein, B.L. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef]
- Mitchell, M.I.; Engelbrecht, A.-M. Metabolic hijacking: A survival strategy cancer cells exploit? Crit. Rev. Oncol. Hematol. 2017, 109, 1–8. [Google Scholar] [CrossRef]
- Pinto de Oliveira, M.; Liesa, M. The role of mitochondrial fat oxidation in cancer cell proliferation and survival. Cells 2020, 9, 2600. [Google Scholar] [CrossRef]
- Zhelev, Z.; Aoki, I.; Lazarova, D.; Vlaykova, T.; Higashi, T.; Bakalova, R. A “weird” mitochondrial fatty acid oxidation as a metabolic “secret” of cancer. Oxidative Med. Cell. Longev. 2022, 2022, 2339584. [Google Scholar] [CrossRef]
- Saha, T.; Dash, C.; Jayabalan, R.; Khiste, S.; Kulkarni, A.; Kurmi, K.; Mondal, J.; Majumder, P.K.; Bardia, A.; Jang, H.L.; et al. Intercellular nanotubes mediate mitochondrial trafficking between cancer and immune cells. Nat. Nanotechnol. 2022, 17, 98–106. [Google Scholar] [CrossRef]
- Zoellner, H.; Chami, B.; Kelly, E.; Moore, M.A.S. Increased cell size, structural complexity and migration of cancer cells acquiring fibroblast organelles by cell-projection pumping. PLoS ONE 2019, 14, e0224800. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sahra, I.; Puissant, A. HER2 signaling hijacks the creatine shuttle to fuel breast cancer cell growth. Cell Metab. 2018, 28, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Sazeides, C.; Le, A. Metabolic relationship between cancer-associated fibroblasts and cancer cells. Adv. Exp. Med. Biol. 2018, 1063, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Verginadis, I.I.; Avgousti, H.; Monslow, J.; Skoufos, G.; Chinga, F.; Kim, K.; Leli, N.M.; Karagounis, I.V.; Bell, B.I.; Velalopoulou, A.; et al. A stromal integrated stress response activates perivascular cancer-associated fibroblasts to drive angiogenesis and tumour progression. Nat. Cell Biol. 2022, 24, 940–953. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Liu, F. Cancer-associated fibroblasts: Versatile mediators in remodeling the tumor microenvironment. Cell. Signal. 2023, 103, 110567. [Google Scholar] [CrossRef]
- Tan, J.; Buache, E.; Chenard, M.-P.; Dali-Youcef, N.; Rio, M.-C. Adipocyte is a non-trivial, dynamic partner of breast cancer cells. Int. J. Dev. Biol. 2011, 55, 851–859. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, W.; Sheng, T.; He, X.; Xiong, X. Overview of the molecular mechanisms contributing to the formation of cancer-associated adipocytes. Mol. Med. Rep. 2021, 24, 768. [Google Scholar] [CrossRef]
- Zhou, C.; He, X.; Tong, C.; Li, H.; Xie, C.; Wu, Y.; Wang, L.; Yan, X.; Luo, D.; Tang, Y.; et al. Cancer-associated adipocytes promote the invasion and metastasis in breast cancer through LIF/CXCLs positive feedback loop. Int. J. Biol. Sci. 2022, 18, 1363–1380. [Google Scholar] [CrossRef]
- Cantini, G.; di Franco, A.; Mannelli, M.; Scime, A.; Maggi, M.; Luconi, M. The role of metabolic changes in shaping the fate of cancer-associated adipose stem cells. Front. Cell Dev. Biol. 2020, 8, 332. [Google Scholar] [CrossRef]
- Xue, C.; Gao, Y.; Li, X.; Zhang, M.; Yang, Y.; Han, Q.; Sun, Z.; Bai, C.; Zhao, R.C. Mesenchymal stem cells derived from adipose accelerate the progression of colon cancer by inducing a MT-CAFs phenotype via TRPC3/NF-κB axis. Stem Cell Res. Ther. 2022, 13, 335. [Google Scholar] [CrossRef]
- Chan, Y.W.; So, C.; Yau, K.L.; Chiu, K.C.; Wang, X.; Chan, F.L.; Tsang, S.Y. Adipose-derived stem cells and cancer cells fuse to generate cancer stem cell-like cells with increased tumorigenicity. J. Cell. Physiol. 2020, 235, 6794–6807. [Google Scholar] [CrossRef]
- Guan, H.; Peng, R.; Fang, F.; Mao, L.; Chen, Z.; Yang, S.; Dai, C.; Wu, H.; Wang, C.; Feng, N.; et al. Tumor-associated macrophages promote prostate cancer progression via exosome-mediated miR-95 transfer. J. Cell. Physiol. 2020, 235, 9729–9742. [Google Scholar] [CrossRef] [PubMed]
- Balta, E.; Wabnitz, G.H.; Samstag, Y. Hijacked immune cells in the tumor microenvironment: Molecular mechanisms of immunosuppression and cues to improve T cell-based immunotherapy of solid tumors. Int. J. Mol. Sci. 2021, 22, 5736. [Google Scholar] [CrossRef]
- Thaker, S.K.; Ch’ng, J.; Christofk, H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019, 17, e59. [Google Scholar] [CrossRef] [PubMed]
- Viscogliosi, G.; Donfrancesco, C.; Palmieri, L.; Giampaoli, S. The metabolic syndrome and 10-year cognitive and functional decline in very old men. A population-based study. Arch. Gerontol. Geriatr. 2017, 70, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, S.M.; Jeong, D.H.; Lee, S.-K.; Ahn, M.-E.; Ryu, O.-H. Associations between metabolic syndrome and type of dementia: Analysis based on the National Health Insurance Service database of Gangwon province in South Korea. Diabetol. Metab. Syndr. 2021, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.B.; Zagler, A.; Engl, K.; Rihs, L.; Musil, R. Prevalence of obesity, metabolic syndrome, diabetes and risk of cardiovascular disease in a psychiatric inpatient sample: Results of the Metabolism in Psychiatry (MiP) Study. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Wang, Q.; Liu, J.; Wang, L.; Chen, Z.; Pang, H.; Tu, J.; Ning, X.; Wang, J.; Fei, S. Relationship between metabolic syndrome and cognitive function: A population-based study of middle-aged and elderly adults in rural China. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Dib Bechara, M.; Portero Sloan, K.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes-Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef] [PubMed]
- Vega, G.L. Obesity, the metabolic syndrome, and cardiovascular disease. Am. Heart J. 2001, 142, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.F.; d’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Artham, S.M.; Lavie, C.J.; Milani, R.V.; Ventura, H.O. The obesity paradox: Impact of obesity on the prevalence and prognosis of cardiovascular diseases. Postgrad. Med. 2008, 120, 34–41. [Google Scholar] [CrossRef] [PubMed]
- McAuley, P.A.; Beavers, K.M. Contribution of cardiorespiratory fitness to the obesity paradox. Prog. Cardiovasc. Dis. 2014, 56, 434–440. [Google Scholar] [CrossRef]
- Cheung, B.M.Y. Hypertension as part of the metabolic syndrome. J. Hum. Hypertens. 2008, 22, 871–874. [Google Scholar] [CrossRef]
- Mulè, G.; Nardi, E.; Cottone, S.; Cusimano, P.; Volpe, V.; Piazza, G.; Mongiovì, R.; Mezzatesta, G.; Andronico, G.; Cerasola, G. Influence of metabolic syndrome on hypertension-related target organ damage. J. Intern. Med. 2005, 257, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.C.; Canani, L.H.; Schvartzman, P.; Gross, J.L. Hypertension is the metabolic syndrome component most strongly associated with microvascular complications and coronary artery calcification in type 1 diabetes. J. Endocrinol. Investig. 2011, 34, E58–E63. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Barone, S.; Luo, H.; Zahedi, K. Pathogenesis of hypertension in metabolic syndrome: The role of fructose and salt. Int. J. Mol. Sci. 2023, 24, 4294. [Google Scholar] [CrossRef]
- Gustafson, B.; Hammarstedt, A.; Andersson, C.X.; Smith, U. Inflamed adipose tissue. A culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2276–2283. [Google Scholar] [CrossRef]
- Luna-Luna, M.; Medina-Urrutia, A.; Vargas-Alarcón, G.; Coss-Rovirosa, F.; Vargas-Barrón, J.; Pérez-Méndez, Ó. Adipose tissue in metabolic syndrome: Onset and progression of atherosclerosis. Arch. Med. Res. 2015, 46, 392–407. [Google Scholar] [CrossRef]
- Kawamoto, R.; Tomita, H.; Oka, Y.; Kodama, A. Metabolic syndrome as a predictor of ischemic stroke in elderly persons. Intern. Med. 2005, 44, 922–927. [Google Scholar] [CrossRef]
- Moghadam-Ahmadi, A.; Soltani, N.; Ayoobi, F.; Jamali, Z.; Sadeghi, T.; Jalali, N.; Vakilian, A.; Lotfi, M.A.; Khalili, P. Association between metabolic syndrome and stroke: A population based cohort study. BMC Endocr. Disord. 2023, 23, e131. [Google Scholar] [CrossRef]
- Ahn, H.-J.; Han, K.-D.; Choi, E.-K.; Jung, J.-H.; Kwon, S.; Lee, S.-R.; Oh, S.; Lip, G.Y.H. Cumulative burden of metabolic syndrome and its components on the risk of atrial fibrillation: A nationwide population-based study. Cardiovasc. Diabetol. 2021, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Umetani, K.; Kodama, Y.; Nakamura, T.; Mende, A.; Kitta, Y.; Kawabata, K.; Obata, J.-E.; Takano, H.; Kugiyama, K. High prevalence of paroxysmal atrial fibrillation and/or atrial flutter in metabolic syndrome. Circ. J. 2007, 71, 252–255. [Google Scholar] [CrossRef]
- Rana, J.S.; Nieuwdorp, M.; Jukema, J.W.; Kastelein, J.J.P. Cardiovascular metabolic syndrome—An interplay of, obesity, inflammation, diabetes and coronary heart disease. Diabetes Obes. Metab. 2007, 9, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Turhan, H.; Erbay, A.R.; Yasar, A.S.; Bicer, A.; Sasmaz, H.; Yetkin, E. Impaired coronary blood flow in patients with metabolic syndrome: Documented by Thrombolysis in Myocardial Infarction (TIMI) frame count method. Am. Heart J. 2004, 148, 789–794. [Google Scholar] [CrossRef]
- Bugger, H.; Abel, E.D. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin. Sci. 2008, 114, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ren, Y.; Guo, Y.-K.; Liu, X.; Xie, L.-J.; Jiang, L.; Shen, M.-T.; Deng, M.-Y.; Yang, Z.-G. Metabolic syndrome and myocardium steatosis in subclinical type 2 diabetes mellitus: A 1H-magnetic resonance spectroscopy study. Cardiovasc. Diabetol. 2020, 19, 70. [Google Scholar] [CrossRef]
- Cheong, K.C.; Lim, K.H.; Ghazali, S.M.; Teh, C.H.; Cheah, Y.K.; Baharudin, A.; Lim, H.L.; Abdul Hamid, A.M.; Mustapha, F.I.; Omar, M.A. Association of metabolic syndrome with risk of cardiovascular disease mortality and all-cause mortality among Malaysian adults: A retrospective cohort study. BMJ Open 2021, 11, e047849. [Google Scholar] [CrossRef]
- Haring, R.; Wallaschofski, H.; Nauck, M.; Felix, S.B.; Schmidt, C.O.; Dörr, M.; Sauer, S.; Wilmking, G.; Völzke, H. Total and cardiovascular disease mortality predicted by metabolic syndrome is inferior relative to its components. Exp. Clin. Endocrinol. Diabetes 2010, 118, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Khadra, D.; Itani, L.; Chebaro, Y.; Obeid, M.; Jaber, M.; Ghanem, R.; Ayton, A.; Kreidieh, D.; Masri, D.E.; Kimura, A.; et al. Association between sarcopenic obesity and metabolic syndrome in adults: A systematic review and meta-analysis. Curr. Cardiol. Rev. 2020, 16, 153–162. [Google Scholar] [CrossRef]
- Kim, S.H.; Jeong, J.B.; Kang, J.; Ahn, D.-W.; Kim, J.W.; Kim, B.G.; Lee, K.L.; Oh, S.; Yoon, S.H.; Park, S.J.; et al. Association between sarcopenia level and metabolic syndrome. PLoS ONE 2021, 16, e0248856. [Google Scholar] [CrossRef]
- Mancia, G.; Bombelli, M.; Facchetti, R.; Casati, A.; Ronchi, I.; Quarti-Trevano, F.; Arenare, F.; Grassi, G.; Sega, R. Impact of different definitions of the metabolic syndrome on the prevalence of organ damage, cardiometabolic risk and cardiovascular events. J. Hypertens. 2010, 28, 999–1006. [Google Scholar] [CrossRef]
- Sciarretta, S.; Ferrucci, A.; Ciavarella, G.M.; de Paolis, P.; Venturelli, V.; Tocci, G.; de Biase, L.; Rubattu, S.; Volpe, M. Markers of inflammation and fibrosis are related to cardiovascular damage in hypertensive patients with metabolic syndrome. Am. J. Hypertens. 2007, 20, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Novo, G.; Corrado, E.; Muratori, I.; Tantillo, R.; Bellia, A.; Galluzzo, A.; Vitale, G.; Novo, S. Markers of inflammation and prevalence of vascular disease in patients with metabolic syndrome. Int. Angiol. 2007, 26, 312–317. [Google Scholar]
- Thomas, G.N.; Schooling, C.M.; McGhee, S.M.; Ho, S.-Y.; Cheung, B.M.Y.; Wat, N.M.S.; Janus, E.D.; Lam, K.S.L.; Lam, T.H. Metabolic syndrome increases all-cause and vascular mortality: The Hong Kong Cardiovascular Risk Factor Study. Clin. Endocrinol. 2007, 66, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-J.; Ha, G.-C.; Ko, K.-J. Association between resting heart rate, metabolic syndrome and cardiorespiratory fitness in Korean male adults. J. Exerc. Sci. Fit. 2017, 15, 27–31. [Google Scholar] [CrossRef]
- Wang, S.C.; Meng, D.; Yang, H.; Wang, X.; Jia, S.; Wang, P.; Wang, Y.-F. Pathological basis of cardiac arrhythmias: Vicious cycle ofimmune-metabolic dysregulation. Cardiovasc. Disord. Med. 2018, 3, 158. [Google Scholar] [CrossRef]
- Kraja, A.T.; Province, M.A.; Arnett, D.; Wagenknecht, L.E.; Tang, W.; Hopkins, P.N.; Djoussé, L.; Borecki, I.B. Do inflammation and procoagulation biomarkers contribute to the metabolic syndrome cluster? Nutr. Metab. 2007, 4, 28. [Google Scholar] [CrossRef]
- Mertens, I.; Verrijken, A.; Michiels, J.J.; van der Planken, M.; Ruige, J.B.; van Gaal, L.F. Among inflammation and coagulation markers, PAI-1 is a true component of the metabolic syndrome. Int. J. Obes. 2006, 30, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Tan, W.; Pan, X.; Tian, E.; Wu, Z.; Yang, J. Metabolic syndrome-related kidney injury: A review and update. Front. Endocrinol. 2022, 13, 904001. [Google Scholar] [CrossRef] [PubMed]
- Prasad, G.V.R. Metabolic syndrome and chronic kidney disease: Current status and future directions. World J. Nephrol. 2014, 3, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.K.; Charles, L.E.; Allison, P.; Violanti, J.M.; Andrew, M.E. Association between the metabolic syndrome and retinal microvascular diameters among police officers. J. Occup. Environ. Med. 2022, 64, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Mbata, O.; Abo El-Magd, N.F.; El-Remessy, A.B. Obesity, metabolic syndrome and diabetic retinopathy: Beyond hyperglycemia. World J. Diabetes 2017, 8, 317–329. [Google Scholar] [CrossRef]
- Alemany, M. Regulation of adipose tissue energy availability through blood flow control in the metabolic syndrome. Free Radic. Biol. Med. 2012, 52, 2108–2119. [Google Scholar] [CrossRef]
- Camastra, S.; Ferrannini, E. Role of anatomical location, cellular phenotype and perfusion of adipose tissue in intermediary metabolism: A narrative review. Rev. Endocr. Metab. Disord. 2022, 23, 43–50. [Google Scholar] [CrossRef]
- Denson, J.L.; Gillet, A.S.; Zu, Y.; Brown, M.; Pham, T.; Yoshida, Y.; Mauvais-Jarvis, F.; Douglas, I.S.; Moore, M.; Tea, K.; et al. Metabolic syndrome and acute respiratory distress syndrome in hospitalized patients with COVID-19. JAMA Netw. Open 2021, 4, e2140568. [Google Scholar] [CrossRef]
- Willson, C.; Watanabe, M.; Tsuji-Hosokawa, A.; Makino, A. Pulmonary vascular dysfunction in metabolic syndrome. J. Physiol. 2019, 597, 1121–1141. [Google Scholar] [CrossRef]
- Chen, W.-L.; Wang, C.-C.; Wu, L.-W.; Kao, T.-W.; Chan, J.Y.-H.; Chen, Y.-J.; Yang, Y.-H.; Chang, Y.-W.; Peng, T.-C. Relationship between lung function and metabolic syndrome. PLoS ONE 2014, 9, e108989. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.-N.; Wang, Z.-H.; Li, S.-H.; Ge, Y.-Y.; Liu, X.; Sui, D.-X. Pulmonary function in metabolic syndrome: A meta-analysis. Metab. Syndr. Relat. Disord. 2022, 20, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.; Dixon, A.E.; Marion, S.; Schaefer, C.; Zhang, Y.; Best, L.G.; Calhoun, D.; Rhoades, E.R.; Lee, E.T. Obesity in adults is associated with reduced lung function in metabolic syndrome and diabetes. The Strong Heart Study. Diabetes Care 2011, 34, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P. Obesity and lung inflammation. J. Appl. Physiol. 2010, 108, 722–728. [Google Scholar] [CrossRef]
- Ghatas, T. The relationship between metabolic syndrome and chronic obstructive pulmonary disease. Egypt. J. Bronchol. 2017, 11, 11–15. [Google Scholar] [CrossRef]
- Karamzad, N.; Izadi, N.; Sanaie, S.; Ahmadian, E.; Eftekhari, A.; Sullman, M.J.M.; Safiri, S. Asthma and metabolic syndrome: A comprehensive systematic review and meta-analysis of observational studies. J. Cardiovasc. Thorac. Res. 2020, 12, 120–128. [Google Scholar] [CrossRef]
- Aziz, R.; Sherwani, A.Y.; Al Mahri, S.; Malik, S.S.; Mohammad, S. Why are obese people predisposed to severe disease in viral respiratory infections? Obesities 2023, 3, 46–58. [Google Scholar] [CrossRef]
- Geerling, E.; Hameed, M.; Weger-Lucarelli, J.; Pinto, A.K. Metabolic syndrome and aberrant immune responses to viral infection and vaccination: Insights from small animal models. Front. Immunol. 2022, 13, 1015563. [Google Scholar] [CrossRef]
- Uzunlulu, M.; Telci Caklili, O.; Oguz, A. Association between metabolic syndrome and cancer. Ann. Nutr. Metab. 2016, 68, 173–179. [Google Scholar] [CrossRef]
- Schwartz, R.A. Acanthosis nigricans. J. Am. Acad. Dermatol. 1994, 31, 1–19. [Google Scholar] [CrossRef]
- Kahn, C.R.; Flier, J.S.; Bar, R.S.; Archer, J.A.; Gorden, P.; Martin, M.M.; Roth, J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N. Engl. J. Med. 1976, 294, 739–745. [Google Scholar] [CrossRef]
- Marchand, L.; Gaimard, M.; Luyton, C. All about skin manifestations of insulin resistance and type 2 diabetes: Acanthosis nigricans and acrochordons. Postgrad. Med. J. 2020, 96, 237. [Google Scholar] [CrossRef]
- Gami, A.S.; Caples, S.M.; Somers, V.K. Obesity and obstructive sleep apnea. Endocrinol. Metab. Clin. N. Am. 2003, 32, 869–894. [Google Scholar] [CrossRef]
- Kim, T.; Kang, J. Relationship between obstructive sleep apnea, insulin resistance, and metabolic syndrome: A nationwide population-based survey. Endocr. J. 2023, 70, 107–119. [Google Scholar] [CrossRef]
- Lin, Q.-C.; Zhang, X.-B.; Chen, G.-P.; Huang, D.-Y.; Din, H.-B.; Tang, A.-Z. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome in nonobese adults. Sleep Breath. 2012, 16, 571–578. [Google Scholar] [CrossRef]
- Ip, M.S.M.; Lam, B.; Ng, M.M.T.; Lam, W.K.; Tsang, K.W.T.; Lam, K.S.L. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med. 2002, 165, 670–676. [Google Scholar] [CrossRef]
- Morgenstern, M.; Wang, J.; Beatty, N.; Batemarco, T.; Sica, A.L.; Greenberg, H. Obstructive sleep apnea. An unexpected cause of insulin resistance and diabetes. Endocrinol. Metab. Clin. N. Am. 2014, 43, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Onat, A.; Hergenc, G.; Uyarel, H.; Yazici, M.; Tuncer, M.; Dogan, Y.; Can, G.; Rasche, K. Obstructive sleep apnea syndrome is associated with metabolic syndrome rather than insulin resistance. Sleep Breath. 2007, 11, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.W.; Tamisier, R.; Liu, Y. Sympathoexcitation and arterial hypertension associated with obstructive sleep apnea and cyclic intermittent hypoxia. J. Appl. Physiol. 2015, 119, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Ambrosetti, M.; Lucioni, A.M.; Conti, S.; Pedretti, R.F.E.; Neri, M. Metabolic syndrome in obstructive sleep apnea and related cardiovascular risk. J. Cardiovasc. Med. 2006, 7, 826–829. [Google Scholar] [CrossRef]
- Liu, L.; Su, X.; Zhao, Z.; Han, J.; Li, J.; Xu, W.; He, Z.; Gao, Y.; Chen, K.; Zhao, L.; et al. Association of metabolic syndrome with long-term cardiovascular risks and all-cause mortality in elderly patients with obstructive sleep apnea. Front. Cardiovasc. Med. 2022, 8, 813280. [Google Scholar] [CrossRef] [PubMed]
- Liew, R. Obstructive sleep apnea, obesity, and atrial fibrillation-What is the mechanistic link? Heart Rhythm 2012, 9, 1417–1418. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Hodge, D.O.; Herges, R.M.; Olson, E.J.; Nykodym, J.; Kara, T.; Somers, V.K. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J. Am. Coll. Cardiol. 2007, 49, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Limberg, J.K.; Morgan, B.J.; Sebranek, J.J.; Proctor, L.T.; Walker, B.J.; Eldridge, M.W.; Schrage, W.G. Altered neurovascular control of the resting circulation in human metabolic syndrome. J. Physiol. 2012, 590, 6109–6119. [Google Scholar] [CrossRef]
- Wiernsperger, N.; Nivoit, P.; Kraemer de Aguiar, L.G.; Bouskela, E. Microcirculation and the metabolic syndrome. Microcirculation 2007, 14, 403–438. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, X.; Luo, W.; Jiang, B.; Lin, Q.; Tang, M.; Li, X.; Xie, L. The causal association between obesity and primary open-angle glaucoma: A two-sample Mendelian randomization study. Front. Genet. 2022, 13, 835524. [Google Scholar] [CrossRef]
- Tesauro, M.; Cardillo, C. Obesity, blood vessels and metabolic syndrome. Acta Physiol. 2011, 203, 279–286. [Google Scholar] [CrossRef]
- Assad, T.R.; Hemnes, A.R. Metabolic dysfunction in pulmonary arterial hypertension. Curr. Hypertens. Rep. 2015, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, J.M.; Davis, K.E.; Scherer, P.E. Mechanisms of obesity and related pathologies: The macro- and microcirculation of adipose tissue. FEBS J. 2009, 276, 5738–5746. [Google Scholar] [CrossRef]
- Forhan, M.; Gill, S.V. Obesity, functional mobility and quality of life. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 129–137. [Google Scholar] [CrossRef]
- Houston, D.K.; Ding, J.; Nicklas, B.J.; Harris, T.B.; Lee, J.S.; Nevitt, M.C.; Rubin, S.M.; Tylavsky, F.A.; Kritchevsky, S.B. Overweight and obesity over the adult life course and incident mobility limitation in older adults. Am. J. Epidemiol. 2009, 169, 927–936. [Google Scholar] [CrossRef]
- Fezeu, L.; Bankir, L.; Hansel, B.; Guerrot, D. Differential circadian pattern of water and Na excretion rates in the metabolic syndrome. Chronobiol. Int. 2014, 31, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Shapiro, S.A.; Bradsell, H.; Frank, R.M. The essential roles of human adipose tissue: Metabolic, thermoregulatory, cellular, and paracrine effects. J. Cartil. Jt. Preserv. 2021, 1, 100023. [Google Scholar] [CrossRef]
- Speakman, J.R. Obesity and thermoregulation. Handb. Clin. Neurol. 2018, 156, 431–443. [Google Scholar] [CrossRef]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef]
- Santos-Marcos, J.A.; Perez-Jimenez, F.; Camargo, A. The role of diet and intestinal microbiota in the development of metabolic syndrome. J. Nutr. Biochem. 2019, 70, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Zwartjes, M.S.Z.; Gerdes, V.E.A.; Nieuwdorp, M. The role of gut microbiota and its produced metabolites in obesity, dyslipidemia, adipocyte dysfunction, and its interventions. Metabolites 2021, 11, 531. [Google Scholar] [CrossRef]
- Duarte, L.; Gasaly, N.; Poblete-Aro, C.; Uribe, D.; Echeverria, F.; Gotteland, M.; Garcia-Diaz, D.F. Polyphenols and their anti-obesity role mediated by the gut microbiota: A comprehensive review. Rev. Endocr. Metab. Disord. 2021, 22, 367–388. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kameyama, K.; Miyauchi, E.; Nakanishi, Y.; Kanaya, T.; Fujii, T.; Kato, T.; Sasaki, T.; Tachibana, N.; Negishi, H.; et al. Fatty acid overproduction by gut commensal microbiota exacerbates obesity. Cell Metab. 2023, 35, 361–375. [Google Scholar] [CrossRef]
- Du, L.; Lei, X.; Wang, J.; Wang, L.; Zhong, Q.; Fang, X.; Li, P.; Du, B.; Wang, Y.; Liao, Z. Lipopolysaccharides derived from Gram-negative bacterial pool of human gut microbiota promote inflammation and obesity development. Int. Rev. Immunol. 2022, 41, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Droz, N.; Vidal, P.M. Selling dreams: An overview of slimming products’ advertisements in Switzerland. Obes. Facts 2014, 7, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. Neglecting major health problems and broadcasting minor, uncertain issues in lifestyle science. J. Am. Med. Assoc. 2019, 322, 2069–2070. [Google Scholar] [CrossRef]
- Lesser, L.I.; Ebbeling, C.B.; Goozner, M.; Wypij, D.; Ludwig, D.S. Relationship between funding source and conclusion among nutrition-related scientific articles. PLoS Med. 2007, 4, e5. [Google Scholar] [CrossRef] [PubMed]
- Nestle, M. Corporate funding of nutrition research. Science or Marketing? JAMA Intern. Med. 2016, 176, 13–14. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Bero, L.A. Corporate manipulation of research: Strategies are similar across five industries. Stanf. Law Policy Rev. 2010, 21, 105–133. [Google Scholar]
- Kearns, C.E.; Glantz, S.A.; Schmidt, L.A. Sugar Industry influence on the scientific agenda of the National Institute of Dental Research’s 1971 National Caries Program: A historical analysis of internal documents. PLoS Med. 2015, 12, e1001798. [Google Scholar] [CrossRef] [PubMed]
- Marzoog, B.A. Recent advances in molecular biology of metabolic syndrome pathophysiology: Endothelial dysfunction as a potential therapeutic target. J. Diabetes Metab. Disord. 2022, 21, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Marsland, A.L.; McCaffery, J.M.; Muldoon, M.F.; Manuck, S.B. Systemic inflammation and the metabolic syndrome among middle-aged community volunteers. Metab. Clin. Exp. 2010, 59, 1801–1808. [Google Scholar] [CrossRef]
- Celsus, A.C.; Serbat, G. De la Médecine; Les Belles Lettres: Paris, France, 1995. [Google Scholar]
- Scott, A.; Khan, K.M.; Cook, J.L.; Duronio, V. What is “inflammation”? Are we ready to move beyond Celsus? Br. J. Sports Med. 2004, 38, 248–249. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Kanneganti, T.-D. The inflammasome: A remote control for metabolic syndrome. Cell Res. 2012, 22, 1095–1098. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Battaglia-Hsu, S.-F.; Arnold, C. Endoplasmic reticulum stress in metabolic disorders. Cells 2018, 7, 63. [Google Scholar] [CrossRef]
- Kirilmaz, B.; Asgun, F.; Alioglu, E.; Ercan, E.; Tengiz, I.; Turk, U.; Saygi, S.; Ozerkan, F. High inflammatory activity related to the number of metabolic syndrome components. J. Clin. Hypertens. 2010, 12, 136–144. [Google Scholar] [CrossRef]
- Scarpellini, E.; Tack, J. Obesity and metabolic syndrome: An inflammatory condition. Dig. Dis. 2012, 30, 148–153. [Google Scholar] [CrossRef]
- Duncan, B.B.; Schmidt, M.I. Chronic activation of the innate immune system may underlie the metabolic syndrome. São Paulo Med. J. 2001, 119, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.H. A modern view of the interstitial space in health and disease. Front. Vet. Sci. 2020, 7, e609583. [Google Scholar] [CrossRef] [PubMed]
- Wiig, H.; Swartz, M.A. Interstitial fluid and lymph formation and transport: Physiological regulation and roles in inflammation and cancer. Physiol. Rev. 2012, 92, 1005–1060. [Google Scholar] [CrossRef] [PubMed]
- Baish, J.W.; Padera, T.P.; Munn, L.L. The effects of gravity and compression on interstitial fluid transport in the lower limb. Sci. Rep. 2022, 12, 4890. [Google Scholar] [CrossRef] [PubMed]
- Maderna, P.; Godson, C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim. Biophys. Acta 2003, 1639, 141–151. [Google Scholar] [CrossRef]
- Cooke, J.P. Inflammation and its role in regeneration and repair. Circ. Res. 2019, 124, 1166–1168. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in tissue repair. In Inflammation: From Molecular and Cellular Mechanisms to the Clinic; Cavaillon, J.-M., Singer, M., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 1517–1538. [Google Scholar] [CrossRef]
- Schett, G.; Neurath, M.F. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef] [PubMed]
- Weaver, W. Molecular biology: Origin of the term. Science 1970, 170, 581–582. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, B.; Manco Urbina, P.A.; Diacci, C.; Borghi, S.; Pinti, M.; Cossarizza, A.; Salvarani, C.; Berggren, M.; Biscarini, F.; Simon, D.T.; et al. Sensing inflammation biomarkers with electrolyte-gated organic electronic transistors. Adv. Healthc. Mater. 2021, 10, 2100955. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Raska, M.; Konttinen, Y.T.; Nich, C.; Goodman, S.B. Innate immunity sensors participating in pathophysiology of joint diseases: A brief overview. J. Long-Term Eff. Med. Implant. 2014, 24, 297–317. [Google Scholar] [CrossRef]
- Beghdadi, W.; Madjène, L.; Benhamou, M.; Charles, N.; Gautier, G.; Launay, P.; Blank, U. Mast cells as cellular sensors in inflammation and immunity. Front. Immunol. 2011, 2, 37. [Google Scholar] [CrossRef]
- Conti, P.; Ronconi, G.; Kritas, S.K.; Caraffa, A.; Theoharides, T.C. Activated mast cells mediate low-grade inflammation in type 2 diabetes: Interleukin-37 could be beneficial. Can. J. Diabetes 2018, 42, 568–573. [Google Scholar] [CrossRef]
- Żelechowska, P.; Agier, J.; Kozłowska, E.; Brzezińska-Błaszczyk, E. Mast cells participate in chronic low-grade inflammation within adipose tissue. Obes. Rev. 2018, 19, 686–697. [Google Scholar] [CrossRef]
- MacGlashan, D. Histamine: A mediator of inflammation. J. Allergy Clin. Immunol. 2003, 112 (Suppl. S1), S53–S59. [Google Scholar] [CrossRef]
- Lago, F.; Diéguez, C.; Gómez-Reino, J.; Gualillo, O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007, 18, 313–325. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Bowker, N.; Shah, R.L.; Sharp, S.J.; Luan, J.A.; Stewart, I.D.; Wheeler, E.; Ferreira, M.A.R.; Baras, A.; Wareham, N.J.; Langenberg, C.; et al. Meta-analysis investigating the role of interleukin-6 mediated inflammation in type 2 diabetes. eBioMedicine 2020, 61, 103062. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am. J. Clin. Nutr. 2006, 83, 447S–455S. [Google Scholar] [CrossRef] [PubMed]
- Soto González, A.; Bellido Guerrero, D.; Buño Soto, M.; Pértega Díaz, S.; Martínez-Olmos, M.; Vidal, O. Metabolic syndrome, insulin resistance and the inflammation markers C-reactive protein and ferritin. Eur. J. Clin. Nutr. 2006, 60, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Tamakoshi, K.; Yatsuya, H.; Kondo, T.; Hori, Y.; Ishikawa, M.; Zhang, H.; Murata, C.; Otsuka, R.; Zhu, S.; Toyoshima, H. The metabolic syndrome is associated with elevated circulating C-reactive protein in healthy reference range, a systemic low-grade inflammatory state. Int. J. Obes. 2003, 27, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Potter, B.J.; Cao, J.-M.; Zhang, C. Interferon-gamma induced adipose tissue inflammation is linked to endothelial dysfunction in type 2 diabetic mice. Basic Res. Cardiol. 2011, 106, 1135–1145. [Google Scholar] [CrossRef]
- Williams, T.J.; Peck, M.J. Role of prostaglandin-mediated vasodilatation in inflammation. Nature 1977, 270, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Dada, L.A.; Sznajder, J.I. Mitochondrial Ca2+ and ROS take center stage to orchestrate TNF-a-mediated inflammatory responses. J. Clin. Investig. 2011, 121, 1683–1685. [Google Scholar] [CrossRef]
- Habib, A.; Finn, A.V. The role of iron metabolism as a mediator of macrophage inflammation and lipid handling in atherosclerosis. Front. Pharmacol. 2014, 5, 195. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.R.; Gao, D.; Pararasa, C. Redox regulation in metabolic programming and inflammation. Redox Biol. 2017, 12, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.T.; Singh, A.K.; Knaus, U.G. Redox regulator network in inflammatory signaling. Curr. Opin. Physiol. 2019, 9, 9–17. [Google Scholar] [CrossRef]
- Derkacz, A.; Olczyk, P.; Olczyk, K.; Komosinska-Vassev, K. The role of extracellular matrix components in inflammatory bowel diseases. J. Clin. Med. 2021, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Riley, H.J.; Bradshaw, A.D. The influence of the extracellular matrix in inflammation: Findings from the SPARC-null mouse. Anat. Rec. 2020, 303, 1624–1629. [Google Scholar] [CrossRef]
- Li, Q.; Hata, A.; Kosugi, C.; Kataoka, N.; Funaki, M. The density of extracellular matrix proteins regulates inflammation and insulin signaling in adipocytes. FEBS Lett. 2010, 584, 4145–4150. [Google Scholar] [CrossRef]
- Moretti, L.; Stalfort, J.; Harrison Barker, T.; Abebayehu, D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J. Biol. Chem. 2022, 298, 101530. [Google Scholar] [CrossRef]
- Grada, A.A.; Phillips, T.J. Lymphedema: Pathophysiology and clinical manifestations. J. Am. Acad. Dermatol. 2017, 77, 1009–1020. [Google Scholar] [CrossRef]
- Mortimer, P.S. The pathophysiology of lymphedema. Cancer 1998, 83 (Suppl. S12B), 2798–2802. [Google Scholar] [CrossRef]
- Eide, P.K.; Vatnehol, S.A.S.; Emblem, K.E.; Ringstad, G. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci. Rep. 2018, 8, 7194. [Google Scholar] [CrossRef]
- Lind-Holm Mogensen, F.; Delle, C.; Nedergaard, M. The glymphatic system (en)during inflammation. Int. J. Mol. Sci. 2021, 22, 7491. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, S.P.; Wen, T.; Zhou, H.N.; Hu, M.; Li, J.X. Postprandial hypertriglyceridemia associated with inflammatory response and procoagulant state after a high-fat meal in hypertensive patients. Coron. Artery Dis. 2008, 19, 145–151. [Google Scholar] [CrossRef]
- Pitsavos, C.; Panagiotakos, D.B.; Chrysohoou, C.; Kavouras, S.; Stefanadis, C. The associations between physical activity, inflammation, and coagulation markers, in people with metabolic syndrome: The ATTICA study. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 12, 151–158. [Google Scholar] [CrossRef]
- Granger, D.N.; Rodrigues, S.F. Microvascular responses to inflammation. In Compendium of Inflammatory Diseases; Parnham, M.J., Ed.; Springer: Basel, Switzerland, 2016; pp. 942–948. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Inflammation and the blood microvascular system. Cold Spring Harb. Perspect. Biol. 2014, 7, a016345. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.; Briet, M.; Empana, J.P.; Cunha, P.G.; Mäki-Petäjä, K.M.; Protogerou, A.D.; Tedgui, A.; Touyz, R.M.; Schiffrin, E.L.; Spronck, B.; et al. Vascular consequences of inflammation: A position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J. Hypertens. 2020, 38, 1682–1698. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.C.; Feng, R.N.; Hou, Y.; Li, K.; Kang, Z.; Wang, J.; Sun, C.H.; Li, Y. Histidine and arginine are associated with inflammation and oxidative stress in obese women. Br. J. Nutr. 2012, 108, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Kluger, M.J. Body temperature changes during inflammation: Their mediation and nutritional significance. Proc. Nutr. Soc. 1989, 48, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Michlewska, S.; Dransfield, I.; Megson, I.L.; Rossi, A.G. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: Key role for TNF-a. FASEB J. 2009, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Kuan, E.L.; Ivanov, S.; Bridenbaugh, E.A.; Victora, G.; Wang, W.; Childs, E.W.; Platt, A.M.; Jakubzick, C.V.; Mason, R.J.; Gashev, A.A.; et al. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J. Immunol. 2015, 194, 5200–5210. [Google Scholar] [CrossRef]
- Blomqvist, A.; Engblom, D. Neural mechanisms of inflammation-induced fever. Neuroscientist 2018, 24, 381–399. [Google Scholar] [CrossRef]
- Bashir, S.; Sharma, Y.; Elahi, A.; Khan, F. Amelioration of obesity-associated inflammation and insulin resistance in c57bl/6 mice via macrophage polarization by fish oil supplementation. J. Nutr. Biochem. 2016, 33, 82–90. [Google Scholar] [CrossRef]
- Xu, L.; Yan, X.; Zhao, Y.; Wang, J.; Liu, B.; Yu, S.; Fu, J.; Liu, Y.; Su, J. Macrophage polarization mediated by mitochondrial dysfunction induces adipose tissue inflammation in obesity. Int. J. Mol. Sci. 2022, 23, 9252. [Google Scholar] [CrossRef]
- Zhang, W.-C.; Qin, F.; Wang, X.-J.; Liu, Z.-F.; Zhu, L.; Zeng, A.; Zhang, M.-Z.; Yu, N.-Z.; Long, X. Adipose-derived stromal cells attenuate adipose inflammation in obesity through adipocyte browning and polarization of M2 macrophages. Mediat. Inflamm. 2019, 2019, 1731540. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Lu, Z.; Wang, B.; Li, L.; You, M.; Wang, L.; Cao, T.; Zhao, Y.; Li, Q.; et al. Mitochondrial dysfunction caused by SIRT3 inhibition drives proinflammatory macrophage polarization in obesity. Obesity 2023, 31, 1050–1063. [Google Scholar] [CrossRef]
- Chen, K.-H.E.; Lainez, N.M.; Coss, D. Sex differences in macrophage responses to obesity-mediated changes determine migratory and inflammatory traits. J. Immunol. 2021, 206, 141–153. [Google Scholar] [CrossRef]
- Kim, B.-S.; Rongisch, R.; Hager, S.; Grieb, G.; Nourbakhsh, M.; Rennekampff, H.-O.; Bucala, R.; Bernhagen, J.; Pallua, N. Macrophage migration inhibitory factor in acute adipose tissue inflammation. PLoS ONE 2015, 10, e0137366. [Google Scholar] [CrossRef] [PubMed]
- Velickovic, N.; Djordjevic, A.; Vasiljevic, A.; Bursac, B.; Milutinovic, D.V.; Matic, G. Tissue-specific regulation of inflammation by macrophage migration inhibitory factor and glucocorticoids in fructose-fed Wistar rats. Br. J. Nutr. 2013, 110, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Hagita, S.; Osaka, M.; Shimokado, K.; Yoshida, M. Adipose inflammation initiates recruitment of leukocytes to mouse femoral artery: Role of adipo-vascular axis in chronic inflammation. PLoS ONE 2011, 6, e19871. [Google Scholar] [CrossRef] [PubMed]
- Kitade, H.; Sawamoto, K.; Nagashimada, M.; Inoue, H.; Yamamoto, Y.; Sai, Y.; Takamura, T.; Yamamoto, H.; Miyamoto, K.-I.; Ginsberg, H.N.; et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes 2012, 61, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.; Inoue, W.; Poole, S.; Luheshi, G.N. Leptin regulates leukocyte recruitment into the brain following systemic LPS-induced inflammation. Mol. Psychiatry 2010, 15, 523–534. [Google Scholar] [CrossRef]
- Granger, D.N.; Kubes, P. The microcirculation and inflammation: Modulation of leukocyte-endothelial cell adhesion. J. Leukoc. Biol. 1994, 55, 662–675. [Google Scholar] [CrossRef]
- Griffin, G.K.; Newton, G.; Tarrio, M.L.; Bu, D.-X.; Maganto-Garcia, E.; Azcutia, V.; Alcaide, P.; Grabie, N.; Luscinskas, F.W.; Croce, K.J.; et al. IL-17 and TNF-a sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J. Immunol. 2012, 188, 6287–6299. [Google Scholar] [CrossRef]
- Söhnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017, 17, 248–261. [Google Scholar] [CrossRef]
- Khatib-Massalha, E.; Bhattacharya, S.; Massalha, H.; Biram, A.; Golan, K.; Kollet, O.; Kumari, A.; Avemaria, F.; Petrovich-Kopitman, E.; Gur-Cohen, S.; et al. Lactate released by inflammatory bone marrow neutrophils induces their mobilization via endothelial GPR81 signaling. Nat. Commun. 2020, 11, 3547. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Neutrophils actively contribute to obesity-associated inflammation and pathological complications. Cells 2022, 11, 1883. [Google Scholar] [CrossRef]
- Toussirot, É. Adipocytokines: Des acteurs del’inflammation dans les rhumatismes inflammatoires chroniques et les maladies auto-immunes? Presse Médicale 2013, 42, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wieser, V.; Moschen, A.R.; Tilg, H. Inflammation, cytokines and insulin resistance: A clinical perspective. Arch. Immunol. Ther. Exp. 2013, 61, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Herbst, K.L.; Calof, O.M.; Hsia, S.H.; Sinha-Hikim, I.; Woodhouse, L.J.; Buchanan, T.A.; Bhasin, S. Effects of transdermal testosterone administration on insulin sensitivity, fat mass and distribution, and markers of inflammation and thrombolysis in human immunodeficiency virus-infected women with mild to moderate weight loss. Fertil. Steril. 2006, 85, 1794–1802. [Google Scholar] [CrossRef]
- Steffens, J.P.; Herrera, B.S.; Coimbra, L.S.; Stephens, D.N.; Rossa, C.; Spolidorio, L.C.; Kantarci, A.; van Dyke, T.E. Testosterone regulates bone response to inflammation. Horm. Metab. Res. 2014, 46, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Adachi, A.; Honda, T.; Egawa, G.; Kanameishi, S.; Takimoto, R.; Miyake, T.; Hossain, M.R.; Komine, M.; Ohtsuki, M.; Gunzer, M.; et al. Estradiol suppresses psoriatic inflammation in mice by regulating neutrophil and macrophage functions. J. Allergy Clin. Immunol. 2022, 150, 909–919. [Google Scholar] [CrossRef]
- Son, H.J.; Kim, N.; Song, C.-H.; Lee, S.M.; Lee, H.-N.; Surh, Y.-J. 17b-Estradiol reduces inflammation and modulates antioxidant enzymes in colonic epithelial cells. Korean J. Intern. Med. 2020, 35, 310–319. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Critchley, H.O.D. Oestrogen and progesterone regulation of inflammatory processes in the human endometrium. J. Steroid Biochem. Mol. Biol. 2010, 120, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Freitas Alves, V.B.; Basso, P.J.; Nardini, V.; Silva, A.; Lazo Chica, J.E.; Ribeiro de Barros Cardoso, C. Dehydroepiandrosterone (DHEA) restrains intestinal inflammation by rendering leukocytes hyporesponsive and balancing colitogenic inflammatory responses. Immunobiology 2016, 221, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, M.; Wang, W.; Chen, H.; Wang, X.; Zhao, K.; Li, Z.; Xu, J.; Tong, F. Dehydroepiandrosterone inhibits vascular proliferation and inflammation by modulating the miR-486a-3p/NLRP3 axis. Am. J. Transl. Res. 2022, 14, 6123–6136. [Google Scholar] [PubMed]
- Feng, X.; Zhao, Y.; Yang, T.; Song, M.; Wang, C.; Yao, Y.; Fan, H. Glucocorticoid-driven NLRP3 inflammasome activation in hippocampal microglia mediates chronic stress-induced depressive-like behaviors. Front. Mol. Neurosci. 2019, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Sacta, M.A.; Tharmalingam, B.; Coppo, M.; Rollins, D.A.; Deochand, D.K.; Benjamin, B.; Yu, L.; Zhang, B.; Hu, X.; Li, R.; et al. Gene-specific mechanisms direct glucocorticoid-receptor-driven repression of inflammatory response genes in macrophages. eLife 2018, 7, e34864. [Google Scholar] [CrossRef] [PubMed]
- Busillo, J.M.; Cidlowski, J.A. The five Rs of glucocorticoid action during inflammation: Ready, reinforce, repress, resolve, and restore. Trends Endocrinol. Metab. 2013, 24, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tu, Y.; Chen, H.; Jackson, A.O.; Patel, V.; Yin, K. Micro-environment and intracellular metabolism modulation of adipose tissue macrophage polarization in relation to chronic inflammatory diseases. Diabetes-Metab. Res. Rev. 2018, 34, e2993. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Jung, B.C.; Kang, S. Epigenetic regulation of inflammatory factors in adipose tissue. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2021, 1866, 159019. [Google Scholar] [CrossRef]
- Kreutzer, C.; Peters, S.; Schulte, D.M.; Fangmann, D.; Tuerk, K.; Wolff, S.; van Eimeren, T.; Ahrens, M.; Beckmann, J.; Schafmayer, C.; et al. Hypothalamic inflammation in human obesity is mediated by environmental and genetic factors. Diabetes 2017, 66, 2407–2415. [Google Scholar] [CrossRef]
- Martinez, J.A.; Cordero, P.; Campion, J.; Milagro, F.I. Interplay of early-life nutritional programming on obesity, inflammation and epigenetic outcomes. Proc. Nutr. Soc. 2012, 71, 276–283. [Google Scholar] [CrossRef]
- Zatterale, F.; Raciti, G.A.; Prevenzano, I.; Leone, A.; Campitelli, M.; de Rosa, V.; Beguinot, F.; Parrillo, L. Epigenetic reprogramming of the inflammatory response in obesity and type 2 diabetes. Biomolecules 2022, 12, 982. [Google Scholar] [CrossRef]
- Michailidou, Z. Fundamental roles for hypoxia signalling in adipose tissue metabolism and inflammation in obesity. Curr. Opin. Physiol. 2019, 12, 39–43. [Google Scholar] [CrossRef]
- Postovalova, E.A.; Makarova, O.V.; Kosyreva, A.M.; Dzhalilova, D.S. Hypoxia is a key mechanism for regulating inflammation in ulcerative colitis. Russ. Open Med. J. 2020, 9, 101. [Google Scholar] [CrossRef]
- Andrei, A.M.; Berbecaru-Iovan, A.; Anghel, F.R.I.D.; Stănciulescu, C.E.; Berbecaru-Iovan, S.; Baniţă, I.M.; Pisoschi, C.G. Interplay between hypoxia, inflammation and adipocyte remodeling in the metabolic syndrome. In Hypoxia and Human Diseases; Zheng, J., Zhou, C., Eds.; InTech: Rijeka, Croatia, 2017; pp. 303–328. [Google Scholar] [CrossRef]
- Bouhamida, E.; Morciano, G.; Perrone, M.; Kahsay, A.E.; della Sala, M.; Wieckowski, M.R.; Fiorica, F.; Pinton, P.; Giorgi, C.; Patergnani, S. The interplay of hypoxia signaling on mitochondrial dysfunction and inflammation in cardiovascular diseases and cancer: From molecular mechanisms to therapeutic approaches. Biology 2022, 11, 300. [Google Scholar] [CrossRef]
- Murdoch, C.; Muthana, M.; Lewis, C.E. Hypoxia regulates macrophage functions in inflammation. J. Immunol. 2005, 175, 6257–6263. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T.; Doherty, G.; Fallon, P.G.; Cummins, E.P. Hypoxia-dependent regulation of inflammatory pathways in immune cells. J. Clin. Investig. 2016, 126, 3716–3724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, X.; Zhang, D.; Deng, B.; Tong, J.; Zhang, M.; Chen, L.; Duan, H.; Niu, W. Hypoxic adipocytes induce macrophages to release inflammatory cytokines that render skeletal muscle cells insulin resistant. Biochem. Biophys. Res. Commun. 2020, 521, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Starks, T. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef]
- Tamaru, T.; Hattori, M.; Ninomiya, Y.; Kawamura, G.; Vares, G.; Honda, K.; Mishra, D.P.; Wang, B.; Benjamin, I.; Sassone-Corsi, P.; et al. ROS stress resets circadian clocks to coordinate pro-survival signals. PLoS ONE 2013, 8, e82006. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.W.; Llanos, R.M.; La Fontaine, S.; Cater, M.A.; Beart, P.M.; Cheung, N.S. Comparative microarray analysis identifies commonalities in neuronal injury: Evidence for oxidative stress, dysfunction of calcium signalling, and inhibition of autophagy-lysosomal pathway. Neurochem. Res. 2016, 41, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Yu, B.P. Alterations in mitochondrial membrane fluidity by lipid peroxidation products. Free Radic. Biol. Med. 1994, 17, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Katz, A. Role of reactive oxygen species in regulation of glucose transport in skeletal muscle during exercise. J. Physiol. 2016, 594, 2787–2794. [Google Scholar] [CrossRef]
- Gronski, M.A.; Kinchen, J.M.; Juncadella, I.J.; Franc, N.C.; Ravichandran, K.S. An essential role for calcium flux in phagocytes for apoptotic cell engulfment and the anti-inflammatory response. Cell Death Differ. 2009, 16, 1323–1331. [Google Scholar] [CrossRef]
- Klein, G.L. The role of calcium in inflammation-associated bone resorption. Biomolecules 2018, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Maier, J.A.M.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef]
- Chowdhury, D.; Gardner, J.C.; Satpati, A.; Nookala, S.; Mukundan, S.; Porollo, A.; Landero Figueroa, J.A.; Subramanian Vignesh, K. Metallothionein 3-zinc axis suppresses caspase-11 inflammasome activation and impairs antibacterial immunity. Front. Immunol. 2021, 12, 755961. [Google Scholar] [CrossRef]
- Xu, R.; Chen, M.-Y.; Liang, W.; Chen, Y.; Guo, M.-Y. Zinc deficiency aggravation of ROS and inflammatory injury leading to renal fibrosis in mice. Biol. Trace Elem. Res. 2021, 199, 622–632. [Google Scholar] [CrossRef]
- Zhang, Q.; Xue, Y.; Fu, Y.; Bao, B.; Guo, M.-Y. Zinc deficiency aggravates oxidative stress leading to inflammation and fibrosis in lung of mice. Biol. Trace Elem. Res. 2021, 200, 4045–4057. [Google Scholar] [CrossRef]
- Vela, D. The dual role of hepcidin in brain iron load and inflammation. Front. Neurosci. 2018, 12, 740. [Google Scholar] [CrossRef]
- Barkas, F.; Liberopoulos, E.; Kei, A.; Elisaf, M. Electrolyte and acid-base disorders in inflammatory bowel disease. Ann. Gastroenterol. 2013, 26, 23–28. [Google Scholar]
- Eisenhut, M. Changes in ion transport in inflammatory disease. J. Inflamm. 2006, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The extracellular matrix in skin inflammation and infection. Front. Cell Dev. Biol. 2021, 9, 682414. [Google Scholar] [CrossRef] [PubMed]
- Rønningen, T.; Shah, A.; Reiner, A.H.; Collas, P.; Moskaug, J.Ø. Epigenetic priming of inflammatory response genes by high glucose in adipose progenitor cells. Biochem. Biophys. Res. Commun. 2015, 467, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Ghosh, A.; Bundschuh, N.; Hinte, L.; Petrosyan, E.; von Meyenn, F.; Bar-Nur, O. Integrative molecular roadmap for direct conversion of fibroblasts into myocytes and myogenic progenitor cells. Sci. Adv. 2022, 8, abj4928. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Hui, X.; Gao, X.; Nie, B.; Mao, L.; Tang, X.; Yuan, R.; Li, K.; Li, P.; Xu, A.; et al. Conversion of non-adipogenic fibroblasts into adipocytes by a defined hormone mixture. Biochem. J. 2015, 467, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Glastonbury, C.A.; Couto Alves, A.; El-Sayed Moustafa, J.S.; Small, K.S. Cell-type heterogeneity in adipose tissue is associated with complex traits and reveals disease-relevant cell-specific eQTLs. Am. J. Hum. Genet. 2019, 104, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Vijay, J.; Gauthier, M.-F.; Biswell, R.L.; Louiselle, D.A.; Johnston, J.J.; Cheung, W.A.; Belden, B.; Pramatarova, A.; Biertho, L.; Gibson, M.; et al. Single-cell analysis of human adipose tissue identifies depot- and disease-specific cell types. Nat. Metab. 2020, 2, 97–109. [Google Scholar] [CrossRef]
- Tálens-Visconti, R.; Bonora, A.; Jover, R.; Mirabet, V.; Carbonell, F.; Castell, J.-V.; Gómez-Lechón, M.J. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World J. Gastroenterol. 2006, 12, 5834–5845. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Posovszky, P.; Wang, Q.A.; Asterholm, I.W.; Rutkowski, J.M.; Scherer, P.E. Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology 2011, 152, 3074–3081. [Google Scholar] [CrossRef] [PubMed]
- Auger, C.; Kajimura, S. Adipose tissue remodeling in pathophysiology. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 71–93. [Google Scholar] [CrossRef]
- Horsley, V. Adipocyte plasticity in tissue regeneration, repair, and disease. Curr. Opin. Genet. Dev. 2022, 76, 101968. [Google Scholar] [CrossRef]
- Constantin-Teodosiu, D.; Constantin, D.; Pelsers, M.M.; Verdijk, L.B.; van Loon, L.; Greenhaff, P.L. Mitochondrial DNA copy number associates with insulin sensitivity and aerobic capacity, and differs between sedentary, overweight middle-aged males with and without type 2 diabetes. Int. J. Obes. 2020, 44, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.-Y.; Jang, J.E.; Lee, S.E.; Koh, E.H.; Lee, K.-U. Mitochondrial dysfunction in adipocytes as a primary cause of adipose tissue inflammation. Diabetes Metab. J. 2019, 43, 247–256. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Wilen, C.B.; Moley, J.R.; Li, Y.; Zou, W.; Malvin, N.P.; Rowen, M.N.; Saunders, B.T.; Ma, H.; Mack, M.R.; et al. Intercellular mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. Cell Metab. 2021, 33, 270–282. [Google Scholar] [CrossRef]
- Lecoutre, S.; Clément, K.; Dugail, I. Obesity-related adipose tissue remodeling in the light of extracellular mitochondria transfer. Int. J. Mol. Sci. 2022, 23, 632. [Google Scholar] [CrossRef]
- Nisoli, E.; Clementi, E.; Carruba, M.O.; Moncada, S. Defective mitochondrial biogenesis—A hallmark of the high cardiovascular risk in the metabolic syndrome? Circ. Res. 2007, 100, 795–806. [Google Scholar] [CrossRef]
- Zamora-Mendoza, R.; Rosas-Vargas, H.; Ramos-Cervantes, M.T.; García-Zúñiga, P.; Pérez-Lorenzana, H.; Mendoza-Lorenzo, P.; Pérez-Ortiz, A.C.; Estrada-Mena, F.J.; Miliar-García, A.; Lara-Padilla, E.; et al. Dysregulation of mitochondrial function and biogenesis modulators in adipose tissue of obese children. Int. J. Obes. 2018, 42, 618–624. [Google Scholar] [CrossRef]
- Cimini, F.A.; Barchetta, I.; Ciccarelli, G.; Leonetti, F.; Silecchia, G.; Chiappetta, C.; di Cristofano, C.; Capoccia, D.; Bertoccini, L.; Ceccarelli, V.; et al. Adipose tissue remodelling in obese subjects is a determinant of presence and severity of fatty liver disease. Diabetes-Metab. Res. Rev. 2021, 37, e3358. [Google Scholar] [CrossRef]
- Lee, M.-J.; Wu, Y.; Fried, S.K. Adipose tissue remodeling in pathophysiology of obesity. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 371–376. [Google Scholar] [CrossRef]
- Alipoor, E.; Hosseinzadeh-Attar, M.J.; Mahdavi-Mazdeh, M.; Yaseri, M.; Zahed, N.S. Comparison of malnutrition inflammation score, anthropometry and biochemical parameters in assessing the difference in protein-energy wasting between normal weight and obese patients undergoing haemodialysis. Nutr. Diet. 2017, 74, 283–290. [Google Scholar] [CrossRef]
- Argilés, J.M. Inflammation as the driving force of muscle wasting in cancer. Clin. Nutr. 2017, 36, 798–803. [Google Scholar] [CrossRef]
- Gougeon, R.; Pencharz, P.B.; Sigal, R.J. Effect of glycemic control on the kinetics of whole-body protein metabolism in obese subjects with non-insulin-dependent diabetes mellitus during iso- and hypoenergetic feeding. Am. J. Clin. Nutr. 1997, 65, 861–870. [Google Scholar] [CrossRef]
- Nardone, O.M.; de Sire, R.; Petito, V.; Testa, A.; Villani, G.; Scaldaferri, F.; Castiglione, F. Inflammatory bowel diseases and sarcopenia: The role of inflammation and gut microbiota in the development of muscle failure. Front. Immunol. 2021, 12, e694217. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Luster, A.D.; Alon, R.; von Andrian, U.H. Immune cell migration in inflammation: Present and future therapeutic targets. Nat. Immunol. 2005, 6, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Dunham-Snary, K.J.; Ballinger, S.W. Mitochondrial genetics and obesity: Evolutionary adaptation and contemporary disease susceptibility. Free Radic. Biol. Med. 2013, 65, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Shannon, S. The role of genetic variation in adaptation and population persistence in a changing environment. Evolution 1996, 49, 434–437. [Google Scholar] [CrossRef]
- Goyal, D.; Limesand, S.W.; Goyal, R. Epigenetic responses and the developmental origins of health and disease. J. Endocrinol. 2019, 242, T105–T119. [Google Scholar] [CrossRef] [PubMed]
- Lecoutre, S.; Petrus, P.; Ryden, M.; Breton, C. Transgenerational epigenetic mechanisms in adipose tissue development. Trends Endocrinol. Metab. 2018, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Franck, M.; de Toro-Martín, J.; Vohl, M.-C. Eco-evolutionary dynamics of the human-gut microbiota symbiosis in a changing nutritional environment. Evol. Biol. 2022, 49, 255–264. [Google Scholar] [CrossRef]
- Shahab, M.; Shahab, N. Coevolution of the human host and gut microbiome: Metagenomics of microbiota. Cureus 2022, 14, e26310. [Google Scholar] [CrossRef]
- Kidwell, C.U.; Casalini, J.R.; Pradeep, S.; Scherer, S.D.; Greiner, D.; Bayik, D.; Watson, D.C.; Olson, G.S.; Lathia, J.D.; Johnson, J.S.; et al. Transferred mitochondria accumulate reactive oxygen species, promoting proliferation. eLife 2023, 12, e85494. [Google Scholar] [CrossRef] [PubMed]
- Torralba, D.; Baixauli, F.; Sánchez-Madrid, F. Mitochondria know no boundaries: Mechanisms and functions of intercellular mitochondrial transfer. Front. Cell Dev. Biol. 2016, 4, 107. [Google Scholar] [CrossRef] [PubMed]
- Valenti, D.; Vacca, R.A.; Moro, L.; Atlante, A. Mitochondria can cross cell boundaries: An overview of the biological relevance, pathophysiological implications and therapeutic perspectives of intercellular mitochondrial transfer. Int. J. Mol. Sci. 2021, 22, 8312. [Google Scholar] [CrossRef]
- Inglis, M.S.; Page, C.M.; Wheatley, D.N. On the essential nature of non-essential amino acids. Mol. Physiol. 1984, 5, 115–122. [Google Scholar]
- Visek, W.J. An update of concepts of essential amino-acids. Annu. Rev. Nutr. 1984, 4, 137–155. [Google Scholar] [CrossRef]
- Alemany, M. Adjustment to dietary energy availability: From starvation to overnutrition. RSC Adv. 2013, 3, 1636–1651. [Google Scholar] [CrossRef]
- Wu, G.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, W.; Liu, C.; Wang, B.; Wang, J.; Yin, Y. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids 2013, 44, 1107–1113. [Google Scholar] [CrossRef]
- Verwijs, M.H.; de van der Schueren, M.A.E.; Ocke, M.C.; Ditewig, J.; Linschooten, J.O.; Roodenburg, A.J.C.; de Groot, L.C.P.G.M. The protein gap-increasing protein intake in the diet of community-dwelling older adults: A simulation study. Public Health Nutr. 2022, 25, 248–256. [Google Scholar] [CrossRef]
- Montiel-Rojas, D.; Nilsson, A.; Santoro, A.; Bazzocchi, A.; de Groot, L.C.P.G.M.; Feskens, E.J.M.; Berendsen, A.A.M.; Madej, D.; Kaluza, J.; Pietruszka, B.; et al. Fighting sarcopenia in ageing European adults: The importance of the amount and source of dietary proteins. Nutrients 2020, 12, 3601. [Google Scholar] [CrossRef]
- Campos-Nonato, I.; Hernandez, L.; Barquera, S. Effect of a high-protein diet versus standard-protein diet on weight loss and biomarkers of metabolic syndrome: A randomized clinical trial. Obes. Facts 2017, 10, 238–251. [Google Scholar] [CrossRef]
- Su, H.Y.; Lee, H.C.; Cheng, W.Y.; Huang, S.Y. A calorie-restriction diet supplemented with fish oil and high-protein powder is associated with reduced severity of metabolic syndrome in obese women. Eur. J. Clin. Nutr. 2015, 69, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U. Protein misfolding diseases. Annu. Rev. Biochem. 2017, 86, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Hong, H. Role of lipids in folding, misfolding and function of integral membrane proteins. Adv. Exp. Med. Biol. 2015, 855, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Liu, Z.-G. Autophagy and the metabolism of misfolding protein. Adv. Exp. Med. Biol. 2019, 1206, 375–420. [Google Scholar] [CrossRef]
- Vidak, E.; Javoršek, U.; Vizovišek, M.; Turk, B. Cysteine cathepsins and their extracellular roles: Shaping the microenvironment. Cells 2019, 8, 264. [Google Scholar] [CrossRef]
- Vizovišek, M.; Fonović, M.; Turk, B. Cysteine cathepsins in extracellular matrix remodeling: Extracellular matrix degradation and beyond. Matrix Biol. 2019, 75, 141–159. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Park, A.; Steitz, J.A. Modulation of mRNA 3′-end processing and transcription termination in virus-infected cells. Front. Immunol. 2022, 13, 828665. [Google Scholar] [CrossRef] [PubMed]
- Cahill, G.F., Jr. Starvation in man. N. Engl. J. Med. 1970, 282, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Felig, P.; Owen, O.E.; Wahren, J.; Cahill, G.F. Amino acid metabolism during prolonged starvation. J. Clin. Investig. 1969, 48, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Jacquet, J. Adaptive reduction in basal metabolic rate in response to food deprivation in humans: A role for feedback signals from fat stores. Am. J. Clin. Nutr. 1998, 68, 599–606. [Google Scholar] [CrossRef]
- Palou, A.; Remesar, X.; Arola, L.; Herrera, E.; Alemany, M. Metabolic effects of short term food deprivation in the rat. Horm. Metab. Res. 1981, 13, 326–330. [Google Scholar] [CrossRef]
- Drenick, E.J.; Swendseid, M.E.; Blahd, W.H.; Tuttle, S.G. Prolonged starvation as treatment for severe obesity. J. Am. Med. Assoc. 1964, 187, 100–105. [Google Scholar] [CrossRef]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Flint, A.J.; Berrington de Gonzalez, A.; Bernstein, L.; Brotzman, M.; MacInnis, R.J.; Moore, S.C.; Robien, K.; Rosenberg, P.S.; Singh, P.N.; et al. Association between class III obesity (BMI of 40–59 kg/m2) and mortality: A pooled analysis of 20 prospective studies. PLoS Med. 2014, 11, e1001673. [Google Scholar] [CrossRef] [PubMed]
- Melzer, K.; Genton, L.; Pichard, C. Régulation de la balance énérgetique: Ajustement de l’apport alimentaire à la dépense énérgetique. Nutr. Clin. Métabol. 2013, 27, 134–138. [Google Scholar] [CrossRef]
- Speakman, J.R. The evolution of body fatness: Trading off disease and predation risk. J. Exp. Biol. 2018, 221 (Suppl. S1), jeb167254. [Google Scholar] [CrossRef] [PubMed]
- Liou, T.-H.; Pi-Sunyer, F.X.; Laferrere, B. Physical disability and obesity. Nutr. Rev. 2005, 63, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Stupfel, M.; Pavely, A. Ultradian, circahoral and circadian structures in endothermic vertebrates and humans. Comp. Biochem. Physiol. A 1990, 96, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Polonsky, K.S.; Scheen, A.J. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr. Rev. 1997, 18, 716–738. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.; Oster, H.; Korf, H.W.; Foster, R.G.; Erren, T.C. Food as a circadian time cue-evidence from human studies. Nat. Rev. Endocrinol. 2020, 16, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Reuter, D.M.; Hopkins, S.S.B.; Price, S.A. What is a mammalian omnivore? Insights into terrestrial mammalian diet diversity, body mass and evolution. Proc. R. Soc. B 2023, 290, 20221062. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Adipose tissue and metabolic syndrome: Too much, too little or neither. Eur. J. Clin. Investig. 2015, 45, 1209–1217. [Google Scholar] [CrossRef]
- Uauy, R.; Díaz, E. Consequences of food energy excess and positive energy balance. Public Health Nutr. 2005, 8, 1077–1099. [Google Scholar] [CrossRef]
- Stock, M.J. Thermogenesis and energy-balance. Int. J. Obes. 1992, 16 (Suppl. S2), S13–S16. [Google Scholar]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kameya, T.; Nakada, K.; Kawai, Y.; Saito, M. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity 2011, 19, 13–16. [Google Scholar] [CrossRef]
- Blackmore, P.F.; Brumley, F.T.; Marks, J.L.; Exton, J.H. Studies on a-adrenergic activation of hepatic glucose output. Relationship between a-adrenergic stimulation of calcium efflux and activation of phosphorylasse in isolated rat liver parenchimal cells. J. Biol. Chem. 1978, 253, 4851–4858. [Google Scholar] [CrossRef]
- Mortimore, G.E. Influence of insulin on the hepatic uptake and release of glucose and amino acids. In Handbook of Physiology; Williams & Wilkins Baltimore: Baltimore, MD, USA, 1972; pp. 495–504. [Google Scholar]
- Sestoft, L.; Marshall, M.O. Regulation of lactate uptake and lactate production in liver from 48-h-starved rats—Effects of pH, flow and glucose- concentration. Clin. Sci. 1988, 74, 403–406. [Google Scholar] [CrossRef]
- Fafournoux, P.; Demigné, C.; Rémésy, C. Mechanisms involved in ketone body release by rat liver cells: Influence of pH and bicarbonate. Am. J. Physiol. 1987, 252, G200–G208. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Ginsberg, H.N. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol. Metab. 2011, 22, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Hardison, W.G.; Proffitt, J.H. Influence of hepatic taurine concentration on bile acid conjugation with taurine. Am. J. Physiol. 1977, 232, E75–E79. [Google Scholar] [CrossRef] [PubMed]
- Omiecinski, C.J.; Vanden Heuvel, J.P.; Perdew, G.H.; Peters, J.M. Xenobiotic metabolism, disposition, and regulation by receptors: From biochemical phenomenon to predictors of major toxicities. Toxicol. Sci. 2011, 120 (Suppl. S1), S49–S75. [Google Scholar] [CrossRef] [PubMed]
- You, L. Steroid hormone biotransformation and xenobiotic induction of hepatic steroid metabolizing enzymes. Chem.-Biol. Interact. 2004, 147, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. 2019, 316, E956–E966. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-J.; Cheng, Y.-C.; Chen, H.-C.; Chao, Y.-K.; Nicholson, M.W.; Yen, E.C.L.; Kamp, T.J.; Hsieh, P.C.H. Commensal gut microbiota-derived acetate and propionate enhance heart adaptation in response to cardiac pressure overload in mice. Theranostics 2022, 12, 7319–7334. [Google Scholar] [CrossRef]
- Bourriaud, C.; Robins, R.J.; Martin, L.; Kozlowski, F.; Tenailleau, E.; Cherbut, C.; Michel, C. Lactate is mainly fermented to butyrate by human intestinal microflora but inter-individual variation is evident. J. Appl. Microbiol. 2005, 99, 201–212. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Hooda, S.; Pieper, R.; Zijlstra, R.T.; van Kessel, A.G.; Mosenthin, R.; Ganzle, M.G. Nonstarch polysaccharides modulate bacterial microbiota, pathways for butyrate production, and abundance of pathogenic Escherichia coli in the pig gastrointestinal tract. Appl. Environ. Microbiol. 2010, 76, 3692–3701. [Google Scholar] [CrossRef]
- Dukić, M.; Radonjić, T.; Jovanović, I.; Zdravković, M.; Todorović, Z.; Kraišnik, N.; Aranđelović, B.; Mandić, O.; Popadić, V.; Nikolić, N.; et al. Alcohol, inflammation, and microbiota in alcoholic liver disease. Int. J. Mol. Sci. 2023, 24, 3735. [Google Scholar] [CrossRef] [PubMed]
- French, S.W. Biochemistry of alcoholic liver disease. Crit. Rev. Clin. Lab. Sci. 1992, 29, 83–115. [Google Scholar] [CrossRef] [PubMed]
- Monte, W.C. While Science Sleeps; CreateSpace: North Charleston, SC, USA, 2011; ISBN 978-1452893679. [Google Scholar]
- Levitt, M.D.; Levitt, D.G. Quantitative evaluation of D-lactate pathophysiology: New insights into the mechanisms involved and the many areas in need of further investigation. Clin. Exp. Gastroenterol. 2020, 13, 321–337. [Google Scholar] [CrossRef]
- Garza, C.; Scrimshaw, N.S.; Young, V.R. Human protein requirements: Interrelationships between energy intake and nitrogen balance in young men consuming the 1973 FAO/WHO safe level of egg protein, with added non-essential amino acids. J. Nutr. 1978, 108, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.A.; Pérez-Sala, D. Mammalian sulfur amino acid metabolism: A nexus between redox regulation, nutrition, epigenetics, and detoxification. Antioxid. Redox Signal. 2018, 29, 408–452. [Google Scholar] [CrossRef] [PubMed]
- Trolle, J.; McBee, R.M.; Kaufman, A.; Pinglay, S.; Berger, H.; German, S.; Liu, L.; Shen, M.J.; Guo, X.; Martin, J.A.; et al. Resurrecting essential amino acid biosynthesis in mammalian cells. eLife 2022, 11, e72847. [Google Scholar] [CrossRef] [PubMed]
- Weiler, M.; Hertzler, S.R.; Dvoretskiy, S. Is it time to reconsider the U.S. recommendations for dietary protein and amino acid intake? Nutrients 2023, 15, 838. [Google Scholar] [CrossRef] [PubMed]
- Bilsborough, S.; Mann, N. A review of issues of dietary protein intake in humans. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 129–152. [Google Scholar] [CrossRef]
- Alemany, M. The problem of nitrogen disposal in the obese. Nutr. Res. Rev. 2012, 25, 18–28. [Google Scholar] [CrossRef]
- Denke, M.A. Metabolic effects of high-protein, low-carbohydrate diets. Am. J. Cardiol. 2001, 88, 59–61. [Google Scholar] [CrossRef]
- Watts, D.P. Meat eating by nonhuman primates: A review and synthesis. J. Hum. Evol. 2020, 149, 102882. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef]
- Mariotti, F.; Gardner, C.D. Dietary protein and amino acids in vegetarian diets—A review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Koh, G. Clinical evidence and mechanisms of high-protein diet-induced weight loss. J. Obes. Metab. Syndr. 2020, 29, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Klaus, S. Increasing the protein: Carbohydrate ratio in a high-fat diet delays the development of adiposity and improves glucose homeostasis in mice. J. Nutr. 2005, 135, 1854–1858. [Google Scholar] [CrossRef] [PubMed]
- Marsset-Baglieri, A.; Fromentin, G.; Tome, D.; Bensaid, A.; Makkarios, L.; Even, P.C. Increasing the protein content in a carbohydrate-free diet enhances fat loss during 35% but not 75% energy restriction in rats. J. Nutr. 2004, 134, 2646–2652. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.I.; Layman, D.K.; Freund, G.G.; Rahn, K.A.; Nakamura, M.T.; Yudell, B.E. A reduced carbohydrate, increased protein diet stabilizes glycemic control and minimizes adipose tissue glucose disposal in rats. J. Nutr. 2006, 136, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; Gaudichon, C.; Fromentin, G.; Even, P.; Tomé, D.; Azzout-Marniche, D. Increasing protein at the expense of carbohydrate in the diet down-regulates glucose utilization as glucose sparing effect in rats. PLoS ONE 2011, 6, e14664. [Google Scholar] [CrossRef]
- Ballesteros-Pomar, M.D.; Calleja-Fernandez, A.R.; Vidal-Casariego, A.; Urioste-Fondo, A.M.; Cano-Rodriguez, I. Effectiveness of energy-restricted diets with different protein: Carbohydrate ratios: The relationship to insulin sensitivity. Public Health Nutr. 2010, 13, 2119–2126. [Google Scholar] [CrossRef]
- Abete, I.; Parra, D.; Martínez de Morentín, B.; Martínez, J.A. Effects of two energy-restricted diets differing in the carbohydrate/protein ratio on weight loss and oxidative changes of obese men. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S3), 1–13. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.K.; Park, M.; Shin, J.E.; Jang, S.-Y.; Shin, J.-Y. Dietary protein to carbohydrate ratio and incidence of metabolic syndrome in Korean adults based on a long-term prospective community-based cohort. Nutrients 2020, 12, 3274. [Google Scholar] [CrossRef] [PubMed]
- Buzelle, S.L.; Santos, M.P.; Baviera, A.M.; Lopes, C.F.; Garofalo, M.A.R.; Navegantes, L.C.C.; Kettelhut, I.C.; Chaves, V.E.; Kawashita, N.H. A low-protein, high-carbohydrate diet increases the adipose lipid content without increasing the glycerol-3-phosphate or fatty acid content in growing rats. Can. J. Physiol. Pharmacol. 2010, 88, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.P.; de Franca, S.A.; dos Santos, J.T.F.; Buzelle, S.L.; Bertolini, G.L.; Garofalo, M.A.R.; do Kettelhut, I.C.; Frasson, D.; Chaves, V.E.; Kawashita, N.H. A low-protein, high-carbohydrate diet increases fatty acid uptake and reduces norepinephrine-induced lipolysis in rat retroperitoneal white adipose tissue. Lipids 2012, 47, 279–289. [Google Scholar] [CrossRef]
- Wiggins, W.D.; Wilder, S.M. Carbohydrates complement high-protein diets to maximize the growth of an actively hunting predator. Ecol. Evol. 2022, 12, e9150. [Google Scholar] [CrossRef] [PubMed]
- Koehnle, T.J.; Russell, M.C.; Gietzen, D.W. Rats rapidly reject diets deficient in essential amino acids. J. Nutr. 2003, 133, 2331–2335. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Takado, Y.; Toyoda, S.; Tsukamoto-Yasui, M.; Minatohara, K.; Takuwa, H.; Urushihata, T.; Takahashi, M.; Shimojo, M.; Ono, M.; et al. Neurodegenerative processes accelerated by protein malnutrition and decelerated by essential amino acids in a tauopathy mouse model. Sci. Adv. 2021, 7, abd5046. [Google Scholar] [CrossRef] [PubMed]
- Coker, R.H.; Miller, S.; Schutzler, S.; Deutz, N.; Wolfe, R.R. Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals. Nutr. J. 2012, 11, 105. [Google Scholar] [CrossRef]
- Jamshidi, A.; Farjam, M.; Ekramzadeh, M.; Homayounfar, R. Evaluating type and amount of dietary protein in relation to metabolic syndrome among Iranian adults: Cross-sectional analysis of Fasa Persian cohort study. Diabetol. Metab. Syndr. 2022, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, K.K.S.; van Nas, A.; Martin, L.J.; Davis, R.C.; Devaskar, S.U.; Lusis, A.J. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes 2009, 58, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Kampman-van de Hoek, E.; Jansman, A.J.; van den Borne, J.J.; van der Peet-Schwering, C.M.; van Beers-Schreurs, H.; Gerrits, W.J. Dietary amino acid deficiency reduces the utilization of amino acids for growth in growing pigs after a period of poor health. J. Nutr. 2016, 146, 51–58. [Google Scholar] [CrossRef] [PubMed]
- López-Tejero, D.; Pastor-Anglada, M.; Remesar, X. Blood amino acid compartmentalization during pregnancy and lactation in the rat. Ann. Nutr. Metab. 1986, 30, 58–65. [Google Scholar] [CrossRef]
- Ma, W.-J.; Zhang, W.; Zhai, J.; Li, H.; Zhao, T.; Han, L.; Wen, Q.; Song, X.; Guo, X.-F. Branched chain and other amino acid intakes are inversely associated with sarcopenia among community elders in Qingdao, China. Asia Pac. J. Clin. Nutr. 2022, 31, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Rafecas, I.; Esteve, M.; Fernández-López, J.A.; Remesar, X.; Alemany, M. Whole rat protein content estimation. Applicability of the N x 6.25 factor. Br. J. Nutr. 1994, 72, 199–209. [Google Scholar] [CrossRef]
- Felton, A.M.; Felton, A.; Raubenheimer, D.; Simpson, S.J.; Krizsan, S.J.; Hedwall, P.-O.; Stolter, C. The nutritional balancing act of a large herbivore: An experiment with captive moose (Alces alces L). PLoS ONE 2016, 11, e0150870. [Google Scholar] [CrossRef]
- Marsh, K.A.; Munn, E.A.; Baines, S.K. Protein and vegetarian diets. Med. J. Aust. 2013, 199 (Suppl. S4), S7–S10. [Google Scholar] [CrossRef]
- Robbins, C.T.; Fortin, J.K.; Rode, K.D.; Farley, S.D.; Shipley, L.A.; Felicetti, L.A. Optimizing protein intake as a foraging strategy to maximize mass gain in an omnivore. Oikos 2007, 116, 1675–1682. [Google Scholar] [CrossRef]
- Spadaro, P.A.; Naug, H.L.; Du Toit, E.F.; Donner, D.; Colson, N.J. A refined high carbohydrate diet is associated with changes in the serotonin pathway and visceral obesity. Genet. Res. 2015, 97, e23. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, K.; Liu, W.; Wu, M.; Wang, Y.; Lv, Y.; Meng, H. Diets enriched in sugar, refined, or whole grain differentially influence plasma cholesterol concentrations and cholesterol metabolism pathways with concurrent changes in bile acid profile and gut microbiota composition in ApoE−/− mice. J. Agric. Food Chem. 2023, 71, 9738–9752. [Google Scholar] [CrossRef]
- Best, C.M.; Hsu, S. Effects of very low carbohydrate ketogenic diets on skeletal health. Curr. Opin. Endocrinol. Diabetes Obes. 2023, 30, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; de Nucci, S.; Castellana, F.; di Chito, M.; Giannuzzi, V.; Shahini, E.; Zupo, R.; Lampignano, L.; Piazzolla, G.; Triggiani, V.; et al. The effects of eight weeks’ very low-calorie ketogenic diet (VLCKD) on liver health in subjects affected by overweight and obesity. Nutrients 2023, 15, 825. [Google Scholar] [CrossRef] [PubMed]
- Alemany, M. Different effects of hyperlipidic diets in human lactation and adulthood: Growth versus the development of obesity. Reprod. Biol. Endocrinol. 2011, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Wieland, O. Ketogenesis and its regulation. Adv. Metab. Disord. 1968, 3, 1–47. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.D.; Foster, D.W. Hormonal control of ketogenesis. In Hormones and Energy Metabolism; Klachko, D.M., Anderson, R.R., Heimberg, M., Eds.; Plenum Press: New York, NY, USA; London, UK, 1979; pp. 79–96. [Google Scholar]
- Scholz, R.; Schwabe, U.; Soboll, S. Influence of fatty acids on energy metabolism. 1. Stimulation of oxygen consumption, ketogenesis and CO2 production following addition of octanoate and olcate in perfused rat liver. Eur. J. Biochem. 1984, 141, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Bae, J.; Jo, D.R.; Lee, M.; Lee, Y.-H.; Kang, E.S.; Cha, B.-S.; Lee, B.-W. Impaired ketogenesis is associated with metabolic-associated fatty liver disease in subjects with type 2 diabetes. Front. Endocrinol. 2023, 14, 1124576. [Google Scholar] [CrossRef] [PubMed]
- Havel, P.J. Dietary fructose: Implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr. Rev. 2005, 63, 133–157. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Meyer, J.G.; Wang, G.-X.; Gupta, M.K.; Batista, T.M.; Lauritzen, H.P.M.M.; Fujisaka, S.; Serra, D.; Herrero, L.; Willoughby, J.; et al. Dietary sugars alter hepatic fatty acid oxidation via transcriptional and post-translational modifications of mitochondrial proteins. Cell Metab. 2019, 30, 735–753. [Google Scholar] [CrossRef]
- Parks, E.J.; Skokan, L.E.; Timlin, M.T.; Dingfelder, C.S. Dietary sugars stimulate fatty acid synthesis in adults. J. Nutr. 2008, 138, 1039–1046. [Google Scholar] [CrossRef]
- Moore, J.B.; Gunn, P.J.; Fielding, B.A. The role of dietary sugars and de novo lipogensis in non-alcoholic fatty liver disease. Nutrients 2014, 6, 5679–5703. [Google Scholar] [CrossRef]
- Bailey, J.W.; Haymond, M.W.; Miles, J.M. Metabolism of ketone bodies by skeletal muscle in starvation and uncontrolled diabetes. Metabolism 1990, 39, 1039–1043. [Google Scholar] [CrossRef]
- Koutnik, A.P.; d’Agostino, D.P.; Egan, B. Anticatabolic effects of ketone bodies in skeletal muscle. Trends Endocrinol. Metab. 2019, 30, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Arsyad, A.; Idris, I.; Rasyid, A.A.; Usman, R.A.; Faradillah, K.R.; Latif, W.O.U.; Lubis, Z.I.; Aminuddin, A.; Yustisia, I.; Djabir, Y.Y. Long-term ketogenic diet induces metabolic acidosis, anemia, and oxidative stress in healthy Wistar rats. J. Nutr. Metab. 2020, 2020, 3642035. [Google Scholar] [CrossRef] [PubMed]
- Biden, T.J.; Taylor, K.W. Effects of ketone bodies on insulin release and islet-cell metabolism in the rat. Biochem. J. 1983, 212, 371–377. [Google Scholar] [CrossRef]
- Ciaraldi, T.P.; Henry, R.R. Insulin regulation of ketone body metabolism. In International Textbook of Diabetes Mellitus; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Yang, S.-L.; Xia, J.-H.; Zhang, Y.-Y.; Fan, J.-G.; Wang, H.; Yuan, J.; Zhao, Z.-Z.; Pan, Q.; Mu, Y.-L.; Xin, L.-L.; et al. Hyperinsulinemia shifted energy supply from glucose to ketone bodies in early nonalcoholic steatohepatitis from high-fat high-sucrose diet induced Bama minipigs. Sci. Rep. 2015, 5, 13980. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; McKenna, C.F.; Skinner, S.K.; Martinez, I.G. Protein-containing food is more than the sum of its constituent amino acids for post-exercise muscle anabolic potential. Sports Sci. Exch. 2019, 32, 1–5. [Google Scholar]
- Corsetti, G.; Romano, C.; Codenotti, S.; Pasini, E.; Fanzani, A.; Dioguardi, F.S. Essential amino acids-rich diet decreased adipose tissue storage in adult mice: A preliminary histopathological study. Nutrients 2022, 14, 2915. [Google Scholar] [CrossRef]
- Xiao, F.; Guo, F. Impacts of essential amino acids on energy balance. Mol. Metab. 2022, 57, 101393. [Google Scholar] [CrossRef]
- Owen, O.E.; Smalley, K.J.; d’Alessio, D.A.; Mozzoli, M.A.; Mozzoli, M.A.; Dawson, E.K. Protein, fat, and carbohydrate requirements during starvation: Anaplerosis and cataplerosis. Am. J. Clin. Nutr. 1998, 68, 12–34. [Google Scholar] [CrossRef]
- Brunengraber, H.; Roe, C.R. Anaplerotic molecules: Current and future. J. Inherit. Metab. Dis. 2006, 29, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Borders, C.B.; Cline, G.W.; Zhang, X.-M.; Alves, T.C.; Petersen, K.F.; Rothman, D.L.; Kibbey, R.G.; Shulman, G.I. Propionate increases hepatic pyruvate cycling and anaplerosis and alters mitochondrial metabolism. J. Biol. Chem. 2016, 291, 12161–12170. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.K.; Finley, L.W.S. Regulation and function of the mammalian tricarboxylic acid cycle. J. Biol. Chem. 2023, 299, 102838. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, D.S.; Ramnanan, C.J.; Grueter, C.A.; Johnson, K.M.S.; Lautz, M.; Neal, D.W.; Williams, P.E.; Cherrington, A.D. Effects of insulin on the metabolic control of hepatic gluconeogenesis in vivo. Diabetes 2009, 58, 2766–2775. [Google Scholar] [CrossRef] [PubMed]
- Cappel, D.A.; Deja, S.; Duarte, J.A.G.; Kucejova, B.; Inigo, M.; Fletcher, J.A.; Fu, X.; Berglund, E.D.; Liu, T.; Elmquist, J.K.; et al. Pyruvate-carboxylase-mediated anaplerosis promotes antioxidant capacity by sustaining TCA cycle and redox metabolism in liver. Cell Metab. 2019, 29, 1291–1305. [Google Scholar] [CrossRef]
- Garrison, J.C.; Haynes, R.C. The hormonal control of gluconeogenesis by regulation of mitochondrial pyruvate carboxylation in isolated rat liver cells. J. Biol. Chem. 1975, 250, 2769–2777. [Google Scholar] [CrossRef]
- Thomas, E.L.; Frost, G.; Taylor-Robinson, S.D.; Bell, J.D. Excess body fat in obese and normal-weight subjects. Nutr. Res. Rev. 2012, 25, 150–161. [Google Scholar] [CrossRef]
- Cortot, A.; Jobin, G.; Ducrot, F.; Aymes, C.; Giraudeaux, V.; Modigliani, R. Gastric emptying and gastrointestinal absorption of alcohol ingested with a meal. Dig. Dis. Sci. 1986, 31, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Guo, Y.; Mashimo, H. Advances in the physiology of gastric emptying. Neurogastroenterol. Motil. 2019, 31, e13546. [Google Scholar] [CrossRef]
- Davis, J.; Camilleri, M.; Eckert, D.; Burton, D.; Joyner, M.; Acosta, A. Physical activity is associated with accelerated gastric emptying and increased ghrelin in obesity. Neurogastroenterol. Motil. 2020, 32, e13879. [Google Scholar] [CrossRef]
- Lawson, M.; Everson, G.T.; Klingensmith, W.; Kern, F. Coordination of gastric and gallbladder emptying after ingestion of a regular meal. Gastroenterology 1983, 85, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Sala-Rabanal, M.; Ghezzi, C.; Hirayama, B.A.; Kepe, V.; Liu, J.; Barrio, J.R.; Wright, E.M. Intestinal absorption of glucose in mice as determined by positron emission tomography. J. Physiol. 2018, 596, 2473–2489. [Google Scholar] [CrossRef] [PubMed]
- Crane, R.K. The physiology of the intestinal absorption of sugars. In Physiological Effects of Food Carbohydrates; Jeanes, A., Hodge, J., Eds.; American Chemical Society: Washington, DC, USA, 1975; pp. 2–19. [Google Scholar]
- Trevaskis, N.L.; Lee, G.; Escott, A.; Phang, K.L.; Hong, J.; Cao, E.; Katneni, K.; Charman, S.A.; Han, S.; Charman, W.N.; et al. Intestinal lymph flow, and lipid and drug transport scale allometrically from pre-clinical species to humans. Front. Physiol. 2020, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Cerda, J.J.; Brooks, F.P.; Prockop, D.J. Intraduodenal hydrolysis of gelatin as a measure of protein digestion in normal subjects and in patients with malabsorption syndromes. Gastroenterology 1968, 54, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Karasov, W.H. Integrative physiology of transcellular and paracellular intestinal absorption. J. Exp. Biol. 2017, 220, 2495–2501. [Google Scholar] [CrossRef]
- Brownlee, I.A.; Forster, D.J.; Wilcox, M.D.; Dettmar, P.W.; Seal, C.J.; Pearson, J.P. Physiological parameters governing the action of pancreatic lipase. Nutr. Res. Rev. 2010, 23, 146–154. [Google Scholar] [CrossRef]
- Randolph, G.J.; Miller, N.E. Lymphatic transport of high-density lipoproteins and chylomicrons. J. Clin. Investig. 2014, 124, 929–935. [Google Scholar] [CrossRef]
- Shibata, S.-J.; Hiramatsu, Y.; Kaseda, M.; Chosa, M.; Ichihara, N.; Amasaki, H.; Hayakawa, T.; Asari, M. The time course of lymph drainage from the peritoneal cavity in beagle dogs. J. Vet. Med. Sci. 2006, 68, 1143–1147. [Google Scholar] [CrossRef]
- Kaur, L.; Mao, B.; Beniwal, A.S.; Abhilasha; Kaur, R.; Chian, F.M.; Singh, J. Alternative proteins vs animal proteins: The influence of structure and processing on their gastro-small intestinal digestion. Trends Food Sci. Technol. 2022, 122, 275–286. [Google Scholar] [CrossRef]
- MacFarlane, G.T.; Cummings, J.H.; Allison, C. Protein degradation by human intestinal bacteria. J. Gen. Microbiol. 1986, 132, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Giezenaar, C.; Lange, K.; Hausken, T.; Jones, K.L.; Horowitz, M.; Chapman, I.; Soenen, S. Acute effects of substitution, and addition, of carbohydrates and fat to protein on gastric emptying, blood glucose, gut hormones, appetite, and energy intake. Nutrients 2018, 10, 1451. [Google Scholar] [CrossRef] [PubMed]
- Silk, D.B.A.; Grimble, G.K.; Rees, R.G. Protein digestion and amino acid and peptide absorption. Proc. Nutr. Soc. 1985, 44, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Peters, T.J. Intestinal peptidases. Gut 1970, 11, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Feracci, H.; Benajiba, A.; Maroux, S. Rôle des protéases des entérocytes dans les dernières étapes de digestion et dans le phénomène d’absorption. Reprod. Nutr. Dév. 1981, 21, 849. [Google Scholar] [CrossRef]
- Higgins, K.A.; Hudson, J.L.; Hayes, A.M.R.; Braun, E.; Cheon, E.; Couture, S.C.; Gunaratna, N.S.; Hill, E.R.; Hunter, S.R.; McGowan, B.S.; et al. Systematic review and meta-analysis on the effect of portion size and ingestive frequency on energy intake and body weight among adults in randomized controlled feeding trials. Adv. Nutr. 2022, 13, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Flieh, S.M.; Miguel-Berges, M.L.; Huybrechts, I.; Castillo, M.J.; Gonzalez-Gross, M.; Marcos, A.; Gottrand, F.; Le Donne, C.; Widhalm, K.; Molnár, D.; et al. Associations between food portion sizes, insulin resistance, VO2 max and metabolic syndrome in European adolescents: The HELENA study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2061–2073. [Google Scholar] [CrossRef]
- Mattes, R. Energy intake and obesity: Ingestive frequency outweighs portion size. Physiol. Behav. 2014, 134, 110–118. [Google Scholar] [CrossRef]
- Erdmann, J.; Tholl, S.; Schusdziarra, V. Effect of carbohydrate- and protein-rich meals on exercise-induced activation of lipolysis in obese subjects. Horm. Metab. Res. 2010, 42, 290–294. [Google Scholar] [CrossRef]
- Saldanha Campello, R.; Teixeira Alves-Wagner, A.B.; Abdulkader, F.; Tieko Mori, R.C.; Fabres Machado, U. Carbohydrate- and lipid-enriched meals acutely disrupt glycemic homeostasis by inducing transient insulin resistance in rats. Can. J. Physiol. Pharmacol. 2012, 90, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Blatt, A.D.; Roe, L.S.; Rolls, B.J. Increasing the protein content of meals and its effect on daily energy intake. J. Am. Diet. Assoc. 2011, 111, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-Y.; Schutzler, S.; Schrader, A.; Spencer, H.J.; Azhar, G.; Ferrando, A.A.; Wolfe, R.R. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am. J. Physiol. 2016, 310, E73–E80. [Google Scholar] [CrossRef] [PubMed]
- Gaiani, S.; Bolondi, L.; Li Bassi, S.; Santi, V.; Zironi, G.; Barbara, L. Effect of meal on portal hemodynamics in healthy humans and in patients with chronic liver disease. Hepatology 1989, 9, 815–819. [Google Scholar] [CrossRef]
- Pischetsrieder, M. Chemistry of glucose and biochemical pathways of biological interest. Perit. Dial. Int. 2000, 20 (Suppl. S2), 26–30. [Google Scholar] [CrossRef]
- Oliva, L.; Fernández-López, J.A.; Remesar, X.; Alemany, M. The anomeric nature of glucose and its implications on its analyses and the influence of diet: Are routine glycaemia measurements reliable enough? J. Endocrinol. Metab. 2019, 9, 63–70. [Google Scholar] [CrossRef]
- Goldstein, D.E.; Curnow, R.T. Effect of starvation on hepatic glycogen metabolism and glucose homeostasis. Metabolism 1978, 27, 315–323. [Google Scholar] [CrossRef]
- Aikawa, T.; Matsutaka, H.; Takezawa, K.; Ishikawa, E. Gluconeogenesis and amino acid metabolism 1. Comparison of various precursors for hepatic gluconeogenesis in vivo. Biochim. Biophys. Acta 1972, 279, 234–244. [Google Scholar] [CrossRef]
- Jungas, R.L.; Halperin, M.L.; Brosnan, J.T. Quantitative analysis of amino acid oxidation and related gluconeogenesis in humans. Physiol. Rev. 1992, 72, 419–448. [Google Scholar] [CrossRef] [PubMed]
- Young, R.A. Fat, energy and mammalian survival. Am. Zool. 1976, 16, 699–710. [Google Scholar] [CrossRef]
- Soeters, M.R.; Soeters, P.B.; Schooneman, M.G.; Houten, S.M.; Romijn, J.A. Adaptive reciprocity of lipid and glucose metabolism in human short-term starvation. Am. J. Physiol. 2012, 303, E1397–E1407. [Google Scholar] [CrossRef]
- Lowell, B.B.; Goodman, M.N. Protein sparing in skeletal-muscle during prolonged starvation: Dependence on lipid fuel availability. Diabetes 1987, 36, 14–19. [Google Scholar] [CrossRef]
- Cahill, G.F., Jr.; Owen, O.E. Body fuels and starvation. Int. Psychiatry Clin. 1970, 7, 25–36. [Google Scholar]
- Van Raaij, J.M.; Schonk, C.M.; Vermaat-Miedema, S.H.; Peek, M.E.; Hautvast, J.G. Energy-cost of lactation, and energy balances of well-nourished Dutch lactating women-reappraisal of the extra energy-requirements of lactation. Am. J. Clin. Nutr. 1991, 53, 612–619. [Google Scholar] [CrossRef]
- Thomson, A.M.; Hytten, F.E.; Billewicz, W.Z. The energy cost of human lactation. Br. J. Nutr. 1970, 24, 565–572. [Google Scholar] [CrossRef]
- Guglielmo, C.G. Move that fatty acid: Fuel selection and transport in migratory birds and bats. Integr. Comp. Biol. 2010, 50, 336–345. [Google Scholar] [CrossRef]
- Price, E.R.; Krokfors, A.; Guglielmo, C.G. Selective mobilization of fatty acids from adipose tissue in migratory birds. J. Exp. Biol. 2008, 211, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Romanyukha, A.A.; Rudnev, S.G.; Sidorov, I.A. Energy cost of infection burden: An approach to understanding the dynamics of host–pathogen interactions. J. Theor. Biol. 2006, 241, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.E. Energy costs of activities in health and disease. AMA Arch. Intern. Med. 1958, 101, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.P.; Farnaud, S.; Evans, W.R. Structure/function overview of proteins involved in iron storage and transport. Curr. Med. Chem. 2005, 12, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Rottensten, K.V. The effect of body stores on the efficiency of calcium utilization. Biochem. J. 1938, 32, 1285–1292. [Google Scholar] [CrossRef]
- Alfrey, A.C.; Miller, N.L.; Butkus, D. Evaluation of body magnesium stores. J. Lab. Clin. Med. 1974, 84, 153–162. [Google Scholar] [CrossRef]
- Heaney, R.P.; Horst, R.L.; Cullen, D.M.; Armas, L.A.G. Vitamin D3 distribution and status in the body. J. Am. Coll. Nutr. 2009, 28, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Sauberlich, H.E. Human requirements and needs. Vitamin C status: Methods and findings. Ann. N. Y. Acad. Sci. 1975, 258, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Zamaria, N. Alteration of polyunsaturated fatty acid status and metabolism in health and disease. Reprod. Nutr. Dev. 2004, 44, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, R. Flux of metabolic fuels during starvation in the rat. Pflüger’s Arch. 1978, 374, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Randle, P.J. Metabolic fuel selection: General integration at the whole-body level. Proc. Nutr. Soc. 1995, 54, 317–327. [Google Scholar] [CrossRef]
- Weber, J.-M. Metabolic fuels: Regulating fluxes to select mix. J. Exp. Biol. 2011, 214, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Reshef, L.; Olswang, Y.; Cassuto, H.; Blum, B.; Croniger, C.M.; Kalhan, S.C.; Tilghman, S.M.; Hanson, R.W. Glyceroneogenesis and the triglyceride/fatty acid cycle. J. Biol. Chem. 2003, 278, 30413–30416. [Google Scholar] [CrossRef]
- Heath, R.B.; Karpe, F.; Milne, R.W.; Burdge, G.C.; Wootton, S.A.; Frayn, K.N. Dietary fatty acids make a rapid and substantial contribution to VLDL-triacylglycerol in the fed state. Am. J. Physiol. 2007, 292, E732–E739. [Google Scholar] [CrossRef]
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hannaford, M.C.; Leiter, L.A.; Josse, R.G.; Goldstein, M.B.; Marliss, E.B.; Halperin, M.L. Protein wasting due to acidosis of prolonged fasting. Am. J. Physiol. 1982, 243, E251–E256. [Google Scholar] [CrossRef] [PubMed]
- Caloin, M. Modeling of lipid and protein depletion during total starvation. Am. J. Physiol. 2004, 287, E790–E798. [Google Scholar] [CrossRef] [PubMed]
- McCue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. A 2010, 156, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.A. Some aspects of the regulation of fuel supply in omnivorous animals. Adv. Enzym. Regul. 1972, 10, 397–420. [Google Scholar] [CrossRef] [PubMed]
- Mittendorfer, B.; Klein, S.; Fontana, L. A word of caution against excessive protein intake. Nat. Rev. Endocrinol. 2020, 16, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Fielding, B. Tracing the fate of dietary fatty acids: Metabolic studies of postprandial lipaemia in human subjects. Proc. Nutr. Soc. 2011, 70, 342–350. [Google Scholar] [CrossRef]
- Teng, K.-T.; Chang, C.-Y.; Chang, L.F.; Nesaretnam, K. Modulation of obesity-induced inflammation by dietary fats: Mechanisms and clinical evidence. Nutr. J. 2014, 13, 12. [Google Scholar] [CrossRef]
- Haag, M.; Dippenaar, N.G. Dietary fats, fatty acids and insulin resistance: Short review of a multifaceted connection. Med. Sci. Monit. 2005, 11, RA359–RA367. [Google Scholar]
- Van Epps-Fung, M.; Williford, J.; Wells, A.; Hardy, R.W. Fatty acid-induced insulin resistance in adipocytes. Endocrinology 1997, 138, 4338–4345. [Google Scholar] [CrossRef]
- Bray, G.A.; Lovejoy, J.C.; Smith, S.R.; DeLany, J.P.; Lefevre, M.; Hwang, D.; Ryan, D.H.; York, D.A. The influence of different fats and fatty acids on obesity, insulin resistance and inflammation. J. Nutr. 2002, 132, 2488–2491. [Google Scholar] [CrossRef]
- La Fleur, S.E.; Luijendijk, M.C.M.; van der Zwaal, E.M.; Brans, M.A.D.; Adan, R.A.H. The snacking rat as model of human obesity: Effects of a free-choice high-fat high-sugar diet on meal patterns. Int. J. Obes. 2014, 38, 643–649. [Google Scholar] [CrossRef]
- Rothwell, N.J.; Saville, M.E.; Stock, M.J. Effects of feeding a cafeteria diet on energy-balance and diet-induced thermogenesis in 4 strains of rat. J. Nutr. 1982, 112, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, F.J.; MacGregor, G.A. High salt intake. Hypertension 2015, 66, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.J. High salt intake as a driver of obesity. Nat. Rev. Nephrol. 2018, 14, 285. [Google Scholar] [CrossRef] [PubMed]
- Erlanson-Albertsson, C. How palatable food disrupts appetite regulation. Basic Clin. Pharmacol. Toxicol. 2005, 97, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Blundell, J.E.; Halford, J.C.G.; Rodgers, R.J. Palatability, food intake and the behavioural satiety sequence in male rats. Physiol. Behav. 2003, 80, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.J.; Whybrow, S. Energy density, diet composition and palatability: Influences on overall food energy intake in humans. Physiol. Behav. 2004, 81, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Staiano, A.E.; Martin, C.K.; Champagne, C.M.; Rood, J.C.; Katzmarzyk, P.T. Sedentary time, physical activity, and adiposity in a longitudinal cohort of nonobese young adults. Am. J. Clin. Nutr. 2018, 108, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Li, T.Y.; Colditz, G.A.; Willett, W.C.; Manson, J.E. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. J. Am. Med. Assoc. 2003, 289, 1785–1791. [Google Scholar] [CrossRef]
- Chaput, J.-P.; Klingenberg, L.; Astrup, A.; Sjodin, A.M. Modern sedentary activities promote overconsumption of food in our current obesogenic environment. Obes. Rev. 2011, 12, e12–e20. [Google Scholar] [CrossRef]
- Pingali, P.L. Green Revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed]
- Sabater, D.; Agnelli, S.; Arriarán, S.; Fernández-López, J.-A.; Romero, M.M.; Alemany, M.; Remesar, X. Altered nitrogen balance and decreased urea excretion in male rats fed cafeteria diet are related to arginine availability. BioMed Res. Int. 2014, 2014, 959420. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Lorente, R.; Fernández-López, J.A.; Alemany, M. Estimation of the metabolizable energy equivalence of dietary proteins. Eur. J. Nutr. 2007, 46, 1–11. [Google Scholar] [CrossRef]
- Livesey, G. The energy equivalents of ATP and the energy values of food proteins and fats. Br. J. Nutr. 1984, 51, 15–28. [Google Scholar] [CrossRef]
- Lam, T.; Poljak, A.; McLean, M.; Bahl, N.; Ho, K.K.Y.; Birzniece, V. Testosterone prevents protein loss via the hepatic urea cycle in human. Eur. J. Endocrinol. 2017, 176, 489–496. [Google Scholar] [CrossRef]
- Agnelli, S.; Arriarán, S.; Oliva, L.; Remesar, X.; Fernández-Lápez, J.-A.; Alemany, M. Modulation of rat liver urea cycle and related ammonium metabolism by sex and cafeteria diet. RSC Adv. 2016, 6, 11278–11288. [Google Scholar] [CrossRef]
- De Chiara, F.; Heeboll, S.; Marrone, G.; Montoliu, C.; Hamilton-Dutoit, S.; Ferrandez, A.; Andreola, F.; Rombouts, K.; Gronbaek, H.; Felipo, V.; et al. Urea cycle dysregulation in non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Denise, C.; Mattheyse, F.J.; Balinsky, J.B. Dietary and hormonal regulation of urea cycle enzymes in rat liver. Enzyme 1981, 26, 113–121. [Google Scholar] [CrossRef]
- Lin, R.C.; Snodgrass, P.J.; Rabier, D. Induction of urea cycle enzymes by glucagon and dexamethasone in monolayer cultures of adult rat hepatocytes. J. Biol. Chem. 1982, 257, 5061–5067. [Google Scholar] [CrossRef]
- Ökun, J.G.; Conway, S.; Schmidt, K.V.; Schumacher, J.; Wang, X.; de Guia, R.; Zota, A.; Klement, J.; Seibert, O.; Peters, A.; et al. Molecular regulation of urea cycle function by the liver glucocorticoid receptor. Mol. Metab. 2015, 4, 732–740. [Google Scholar] [CrossRef]
- Treberg, J.R.; Clow, K.A.; Greene, K.A.; Brosnan, M.E.; Brosnan, J.T. Systemic activation of glutamate dehydrogenase increases renal ammoniagenesis: Implications for the hyperinsulinism/hyperammonemia syndrome. Am. J. Physiol. 2010, 298, E1219–E1225. [Google Scholar] [CrossRef]
- Wergedal, J.E.; Harper, A.E. Metabolic adaptations in higher animals. 10. Glutamic dehydrogenase activity of rats consuming high protein diets. Proc. Soc. Exp. Biol. Med. 1964, 116, 600–604. [Google Scholar] [CrossRef]
- Sandoval, I.V.; Sols, A. Gluconeogenesis from serine by the serine-dehydratase-dependent pathway in rat liver. Eur. J. Biochem. 1974, 43, 609–616. [Google Scholar] [CrossRef]
- Kikuchi, G.; Motokawa, Y.; Yoshida, T.; Hiraga, K. Glycine cleavage system: Reaction mechanism, physiological significance, and hyperglycinemia. Proc. Jpn. Acad. B 2008, 84, 246–263. [Google Scholar] [CrossRef]
- Flanagan, W.F.; Holmes, E.W.; Sabina, R.L.; Swain, J.L. Importance of purine nucleotide cycle to energy production in skeletal muscle. Am. J. Physiol. 1986, 251, C795–C802. [Google Scholar] [CrossRef] [PubMed]
- Tornheim, K.; Lowenstein, J.M. The purine nucleotide cycle. The production of ammonia from aspartate by extracts of rat skeletal muscle. J. Biol. Chem. 1972, 247, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Witalison, E.E.; Thompson, P.R.; Hofseth, L.J. Protein arginine deiminases and associated citrullination: Physiological functions and diseases associated with dysregulation. Curr. Drug Targets 2015, 16, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M. Regulation of enzymes of the urea cycle and arginine metabolism. Annu. Rev. Nutr. 2002, 22, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, F.; Sanz, T.; Fernández-López, J.A.; Alemany, M.; Remesar, X. Stable isotope analysis of dietary arginine accrual and disposal efficiency in male rats fed diets of different protein content. RSC Adv. 2016, 6, 69177–69184. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef]
- Peng, C.; Wong, C.C. The story of protein arginine methylation: Characterization, regulation, and function. Expert Rev. Proteom. 2017, 14, 157–170. [Google Scholar] [CrossRef]
- Costa, G.; Ullrich, L.; Kantor, F.; Holland, J.F. Production of elemental nitrogen by certain mammals including man. Nature 1968, 218, 546–551. [Google Scholar] [CrossRef]
- Cissik, J.H.; Johnson, R.E.; Hertig, B.A. Production of gaseous nitrogen during human steady state exercise. Aerosp. Med. 1972, 43, 1245–1250. [Google Scholar] [PubMed]
- Oliva, L.; Alemany, M.; Remesar, X.; Fernández-López, J.A. The food energy/protein ratio regulates the rat urea cycle but not total nitrogen losses. Nutrients 2019, 11, 316. [Google Scholar] [CrossRef]
- Esteve, M.; Rafecas, I.; Remesar, X.; Alemany, M. Nitrogen balance discrepancy in Wistar rats fed a cafeteria diet. Biochem. Int. 1992, 26, 687–694. [Google Scholar]
- Dudka, L.T.; Inglis, H.J.; Johnson, R.E.; Pechinski, J.M.; Plowman, S. Inequality of inspired and expired gaseous nitrogen in man. Nature 1971, 232, 265–268. [Google Scholar] [CrossRef] [PubMed]
- De la Garza, A.L.; Martínez-Tamez, A.M.; Mellado-Negrete, A.; Arjonilla-Becerra, S.; Peña-Vázquez, G.I.; Marín-Obispo, L.M.; Hernández-Brenes, C. Characterization of the cafeteria diet as simulation of the human Western diet and its impact on the lipidomic profile and gut microbiota in obese rats. Nutrients 2023, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Ota, T. Molecular mechanisms of nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH). Adv. Exp. Med. Biol. 2021, 1261, 223–229. [Google Scholar] [CrossRef]
- Paschos, P.; Paletas, K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia 2009, 13, 9–19. [Google Scholar]
- Sims, E.A.; Danforth, E., Jr.; Horton, E.S.; Bray, G.A.; Glennon, J.A.; Salans, L.B. Endocrine and metabolic effects of experimental obesity in man. Recent Prog. Horm. Res. 1973, 29, 457–496. [Google Scholar] [CrossRef]
- Kaufman, F.R. Diabesity: The Obesity-Diabetes Epidemic that Threatens America—And What We Must Do to Stop It; Bentham Dell: New York, NY, USA, 2005; ISBN 978-0-553-90084-2. [Google Scholar]
- Van Harmelen, V.; Röhrig, K.; Hauner, H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism 2004, 53, 632–637. [Google Scholar] [CrossRef]
- Michailidou, Z.; Gomez-Salazar, M.; Alexaki, V.I. Innate immune cells in the adipose tissue in health and metabolic disease. J. Innate Immun. 2021, 14, 4–30. [Google Scholar] [CrossRef]
- Ferrante, A.W., Jr. The immune cells in adipose tissue. Diabetes Obes. Metab. 2013, 15 (Suppl. S3), 34–38. [Google Scholar] [CrossRef]
- Mienaltowski, M.J.; Gonzales, N.L.; Beall, J.M.; Pechanec, M.Y. Basic structure, physiology, and biochemistry of connective tissues and extracellular matrix collagens. Adv. Exp. Med. Biol. 2021, 1348, 5–43. [Google Scholar] [CrossRef]
- Oxlund, H. Relationships between the biomechanical properties, composition and molecular structure of connective tissues. Connect. Tissue Res. 1986, 15, 65–72. [Google Scholar] [CrossRef]
- Maximow, A.A. Relation of blood cells to connective tissues and endothelium. Physiol. Rev. 1924, 4, 533–563. [Google Scholar] [CrossRef]
- Meyer, K.; Rapport, M.M. The mucopolysaccharides of the ground substance of connective tissue. Science 1951, 113, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Young, R.A. The ground substance of connective tissue. J. Physiol. 1894, 16, 325–350. [Google Scholar] [CrossRef] [PubMed]
- Oschman, J.L. Structure and properties of ground substances. Am. Zool. 1984, 24, 199–215. [Google Scholar] [CrossRef]
- Ushiki, T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch. Histol. Cytol. 2002, 65, 109–126. [Google Scholar] [CrossRef]
- Heino, J. The collagen family members as cell adhesion proteins. BioEssays 2007, 29, 1001–1010. [Google Scholar] [CrossRef]
- Ross, R. The elastic fiber a review. J. Histochem. Cytochem. 1973, 21, 199–208. [Google Scholar] [CrossRef]
- Bich, L.; Pradeu, T.; Moreau, J.-F. Understanding multicellularity: The functional organization of the intercellular space. Front. Physiol. 2019, 10, 1170. [Google Scholar] [CrossRef]
- Cenaj, O.; Allison, D.H.R.; Imam, R.; Zeck, B.; Drohan, L.M.; Chiriboga, L.; Llewellyn, J.; Liu, C.Z.; Park, Y.N.; Wells, R.G.; et al. Evidence for continuity of interstitial spaces across tissue and organ boundaries in humans. Commun. Biol. 2021, 4, 436. [Google Scholar] [CrossRef]
- Bich, L.; Pradeu, T.; Moreau, J.-F.; Boyan, B.D.; Hart, D.A.; Enoka, R.M.; Nicolella, D.P.; Resnick, E.; Berkley, K.J.; Sluka, K.A.; et al. Hormonal modulation of connective tissue homeostasis and sex differences in risk for osteoarthritis of the knee. Biol. Sex Differ. 2013, 4, 3. [Google Scholar] [CrossRef]
- Cinti, S. The adipose organ: Morphological perspectives of adipose tissues. Proc. Nutr. Soc. 2001, 60, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Daemen, S.; van Polanen, N.; Hesselink, M.K.C. The effect of diet and exercise on lipid droplet dynamics in human muscle tissue. J. Exp. Biol. 2018, 221 (Suppl. S1), jeb167015. [Google Scholar] [CrossRef] [PubMed]
- Lally, J.S.V.; Snook, L.A.; Han, X.X.; Chabowski, A.; Bonen, A.; Holloway, G.P. Subcellular lipid droplet distribution in red and white muscles in the obese Zucker rat. Diabetologia 2012, 55, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Enjoji, M.; Kohjima, M.; Sugimoto, R.; Ohta, S.; Kotoh, K.; Kuniyoshi, M.; Kobayashi, K.; Imamura, M.; Inoguchi, T.; et al. Adipose differentiation related protein induces lipid accumulation and lipid droplet formation in hepatic stellate cells. In Vitro Cell. Dev. Biol. Anim. 2005, 41, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Zembroski, A.S.; d’Aquila, T.; Buhman, K.K. Characterization of cytoplasmic lipid droplets in each region of the small intestine of lean and diet-induced obese mice in response to dietary fat. Am. J. Physiol. 2021, 321, G75–G86. [Google Scholar] [CrossRef]
- Bosch, M.; Sánchez-Álvarez, M.; Fajardo, A.; Kapetanovic, R.; Steiner, B.; Dutra, F.; Moreira, L.; López, J.A.; Campo, R.; Marí, M.; et al. Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science 2020, 370, aay8085. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.N.; Weller, P.F. Lipid droplets in leukocytes: Organelles linked to inflammatory responses. Exp. Cell Res. 2016, 340, 193–197. [Google Scholar] [CrossRef]
- Chen, L.; Jin, Y.; Wu, J.; Ren, Z. Lipid droplets: A cellular organelle vital for thermogenesis. Int. J. Biol. Sci. 2022, 18, 6176–6188. [Google Scholar] [CrossRef] [PubMed]
- Renne, M.F.; Hariri, H. Lipid droplet-organelle contact sites as hubs for fatty acid metabolism, trafficking, and metabolic channeling. Front. Cell Dev. Biol. 2021, 9, 726261. [Google Scholar] [CrossRef]
- Welte, M.A.; Gould, A.P. Lipid droplet functions beyond energy storage. Biochim. Biophys. Acta 2017, 1862 Pt B, 1260–1272. [Google Scholar] [CrossRef]
- Jüngst, C.; Klein, M.; Zumbusch, A. Long-term live cell microscopy studies of lipid droplet fusion dynamics in adipocytes. J. Lipid Res. 2013, 54, 3419–3429. [Google Scholar] [CrossRef] [PubMed]
- Moren, B.; Stenkula, K.G. Dispersed lipid droplets: An intermediate site for lipid transport and metabolism in primary human adipocytes. J. Lipid Res. 2020, 61, 1141. [Google Scholar] [CrossRef]
- Rotondo, F.; Ho-Palma, A.-C.; Romero, M.M.; Remesar, X.; Fernández-López, J.A.; Alemany, M. Higher lactate production from glucose in cultured adipose nucleated stromal cells than for rat adipocytes. Adipocyte 2019, 8, 61–76. [Google Scholar] [CrossRef]
- Akter, M.H.; Yamaguchi, T.; Hirose, F.; Osumi, T. Perilipin, a critical regulator of fat storage and breakdown, is a target gene of estrogen receptor-related receptor α. Biochem. Biophys. Res. Commun. 2008, 368, 563–568. [Google Scholar] [CrossRef]
- Londos, C.; Brasaemle, D.L.; Schultz, C.J.; Segrest, J.P.; Kimmel, A.R. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin. Cell Dev. Biol. 1999, 10, 51–58. [Google Scholar] [CrossRef]
- Tansey, J.T.; Sztalryd, C.; Hlavin, E.M.; Kimmel, A.R.; Londos, C. The central role of perilipin A in lipid metabolism and adipocyte lipolysis. IUBMB Life 2004, 56, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Begaye, L.; Simcox, J.A. Intramuscular adipocytes: A buried adipose tissue depot deserving more exploration. J. Lipid Res. 2019, 60, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, M.; Weiss, R.; Leizerman, I.; Anaby, D.; Golomb, E.; Leibowitz, G.; Kaiser, N. Scanning electron microscopic analysis of intramyocellular lipid droplets in an animal model of type 2 diabetes. Obesity 2008, 16, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Hong, S.J.; Park, C.-Y.; Park, J.H.; Choi, S.-C.; Woo, S.-K.; Yu, J.W.; Cheon, G.J.; Joo, H.J.; Lim, D.-S. Intramyocardial adipose-derived stem cell transplantation increases pericardial fat with recovery of myocardial function after acute myocardial infarction. PLoS ONE 2016, 11, e0158067. [Google Scholar] [CrossRef]
- Al-Dibouni, A.; Gaspar, R.; Ige, S.; Boateng, S.; Cagampang, F.R.; Gibbins, J.; Cox, R.D.; Sellayah, D. Unique genetic and histological signatures of mouse pericardial adipose tissue. Nutrients 2020, 12, 1855. [Google Scholar] [CrossRef] [PubMed]
- Eringa, E.C.; Bakker, W.; Smulders, Y.M.; Serne, E.H.; Yudkin, J.S.; Stehouwer, C.D.A. Regulation of vascular function and insulin sensitivity by adipose tissue: Focus on perivascular adipose tissue. Microcirculation 2007, 14, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alfonso, M.S.; Somoza, B.; Tsvetkov, D.; Kuczmanski, A.; Dashwood, M.; Gil-Ortega, M. Role of perivascular adipose tissue in health and disease. Compr. Physiol. 2018, 8, 23–59. [Google Scholar] [CrossRef]
- Ambrosi, T.H.; Schulz, T.J. The emerging role of bone marrow adipose tissue in bone health and dysfunction. J. Mol. Med. 2017, 95, 1291–1301. [Google Scholar] [CrossRef]
- Cawthorn, W.P.; Scheller, E.L. Bone marrow adipose tissue: Formation, function, and impact on health and disease. Front. Endocrinol. 2017, 8, 112. [Google Scholar] [CrossRef]
- Wang, Q.A.; Scherer, P.E. Remodeling of murine mammary adipose tissue during pregnancy, lactation, and involution. J. Mammary Gland Biol. Neoplasia 2019, 24, 207–212. [Google Scholar] [CrossRef]
- Bederman, I.R.; Foy, S.; Chandramouli, V.; Alexander, J.C.; Previs, S.F. Triglyceride synthesis in epididymal adipose tissue Contribution of glucose and non-glucose carbon sources. J. Biol. Chem. 2009, 284, 6101–6108. [Google Scholar] [CrossRef]
- Narita, T.; Kobayashi, M.; Itakura, K.; Itagawa, R.; Kabaya, R.; Sudo, Y.; Okita, N.; Higami, Y. Differential response to caloric restriction of retroperitoneal, epididymal, and subcutaneous adipose tissue depots in rats. Exp. Gerontol. 2018, 104, 127–137. [Google Scholar] [CrossRef]
- Flechtner-Mors, M.; Ditschuneit, H.H.; Yip, I.; Adler, G. Sympathetic modulation of lipolysis in subcutaneous adipose tissue: Effects of gender and energy restriction. J. Lab. Clin. Med. 1999, 134, 33–41. [Google Scholar] [CrossRef]
- Sbierski-Kind, J.; Mai, K.; Kath, J.; Jürisch, A.; Streitz, M.; Kuchenbecker, L.; Babel, N.; Nienen, M.; Jurchott, K.; Spranger, L.; et al. Association between subcutaneous adipose tissue inflammation, insulin resistance, and calorie restriction in obese females. J. Immunol. 2020, 205, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Lovejoy, J.C.; Greenway, F.; Ryan, D.; Dejonge, L.; de la Bretonne, J.; Volafova, J.; Bray, G.A. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 2001, 50, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Grigoraş, A.; Anca Balan, R.; Cǎruntu, I.-D.; Giuşcǎ, S.E.; Lozneanu, L.; Avadanei, R.E.; Rusu, A.; Riscanu, L.A.; Amalinei, C. Perirenal adipose tissue-current knowledge and future opportunities. J. Clin. Med. 2021, 10, 1291. [Google Scholar] [CrossRef] [PubMed]
- Catalano, K.J.; Stefanovski, D.; Bergman, R.N. Critical role of the mesenteric depot versus other intra-abdominal adipose depots in the development of insulin resistance in young rats. Diabetes 2010, 59, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Tan, J.; Chi, Y.; Zhang, F.; Xu, J.; Song, Y.; Cong, X.; Wu, N.; Liu, Y. Mesenteric adipose tissue contributes to intestinal barrier integrity and protects against nonalcoholic fatty liver disease in mice. Am. J. Physiol. 2018, 315, G659–G670. [Google Scholar] [CrossRef]
- Eisenstein, A.; Chitalia, S.V.; Ravid, K. Bone marrow and adipose tissue adenosine receptors effect on osteogenesis and adipogenesis. Int. J. Mol. Sci. 2020, 21, 7470. [Google Scholar] [CrossRef]
- Turaihi, A.H.; Bakker, W.; van Hinsbergh, V.W.M.; Serne, E.H.; Smulders, Y.M.; Niessen, H.W.M.; Eringa, E.C. Insulin receptor substrate 2 controls insulin-mediated vasoreactivity and perivascular adipose tissue function in muscle. Front. Physiol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ding, Y.; Zeng, Q.; Wang, D.; Liu, G.; Hussain, Z.; Xiao, B.; Liu, W.; Deng, T. Characteristics of mesenteric adipose tissue attached to different intestinal segments and their roles in immune regulation. Am. J. Physiol. 2022, 322, G310–G326. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.M.; Roy, S.; Pouillot, K.; Feito, M.; Esteve, M.; Grasa, M.M.; Fernández-López, J.-A.; Alemany, M.; Remesar, X. Treatment of rats with a self-selected hyperlipidic diet, increases the lipid content of the main adipose tissue sites in a proportion similar to that of the lipids in the rest of organs and tissues. PLoS ONE 2014, 9, e90995. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Smorlesi, A.; Frontini, A.; Barbatelli, G.; Cinti, S. White, brown and pink adipocytes: The extraordinary plasticity of the adipose organ. Eur. J. Endocrinol. 2014, 170, R159–R171. [Google Scholar] [CrossRef] [PubMed]
- Labbé, S.M.; Caron, A.; Bakan, I.; Laplante, M.; Carpentier, A.C.; Lecomte, R.; Richard, D. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J. 2015, 29, 2046–2058. [Google Scholar] [CrossRef]
- Rothwell, N.J.; Stock, M.J. Similarities between cold-induced and diet-induced thermogenesis in the rat. Can. J. Physiol. Pharmacol. 1980, 58, 842–848. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Li, M.; Speakman, J.R. Switching on the furnace: Regulation of heat production in brown adipose tissue. Mol. Asp. Med. 2019, 68, 60–73. [Google Scholar] [CrossRef]
- Rothwell, N.J.; Stock, M.J. A role for brown adipose tissue in diet-induced thermogenesis. Nature 1979, 281, 31–35. [Google Scholar] [CrossRef]
- Zafrir, B. Brown adipose tissue: Research milestones of a potential player in human energy balance and obesity. Horm. Metab. Res. 2013, 45, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.O.; Frydman, M.L. Tissue distribution of cold-induced thermogenesis in conscious warm-or cold-acclimated rats reevaluated from changes in tissue blood flow: The dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can. J. Physiol. Pharmacol. 1978, 57, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Lesná, I.; Vybíral, S.; Janský, L.; Zeman, V. Human nonshivering thermogenesis. J. Therm. Biol. 1999, 24, 63–69. [Google Scholar] [CrossRef]
- Van Marken Lichtenbelt, W.D.; Schrauwen, P. Implications of nonshivering thermogenesis for energy balance regulation in humans. Am. J. Physiol. 2011, 301, R285–R296. [Google Scholar] [CrossRef]
- Mengel, L.A.; Nemati Moud, B.; Seidl, H.; Mesas-Fernández, A.; Seeliger, C.; Brandl, B.; Skurk, T.; Holzapfel, C.; Claussnitzer, M.; Hauner, H. Effect of BMI on the thermogenic response to cold exposure and associated changes in metabolism and browning markers in adult humans. Obes. Facts 2022, 15, 405–415. [Google Scholar] [CrossRef]
- Norgan, N.G. Thermogenesis above maintenance in humans. Proc. Nutr. Soc. 1990, 49, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Brychta, R.J.; Chen, K.Y. Cold-induced thermogenesis in humans. Eur. J. Clin. Nutr. 2017, 71, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Leibel, R.L. Adaptive thermogenesis in humans. Int. J. Obes. 2010, 34 (Suppl. S1), S47–S55. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Filho, A.B.; Peixoto, A.S.; Castro, É.; Oliveira, T.E.; Perandini, L.A.; Moreira, R.J.; da Silva, R.P.; da Silva, B.P.; Moretti, E.H.; Steiner, A.A.; et al. Futile cycle of β-oxidation and de novo lipogenesis are associated with essential fatty acids depletion in lipoatrophy. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159264. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Rognstad, R. Futile cycles in the metabolism of glucose. Curr. Top. Cell. Regul. 1976, 10, 237–289. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, T.; Lix, L.; Giesbrecht, G. Shivering heat production and body fat protect the core from cooling during body immersion, but not during head submersion: A structural equation model. Comput. Biol. Med. 2011, 41, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, E.; Haman, F.; Weber, J.-M. Fuel selection in Wistar rats exposed to cold: Shivering thermogenesis diverts fatty acids from re-esterification to oxidation. J. Physiol. 2009, 587, 4349–4359. [Google Scholar] [CrossRef]
- Ma, S.W.Y.; Nadeau, B.E.; Foster, D.O. Evidence for liver as the major site of the diet-induced thermogenesis of rats fed a cafeteria diet. Can. J. Physiol. Pharmacol. 1987, 65, 1802–1804. [Google Scholar] [CrossRef]
- Simcox, J.; Geoghegan, G.; Maschek, J.A.; Bensard, C.L.; Pasquali, M.; Miao, R.; Lee, S.; Jiang, L.; Huck, I.; Kershaw, E.E.; et al. Global analysis of plasma lipids identifies liver-derived acylcarnitines as a fuel source for brown fat thermogenesis. Cell Metab. 2017, 26, 509–522. [Google Scholar] [CrossRef]
- Aydin, J.; Shabalina, I.G.; Place, N.; Reiken, S.; Zhang, S.J.; Bellinger, A.M.; Nedergaard, J.; Cannon, B.; Marks, A.R.; Bruton, J.D.; et al. Nonshivering thermogenesis protects against defective calcium handling in muscle. FASEB J. 2008, 22, 3919–3924. [Google Scholar] [CrossRef]
- Jansky, L.; Hart, J.S. Participation of skeletal muscle and kidney during nonshivering thermogenesis in cold-acclimated rats. Can. J. Biochem. Physiol. 1963, 41, 953–964. [Google Scholar] [CrossRef]
- Astrup, A. Thermogenesis in human brown adipose-tissue and skeletal-muscle induced by sympathomimetic stimulation. Acta Endocrinol. 1986, 112 (Suppl. S278), 7–32. [Google Scholar] [CrossRef]
- Blondin, D.P. Human thermogenic adipose tissue. Curr. Opin. Genet. Dev. 2023, 80, 102054. [Google Scholar] [CrossRef] [PubMed]
- Székely, M.; Kellermayer, M.; Cholnoky, G.; Sümegi, I. Thermoregulatory heat production by periaortic brown adipose tissue in the non-cold-acclimatized rat. Experientia 1970, 26, 1314–1315. [Google Scholar] [CrossRef] [PubMed]
- Chechi, K.; van Marken Lichtenbelt, W.; Richard, D. Brown and beige adipose tissues: Phenotype and metabolic potential in mice and men. J. Appl. Physiol. 2018, 124, 482–496. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Forner, F.; Kumar, C.; Luber, C.A.; Fromme, T.; Klingenspor, M.; Mann, M. Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell Metab. 2009, 10, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Hammad Mirza, A.; Cui, L.; Zhang, S.; Liu, P. Comparative proteomics reveals that lipid droplet-anchored mitochondria are more sensitive to cold in brown adipocytes. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2021, 1866, 158992. [Google Scholar] [CrossRef]
- Rosina, M.; Ceci, V.; Turchi, R.; Chuan, L.; Borcherding, N.; Sciarretta, F.; Sánchez-Díaz, M.; Tortolici, F.; Karlinsey, K.; Chiurchiù, V.; et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 2022, 34, 533–548. [Google Scholar] [CrossRef]
- Cannon, B.; Hedin, A.; Nedergaard, J. Exclusive occurrence of thermogenin antigen in brown adipose tissue. FEBS Lett. 1982, 150, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Carrière, A.; Jeanson, Y.; Berger-Müller, S.; André, M.; Chenouard, V.; Arnaud, E.; Barreau, C.; Walther, R.; Galinier, A.; Wdziekonski, B.; et al. Browning of white adipose cells by intermediate metabolites: An adaptive mechanism to alleviate redox pressure. Diabetes 2014, 63, 3253–3265. [Google Scholar] [CrossRef]
- Jankovic, A.; Otasevic, V.; Stancic, A.; Buzadzic, B.; Korac, A.; Korac, B. Physiological regulation and metabolic role of browning in white adipose tissue. Horm. Mol. Biol. Clin. Investig. 2017, 31, 20170034. [Google Scholar] [CrossRef] [PubMed]
- McKie, G.L.; Wright, D.C. Biochemical adaptations in white adipose tissue following aerobic exercise: From mitochondrial biogenesis to browning. Biochem. J. 2020, 477, 1061–1081. [Google Scholar] [CrossRef]
- Oliveira Pinto, Y.; Lara Festuccia, W.T.; Magdalon, J. The involvement of the adrenergic nervous system in activating human brown adipose tissue and browning. Hormones 2022, 21, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Zuriaga, M.A.; Fuster, J.J.; Gokce, N.; Walsh, K. Humans and mice display opposing patterns of “browning” gene expression in visceral and subcutaneous white adipose tissue depots. Front. Cardiovasc. Med. 2017, 4, 27. [Google Scholar] [CrossRef]
- Arriarán, S.; Agnelli, S.; Sabater, D.; Remesar, X.; Fernández-López, J.A.; Alemany, M. Evidences of basal lactate production in the main white adipose tissue sites of rats. Effects of sex and a cafeteria diet. PLoS ONE 2015, 10, e0119572. [Google Scholar] [CrossRef]
- Hagström, E.; Arner, P.; Ungerstedt, U.; Bolinder, J. Subcutaneous adipose tissue: A source of lactate production after glucose ingestion in humans. Am. J. Physiol. 1990, 258, E888–E893. [Google Scholar] [CrossRef]
- Muñoz, S.; Franckhauser, S.; Elias, I.; Ferré, T.; Hidalgo, A.; Monteys, A.M.; Molas, M.; Cerdán, S.; Pujol, A.; Ruberte, J.; et al. Chronically increased glucose uptake by adipose tissue leads to lactate production and improved insulin sensitivity rather than obesity in the mouse. Diabetologia 2010, 53, 2417–2430. [Google Scholar] [CrossRef]
- Jansson, P.A.; Larsson, A.; Smith, U.; Lönnroth, P. Glycerol production in subcutaneous adipose tissue of lean and obese humans. J. Clin. Investig. 1992, 89, 1610–1617. [Google Scholar] [CrossRef]
- Rotondo, F.; Ho-Palma, A.C.; Remesar, X.; Fernandez-Lopez, J.A.; Romero, M.M.; Alemany, M. Glycerol is synthesized and secreted by adipocytes to dispose of excess glucose, via glycerogenesis and increased acyl-glycerol turnover. Sci. Rep. 2017, 7, 8983. [Google Scholar] [CrossRef] [PubMed]
- Snell, K.; Duff, D.A. Alanine release by rat adipose tissue in vitro. Biochem. Biophys. Res. Commun. 1977, 77, 925–931. [Google Scholar] [CrossRef]
- Arriarán, S.; Agnelli, S.; Remesar, X.; Fernández-López, J.-A.; Alemany, M. The urea cycle of rat white adipose tissue. RSC Adv. 2015, 5, 93403–93414. [Google Scholar] [CrossRef]
- Halama, A.; Horsch, M.; Kastenmüller, G.; Moller, G.; Kumar, P.; Prehn, C.; Laumen, H.; Hauner, H.; Hrabe de Angelis, M.; Beckers, J.; et al. Metabolic switch during adipogenesis: From branched chain amino acid catabolism to lipid synthesis. Arch. Biochem. Biophys. 2016, 589, 93–107. [Google Scholar] [CrossRef]
- Herman, M.A.; She, P.; Peroni, O.D.; Lynch, C.J.; Kahn, B.B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem. 2010, 285, 11348–11356. [Google Scholar] [CrossRef]
- Hodson, L. Adipose tissue oxygenation: Effects on metabolic function. Adipocyte 2014, 3, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Lempesis, I.G.; van Meijel, R.L.J.; Manolopoulos, K.N.; Goossens, G.H. Oxygenation of adipose tissue: A human perspective. Acta Physiol. 2020, 228, e13298. [Google Scholar] [CrossRef]
- Sabater, D.; Arriarán, S.; Romero, M.M.; Agnelli, S.; Remesar, X.; Fernández-López, J.A.; Alemany, M. Cultured 3T3L1 adipocytes dispose of excess medium glucose as lactate under abundant oxygen availability. Sci. Rep. 2014, 4, 3663. [Google Scholar] [CrossRef]
- Cinti, S. Transdifferentiation properties of adipocytes in the adipose organ. Am. J. Physiol. 2009, 297, E977–E986. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, A.; Oh, K.-J.; Lee, S.C.; Kim, W.K.; Bae, K.-H. The role of adipose tissue mitochondria: Regulation of mitochondrial function for the treatment of metabolic diseases. Int. J. Mol. Sci. 2019, 20, 4924. [Google Scholar] [CrossRef]
- Yin, X.; Lanza, I.R.; Swain, J.M.; Sarr, M.G.; Nair, K.S.; Jensen, M.D. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J. Clin. Endocrinol. Metab. 2014, 99, E209–E216. [Google Scholar] [CrossRef]
- Cheong, L.Y.; Xu, A. Intercellular and inter-organ crosstalk in browning of white adipose tissue: Molecular mechanism and therapeutic complications. J. Mol. Cell Biol. 2021, 13, 466–479. [Google Scholar] [CrossRef]
- Smorlesi, A.; Frontini, A.; Giordano, A.; Cinti, S. The adipose organ: White-brown adipocyte plasticity and metabolic inflammation. Obes. Rev. 2012, 13 (Suppl. S2), 83–96. [Google Scholar] [CrossRef]
- Dong, L.-F.; Rohlena, J.; Zobalova, R.; Nahacka, Z.; Rodriguez, A.-M.; Berridge, M.V.; Neuzil, J. Mitochondria on the move: Horizontal mitochondrial transfer in disease and health. J. Cell Biol. 2023, 222, e202211044. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Havel, P.J. Role of adipose tissue in body-weight regulation: Mechanisms regulating leptin production and energy balance. Proc. Nutr. Soc. 2000, 59, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Rhode, S.C.; Beier, J.P.; Ruhl, T. Adipose tissue stem cells in peripheral nerve regeneration in vitro and in vivo. J. Neurosci. Res. 2021, 99, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Tchernof, A.; Mansour, M.F.; Pelletier, M.; Boulet, M.-M.; Nadeau, M.; Luu-The, V. Updated survey of the steroid-converting enzymes in human adipose tissues. J. Steroid Biochem. Mol. Biol. 2015, 147, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Killinger, D.W.; Strutt, B.J.; Roncari, D.A.; Khalil, M.W. Estrone formation from dehydroepiandrosterone in cultured human breast adipose stromal cells. J. Steroid Biochem. Mol. Biol. 1995, 52, 195–201. [Google Scholar] [CrossRef]
- Andrew, R.; Westerbacka, J.; Wahren, J.; Yki-Jarvinen, H.; Walker, B.R. The contribution of visceral adipose tissue to splanchnic cortisol production in healthy humans. Diabetes 2005, 54, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.A.; Manolopoulos, K.N.; Iqbal, J.; Cruden, N.L.; Stimson, R.H.; Reynolds, R.M.; Newby, D.E.; Andrew, R.; Karpe, F.; Walker, B.R. Recycling between cortisol and cortisone in human splanchnic, subcutaneous adipose, and skeletal muscle tissues in vivo. Diabetes 2012, 61, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Fried, S.K.; Mundt, S.S.; Wang, Y.; Sullivan, S.; Stefanni, A.; Daugherty, B.L.; Hermanowski-Vosatka, A. Depot-specific regulation of the conversion of cortisone to cortisol in human adipose tissue. Obesity 2008, 16, 1178–1185. [Google Scholar] [CrossRef]
- Ohlsson, C.; Hammarstedt, A.; Vandenput, L.; Saarinen, N.; Ryberg, H.; Windahl, S.H.; Farman, H.H.; Jansson, J.-O.; Moverare-Skrtic, S.; Smith, U.; et al. Increased adipose tissue aromatase activity improves insulin sensitivity and reduces adipose tissue inflammation in male mice. Am. J. Physiol. 2017, 313, E450–E462. [Google Scholar] [CrossRef]
- Ostinelli, G.; Laforest, S.; Denham, S.G.; Gauthier, M.-F.; Drolet-Labelle, V.; Scott, E.; Hould, F.-S.; Marceau, S.; Homer, N.Z.M.; Bégin, C.; et al. Increased adipose tissue indices of androgen catabolism and aromatization in women with metabolic dysfunction. J. Clin. Endocrinol. Metab. 2022, 107, E3330–E3342. [Google Scholar] [CrossRef]
- Perel, E.; Killinger, D.W. The interconversion and aromatization of androgens by human adipose tissue. J. Steroid Biochem. 1979, 10, 623–627. [Google Scholar] [CrossRef]
- Esteve, M.; Virgili, J.; Aguilar, H.; Balada, F.; Fernández-López, J.A.; Remesar, X.; Alemany, M. Leptin enhances the synthesis of oleoyl-estrone from estrone in white adipose tissue. Eur. J. Nutr. 1999, 38, 99–104. [Google Scholar] [CrossRef]
- Hetemäki, N.; Mikkola, T.S.; Tikkanen, M.J.; Wang, F.; Hämäläinen, E.; Turpeinen, U.; Haanpää, M.; Vihma, V.; Savolainen-Peltonen, H. Adipose tissue estrogen production and metabolism in premenopausal women. J. Steroid Biochem. Mol. Biol. 2021, 209, e105849. [Google Scholar] [CrossRef]
- Kuryłowicz, A. Estrogens in adipose tissue physiology and obesity-related dysfunction. Biomedicines 2023, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N.; Karpe, F.; Fielding, B.A.; Macdonald, I.A.; Coppack, S.W. Integrative physiology of human adipose tissue. Int. J. Obes. 2003, 27, 875–888. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Scherer, T.; O’Hare, J.; Diggs-Andrews, K.; Schweiger, M.; Cheng, B.; Lindtner, C.; Zielinski, E.; Vempati, P.; Su, K.; Dighe, S.; et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011, 13, 183–194. [Google Scholar] [CrossRef]
- Abreu-Vieira, G.; Hagberg, C.E.; Spalding, K.L.; Cannon, B.; Nedergaard, J. Adrenergically stimulated blood flow in brown adipose tissue is not dependent on thermogenesis. Am. J. Physiol. 2015, 308, E822–E829. [Google Scholar] [CrossRef]
- Cero, C.; Lea, H.J.; Zhu, K.Y.; Shamsi, F.; Tseng, Y.-H.; Cypess, A.M. β3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. JCI Insight 2021, 6, e139160. [Google Scholar] [CrossRef]
- Conde, J.; Scotece, M.; Gomez, R.; Lopez, V.; Gomez-Reino, J.J.; Lago, F.; Gualillo, O. Adipokines: Biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity. BioFactors 2011, 37, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, L.; Liu, S.; Shan, T. Adipose tissue adipokines and lipokines: Functions and regulatory mechanism in skeletal muscle development and homeostasis. Metabolism 2023, 139, 155379. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T. Perivascular adipose tissue, a janus-faced regulator of vascular function. Circ. J. 2010, 74, 1300–1301. [Google Scholar] [CrossRef]
- Pulgar, V.M. Neurovascular effects of perivascular adipose tissue: Regulation of sympathetic-sensory communication. J. Cardiovasc. Pharmacol. 2020, 75, 18–20. [Google Scholar] [CrossRef]
- Barthelemy, J.; Bogard, G.; Wolowczuk, I. Beyond energy balance regulation: The underestimated role of adipose tissues in host defense against pathogens. Front. Immunol. 2023, 14, 1083191. [Google Scholar] [CrossRef]
- Caspar-Bauguil, S.; Cousin, B.; Galinier, A.; Segafredo, C.; Nibbelink, M.; Andre, A.; Casteilla, L.; Pénicaud, L. Adipose tissues as an ancestral immune organ: Site-specific change in obesity. FEBS Lett. 2005, 579, 3487–3492. [Google Scholar] [CrossRef]
- Song, Y.C.; Lee, S.E.; Jin, Y.; Park, H.W.; Chun, K.-H.; Lee, H.-W. Classifying the linkage between adipose tissue inflammation and tumor growth through cancer-associated adipocytes. Mol. Cells 2020, 43, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Vallega, K.A.; Bosco, D.B.; Ren, Y.; Sang, Q.-X.A. Macrophage-conditioned media promotes adipocyte cancer association, which in turn stimulates breast cancer proliferation and migration. Biomolecules 2022, 12, 1757. [Google Scholar] [CrossRef] [PubMed]
- Bartness, T.J.; Song, C.K. Brain-adipose tissue neural crosstalk. Physiol. Behav. 2007, 91, 343–351. [Google Scholar] [CrossRef]
- Caron, A.; Lee, S.; Elmquist, J.K.; Gautron, L. Leptin and brain-adipose crosstalks. Nat. Rev. Neurosci. 2018, 19, 153–165. [Google Scholar] [CrossRef]
- Little, J.P.; Safdar, A. Adipose-brain crosstalk: Do adipokines have a role in neuroprotection? Neural Regen. Res. 2015, 10, 1381–1382. [Google Scholar] [CrossRef] [PubMed]
- Pénicaud, L. Relationships between adipose tissues and brain: What do we learn from animal studies? Diabetes Metab. 2010, 36, S39–S44. [Google Scholar] [CrossRef]
- Parimisetty, A.; Dorsemans, A.-C.; Awada, R.; Ravanan, P.; Diotel, N.; Lefebre d’Hellencourt, C. Secret talk between adipose tissue and central nervous system via secreted factors-an emerging frontier in the neurodegenerative research. J. Neuroinflammation 2016, 13, 67. [Google Scholar] [CrossRef]
- Patist, C.M.; Stapelberg, N.J.C.; du Toit, E.F.; Headrick, J.P. The brain-adipocyte-gut network: Linking obesity and depression subtypes. Cogn. Affect. Behav. Neurosci. 2018, 18, 1121–1144. [Google Scholar] [CrossRef]
- Malinici, E.; Sirbu, A.; Popa, M.; Fica, S. Linking the brain and bone through fat. J. Mind Med. Sci. 2021, 8, 17–26. [Google Scholar] [CrossRef]
- Gahete, M.D.; Granata, R.; Luque, R.M. Editorial: Pathophysiological interrelationship between obesity, metabolic diseases, and cancer. Front. Oncol. 2021, 11, 755735. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Zhou, Z.; Song, L.; Chen, S.; Yang, Y.; Hu, Y.; Wang, Y.; Wu, S.; Tian, Y. Lifetime risk of cardiovascular disease and life expectancy with and without cardiovascular disease according to changes in metabolic syndrome status. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 373–381. [Google Scholar] [CrossRef]
- Li, W.; Qiu, X.; Ma, H.; Geng, Q. Incidence and long-term specific mortality trends of metabolic syndrome in the United States. Front. Endocrinol. 2023, 13, 1029736. [Google Scholar] [CrossRef]
- Paramio Rodríguez, A.; Letrán Sarría, Y.; Requesén Gálvez, R.L. Síndrome metabólico en personas mayores de 40 años del primer nivel de atención. Finlay 2022, 12, 21–28. [Google Scholar]
- Zhang, L.; Zuo, L.; Wang, F.; Wang, M.; Wang, S.; Liu, L.; Wang, H. Metabolic syndrome and chronic kidney disease in a Chinese population aged 40 years and older. Mayo Clin. Proc. 2007, 82, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Bosello, O.; Zamboni, M. Visceral obesity and metabolic syndrome. Obes. Rev. 2000, 1, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P.G.; Albon, L. Obesity, non-insulin-dependent diabetes mellitus and the metabolic syndrome. Br. Med. Bull. 1997, 53, 322–340. [Google Scholar] [CrossRef] [PubMed]
- Salamone, F.; Bugianesi, E. Nonalcoholic fatty liver disease: The hepatic trigger of the metabolic syndrome. J. Hepatol. 2010, 53, 1146–1147. [Google Scholar] [CrossRef]
- Branth, S.; Ronquist, G.; Stridsberg, M.; Hambraeus, L.; Kindgren, E.; Olsson, R.; Carlander, D.; Arnetz, B. Development of abdominal fat and incipient metabolic syndrome in young healthy men exposed to long-term stress. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 427–435. [Google Scholar] [CrossRef]
- Prescott, E.; Godtfredsen, N.; Osler, M.; Schnohr, P.; Barefoot, J. Social gradient in the metabolic syndrome not explained by psychosocial and behavioural factors: Evidence from the Copenhagen City Heart Study. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, S.E.; Whincup, P.H.; Morris, R.; Lennon, L.; Wannamethee, S.G. Is socioeconomic position related to the prevalence of metabolic syndrome? Influence of social class across the life course in a population-based study of older men. Diabetes Care 2008, 31, 2380–2382. [Google Scholar] [CrossRef] [PubMed]
- Junquero, D.; Rival, Y. Syndrome métabolique: Quelle définition pour quel(s) traitement(s)? M S-Med. Sci. 2005, 21, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Drug therapy of the metabolic syndrome: Minimizing the emerging crisis in polypharmacy. Nat. Rev. Drug Discov. 2006, 5, 294–309. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Quarti-Trevano, F.; Mineo, C.; Lonati, L.; Facchetti, R.; Mancia, G. Reinforcement of the adrenergic overdrive in the metabolic syndrome complicated by obstructive sleep apnea. J. Hypertens. 2010, 28, 1313–1320. [Google Scholar] [CrossRef]
- Hsu, C.H. Different impacts of metabolic syndrome components on insulin resistance in type 2 diabetes. Int. J. Endocrinol. 2013, 2013, 740419. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, B.; Gluvic, Z.M.; Obradovic, M.; Radunovic, M.; Rizzo, M.; Banach, M.; Isenovic, E.R. Non-alcoholic fatty liver disease, metabolic syndrome, and type 2 diabetes mellitus: Where do we stand today? Arch. Med. Sci. 2023, 19, 884–894. [Google Scholar] [CrossRef]
- Pajunen, P.; Rissanen, H.; Härkänen, T.; Jula, A.; Reunanen, A.; Salomaa, V. The metabolic syndrome as a predictor of incident diabetes and cardiovascular events in the Health 2000 Study. Diabetes Metab. 2010, 36, 395–401. [Google Scholar] [CrossRef]
- Dunbar, J.A.; Reddy, P.; Davis-Lameloise, N.; Philpot, B.; Laatikainen, T.; Kilkkinen, A.; Bunker, S.J.; Best, J.D.; Vartiainen, E.; Kai Lo, S.; et al. Depression: An important comorbidity with metabolic syndrome in a general population. Diabetes Care 2008, 31, 2368–2373. [Google Scholar] [CrossRef]
- Pan, A.; Keum, N.; Okereke, O.I.; Sun, Q.; Kivimaki, M.; Rubin, R.R.; Hu, F.B. Bidirectional association between depression and metabolic syndrome. A systematic review and meta-analysis of epidemiological studies. Diabetes Care 2012, 35, 1171–1180. [Google Scholar] [CrossRef]
- Hildrum, B.; Mykletun, A.; Midthjell, K.; Ismail, K.; Dahl, A.A. No association of depression and anxiety with the metabolic syndrome: The Norwegian HUNT study. Acta Psychiatr. Scand. 2009, 120, 14–22. [Google Scholar] [CrossRef]
- Takeuchi, T.; Nakao, M.; Nomura, K.; Yano, E. Association of metabolic syndrome with depression and anxiety in Japanese men. Diabetes Metab. 2009, 35, 32–36. [Google Scholar] [CrossRef]
- James, W.P.T.; Rigby, N.; Leach, R. Obesity and the metabolic syndrome. The stress on society. Ann. N. Y. Acad. Sci. 2006, 1083, 1–10. [Google Scholar] [CrossRef]
- Türkoglu, C.; Duman, B.S.; Günay, D.; Cagatay, P.; Özcan, R.; Büyükdevrim, A.S. Effect of abdominal obesity on insulin resistance and the components of the metabolic syndrome: Evidence supporting obesity as the central feature. Obes. Surg. 2003, 13, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Beigh, S.H.; Jain, S. Prevalence of metabolic syndrome and gender differences. Bioinformation 2012, 8, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-E.; Zhang, F.-L.; Zhang, P.; Liu, D.; Liu, H.-Y.; Guo, Z.-N.; Yang, Y. Sex-based differences in and risk factors for metabolic syndrome in adults aged 40 years and above in Northeast China: Results from the cross-sectional China national stroke screening survey. BMJ Open 2021, 11, e038671. [Google Scholar] [CrossRef] [PubMed]
- Henstridge, D.C.; Abildgaard, J.; Lindegaard, B.; Febbraio, M.A. Metabolic control and sex: A focus on inflammatory-linked mediators. Br. J. Pharmacol. 2019, 176, 4193–4207. [Google Scholar] [CrossRef] [PubMed]
- Matsiukevich, D.; Kovacs, A.; Li, T.; Kokkonen-Simon, K.; Matkovich, S.J.; Oladipupo, S.S.; Ornitz, D.M. Characterization of a robust mouse model of heart failure with preserved ejection fraction. Am. J. Physiol. 2023, 325, H203–H231. [Google Scholar] [CrossRef] [PubMed]
- Arganini, C.; Saba, A.; Comitato, R.; Virgili, F.; Turrini, A. Gender differences in food choice and dietary intake in modern western societies. In Public Health Social and Behavioral Health; Maddock, J., Ed.; In Tech: Rijeka, Croatia; Shanghai, China, 2012; pp. 83–102. [Google Scholar] [CrossRef]
- Ozdemir, C.; Akbas Gunes, N. The effect of diet and regular exercise on psychological resilience in obese or overweight women. Int. J. Clin. Pract. 2021, 75, e14320. [Google Scholar] [CrossRef]
- Luine, V. Estradiol: Mediator of memories, spine density and cognitive resilience to stress in female rodents. J. Steroid Biochem. Mol. Biol. 2016, 160, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Link, J.C.; Hasin-Brumshtein, Y.; Cantor, R.M.; Chen, X.; Arnold, A.P.; Lusis, A.J.; Reue, K. Diet, gonadal sex, and sex chromosome complement influence white adipose tissue miRNA expression. BMC Genom. 2017, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Reue, K. Sex differences in obesity: X chromosome dosage as a risk factor for increased food intake, adiposity and co-morbidities. Physiol. Behav. 2017, 176, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Sergi, G.; Dianin, M.; Bertocco, A.; Zanforlini, B.M.; Curreri, C.; Mazzochin, M.; Simons, L.A.; Manzato, E.; Trevisan, C. Gender differences in the impact of metabolic syndrome components on mortality in older people: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.A.; Meehan, A.G.; Shah, A. The age related decrease in testosterone is significantly exacerbated in obese men with the metabolic syndrome. What are the implications for the relatively high incidence of erectile dysfunction observed in these men? J. Urol. 2006, 176, 1524–1527. [Google Scholar] [CrossRef]
- Guay, A.; Jacobson, J. The relationship between testosterone levels, the metabolic syndrome (by two criteria), and insulin resistance in a population of men with organic erectile dysfunction. J. Sex. Med. 2007, 4, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Munhos Hermoso, D.A.; Veiga Bizerra, P.F.; Polimeni Constantin, R.; Ishii-Iwamoto, E.L.; Hideo Gilglioni, E. Association between metabolic syndrome, hepatic steatosis, and testosterone deficiency: Evidences from studies with men and rodents. Aging Male 2021, 23, 1296–1315. [Google Scholar] [CrossRef]
- Pospíšilová, H.; Vaňková, M.; Hill, M.; Meloun, M.; Bendlová, B.; Dušková, M.; Stárka, L. The differences between aromatizable and non-aromatizable androgens in relation to body composition and metabolic syndrome risk factors in men. J. Steroid Biochem. Mol. Biol. 2012, 132, 105–111. [Google Scholar] [CrossRef]
- Camporez, J.P.; Lyu, K.; Goldberg, E.L.; Zhang, D.; Cline, G.W.; Jurczak, M.J.; Dixit, V.D.; Petersen, K.F.; Shulman, G.I. Anti-inflammatory effects of oestrogen mediate the sexual dimorphic response to lipid-induced insulin resistance. J. Physiol. 2019, 597, 3885–3903. [Google Scholar] [CrossRef]
- Remesar, X.; Oliva, L.; Fernández-López, J.A.; Alemany, M. Diet carbohydrate, protein content and circulating 17-β estradiol are critical factors for energy partition in the rat. Preprint 2023, 2023100281. [Google Scholar] [CrossRef]
- Brodsky, I.G.; Balagopal, P.; Nair, K.S. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men. A clinical research center study. J. Clin. Endocrinol. Metab. 1996, 81, 3469–3475. [Google Scholar] [CrossRef] [PubMed]
- Van den Beld, A.W.; de Jong, F.H.; Grobbee, D.E.; Pols, H.A.; Lamberts, S.W.J. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J. Clin. Endocrinol. Metab. 2000, 85, 3276–3282. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Smith, G.I.; Patterson, B.W.; Reeds, D.N.; Kampelman, J.; Magkos, F.; Mittendorfer, B. Testosterone increases the muscle protein synthesis rate but does not affect very-low-density lipoprotein metabolism in obese premenopausal women. Am. J. Physiol. 2012, 302, E740–E746. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Wang, X.; Sheng, Y.; Chen, S.; Lai, B.; Lv, R.; Yu, J. Metabolic syndrome and its association with components of sarcopenia in older community-dwelling Chinese. J. Biomed. Res. 2022, 36, 120–126. [Google Scholar] [CrossRef]
- Chao, W.-C.; Wang, S.-Y. Sarcopenia and frailty in elderly: Manifestations, impacts on diseases, and management. Int. J. Gerontol. 2020, 14, 2–5. [Google Scholar] [CrossRef]
- Scott, D.; Blyth, F.; Naganathan, V.; Le Couteur, D.G.; Handelsman, D.J.; Waite, L.M.; Hirani, V. Sarcopenia prevalence and functional outcomes in older men with obesity: Comparing the use of the EWGSOP2 sarcopenia versus ESPEN-EASO sarcopenic obesity consensus definitions. Clin. Nutr. 2023, 42, 1610–1618. [Google Scholar] [CrossRef]
- Borges, R.; Temido, P.; Sousa, L.; Azinhais, P.; Conceicao, P.; Pereira, B.; Leao, R.; Retroz, E.; Brandao, A.; Cristo, L.; et al. Metabolic syndrome and sexual (dys)function. J. Sex. Med. 2009, 6, 2958–2975. [Google Scholar] [CrossRef]
- Esposito, K.; Ciotola, M.; Marfella, R.; di Tommaso, D.; Cobellis, L.; Giugliano, D. The metabolic syndrome: A cause of sexual dysfunction in women. Int. J. Impot. Res. 2005, 17, 224–226. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, D. Obesity, the metabolic syndrome, and sexual dysfunction in men. Clin. Pharmacol. Ther. 2011, 90, 169–173. [Google Scholar] [CrossRef]
- Yuki, A.; Otsuka, R.; Kozakai, R.; Kitamura, I.; Okura, T.; Ando, F.; Shimokata, H. Relationship between low free testosterone levels and loss of muscle mass. Sci. Rep. 2013, 3, 1818. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Islam, R.M.; Bell, R.J.; Hemachandra, C.; Davis, S.R. Endogenous testosterone concentrations and muscle mass, strength and performance in women, a systematic review of observational studies. Clin. Endocrinol. 2023, 98, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, O.; Banga, S.; Heinze-Milne, S.; Rose, R.A.; Pyle, W.G.; Howlett, S.E. Long-term testosterone deficiency modifies myofilament and calcium-handling proteins and promotes diastolic dysfunction in the aging mouse heart. Am. J. Physiol. 2019, 316, H768–H780. [Google Scholar] [CrossRef]
- Pal, M.; Khan, J.; Kumar, R.; Surolia, A.; Gupta, S. Testosterone supplementation improves insulin responsiveness in HFD fed male T2DM mice and potentiates insulin signaling in the skeletal muscle and C2C12 myocyte cell line. PLoS ONE 2019, 14, e0224162. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, L.; Højlund, K.; Hougaard, D.M.; Brixen, K.; Andersen, M. Testosterone therapy increased muscle mass and lipid oxidation in aging men. Age 2012, 34, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Høst, C.; Gormsen, L.C.; Christensen, B.; Jessen, N.; Hougaard, D.M.; Christiansen, J.S.; Pedersen, S.B.; Jensen, M.D.; Nielsen, S.; Gravholt, C.H. Independent effects of testosterone on lipid oxidation and VLDL-TG production a randomized, double-blind, placebo-controlled, crossover study. Diabetes 2013, 62, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, L.V.; Glintborg, D.; Hermann, P.; Hougaard, D.M.; Højlund, K.; Andersen, M. Effect of testosterone on insulin sensitivity, oxidative metabolism and body composition in aging men with type 2 diabetes on metformin monotherapy. Diabetes Obes. Metab. 2016, 18, 980–989. [Google Scholar] [CrossRef]
- Chen, Z.; Shen, X.; Tian, K.; Liu, Y.; Xiong, S.; Yu, Q.; Dai, L.; Shi, Y.; Zhang, R.; Zeng, R.; et al. Bioavailable testosterone is associated with symptoms of depression in adult men. J. Int. Med. Res. 2020, 48, 0300060520941715. [Google Scholar] [CrossRef]
- Hemachandra, C.; Islam, R.M.; Bell, R.J.; Sultana, F.; Davis, S.R. The association between testosterone and depression in postmenopausal women: A systematic review of observational studies. Maturitas 2023, 168, 62–70. [Google Scholar] [CrossRef]
- Zarrouf, F.A.; Artz, S.; Griffith, J.; Sirbu, C.; Kommor, M. Testosterone and depression: Systematic review and meta-analysis. J. Psychiatr. Pract. 2009, 15, 289–305. [Google Scholar] [CrossRef]
- Braca, F.; Betancort, J.C.; Pérez-Martín, N.; Cabrera Argany, A.; Boronat, M. Recovery of hypothalamic-pituitary-gonadal function with low dose testosterone treatment in a male with congenital hypogonadotropic hypogonadism. Andrologia 2022, 54, e14628. [Google Scholar] [CrossRef]
- Seale, J.V.; Wood, S.A.; Atkinson, H.C.; Lightman, S.L.; Harbuz, M.S. Organizational role for testosterone and estrogen on adult hypothalamic-pituitary-adrenal axis activity in the male rat. Endocrinology 2005, 146, 1973–1982. [Google Scholar] [CrossRef]
- Austad, S.N. Why women live longer than men: Sex differences in longevity. Gend. Med. 2006, 3, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Ginter, E.; Simko, V. Women live longer than men. Bratisl. Med. J. 2013, 114, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Zarulli, V.; Barthold Jones, J.A.; Oksuzyan, A.; Lindahl-Jacobsen, R.; Christensen, K.; Vaupel, J.W. Women live longer than men even during severe famines and epidemics. Proc. Natl. Acad. Sci. USA 2018, 115, E832–E840. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, Z.; Rostami, M.; Whiting, S.J.; Vatanparast, H. Fast-food dietary pattern is linked to higher prevalence of metabolic syndrome in older canadian adults. J. Nutr. Metab. 2021, 2021, 5712844. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.A.; Drewnowski, A.; Christakis, D.A. Dietary energy density is associated with obesity and the metabolic syndrome in U.S. adults. Diabetes Care 2007, 30, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: Comparison to high-fat diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; Harris, C.A.; Wang, J.-C. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol. Cell. Endocrinol. 2013, 380, 79–88. [Google Scholar] [CrossRef]
- Ohtsuka, A.; Tanaka, H.; Kizaki, K.; Hayashi, K. Chick skeletal muscle proteolysis in vitro increased by corticosterone. Biosci. Biotechnol. Biochem. 1998, 62, 1781–1783. [Google Scholar] [CrossRef]
- Sheffield-Moore, M. Androgens and the control of skeletal muscle protein synthesis. Ann. Med. 2000, 32, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Burdette, J.H.; Laurienti, P.J.; Miron, L.L.; Bahrami, M.; Simpson, S.L.; Nicklas, B.J.; Fanning, J.; Rejeski, W.J. Functional brain networks: Unique patterns with hedonic appetite and confidence to resist eating in older adults with obesity. Obesity 2020, 28, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Morales, I. Brain regulation of hunger and motivation: The case for integrating homeostatic and hedonic concepts and its implications for obesity and addiction. Appetite 2022, 177, 106146. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. Taste, olfactory and food texture reward processing in the brain and the control of appetite. Proc. Nutr. Soc. 2012, 71, 488–501. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Veras, M.; Parenti, E.; Neiva, S.D.S. Food security: Conceptual history and pillars. In Zero Hunger; Leal Filho, W., Azul, A.M., Brandli, L., Özuyar, P.G., Wall, T., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 1–10. [Google Scholar] [CrossRef]
- Maletta, H. From hunger to food security: A conceptual history. SSRN Electron. J. 2014. [Google Scholar] [CrossRef]
- Dzubur, E.; Semborski, S.; Redline, B.; Hedeker, D.; Dunton, G.F.; Henwood, B.F. Food insecurity, hunger, stress, and homelessness among young adults. Health Psychol. 2022, 41, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Gyasi, R.M.; Obeng, B.; Yeboah, J.Y. Impact of food insecurity with hunger on mental distress among community-dwelling older adults. PLoS ONE 2020, 15, e0229840. [Google Scholar] [CrossRef]
- Herman, J.P. The neuroendocrinology of stress: Glucocorticoid signaling mechanisms. Psychoneuroendocrinology 2022, 137, 105641. [Google Scholar] [CrossRef]
- Moeller, S.J.; Couto, L.; Cohen, V.; Lalazar, Y.; Makotkine, I.; Williams, N.; Yehuda, R.; Goldstein, R.Z.; Geer, E.B. Glucocorticoid regulation of food-choice behavior in humans: Evidence from Cushing’s syndrome. Front. Neurosci. 2016, 10, 21. [Google Scholar] [CrossRef]
- Balsevich, G.; Abizaid, A.; Chen, A.; Karatsoreos, I.N.; Schmidt, M.V. Stress and glucocorticoid modulation of feeding and metabolism. Neurobiol. Stress 2019, 11, 100171. [Google Scholar] [CrossRef]
- Izzi-Engbeaya, C.; Ma, Y.; Buckley, N.W.; Ratnasabapathy, R.; Richardson, E.; Counsell, J.R.; Fernandes-Freitas, I.; Norton, M.; Farooq, G.; Mirza, Z.; et al. Effects of corticosterone within the hypothalamic arcuate nucleus on food intake and body weight in male rats. Mol. Metab. 2020, 36, 100972. [Google Scholar] [CrossRef]
- Kuckuck, S.; van der Valk, E.S.; Scheurink, A.J.W.; van der Voorn, B.; Iyer, A.M.; Visser, J.A.; Delhanty, P.J.D.; van den Berg, S.A.A.; van Rossum, E.F.C. Glucocorticoids, stress and eating: The mediating role of appetite-regulating hormones. Obes. Rev. 2023, 24, e13539. [Google Scholar] [CrossRef]
- Hupkens, C.L.H.; Knibbe, R.A.; Drop, M.J. Social class differences in food consumption: The explanatory value of permissiveness and health and cost considerations. Eur. J. Public Health 2000, 10, 108–113. [Google Scholar] [CrossRef]
- Kearney, J. Food consumption trends and drivers. Philos. Trans. R. Soc. B 2010, 365, 2793–2807. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.; Wood, E. Food taboos, health beliefs, and gender: Understanding household food choice and nutrition in rural Tajikistan. J. Health Popul. Nutr. 2019, 38, 17. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Larsen, P. Food availability/convenience and obesity. Adv. Nutr. 2014, 5, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D. Did the food environment cause the obesity epidemic? Obesity 2018, 26, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J.; Rowe, E.A.; Rolls, E.T.; Kingston, B.; Megson, A.; Gunary, R. Variety in a meal enhances food intake in man. Physiol. Behav. 1981, 26, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, M.S.; Bhavani, R.V. Food production and availability--essential prerequisites for sustainable food security. Indian J. Med. Res. 2013, 138, 383–391. [Google Scholar] [PubMed]
- Rask, K.J.; Rask, N. Economic development and food production–consumption balance: A growing global challenge. Food Policy 2011, 36, 186–196. [Google Scholar] [CrossRef]
- Misra, A.; Khurana, L. Obesity and the metabolic syndrome in developing countries. J. Clin. Endocrinol. Metab. 2008, 93 (Suppl. S1), S9–S30. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.M. The emerging epidemic of obesity in developing countries. Int. J. Epidemiol. 2006, 35, 93–99. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, P.; Füllgrabe, J.; Joseph, B. The hunger strikes back: An epigenetic memory for autophagy. Cell Death Differ. 2023, 30, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Lushchak, O. Prenatal famine exposure and adult health outcomes: An epigenetic link. Environ. Epigenetics 2021, 7, dvab013. [Google Scholar] [CrossRef]
- Russell, C.G.; Taki, S.; Laws, R.; Azadi, L.; Campbell, K.J.; Elliott, R.; Lynch, J.; Ball, K.; Taylor, R.; Denney-Wilson, E. Effects of parent and child behaviours on overweight and obesity in infants and young children from disadvantaged backgrounds: Systematic review with narrative synthesis. BMC Public Health 2016, 16, e151. [Google Scholar] [CrossRef]
- Buksh, S.M.; de Wit, J.B.F.; Hay, P. Sociocultural influences contribute to overeating and unhealthy eating: Creating and maintaining an obesogenic social environment in indigenous communities in urban Fiji. Nutrients 2022, 14, 2803. [Google Scholar] [CrossRef]
- Dunbar, R.I.M. Breaking bread: The functions of social eating. Adapt. Hum. Behav. Physiol. 2017, 3, 198–211. [Google Scholar] [CrossRef]
- Herman, C.P. The social facilitation of eating or the facilitation of social eating? J. Eat. Disord. 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Embling, R.; Pink, A.F.; Gatzemeier, J.; Price, M.; Lee, M.D.; Wilkinson, L.L. Effect of food variety on intake of a meal: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2021, 113, 716–741. [Google Scholar] [CrossRef] [PubMed]
- Broustet, J.P. Conséquences cardiorespiratoires de la surcharge pondérale. Arch. Mal. Coeur Vaiss. 2003, 96 (Suppl. S6), 5–6. [Google Scholar] [PubMed]
- Lin, C.-K.; Lin, C.-C. Work of breathing and respiratory drive in obesity. Respirology 2012, 17, 402–411+1323–7799. [Google Scholar] [CrossRef]
- Alpert, M.A.; Omran, J.; Bostick, B.P. Effects of obesity on cardiovascular hemodynamics, cardiac morphology, and ventricular function. Curr. Obes. Rep. 2016, 5, 424–434. [Google Scholar] [CrossRef]
- Lavie, C.J.; Milani, R.V. Obesity and cardiovascular disease: The hippocrates paradox? J. Am. Coll. Cardiol. 2003, 42, 677–679. [Google Scholar] [CrossRef]
- Hydes, T.; Alam, U.; Cuthbertson, D.J. The impact of macronutrient intake on non-alcoholic fatty liver disease (NAFLD): Too much fat, too much carbohydrate, or just too many calories? Front. Nutr. 2021, 8, 640557. [Google Scholar] [CrossRef]
- Pan, X.; Wang, P.; Luo, J.; Wang, Z.; Song, Y.; Ye, J.; Hou, X. Adipogenic changes of hepatocytes in a high-fat diet-induced fatty liver mice model and non-alcoholic fatty liver disease patients. Endocrine 2015, 48, 834–847. [Google Scholar] [CrossRef]
- Lucero, D.; Zago, V.; López, G.I.; Graffigna, M.; López, G.H.; Fainboim, H.; Miksztowicz, V.; Gómez Rosso, L.; Belli, S.; Levalle, O.; et al. Does non-alcoholic fatty liver impair alterations of plasma lipoproteins and associated factors in metabolic syndrome? Clin. Chim. Acta 2011, 412, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Liu, Z.-X.; Qu, X.; Elder, B.D.; Bilz, S.; Befroy, D.; Romanelli, A.J.; Shulman, G.I. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004, 279, 32345–32353. [Google Scholar] [CrossRef] [PubMed]
- Magalhães Demaria, T.; Diniz Crepaldi, L.; Costa-Bartuli, E.; Ristow Branco, J.; Zancan, P.; Sola-Penna, M. Once a week consumption of Western diet over twelve weeks promotes sustained insulin resistance and non-alcoholic fat liver disease in C57BL/6 J mice. Sci. Rep. 2023, 13, 3058. [Google Scholar] [CrossRef]
- Tian, G.X.; Sun, Y.; Pang, C.J.; Tan, A.H.; Gao, Y.; Zhang, H.Y.; Yang, X.B.; Li, Z.X.; Mo, Z.N. Oestradiol is a protective factor for non-alcoholic fatty liver disease in healthy men. Obes. Rev. 2012, 13, 381–387. [Google Scholar] [CrossRef]
- Yim, J.Y.; Kim, J.; Kim, D.; Ahmed, A. Serum testosterone and non-alcoholic fatty liver disease in men and women in the US. Liver Int. 2018, 38, 2051–2059. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, C.; Wang, Y.; Wang, N.; Chen, Y.; Lu, Y.; Xia, F. The associations of total testosterone with probable nonalcoholic steatohepatitis and nonalcoholic fatty liver disease fibrotic progression in men with type 2 diabetes: A cross-sectional study. Eur. J. Med. Res. 2022, 27, 307. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, E.-H. Association between metabolic syndrome and age-related cataract. Int. J. Ophthalmol. 2015, 8, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.M.; Zoungas, S.; Rossing, P.; Groop, P.-H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef]
- De Jongh, R.T.; Serne, E.H.; IJzerman, R.G.; de Vries, G.; Stehouwer, C.D.A. Impaired microvascular function in obesity-Implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation 2004, 109, 2529–2535. [Google Scholar] [CrossRef]
- Qi, D.; Rodrigues, B. Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolism. Am. J. Physiol. 2007, 292, E654–E667. [Google Scholar] [CrossRef] [PubMed]
- Rafacho, A.; Ortsater, H.; Nadal, A.; Quesada, I. Glucocorticoid treatment and endocrine pancreas function: Implications for glucose homeostasis, insulin resistance and diabetes. J. Endocrinol. 2014, 223, R49–R62. [Google Scholar] [CrossRef]
- Alonso, A.; Fernández, R.; Moreno, M.; Ordóñez, P.; González-Pardo, H.; Conejo, N.M.; Díaz, F.; González, C. Positive effects of 17 beta-estradiol on insulin sensitivity in aged ovariectomized female rats. J. Gerontol. A 2006, 61, 419–426. [Google Scholar] [CrossRef]
- Donin, A.S.; Nightingale, C.M.; Owen, C.G.; Rudnicka, A.R.; Jebb, S.A.; Ambrosini, G.L.; Stephen, A.M.; Cook, D.G.; Whincup, P.H. Dietary energy intake is associated with type 2 diabetes risk markers in children. Diabetes Care 2014, 37, 116–123. [Google Scholar] [CrossRef]
- Friedman, M.I. Fuel partitioning and food intake. Am. J. Clin. Nutr. 1998, 67 (Suppl. S3), 513S–518S. [Google Scholar] [CrossRef]
- Ayyobi, A.F.; Brunzell, J.D. Lipoprotein distribution in the metabolic syndrome, type 2 diabetes mellitus, and familial combined hyperlipidemia. Am. J. Cardiol. 2003, 92, 27J–33J. [Google Scholar] [CrossRef]
- Golay, A.; Ybarra, J. Link between obesity and type 2 diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 649–663. [Google Scholar] [CrossRef] [PubMed]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular matrix composition of connective tissues: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, M.A.R.; Wünderlich, F.T. Macrophage function in obesity-induced inflammation and insulin resistance. Pflüger’s Arch-Eur. J. Physiol. 2017, 469, 385–396. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Xu, D. The roles of T cells in obese adipose tissue inflammation. Adipocyte 2021, 10, 435–445. [Google Scholar] [CrossRef]
- Szucs, D.; Miklos, V.; Monostori, T.; Guba, M.; Kun-Varga, A.; Poliska, S.; Kis, E.; Bende, B.; Kemeny, L.; Vereb, Z. Effect of inflammatory microenvironment on the regenerative capacity of adipose-derived mesenchymal stem cells. Cells 2023, 12, 1966. [Google Scholar] [CrossRef]
- Miki, H.; Manresa, M.C. Novel fibroblast phenotypes in homeostasis and chronic inflammation: From functions to potential regulators. J. Physiol. 2023, 601, 2273–2291. [Google Scholar] [CrossRef] [PubMed]
- Karra, P.; Winn, M.; Pauleck, S.; Bulsiewicz-Jacobsen, A.; Peterson, L.; Coletta, A.; Doherty, J.; Ulrich, C.M.; Summers, S.A.; Gunter, M.; et al. Metabolic dysfunction and obesity-related cancer: Beyond obesity and metabolic syndrome. Obesity 2022, 30, 1323–1334. [Google Scholar] [CrossRef]
- Recalde, M.; Pistillo, A.; Davila-Batista, V.; Leitzmann, M.; Romieu, I.; Viallon, V.; Freisling, H.; Duarte-Salles, T. Longitudinal body mass index and cancer risk: A cohort study of 2.6 million Catalan adults. Nat. Commun. 2023, 14, 3816. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Z.; Li, L.-L.; Fu, Z.-P.; Xu, C.-H. Clinical significance of adipose tissue invasion in ER-positive breast cancer. Acta Medica Mediterr. 2022, 38, 3383–3387. [Google Scholar] [CrossRef]
- Sarkar, M.; Nguyen, T.; Gundre, E.; Ogunlusi, O.; El-Sobky, M.; Giri, B.; Sarkar, T.R. Cancer-associated fibroblasts: The chief architect in the tumor microenvironment. Front. Cell Dev. Biol. 2023, 11, 1089068. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Niu, Y.-R.; Wang, Z.-H.; Ye, L.-L.; Peng, W.-B.; Zhou, Q. Cancer-associated fibroblasts: Vital suppressors of the immune response in the tumor microenvironment. Cytokine Growth Factor Rev. 2022, 67, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sun, S.; Li, J.-J.; Yuan, J.-P.; Sun, S.-R.; Wu, Q. Adipose tissue macrophages: Implications for obesity-associated cancer. Mil. Med. Res. 2023, 10, 1. [Google Scholar] [CrossRef]
- Wheeler, J.B.; Ikonomidis, J.S.; Jones, J.A. Connective tissue disorders and cardiovascular complications: The indomitable role of transforming growth factor-β signaling. Adv. Exp. Med. Biol. 2021, 1348, 161–184. [Google Scholar] [CrossRef] [PubMed]
- Igumnov, I.A.; Kurashova, N.A.; Belenkaya, V.L.; Vilson, N.I.; Suturina, V.L. Prevalence of metabolic syndrome in PCOS patients. Int. J. Biomed. 2022, 12, 95–99. [Google Scholar] [CrossRef]
- Lazar, A.-L.; Vulturar, R.; Fodor, A.; Orasan, O.H.; Crişan, C.-H.-E.; Login, C.; Para, I.; Negrean, V.; Tiperciuc, B.; Cozma, A. The molecular mechanisms linking metabolic syndrome to endometrial and breast cancers. J. Mind Med. Sci. 2021, 8, 167–178e2. [Google Scholar] [CrossRef]
- Nobes, J.P.; Langley, S.E.M.; Laing, R.W. Metabolic syndrome and prostate cancer: A review. Clin. Oncol. 2009, 21, 183–191. [Google Scholar] [CrossRef]
- Holanda Torres Pinheiro, L.C.; Rossi, M.; Freitas dos Santos, C.A.; Franco Oliveira, L.V.; Vencio, S.; de Paula Vieira, R.; Juliano, Y.; Armond, J.; Hassel Mendes Silva, C.; Fonseca, A.L.; et al. Prevalence of associations among sarcopenia, obesity, and metabolic syndrome in Brazilian older adults. Front. Med. 2023, 10, 1206545. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, X.; Ding, L.; Lu, J.; Zhu, H.; Zhao, K.; Zhu, S.; Xu, Q. The association between metabolic syndrome and presence of frailty: A systematic review and meta-analysis. Eur. Geriatr. Med. 2022, 13, 1047–1056. [Google Scholar] [CrossRef]
- Yates, K.F.; Sweat, V.; Yau, P.L.; Turchiano, M.M.; Convit, A. Impact of metabolic syndrome on cognition and brain. A selected review of the literature. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2060–2067. [Google Scholar] [CrossRef]
- Neergaard, J.S.; Dragsbaek, K.; Christiansen, C.; Nielsen, H.B.; Brix, S.; Karsdal, M.A.; Henriksen, K. Metabolic syndrome, insulin resistance, and cognitive dysfunction: Does your metabolic profile affect your brain? Diabetes 2017, 66, 1957–1963. [Google Scholar] [CrossRef]
- Birdsill, A.C.; Carlsson, C.M.; Willette, A.A.; Okonkwo, O.C.; Johnson, S.C.; Xu, G.; Oh, J.M.; Gallagher, C.L.; Koscik, R.L.; Jonaitis, E.M.; et al. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity 2013, 21, 1313–1320. [Google Scholar] [CrossRef]
- Fakih, W.; Zeitoun, R.; AlZaim, I.; Eid, A.H.; Kobeissy, F.; Abd-Elrahman, K.S.; El-Yazbi, A.F. Early metabolic impairment as a contributor to neurodegenerative disease: Mechanisms and potential pharmacological intervention. Obesity 2022, 30, 982–993. [Google Scholar] [CrossRef]
- Boleti, A.P.d.A.; Almeida, J.A.; Migliolo, L. Impact of the metabolic syndrome on the evolution of neurodegenerative diseases. Neural Regen. Res. 2021, 16, 688–689. [Google Scholar] [CrossRef]
- Kordestani-Moghadam, P.; Assari, S.; Nouriyengejeh, S.; Mohammadipour, F.; Pourabbasi, A. Cognitive impairments and associated structural brain changes in metabolic syndrome and implications of neurocognitive intervention. J. Obes. Metab. Syndr. 2020, 29, 174–179. [Google Scholar] [CrossRef]
- Benedito-Silva, A.A.; Evans, S.; Viana Mendes, J.; Castro, J.; da Silva, B.; Gonçalves, B.; Ruiz, F.S.; Beijamini, F.; Evangelista, F.S.; Vallada, H.; et al. Association between light exposure and metabolic syndrome in a rural Brazilian town. PLoS ONE 2020, 15, e0238772. [Google Scholar] [CrossRef]
- Zhang, C.; Tait, C.; Minacapelli, C.D.; Bhurwal, A.; Gupta, K.; Amin, R.; Rustgi, V.K. The role of race, sex, and age in circadian disruption and metabolic disorders. Gastro Hep Adv. 2022, 1, 471–479. [Google Scholar] [CrossRef]
- Garvey, J.F.; Pengo, M.F.; Drakatos, P.; Kent, B.D. Epidemiological aspects of obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 920–929. [Google Scholar] [CrossRef]
- Foldvary-Schaefer, N.R.; Waters, T.E. Sleep-disordered breathing. Continuum 2017, 23, 1093–1116. [Google Scholar] [CrossRef]
- Locke, B.W.; Lee, J.J.; Sundar, K.M. OSA and chronic respiratory disease: Mechanisms and epidemiology. Int. J. Environ. Res. Public Health 2022, 19, 5473. [Google Scholar] [CrossRef]
- Osman, A.M.; Carter, S.G.; Carberry, J.C.; Eckert, D.J. Obstructive sleep apnea: Current perspectives. Nat. Sci. Sleep 2018, 10, 21–34. [Google Scholar] [CrossRef]
- Joosten, S.A.; Edwards, B.A.; Wellman, A.; Turton, A.; Skuza, E.M.; Berger, P.J.; Hamilton, G.S. The effect of body position on physiological factors that contribute to obstructive sleep apnea. Sleep 2015, 38, 1469–1478. [Google Scholar] [CrossRef]
- Eckert, D.J.; Malhotra, A. Pathophysiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Pavwoski, P.; Shelgikar, A.V. Treatment options for obstructive sleep apnea. Neurol. Clin. Pract. 2017, 7, 77–85. [Google Scholar] [CrossRef]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef]
- Clark, K.C.; Kwitek, A.E. Multi-omic approaches to identify genetic factors in metabolic syndrome. Compr. Physiol. 2022, 12, 3045–3084. [Google Scholar] [CrossRef]
- Rottenkolber, M.; Gar, C.; Then, C.; Wanger, L.; Sacco, V.; Banning, F.; Potzel, A.L.; Kern-Matschilles, S.; Nevinny-Stickel-Hinzpeter, C.; Grallert, H.; et al. A pathophysiology of type 2 diabetes unrelated to metabolic syndrome. J. Clin. Endocrinol. Metab. 2021, 106, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Holman Rury, R. Normal weight individuals who develop Type 2 diabetes: The personal fat threshold. Clin. Sci. 2014, 128, 405–410. [Google Scholar] [CrossRef]
- Biondi, B. Thyroid and obesity: An intriguing relationship. J. Clin. Endocrinol. Metab. 2010, 95, 3614–3617. [Google Scholar] [CrossRef] [PubMed]
- Després, J.P. What is “metabolically healthy obesity”?: From epidemiology to pathophysiological insights. J. Clin. Endocrinol. Metab. 2012, 97, 2283–2285. [Google Scholar] [CrossRef]
- Hoddy, K.K.; Axelrod, C.L.; Mey, J.T.; Hari, A.; Beyl, R.A.; Blair, J.B.; Dantas, W.S.; Kirwan, J.P. Insulin resistance persists despite a metabolically healthy obesity phenotype. Obesity 2022, 30, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y.; Segev, E.; Shefer, G.; Eilam, D.; Shenkerman, G.; Buch, A.; Shenhar-Tsarfaty, S.; Zeltser, D.; Shapira, I.; Berliner, S.; et al. Metabolically healthy obesity is a misnomer: Components of the metabolic syndrome linearly increase with BMI as a function of age and gender. Biology 2023, 12, 719. [Google Scholar] [CrossRef]
- Man, S.; Lv, J.; Yu, C.; Deng, Y.; Yin, J.; Wang, B.; Li, L.; Liu, H. Association between metabolically healthy obesity and non-alcoholic fatty liver disease. Hepatol. Int. 2022, 16, 1412–1423. [Google Scholar] [CrossRef]
- Namgoung, S.; Chang, Y.; Woo, C.-Y.; Kim, Y.; Kang, J.; Kwon, R.; Lim, G.-Y.; Choi, H.R.; Kim, K.-H.; Kim, H.; et al. Metabolically healthy and unhealthy obesity and risk of vasomotor symptoms in premenopausal women: Cross-sectional and cohort studies. Br. J. Obstet. Gynaecol. 2022, 129, 1926–1934. [Google Scholar] [CrossRef]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef]
- Oyovwi Mega, O.; Ben-Azu, B.; Ovuakporaye Irikefe, S.; Onome Bright, O.; Emojevwe, V.; Falajiki, Y.F.; Patrick Godwin, O.; Rotu Arientare, R.; Okoro Ogheneyebrorue, G.; Nwangwa Eze, K. Testosterone: The male sex hormone. In Testosterone; Hirokazu, D., Ed.; IntechOpen: Rijeka, Croatia, 2023; pp. 1–21. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef]
- Boullu-Ciocca, S.; Verger, P.; Bocquier, A.; Oliver, C. Axe corticotrope et stress chronique dans l’obésité abdominale et le syndrome métabolique. Presse Médicale 2005, 34, 1646–1653. [Google Scholar] [CrossRef]
- Black, P.H. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav. Immun 2003, 17, 350–364. [Google Scholar] [CrossRef]
- Chrousos, G.P. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: Neuro-endocrine and target tissue-related causes. Int. J. Obes. 2000, 24 (Suppl. S2), S50–S55. [Google Scholar] [CrossRef] [PubMed]
- Rosmond, R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology 2005, 30, 1–10. [Google Scholar] [CrossRef]
- Arage, G.; Belachew, T.; Hassen, H.; Abera, M.; Abdulhay, F.; Abdulahi, M.; Hassen Abate, K. Effects of prenatal exposure to the 1983–1985 Ethiopian great famine on the metabolic syndrome in adults: A historical cohort study. Br. J. Nutr. 2020, 124, 1052–1060. [Google Scholar] [CrossRef]
- De Rooij, S.R.; Painter, R.C.; Holleman, F.; Bossuyt, P.M.; Roseboom, T.J. The metabolic syndrome in adults prenatally exposed to the Dutch famine. Am. J. Clin. Nutr. 2007, 86, 1219–1224. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, H.; Hu, C.; Wang, S.; Zhu, Y.; Lin, H.; Lin, L.; Zhang, J.; Wang, T.; Zhao, Z.; et al. Association between early life famine exposure and risk of metabolic syndrome in later life. J. Diabetes 2022, 14, 685–694. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, E.; le Cléssiau, H.; Valensi, P. Metabolic syndrome and psychosocial deprivation. Data collected from a Paris suburb. Diabetes Metab 2008, 34, 155–161. [Google Scholar] [CrossRef]
- Eftekhari, S.; Alipour, F.; Aminian, O.; Saraei, M. The association between job stress and metabolic syndrome among medical university staff. J. Diabetes Metab. Disord 2021, 20, 321–327. [Google Scholar] [CrossRef]
- Berthoud, H.-R. Neural control of appetite: Cross-talk between homeostatic and non-homeostatic systems. Appetite 2004, 43, 315–317. [Google Scholar] [CrossRef]
- Sternson, S.M.; Eiselt, A.-K. Three pillars for the neural control of appetite. Annu. Rev. Physiol. 2017, 79, 401–423. [Google Scholar] [CrossRef] [PubMed]




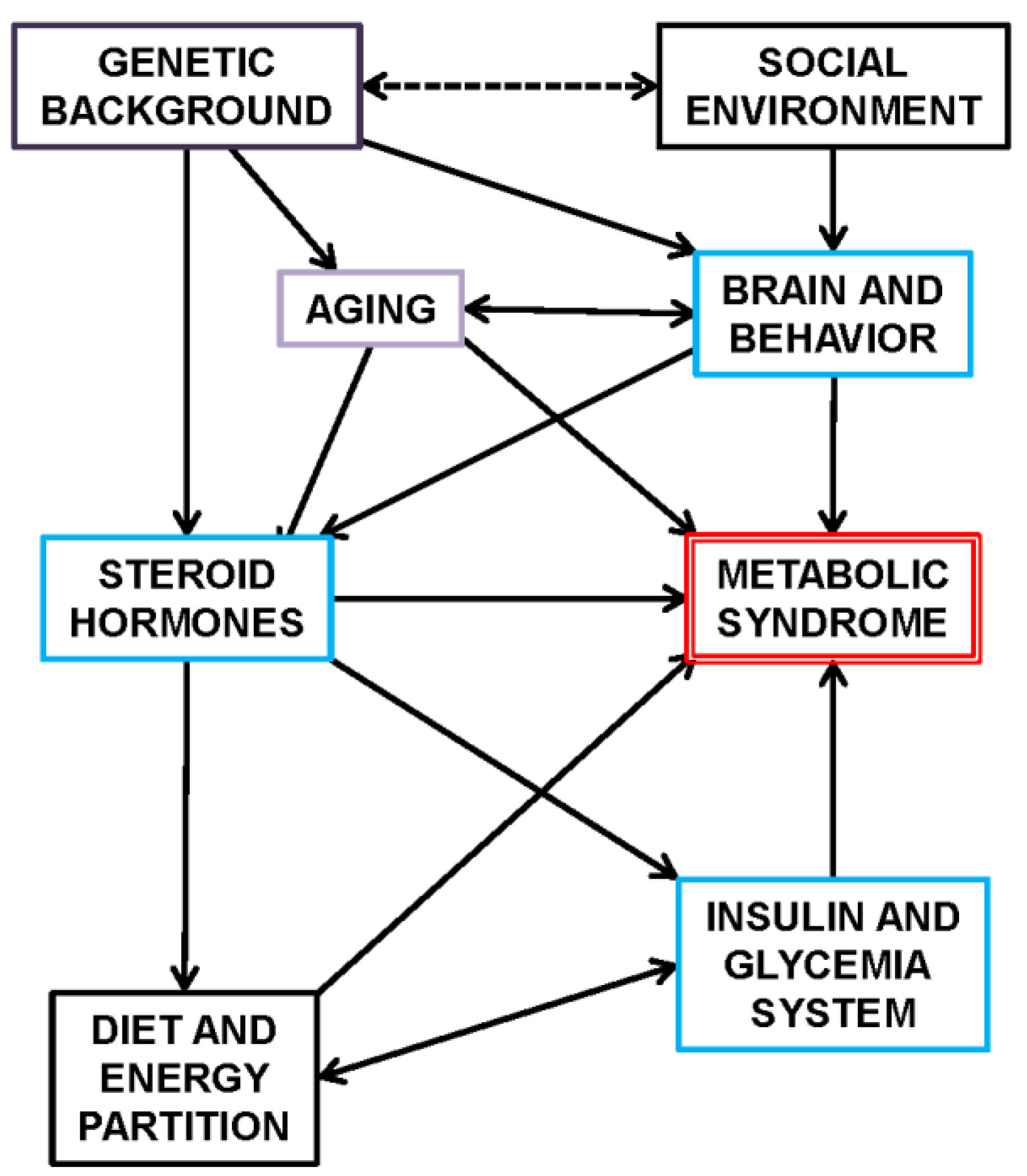
| MS Main Specific Pathology Family | MS-Related Agent, Effect, Action, or Pathology | Organ/System Implicated | Comments |
|---|---|---|---|
| Genetic/epigenetic predisposition factors | monogenic and polygenic obesity diabetes and other disorders | all systems, adipose depots, energy balance-systems failure | gene-defective signals/altered systems |
| genetic predisposition to cancer | reproductive, interstitial, other | gene-related cancer | |
| inherited predisposition to system or organ failure | cardiovascular, liver, kidney, hematopoietic, interstitial, other | defective gene-related alterations | |
| Nervous system(s) | bulimic/anorexic disorders | nervous and endocrine systems | appetite + behavioral |
| depression | nervous signal disorder | ||
| hypothalamic disorders | adrenals/gonads | ||
| parasympathetic predominance | phased-out (?) affectation | ||
| neuropathy (central, peripheral, sensory), chemical senses, glaucoma | nerves from central, autonomic and enteric nervous systems, sensory systems | wide range of peripheral- and senses affectation | |
| Muscle and nervous signal disorders | fibrillation, disordered heart rhythm | heart, muscle and circulatory, brain | |
| Altered energy expenditure | sedentary behaviors, lack of exercise | muscle, skeleton, splanchnic bed, interstitial | essentially related to insufficient energy expenditure with respect to intake, also psychogenic causes |
| immobilization, extreme rest | muscle, skeleton, brain | bed rest, paralysis, etc. | |
| body heat and thermogenesis-related disorders | liver, brown adipose tissue, muscle, brain | insufficient thermogenic capability or regulation. Hyperthermia/fever | |
| Mitochondrial function disorders | mitochondrial cycle failure | liver, nervous system | |
| Rheumatic-related disorders (immune and inflammatory) | rheumatoid arthritis (and osteoarthritis) | interstitial, skeletal, intestine, nervous, all systems | additional large and varied pathologic relationships |
| altered redox states and purine metabolism, hyperuricemia, gout | interstitial, all systems | ||
| atopia, psoriasis | skin, intestine, nervous, immune | relationships with cancer and other MS conditions (e.g., obesity) | |
| Diet; excess energy intake vs. losses (and nutrient/energy triage/partition) | excess energy intake (uncompensated) | liver, kidney, muscle, all systems | includes the dietary protein paradox |
| liver steatosis (and fibrosis) | liver, circulatory, brain, interstitial | probably a primordial cause of MS | |
| relative excess of carbohydrate | liver, muscle, interstitial, endocrine | ||
| insufficient (relative) N intake | liver, kidney, muscle, all systems | includes the dietary protein conundrum | |
| obesogenic (lipid, sugars) diets | liver, interstitial, endocrine | ||
| excess energy available and ectopic deposition (obesity) | liver, muscle, interstitial, skin, all systems | a main cause of obesity | |
| purified/ultra-processed diets | liver, muscle, interstitial, endocrine | probably a mix of relative excess of carbohydrate and obesogenic diets | |
| essential nutrient deficits | liver, nervous system, all systems | deficits affecting regulation | |
| Excess body lipids | obesity, all causes | interstitial, all systems | relative and absolute excess of lipid in the body including ectopic depots |
| altered adipose metabolic control-related depots | heart and vessels-circulatory control-related depots | control of heart and vessel blood dynamics | |
| disordered tissue fat deposition | interstitial, muscle, all systems | fat depots for direct organ use | |
| hyperlipidemia | liver, peripheral organs/tissues, blood | hypertriacylglycerolemia, hypercholesterolemia, and apolipoprotein-related disorders | |
| Secondary to excess body lipids | atherosclerosis | circulatory, liver, blood vessels | altered blood flow and heart overburden |
| arterial hypertension | circulatory, kidney, brain, liver, other | vessel endothelial damage, increased blood flow resistance, | |
| body mass-related respiratory and hemodynamic disorders | heart-circulatory, interstitial, respiratory, nervous, all systems | disordered supply of oxygen and nutrients, altered blood flow and renal control of water and electrolytes. Hypoxia | |
| hypotension/heart insufficiency | chronic hypoxia, edema, lymphedema, inflammation | ||
| obesity-related cancer | liver, other splanchnic, nervous, all systems | fibroblasts and adipocytes as necessary energy support for cancer types | |
| Toxic-induced disorders | chronic and acute toxicity | liver, interstitial, all systems | includes alcohol and other drugs and iatrogenesis (i.e., thyroid) |
| carcinogen-induced cancer | liver, interstitial, brain, reproductive system | not part of MS but both/either favoring MS or facilitated by MS | |
| Insulin and diabetes mellitus | prediabetes | all systems, liver | |
| insulin resistance | insulin function, all organs | a main recognized factor of MS | |
| gestational diabetes | all systems, liver, placenta | temporary | |
| type 2 diabetes | pancreas, liver, interstitial, all systems | close relationship with obesity and other endocrine disorders of energy handling | |
| polycystic ovary syndrome | ovary, adrenals, liver, all systems | ||
| Other hormone disorders | hypothyroidism | thyroid, interstitial, liver, all systems | |
| hypercortisolism | adrenals, brain, endocrine, interstitial, circulatory, all systems | often not defined within MS but as separate entities | |
| testosterone deficit, andropause | testicle, muscle, bone, liver, endocrine, brain, all systems | a main factor in developing MS | |
| DHEA deficit | adrenals, brain, liver, reproductive, all systems | ||
| estrogen deficit, menopause | adrenals, reproductive, liver, interstitial, all systems | related to testosterone deficit | |
| altered handling of water/ions | adrenals, kidney, brain, circulatory, all systems | unclear association with BW; ion handling effects on diet or metabolic regulation | |
| hormone-related cancer | reproductive, endocrine, all systems | ovary, endometrial, breast, prostate cancers as main examples | |
| Sleep apnea | so far unknown, albeit syndromic with obesity and inflammation | cartilage, respiratory, circulatory | mechanism of pathogenesis not yet related to MS, but syndromic with obesity |
| Microbiota-related disorders | altered microbiota states | microbiota, intestine, liver, interstitial, nervous, skin, all systems | loss of digestive efficiency; infection and forfeiting of the host-biota immune status quo |
| interspecies gene transfer | all systems | integration of viral and bacterial genes into the human genome, with quite different long-term effects (part of evolution?) | |
| Infection | virus infection and artificial gene transfer | immune, interstitial, all systems | virus-transmitted obesity |
| parasites, sepsis | immune, intestine, interstitial, all systems | MS as a sequel or consequence of infection/recovery or overreaction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alemany, M. The Metabolic Syndrome, a Human Disease. Int. J. Mol. Sci. 2024, 25, 2251. https://doi.org/10.3390/ijms25042251
Alemany M. The Metabolic Syndrome, a Human Disease. International Journal of Molecular Sciences. 2024; 25(4):2251. https://doi.org/10.3390/ijms25042251
Chicago/Turabian StyleAlemany, Marià. 2024. "The Metabolic Syndrome, a Human Disease" International Journal of Molecular Sciences 25, no. 4: 2251. https://doi.org/10.3390/ijms25042251






