Different Forms of TFF3 in the Human Endocervix, including a Complex with IgG Fc Binding Protein (FCGBP), and Further Aspects of the Cervico-Vaginal Innate Immune Barrier
Abstract
:1. Introduction
2. Results
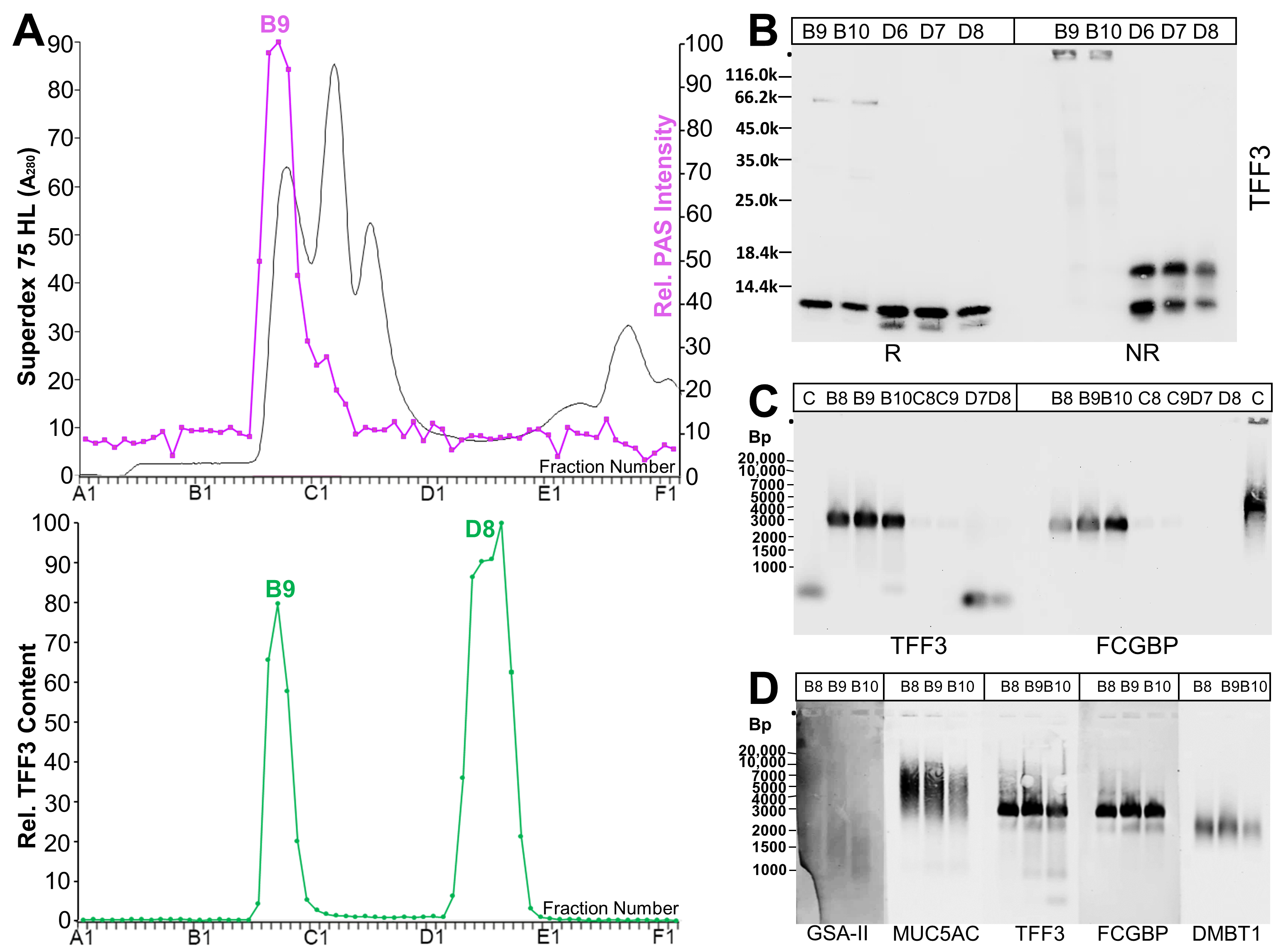
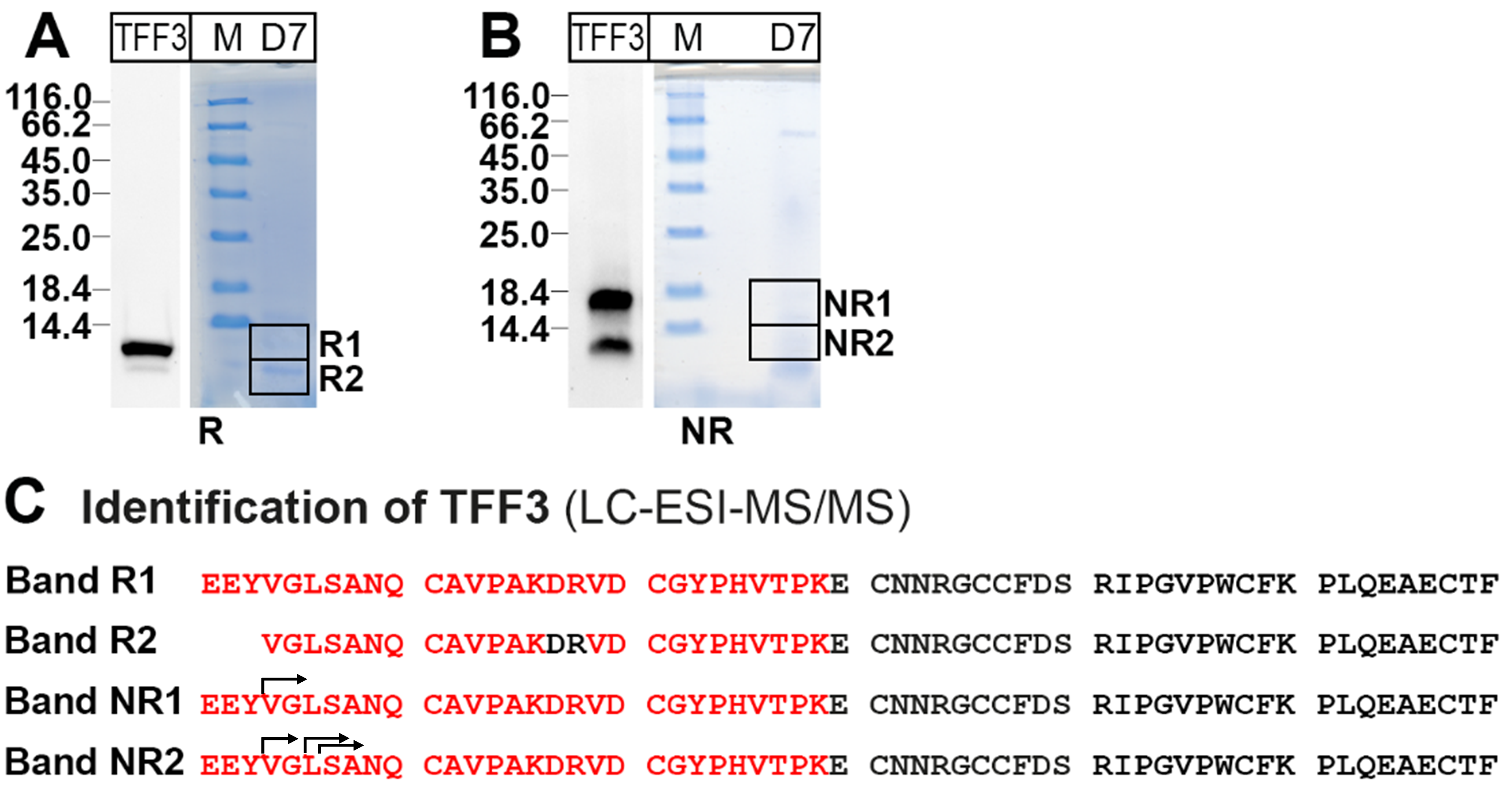
2.1. Characterization of TFF3 Forms in Human Endocervix Specimens
2.2. Transcriptional Profiling of Human Endocervical and Vaginal Specimens (RT-PCR Analyses)
2.3. Analysis of Endocervical Mucus Fractions concerning Fucosylation
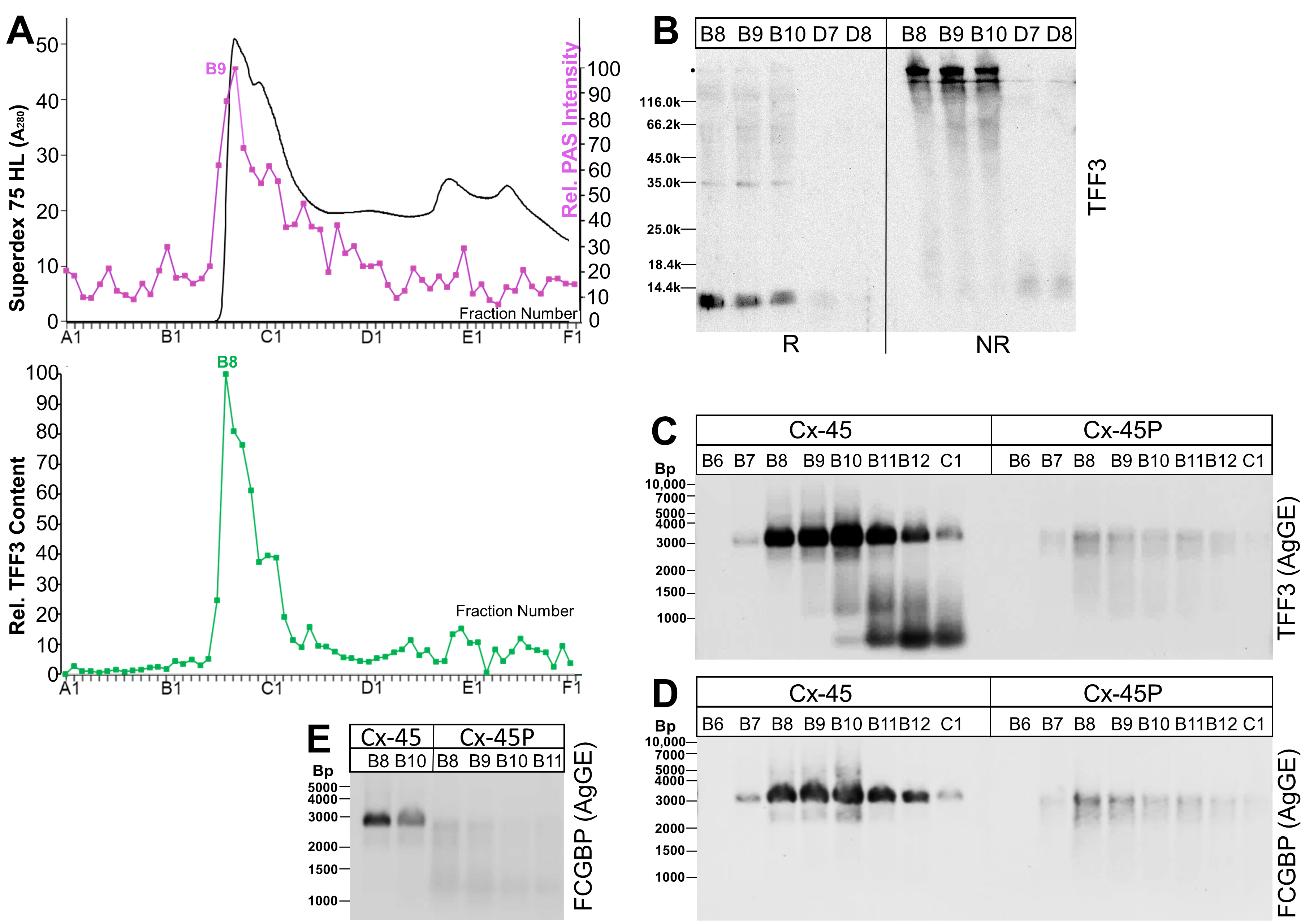
2.4. Analysis of Human Endocervical and Vaginal Mucus Specimens during the Menstrual Cycle (Western Blot Analyses)
2.5. Localization of FCGBP and TFF3 in the Human Endocervix
3. Discussion
3.1. TFF3 from the Human Endocervix Forms High-Molecular-Mass Heterodimers with FCGBP
3.2. Low-Molecular-Mass Forms of TFF3 in the Human Endocervix
3.3. Transcriptional Profiling of the Human Endocervix and Vagina: Specific Aspects of the Vaginal Innate Immune Barrier
3.4. TFF3 and FCGBP Content in the Human Endocervical and Vaginal Mucus during the Proliferative and Secretory Phases of the Menstrual Cycle
4. Materials and Methods
4.1. Human Specimens
4.2. Extraction of Proteins, Protein Purification by SEC
4.3. SDS-PAGE, AgGE, and Western Blot Analysis
4.4. Identification of Proteins by Bottom-Up Proteomics
4.5. RNA Extraction, PCR Analysis
4.6. Immunohistochemistry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| AgGE | Agarose gel electrophoresis |
| FCGBP | IgG Fc binding protein |
| FUT | Fucosyltransferase |
| LYZ | Lysozyme |
| PAS | Periodic acid-Schiff |
| SEC | Size exclusion chromatography |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| TFF | Trefoil factor family |
References
- Lacroix, G.; Gouyer, V.; Gottrand, F.; Desseyn, J.-L. The Cervicovaginal Mucus Barrier. Int. J. Mol. Sci. 2020, 21, 8266. [Google Scholar] [CrossRef]
- Andersch-Björkman, Y.; Thomsson, K.A.; Holmén Larsson, J.M.; Ekerhovd, E.; Hansson, G.C. Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol. Cell. Proteom. 2007, 4, 708–716. [Google Scholar] [CrossRef]
- Shaw, J.L.; Smith, C.R.; Diamandis, E.P. Proteomic analysis of human cervico-vaginal fluid. J. Proteome Res. 2007, 7, 2859–2865. [Google Scholar] [CrossRef]
- Panicker, G.; Ye, Y.; Wang, D.; Unger, E.R. Characterization of the Human Cervical Mucous Proteome. Clin. Proteom. 2010, 1, 18–28. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ogata, H.; Morikawa, M.; Iijima, S.; Harada, N.; Yoshida, T.; Brown, W.R.; Inoue, N.; Hamada, Y.; Ishii, H.; et al. Distribution and partial characterisation of IgG Fc binding protein in various mucin producing cells and body fluids. Gut 2002, 51, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Hermida, Y.; Vincenzoni, F.; Milardi, D.; Astorri, A.L.; Urbani, A.; Grande, G.; Azagra, R. Light Microscopy and Proteomic Patterns of Ovulation in Cervical Mucus. Life 2022, 12, 1815. [Google Scholar] [CrossRef] [PubMed]
- Becher, N.; Adams Waldorf, K.; Hein, M.; Uldbjerg, N. The cervical mucus plug: Structured review of the literature. Acta Obstet. Gynecol. Scand. 2009, 88, 502–513. [Google Scholar] [CrossRef]
- Lee, D.-C.; Hassan, S.S.; Romero, R.; Tarca, A.L.; Bhatti, G.; Gervasi, M.T.; Caruso, J.A.; Stemmer, P.M.; Kim, C.J.; Hansen, L.K.; et al. Protein profiling underscores immunological functions of uterine cervical mucus plug in human pregnancy. J. Proteom. 2011, 74, 817–828. [Google Scholar] [CrossRef]
- Vornhagen, J.; Quach, P.; Santana-Ufret, V.; Alishetti, V.; Brokaw, A.; Armistead, B.; Qing Tang, H.; MacDonald, J.W.; Bammler, T.K.; Adams Waldorf, K.M.; et al. Human Cervical Mucus Plugs Exhibit Insufficiencies in Antimicrobial Activity towards Group B Streptococcus. J. Infect. Dis. 2018, 217, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, G.; Gouyer, V.; Rocher, M.; Gottrand, F.; Desseyn, J.-L. A porous cervical mucus plug leads to preterm birth induced by experimental vaginal infection in mice. iScience 2022, 25, 104526. [Google Scholar] [CrossRef]
- Hoffmann, W. Salivary Trefoil Factor Family (TFF) Peptides and Their Roles in Oral and Esophageal Protection: Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 12221. [Google Scholar] [CrossRef]
- Salm, F.; Znalesniak, E.B.; Laskou, A.; Harder, S.; Schlüter, H.; Hoffmann, W. Expression Profiling along the Murine Intestine: Different Mucosal Protection Systems and Alterations in Tff1-Deficient Animals. Int. J. Mol. Sci. 2023, 24, 12684. [Google Scholar] [CrossRef]
- Hein, M.; Valore, E.V.; Helmig, R.B.; Uldbjerg, N.; Ganz, T. Antimicrobial factors in the cervical mucus plug. Am. J. Obstet. Gynecol. 2002, 187, 137–144. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Bae, Y.S.; Choi, M.K.; Lee, W.-J. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends Immunol. 2010, 31, 278–287. [Google Scholar] [CrossRef]
- Goto, Y.; Obata, T.; Kunisawa, J.; Sato, S.; Ivanov, I.I.; Lamichhane, A.; Takeyama, N.; Kamioka, M.; Sakamoto, M.; Matsuki, T.; et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 2014, 345, 1254009. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Diverse Molecular Functions in Mucus Barrier Protection and More: Changing the Paradigm. Int. J. Mol. Sci. 2020, 21, 4535. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Links to Inflammation: A Re-evaluation and New Medical Perspectives. Int. J. Mol. Sci. 2021, 22, 4909. [Google Scholar] [CrossRef] [PubMed]
- Wiede, A.; Hinz, M.; Canzler, E.; Franke, K.; Quednow, C.; Hoffmann, W. Synthesis and localization of the mucin-associated TFF-peptides in the human uterus. Cell Tissue Res. 2001, 303, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.H.; Chaiyarit, P.; Nortvig, H.; Vestergaard, E.M.; Ernst, E.; Nexo, E. Trefoil factor family peptides in human saliva and cyclical cervical mucus. Method evaluation and results on healthy individuals. Clin. Chem. Lab. Med. 2011, 49, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Pluta, K.; McGettigan, P.A.; Reid, C.J.; Browne, J.A.; Irwin, J.A.; Tharmalingam, T.; Corfield, A.; Baird, A.; Loftus, B.J.; Evans, A.C.; et al. Molecular aspects of mucin biosynthesis and mucus formation in the bovine cervix during the periestrous period. Physiol. Genom. 2012, 44, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Bastholm, S.K.; Samson, M.H.; Becher, N.; Hansen, L.K.; Stubbe, P.R.; Chronakis, I.S.; Nexo, E.; Uldbjerg, N. Trefoil factor peptide 3 is positively correlated with the viscoelastic properties of the cervical mucus plug. Acta Obstet. Gynecol. Scand. 2017, 96, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Albert, T.K.; Laubinger, W.; Müller, S.; Hanisch, F.-G.; Kalinski, T.; Meyer, F.; Hoffmann, W. Human intestinal TFF3 forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J. Proteome Res. 2010, 9, 3108–3117. [Google Scholar] [CrossRef]
- Houben, T.; Harder, S.; Schlüter, H.; Kalbacher, H.; Hoffmann, W. Different Forms of TFF3 in the Human Saliva: Heterodimerization with IgG Fc Binding Protein (FCGBP). Int. J. Mol. Sci. 2019, 20, 5000. [Google Scholar] [CrossRef] [PubMed]
- Weste, J.; Houben, T.; Harder, S.; Schlüter, H.; Lücke, E.; Schreiber, J.; Hoffmann, W. Different Molecular Forms of TFF3 in the Human Respiratory Tract: Heterodimerization with IgG Fc Binding Protein (FCGBP) and Proteolytic Cleavage in Bronchial Secretions. Int. J. Mol. Sci. 2022, 23, 15359. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.L.; Diamandis, E.P. A potential role for tissue kallikrein-related peptidases in human cervico-vaginal physiology. Biol. Chem. 2008, 389, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Turinetto, M.; Valsecchi, A.A.; Tuninetti, V.; Scotto, G.; Borella, F.; Valabrega, G. Immunotherapy for Cervical Cancer: Are We Ready for Prime Time? Int. J. Mol. Sci. 2022, 23, 3559. [Google Scholar] [CrossRef]
- Mei, L.; Wang, T.; Chen, Y.; Wei, D.; Zhang, Y.; Cui, T.; Meng, J.; Zhang, X.; Liu, Y.; Ding, L.; et al. Dysbiosis of vaginal microbiota associated with persistent high-risk human papilloma virus infection. J. Transl. Med. 2022, 20, 12. [Google Scholar] [CrossRef]
- Ehrencrona, E. The Role of FCGBP in Mucus: Structure, Processing and Function. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 2021. [Google Scholar]
- Menheniott, T.R.; Kurklu, B.; Giraud, A.S. Gastrokines: Stomach-specific proteins with putative homeostatic and tumor suppressor roles. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G109–G121. [Google Scholar] [CrossRef]
- Schroeder, B.W.; Verhaeghe, C.; Park, S.W.; Nguyenvu, L.T.; Huang, X.; Zhen, G.; Erle, D.J. AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am. J. Respir. Cell. Mol. Biol. 2012, 47, 178–185. [Google Scholar] [CrossRef]
- Gupta, A.; Wodziak, D.; Tun, M.; Bouley, D.M.; Lowe, A.W. Loss of anterior gradient 2 (Agr2) expression results in hyperplasia and defective lineage maturation in the murine stomach. J. Biol. Chem. 2013, 288, 4321–4333. [Google Scholar] [CrossRef]
- Hoffmann, W. TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more. Int. J. Oncol. 2015, 47, 806–816. [Google Scholar] [CrossRef]
- Fattman, C.L.; Schaefer, L.M.; Oury, T.D. Extracellular superoxide dismutase in biology and medicine. Free Radic. Biol. Med. 2003, 35, 236–256. [Google Scholar] [CrossRef]
- Rüdiger, H.; Gabius, H.-J. The Biochemical Basis and Coding Capacity of the Sugar Code. In The Sugar Code, 1st ed.; Gabius, H.-J., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 3–13. [Google Scholar]
- Hoffmann, W.; Jagla, W. Cell type specific expression of secretory TFF peptides: Colocalization with mucins and synthesis in the brain. Int. Rev. Cytol. 2002, 213, 147–181. [Google Scholar]
- Kouznetsova, I.; Gerlach, K.L.; Zahl, C.; Hoffmann, W. Expression analysis of human salivary glands by laser microdissection: Differences between submandibular and labial glands. Cell. Physiol. Biochem. 2010, 26, 375–382. [Google Scholar] [CrossRef]
- Stürmer, R.; Harder, S.; Schlüter, H.; Hoffmann, W. Commercial Porcine Gastric Mucin Preparations, also Used as Artificial Saliva, are a Rich Source for the Lectin TFF2: In Vitro Binding Studies. ChemBioChem 2018, 19, 2598–2608. [Google Scholar] [CrossRef]
- Znalesniak, E.B.; Laskou, A.; Salm, F.; Haupenthal, K.; Harder, S.; Schlüter, H.; Hoffmann, W. The Forms of the Lectin Tff2 Differ in the Murine Stomach and Pancreas: Indications for Different Molecular Functions. Int. J. Mol. Sci. 2023, 24, 7059. [Google Scholar] [CrossRef]
- Madsen, J.; Sorensen, G.L.; Nielsen, O.; Tornoe, I.; Thim, L.; Fenger, C.; Mollenhauer, J.; Holmskov, U. A variant form of human deleted in malignant brain tumor 1 (DMBT1) gene shows increased expression in inflammatory bowel diseases and interacts with dimeric trefoil factor 3 (TFF3). PLoS ONE 2013, 8, e64441. [Google Scholar] [CrossRef] [PubMed]
- Kutteh, W.H.; Mestecky, J.; Wira, C.R. Mucosal Immunity in the Human Female Reproductive Tract. In Mucosal Immunology, 3rd ed.; Mestecky, J., Bienenstock, J., Lamm, M.E., Mayer, L., McGhee, J.R., Strober, W., Eds.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2005; pp. 1631–1646. [Google Scholar]
- Fahrbach, K.M.; Malykhina, O.; Stieh, D.J.; Hope, T.J. Differential binding of IgG and IgA to mucus of the female reproductive tract. PLoS ONE 2013, 8, e76176. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Palaniyandi, S.; Zeng, R.; Tuo, W.; Roopenian, D.C.; Zhu, X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc. Natl. Acad. Sci. USA 2011, 108, 4388–4393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Kannan, A.; Nunn, K.L.; Murphy, M.A.; Subramani, D.B.; Moench, T.; Cone, R.; Lai, S.K. IgG in cervicovaginal mucus traps HSV and prevents vaginal herpes infections. Mucosal Immunol. 2014, 7, 1036–1044. [Google Scholar] [CrossRef]
- Schroeder, H.A.; Nunn, K.L.; Schaefer, A.; Henry, C.E.; Lam, F.; Pauly, M.H.; Whaley, K.J.; Zeitlin, L.; Humphrys, M.S.; Ravel, J.; et al. Herpes simplex virus-binding IgG traps HSV in human cervicovaginal mucus across the menstrual cycle and diverse vaginal microbial composition. Mucosal Immunol. 2018, 11, 1477–1486. [Google Scholar] [CrossRef]
- Schaefer, A.; Yang, B.; Schroeder, H.A.; Harit, D.; Humphry, M.S.; Ravel, J.; Lai, S.K. Broadly neutralizing antibodies consistently trap HIV-1 in fresh cervicovaginal mucus from select individuals. Acta Biomater. 2023, 169, 387–397. [Google Scholar] [CrossRef]
- Schaefer, A.; Lai, S.K. The biophysical principles underpinning muco-trapping functions of antibodies. Hum. Vaccin. Immunother. 2022, 18, 1939605. [Google Scholar] [CrossRef]
- Gupta, S.; Gach, J.S.; Becerra, J.C.; Phan, T.B.; Pudney, J.; Moldoveanu, Z.; Joseph, S.B.; Landucci, G.; Supnet, M.J.; Ping, L.-H.; et al. The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 2013, 9, e1003776. [Google Scholar] [CrossRef]
- Schwartz, J.L. Fcgbp—A Potential Viral Trap in RV144. Open AIDS J. 2014, 8, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Tachibana, M.M.; Tsutsumi, Y. Neglected roles of IgG Fc-binding protein secreted from airway mucin-producing cells in protecting against SARS-CoV-2 infection. Innate Immun. 2021, 27, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Kalinska, M.; Meyer-Hoffert, U.; Kantyka, T.; Potempa, J. Kallikreins—The melting pot of activity and function. Biochimie 2016, 122, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Chen, D.; Chen, X.; Yang, H.; Wei, Y. Overexpression of trefoil factor 3 (TFF3) contributes to the malignant progression in cervical cancer cells. Cancer Cell Int. 2017, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Dieckow, J.; Brandt, W.; Hattermann, K.; Schob, S.; Schulze, U.; Mentlein, R.; Ackermann, P.; Sel, S.; Paulsen, F.P. CXCR4 and CXCR7 Mediate TFF3-Induced Cell Migration Independently from the ERK1/2 Signaling Pathway. Investig. Ophthalmol. Vis. Sci. 2016, 57, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Tian, D.; Chen, J.; Tan, Y.; Cheng, Y.; Ye, L.; Deng, G.; Liu, B.; Yuan, F.; Zhang, S.; et al. FCGBP Is a Prognostic Biomarker and Associated with Immune Infiltration in Glioma. Front. Oncol. 2022, 11, 769033. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, H.; Wu, D.-C.; Podolsky, D.K.; Fishman, M.C. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 1996, 274, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Blaskewicz, C.D.; Pudney, J.; Anderson, D.J. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol. Reprod. 2011, 85, 97–104. [Google Scholar] [CrossRef] [PubMed]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Pigny, P.; Guyonnet-Duperat, V.; Hill, A.S.; Pratt, W.S.; Galiegue-Zouitina, S.; d’Hooge, M.C.; Laine, A.; Van-Seuningen, I.; Degand, P.; Gum, J.R.; et al. Human mucin genes assigned to 11p15.5: Identification and organization of a cluster of genes. Genomics 1996, 38, 340–352. [Google Scholar] [CrossRef]
- Ueda, Y.; Mogami, H.; Kawamura, Y.; Takakura, M.; Inohaya, A.; Yasuda, E.; Matsuzaka, Y.; Chigusa, Y.; Ito, S.; Mandai, M.; et al. Cervical MUC5B and MUC5AC are Barriers to Ascending Pathogens During Pregnancy. J. Clin. Endocrinol. Metab. 2022, 107, 3010–3021. [Google Scholar] [CrossRef]
- Andrews, A.R.; Kakadekar, A.; Greene, D.N.; Khalifa, M.A.; Santiago, V.; Schmidt, R.L. Histologic Findings in Surgical Pathology Specimens from Individuals Taking Masculinizing Hormone Therapy for the Purpose of Gender Transition. Arch. Pathol. Lab. Med. 2022, 146, 766–779. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, S.; Son, M.J.; Kim, G.; Singh, P.; Kim, H.N.; Choi, H.G.; Yoo, H.J.; Ko, Y.B.; Lee, B.S.; et al. Dual oxidase 1 and NADPH oxidase 2 exert favorable effects in cervical cancer patients by activating immune response. BMC Cancer 2019, 19, 1078. [Google Scholar] [CrossRef]
- Lehtoranta, L.; Ala-Jaakkola, R.; Laitila, A.; Maukonen, J. Healthy Vaginal Microbiota and Influence of Probiotics Across the Female Life Span. Front Microbiol. 2022, 13, 819958. [Google Scholar] [CrossRef]
- Baud, A.; Hillion, K.-H.; Plainvert, C.; Tessier, V.; Tazi, A.; Mandelbrot, L.; Poyart, C.; Kennedy, S.P. Microbial diversity in the vaginal microbiota and its link to pregnancy outcomes. Sci. Rep. 2023, 13, 9061. [Google Scholar] [CrossRef]
- Verstraelen, H.; Swidsinski, A. The biofilm in bacterial vaginosis: Implications for epidemiology, diagnosis and treatment: 2018 update. Curr. Opin. Infect. Dis. 2019, 32, 38–42. [Google Scholar] [CrossRef]
- Vandenbroucke, K.; Hans, W.; Van Huysse, J.; Neirynck, S.; Demetter, P.; Remaut, E.; Rottiers, P.; Steidler, L. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology 2004, 127, 502–513. [Google Scholar] [CrossRef]
- Chan, D.; Bennett, P.R.; Lee, Y.S.; Kundu, S.; Teoh, T.G.; Adan, M.; Ahmed, S.; Brown, R.G.; David, A.L.; Lewis, H.V.; et al. Microbial-driven preterm labour involves crosstalk between the innate and adaptive immune response. Nat. Commun. 2022, 13, 975. [Google Scholar] [CrossRef]
- Zhu, X.; Meng, G.; Dickinson, B.L.; Li, X.; Mizoguchi, E.; Miao, L.; Wang, Y.; Robert, C.; Wu, B.; Smith, P.D.; et al. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J. Immunol. 2001, 166, 3266–3276. [Google Scholar] [CrossRef]
- Kao, L.C.; Tulac, S.; Lobo, S.; Imani, B.; Yang, J.P.; Germeyer, A.; Osteen, K.; Taylor, R.N.; Lessey, B.A.; Giudice, L.C. Global gene profiling in human endometrium during the window of implantation. Endocrinology 2002, 143, 2119–2138. [Google Scholar] [CrossRef]
- Borthwick, J.M.; Charnock-Jones, D.S.; Tom, B.D.; Hull, M.L.; Teirney, R.; Phillips, S.C.; Smith, S.K. Determination of the transcript profile of human endometrium. Mol. Hum. Reprod. 2003, 9, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Paiva, P.; Lockhart, M.G.; Girling, J.E.; Olshansky, M.; Woodrow, N.; Marino, J.L.; Hickey, M.; Rogers, P.A. Identification of genes differentially expressed in menstrual breakdown and repair. Mol. Hum. Reprod. 2016, 22, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Henze, D.; Doecke, W.-D.; Hornung, D.; Agueusop, I.; von Ahsen, O.; Machens, K.; Schmitz, A.A.; Gashaw, I. Endometriosis Leads to an Increased Trefoil Factor 3 Concentration in the Peritoneal Cavity but Does Not Alter Systemic Levels. Reprod. Sci. 2017, 24, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Znalesniak, E.B.; Salm, F.; Hoffmann, W. Molecular alterations in the stomach of Tff1-deficient mice: Early steps in antral carcinogenesis. Int. J. Mol. Sci. 2020, 21, 644. [Google Scholar] [CrossRef] [PubMed]
- Thornton, D.J.; Howard, M.; Devine, P.L.; Sheehan, J.K. Methods for separation and deglycosylation of mucin subunits. Anal. Biochem. 1995, 227, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Katsuyama, T. Alternating laminated array of two types of mucin in the human gastric surface mucous layer. Histochem. J. 1992, 24, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Nexoe, A.B.; Pedersen, A.A.; von Huth, S.; Detlefsen, S.; Hansen, P.L.; Holmskov, U. Immunohistochemical Localization of Deleted in Malignant Brain Tumors 1 in Normal Human Tissues. J. Histochem. Cytochem. 2020, 68, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsova, I.; Kalinski, T.; Meyer, F.; Hoffmann, W. Self-renewal of the human gastric epithelium: New insights from expression profiling using laser microdissection. Mol. Biosyst. 2011, 7, 1105–1112. [Google Scholar] [CrossRef] [PubMed]





| Genes Accession No. | Primer No. | Primer Pairs | Nucleotide Positions | Annealing T Size (bp) |
| ACTB NM_001101.5 | MB2931 MB2932 | GGATTCCTATGTGGGCGACGA GCGTACAGGGATAGCACAGC | 234–254 515–496 | 60 °C 282 |
| AGR2 NM_006408.4 | MB3019 MB3020 | AAGGCAGGTGGGTGAGGAAA AGGACAAACTGCTCTGCCAA | 41–60 389–370 | 60 °C 349 |
| A4GNT NM_016161.3 | MB3009 MB3010 | CCGATGCCCTCAAACTCCAC ATTCCCACAAAAAGGGGTGGT | 488–507 824–804 | 60 °C 337 |
| DMBT1 NM_004406.3 | MB3015 MB3016 | TGCGCTGCTCAGGCTA TGATGGTCGGCAATGTGTCT | 1321–1306 1455–1436 | 60 °C 150 |
| DUOX1 NM_175940.3 | MB3033 MB3034 | ACTTCTGGTTGGGGCATGGA TTGCTAAGGTCTCGGGGGTT | 184–203 393–374 | 60 °C 210 |
| DUOX2 NM_014080.4 | MB1577 MB1578 | GATGGTGACCGCTACTGGTT GCCACCACTCCAGAGAGAAG | 1751–1770 2073–2054 | 60 °C 323 |
| FCGBP NM_003890.2 | MB2923 MB2924 | CCTACAGCCACTCTGTGTCG TCCAGCTACTTGCGAACTCC | 1612–1631 1929–1910 | 60 °C 318 |
| FCGRT NM_001136019.3 | MB3351 MB3352 | CTCTCCCTCCTGTACCACCTTACC ATAGCAGGAAGGTGAGCTCCTTGT | 186–209 642–619 | 60 °C 457 |
| FUT2 NM_000511.5 | MB1994 MB1995 | CACTGAGGTGCCTGCCCAACC GCAGCACCGGCAGGGTGATT | 58–78 464–445 | 60 °C 407 |
| GKN1NM_019617.3 | MB2392 MB2393 | CCTCTGTCCACTGCTTTCGT CTGGTTGCAGCAAAGCCATT | 77–96 326–307 | 60 °C 250 |
| GKN2 NM_182536.2 | MB2264 MB2265 | ATCCACATCTTCAAGCCCATA CAACCACTTCCCCCTTATACA | 27–47 572–552 | 60 °C 546 |
| LYZ NM_000239.3 | MB3021 MB3022 | GGGGAATCAGCCTAGCAAACT GGATCACGGACAACCCTCTTT | 144–164 390–370 | 60 °C 247 |
| MUC2 NM_002457.2 | MB2260 MB2261 | CTGAGGGCACCATGAACTAC GGGCCGTTTGATGATACAGT | 14,439–14,420 15,027–15,008 | 60 °C 608 |
| MUC5AC NM_001304359.2 | MB2929 MB2930 | TGCCCCAACATCAGGAACAG AGTGGTCATAGGCTTCGTGC | 1863–1882 2156–2137 | 60 °C 294 |
| MUC5B NM_002458.3 | MB326 MB327 | CTGCGAGACCGAGGTCAACATC TGGGCAGCAGGAGCACGGAG | 17,071–17,092 17,485–17,466 | 60 °C 415 |
| MUC6 NM_005961.3 | MB2927 MB2928 | CACCCGAGTTCCCACATCAG CATGCACCCCTTGAACGTGA | 6894–6913 7155–7136 | 60 °C 262 |
| NOX1 NM_007052.5 | MB2885 MB2886 | GCTCCAAACCACCTCTTGAC CAGATTGCGACACACAGGAAG | 200–219 445–425 | 60 °C 246 |
| CYBB(NOX2) NM_000397.4 | MB3023 MB3024 | TTCTGGTTTGGCTGGGGTTG TCGGGCATTCACACACCATT | 63–82 409–390 | 60 °C 347 |
| NOX5 NM_024505.4 | MB3025 MB3026 | CCCTGAAGGCTGTAGAGGCA CATGGATGAGCAGGGTCAGT | 74–93 315–296 | 60 °C 242 |
| PDIA3 NM_005313.5 | MB3027 MB3028 | GCAAGCAGCGGGTTAGT ACAGGTGTTAGTGTTGGCAGT | 13–29 378–358 | 60 °C 366 |
| PIGR NM_002644.4 | MB3355 MB3356 | GCCAATGACAACATGGGAGC GATTGTCATGGGTGCAGGGA | 2264–2283 2516–2497 | 60 °C 253 |
| SOD3 NM_003102.4 | MB2863 MB2864 | GGTGCAGCTCTCTTTTCAGGA ATCTCCGTGACCTTGGCGTA | 30–50 229–210 | 60 °C 200 |
| TFF2 NM_005423.4 | MB2228 MB2229 | ATAACAGGACGAACTGCGG ATGAAGCTGATAAGGCGAAGT | 252–270 612–592 | 60 °C 361 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskou, A.; Znalesniak, E.B.; Harder, S.; Schlüter, H.; Jechorek, D.; Langer, K.; Strecker, C.; Matthes, C.; Tchaikovski, S.N.; Hoffmann, W. Different Forms of TFF3 in the Human Endocervix, including a Complex with IgG Fc Binding Protein (FCGBP), and Further Aspects of the Cervico-Vaginal Innate Immune Barrier. Int. J. Mol. Sci. 2024, 25, 2287. https://doi.org/10.3390/ijms25042287
Laskou A, Znalesniak EB, Harder S, Schlüter H, Jechorek D, Langer K, Strecker C, Matthes C, Tchaikovski SN, Hoffmann W. Different Forms of TFF3 in the Human Endocervix, including a Complex with IgG Fc Binding Protein (FCGBP), and Further Aspects of the Cervico-Vaginal Innate Immune Barrier. International Journal of Molecular Sciences. 2024; 25(4):2287. https://doi.org/10.3390/ijms25042287
Chicago/Turabian StyleLaskou, Aikaterini, Eva B. Znalesniak, Sönke Harder, Hartmut Schlüter, Dörthe Jechorek, Kathrin Langer, Carina Strecker, Claudia Matthes, Svetlana N. Tchaikovski, and Werner Hoffmann. 2024. "Different Forms of TFF3 in the Human Endocervix, including a Complex with IgG Fc Binding Protein (FCGBP), and Further Aspects of the Cervico-Vaginal Innate Immune Barrier" International Journal of Molecular Sciences 25, no. 4: 2287. https://doi.org/10.3390/ijms25042287
APA StyleLaskou, A., Znalesniak, E. B., Harder, S., Schlüter, H., Jechorek, D., Langer, K., Strecker, C., Matthes, C., Tchaikovski, S. N., & Hoffmann, W. (2024). Different Forms of TFF3 in the Human Endocervix, including a Complex with IgG Fc Binding Protein (FCGBP), and Further Aspects of the Cervico-Vaginal Innate Immune Barrier. International Journal of Molecular Sciences, 25(4), 2287. https://doi.org/10.3390/ijms25042287








