The Thiol Group Reactivity and the Antioxidant Property of Human Serum Albumin Are Controlled by the Joint Action of Fatty Acids and Glucose Binding
Abstract
1. Introduction
2. Results
2.1. The Structural Changes of HSA during Glycation
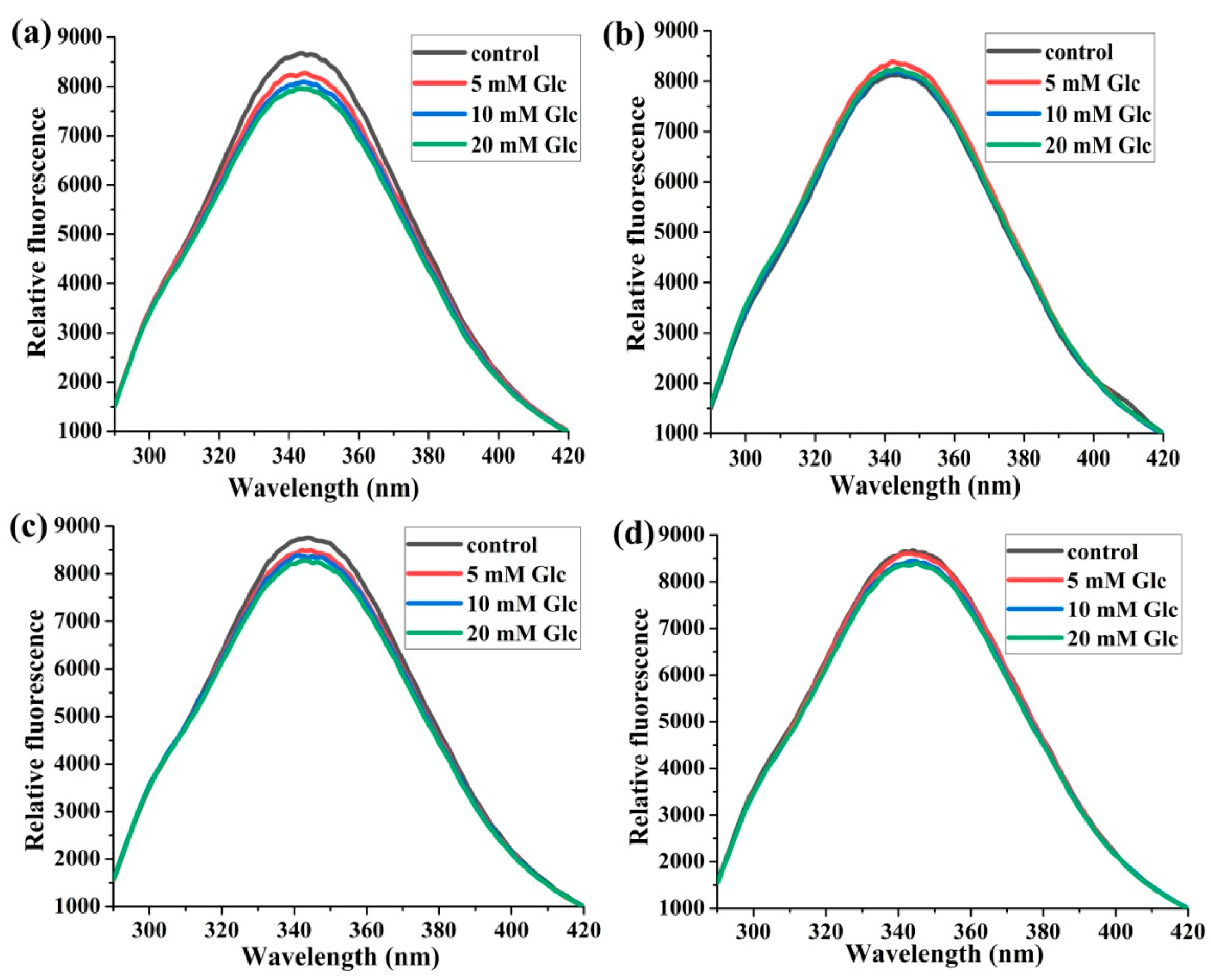
2.1.1. The Effects of Glucose Concentrations on Trp214 Fluorescence
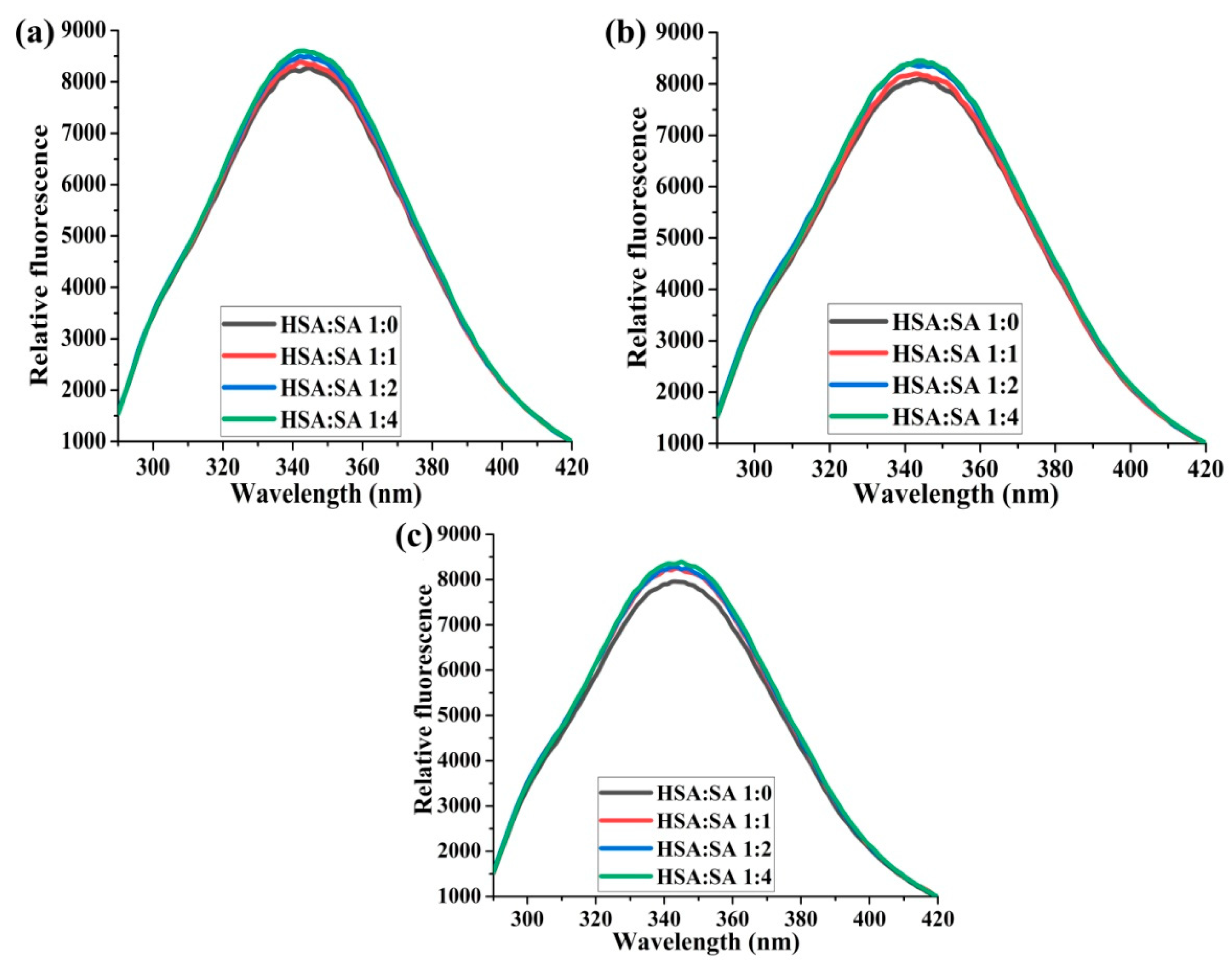
2.1.2. The Effects of the Molar Ratios of HSA:SA on Trp214 Fluorescence
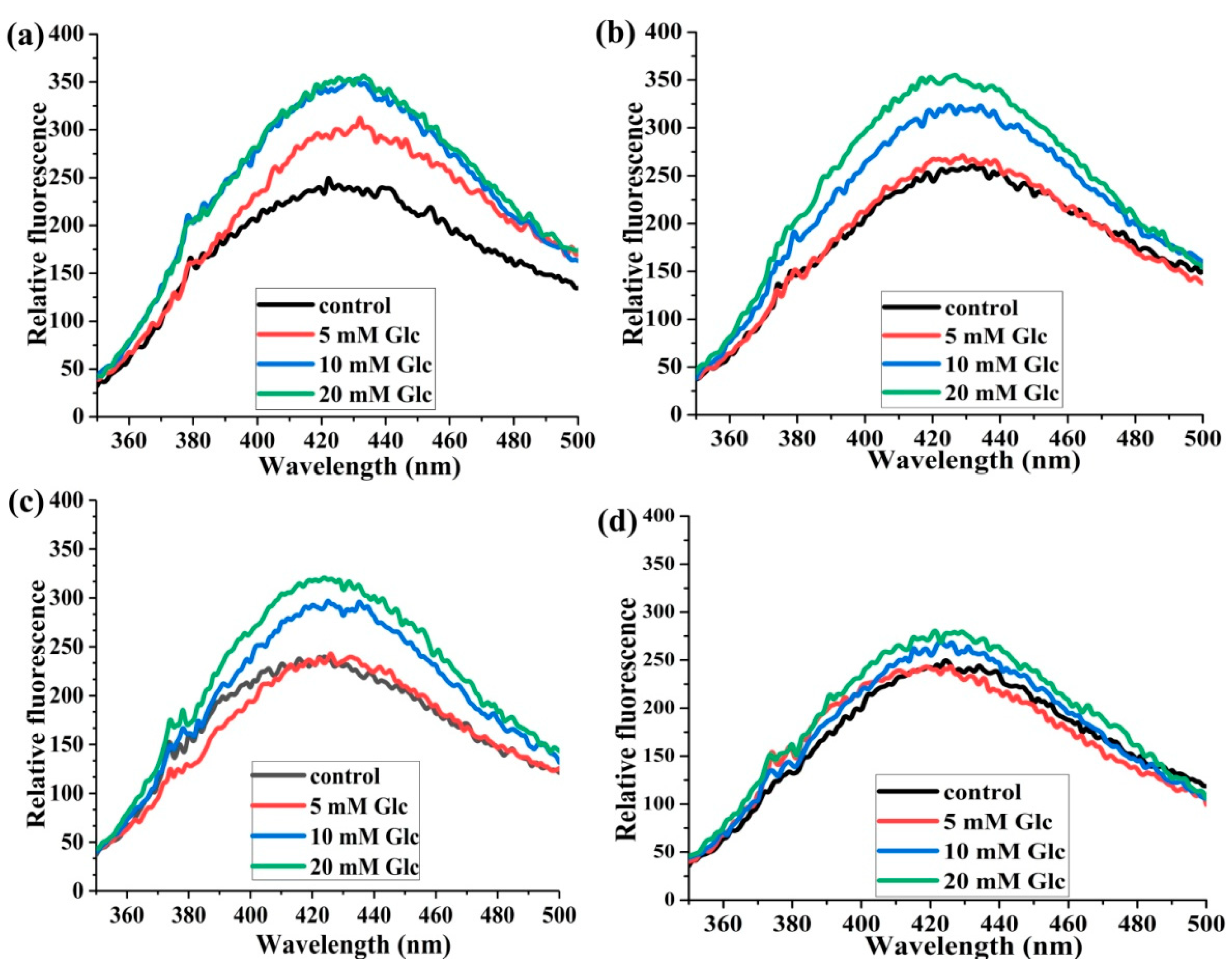
2.1.3. The Effects of Glucose Concentration on the Formation of AGEs
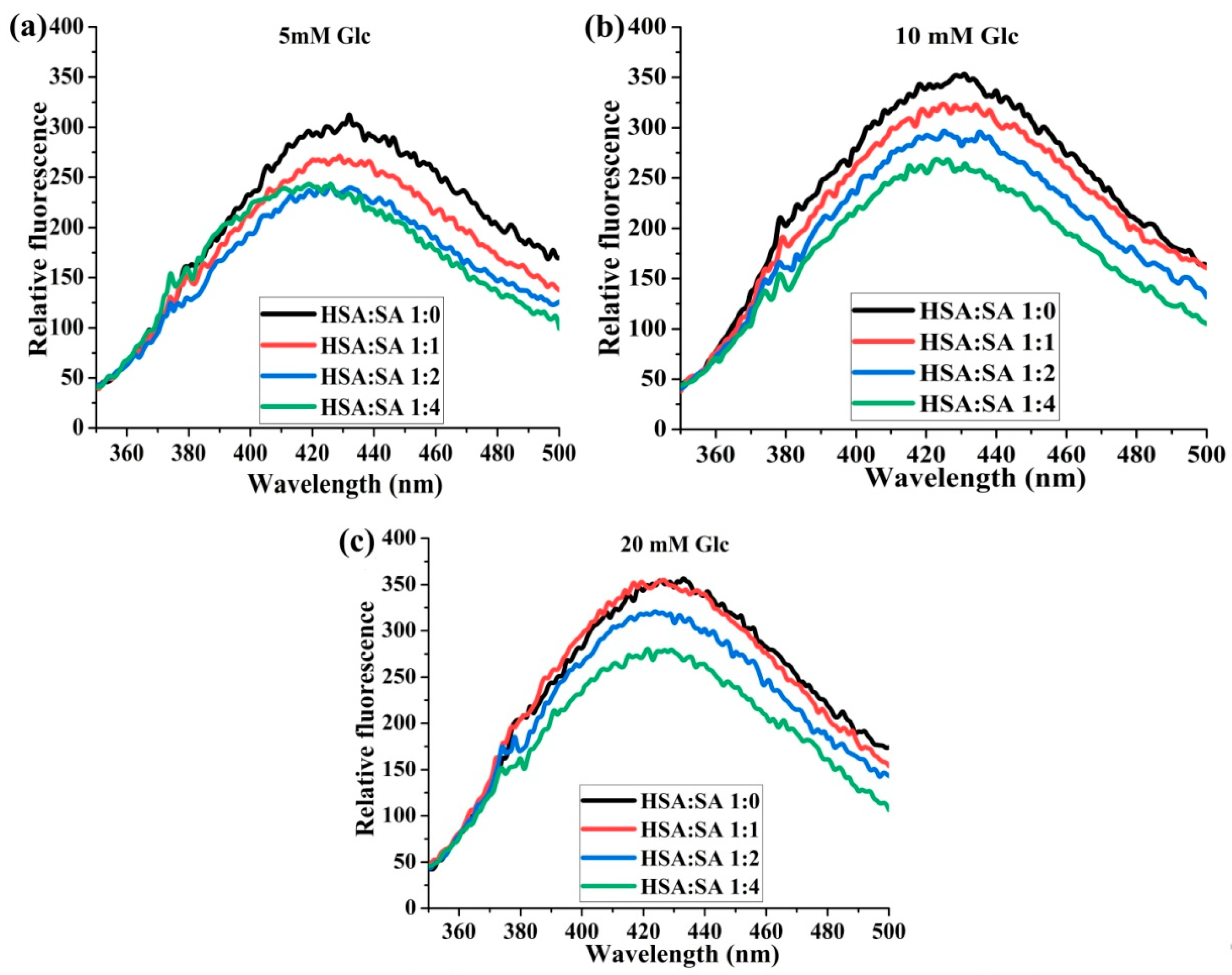
2.1.4. The Effects of the Molar Ratios of HSA:SA on the Formation of AGEs
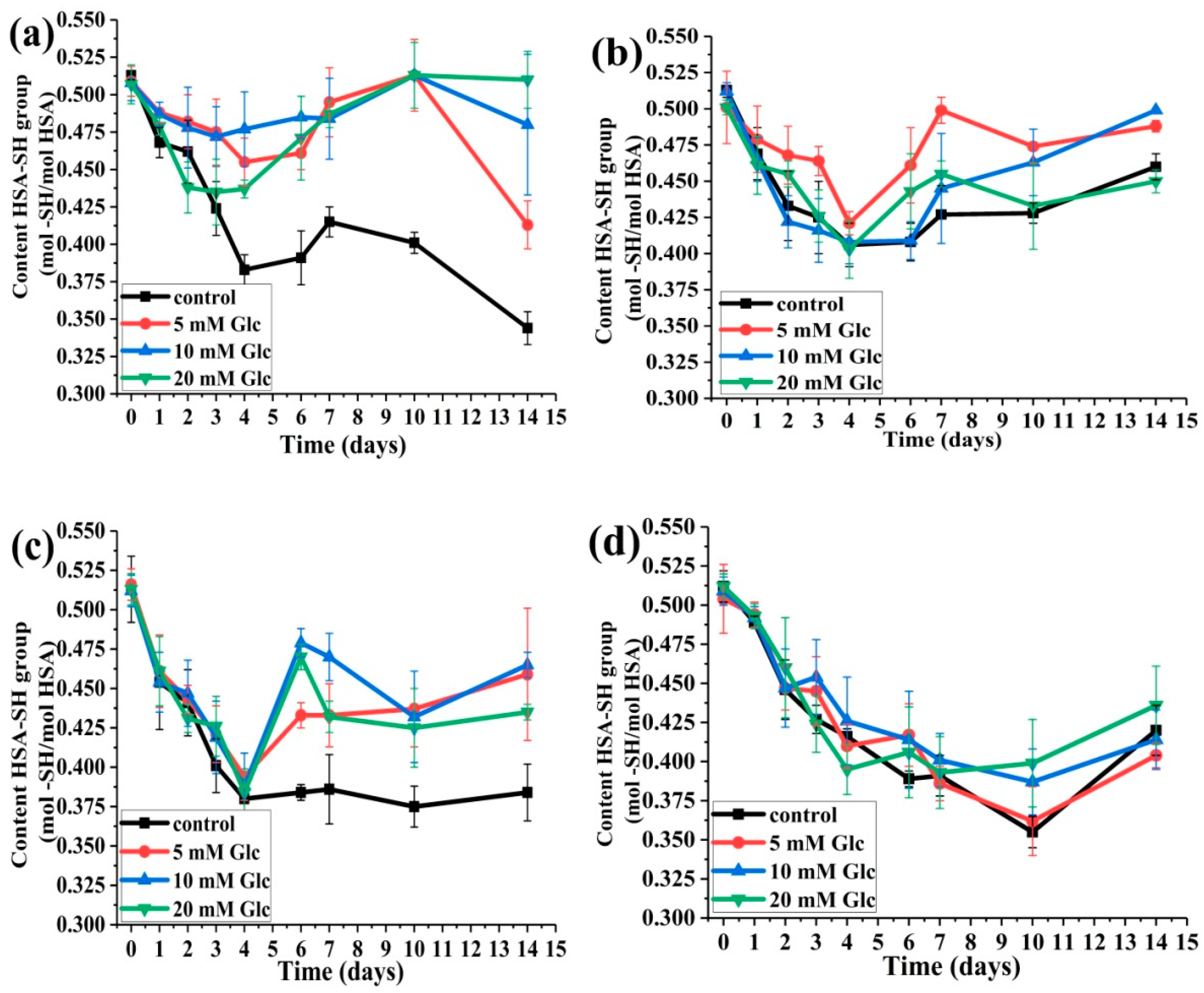
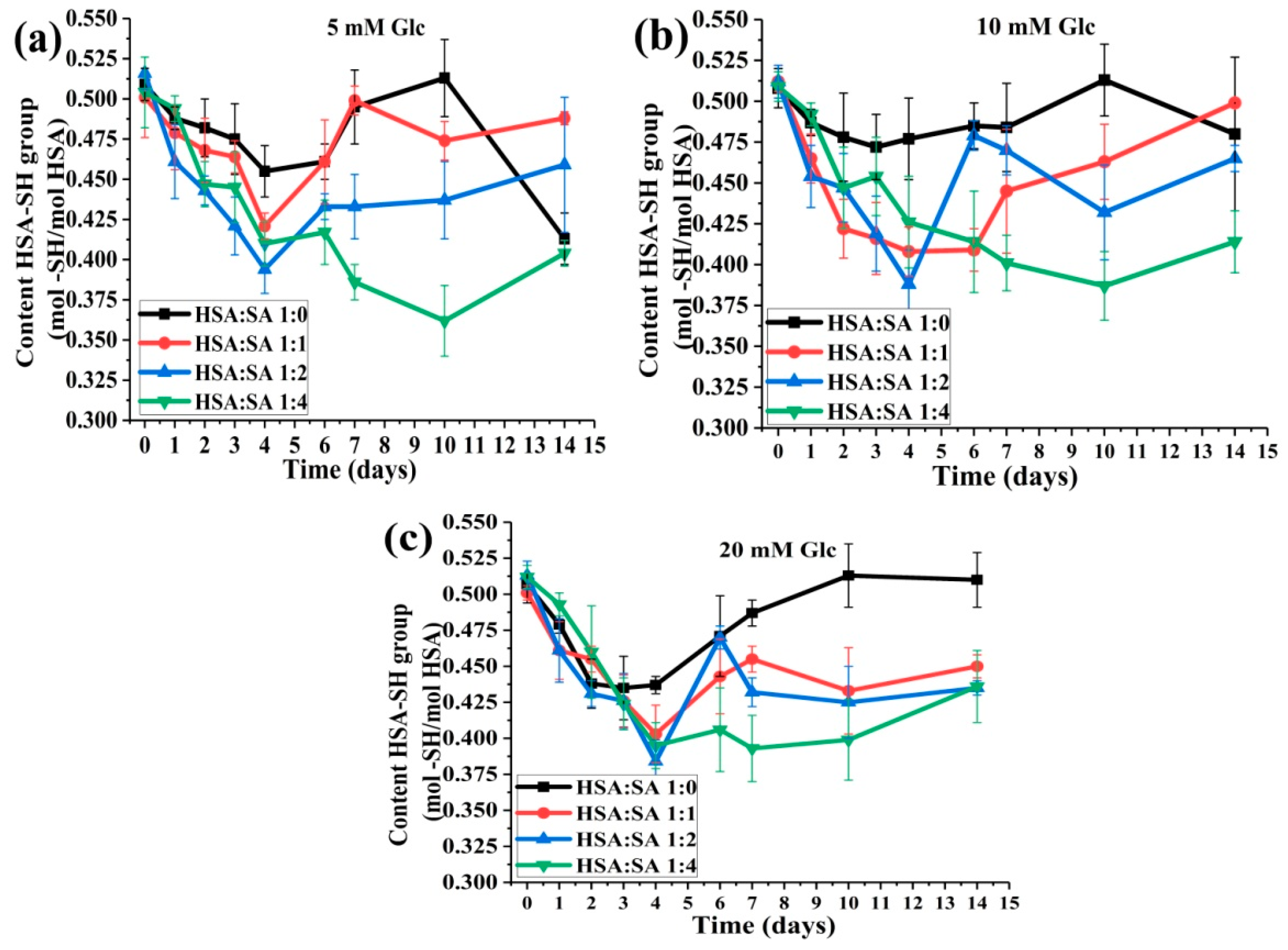
2.2. Changes in HSA-SH Content during 14 Days of Incubation
2.2.1. The Effects of Glucose Concentration on HSA-SH Content
2.2.2. The Effects of the Molar Ratios of HSA:SA on HSA:SH Content
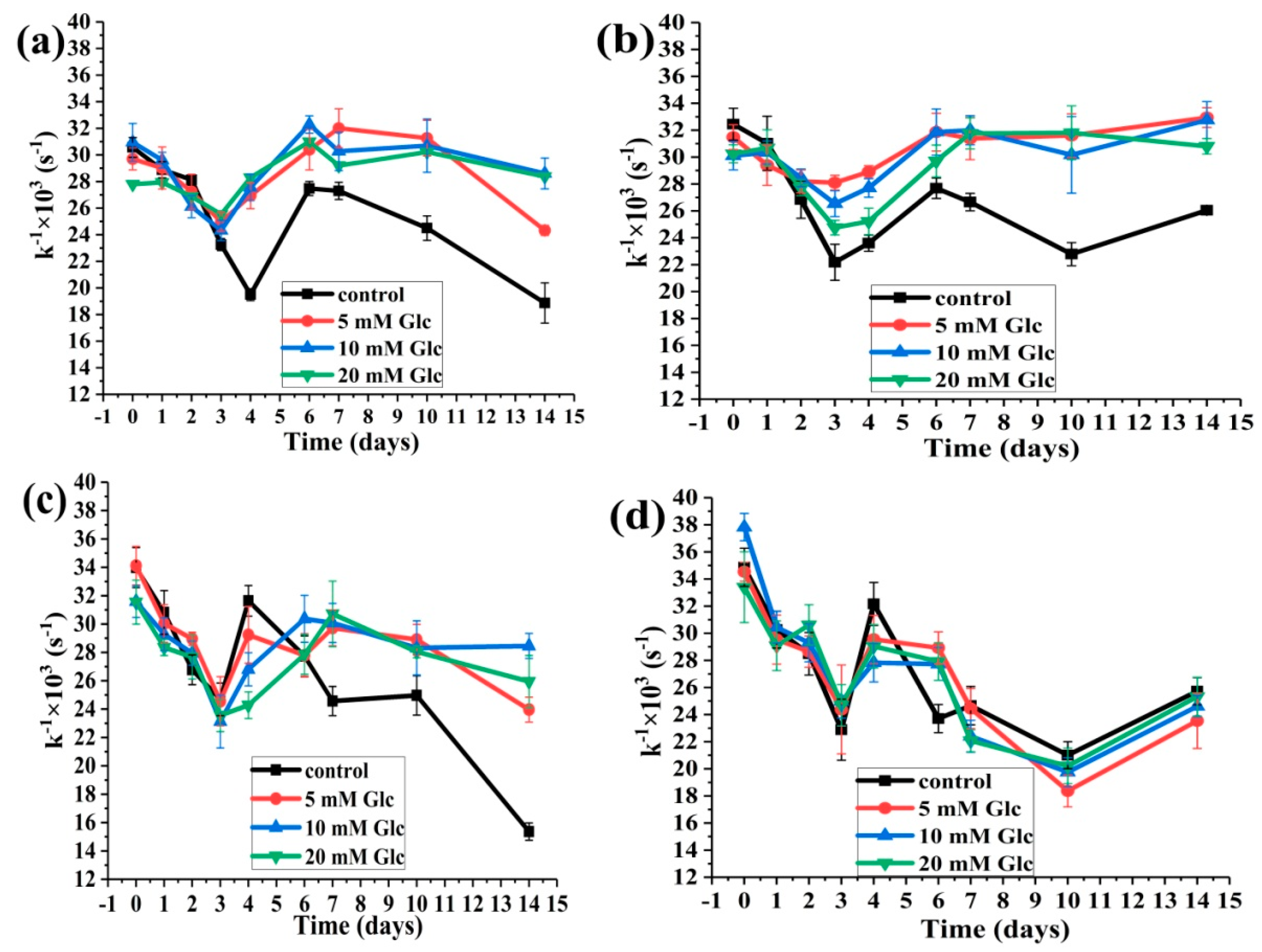
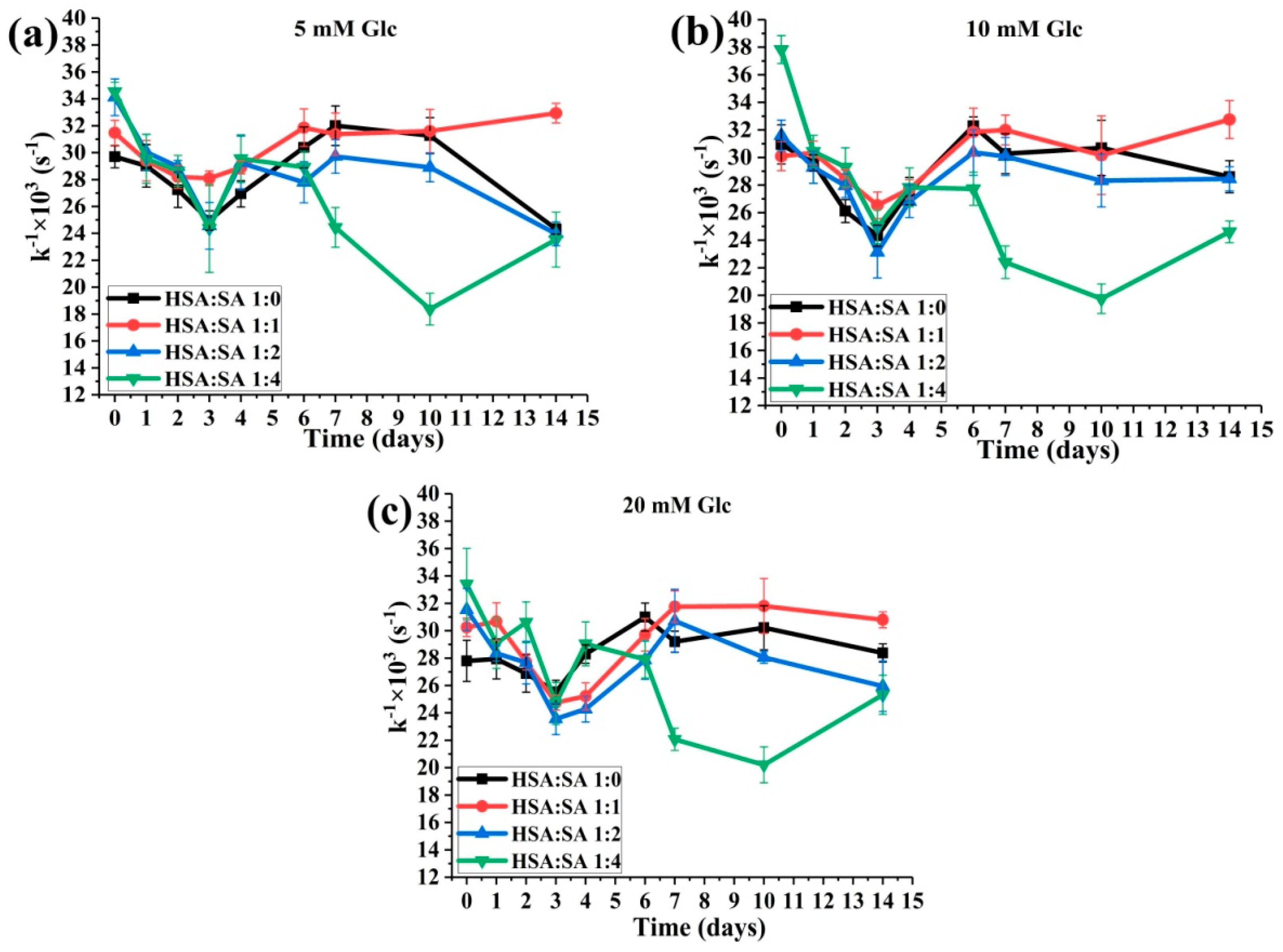
2.3. Changes in HSA-SH Reactivity during Glycation
2.3.1. The Effects of Glucose Concentration on the HSA-SH Reactivity
2.3.2. The Effects of the Molar Ratios of HSA:SA on the HSA-SH Reactivity
2.4. The Influence of Glucose on the Quenching Constant of HSA Trp214 with SA
3. Discussion
4. Materials and Methods
4.1. Chemicals and Instrumentation
4.2. Preparation of FA-Free HSA
4.3. Preparation of Highly Reduced HSA
4.4. Preparation of FA-Bound HSA
4.5. In Vitro Glycation of SA-Bound and SA-Free HSA
4.6. Quantification of HSA and HSA-SH Group Content
4.7. Determination of the Pseudo-First-Order Constant for the Reaction of HSA-SH with DTNB
4.8. Fluorometric Determination of HSA Structure Changes during In Vitro Glycation
4.9. Determination of the Quenching Constant of HSA Trp214 with SA
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, V.; Heath, R.J. Structural and Biochemical Features of Human Serum Albumin Essential for Eukaryotic Cell Culture. Int. J. Mol. Sci. 2021, 22, 8411. [Google Scholar] [CrossRef]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Merlot, A.M.; Kalinowsk, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin—More than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef]
- Pavićević, I.D.; Jovanović, V.B.; Takić, M.M.; Penezić, A.Z.; Aćimović, J.M.; Mandić, L.M. Fatty acids binding to human serum albumin: Changes of reactivity and glycation level of Cysteine-34 free thiol group with methylglyoxal. Chem. Biol. Interact. 2014, 224, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Penezić, A.Z.; Aćimović, J.M.; Pavićević, I.D.; Jovanović, V.B.; Takić, M.; Mandić, L.M. The interplay between copper (II), human serum albumin, fatty acids, and carbonylating agent interferes with Cys 34 thiol reactivity and copper binding. J. Biol. Inorg. Chem. 2019, 24, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Takić, M.M.; Jovanović, V.B.; Pavićević, I.D.; Uzelac, T.N.; Aćimović, J.M.; Ristić-Medić, D.K.; Mandić, L.M. Binding of enterolactone and enterodiol to human serum albumin: Increase of cysteine-34 thiol group reactivity. Food Funct. 2016, 7, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Uzelac, T.N.; Nikolić-Kokić, A.L.; Spasić, S.D.; Mačvanin, M.T.; Nikolić, M.R.; Mandić, L.M.; Jovanović, V.B. Opposite clozapine and ziprasidone effects on the reactivity of plasma albumin SH-group are the consequence of their different binding properties dependent on protein fatty acids content. Chem. Biol. Interact. 2019, 311, 108787. [Google Scholar] [CrossRef] [PubMed]
- Taverna, M.; Marie, A.L.; Mira, J.P.; Guidet, B. Specific antioxidant properties of human serum albumin. Ann. Intensive Care 2013, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Turell, L.; Botti, H.; Carballal, S.; Radi, R.; Alvarez, B. Sulfenic acid-a key intermediate in albumin thiol oxidation. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 3384–3392. [Google Scholar] [CrossRef]
- De Simone, G.; di Masi, A.; Ascenzi, P. Serum Albumin: A Multifaced Enzyme. Int. J. Mol. Sci. 2021, 22, 10086. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; Fasano, M. Allostery in a monomeric protein: The case of human serum albumin. Biophys. Chem. 2010, 148, 16–22. [Google Scholar] [CrossRef]
- Leboffe, L.; di Masi, A.; Polticelli, F.; Trezza, V.; Ascenzi, P. Structural basis of drug recognition by human serum albumin. Curr. Med. Chem. 2020, 27, 4907–4931. [Google Scholar] [CrossRef]
- Bhattacharya, A.A.; Grune, T.; Curry, S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J. Mol. Biol. 2000, 303, 721–732. [Google Scholar] [CrossRef]
- Curry, S. Plasma albumin as a fatty acid carrier. Adv. Mol. Cell Biol. 2004, 33, 29–46. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Shi, X.; Luo, Z.; Lin, D.; Huang, M. Structural mechanism of ring-opening reaction of glucose by human serum albumin. J. Biol. Chem. 2013, 288, 15980–15987. [Google Scholar] [CrossRef] [PubMed]
- Pongprayoon, P.; Mori, T. The critical role of dimer formation in monosaccharides binding to human serum albumin. Phys. Chem. Chem. Phys. 2018, 20, 3249–3257. [Google Scholar] [CrossRef] [PubMed]
- Awang, T.; Wiriyatanakorn, N.; Saparpakorn, P.; Japrung, D.; Pongprayoon, P. Understanding the effects of two bound glucose in Sudlow site I on structure and function of human serum albumin: Theoretical studies. J. Biomol. Struc. Dynam. 2017, 35, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Bonanata, J.; Turell, L.; Antmann, L.; Ferrer-Sueta, G.; Botasini, S.; Méndez, E.; Alvarez, B.; Coitiño, E.L. The thiol of human serum albumin: Acidity, microenvironment and mechanistic insights on its oxidation to sulfenic acid. Free. Radic. Biol. Med. 2017, 108, 952–962. [Google Scholar] [CrossRef]
- Torres, M.J.; Turell, L.; Botti, H.; Antmann, L.; Carballal, S.; Ferrer-Sueta, G.; Radi, R.; Alvarez, B. Modulation of the reactivity of the thiol of human serum albumin and its sulfenic derivative by fatty acids. Arch. Biochem. Biophys. 2012, 521, 102–110. [Google Scholar] [CrossRef]
- Blache, D.; Bourdon, E.; Salloignon, P.; Lucchi, G.; Ducoroy, P.; Petit, J.M.; Verges, B.; Lagrost, L. Glycated albumin with loss of fatty acid binding capacity contributes to enhanced arachidonate oxygenation and platelet hyperactivity: Relevance in patients with type 2 diabetes. Diabetes 2015, 64, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, E.; Inagaki, M.; Kurita, O.; Inoue, T. Kinetics of fatty acid binding ability of glycated human serum albumin. J. Biosci. 2005, 30, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Lautenslager, G.T.; Shearman, C.W.; Hud, E.; Cohen, M.P. Effects of nonenzymatic glycation and fatty acids on functional properties of human albumin. Metabolism 2011, 60, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Szkudlarek, A.; Pozycka, J.; Kulig, K.; Owczarzy, A.; Rogóz, W.; Maciazek-Jurczyk, M. Changes in glycated human serum albumin binding affinity for losartan in the presence of fatty acids in vitro spectroscopic analysis. Molecules 2022, 27, 401. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Cate, S.A.; Dominguez, M.; Osborn, W.; Özpolat, T.; Konkle, B.A.; Chen, J.; López, J.A. Cysteine disulfides (Cys-ss-X) as sensitive plasma biomarkers of oxidative stress. Sci. Rep. 2019, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Paar, M.; Cvirn, G.; Hoerl, G.; Reibnegger, G.; Sourij, H.; Sourij, C.; Kojzar, H.; Oettl, K. Albumin of people with diabetes mellitus is more reduced at low HbA1c. Int. J. Mol. Sci. 2023, 24, 16256. [Google Scholar] [CrossRef]
- Szkudlarek, A. Effect of palmitic acid on tertiary structure of glycated human serum albumin. Processes 2023, 11, 2746. [Google Scholar] [CrossRef]
- Rao, S.S.; Disraeli, P.; McGregor, T. Impaired glucose tolerance and impaired fasting glucose. Am. Fam. Physician 2004, 69, 1961–1968. [Google Scholar] [PubMed]
- Tupe, R.S.; Agte, V.V. Role of zinc along with ascorbic acid and folic acid during long-term in vitro albumin glycation. Br. J. Nutr. 2010, 103, 370–377. [Google Scholar] [CrossRef]
- Shetty, S.S.; Kumari, S. Fatty acids and their role in type-2 diabetes. Exp. Ther. Med. 2021, 22, 706. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Xiao, A.; Mei, S.; Xie, Y. Influence of human serum albumin glycation on the binding affinities for natural flavonoids. Open Chem. 2019, 17, 806–812. [Google Scholar] [CrossRef]
- Anguizola, J.; Matsuda, R.; Barnaby, O.S.; Hoy, K.S.; Wa, C.; DeBolt, E.; Koke, M.; Hage, D.S. Glycation of human serum albumin. Clin. Chim. Acta 2013, 425, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Setoyama, H.; Tanaka, M.; Nagumo, K.; Naoe, H.; Watanabe, T.; Yoshimaru, Y.; Tateyama, M.; Sasaki, M.; Watanabe, H.; Otagiri, M.; et al. Oral branched-chain amino acid granules improve structure and function of human serum albumin in cirrhotic patients. J. Gastroenterol. 2017, 52, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Oettl, K.; Marsche, G. Redox state of human serum albumin in terms of cysteine-34 in health and disease. Methods Enzymol. 2010, 474, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Mera, K.; Anraku, M.; Kitamura, K.; Nakajou, K.; Maruyama, T.; Otagiri, M. The structure and function of oxidized albumin in hemodialysis patients: Its role in elevated oxidative stress via neutrophil burst. Biochem. Biophys. Res. Commun. 2005, 334, 1322–1328. [Google Scholar] [CrossRef]
- Paar, M.; Seifried, K.; Cvirn, G.; Buchmann, A.; Khalil, M.; Oettl, K. Redox state of human serum albumin in multiple sclerosis: A pilot study. Int. J. Mol. Sci. 2022, 23, 15806. [Google Scholar] [CrossRef]
- Sobczaka, A.I.S.; Pitt, S.J.; Smith, T.K.; Ajjan, R.A.; Stewart, A.J. Lipidomic profiling of plasma free fatty acids in type-1 diabetes highlights specific changes in lipid metabolism. BBA Mol. Cell Biol. Lipids 2021, 1866, 158823. [Google Scholar] [CrossRef]
- Jovanovic, V.B.; Pavicevic, I.D.; Takic, M.M.; Penezic-Romanjuk, A.Z.; Acimovic, J.M.; Mandic, L.M. The influence of fatty acids on determination of human serum albumin thiol group. Anal. Biochem. 2014, 448, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, V.B.; Penezic-Romanjuk, A.Z.; Pavicevic, I.D.; Acimovic, J.M.; Mandic, L.M. Improving the reliability of human serum albumin-thiol group determination. Anal. Biochem. 2013, 439, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Szkudlarek, A.; Maciazek-Jurczyk, M.; Chudzik, M.; Rownicka-Zubik, J.; Sulkowska, A. Alteration of human serum albumin tertiary structure induced by glycation. Spectroscopic study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 153, 560–565. [Google Scholar] [CrossRef]
- Christodoulou, J.; Sadler, P.J.; Tucker, A. 1H NMR of albumin in human blood plasma: Drug binding and redox reactions at Cys34. FEBS Lett. 1995, 376, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.F. Removal of fatty acids from serum albumin by charcoal treatment. J. Biol. Chem. 1967, 242, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.W.; Hogden, C.G. The biuret reaction in the determination of serum proteins: II Measurments made by the Kjeldahl procedure. J. Biol. Chem. 1940, 135, 727–731. [Google Scholar] [CrossRef]
- Bulaj, G.; Kortemme, T.; Goldenberg, D.P. Ionization-reactivity relationships for cysteine thiols in polypeptides. Biochemistry 1998, 37, 8965–8972. [Google Scholar] [CrossRef]
- Lakovicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006. [Google Scholar]
| Ksv × 104 (mol −1 × L) | ||||
|---|---|---|---|---|
| Control | HSA + 5 mM Glc | HSA + 10 mM Glc | HSA + 20 mM Glc | |
| Experiment 1 | 1.28 | 1.98 | 1.60 | 1.42 |
| Experiment 2 | 1.24 | 1.83 | 1.65 | 1.14 |
| Mean ± SD | 1.26 ± 0.03 | 1.91 ± 0.10 * | 1.63 ± 0.04 ** | 1.28 ± 0.19 + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzelac, T.; Smiljanić, K.; Takić, M.; Šarac, I.; Oggiano, G.; Nikolić, M.; Jovanović, V. The Thiol Group Reactivity and the Antioxidant Property of Human Serum Albumin Are Controlled by the Joint Action of Fatty Acids and Glucose Binding. Int. J. Mol. Sci. 2024, 25, 2335. https://doi.org/10.3390/ijms25042335
Uzelac T, Smiljanić K, Takić M, Šarac I, Oggiano G, Nikolić M, Jovanović V. The Thiol Group Reactivity and the Antioxidant Property of Human Serum Albumin Are Controlled by the Joint Action of Fatty Acids and Glucose Binding. International Journal of Molecular Sciences. 2024; 25(4):2335. https://doi.org/10.3390/ijms25042335
Chicago/Turabian StyleUzelac, Tamara, Katarina Smiljanić, Marija Takić, Ivana Šarac, Gordana Oggiano, Milan Nikolić, and Vesna Jovanović. 2024. "The Thiol Group Reactivity and the Antioxidant Property of Human Serum Albumin Are Controlled by the Joint Action of Fatty Acids and Glucose Binding" International Journal of Molecular Sciences 25, no. 4: 2335. https://doi.org/10.3390/ijms25042335
APA StyleUzelac, T., Smiljanić, K., Takić, M., Šarac, I., Oggiano, G., Nikolić, M., & Jovanović, V. (2024). The Thiol Group Reactivity and the Antioxidant Property of Human Serum Albumin Are Controlled by the Joint Action of Fatty Acids and Glucose Binding. International Journal of Molecular Sciences, 25(4), 2335. https://doi.org/10.3390/ijms25042335







